Abstract
Although evidence indicates that activation of presympathetic paraventricular nucleus (PVN) neurons contributes to the pathogenesis of salt-sensitive hypertension, the underlying cellular mechanisms are not fully understood. Recent evidence indicates that small conductance Ca2+-activated K+ (SK) channels play a significant role in regulating the excitability of a key group of sympathetic regulatory PVN neurons, those with axonal projections to the rostral ventrolateral medulla (RVLM; i.e., PVN-RVLM neurons). In the present study, rats consuming a high salt (2% NaCl) diet were made hypertensive by systemic infusion of angiotensin II (AngII), and whole cell patch-clamp recordings were made in brain slice from retrogradely labeled PVN-RVLM neurons. To determine if the amplitude of SK current was altered in neurons from hypertensive rats, voltage-clamp recordings were performed to isolate SK current. Results indicate that SK current amplitude (P < 0.05) and density (P < 0.01) were significantly smaller in the hypertensive group. To investigate the impact of this on intrinsic excitability, current-clamp recordings were performed in separate groups of PVN-RVLM neurons. Results indicate that the frequency of spikes evoked by current injection was significantly higher in the hypertensive group (P < 0.05–0.01). Whereas bath application of the SK channel blocker apamin significantly increased discharge of neurons from normotensive rats (P < 0.05–0.01), no effect was observed in the hypertensive group. In response to ramp current injections, subthreshold depolarizing input resistance was greater in the hypertensive group compared with the normotensive group (P < 0.05). Blockade of SK channels increased depolarizing input resistance in normotensive controls (P < 0.05) but had no effect in the hypertensive group. On termination of current pulses, a medium afterhyperpolarization potential (mAHP) was observed in most neurons of the normotensive group. In the hypertensive group, the mAHP was either small or absent. In the latter case, an afterdepolarization potential (ADP) was observed that was unaffected by apamin. Apamin treatment in the normotensive group blocked the mAHP and revealed an ADP resembling that seen in the hypertensive group. We conclude that diminished SK current likely underlies the absence of mAHPs in PVN-RVLM neurons from hypertensive rats. Both the ADP and greater depolarizing input resistance likely contribute to increased excitability of PVN-RVLM neurons from rats with AngII-Salt hypertension.
INTRODUCTION
Low-dose systemic infusion of angiotensin II in combination with a high salt (2% NaCl) diet (AngII-Salt) leads to development of arterial hypertension in rats. Like many forms of hypertension in humans, the AngII-Salt model is salt-sensitive and dependent on sustained activation of the sympathetic nervous system (King and Fink 2006; King et al. 2008; Kline et al. 1990; Osborn et al. 2007; Toney et al. 2010). At present, the CNS sites and cellular mechanisms that mediate the increase of sympathetic nerve activity are unknown.
Studies have demonstrated that acute central administration of NaCl and AngII activate sympathetic-regulatory neurons in the hypothalamic PVN via inputs they receive from forebrain circumventricular organs (CVOs). Because forebrain CVOs lack a blood-brain barrier, their neurons are capable of sensing and responding to elevated levels of plasma Na+/osmolality and circulating AngII (Barth and Gerstberger 1999; Bourque et al. 2007; Brooks et al. 2001; Ferguson and Bains 1997; Gutman et al. 1998; Shi et al. 2007, 2008; Stocker and Toney 2005; Toney et al. 2003). Synaptic activation of presympathetic PVN neurons thereby translates increases of plasma AngII and Na+ concentration into elevations of sympathetic nerve activity (Chen and Toney 2001; Schad and Seller 1975; Shi et al. 2007, 2008; Yasuda et al. 2000). Increases of sympathetic activity and arterial pressure (Chen and Toney 2001; Martin and Haywood 1992; Porter and Brody 1985) that result from PVN neuronal activation are mediated through a variety of efferent pathways (Chen and Toney 2003, 2010; Kannan and Yamashita 1983; Stocker et al. 2004, 2006; Yang and Coote 1998; Yang et al. 2001), including monosynaptic connections with sympathoexcitatory neurons in the rostral ventrolateral medulla (RVLM) (Stocker et al. 2006; Yang et al. 2001), the main source of excitatory synaptic input to sympathetic preganglionic neurons in the spinal cord (Guyenet et al. 2001).
