Abstract
Background
More than 80% of the wheat genome is composed of transposable elements (TEs). Since active TEs can move to different locations and potentially impose a significant mutational load, their expression is suppressed in the genome via small non-coding RNAs (sRNAs). sRNAs guide silencing of TEs at the transcriptional (mainly 24-nt sRNAs) and post-transcriptional (mainly 21-nt sRNAs) levels. In this study, we report the distribution of these two types of sRNAs among the different classes of wheat TEs, the regions targeted within the TEs, and their impact on the methylation patterns of the targeted regions.
Results
We constructed an sRNA library from hexaploid wheat and developed a database that included our library and three other publicly available sRNA libraries from wheat. For five completely-sequenced wheat BAC contigs, most perfectly matching sRNAs represented TE sequences, suggesting that a large fraction of the wheat sRNAs originated from TEs. An analysis of all wheat TEs present in the Triticeae Repeat Sequence database showed that sRNA abundance was correlated with the estimated number of TEs within each class. Most of the sRNAs perfectly matching miniature inverted repeat transposable elements (MITEs) belonged to the 21-nt class and were mainly targeted to the terminal inverted repeats (TIRs). In contrast, most of the sRNAs matching class I and class II TEs belonged to the 24-nt class and were mainly targeted to the long terminal repeats (LTRs) in the class I TEs and to the terminal repeats in CACTA transposons. An analysis of the mutation frequency in potentially methylated sites revealed a three-fold increase in TE mutation frequency relative to intron and untranslated genic regions. This increase is consistent with wheat TEs being preferentially methylated, likely by sRNA targeting.
Conclusions
Our study examines the wheat epigenome in relation to known TEs. sRNA-directed transcriptional and post-transcriptional silencing plays important roles in the short-term suppression of TEs in the wheat genome, whereas DNA methylation and increased mutation rates may provide a long-term mechanism to inactivate TEs.
Background
The genome of hexaploid wheat (2n = 6X = 42; genomes AABBDD) is one of the largest in the grass family. The 2C DNA content of hexaploid wheat is 33.1 pg, about 37 and 165 times the genome size of rice (Oryza sativa) and Arabidopsis thaliana, respectively [1]. Based on DNA re-association studies the non-repetitive DNA fraction is estimated to be about 17% of the wheat genome [2], or hypothesized to be as low as 1% based on available sequence data analysis and genome size in relation to other plant genomes [3]. The repetitive, non-genic regions of wheat, as in many plant genomes, primarily consist of transposable elements (TEs) [4-7] and to a much lesser extent of pseudogenes [8-11]. During the past few years, about 1,500 Triticeae TE sequences have been discovered and deposited in the database for Triticeae repeats (TREP; http://wheat.pw.usda.gov/ITMI/Repeats).
First discovered by Barbara McClintock (1950) in maize, TEs have been reported to be present in all genomes analyzed, with similarities even among life kingdoms [12]. TEs are discrete sequences in the genome that can multiply and/or move within a host genome [13]. Class I TEs, which include long terminal repeat (LTR) retrotransposons and non-LTR transposons, are transcribed into mRNA that is subsequently reverse transcribed into DNA by a reverse transcriptase. Class II TEs, which are DNA transposons, including terminal inverted repeats (TIR) transposons, miniature inverted repeat transposable elements (MITEs) and Helitrons, move as DNA molecules that are excised from a genomic position and integrate elsewhere [14]. TEs are now recognized as important contributors to genomic organization and as major drivers of genome evolution. Centromeric and pericentromeric regions mainly consist of TEs [15-17], which may play an important role in centromeric stability and heterochromatin maintenance [18,19]. Induced activation of TEs resulted in altered chromosome segregation and meiotic disruption in mouse [20], loss of sister chromatid cohesion in yeast [21] and loss of centromere condensation in A. thaliana [22].
Active TEs constitute a major source of mutations in the genome. Transposition of a TE can result in altered gene expression [23-30], generation of novel regulatory networks [31], gene deletions [32,33], gene duplications [34], increases in genome size [6,35,36], illegitimate recombination [37] and chromosome breaks and rearrangements [38,39]. Because of the potential harmful effects of active TEs, the expression of most TEs in the genome is suppressed so that, even if whole and capable of autonomous transposition, most TEs remain silent throughout the plant's life cycle [19]. Only few naturally active TEs have been identified so far [12,40]. Nonetheless, TE-derived sequences are abundant in wheat cDNA libraries [41] and activation of TEs has been observed under conditions of biotic and abiotic stresses [42,43]. TE expression is silenced both at transcription and after transcription through epigenetic mechanisms [19].
TEs can be transcriptionally silenced by DNA methylation and repressive chromatin formation, involving modifications of histone tails and altered chromatin packing [12,44,45]. Post-transcriptional silencing of TEs is achieved by the degradation of TE transcripts by RNA-degrading complexes [12,46-48]. Small non-coding RNAs (sRNAs), generated when double-stranded RNA (dsRNA) is cleaved by proteins belonging to the Dicer family, guide the sequence-specific silencing after transcription [49]. sRNAs are also involved in DNA methylation of homologous DNA sequences in the nucleus (RNA-directed DNA methylation) and heterochromatin formation, guiding the silencing of TE at the transcriptional level [50,51]. The function of sRNAs is related to their length: if 21-nt long, silencing is post-transcriptional, whereas if 24-nt long, silencing is mediated by RNA-dependent DNA methylation and heterochromatin maintenance [19,51]. TEs are mobilized in Caenorhabditis elegans mutants that are defective in RNAi [52,53] and in mutants of A. thaliana that are deficient in DNA methylation and chromatin structure regulation [45,54-56]. Beside TE-silencing, the sRNAs are involved in a wide variety of biological phenomena, ranging from developmental processes to responses to biotic and abiotic stresses [57].
High-throughput sequencing has greatly facilitated the analysis of sRNA sequences. Massively-parallel sequencing platforms allow the identification of hundreds of thousands of sRNAs in any organism [58-66]. Profiles of sRNA collected from 22 species of higher plants, including wheat, are now publically available http://smallrna.udel.edu/.
In wheat, fast rates of TE insertion and deletion result in rapid turnover of intergenic regions, which can affect neighbouring genes [67]. This fast mutation frequency, together with the high tolerance to mutations of a polyploid genome, accounts for the genomic dynamism and adaptability of wheat [67]. Regulation of TE expression in the wheat genome has not been studied in detail. In this study, we report the analysis of the different classes of sRNAs originated from the different known classes of TEs in wheat, their target regions within the repetitive elements, and their impact on the methylation patterns of the targeted regions.
Results
Sequencing of sRNAs and comparison to extant public libraries
To investigate the relationship between sRNAs and TEs in wheat, we constructed an sRNA library from leaves of T. aestivum and developed a database that included our library plus the three libraries of sRNAs from T. aestivum that were publicly available at http://smallrna.udel.edu/. We sequenced 1,074,691 sRNAs (TAE4 library; GEO accession: GSM548032), which were then combined with 3,570,129 sRNAs from T. aestivum leaves (TAE1 library), as well as 2,916,955 and 2,968,383 sRNAs from T. aestivum healthy (TAE2 library) and Fusarium-infected spikelets (TAE3 library), respectively (Additional File 1 Table S1; the python program "dbmanager.py" written for the sRNA database setup is in Additional File 2). The resulting database is composed of 10,530,158 sRNA sequences (3,755,852 distinct sRNAs), with a bimodal size distribution with peaks at 21-nt (17.7 ± 4.6%) and 24-nt (28.7 ± 9.2%). Since libraries were from different tissues and developmental stages some variability was observed in the abundance of the 21 and 24-nt classes, which are summarized in the Additional File 1 Table S1.
The hexaploid wheat used to construct the TAE4 sRNA library expressed an RNAi construct under the 35S promoter that targeted the endogenous NO APICAL MERISTEM (NAM) gene [68]. The presence of this RNAi transgene caused a 40% reduction in expression of the target genes as measured by quantitative RT-PCR [68]. Out of 1,074,691 sRNAs in the TAE4 library, 4,105 (88.1% 21-nt and 6.3% 24-nt) perfectly matched the targeted NAM sequence in the RNAi construct reflecting the efficacy of the silencing construct (Additional File 1 Table S3). No sRNA from the other libraries made from non-transgenic materials matched the target NAM gene. We cannot rule out the possibility that the RNAi may cause other effect on the sRNA population.
