Abstract
Oxidative stress plays a pivotal role in the development of diabetes complications, both microvascular and cardiovascular. The metabolic abnormalities of diabetes cause mitochondrial superoxide overproduction in endothelial cells of both large and small vessels, and also in the myocardium. This increased superoxide production causes the activation of five major pathways involved in the pathogenesis of complications: polyol pathway flux, increased formation of advanced glycation end-products (AGEs), increased expression of the receptor for AGEs and its activating ligands, activation of protein kinase C (PKC) isoforms, and overactivity of the hexosamine pathway. It also directly inactivates two critical antiatherosclerotic enzymes, eNOS and prostacyclin synthase. Through these pathways, increased intracellular ROS cause defective angiogenesis in response to ischemia, activate a number of pro-inflammatory pathways, and cause long-lasting epigenetic changes which drive persistent expression of proinflammatory genes after glycemia is normalized (‘hyperglycemic memory’). Atherosclerosis and cardiomyopathy in type 2 diabetes are caused in part by pathway-selective insulin resistance, which increases mitochondrial ROS production from free fatty acids and by inactivation of anti-atherosclerosis enzymes by ROS. Overexpression of superoxide dismutase in transgenic diabetic mice prevents diabetic retinopathy, nephropathy, and cardiomyopathy. The aim of this review is to highlight advances in understanding the role of metabolite-generated ROS in the development of diabetic complications.
Keywords: Hyperglycemia, Mitochondria, Metabolic Memory, Epigenetic modifications, insulin resistance
Introduction
All forms of diabetes are characterized by hyperglycemia, a relative or absolute lack of insulin action, pathway-selective insulin resistance, and the development of diabetes-specific pathology in the retina, renal glomerulus, and peripheral nerve. Diabetes is also associated with accelerated atherosclerotic disease affecting arteries that supply the heart, brain, and lower extremities. In addition, diabetic cardiomyopathy is a major diabetic complication.
Diabetic accelerated lower extremity arterial disease in conjunction with neuropathy accounts for 60% of all nontraumatic amputations in the United States [1]. Diabetes and impaired glucose tolerance increase cardiovascular disease risk three to eight-fold. Thus, over 30% of patients hospitalized with acute myocardial infarction have diabetes, and 35% have impaired glucose tolerance[2]. Finally, new blood vessel growth in response to ischemia is impaired in diabetes, resulting in decreased collateral vessel formation in ischemic hearts, and in non-healing foot ulcers [3]. Hyperglycemia causes tissue damage through five major mechanisms: Increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end-products (AGEs), increased expression of the receptor for advanced glycation end products and its activating ligands, activation of protein kinase C (PKC) isoforms and overactivity of the hexosamine pathway. Several lines of evidence indicate that all five mechanisms are activated by a single upstream event [4]: mitochondrial overproduction of reactive oxygen species. In the diabetic microvasculature, this is a consequence of intracellular hyperglycemia. In the diabetic macrovascular and in the heart, in contrast, this appears to be a consequence of increased oxidation of fatty acids, resulting in part from pathway-specific insulin resistance.
Role of hyperglycemia in microvascular complications
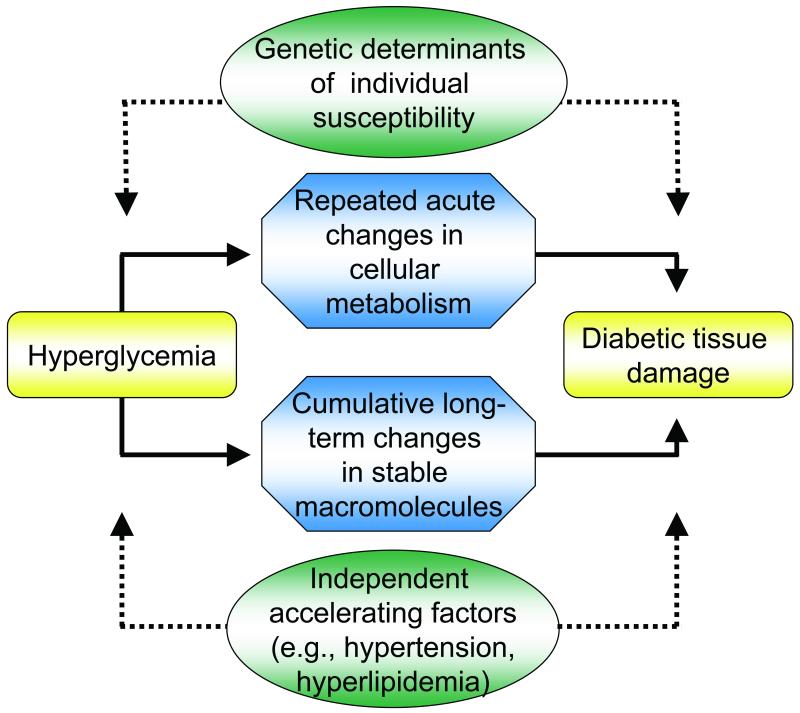
Overall, diabetic microvascular complications are caused by prolonged exposure to high glucose levels (Fig 1). The extent of diabetic tissue damage is also determined by genetic determinants of individual susceptibility and, as with atherosclerosis, by the presence of such independent accelerating factors as hypertension and dyslipidemia. This role of hyperglycemia has been established by large-scale prospective studies for both type 1 and type 2 diabetes, the Diabetes Control and Complications Trial (DCCT/EDIC) [5] and the UK Prospective Diabetes Study (UKPDS) [6]. Similar data have been reported by the Steno-2 study [7]. However, further analysis of the DCCT data shows that while intensive therapy reduced the risk of sustained retinopathy progression by 73% compared to standard treatment, HbA1C and duration of diabetes (glycemic exposure) explained only about ~ 11% of the variation in retinopathy risk for the entire study population, suggesting that the remaining 89% of the variation in risk is presumably explained by aspects of glycemia not captured by HbA1c [8].
Fig.1.
General features of hyperglycemia-induced tissue damage.
Reprinted with permission from the American Diabetes Association via the Copyright Clearance Center from Brownlee [4].
Since every cell in the body of people with diabetes is exposed to abnormally high glucose concentrations, why does hyperglycemia selectively damage some cell types, and not others? The targeting of specific cell types by generalized hyperglycemia reflects the failure of those cells to down-regulate their uptake of glucose when extracellular glucose concentrations are elevated. Cells that are not directly susceptible to direct hyperglycemic damage show an inverse relationship between extracellular glucose concentrations and glucose transport. In contrast, vascular endothelial cells, a major target of hyperglycemic damage, show no significant change in glucose transport rate when glucose concentration is elevated, resulting in intracellular hyperglycemia [9].
Mechanisms of hyperglycemia-induced damage
The vast majority of publications about the mechanisms underlying hyperglycemia-induced diabetic vascular damage focus on five major mechanisms: increased flux of glucose and other sugars through the polyol pathway, increased intracellular formation of advanced glycation end-products (AGEs), increased expression of the receptor for advanced glycation end products and its activating ligands, activation of protein kinase C (PKC) isoforms, and overactivity of the hexosamine pathway. However, the results of clinical studies in which only one of these pathways is blocked have been disappointing [10,11].
This led to the hypothesis in 2000 that all five mechanisms are activated by a single upstream event: mitochondrial overproduction of the reactive oxygen species (ROS).
Increased polyol pathway flux
The polyol pathway is based on a family of aldo-keto reductase enzymes which can utilize as substrates a wide variety of carbonyl compounds, and reduce these by nicotinic acid adenine dinucleotide phosphate (NADPH) to their respective sugar alcohols (polyols). It was first thought that glucose is converted to sorbitol by the enzyme aldose reductase, with sorbitol then oxidized to fructose by the enzyme sorbitol dehydrogenase (SDH), with NAD+ as a cofactor.
Aldose reductase is found in tissues such as nerve, retina, lens, glomerulus and vascular cells [11]. In many of these tissues, glucose uptake is mediated by insulin independent GLUTs; intracellular glucose concentrations therefore rise in parallel with hyperglycemia. Several mechanisms have been proposed to explain how hyperglycemia-induced increases in polyol pathway flux could damage the tissues involved. The most cited is an increase in redox stress due to the consumption of NADPH. Since NADPH is a cofactor required to regenerate reduced glutathione (GSH), and GSH is an important scavenger of reactive oxygen species (ROS), this could induce or exacerbate intracellular oxidative stress. Indeed, overexpression of human aldose ruductase increased atherosclerosis in diabetic mice and reduced the expression of genes that regulate regeneration of glutathione [12]. Reduced GSH is also depleted in the lens of transgenic mice that overexpress aldose reductase and in diabetic rat lens compared to non-diabetic lens [13-16]. It has been also demonstrated that decreased glutathiolation of cellular proteins is related to decreased NO availability in diabetic rats which would decrease GSNO. Restoring the NO levels in diabetic animals increases glutathiolation of cellular proteins, inhibits aldose ruductase activity and prevents sorbitol accumulation.