Studies performed to date indicate that changes in both synaptic inputs to and intrinsic membrane properties of PVN-RVLM neurons contribute to sustained activation of sympathetic activity in hypertension. For example, Li and Pan reported that in vitro spontaneous discharge of PVN-RVLM neurons from spontaneously hypertensive rats is greater than that of neurons from normotensive controls. The latter appears to involve both greater glutamatergic and reduced GABAergic activity (Li and Pan 2006; Li et al. 2008). Similarly, Sonner et al. reported that potassium A-current is diminished among PVN-RVLM neurons from rats with renal-vascular hypertension. They further demonstrated that the diminution of A-current contributes to enhanced in vitro spontaneous discharge (Sonner et al. 2008). In further exploring ion channel mechanisms controlling PVN-RVLM neuronal excitability, we recently reported that PVN-RVLM neurons express a prominent current mediated by small conductance Ca2+-activated K+ (SK) channels. Because this current was determined to potently suppress the intrinsic excitability of PVN-RVLM neurons (Chen and Toney 2009), the present study was performed to determine if PVN-RVLM neurons from rats with AngII-Salt hypertension exhibit diminished SK current and if this contributes to enhancement of their excitability.
METHODS
Animal preparation and AngII-Salt hypertension
Male Sprague-Dawley rats (n = 25, 225-250g, Charles River Labs, Wilmington, MA) were individually housed in a temperature controlled room (22–23°C) with a 14 h:10 h light-dark cycle. Rats in the normotensive (NT) group were placed on a normal salt diet (0.4% NaCl), and rats in the AngII-Salt hypertensive (HT) group were placed on a high salt diet (2% NaCl). Diets were otherwise identical in calories from fat and protein as well as total carbohydrate and sucrose (Research Diets, New Brunswick, NJ). After 1 wk on each diet, animals were anesthetized with isoflurane (3% in O2) and instrumented with a telemetry transmitter (Data Science) for recording arterial blood pressure and heart rate. Each animal received daily injections of penicillin G (30,000 U/100 g body wt sc) and buprenorphine (0.05 mg/kg sc) for 3 days after surgery. After recovering for 5–7 days, baseline values of arterial pressure and heart rate were recorded for 7 days. Then an osmotic mini-pump (2ML2, Alzet) was implanted subcutaneously to deliver AngII (150 ng · kg−1 · min−1) in the HT group or vehicle (saline) in the NT group. AngII and saline were infused for 14 days prior to performing electrophysiological studies. All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee of The University of Texas Health Science Center at San Antonio.
Retrograde labeling of PVN-RVLM neurons
Two to 3 days before implantation of osmotic mini-pumps (see preceding text), PVN neurons were retrogradely labeled from the ipsilateral RVLM as previously described (Cato and Toney 2005; Chen and Toney 2009). Briefly, rats were anesthetized with isoflurane (3% in O2), placed in a stereotaxic frame, and a small burr hole was made to expose the cerebellum. A glass micropipette was lowered into the pressor region of RVLM (coordinates: −12.7 mm caudal to bregma, 1.8 mm lateral to midline and 8.9 mm below the skull) and rhodamine-containing microspheres were microinjected in a volume of 50 nl. Location of the tracer was verified postmortem in histological sections through the RVLM (Cato and Toney 2005; Chen and Toney 2009; Stocker et al. 2006).
Electrophysiology
After 2 wk of continuous AngII or saline infusion, rats were anesthetized with isoflurane (3% in O2) and decapitated. Brains were removed and placed in ice-cold cutting solution containing (in mM) 206 sucrose, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 1 CaCl2, 1 MgCl2, 10 d-glucose, and 0.4 ascorbic acid. Osmolality and pH were adjusted to 290–295 mosmol l−1 and 7.4, respectively. Solution pH and pO2 were maintained by equilibration with a 95% O2-5% CO2 gas mixture. Coronal slices through the PVN were cut to a thickness of 300 μm on a vibrating microtome (Leica VT 1000S; Leica, Nussloch, Germany). Slices were incubated at room temperature (24–26°C) for ≥2 h in continuously gassed artificial cerebrospinal fluid (ACSF) containing (in mM) 125 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 10 d-glucose, and 0.4 ascorbic acid (osmolality: 295–300 mosmol l−1; pH 7.4). Slices were transferred to a glass-bottomed recording chamber and viewed through an upright microscope (E600FN, Nikon) equipped with DIC optics, epi-fluorescence, an infrared (IR) filter and an IR-sensitive video camera (C2400, Hamamatsu, Bridgewater, NJ). An appropriate filter was used to visualize neurons retrogradely labeled with rhodamine beads.