Distribution of sRNA counts within annotated BAC sequences
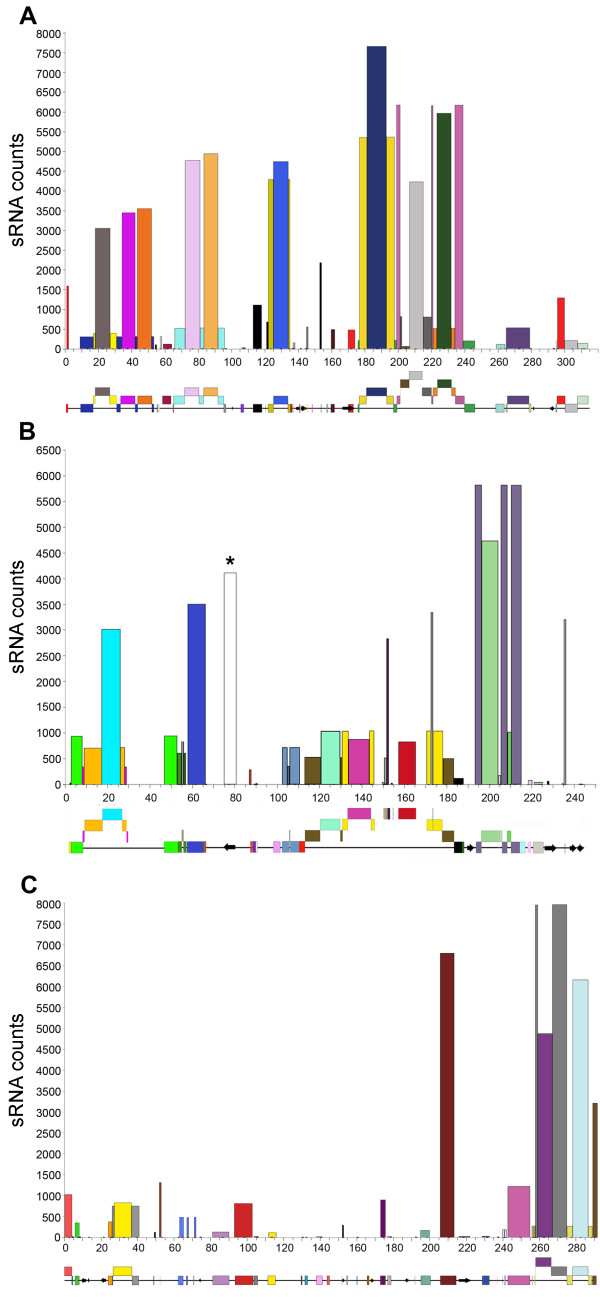
To explore the distribution of sRNAs in relation to both the sequences from which they originated and their potential targets, we mapped the sRNAs from the four libraries present in our database onto five completely-sequenced genomic regions, three from tetraploid wheat (T. turgidum) and two from hexaploid wheat (T. aestivum). Five entirely annotated genomic regions, EU835198 (314,057 bp) [69], DQ871219 (245,486 bp) [68], EF540321 (291,163 bp) [70], EF567062 (137,614 bp) [71], and DQ537335 (292,102 bp) [72] obtained by sequencing 10 bacterial artificial chromosomes (BACs) were chosen. Altogether, these regions (1,280,422 bp) include 190 (63% of the total genomic region analyzed) TEs (65% class I and 35% class II) and 26 (6% of the total genomic region analyzed) genes. The gene density of these genomic regions ranges from 1 gene per 34 kb in EF567062 to 1 gene per 63 kb in EU835198, similar to that observed in other wheat gene-rich regions [6,73-75].
A scrolling window analysis (Additional Files 3 and 4) was done to identify all sRNAs that perfectly matched the genomic sequences. Table 1 shows the distributions of the counts of sRNA mapping to the annotated TEs and genes in the three T. turgidum genomic regions, EU835198, DQ871219, and EF540321 (Figures 1A to 1C respectively) and the two T. aestivum sequences EF567062 and DQ537335. TEs and gene coordinates within each of the five regions and the relative sRNA counts are reported in Additional File 1 Tables S2-6. The number of perfectly matching sRNAs ranged from 71,868 (0.23 sRNA counts/bp for DQ871219) to 17,447 sRNAs (0.13 sRNA counts/bp for EF567062) (Table 1). Similar profiles of sRNA distribution on TEs and gene-encoding regions was observed in the three genomic regions from T. turgidum and the two of T. aestivum: ninety three percent of the total sRNAs (92% in T. turgidum; 94% in T. aestivum) that matched these genomic regions matched TE sequences (average 0.29 sRNA counts/bp), whereas only 0.07% (0.05% in T. turgidum; 0.1% in T. aestivum) matched the gene-encoding regions (average 0.001 sRNA counts/bp, excluding the sRNA that matched the NAM RNAi region in the GPC_RNAi library). A statistical comparison of the sRNA/bp in TEs and gene regions showed highly significant differences (P < 0.001).
Table 1.
Distribution of sRNA matches between transposable elements and genes in three annotated genomic regions
| Total | Transposable elements | Genes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Organism | Length (bp) | sRNA counts | sRNA counts/bp | Length (bp) | sRNA counts | sRNA counts/bp | Length (bp) | sRNA counts | sRNA counts/bp |
| EU835198 | T. turgidum | 314,057 | 71,868 | 0.23 | 220,511 | 70,808 | 0.32 | 18,298 | 8 | 0.00 |
| DQ871219 | T. turgidum | 245,486 | 43,789 | 0.18 | 144,831 | 37,374 | 0.26 | 15,324 | 59 * | 0.00 |
| EF540321 | T. turgidum | 291,163 | 43,074 | 0.15 | 131,089 | 38,216 | 0.29 | 16,412 | 13 | 0.00 |
| EF567062 | T. aestivum | 137,614 | 17,447 | 0.13 | 83,355 | 17,008 | 0.20 | 14,335 | 30 | 0.00 |
| DQ537335 | T. aestivum | 292,102 | 41,761 | 0.14 | 231,351 | 39,091 | 0.17 | 11,096 | 32 | 0.00 |
* 54 of these match to a single gene annotated as a predicted leucine rich repeat gene.
Figure 1.
sRNA counts over annotated genomic regions. Bar graphs representing the total counts of sRNA that perfectly matched annotated TEs and genes in EU835198 (A), DQ871219 (B), and EF540321 (C). sRNA counts and size of the annotated locus to which the sRNAs match correspond to bar height and bar width, respectively. A graphical representation of the structure of the genomic regions is provided below each bar graph. TEs are shown as colored boxes and genes as arrows. More recent insertions of repetitive elements are shown as boxes nested above the elements into which they were inserted. Boxes of the same colour and at the same level are part of the same element. In Figure 1B the bar with asterisk corresponds to the total counts of sRNAs from library TAE4 matching the RNAi target (TaNAM) gene.
Within the TEs, class I and class II TEs showed a similar sRNA density (class I: 0.24 sRNA counts/bp; class II: 0.26 sRNA counts/bp). However, 74% of the sRNAs that matched the class II TEs, correspond to the miniature inverted repeats transposable elements (MITEs), which account for only 4.2% of the class II TEs and, thus, have a significantly higher sRNA density than class I and the rest of the class II TEs (4.65 sRNA counts/bp; P = 7.0 × 10 -7).
Many of the TEs present in the analyzed regions are organized in nested structures that include up to four layers of nested insertions, with the relative position in the nested structure providing an estimate of their relative insertion times [6,36]. We observed a significantly higher number of sRNAs matching the TEs that were at the top of the nested structures compared to the number of sRNAs that were associated with elements at the lower, more ancient layers of the nested structures (P = 0.047).
Comparison between the predicted methylation pattern of TEs and genic regions
Because TEs accounted for most of the sRNAs in the genomic regions analyzed and transcriptional silencing, including cytosine methylation, is directed by sRNAs [76], we hypothesized that a higher level of cytosine methylation would be present in the repetitive elements than in the single-copy regions associated with genes, such as untranslated regions (UTRs) and introns. We used an in silico approach that took advantage of the different mutation rates of methylated C to infer the methylated regions [77]. Methylated cytosines display higher frequency of mutation than non-methylated cytosines because of a 10-fold increase in transition rate due to the passive deamination of methylated cytosine into thymine [78]. In plants, C methylation can occur not only in CG di-nucleotides , as observed in mammals, but also in CHG and, less frequently, in CHH tri-nucleotides, where H is A, T, or C [79].
We were able to use this approach only for the 80 kb VRN2 region, for which we had orthologous sequences from the T. monococcum A m genome BAC AY485644 and the T. turgidum A genome BAC EF540321 (these two genomes are >96% identical and diverged approximately 1 million years ago from each other [67]). The two orthologous regions were aligned and compared to count the number of mutations in potentially methylated sites (PMS).
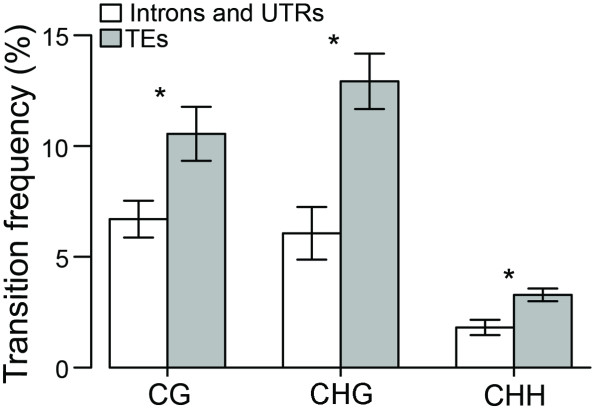
We developed a computer program, "Cmet scan" (Additional File 5), to classify all the C and G sites as CG, CHG, and CHH and to count the transitions that occurred at these sites between the two aligned sequences. The percent of mutations in PMS is used here as a proxy to infer the prevalence of methylation in a genomic region. Exonic sequences were excluded from the analysis to minimize the effect of selection on mutation frequencies. Our analysis confirmed that the overall frequency of mutations (transitions and transversions) was higher in PMS than in non-PMS sites. The observed number of mutations per nucleotide was approximately 20-fold and 11-fold higher in PMS than in non-PMS sites in TEs and in intronic and untranslated regions, respectively. The average percentage of CG, CHG, and CHH sites that underwent transitions was 2-fold higher (P < 0.05) in TEs than in intronic and untranslated regions (Figure 2). This difference between TEs and intronic and untranslated regions was also reflected in a higher transition to transversion ratio in the TEs (2.8) relative to the intronic and untranslated regions (1.9). This difference was the result of a higher transition frequency in the TEs versus the intronic and untranslated regions (P = 0.002; Figure 3A), with no apparent difference in the frequency of transversion (P = 0.59).