In diabetic vascular cells glucose does not appear to be the substrate for aldose reductase, since the Km of aldose reductase for glucose in 100mM [17], while the intracellular concentration of glucose in diabetic endothelial cells is 30 nmols/mg protein [18].
Although the aldehyde form of glucose is a much better substrate for aldose reductase than are the ring forms [19], with a Km of 0.66 μM for the aldehyde form of glucose, the aldehyde form of glucose represents only 0.002% of the total glucose [20]. Thus, a substrate concentration required for effective catalysis to occur may not be common. Glycolytic metabolites of glucose such as glyceraldehyde 3-phosphate, for which aldose reductase has a much higher affinity, may be the physiologically relevant substrate.
Increased intracellular AGE formation
Advanced glycation end products (AGEs) are formed by the non-enzymatic reaction of glucose and other glycating compounds derived both from glucose and from increased fatty acid oxidation in arterial endothelial cells and most likely heart (e.g. dicarbonyls such as 3-deoxyglucosone, methylglyoxal and glyoxal) with proteins [21,22]. In diabetes, AGEs are found in increased amounts in extracellular matrix [23-27]. Intracellular production of AGE precursors can damage cells by three general mechanisms. Firstly, intracellular proteins modified by AGEs have altered function. Secondly, extracellular matrix components modified by AGE precursors interact abnormally with other matrix components and with matrix receptors (integrins) that are expressed on the surface of cells. Finally, plasma proteins modified by AGE precursors bind to AGE receptors on cells such as macrophages, vascular endothelial cells and vascular smooth muscle cells. RAGE binding induces the production of ROS, which in turn activates the pleiotropic transcription factor, nuclear factor kappa B (NFκB), causing multiple pathological changes in gene expression [28]. In addition, increased metabolite-induced methylglyoxal in arterial endothelial cells and in mouse kidney endothelial cells increases methylglyoxal modification of the corepressor mSin3A. Methylglyoxal modification of mSin3A results in increased recruitment of O-GlcNAc-transferase, with consequent increased modification of Sp3 by O-linked N-Acetylglucosamine. This modification of Sp3 causes decreased binding to a glucose-responsive GC-box in the angiopoietin-2 (Ang-2) promoter, resulting in increased Ang-2 expression. Increased Ang-2 expression induced by high glucose in renal endothelial cells increased expression of intracellular adhesion molecule 1 and vascular cell adhesion molecule. In cells and in kidneys from diabetic mice, increased expression of Ang-2 sensitized microvascular endothelial cells to the proinflammatory effects of tumor necrosis factor alpha [29]. It has been also recently demonstrated that methylglyoxal covalently modifies the 20S proteasome, decreasing its activity in the diabetic kidney, and reduces the polyubiquitin receptor 19S-S5a, indicating a new link between hyperglycemia and impairment of cell function [30].
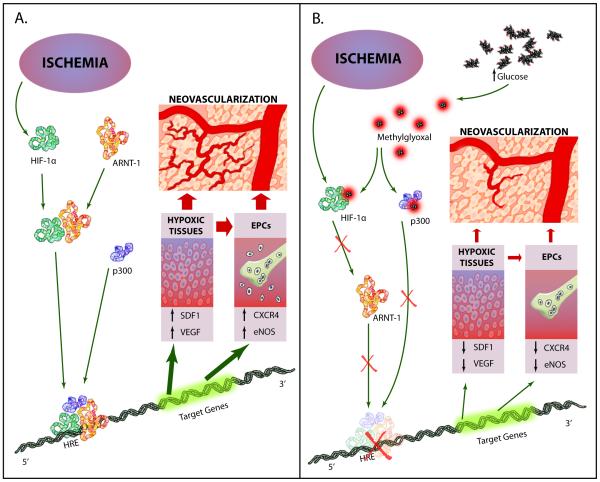
Clinically, diabetes is associated with poor outcomes following acute vascular occlusive events. Diabetic animals have a decreased vascular density following hind limb ischemia [31, 32] and impaired wound healing [33]. Human angiograms demonstrate fewer collateral vessels in diabetic patients compared with non-diabetic controls [3]. Clinically, this contributes to increased rates of lower limb amputation, heart failure, and increased mortality after ischemic events. These defects result in part from a failure to form adequate compensatory vasculogenesis in response to ischemia. AGEs appear to play a central role in this failure. High glucose induces a decrease in transactivation by the transcription factor hypoxia-inducible factor-1alpha (HIF-1alpha), which mediates hypoxia-stimulated chemokine and VEGF production by hypoxic tissue, and chemokine receptor and eNOS expression in endothelial precursor cells in the bone marrow. Decreasing superoxide in diabetic mice by either transgenic expression of manganese superoxide dismutase or by administration of a superoxide dismutase mimetic corrected post-ischemic defects in neovascularization, oxygen delivery, and chemokine expression, and normalized tissue survival. Decreased HIF-1alpha functional activity was specifically caused by impaired HIF-1alpha binding to the coactivator p300. Hyperglycemia-induced covalent modification of p300 by the dicarbonyl metabolite methylglyoxal (MG) is responsible for this decreased association (Fig 2). In diabetic mouse models of impaired angiogenesis and wound healing, decreasing mitochondria ROS formation normalizes both ischemia-induced new vessel formation and wound healing [34,35].
Fig 2.
Model of ischemia-induced neovascularization in normal and high glucose. A. In the presence of normal glucose concentration, ischemia-stabilized HIF1α forms heterodimers with ARNT which bind the coactivator p300. This complex binds to the hypoxia response element (HRE) and activates expression of genes required for neovascularization. B. High glucose-induced methylglyoxal (MG) modifies HIF1α and p300, inhibiting complex binding to the HREs of genes required for neovascularization (Data from Thangarajah et al. [34] and Ceradini, et al.[35]) (Illustration Credit: Ben Smith/Cosmocyte)
AGE-modified proteins in the circulation can affect a range of cells and tissues. A specific receptor for AGEs (RAGE) has been shown to mediate signal transduction via generation of ROS, activation of NFκB, and p21 ras [36-39]. AGE signaling can be blocked in cells by expression of RAGE antisense cDNA [38] or anti-RAGE ribozyme [39]. It has been also recently demonstrated that the RAGE-NFκB axis operates in diabetic neuropathy, mediating functional sensory deficits [40]. In endothelial cells, AGE binding to its receptor alters the expression of several genes, including thrombomodulin, tissue factor and vascular cell adhesion molecule-1 (VCAM-1) [41-44]. These effects induce procoagulatory changes on the endothelial cell surface and increase the adhesion of inflammatory cells to the endothelium. In addition, endothelial AGE receptor binding appears to mediate in part the increased vascular permeability induced by diabetes, probably through the induction of vascular endothelial growth factor (VEGF) [45-48]. RAGE deficiency attenuates the development of atherosclerosis in the diabetic apoE(−/−) model of accelerated atherosclerosis. Diabetic RAGE(−/−)/apoE(−/−) mice had significantly reduced atherosclerotic plaque area. These beneficial effects on the vasculature were associated with attenuation of leukocyte recruitment, decreased expression of proinflammatory mediators, including the nuclear factor-kappaB subunit p65, VCAM-1, and MCP-1, and reduced oxidative stress [49].
It is important to note that more recent studies indicate that AGEs at the concentrations found in diabetic sera may not be the major ligand for RAGE. Rather, several endogenously produced proinflammatory protein ligands have been identified, which activate RAGE at low concentrations. These include several members of the S100 calgranulin family and high mobility group box 1 (HMGB1). Each of these is increased by diabetic hyperglycemia [50]. Ligation of these ligands with RAGE causes cooperative interaction with the innate immune system signaling molecule toll like receptor 4 (TLR-4)[51-52]. Expression of RAGE, S100A8, S100A12, and HMGB1 are all increased by high glucose in cell culture and in diabetic animals. This hyperglycemia-induced overexpression is mediated by ROS-induced methylglyoxal, which increases binding of the transcription factors NFκB and AP-1 to the promoters of RAGE and RAGE ligands, respectively [48].
Increased protein kinase C activation
PKC are a family of at least 11 isoforms that are widely distributed in mammalian tissues. The enzyme phosphorylates various target proteins. The activity of the classic isoforms is dependent on both Ca2+ ions and phosphatidylserine, and is greatly enhanced by diacylglycerol (DAG) [10].
Persistent and excessive activation of several PKC isoforms operates as a third common pathway mediating tissue injury induced by diabetes-induced ROS. This results primarily from enhanced de-novo synthesis of DAG from glucose via triose phosphate, whose availability is increased because increased ROS inhibit activity of the glycolytic enzyme GAPDH, raising intracellular levels of the DAG precursor triose phosphate [53-56]. Evidence suggests that the enhanced activity of PKC isoforms could also result from the interaction between AGEs and their cell-surface receptors [57]. Hyperglycemia primarily activates the β and δ isoforms of PKC in cultured vascular cells [58-60]. In the diabetic retina, hyperglycemia persistently activates protein kinase C and p38α mitogen-activated protein kinase (MAPK) to increase the expression of a previously unknown target of PKC signaling, Src homology-2 domain–containing phosphatase-1 (SHP-1), a protein tyrosine phosphatase. This signaling cascade leads to PDGF receptor-βdephosphorylation and a reduction in downstream signaling from this receptor, resulting in pericyte apoptosis [61]. The same pathway, activated by increased fatty acid oxidation in insulin-resistant arterial endothelial cells and heart, may play an equally important role in diabetic atherosclerosis and cardiomyopathy. Overactivity of PKC has been implicated in the decreased NO production in smooth-muscle cells [62], and has been shown to inhibit insulin-stimulated expression of endothelial NO synthase (eNOS) in cultured endothelial cells [63]. Activation of PKC by high glucose also induces expression of the permeability-enhancing factor VEGF in vascular smooth-muscle cells [64].