Patch electrodes were pulled (Flaming/Brown P-97, Sutter Instrument, Novato, CA) from borosilicate glass capillaries and polished to a tip resistance of 4–5 MΩ. Electrodes were filled with a solution containing (in mM) 135 K-gluconate, 10 HEPES, 0.1 EGTA, 1.0 MgCl2, 1.0 NaCl, 2.0 Na2ATP, and 0.5 Na2GTP (osmolality: 280–285 mosmol l−1; pH 7.3). Note that a relatively low concentration of EGTA (0.1 mM) was used to allow intracellular free Ca2+ to accumulate and activate SK channels during membrane potential depolarization (Brenner et al. 2005; Chen and Toney 2009). Records were not corrected for a liquid junction potential of −15 mV. After achieving a GΩ seal and whole cell configuration, cell capacitance, access resistance, and resting membrane potential (Vm) were monitored until stable. In voltage-clamp recordings, uncompensated series resistance averaged 16 ± 2 MΩ. Cells that met the following criteria were included in the analysis: action potential amplitude ≥50 mV from threshold to peak, input resistance (Rinput) >0.5 GΩ (determined by injection of −20 pA from a holding potential of −80 mV), resting Vm negative to −50 mV, and <20% change in series resistance during the recording. Recordings were made using an Axopatch 200B amplifier and pCLAMP software (v10.0, Axon Instruments, Union City, CA). Signals were filtered at 1 kHz, digitized at 10 kHz (Digidata 1322A, Axon Instruments), and saved on a computer for off-line analysis.
Recording SK current
To study SK current, voltage-clamp recordings were performed with intracellular solution containing the cAMP analogue 8-(4-chlorophenylthio) 3′,5′-cyclic adenosine monophosphate (8CPT-cAMP, 50 μM) to block the slow afterhyperpolarization current (Stocker et al. 1999). Recordings were performed in the presence of extracellular tetrodotoxin (TTX, 1.0 μM) and tetraethylammonium (TEA, 1.0 mM). Membrane potential was clamped at −60 mV and stepped to +10 mV for 100 ms. On returning Vm to −60 mV, an outward tail current was recorded. After recording a control tail current, apamin (100 nM) was bath applied to selectively block SK channels. The tail current recorded during treatment was subtracted from the corresponding control tail current to isolate the SK current. Decay of the SK current was analyzed by fitting the subtracted current with a one-phase exponential.
Testing neuronal excitability
Excitability of neurons from NT and HT rats was studied in current-clamp mode in the absence of TTX, TEA, or 8CPT-cAMP. With Vm adjusted to-80 mV by continuous negative current injection, a series of square-wave current injections was delivered in steps of +25 pA, each for a duration of 800 ms. To study the medium afterhyperpolarization potential (mAHP) and afterdepolarization potential (ADP), +150 pA current injections (500 ms) were made from a potential of −60 mV. Between current injections, Vm was returned to −60 mV for 5 s. Amplitude of the slow AHP was quantified 1.5 s after termination of each current pulse. To determine the action potential voltage threshold (Vt) and depolarizing Rinput below Vt, ramp current injections (0.2 pA ms−1, 1,000 ms) were made from a potential of −80 mV. Square-wave and ramp current injections were made in the same neurons. Effects of SK channel blockade on neuronal excitability were determined by comparing current evoked Vm and spike frequency responses under control conditions with responses recorded from separate groups of neurons exposed to bath applied apamin (100 nM). Note that current- and voltage-clamp recordings were made from different groups of PVN-RVLM neurons.
Chemicals
All chemicals were obtained from Sigma-Aldrich (St Louis, MO) except for TTX (Tocris Bioscience) and TEA (Fluka BioChemika).
Statistical analysis
Amplitude of the apamin-sensitive (SK) current was compared across groups of neurons from NT and HT rats using an unpaired t-test. Passive membrane properties of neurons from NT and HT rats under control conditions were compared with corresponding values determined in separate groups of neurons exposed to apamin using a one-way ANOVA. Excitability tested with graded square-wave current injections was compared across groups using a two-way ANOVA. A similar approach was used for group comparisons of ISI data for assessment of spike-frequency adaptation. When significant main effects were found, Dunn's post hoc tests were used for multiple pair-wise comparisons. All statistical tests were performed using Prism software (v5.0, GraphPad). Differences between means were considered significant at P < 0.05. Summary data are reported as means ± SE.
RESULTS
Recordings were made from 46 PVN-RVLM neurons; 22 from NT rats (n = 12) and 24 from HT rats (n = 13). Before performing brain slice studies, telemetric recordings in conscious rats revealed that baseline mean arterial pressure (MAP) and heart rate (HR) were not different (P > 0.05) between NT (MAP: 97 ± 2 mmHg, HR: 412 ± 7 bpm) and HT (MAP: 101 ± 2 mmHg, HR: 393 ± 5 bpm) rats. On day 14 of AngII administration, MAP was significantly (P < 0.001) higher in HT (134 ± 5 mmHg) than NT (101 ± 1 mmHg) rats. Throughout the infusion protocols, there was no difference in HR between groups (NT: 416 ± 4 bpm; HT: 402 ± 5 bpm).