Figure 2.
Transition frequencies in potentially methylated sites (CG, CHG, and CHH). White bars represent introns and untranslated (UTR) gene regions. Exons were excluded. Gray bars represent transposable elements (TEs). Frequencies were calculated using the program "Cmet scan" (Additional File 5). Bars represent standard errors of the means.
Figure 3.
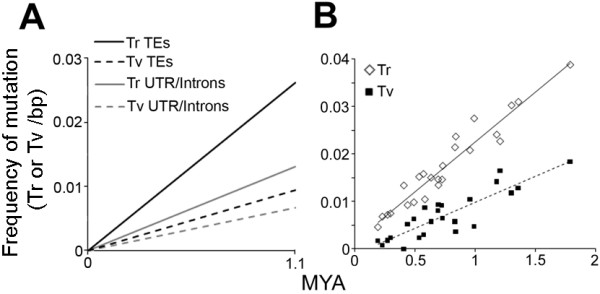
Rate of transition and transversion in UTR/Introns and TEs. (A) Number of transitions (Tr) and transversion (Tv) per bp that occurred in the orthologous VRN2 regions in T. monococcum and T. turgidum A genome during their 1.1 million year (MYA) of divergence. (B) Scatterplot representing the relation between the estimated insertion time (MYA) of LTR retrotransposons present in the VRN2 locus of T. monococcum and T. turgidum (LTRs are identical at the time of insertion) and the frequency of Tr and Tv in the LTRs. The slopes of the estimated linear trends represent the transition and transversions rates.
To analyze how the different rates of mutation affected the sequence divergence of the repetitive elements with time, we calculated the divergence rate per MYA using a previous estimate of 1.1 MYA of divergence between the T. monococcum and wheat A genome in the VRN2 region [67]. This estimate was based on the Kimura two-parameter method (K2P) [6,36,80] and a mutation rate of 5.5 x 10 -9 substitutions per synonymous site per year for the intronic and low copy number regions (10,397 aligned bp, 76 transitions and 51 transversions). Analysis of the orthologous TEs adjusted for the same divergence time resulted in a mutation rate of 1.67 x 10 -8 substitutions per synonymous site per year.
Using the previous rate, we calculated the hypothetical insertion times of the LTR retrotransposons in both of the homologous regions of T. monococcum and T. turgidum, assuming that the two LTRs are identical copies of the same template at the time of insertion [36]. Estimates of insertion time were possible for 24 elements with intact pairs of LTRs, 7 in T. turgidum and 17 in T. monococcum (Additional File 1 Table S8). Similarly to the comparison between orthologous transposable elements in the VRN2 the transition rate was approximately 2-fold higher than the transversion rate.
Distribution of sRNAs among TEs
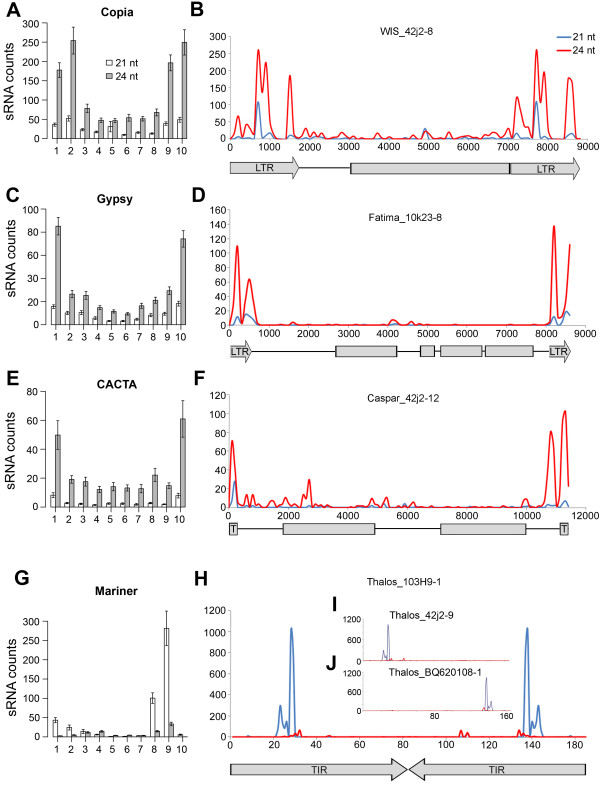
To extend our study to more TEs than those present in the five sequenced genomic regions, we analyzed the targets of the sRNA among the repetitive DNA sequences deposited in the Triticeae Repeat Sequence Database (TREP, http://wheat.pw.usda.gov/ITMI/Repeats/). The complete TREP database (Release 10, July 2008) contains sequences for 1,562 Triticeae TEs, of which 1,005 are complete elements. From the complete elements, we selected 918 that belong to the genera Triticum and Aegilops, and, among those, the 877 that include no ambiguous base calls (e.g., N). The results from the query of the sequences of these 877 elements for perfect matches to our sRNA database are detailed in Additional File 1 Table S7 and summarized graphically in Figure 4 (major TE superfamilies) and Additional File 1 Figure S1 (major TE families within major superfamilies). Copia elements showed the highest sRNA counts, about 2-fold higher than Mariner TEs and about 3-fold higher than Gypsy TEs. Of the sRNA perfectly matching Copia TEs, 93% matched Angela (62%) and WIS (31%) TEs, which also showed the highest median sRNA counts per element (Angela 3,228 and WIS 3,648). Among the Gypsy TEs, the ones with highest median sRNA counts where Wham (972) and Sabrina (925), but together accounted only for 38% of the total sRNA that matched Gypsy TEs, reflecting the higher diversity of abundant Gypsy TEs. Caspar, Clifford, Hamlet, and Jorge TEs accounted for 69% of the sRNAs perfectly matching CACTA TEs. Among Mariner TEs, Thalos MITEs showed the highest median sRNA count per element (614) and alone accounted for 44% of the sRNA matching its superfamily.
Figure 4.
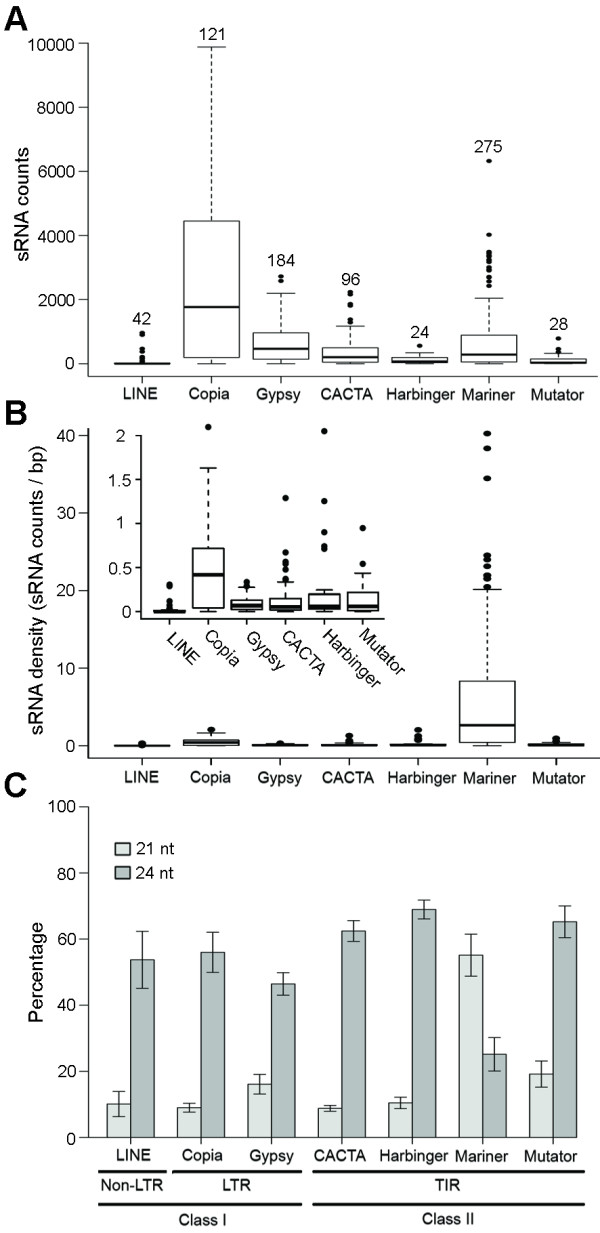
Distribution of sRNAs among TEs. (A) sRNA counts. Box plots represent the distribution of the total counts of sRNA perfectly matching wheat TEs of the seven major superfamilies deposited in the TREP database. Numbers above the whiskers represent the number of TREP elements within each superfamily considered.
(B) sRNA density. Same data as before but adjusted by the size of the TEs. The inset represents all superfamiles excluding Mariner and an expanded scale.
(C) Bar graph representing the percentage of 21 and 24 nucleotide sRNAs out of the total number that perfectly matched each superfamily of wheat TEs. Bars represent standard errors of the means.