In addition to mediating hyperglycemia-induced abnormalities of blood flow and permeability, activation of PKC may contribute to the accumulation of microvascular matrix protein by inducing expression of TGF-β1, fibronectin and type IV collagen in both cultured mesangial cells [65,66] and in glomeruli of diabetic rats [67]. This effect also appears to be mediated through PKC’s inhibition of NO production [65]. Hyperglycemia-induced activation of PKC has also been implicated in the overexpression of the fibrinolytic inhibitor, plasminogen activator inhibitor-1 (PAI-1) [68], and in the activation of NFκB in cultured endothelial cells and vascular smooth-muscle cells [69,70].
Increased hexosamine pathway flux
Hyperglycemia and insulin resistance-induced excess fatty acid oxidation also appear to contribute to the pathogenesis of diabetic complications by increasing the flux of fructose 6-phosphate into the hexosamine pathway [71-74]. In this pathway, fructose 6-phosphate is diverted from glycolysis to provide substrate for the rate-limiting enzyme of this pathway, glutamine: fructose 6-phosphate amidotransferase (GFAT). GFAT converts fructose 6-phosphate to Glucosamine 6-phosphate, which is then converted to UDP-N-Acetylglucosamine. Specific O-GlcNAc transferases use this for post-translational modification of specific serine and threonine residues on cytoplasmic and nuclear proteins by O-linked N-Acetylglucosamine. Inhibition of GFAT blocks hyperglycemia-induced increases in the transcription of both TGF-α [71] and TGF-β1 [72].
While it is not entirely clear how increased flux through the hexosamine pathway mediates hyperglycemia-induced increases in the gene transcription of key genes such as TGF-α, TGF-β1 and PAI-1, it has been shown that hyperglycemia causes a four-fold increase in O-GlcNAcylation of the transcription factor Sp1, which mediates hyperglycemia-induced activation of the PAI-1 promoter in vascular smooth-muscle cells [73] and of TGF-β1 and PAI-1 in arterial endothelial cells [74].
Of particular relevance to diabetic vascular complications is the inhibition of endothelial nitric oxide synthase (eNOS) activity in arterial endothelial cells by O-GlcNAcylation at the Akt activation site of eNOS protein [75-77]. Hyperglycemia also increases GFAT activity in aortic smooth muscle cells, which increases O-GlcNAc-modification of several proteins in these cells [78].
Finally, diabetic hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation, which reduced sarcoendoplasmic reticulum Ca(2+)-ATPase 2a (SERCA2a) mRNA and protein expression, and decreased SERCA2a promoter activity [79].In isolated perfused rat hearts, increased GlcNAcyation inhibited phenylephrine-induced inotropy by impairing capacitative Ca2+ entry (CCE), the influx of Ca2+ through plasma membrane channels activated in response to depletion of endoplasmic or sarcoplasmic reticulum Ca2+ stores [80].
A single process underlies different hyperglycemia-induced pathogenic mechanisms: Mitochondrial superoxide production
Specific inhibitors of aldose reductase activity, AGE formation, RAGE ligand binding, PKC activation, and hexosamine pathway flux each ameliorate various diabetes-induced abnormalities in cell culture or animal models, but it has not been clear whether these processes are interconnected or might have a common cause [62;81-84]. Moreover, all the above abnormalities are rapidly corrected when euglycemia is restored—which makes the phenomenon of hyperglycemic memory conceptually difficult to explain (see below). It has now been established that all of the different pathogenic mechanisms described above stem from a single hyperglycemia-induced process, namely overproduction of superoxide by the mitochondrial electron-transport chain [85,74]. Superoxide is the initial oxygen free radical formed by the mitochondria, which is then converted to other more reactive species that can damage cells in numerous ways [86].
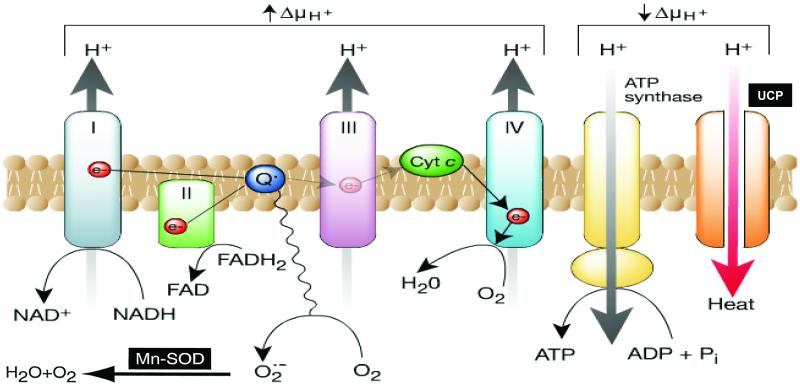
Normally, electron transfer through Complexes I, III and IV extrudes protons outwards into the intermembrane space, generating a proton gradient that drives ATP synthase (Complex V) as protons pass back through the inner membrane into the matrix. In contrast, in diabetic cells with high intracellular glucose concentration, there is more glucose-derived pyruvate being oxidized in the TCA cycle, increasing the flux of electron donors (NADH and FADH2) into the electron transport chain. As a result, the voltage gradient across the mitochondrial membrane increases until a critical threshold is reached. At this point, electron transfer inside complex III is blocked [87], causing the electrons to back up to coenzyme Q, which donates the electrons one at a time to molecular oxygen, thereby generating superoxide (Fig. 3). The mitochondrial isoform of the enzyme superoxide dismutase degrades this oxygen free radical to hydrogen peroxide, which is then converted to H2O and O2 by other enzymes. In primary arterial endothelial cells in culture, intracellular hyperglycemia increases the voltage across the mitochondrial membrane above the critical threshold necessary to increase superoxide formation [88] and, subsequently, increases production of ROS. It has been also demonstrated that dynamic changes in mitochondrial morphology are associated with high glucose-induced overproduction of ROS. Inhibition of mitochondrial fission prevented periodic fluctuation of ROS production during high glucose exposure [89]. Neither hyperglycemia nor increased fatty acid oxidation in vascular endothelium increases ROS nor activates any of the pathways when either the voltage gradient across the mitochondrial membrane is collapsed by uncoupling protein 1 (UCP-1) or when the superoxide produced is degraded by MnSOD [90]. Moreover hyperglycemia does not induce ROS formation in so-called rho zero endothelial cells, where the mitochondrial electron transport chain has been inhibited [4]. While it thus appears that the mitochondria are required for the initiation of hyperglycemia-induced superoxide production, much evidence indicates that this, in turn, can activate a number of other superoxide production pathways that may amplify the original damaging effect of hyperglycemia. These include redox changes, NADPH oxidases, and uncoupled eNOS.
Fig.3.
Production of ROS by the mitochondrial electron transport chain. In cultured endothelial cells the electron donors NADH and FADH2 are generated by the oxidation of glucose-derived pyruvate. The flow of the donated electrons (e−) through the electron-transport chain in the inner mitochondrial membrane pumps H+ ions into the intermembrane space. When the voltage gradient is high due to increased flux of electron donors, more superoxide is generated. H+ ions can pass back across the inner membrane along their concentration gradient, either via ATP synthase (to produce ATP) or via uncoupling proteins (UCP), which dissipate the energy of the proton gradient as heat. ADP, adenosine diphosphate; Cyt c, cytochrome c; FAD, flavin adenine dinucleotide; NAD, nicotinamide adenine dinucleotide (Adapted From Brownlee [4]).
In the diabetic heart, overexpression of MnSOD or catalase protects cardiac mitochondria from oxidative damage, improves respiration and normalizes mass in diabetic mitochondria. MnSOD also prevents the morphological changes in diabetic hearts and completely normalizes contractility in diabetic cardiomyocytes [91,92]. In endothelial cells increased MnSOD or UCP-2 expression inhibits both hyperglycemia- and fatty acid-induced inactivation of the anti-atherosclerosis endothelial enzyme prostacyclin synthase by nitration in diabetes [93-95]. Overexpression of either MnSOD and UCP-1 also prevents inhibition of eNOS activity by these metabolites [90].
In humans, skin fibroblast gene expression profiles from two groups of type 1 diabetic patients---20 years duration with a very fast (fast track) versus 20 duration with a very slow (slow track) rate of development of diabetic nephropathy lesions---showed that the fast-track group has increased expression of oxidative phosporylation genes, electron transport system complex II and TCA cycle genes compared to that of the slow track group. This association is consistent with a central role for mitochondrial ROS production in the pathogenesis of diabetic complications [96].
Hyperglycemia-induced mitochondrial superoxide production activates the five damaging pathways by inhibiting GAPDH
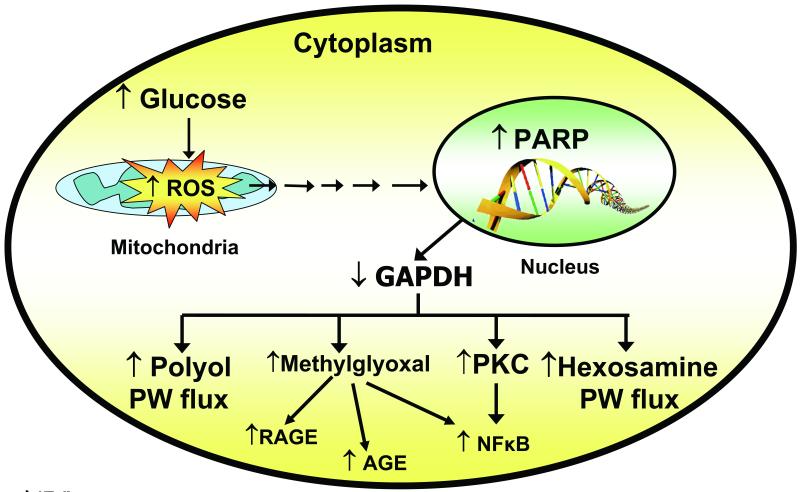
Diabetes in animals and patients, and hyperglycemia in cells, all decrease the activity of the key glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in cell types that develop intracellular hyperglycemia. Inhibition of GAPDH activity by hyperglycemia does not occur when mitochondrial overproduction of superoxide is prevented by either UCP-1 or MnSOD [95]. When GAPDH activity is inhibited, the level of all the glycolytic intermediates that are upstream of GAPDH increase. This then increases the flux into the 5 pathways described earlier. Increased levels of the upstream glycolytic metabolite glyceraldehyde 3-phosphate activates two major pathways. It activates the AGE pathway because the major intracellular AGE precursor methylglyoxal is formed non-enzymatically from glyceraldehyde 3-phosphate. Hyperglycemia-induced methylglyoxal formation has recently been shown to cause both increased expression of the receptor for advanced glycation end products (RAGE) and its activating ligands S100 calgranulins and HMGB1[48].
Increased glyceraldehyde 3-phosphate also activates the classic PKC pathway, since the physiologic activator of PKC, diacylglycerol, is also formed from glyceraldehyde 3-phosphate. Further upstream, levels of the glycolytic metabolite fructose 6-phosphate increase, which then increases flux through the hexosamine pathway, where fructose 6-phosphate is converted by the enzyme GFAT to UDP–N-Acetylglucosamine (UDP-GlcNAc). Finally, inhibition of GAPDH increases intracellular levels of the first glycolytic metabolite, glucose. This increases flux through the polyol pathway, where the enzyme aldose reductase reduces it (or glyceraldehyde 3-phosphate), consuming NADPH in the process. Inhibition of GAPDH activity in 5 mmol/l glucose using antisense DNA elevates the activity of each of the major pathways of hyperglycemic damage to the same extent as that induced by hyperglycemia [97] (Fig 4).
Fig. 4.
Schematic showing elements of the unifying mechanism of hyperglycemia-induced cellular damage (Adapted form Brownlee [4]).
Hyperglycemia-induced superoxide inhibits GAPDH activity in vivo by modifying the enzyme with polymers of ADP-ribose [97]. By inhibiting mitochondrial superoxide production with either UCP-1 or MnSOD, both modification of GAPDH by poly(ADP-ribose) and reduction of its activity by hyperglycemia were prevented. Most importantly, both modification of GAPDH by poly(ADP-ribose) and reduction of its activity by hyperglycemia were also prevented by a specific inhibitor of poly(ADP-ribose) polymerase (PARP), the enzyme that makes these polymers of ADP-ribose. Normally, PARP resides in the nucleus in an inactive form, waiting for DNA damage to activate it. When increased intracellular glucose generates increased ROS in the mitochondria, free radicals induce DNA strand breaks, thereby activating PARP. Both hyperglycemia-induced processes are prevented by either UCP-1 or MnSOD[97,74]. Once activated, PARP splits the NAD+ molecule into its two component parts: nicotinic acid and ADP-ribose. PARP then proceeds to make polymers of ADP-ribose, which accumulate on GAPDH and other nuclear proteins. GAPDH is commonly thought to reside exclusively in the cytosol. However, it normally shuttles in and out of the nucleus, where it plays a critical role in DNA repair [97-98] (Fig 4).
Glycemic memory
In 1993, the results of the landmark Diabetes Control and Complications Trial (DCCT) showed that in people with short-duration type 1 diabetes, intensive glycemic control dramatically reduced the occurrence and severity of diabetic microvascular complications. After the announcement of the DCCT results, many patients who had been in the standard therapy group adopted more intensive therapeutic regimens, and their level of glycemic control improved, as measured by the hemoglobin A1c (HbA1c) test. At the same time, the mean level of HbA1c worsened for patients who had been in the intensive therapy group. The post-DCCT HbA1c values for both groups have become statistically identical during the approximate 14 years of follow-up in the ongoing Epidemiology of Diabetes Interventions and Complications Study (EDIC) [99]. Surprisingly and provocatively, however, the effects of a 6.5-year difference in HbA1c during the DCCT on the incidence of retinopathy and nephropathy persisted, and have even become greater over the subsequent 14 years of follow-up. People in the standard therapy group continue to have a higher incidence of complications, even with an improvement in glycemic control during the 14-years of EDIC, while people in the intensive therapy group continue to have a lower incidence of both microvascular complications and cardiovascular disease, even with a deterioration in glycemic control during the EDIC years. Intensive treatment reduced the risk of any cardiovascular disease event by 42 percent and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57 percent [100]. This phenomenon has been given the name “glycemic memory”.
More recent data indicate that glycemic memory also occurs in type 2 diabetic patients. Indeed the tight glucose control group from the UKPDS demonstrated a continued reduction in microvascular risk and emergent risk reductions for myocardial infarction and death from any cause, despite an early loss of glycaemic differences (also termed “the legacy effect”). A continued benefit was evident during the ten-year post-trial follow-up among overweight patients [101]. Glycemic memory has several important clinical implications: 1) early tight control is very important; 2) cure of diabetes may not prevent subsequent development of complications; and 3) novel therapies that reverse hyperglycemic memory may be needed. The continuing progression of tissue damage after the correction of hyperglycemia (‘hyperglycemic memory’) may be explained in part by persistent epigenetic changes caused by hyperglycemia-induced mitochondrial superoxide production.
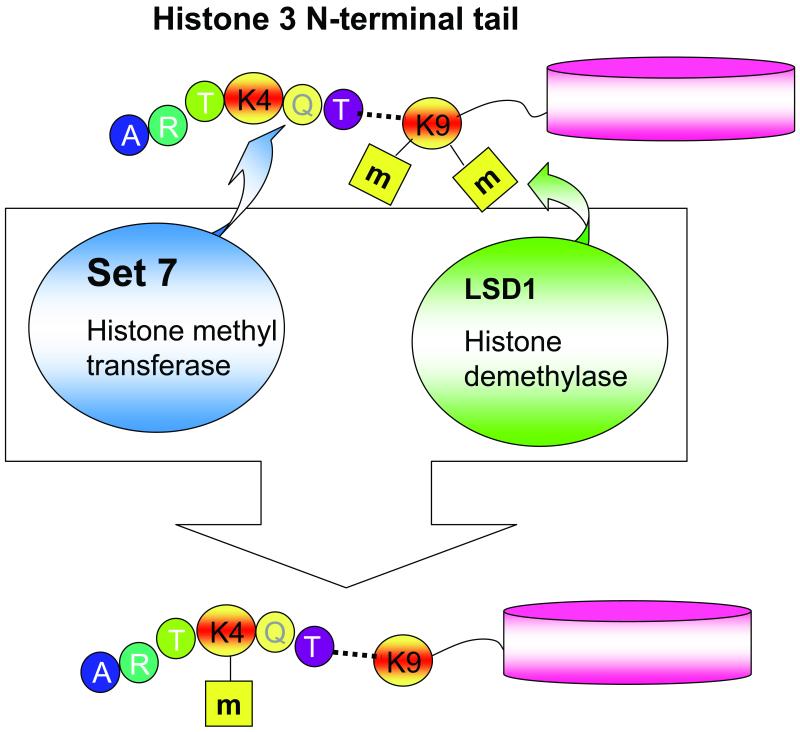
Post-translational modifications of histones cause chromatin remodeling and changes in levels of gene expression [102-104]. Since these modifications do not involve differences in DNA sequence, they are called “epigenetic”. Transient hyperglycemia, at a level sufficient to increase mitochondrial ROS production, induces long-lasting activating epigenetic changes in the proximal promoter of the nuclear factor kappaB (NF-kappaB) subunit p65 in human aortic endothelial cells (16 hrs exposure) and in aortic cells in vivo in non-diabetic mice (6 hrs exposure). These epigenetic changes (recruitment of Set 7 and H3K4 monomethylation) cause sustained increases in p65 gene expression and in the expression of p65-dependent proinflammatory genes. Both the epigenetic changes and the gene expression changes persist for at least 6 days of subsequent normal glycemia in cultured cells, and for months in previously diabetic mice whose beta cell function recovered [105]. Hyperglycemia-induced epigenetic changes and increased p65 expression are prevented by normalizing mitochondrial superoxide production or superoxide-induced methylglyoxal [105]. These results highlight the dramatic and long-lasting effects that short-term hyperglycemic spikes can have on vascular cells and suggest that transient spikes of hyperglycemia may be an HbA1c-independent risk factor for diabetic complications. Demethylation of another histone lysine residue in the proximal p65 promoter, H3K9, is also induced by hyperglcycemia-induced overproduction of ROS. This reduces inhibition of p65 gene expression, and thus acts synergistically with the activating methylation of histone 3 lysine 4 [106] (Fig. 5). Consistent with these observations, others have shown similar epigenetic changes in lymphocytes from patients with type 1 diabetes [107] and in vascular smooth muscle cells derived from db/db mice [108,109].