To see if the differences in sRNA counts were correlated with the abundance of the elements, we estimated the number of TEs from the different classes in the wheat genome using the TREP database. In the complete TREP database, the class I LTR retrotransposons Copia (14%) and Gypsy (21%), and the class II elements Mariner (32%) and CACTA (11%) are the most abundant TE superfamilies, accounting together for about 80% of all TEs (Table 2). Accordingly, the TEs belonging to these superfamilies displayed the largest number of sRNAs counts, accounting for 97% of the 644,720 sRNAs that perfectly matched the 877 selected TE sequences. These perfect matches included 2,474 sRNAs per Copia element in TREP, 614 per Gypsy, 377 sRNAs per CACTA, and 655 sRNAs per Mariner, suggesting an excess of sRNA matching Copia elements. The correlation between element abundance in the TREP database and total sRNA counts per element was r = 0.63 (P = 0.129), and increased markedly to r = 0.99 (P < 0.0001) when Copia TEs were excluded from the analysis.
Table 2.
Total sRNA counts and element abundance in 21 annotated BACs, TREP database and blastn hits in T. aestivum NCBI EST collection
| Total sRNA (%) | 21 BAC counts (%) | TREP counts (%) | EST hits (%) | |
|---|---|---|---|---|
| Class I | ||||
| Gypsy | 19.9 | 38.2 | 23.9 | 43.1 |
| Copia | 57.9 | 19.4 | 15.7 | 26.2 |
| LINE | 0.3 | 4.3 | 5.4 | 1.3 |
| Class II | ||||
| CACTA | 3.6 | 14.3 | 12.5 | 9.3 |
| Harbinger | 0.3 | 0.9 | 3.1 | 0.9 |
| Mutator | 0.3 | 0.9 | 3.6 | 0.7 |
| Mariner | 17.7 | 22.0 | 35.7 | 18.5 |
Data are expressed as percentage of the total values per column.
To determine whether Copia TEs were underrepresented in the TREP database (121 TEs) compared to other TE superfamilies, such as Mariner (274 TEs) and Gypsy (184), we estimated the abundance of TEs in Triticum by analyzing 21 annotated BACs (NCBI; http://www.ncbi.nlm.nih.gov/; Table 2). In the 3,356,076 bp analyzed, Gypsy TEs were the most abundant (205 instances) followed by Mariner (118) and Copia (104). This frequency distribution was very similar to the one found in the TREP database (r = 0.79, P = 0.033). The higher abundance of Gypsy relative to Copia was confirmed in a 1X shotgun sequencing of the complete hexaploid wheat genome (http://www.cerealsdb.uk.net/search_reads.htm; K.J. Edwards, personal communication). In summary, all the different estimates of TE copy number confirmed that at least six times more sRNAs matched individual Copia than Gypsy elements.
We also studied the representation of the different TE superfamilies in the NCBI collection of T. aestivum ESTs (Table 2). The number of TEs in the EST libraries is expected to be proportional to their abundance in the RNA population and, thus, related to their transcriptional activity. Database searches were carried out using the blastn search tool with an E-value threshold of 1e -10 for CACTA, Copia, LINE, Gypsy, and of 1e -6 for Mariner, Harbinger, and Mutator TEs to account for the smaller sizes of the latter. Correlations between sRNA matches and TE representation in the EST database showed a similar pattern to what was observed in the correlations with the genomic data: overall, sRNA counts significantly correlated with BLASTn hits in the EST library only if Copia were excluded from the analysis (with Copia: r = 0.64, P = 0.12; without Copia: r = 0.91, P <0.01).
When the total number of counts was replaced by the counts per bp (match density), the Stowaway MITEs and the Mariner superfamily showed the highest density of sRNA counts (average 5.6 sRNA counts/bp), which was about 10-fold higher than Copia TEs, 65-fold higher than Gypsy TEs, and 47-fold higher than CACTA TEs (Figure 4B). In addition to the highest match density, the Mariner TEs presented a different pattern of sRNA sizes than the other groups (Figure 4C). Mariner TEs were the only class for which 21-nt sRNAs (54%) matches exceeded 24-nt sRNAs matches (27%), whereas for all other classes of TEs 24-nt matches were higher than 21-nt matches.
Distribution of sRNAs within TEs
To explore whether sRNAs preferentially matched specific regions of each TE, we divided the nucleotide sequence of each element of the Copia, Gypsy, CACTA and Mariner superfamilies present in the TREP database, into ten equal sections and determined the number of sRNAs that perfectly matched each section (Figure 5A,C, E, G). About 70% of the 24-nt sRNA matching the large LTR TEs was concentrated in the first and last 10% (Gypsy) and 20% (Copia) of these elements, corresponding to the LTRs. This observation was supported by the analysis of single elements (Figure 5B, D). Chi square tests averaging the duplicated LTR classes showed that the distribution of sRNA matches over the ten intervals differed significantly from a uniform distribution (df = 7 for Copia anddf = 8 for Gypsy; P < 0.0001).
Figure 5.
Distribution of sRNAs within TEs. The nucleotide sequence of each element of the Copia, Gypsy, CACTA and Mariner superfamilies present in the complete TREP database were divided in ten fractions, each representing 10% of the total element. (A, C, E, G). Bar graphs represent the mean number of total sRNAs that perfectly matched each of the ten fractions within each TE superfamily. Bars represent standard errors of the means.
(B, D, F, H) Distribution of perfectly matching sRNAs in representative elements from each TE superfamily. A graphical representation of the elements is provided below each graph; arrows and boxes correspond to repeats and open reading frames, respectively. (I, J) Distribution of perfectly matching sRNAs in MITEs with mutations in the 5' (I) and 3' (J) regions.
Complete CACTA elements are flanked by short terminal repeats (TIRs) that terminate in the CACTA motifs [81]. CACTA elements also contain sub-terminal repeats in direct and inverted orientation (TRs). Sub-terminal repeats typically lack sequence conservation between different families. About 50% of the sRNAs matching the CACTA elements matched the first and last 10% of these elements, which was also significantly different from a uniform distribution (sections 1 and 10 were averaged, df = 8, P < 0.0001; Figure 5E). The distal 10% corresponds to the TRs and adjacent sequences as suggested by the analysis of individual Caspar TEs (Figure 5F).
Wheat Stowaway MITEs represent the largest group within the Mariner superfamily. They are small (50-500 bp), non-autonomous elements that end in well conserved TIRs that comprise the majority of their structure. About 93% of the sRNAs matching this group of TEs are targeted to the TIRs. The higher frequency of sRNA in the 3'-TIR is likely due to a larger number of 5'-truncated elements in the TREP database (Figure 5G-J). The analysis of the individual MITE Stowaway Thalos 103H9-1 (composed of only two TIRs) shows a symmetric distribution of the 21-nt sRNA matches (Figure 5H). In this element, the perfectly paired regions of the TIRs are the main target of the sRNA. In contrast, the different MITE Thalos 42j2-9 has two nucleotide changes in the 3'-TIR (Figure 5I) that greatly reduce the sRNA counts in this region. In Thalos BQ620108-1 a single nucleotide change in the 5'-TIR also reduces sRNA counts (Figure 5J).
Discussion
Many wheat sRNAs target transposable elements
The profile of sRNAs perfectly matching the five wheat genomic regions analyzed here suggests that many wheat sRNAs are produced from TEs. These results are in general agreement with whole-genome studies of other plant species, in spite of the fact that the five analyzed regions comprise only 7.5 × 10 -5 % of the wheat genome (~17 Gb) and were selected from gene rich regions providing only a partial view of the wheat genome. In Arabidopsis thaliana most of the sRNAs correspond to transposons and repeats, and the highest densities of 24-nt sRNA-matching regions were detected in the centromeric and pericentromeric regions, where DNA transposons and retrotransposons are highly abundant [22,64,82]. In rice, large numbers of sRNAs originate from retrotransposon or transposon-related sequences [83]. Our analysis is limited to gene-rich regions of the wheat genome and we cannot rule out that a different profile of perfectly matching sRNAs is present in gene-poor regions, which represent most of the wheat genome.
In spite of the preponderance of sRNAs matching TEs in the five genomic regions analyzed here, a search of the TREP database perfectly matched only 6% of the sRNAs in our consolidated wheat sRNA database. Many of the non-matching sRNAs likely originate from intergenic regions [64], pseudogenes [84], or gene coding loci [64], but many may match undiscovered TE families or members of known families that have sufficiently diverged from their representatives in TREP. Most of the wheat genomic regions currently deposited in GenBank are the result of map-based positional cloning efforts and, therefore, are focused on gene-rich regions, whereas a large proportion of TEs are present in gene-poor regions [85,86]. Although this bias is likely reflected in the TEs currently present in TREP, the relative abundance of the different superfamilies in TREP was confirmed by the analysis of 1X shotgun sequencing of the complete hexaploid wheat genome (http://www.cerealsdb.uk.net/search_reads.htm; K.J. Edwards, personal communication). In addition, by considering only perfect matches, our analysis may not include an adequate sampling of the diversity of TE sequences within families and may underestimate the sRNA targets, since some sRNAs can be also effective against imperfectly matched targets [87,88]. The wheat TEs with the highest number of perfectly matching sRNAs include the class I Copia and Gypsy retrotransposons and the class II MITEs, which are also likely the most abundant TEs in the wheat genome (Table 2). A similar situation is observed in barley where the sRNAs matching the Copia, Gypsy and CACTA TEs account for 83% of the perfect matching barley sRNAs and together represent more than 50% of the matches to the random sequencing of 1% of the haploid barley genome [86]. However, the correspondence between TE abundance and number of perfect matches to the sRNAs is not perfect. In the sampled genomes of both wheat and barley, Gypsy TEs (38% in wheat and 48% in barley) are more abundant than Copia TEs (19% in wheat and 27% in barley), but the number of sRNAs with perfect matches is higher in Copia (47% in wheat and 53% in barley) than in Gypsy (18% in wheat and 15% in barley) TEs. Based on the different estimates of TE abundance it can be estimated that 6- to 8-fold more sRNAs match Copia than Gypsy TEs. Their relative levels of expression cannot explain this difference since the abundance of TEs from these two classes in the EST collections seems to be proportional to the number of copies (Table 2). Since most of the sRNAs are targeted to the LTRs we speculated that longer LTRs in the Copia relative to the Gypsy TEs could provide an explanation. However, this was not the case since, although very variable in size (Additional File 1 Figure S2), average LTR lengths were longer in Gypsy (2,045 bp) than Copia (1,110 bp) TEs. In summary, the excess of sRNAs matching the Copia TEs remains to be explained.
Different classes of wheat TEs are targeted by different classes of sRNAs
The 24-nt sRNA are involved in RNA-dependent DNA methylation and heterochromatin maintenance and thus suppress transcription from DNA, whereas the 21-nt sRNAs regulate the half life and translation of related mRNAs [49,51,89]. It is interesting that most of the TE-matching sRNAs in wheat belong to the 24-nt group, whereas MITEs are preferentially matched by 21-nt sRNAs.
These results suggest that the activity of MITE TEs is regulated primarily after transcription, while the activity of all the other TE families is regulated by repression of transcription. In addition, the restricted targeting of the 21-nt sRNAs observed within the TIR regions of MITEs is more similar to the pattern of regulatory sRNAs in A. thaliana and rice than to the more dispersed distribution of 24-nt sRNAs [64,83]. Unlike other TEs, MITEs often occur in 5' or 3' UTRs of genes and sometimes even integrate in the coding sequences [13]. In consequence, MITEs are often expressed as read-through transcripts [46]. MITEs are flanked by short TIRs joined by little or no spacer DNA [46] that when expressed as RNA form a highly stable hairpin loop, which can then be recognized and processed by the RNA interference enzymes into mature 21-nt RNA [46].
The preponderance of 21-nt MITE sRNAs in polyploid wheat contrasts with the preponderance of 24-nt MITE sRNAs reported in diploid plant species [90]. Wheat is a recent polyploid with a high level of gene redundancy, and therefore, has a high tolerance to genic mutations [67], which may allow the accumulation of MITEs in genic regions. Besides introducing a target site for silencing, the insertion of a TE in a coding region may introduce an alternative polyadenylation site when located in the 3' UTR, affect mRNA stability and translation initiation, or interfere with the normal splicing pattern and perturb the functionality of the resulting protein [46].
Retrotransposons and DNA transposons other than MITEs were preferentially associated with 24-nt sRNAs. As in the case of MITEs, in DNA transposons such as the CACTA elements, read-though transcription and intramolecular pairing of inverted repeats may underlie the generation of dsRNA and consequently of sRNAs [19]. The higher sRNA counts matching the terminal repeats, including the subterminal inverted repeats may reflect not only the higher degree of conservation of these regions [81], but also a mechanism of sRNA generation based on the formation of terminal dsRNA loops.
In the case of the class I TEs, dsRNA can be generated by RNA-dependent RNA polymerase (RdRP) [91] or by intermolecular pairing of antiparallel transcripts [19]. For example, the bidirectional transcription of retrotransposons leads to the generation of sRNAs and to silencing in human cells [92] and in Drosophila melanogaster [93].
sRNAs match specific areas of the repetitive elements
The sRNAs matching wheat Copia and Gypsy TEs were concentrated in the LTRs (Figure 5), a pattern also observed in maize [94]. LTRs do not encode for known proteins, but contain the promoters and terminators required for the transcription of the retroelement and are partially transcribed [95].
The significantly larger proportion of sRNAs matching LTRs may simply reflect the higher abundance of LTRs relative to the internal domain region in the genome. In addition to the natural duplication of the LTR at both ends of the TE, the inter-LTR region is eliminated in a large proportion of TEs resulting in solo-LTRs. For example, barley contains an average of 15 solo-LTRs per internal domain [96]. The significantly larger proportion of sRNAs matching LTRs might also indicate a higher chance of antiparallel pairing of these repetitive regions, or that LTRs are targeted by RdRP, or that the plant-specific DNA dependent RNA polymerase IVa (PolIVa) at the DNA level uses LTRs as template to generate sRNAs [97-100].
Regions targeted by sRNAs have higher rates of mutation
The presence of abundant sRNAs matching TEs suggests that epigenetic mechanisms are involved in the silencing of their expression and motility in wheat. The sRNA-metabolic pathway guides both the de novo methyltransferases to initiate DNA methylation at direct repeats [101] and the maintenance methyltransferases responsible for remethylation and the maintenance of the transgenerational stability of the heavily methylated repetitive elements [102]. In A. thaliana, 24-nt sRNAs are generated by the DICER-LIKE 3 protein and, when loaded in one of the ten argonaute proteins, AGO4, target DNA methylation [50].
In plants, sRNAs induce methylation of not only CG dinucleotides, which are the primary sites of methylation in mammals, but also cytosines in the CHG and CHH sequence contexts [79]. Our estimations of DNA methylation based on transition rates parallel the higher methylation of cytosines in the CG and CHG contexts compared to CHH observed in Arabidopsis [79]. In wheat, TEs are preferentially methylated compared to introns and untranslated genic regions in agreement with studies in A. thaliana, maize, and primates [77,79,103]. The importance of DNA methylation is evidenced by the dramatic increase in TE transcription observed in methylation-deficient mutants of A. thaliana [54,79,104]. Methylated cytosine may affect TE expression either directly by interfering with the proper binding of proteins involved in transposition or indirectly by recruiting methylcytosine binding proteins that in turn associate with complexes containing co-repressors and histone deacetylases that modify chromatin structure [105].
In addition to the rapid and reversible TE repression, DNA methylation can irreversibly inactivate TEs by increasing the mutation frequency of the methylated sites. Methylated cytosines spontaneously deaminate to form thymine at a faster rate than non-methylated cytosines in the same sequence context [78]. Based on the estimated divergence time between T. monococcum and T. turgidum of 1.1 MYA [67] and an estimated nucleotide substitution rate in the introns and untranslated regions of 5.5 x 10 -9 nt -1 year -1 [106], we estimated that the substitution rate was 1.6 x 10 -8 nt -1 year -1 in the TEs (1,643 nucleotides considered, including 431 transitions and 156 transversions), which is about three times faster than the substitution rate in the untranslated genic regions. This estimate is almost identical to the one obtained previously for a different wheat genomic region [107]. This higher substitution rate in TEs is paralleled by a significantly higher transition rates but not any difference in the rate of transversions. Thus cytosine methylation and subsequent transition account for the faster substitution rate observed in the TEs.
The sequence erosion initiated by DNA methylation may account for the smaller number of sRNAs derived from older TEs relative to the number from more recently inserted TEs (Figure 1). The higher mutation rate in methylated TEs together with high rates of deletions in the intergenic regions may contribute to the permanent inactivation of TEs, [67,81].
Conclusions
Our study provides a first exploration of the wheat epigenome and its close connection with the TEs that compose the vast majority of the wheat genome. Our findings suggest that sRNA-directed transcriptional and post-trascriptional silencing suppress TE activity in the wheat genome. DNA methylation and the consequent increase in the mutation rate at the methylated sites may silence TEs more permanently.
Methods
sRNA database construction
For the TAE4 library, transgenic plants for the TaNAM RNAi construct [68] were grown under long-days (16 h light 8 h dark). The experiment was originally performed to characterize the production of sRNAs from the NAM-RNAi transgene, but it then expanded beyond the original objective. At anthesis, spikes were labelled and after 12 days, flag-leaves samples from four plants were pooled and used for RNA extraction. Total RNA was prepared using the TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and its integrity was evaluated by gel electrophoresis. The TAE4 sRNA library was prepared using Illumina Small RNA Library Sample Prep protocol version 1.5. Total RNA (10 ug) was used as input material. The 3' adaptor (Illumina, San Diego, CA, USA) was ligated to RNAs. The v1.5 3' adapter predominantly ligates to microRNAs and other small RNAs that have a 3' hydroxyl group. The 3' ligation products were then ligated to the 5' small RNA adapter (Illumina). The ligation products were reverse transcribed followed by PCR amplification. The amplification products from 18 - 30 nt long RNAs were excised from a Novex 6% TBE PAGE gel (Invitrogen). The purified DNA fragments were submitted for 45 cycles of sequencing on the Illumina Genome Analyzer. The resulting sequencing reads were filtered for quality, and then trimmed to remove the sequence of 3' adapters. Filtering and trimming scripts are available from http://code.google.com/p/atgc-illumina/. Only the high quality reads with detectable 3' adapter were used for the analysis. The sequence data were deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO; [108]) and are accessible through GEO (accession n. GSM548032; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc= GSM548032). TAE1, TAE2, and TAE3 sRNA libraries were obtained from the comparative sequencing project described on http://smallrna.udel.edu, where details on the libraries are available [94]. TAE1, TAE2, TAE3, and TAE4 sRNAs libraries were integrated in a single database using MySQL5.1 (MySQL AB; http://www.mysql.com/). For the analysis of barley sRNAs we used the barley sRNA libraries HVU1, HVU2, and HVU3, obtained from the comparative sequencing project http://smallrna.udel.edu.