Fig. 5.
Hyperglycemia-induced activating modifications of histone 3 lysine 4 and derepressing modifications of histone 3 lysine 9 at the NFκB p65 proximal promoter (Data from El-Osta et al. [105] and Brasacchio et al. [106])
Insulin resistance, ROS, and atherosclerosis
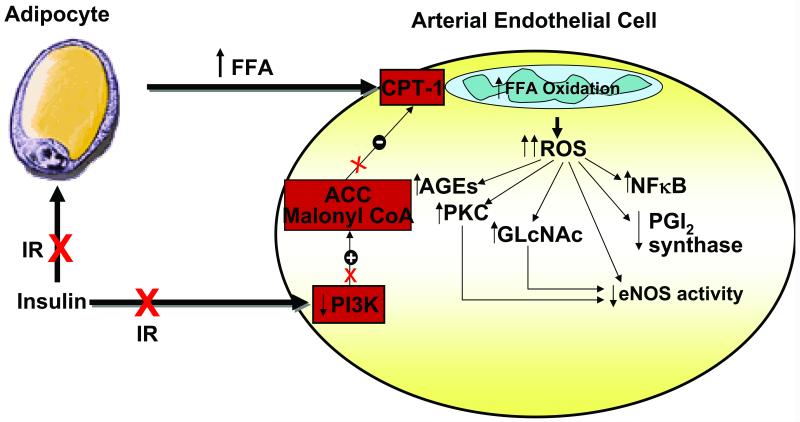
Insulin resistance (IR) occurs in the majority of patients with type 2 diabetes, and in two-thirds of subjects with impaired glucose tolerance [110]. Both these groups have a significantly higher risk of developing cardiovascular disease (CVD) [111-114]. In order to isolate the effects of IR from those of hyperglycemia and diabetes, several studies have evaluated subjects with normal glucose tolerance. In nonobese subjects without diabetes, IR predicted the development of CVD independently of other known risk factors [115]. In another group of subjects without diabetes or impaired glucose tolerance, those in the highest quintile of IR had a 2.0-fold increase in CVD risk compared with those in the lowest quintile after adjustment for 11 known cardiovascular risk factors, including LDL, triglycerides, HDL, systolic blood pressure, and smoking. These data indicate that IR itself promotes atherogenesis in the absence of hyperglycemia. IR in adipocytes increases release of free fatty acids from stored triglyceride, and increased oxidation of FFAs in aortic endothelial cells due to lack of insulin stimulation of malonyl CoA production causes increased production of superoxide by the mitochondrial electron transport chain. By activating the same mechanisms as hyperglycemia-induced ROS, FFA-induced overproduction of superoxide activates a variety of proinflammatory signals and inactivates two important antiatherogenic enzymes-- prostacyclin synthase and eNOS. In two non-diabetic rodent models of insulin resistance, inactivation of prostacyclin synthase and eNOS was prevented by inhibition of FFA release from adipose tissue, by inhibition of the rate-limiting enzyme for fatty acid oxidation in mitochondria (carnitine palmitoyltransferase I), and by reduction of superoxide levels [116] (Fig 6). Human atherosclerotic samples obtained during vascular surgery show greater mitochondrial DNA damage than nonatherosclerotic samples obtained from age-matched transplant donors, consistent with increased ROS production. Mitochondrial damage precedes the development of atherosclerosis and tracks with lesion extent in apoE-null mice, and mitochondrial dysfunction caused by heterozygous deficiency of a superoxide dismutase (SOD2) increases atherosclerosis and vascular mitochondrial damage in the same model [117,118].
Fig.6.
Role of insulin resistance and free fatty acids in macrovascular endothelial cell ROS formation and atherogenesis (Data form Du X, et al [116]).
While the association of insulin resistance with CVD risk is clear, data concerning the relative role of hyperglycemia in promoting cardiovascular disease in diabetes appear to be somewhat contradictory. In Type 1 diabetes, where severe insulin resistance is not a major abnormality, lowering of HbA1c levels with more intensive insulin treatment during the Diabetes Control and Complications Trial reduced both atherosclerosis surrogates during the trial and actual CVD events years after the trial concluded. Intensive treatment reduced the risk of any cardiovascular disease event by 42 percent and the risk of nonfatal myocardial infarction, stroke, or death from cardiovascular disease by 57 percent [100]. In contrast, intensive insulin treatment during the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial was associated with an unexpected excess of cardiovascular mortality in the intensive arm that caused the study to end early. Patients in this group were treated with a goal of lowering HbA1c to 6.0%. Overall a higher average on-treatment A1C was a stronger predictor of mortality than the A1C for the last interval of follow-up or the decrease of A1C in the first year. Of note, the excess risk associated with intensive glycemic treatment occurred among those participants whose average A1C, contrary to the intent of the strategy, was >7% [119]. How might these apparently conflicting results be explained? One possibility is that intensive therapy in extremely insulin resistant diabetic patients causes pro-atherogenic effects through overstimulation of insulin signaling pathways not affected by insulin resistance. In the liver, insulin exerts two predominant actions: it reduces glucose production (gluconeogenesis) and it increases the synthesis of fatty acids and triglycerides (lipogenesis). In the insulin-resistant state, only one of these actions is blocked in liver. Insulin loses its ability to reduce gluconeogenesis but it retains its ability to enhance lipogenesis [120,121].
Thus, increasing insulin levels to overcome the hyperglycemia caused by pathway-selective insulin resistance likely overdrive non-resistant pathways of insulin signaling, including MAPK. In arterial endothelial cells, such selective overdrive of the MAPK pathway by insulin would stimulate cellular growth and migration and the production of prothrombotic and profibrotic factors. Insulin stimulates production of the vasoconstrictor endothelin-1 (ET-1), and increases cellular adhesion molecule expression. In arterial smooth muscle cells, overdrive of the MAPK pathway by high insulin levels would stimulate VSMC proliferation and migration and expression of angiotensinogen and AT1R [122,123].
In rodent models of type 1 and type 2 diabetes, accumulation of triglyceride in the heart is associated with cardiac dysfunction and oxidative stress [124,125]. Expression of antioxidant enzymes or treatment with antioxidant compounds can ameliorate both triglyceride-associated oxidative stress and diabetic cardiomyopathy [126,127]. In vitro studies of cultured myocytes and endothelial cells have shown that excess saturated long chain fatty acids promote generation of ROS [128-129]. Several mechanisms have been implicated including lipid activation of NADPH oxidase activity through the downstream production of ceramides, and signaling through pathways that converge on NFkB [130-131]. Oxidative stress may also be intimately linked to lipid-induced endoplasmic reticulum stress [132].
Recently, a genetic screen for mutations that abrogate lipotoxic cell death led to the identification of a long non-coding RNA, gadd7, which functions as a feed forward amplifier of lipid-induced and generalized oxidative stress [133].
Mechanism-based therapeutic approaches
Because current methods of treating diabetes do not prevent diabetic complications, new mechanism-based therapeutic strategies are needed. One new class of potential therapeutic agents is transketolase activators. When increased superoxide inhibits GAPDH activity, the concentration of glycolytic intermediates above the enzyme accumulates which increases the flux into the five pathways of hyperglycemic damage. Two of these glycolytic intermediates, fructose 6-phosphate and glyceraldehyde 3-phosphate, are also the final products of the transketolase reaction, which is the rate-limiting enzyme in the non-oxidative part of the pentose phosphate pathway. Although the function of this enzyme is normally taught to be the conversion of pentose phosphates to glycolytic intermediates, it can also convert glycolytic intermediates to pentose phosphates. Since in diabetes the concentration of these two glycolytic intermediates is high, transketolase could reduces their concentration and thereby divert their flux away from three of the damaging pathways activated by hyperglycemia. This enzyme requires the vitamin thiamine as a cofactor. Although thiamine itself only activated transketolase ~ 25% in arterial endothelial cells, the thiamine derivative benfotiamine was found to activate transketolase 250% in arterial endothelial cells. Based on those findings, several groups have demonstrated the ability of benfotiamine to counteract glucose-mediated toxicity in cultured endothelial cells, endothelial progenitor endothelial cells, and mouse models of diabetes [134-136]. Most importantly, it has been recently demonstrated that beneficial effects of benfotiamine on complication-causing pathways in rodent models of diabetic complications also occur in humans with type 1 diabetes [137]. Benfotiamine treatment prevented experimental diabetic retinopathy and nephropathy in mice, and treatment with high-dose thiamine reduced albuminuria in patients with Type 2 diabetes. Diabetes has recently been found to cause intracellular thiamine depletion, which would impair normal transketolase conversion of hyperglycemia-induced elevation of glycolytic intermediates to pentose phosphates, and further exacerbate activation of three of the damaging pathways [138-140].
A second new class of mechanism-based potential therapeutic agents are PARP inhibitors. In cultured arterial endothelial cells, a specific PARP inhibitor prevents hyperglycemia-induced activation of PKC, NFκB, intracellular AGE formation, and the hexosamine pathway. In animal models of diabetes, PARP inhibition prevents arterial endothelial cell injury and podocyte apoptosis, ameliorates nephropathy, and alleviates sensory neuropathy [141-143].
A third class of mechanism-based therapeutics are SOD/catalase mimetics. Excess superoxide itself directly inhibits critical anti-atherosclerosis endothelial enzymes independent of activating the five damaging pathways implicated in metabolite-induced diabetic complications. Both of these enzymes-- endothelial nitric oxide synthase (eNOS) and prostacyclin synthase--are inhibited in diabetic patients and diabetic animals. To prevent oxidative inactivation of these key enzymes, in addition to preventing activation of the pathways discussed above, it is necessary to directly reduce the amount of superoxide. Conventional antioxidants are unlikely to do this effectively because conventional antioxidants neutralize reactive oxygen molecules on a one-for-one basis, while hyperglycemia-induced overproduction of superoxide is a continuous process. Based on observations of the beneficial effects of overexpression of antioxidant enzymes in mouse models, what is needed is a new type of antioxidant, a catalytic antioxidant, such as an SOD/catalase mimetic [144]. Hyperglycemia-induced reactive oxygen overproduction directly reduces eNOS activity in diabetic aortas by 65%. However, when these diabetic animals are treated with an SOD/catalase mimetic, there is no reduction in activity of this antiatherogenic enzyme [4]. Similarly, but more dramatically, hyperglycemia-induced reactive oxygen overproduction directly reduces prostacyclin synthase activity in diabetic aortas by 95%. Treatment of these diabetic animals with an SOD/catalase mimetic completely prevents diabetes-induced oxidative inactivation of aortic prostacyclin synthase, and also normalizes all five of the pathways implicated in hyperglycemic damage [4]. Inhibition of hyperglycemia-induced ROS production in diabetic mice using either transgenic antioxidant enzyme expression or combinations of antioxidant compounds prevents the development of experimental diabetic retinopathy, nephropathy, neuropathy and cardiomyopathy [145-149]. Together, these data strongly suggest that therapeutic correction of diabetes-induced superoxide overproduction may be a powerful approach for preventing diabetic complications.
Summary
Oxidative stress plays a pivotal role in the development of diabetes complications, both microvascular and cardiovascular. The experimental data discussed in this review demonstrate that the metabolic abnormalities of diabetes cause mitochondrial superoxide overproduction. This increased superoxide production is the central and major mediator of diabetes tissue damage, causing the activation of five pathways involved in the pathogenesis of complications and direct inactivation of two antiatherosclerotic enzymes, eNOS and prostacyclin synthase. This model is strongly supported by in vitro and vivo experiments. However, rodent models of diabetic atherosclerosis, cardiomyopathy, and indeed, any diabetic microvascular complication do not recapitulate major aspects of the phenotypes of human diabetic complications. Large animal models such as pigs or non-human primates where diabetic cardiovascular disease more closely resembles that in man have been generated [150,151] and future testing of ROS involvement in these models is the critical next step. While conventional antioxidants such as vitamin E have not shown any benefit in human CVD, these conventional antioxidants neutralize reactive oxygen molecules on a one-for-one basis, while diabetes-induced overproduction of superoxide is a continuous process. Based on observations of the beneficial effects of overexpression of antioxidant enzymes in transgenic mouse models of CVD, catalytic antioxidants such as the family of SOD/catalase mimetic compounds are the most logical choice for reducing diabetes-induced ROS in the large animal models mentioned above.
This article is part of a thematic series on Cardiovascular Complications of Diabetes and Obesity which includes the following articles:
The Impact of Macrophage Insulin Resistance on Advanced Atherosclerotic Plaque Progression [2010;106:58-67]
The RAGE Axis: a Fundamental Mechanism Signaling Danger to the Vulnerable Vasculature [2010;106:842-853]
The Promise of Cell-Based Therapies for Diabetic Complications: Challenges and Solutions [2010;106:854-869]
Activiation of Protein Kinase C Isoforms and Its Impact on Diabetic Complications [2010;106:1319-1331]
Aldose Reductase and Cardiovascular Diseases, Creating Human-Like Diabetic Complications In An Experimental Model [2010;106:1449-1458]
Endoplasmic Reticulum Stress and Inflammation in Obesity and Diabetes [2010;107:579-591]
Oxidative Stress and Diabetic Complications
Epigenetics – Mechanisms and Implications for Diabetic Complications
Ann Marie Schmidt, Guest Editor
Acknowledgments
Source of founding
Work performed in the laboratory of the authors was supported by NIH grant R01-DK07453, 2P60-DK020541 (Diabetes Research and Training Center Grant) and the Juvenile Diabetes Research Foundation 4-2004-804 (to M.B.) and a European Federation for the Study of Diabetes postdoctoral fellowship (to F.G.)
Non-standard Abbreviations and Acronyms
- AGE
advanced glycation end products
- eNOS
endothelial nitric oxide synthase
- FFAs
free fatty acids
- HMGB
high-mobility group box
- IR
Insulin resistance
- MG
methylglyoxal
- MnSOD
Manganese Superoxide Dismutase
- NO
Nitric oxide
- O-GlcNAc
O-linked N-Acetylglucosamine
- PKC
protein kinase C
- PPAR
peroxisome proliferator-activated receptor
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- TCA cycle
tricarboxylic acid cycle
- UCP1
uncoupling protein 1
Footnotes
Financial disclosure
The authors have no conflicts.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC National Diabetes fact sheet. 2007 [Google Scholar]
- 2.Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc S, Rydén L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140–4. doi: 10.1016/S0140-6736(02)09089-X. [DOI] [PubMed] [Google Scholar]
- 3.Abaci A, Oğuzhan A, Kahraman S, Eryol NK, Unal S, Arinç H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–42. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 4.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 7.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 8.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—revisted. Diabetes. 2008;57:995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–9. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31. doi: 10.1161/CIRCRESAHA.110.217117. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramasamy R, Goldberg IJ. Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model. Circ Res. 2010;106:1449–58. doi: 10.1161/CIRCRESAHA.109.213447. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005:2434–43. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 14.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233–6. doi: 10.1097/01.asn.0000077408.15865.06. Review. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Apse K, Pang J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem. 2000;275:40042–7. doi: 10.1074/jbc.M007505200. [DOI] [PubMed] [Google Scholar]
- 16.Ii S, Ohta M, Kudo E, Yamaoka T, Tachikawa T, Moritani M, Itakura M, Yoshimoto K. Redox state-dependent and sorbitol accumulation-independent diabetic albuminuria in mice with transgene-derived human aldose reductase and sorbitol dehydrogenase deficiency. Diabetologia. 2004;47:541–8. doi: 10.1007/s00125-004-1325-7. [DOI] [PubMed] [Google Scholar]
- 17.Bohren KM, Grimshaw CE, Gabbay KH. Catalytic effectiveness of human aldose reductase. Critical role of C-terminal domain. J Biol Chem. 1992;267:20965–70. [PubMed] [Google Scholar]
- 18.Zhang JZ, Gao L, Widness M, Xi X, Kern TS. Captopril inhibits glucose accumulation in retinal cells in diabetes. Invest Ophthalmol Vis Sci. 2003;44:4001–5. doi: 10.1167/iovs.02-1193. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki K, Miwa I, Okuda J. Affinity purification and glucose specificity of aldose reductase from bovine lens. Arch Biochem Biophys. 1982;216:337–344. doi: 10.1016/0003-9861(82)90219-3. [DOI] [PubMed] [Google Scholar]