The program "dbmanager.py" written in Python 2.6 for the database setup is provided in Additional File 1 Text S1.
Analysis of perfect matching sRNAs
A computer program,"srna_seeker.py" (Additional File 4) written in Python 2.6 was used to count the perfectly matching sRNAs to both BAC sequences and TEs. The program consisted in a scrolling window with frame size between 18 and 33 nt that scans through the entire query sequence with a 1 nt increment after each read ("scanner.py", Additional File 3). When the scanner finds a region of the query sequence that exactly matches a sRNA in the database, "srna_seeker.py" returns the matching sRNA sequence, the length of the sequence (18 - 33 nt), the coordinate within the queried region, the library of origin, and the total counts in the sRNA database.
Estimation of cytosine methylation
A computer program, "Cmet_scan.py" (Additional File 5) written in Python 2.5, was used to estimate the frequency of mutations in potentially methylated sites (CG, CHG and CHH). This measure is used as a proxy to infer the methylation state of these regions, since methylated C mutates three-times more frequently than unmethylated C (see main text). The program was used to compare repetitive and low copy number regions in orthologous genomic sequences of T. monococcum and T. turgidum and 5' and 3' long tandem repeats (LTRs) flanking Copia and Gypsy retroelements. Orthologous regions were aligned with ClustalW which generates outputs with "-" characters in the indels. The aligned FASTA file sequences were used as input for Cmet_scan.py. All Cs or Gs in both genomes in any of the CG, CHG, and CHH nucleotide contexts were considered as potentially methylated sites (PMS). G sites in one DNA strand are C in the opposite strand, and can be methylated. Therefore, both C and G sites were counted as potentially methylated sites. Bases paired with "-" (indels) were excluded from all calculations. C and G sites were classified as CG, CHG, or CHH. Presence of a CG or CHG in one genome was considered sufficient to count both the C and the G as potentially methylated. Detailed examples are included in the program file (Additional File 5). Cmet_scan.py counts all nucleotide substitutions, either transitions or transversions, and returns separately the percentage of transitions and transversions from CG, CHG, and CHH, for the complete sequence and for the total PMS.
Authors' contributions
DC, LSV, MM, and AD carried out the experiments; DC, LSV, MM, and JD performed data analysis; DC, AS, MD, and JD developed the computer programs; DC, LSV, JD performed the statistical analyses; JD, RWM, and MM helped with the interpretation of the results; DC drafted the manuscript; JD and RWM were involved in improving the manuscript. All the authors approved the final version of the manuscript.
Supplementary Material
Figure S1 - sRNA counts in TE families. Box plots represent the distribution of the total counts of sRNA perfectly matching wheat TEs of each family in the seven major superfamilies deposited in the TREP database. Numbers above the whiskers represent the number of TREP elements within each superfamily considered. Figure S2 - LTR length in Copia and Gypsy TEs. Box plots represent the distribution of LTR lengths in the Copia and Gypsy elements deposited in the TREP database. Table S1 - Summary of sRNA libraries. Table S2 - Distribution of sRNA counts in the EU835198 genomic region (T. turgidum). Table S3 - Distribution of sRNA counts in the DQ871219 genomic region (T. turgidum). Table S4 - Distribution of sRNA counts in the EF540321 genomic region (T. turgidum). Table S5 - Distribution of sRNA counts in the EF567062 genomic region (T. aestivum). Table S6 - Distribution of sRNA counts in the DQ537335 genomic region (T. aestivum). Table S7 - sRNA counts in the different TE families. Table S8 - Estimates of LTR age of insertion and cytosine methylation in the CG, CHG, and CHH contexts.
dbmanager.py - python program for sRNA database setup.
scanner.py - python program for scrolling window analysis with frame size between 18 and 33 nt that scans through the entire query sequence with a 1 nt increment after each read.
srna_seeker.py - program for scanning nucleotide sequences for perfect matching sRNAs using "dbmanager.py" to access the sRNA database and "scanner.py" to analyze the query sequences.
Cmet_scan.py - python program for counting mutations in potentially methylated sites.
Contributor Information
Dario Cantu, Email: dacantu@ucdavis.edu.
Leonardo S Vanzetti, Email: lvanzetti@mjuarez.inta.gov.ar.
Adam Sumner, Email: assumner@ucdavis.edu.
Martin Dubcovsky, Email: dubcovsky@ucdavis.edu.
Marta Matvienko, Email: matvienko@gmail.com.
Assaf Distelfeld, Email: adistel@ucdavis.edu.
Richard W Michelmore, Email: rwmichelmore@ucdavis.edu.
Jorge Dubcovsky, Email: jdubcovsky@ucdavis.edu.
Acknowledgements
We thank Dr. Alex Kozik for Illumina data filtering, trimming, and sequence conversion to FASTA files and Drs. Blake Meyers and Pamela Green (University of Delaware) for the access to the wheat and barley sRNA data http://smallrna.udel.edu generated by NSF grant #0638525. This project was supported in part by the National Research Initiative Competitive Grant no. 2009-65300-05640 from the USDA National Institute of Food and Agriculture.
References
- Arumuganathan K, Earle E. Estimation of nuclear DNA contect of plant by flow cytometry. Plant Mol Biol Rep. 1991;9:229–233. doi: 10.1007/BF02672073. [DOI] [Google Scholar]
- Flavell R, Bennett M, Smith J, Smith D. Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet. 1974;12:257–269. doi: 10.1007/BF00485947. [DOI] [PubMed] [Google Scholar]
- Sandhu D, Gill KS. Gene-containing regions of wheat and the other grass genomes. Plant Physiol. 2002;128:803–811. doi: 10.1104/pp.010745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen JL, SanMiguel P, Chen MS, Tikhonov A, Francki M, Avramova Z. Grass genomes. Proc Natl Acad Sci USA. 1998;95:1975–1978. doi: 10.1073/pnas.95.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagudah ES, Dubcovsky J, Powell W. Wheat genomics. Plant Physiol Biochem. 2001;39:335–344. doi: 10.1016/S0981-9428(00)01233-X. [DOI] [Google Scholar]
- SanMiguel P, Ramakrishna W, Bennetzen J, Busso C, Dubcovsky J. Transposable elements, genes and recombination in a 215-kb contig from wheat chromosome 5A m. Funct Integr Genomics. 2002;2:70–80. doi: 10.1007/s10142-002-0056-4. [DOI] [PubMed] [Google Scholar]
- Shirasu K, Schulman AH, Lahaye T, Schulze-Lefert P. A contiguous 66-kb barley DNA sequence provides evidence for reversible genome expansion. Genome Research. 2000;10:908–915. doi: 10.1101/gr.10.7.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili G, Feldman M. Intergenomic suppression of endosperm protein genes in common wheat. Can J Genet Cytol. 1983;26:651–656. [Google Scholar]
- Watterson GA. On the time for gene silencing at duplicate loci. Genetics. 1983;105:745–766. doi: 10.1093/genetics/105.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Mol Biol. 2000;42:225–249. doi: 10.1023/A:1006392424384. [DOI] [PubMed] [Google Scholar]
- Zhu T, Schupp JM, Oliphant A, Keim P. Hypomethylated sequences - characterization of the duplicate soybean genome. Mol Gen Genet. 1994;244:638–645. doi: 10.1007/BF00282754. [DOI] [PubMed] [Google Scholar]
- Feschotte C, Jiang N, Wessler SR. Plant transposable elements: where genetics meets genomics. Nat Rev Genet. 2002;3:329–341. doi: 10.1038/nrg793. [DOI] [PubMed] [Google Scholar]
- Sabot F, Simon D, Bernard M. Plant transposable elements, with an emphasis on grass species. Euphytica. 2004;139:227–247. doi: 10.1007/s10681-004-3179-y. [DOI] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O. et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Dong FG, Miller JT, Jackson SA, Wang GL, Ronald PC, Jiang JM. Rice (Oryza sativa) centromeric regions consist of complex DNA. Proc Natl Acad Sci USA. 1998;95:8135–8140. doi: 10.1073/pnas.95.14.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui KN, Suzuki G, Lagudah ES, Rahman S, Appels R, Yamamoto M, Mukai Y. Physical arrangement of retrotransposon-related repeats in centromeric regions of wheat. Plant Cell Physiol. 2001;42:189–196. doi: 10.1093/pcp/pce026. [DOI] [PubMed] [Google Scholar]
- Kishii M, Nagaki K, Tsujimoto H. A tandem repetitive sequence located in the centromeric region of common wheat (Triticum aestivum) chromosomes. Chromosome Res. 2001;9:417–428. doi: 10.1023/A:1016739719421. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL. Transposable element contributions to plant gene and genome evolution. Plant Mol Biol. 2000;42:251–269. doi: 10.1023/A:1006344508454. [DOI] [PubMed] [Google Scholar]
- Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nature Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- Bourchis D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- Coen ES, Carpenter R, Martin C. Transposable elements generate novel spatial patterns of gene-expression in Antirrhinum majus. Cell. 1986;47:285–296. doi: 10.1016/0092-8674(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Fu DL, Szucs P, Yan LL, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat (vol 273, pg 54, 2005) Mol Genet Genomics. 2005;274:442–443. doi: 10.1007/s00438-005-0045-0. [DOI] [PubMed] [Google Scholar]