- 20.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–4. doi: 10.1126/science.12192669. [DOI] [PubMed] [Google Scholar]
- 21.Wautier JL, Schmidt AM. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–8. doi: 10.1161/01.RES.0000137876.28454.64. Review. [DOI] [PubMed] [Google Scholar]
- 22.Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–92. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- 23.Stitt AW, Moore JE, Sharkey JA, Murphy G, Simpson DA, Bucala R, Vlassara H, Archer DB. Advanced glycation end products in vitreous: structural and functional implications for diabetic vitreopathy. Invest Ophthalmol Vis Sci. 1998;39:2517–23. [PubMed] [Google Scholar]
- 24.Stitt AW, Li YM, Gardiner TA, Bucala R, Archer DB, Vlassara H. Advanced glycation end products (AGEs) co-localize with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am J Pathol. 1997;150:523–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino T, Horii Y, Shiiki H, Yamamoto H, Makita Z, Bucala R, Dohi K. Immunohistochemical detection of advanced glycosylation end products within the vascular lesions and glomeruli in diabetic nephropathy. Hum Pathol. 1995;26:308–13. doi: 10.1016/0046-8177(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 26.Horie K, Miyata T, Maeda K, Miyata S, Sugiyama S, Sakai H, van Ypersole de Strihou C, Monnier VM, Witztum JL, Kurokawa K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions: implication for glycoxidative stress in the pathogenesis of diabetic nephro-pathy. J Clin Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa T, Katsuzaki T, Miyazaki S, Miyazaki T, Ishizaki Y, Hayase F, Tatemichi N, Takei Y. Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J Clin Invest. 1997;99:1272–80. doi: 10.1172/JCI119285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products:sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. Review. [DOI] [PubMed] [Google Scholar]
- 29.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M. High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem. 2007;282:31038–45. doi: 10.1074/jbc.M704703200. [DOI] [PubMed] [Google Scholar]
- 30.Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, Brownlee M, Preissner KT. Hyperglycemia Impairs Proteasome Function by Methylglyoxal. Diabetes. 2010;59:670–8. doi: 10.2337/db08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Am. J. Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schatteman GC, Hanlon HD, Jiao C, Dodds SG, Christy BA. J. Clin. Investig. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. J. Clin. Investig. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thangarajah H, Yao D, Chang EI, Shi Y, Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, Januszyk M, Brownlee M, Gurtner GC. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A. 2009;106:13505–10. doi: 10.1073/pnas.0906670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–8. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor foe advanced glycation end products triggers a p21(ras)-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–14. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- 38.Yamagishi S, Fujimori H, Yonekura H, Yamamoto Y, Yamamoto H. Advanced glycation endproducts inhibit prostacyclin production and induce plasminogen activator inhibitor-1 in human microvascular endothelial cells. Diabetologia. 1998;41:1435–41. doi: 10.1007/s001250051089. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji H, Iehara N, Masegi T, Imura M, Ohkawa J, Arai H, Ishii K, Kita T, Doi T. Ribozyme targeting of receptor for advanced glycation end products in mouse mesangial cells. Biochem Biophys Res Commun. 1998;245:583–8. doi: 10.1006/bbrc.1998.8489. [DOI] [PubMed] [Google Scholar]
- 40.Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundörfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–51. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlassara H, Fuh H, Donnelly T, Cybulsky M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol Med. 1995;1:447–56. [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt AM, Crandall J, Hori O, Cao R, Lakatta E. Elevated plasma levels of vascular cell adhesion molecule-1 (VCAM-1) in diabetic patients with microalbuminuria: a marker of vascular dysfunction and progressive vascular disease. Br J Haematol. 1996;92:747–50. doi: 10.1046/j.1365-2141.1996.379915.x. [DOI] [PubMed] [Google Scholar]
- 44.Sengoelge G, Födinger M, Skoupy S, Ferrara I, Zangerle C, Rogy M, Hörl WH, Sunder-Plassmann G, Menzel J. Endothelial cell adhesion molecule and PMNL response to inflammatory stimuli and AGE-modified fibronectin. Kidney Int. 1998;54:1637–51. doi: 10.1046/j.1523-1755.1998.00157.x. [DOI] [PubMed] [Google Scholar]
- 45.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–43. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest. 1998;101:1219–24. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirata C, Nakano K, Nakamura N, Kitagawa Y, Shigeta H, Hasegawa G, Ogata M, Ikeda T, Sawa H, Nakamura K, Ienaga K, Obayashi H, Kondo M. Advanced glycation end products induce expression of vascular endothelial growth factor by retinal Muller cells. Biochem Biophys Res Commun. 1997;236:712–15. doi: 10.1006/bbrc.1997.7036. [DOI] [PubMed] [Google Scholar]
- 48.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–55. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–9. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–86. doi: 10.1007/s00109-005-0688-7. Review. [DOI] [PubMed] [Google Scholar]
- 51.Rong LL, Gooch C, Szabolcs M, Herold KC, Lalla E, Hays AP, Yan SF, Yan SS, Schmidt AM. RAGE: a journey from the complications of diabetes to disorders of the nervous system - striking a fine balance between injury and repair. Restor Neurol Neurosci. 2005;23:355–65. Review. [PubMed] [Google Scholar]
- 52.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 53.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–63. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craven PA, Davidson CM, DeRubertis FR. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990;39:667–74. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- 55.Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265:E783–93. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- 56.Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-beta (II) in neonatal mesangial cells. Am J Physiol. 2000;278:F676–83. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 57.Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes: mechanism and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- 58.Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–9. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 59.Ayo SH, Radnik R, Garoni JA, Troyer DA, Kreisberg JI. High glucose increases diacylglycerol mass and activates protein kinase C in mesangial cell cultures. Am J Physiol. 1991;261:F571–7. doi: 10.1152/ajprenal.1991.261.4.F571. [DOI] [PubMed] [Google Scholar]
- 60.Koya D, Jirousek MR, Lin YW, Ishii H, Kuboki K, King GL. Characterization of protein kinase C beta isoform activation on the gene expression of transforming growth factor-beta, extracellular matrix components, and prostanoids in the glomeruli of diabetic rats. J Clin Invest. 1997;100:115–26. doi: 10.1172/JCI119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganz MB, Seftel A. Glucose-induced changes in protein kinase C and nitric oxide are prevented by vitamin E. Am J Physiol Endocrinol Metab. 2000;278:E146–52. doi: 10.1152/ajpendo.2000.278.1.E146. [DOI] [PubMed] [Google Scholar]
- 63.Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–81. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- 64.Williams B, Gallacher B, Patel H, Orme C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes. 1997;46:1497–503. doi: 10.2337/diab.46.9.1497. [DOI] [PubMed] [Google Scholar]
- 65.Pugliese G, Pricci F, Pugliese F, Mene P, Lenti L, Andreani D, Galli G, Casini A, Bianchi S, Rotella CM. Mechanisms of glucose-enhanced extracellular matrix accumulation in rat glomerular mesangial cells. Diabetes. 1994;43:478–90. doi: 10.2337/diab.43.3.478. [DOI] [PubMed] [Google Scholar]
- 66.Craven PA, Studer RK, Felder J, Phillips S, DeRubertis FR. Nitric oxide inhibition of transforming growth factor-beta and collagen synthesis in mesangial cells. Diabetes. 1997;46:671–81. doi: 10.2337/diab.46.4.671. [DOI] [PubMed] [Google Scholar]
- 67.Kikkawa R, Haneda M, Uzu T, Koya D, Sugimoto T, Shigeta Y. Translocation of protein kinase C alpha and zeta in rat glomerular mesangial cells cultured under high glucose conditions. Diabetologia. 1994;37:838–41. doi: 10.1007/BF00404342. [DOI] [PubMed] [Google Scholar]
- 68.Feener EP, Xia P, Inoguchi T, Shiba T, Kunisaki M, King GL. Role of protein kinase C in glucose- and angiotensin II-induced plasminogen activator inhibitor expression. Contrib Nephrol. 1996;118:180–7. doi: 10.1159/000425092. [DOI] [PubMed] [Google Scholar]
- 69.Pieper GM, Riaz-ul-Haq Activation of nuclear factor-kappaB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–32. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 70.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–64. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 71.Sayeski PP, Kudlow JE. Glucose metabolism to glucosamine is necessary for glucose stimulation of transforming growth factor-alpha gene transcription. J Biol Chem. 1996;271:15237–43. doi: 10.1074/jbc.271.25.15237. [DOI] [PubMed] [Google Scholar]
- 72.Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher ED. High glucose-induced transforming growth factor beta1 production is mediated by hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160–9. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen YQ, Su M, Walia RR, Hao Q, Covington JW, Vaughan DE. Sp1 sites mediate activation of the plasminogen activator inhibitor-1 promoter by glucose in vascular smooth muscle cells. J Biol Chem. 1998;273:8225–31. doi: 10.1074/jbc.273.14.8225. [DOI] [PubMed] [Google Scholar]
- 74.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA. 2000;97:12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamagishi SI, Edelstein D, Du XL, Brownlee M. Hyperglycemia potentiates collagen-induced platelet activation through mitochondrial superoxide overproduction. Diabetes. 2001;50:1491–4. doi: 10.2337/diabetes.50.6.1491. [DOI] [PubMed] [Google Scholar]
- 76.Hart GW. Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–35. doi: 10.1146/annurev.biochem.66.1.315. Review. [DOI] [PubMed] [Google Scholar]
- 77.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870–5. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akimoto Y, Kreppel LK, Hirano H, Hart GW. Hyperglycemia and the O-GlcNAc transferase in rat aortic smooth muscle cells: elevated expression and altered patterns of O-GlcNAcylation. Arch Biochem Biophys. 2001;389:166–75. doi: 10.1006/abbi.2001.2331. [DOI] [PubMed] [Google Scholar]