- Kloeckener-Gruissem B, Freeling M. Transposon-induced promoter scrambling: a mechanism for the evolution of new alleles. Proc Natl Acad Sci USA. 1995;92:1836–1840. doi: 10.1073/pnas.92.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukoianov A, Yan LL, Blechl A, Sanchez A, Dubcovsky J. Regulation of VRN-1 vernalization genes in normal and transgenic polyploid wheat. Plant Physiol. 2005;138:2364–2373. doi: 10.1104/pp.105.064287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcginnis W, Shermoen AW, Beckendorf SK. A transposable element inserted just 5' to a Drosophila glue protein gene alters gene-expression and chromatin structure. Cell. 1983;34:75–84. doi: 10.1016/0092-8674(83)90137-X. [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA. 2006;103:19581–19586. doi: 10.1073/pnas.0607142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA. 2003;100:6263–6268. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Saier MH. A Novel Mechanism of Transposon-Mediated Gene Activation. Plos Genetics. 2009;5:e1000689. doi: 10.1371/journal.pgen.1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K, Zhang F, Tsukiyama T, Saito H, Hancock CN, Richardson AO, Okumoto Y, Tanisaka T, Wessler SR. Unexpected consequences of a sudden and massive transposon amplification on rice gene expression. Nature. 2009;461:1130–1134. doi: 10.1038/nature08479. [DOI] [PubMed] [Google Scholar]
- Chopra S, Brendel V, Zhang J, Axtell JD, Peterson T. Molecular characterization of a mutable pigmentation phenotype and isolation of the first active transposable element from Sorghum bicolor. Proc Natl Acad Sci USA. 1999;96:15330–15335. doi: 10.1073/pnas.96.26.15330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Flavell RB, Thompson RD. Identification of a transposon-like insertion in a Glu-1 allele of wheat. Molecular & General Genetics. 1987;209:326–332. doi: 10.1007/BF00329661. [DOI] [PubMed] [Google Scholar]
- Akhunov ED, Akhunova AR, Dvorak J. Mechanisms and rates of birth and death of dispersed duplicated genes during the evolution of a multigene family in diploid and tetraploid wheats. Mol Biol Evol. 2007;24:539–550. doi: 10.1093/molbev/msl183. [DOI] [PubMed] [Google Scholar]
- Kalendar R, Tanskanen J, Immonen S, Nevo E, Schulman AH. Genome evolution of wild barley (Hordeum spontaneum) by BARE-1 retrotransposon dynamics in response to sharp microclimatic divergence. Proc Natl Acad Sci USA. 2000;97:6603–6607. doi: 10.1073/pnas.110587497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL. The paleontology of intergene retrotransposons of maize. Nat Genet. 1998;20:43–45. doi: 10.1038/1695. [DOI] [PubMed] [Google Scholar]
- Lönnig W-E, Saedler H. Chromosome rearrangements and transposable elements. Annu Rev Genet. 2003;36:389–410. doi: 10.1146/annurev.genet.36.040202.092802. [DOI] [PubMed] [Google Scholar]
- McClintock B. The origin and behavior of mutable loci in maize. Proc Natl Acad Sci USA. 1950;36:344–355. doi: 10.1073/pnas.36.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Grandbastien MA, Spielmann A, Caboche M. Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature. 1989;337:376–380. doi: 10.1038/337376a0. [DOI] [PubMed] [Google Scholar]
- Echenique V, Stamova B, Wolters P, Lazo G, Carollo V, Dubcovsky J. Frequencies of Ty1-copia and Ty3-gypsy retroelements within the Triticeae EST databases. Theor Appl Genet. 2002;104:840–844. doi: 10.1007/s00122-001-0849-1. [DOI] [PubMed] [Google Scholar]
- Hirochika H. Modification of gene expression and non-mendelian inheritance. Japan: NIAR; 1995. Activation of plant retrotransposons by stress; pp. 15–21. [Google Scholar]
- Wendel JF, Wessler SR. Retrotransposon-mediated genome evolution on a local ecological scale. Proc Natl Acad Sci USA. 2000;97:6250–6252. doi: 10.1073/pnas.97.12.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer A. Endogenous targets of transcriptional gene silencing in Arabidopsis. Plant Cell. 2000;12:1165–1178. doi: 10.1105/tpc.12.7.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompa R, McCallum CM, Delrow J, Henikoff JG, van Steensel B, Henikoff S. Genome-wide profiling of DNA methylation reveals transposon targets of CHROMOMETHYLASE3. Curr Biol. 2002;12:65–68. doi: 10.1016/S0960-9822(01)00622-4. [DOI] [PubMed] [Google Scholar]
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nature Reviews Genetics. 2008;9:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S, Gassama M-P, Heidmann T. Taming of transposable elements by homology-dependent gene silencing. Nat Genet. 1999;21:209–212. doi: 10.1038/5997. [DOI] [PubMed] [Google Scholar]
- Wu-Scharf D, Jeong B-R, Zhang C, Cerutti H. Transgene and transposon silencing in Chlamydomonas reinhardtii by a DEAH-Box RNA helicase. Science. 2000;290:1159–1162. doi: 10.1126/science.290.5494.1159. [DOI] [PubMed] [Google Scholar]
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. doi: 10.1038/nature02874. [DOI] [PubMed] [Google Scholar]
- Qi Y, He X, Wang X-J, Kohany O, Jurka J, Hannon GJ. Distinct catalytic and non-catalytic roles of ARGONAUTE4 in RNA-directed DNA methylation. Nature. 2006;443:1008–1012. doi: 10.1038/nature05198. [DOI] [PubMed] [Google Scholar]
- Schwach F, Moxon S, Moulton V, Dalmay T. Deciphering the diversity of small RNAs in plants: the long and short of it. Brief Funct Genomic Proteomic. 2009;8:472–481. doi: 10.1093/bfgp/elp024. [DOI] [PubMed] [Google Scholar]
- Ketting RF, Haverkamp THA, van Luenen HGAM, Plasterk RHA. mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell. 1999;99:133–141. doi: 10.1016/S0092-8674(00)81645-1. [DOI] [PubMed] [Google Scholar]
- Tabara H, Sarkissian M, Kelly WG, Fleenor J, Grishok A, Timmons L, Fire A, Mello CC. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/S0092-8674(00)81644-X. [DOI] [PubMed] [Google Scholar]
- Miura A. Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature. 2001;411:212–214. doi: 10.1038/35075612. [DOI] [PubMed] [Google Scholar]
- Singer T, Yordan C, Martienssen RA. Robertson's Mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1) Genes Dev. 2001;15:591–602. doi: 10.1101/gad.193701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Kobayashi A, Kawabe A, Mathieu O, Miura A, Kakutani T. Bursts of retrotransposition reproduced in Arabidopsis. Nature. 2009;461:423–426. doi: 10.1038/nature08351. [DOI] [PubMed] [Google Scholar]
- He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RHA. Diversity of microRNAs in human and chimpanzee brain. Nat Genet. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- Burnside J, Ouyang M, Anderson A, Bernberg E, Lu C, Meyers B, Green P, Markis M, Isaacs G, Huang E. et al. Deep sequencing of chicken microRNAs. BMC Genomics. 2008;9:185. doi: 10.1186/1471-2164-9-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL. et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS ONE. 2007;2:e219. doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson IR, Zhang X, Lu C, Johnson L, Meyers BC, Green PJ, Jacobsen SE. Dissecting Arabidopsis thaliana DICER function in small RNA processing, gene silencing and DNA methylation patterning. Nat Genet. 2006;38:721–725. doi: 10.1038/ng1804. [DOI] [PubMed] [Google Scholar]
- Klevebring D, Street N, Fahlgren N, Kasschau K, Carrington J, Lundeberg J, Jansson S. Genome-wide profiling of Populus small RNAs. BMC Genomics. 2009;10:620. doi: 10.1186/1471-2164-10-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Kulkarni K, Souret FF, MuthuValliappan R, Tej SS, Poethig RS, Henderson IR, Jacobsen SE, Wang W, Green PJ. et al. MicroRNAs and other small RNAs enriched in the Arabidopsis RNA-dependent RNA polymerase-2 mutant. Genome Res. 2006;16:1276–1288. doi: 10.1101/gr.5530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Tej SS, Luo S, Haudenschild CD, Meyers BC, Green PJ. Elucidation of the small RNA component of the transcriptome. Science. 2005;309:1567–1569. doi: 10.1126/science.1114112. [DOI] [PubMed] [Google Scholar]
- Ruby JG, Jan C, Player C, Axtell MJ, Lee W, Nusbaum C, Ge H, Bartel DP. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127:1193–1207. doi: 10.1016/j.cell.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Szittya G, Moxon S, Santos D, Jing R, Fevereiro M, Moulton V, Dalmay T. High-throughput sequencing of Medicago truncatula short RNAs identifies eight new miRNA families. BMC Genomics. 2008;9:593. doi: 10.1186/1471-2164-9-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubcovsky J, Dvorak J. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science. 2007;316:1862–1866. doi: 10.1126/science.1143986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC Gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J. A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science. 2009;323:1357–1360. doi: 10.1126/science.1166289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004;303:1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier S, McCallum B, Loutre C, Banks T, Wicker T, Feuillet C, Keller B, Jordan M. Leaf rust resistance gene Lr1 , isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Molecular Biology. 2007;65:93–106. doi: 10.1007/s11103-007-9201-8. [DOI] [PubMed] [Google Scholar]