- 79.Clark RJ, McDonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–7. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 80.Pang Y, Bounelis P, Chatham JC, Marchase RB. Hexosamine pathway is responsible for inhibition by diabetes of phenylephrine-induced inotropy. Diabetes. 2004;53:1074–81. doi: 10.2337/diabetes.53.4.1074. [DOI] [PubMed] [Google Scholar]
- 81.Engerman RL, Kern TS, Larson ME. Nerve conduction and aldose reductase inhibition during 5 years of diabetes or galactosaemia in dogs. Diabetologia. 1994;37:141–4. doi: 10.1007/s001250050084. [DOI] [PubMed] [Google Scholar]
- 82.Sima AA, Prashar A, Zhang WX, Chakrabarti S, Greene DA. Preventive effect of long-term aldose reductase inhibition (ponalrestat) on nerve conduction and sural nerve structure in the spontaneously diabetic Bio-Breeding rat. J Clin Invest. 1990;85:1410–20. doi: 10.1172/JCI114585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci USA. 1995;92:2780–4. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 85.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 86.Wallace DC. Disease of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 87.Trumpower BL. The protonmotive Q cycle: energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–12. [PubMed] [Google Scholar]
- 88.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 89.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci U S A. 2006;103:2653–8. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 92.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 93.Xie Z, Zhang J, Wu J, Viollet B, Zou MH. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57:3222–30. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB. Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int. 2005;67:1762–71. doi: 10.1111/j.1523-1755.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 95.Nascimento NR, Lessa LM, Kerntopf MR, Sousa CM, Alves RS, Queiroz MG, Price J, Heimark DB, Larner J, Du X, Brownlee M, Gow A, Davis C, Fonteles MC. Inositols prevent and reverse endothelial dysfunction in diabetic rat and rabbit vasculature metabolically and by scavenging superoxide. Proc Natl Acad Sci U S A. 2006;103:218–23. doi: 10.1073/pnas.0509779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang C, Kim Y, Caramori ML, Moore JH, Rich SS, Mychaleckyj JC, Walker PC, Mauer M. Diabetic nephropathy is associated with gene expression levels of oxidative phosphorylation and related pathways. Diabetes. 2006;55:1826–31. doi: 10.2337/db05-1438. [DOI] [PubMed] [Google Scholar]
- 97.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94:11669–11674. doi: 10.1073/pnas.94.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–9. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 102.Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–8. doi: 10.1016/j.mrrev.2008.02.004. Review. [DOI] [PubMed] [Google Scholar]
- 103.Probst AV, Dunleavy E, Almousni G. Epigenetic inheritance during the cell cycle. Nature Reviews/Molecular Cell Biology. 2009;10:192–206. doi: 10.1038/nrm2640. 2009. [DOI] [PubMed] [Google Scholar]
- 104.Göndör A, Ohlsson R. Replication timing and epigenetic reprogramming of gene expression: a two-way relationship? Nat Rev Genet. 2009;10:269–76. doi: 10.1038/nrg2555. [DOI] [PubMed] [Google Scholar]
- 105.El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205:2409–17. doi: 10.1084/jem.20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brasacchio D, Okabe J, Tikellis C, Balcerczyk A, George P, Baker EK, Calkin AC, Brownlee M, Cooper ME, El-Osta A. Hyperglycemia induces a Dynamic Cooperativity of Histone Methylase and Demethylase Enzymes associated with Gene-Activating Epigenetic Marks that co-exist on the Lysine Tail. Diabetes. 2009;58:1229–36. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes. 2008;57:3189–98. doi: 10.2337/db08-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reddy MA, Villeneuve LM, Wang M, Lanting L, Natarajan R. Role of the lysine-specific demethylase 1 in the proinflammatory phenotype of vascular smooth muscle cells of diabetic mice. Circ Res. 2008;103:615–23. doi: 10.1161/CIRCRESAHA.108.175190. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci U S A. 2008;105:9047–52. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. Prevalence of IR in metabolic disorders: the Bruneck Study. Diabetes. 1988;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 111.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 112.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 24:1397–1402. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 113.National Diabetes Data Group . Diabetes in America. 2nd edition. NIH; Bethesda, Maryland, USA: 1995. p. 782. National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 114.DECODE Study Group. the European Diabetes Epidemiology Group vascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001;161:397–405. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 115.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J. Clin. Endocrinol. Metab. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 116.Du X, Edelstein D, Obici S, Higham N, Zou MH, Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–80. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–9. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 118.Semenkovich CF. Insulin resistance and atherosclerosis. J Clin Invest. 2006;116:1813–22. doi: 10.1172/JCI29024. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. Action to Control Cardiovascular Risk in Diabetes Investigators. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–90. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–6. doi: 10.1016/j.cmet.2007.12.009. Review. [DOI] [PubMed] [Google Scholar]
- 121.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–6. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–57. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulman IH, Zhou MS. Vascular insulin resistance: a potential link between cardiovascular and metabolic diseases. Curr Hypertens Rep. 2009;11:48–55. doi: 10.1007/s11906-009-0010-0. Review. [DOI] [PubMed] [Google Scholar]
- 124.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thandavarayan RA, Watanabe K, Ma M, Gurusamy N, Veeraveedu PT, Konishi T, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H911–9. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 126.Lee Y, Naseem RH, Park BH, Garry DJ, Richardson JA, Schaffer JE, Unger RH. Alpha-lipoic acid prevents lipotoxic cardiomyopathy in acyl CoA-synthase transgenic mice. Biochem Biophys Res Commun. 2006;344:446–52. doi: 10.1016/j.bbrc.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 127.Liang Q, Carlson EC, Donthi RV, Kralik PM, Shen X, Epstein PN. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–81. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 128.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–45. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 129.Borradaile NM, Buhman KK, Listenberger LL, Magee CJ, Morimoto ET, Ory DS, Schaffer JE. A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death. Mol Biol Cell. 2006;17:770–8. doi: 10.1091/mbc.E05-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang DX, Zou AP, Li PL. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am J Physiol Heart Circ Physiol. 2003;284:H605–12. doi: 10.1152/ajpheart.00697.2002. [DOI] [PubMed] [Google Scholar]
- 131.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 132.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 133.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem. 2009;284:7446–54. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 135.Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281:9307–13. doi: 10.1074/jbc.M600418200. [DOI] [PubMed] [Google Scholar]
- 136.Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, Fukamizu A, Lauro R, Federici M. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231–7. doi: 10.2337/db06-0369. [DOI] [PubMed] [Google Scholar]
- 137.Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia. 2008;51:1930–2. doi: 10.1007/s00125-008-1100-2. [DOI] [PubMed] [Google Scholar]
- 138.Rabbani N, Alam SS, Riaz S, Larkin JR, Akhtar MW, Shafi T, Thornalley PJ. High-dose thiamine therapy for patients with type 2 diabetes andmicroalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia. 2009;52:208–12. doi: 10.1007/s00125-008-1224-4. [DOI] [PubMed] [Google Scholar]
- 139.Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1:287–98. doi: 10.2174/157339905774574383. Review. [DOI] [PubMed] [Google Scholar]
- 140.Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164–70. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szabó C, Biser A, Benko R, Böttinger E, Suszták K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55:3004–12. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 142.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–94. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garcia Soriano F, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabó C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–13. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 144.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 145.Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol. 2007;208:216–27. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Otero P, Bonet B, Herrera E, Rabano A. Development of atherosclerosis in the diabetic BALB/c mice. Prevention with Vitamin E administration. Atherosclerosis. 2005;182:259–65. doi: 10.1016/j.atherosclerosis.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y, Wada J, Hashimoto I, Eguchi J, Yasuhara A, Kanwar YS, Shikata K, Makino H. Therapeutic approach for diabetic nephropathy using gene delivery of translocase of inner mitochondrial membrane 44 by reducing mitochondrial superoxide production. J Am Soc Nephrol. 2006;17:1090–101. doi: 10.1681/ASN.2005111148. [DOI] [PubMed] [Google Scholar]
- 148.Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006;41:1191–6. doi: 10.1016/j.freeradbiomed.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 149.DeRubertis FR, Craven PA, Melhem MF. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: effects of tempol. Metabolism. 2007;56(9):1256–64. doi: 10.1016/j.metabol.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 150.McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN, Chait A, O’Brien KD. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149–57. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Clarkson TB, Koritnik DR, Weingand KW, Miller LC. Nonhuman primate models of atherosclerosis: potential for the study of diabetes mellitus and hyperinsulinemia. Metabolism. 1985;34:51–9. doi: 10.1016/s0026-0495(85)80010-x. Review. [DOI] [PubMed] [Google Scholar]