- Gu YQ, Salse J, Coleman-Derr D, Dupin A, Crossman C, Lazo GR, Huo N, Belcram H, Ravel C, Charmet G. et al. Types and rates of sequence evolution at the high-molecular-weight glutenin locus in hexaploid wheat and its ancestral genomes. Genetics. 2006;174:1493–1504. doi: 10.1534/genetics.106.060756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris JD, Haen KM, Gill BS. Saturation mapping of a gene-rich recombination hot spot region in wheat. Genetics. 2000;154:823–835. doi: 10.1093/genetics/154.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KS, Gill BS, Endo TR, Boyko EV. Identification and high-density mapping of gene-rich regions in chromosome group 5 of wheat. Genetics. 1996;143:1001–1012. doi: 10.1093/genetics/143.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Stein N, Albar L, Feuillet C, Schlagenhauf E, Keller B. Analysis of a contiguous 211 kb sequence in diploid wheat (Triticum monococcum L.) reveals multiple mechanisms of genome evolution. Plant J. 2001;26:307–316. doi: 10.1046/j.1365-313X.2001.01028.x. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Naveh-Many T, Cedar H, Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981;292:860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Meunier J, Khelifi A, Navratil V, Duret L. Homology-dependent methylation in primate repetitive DNA. Proc Natl Acad Sci USA. 2005;102:5471–5476. doi: 10.1073/pnas.0408986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker E. Premeiotic instability of repeated sequences in Neurospora crassa. Ann Rev Genet. 1990;24:579–613. doi: 10.1146/annurev.ge.24.120190.003051. [DOI] [PubMed] [Google Scholar]
- Lister R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Wicker T, Guyot R, Yahiaoui N, Keller B. CACTA transposons in Triticeae. A diverse family of high-copy repetitive elements. Plant Physiol. 2003;132:52–63. doi: 10.1104/pp.102.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasschau KD, Fahlgren N, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Carrington JC. Genome-wide profiling and analysis of Arabidopsis siRNAs. PLoS Biol. 2007;5:e57. doi: 10.1371/journal.pbio.0050057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuta K, Venu RC, Lu C, Belo A, Vemaraju K, Kulkarni K, Wang W, Pillay M, Green PJ, Wang G-l. et al. An expression atlas of rice mRNAs and small RNAs. Nat Biotech. 2007;25:473–477. doi: 10.1038/nbt1291. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang Z, Gerstein MB, Zheng D. Small RNAs originated from pseudogenes: cis- or trans-acting? PLoS Comput Biol. 2009;5:e1000449. doi: 10.1371/journal.pcbi.1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LL, Echalier B, Chao S, Lazo GR, Butler GE, Anderson OD, Akhunov ED, Dvorak J, Linkiewicz AM, Ratnasiri A. et al. A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics. 2004;168:701–712. doi: 10.1534/genetics.104.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker T, Taudien S, Houben A, Keller B, Graner A, Platzer M, Stein N. A whole-genome snapshot of 454 sequences exposes the composition of the barley genome and provides evidence for parallel evolution of genome size in wheat and barley. Plant J. 2009;59:712–722. doi: 10.1111/j.1365-313X.2009.03911.x. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Linsley PS. Noise amidst the silence: off-target effects of siRNAs? Trends Genet. 2004;20:521–524. doi: 10.1016/j.tig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Voinnet O, Chappell L, Baulcombe D. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002;21:4671–4679. doi: 10.1093/emboj/cdf464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang H, Padmanabhan C, Li F, Kamei A, Bhaskar PB, Ouyang S, Jiang J, Buell CR, Baker B. Identification of miniature inverted-repeat transposable elements (MITEs) and biogenesis of their siRNAs in the Solanaceae: New functional implications for MITEs. Genome Res. 2009;19:42–56. doi: 10.1101/gr.078196.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Fire A. Recognition and silencing of repeated DNA. Annu Rev Genet. 2000;34:187–204. doi: 10.1146/annurev.genet.34.1.187. [DOI] [PubMed] [Google Scholar]
- Yang N, Kazazian HH. L1 retrotransposition is suppressed by endogenously encoded small interfering RNAs in human cultured cells. Nat Struct Mol Biol. 2006;13:763–771. doi: 10.1038/nsmb1141. [DOI] [PubMed] [Google Scholar]
- Ronsseray S, Josse T, Boivin A, Anxolabéhère D. Telomeric transgenes and trans-silencing in Drosophila. Genetica. 2003;117:327–335. doi: 10.1023/A:1022929121828. [DOI] [PubMed] [Google Scholar]
- Nobuta K, Lu C, Shrivastava R, Pillay M, De Paoli E, Accerbi M, Arteaga-Vazquez M, Sidorenko L, Jeong D-H, Yen Y. et al. Distinct size distribution of endogenous siRNAs in maize: Evidence from deep sequencing in the mop1-1 mutant. Proc Natl Acad Sci USA. 2008;105:14958–14963. doi: 10.1073/pnas.0808066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bennetzen JL. Plant retrotransposons. Annu Rev Genet. 1999;33:479–532. doi: 10.1146/annurev.genet.33.1.479. [DOI] [PubMed] [Google Scholar]
- Vicient C, Kalendar R, Anamthawat-Jonsson K, Suoniemi A, Schulman A. Structure, functionality, and evolution of the BARE-1 retrotransposon of barley. Genetica. 1999;107:53–63. doi: 10.1023/A:1003929913398. [DOI] [PubMed] [Google Scholar]
- Almeida R, Allshire RC. RNA silencing and genome regulation. Trends in Cell Biology. 2005;15:251–258. doi: 10.1016/j.tcb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Herr AJ, Jensen MB, Dalmay T, Baulcombe DC. RNA polymerase IV directs silencing of endogenous DNA. Science. 2005;308:118–120. doi: 10.1126/science.1106910. [DOI] [PubMed] [Google Scholar]
- Huettel B, Kanno T, Daxinger L, Aufsatz W, Matzke AJM, Matzke M. Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO J. 2006;25:2828–2836. doi: 10.1038/sj.emboj.7601150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell. 2005;120:613–622. doi: 10.1016/j.cell.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Chan SWL, Zilberman D, Xie Z, Johansen LK, Carrington JC, Jacobsen SE. RNA silencing genes control de novo DNA methylation. Science. 2004;303:1336. doi: 10.1126/science.1095989. [DOI] [PubMed] [Google Scholar]
- Teixeira FK, Heredia F, Sarazin A, Roudier F, Boccara M, Ciaudo C, Cruaud C, Poulain J, Berdasco M, Fraga MF. et al. A role for RNAi in the selective correction of DNA methylation defects. Science. 2009;323:1600–1604. doi: 10.1126/science.1165313. [DOI] [PubMed] [Google Scholar]
- Rabinowicz PD, Palmer LE, May BP, Hemann MT, Lowe SW, McCombie WR, Martienssen RA. Genes and transposons are differentially methylated in plants, but not in mammals. Genome Res. 2003;13:2658–2664. doi: 10.1101/gr.1784803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S. Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet. 2007;39:61–69. doi: 10.1038/ng1929. [DOI] [PubMed] [Google Scholar]
- Springer NM, Kaeppler SM. Evolutionary divergence of monocot and dicot methyl-CpG-binding domain proteins. Plant Physiol. 2005;138:92–104. doi: 10.1104/pp.105.060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J, Akhunov ED. Tempos of gene locus deletions and duplications and their relationship to recombination rate during diploid and polyploid evolution in the Aegilops-Triticum alliance. Genetics. 2005;171:323–332. doi: 10.1534/genetics.105.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J, Akhunov ED, Akhunov AR, Deal KR, Luo MC. Molecular characterization of a diagnostic DNA marker for domesticated tetraploid wheat provides evidence for gene flow from wild tetraploid wheat to hexaploid wheat. Mol Biol Evol. 2006;23:1386–1396. doi: 10.1093/molbev/msl004. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucl Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 - sRNA counts in TE families. Box plots represent the distribution of the total counts of sRNA perfectly matching wheat TEs of each family in the seven major superfamilies deposited in the TREP database. Numbers above the whiskers represent the number of TREP elements within each superfamily considered. Figure S2 - LTR length in Copia and Gypsy TEs. Box plots represent the distribution of LTR lengths in the Copia and Gypsy elements deposited in the TREP database. Table S1 - Summary of sRNA libraries. Table S2 - Distribution of sRNA counts in the EU835198 genomic region (T. turgidum). Table S3 - Distribution of sRNA counts in the DQ871219 genomic region (T. turgidum). Table S4 - Distribution of sRNA counts in the EF540321 genomic region (T. turgidum). Table S5 - Distribution of sRNA counts in the EF567062 genomic region (T. aestivum). Table S6 - Distribution of sRNA counts in the DQ537335 genomic region (T. aestivum). Table S7 - sRNA counts in the different TE families. Table S8 - Estimates of LTR age of insertion and cytosine methylation in the CG, CHG, and CHH contexts.
dbmanager.py - python program for sRNA database setup.
scanner.py - python program for scrolling window analysis with frame size between 18 and 33 nt that scans through the entire query sequence with a 1 nt increment after each read.
srna_seeker.py - program for scanning nucleotide sequences for perfect matching sRNAs using "dbmanager.py" to access the sRNA database and "scanner.py" to analyze the query sequences.
Cmet_scan.py - python program for counting mutations in potentially methylated sites.