Comparison of passive membrane properties
Table 1 shows resting Vm, Rinput, and whole cell capacitance (Cm) of PVN-RVLM neurons from NT and HT rats. No significant differences were identified either in the absence or presence of bath applied apamin (100 nM). Note that values under control conditions and during bath application of apamin were determined from separate groups of neurons.
Table 1.
Passive membrane properties of PVN-RVLM neurons
| Group | n | Vm, mV | Rinput, GΩ | Cm, pF |
|---|---|---|---|---|
| NT-control | 8 | −62 ± 2 | 1.2 ± 0.1 | 25 ± 2 |
| NT-apamin | 8 | −63 ± 2 | 1.2 ± 0.1 | 24 ± 2 |
| HT-control | 10 | −66 ± 2 | 1.3 ± 0.1 | 28 ± 2 |
| HT-apamin | 7 | −60 ± 2 | 1.3 ± 0.1 | 23 ± 2 |
PVN-RVLM, paraventricular nucleus - rostral ventrolateral medulla; Vm, resting membrane potential; Cm, membrane capacitance; Rinput, input resistance; NT, normotensive; HT, hypotensive.
Comparison of SK current
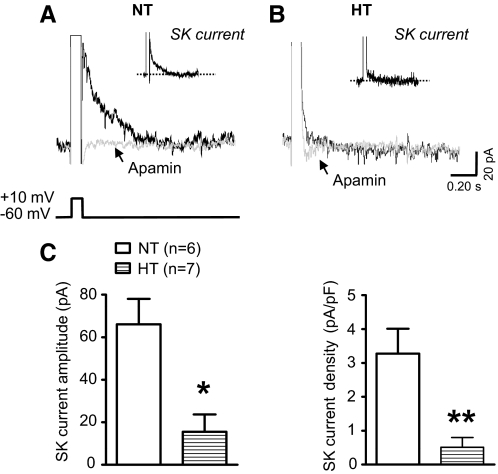
PVN-RVLM neurons express a prominent SK current (Chen and Toney 2009). Here an apamin-sensitive SK current was recorded in six of six neurons tested from NT rats. Figure 1A shows representative tail current traces before and after bath application of apamin (100 nM). Digital subtraction of these traces yielded the SK current (inset). Peak SK current amplitude and density averaged 66 ± 12 pA (Fig. 1C, left) and 3.3 ± 0.8 pA/pF (C, right), respectively. Figure 1B shows representative tail current traces before and after apamin and the SK current (inset) of a neuron from an HT rat. In the HT group (n = 7), peak current amplitude and density were significantly less than corresponding values in the NT group and averaged 16 ± 8 pA (Fig. 1C, left, P < 0.05) and 0.5 ± 0.3 pA/pF (C, right, P < 0.01), respectively.
Fig. 1.
Comparison of SK current among PVN-RVLM neurons from NT and HT rats. A: representative traces from a neuron of a NT rat showing outward tail current following step (100 ms) depolarization of Vm (−60 to +10 mV) in the absence (black trace) and presence (gray trace) of apamin (100 nM). B: outward tail current in a neuron from a HT rat in the absence (black trace) and presence (gray trace) of apamin (100 nM). Note: Insets in A and B show the net SK current obtained by subtraction (control - apamin). C: summary data show the amplitude (left) and density (right) of SK current for cells in each treatment group. All recordings were performed with TTX (0.5 μM) and TEA (1.0 mM) in the bath and with 8-(4-chlorophenylthio) 3′,5′-cyclic adenosine monophosphate (8CPT-cAMP, 50 μM) in the intracellular solution. *P < 0.05, **P < 0.01 vs. NT (unpaired Mann-Whitney test).
SK current regulates PVN-RVLM neuronal excitability
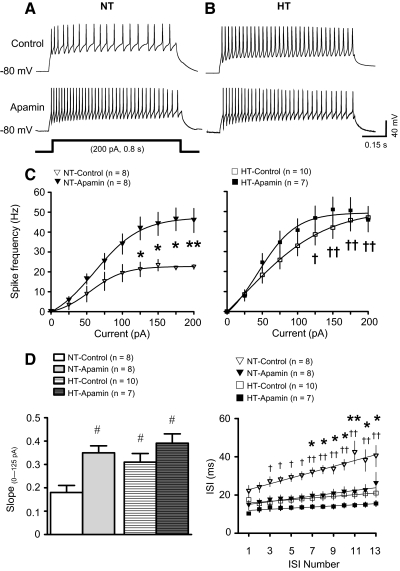
As reported earlier (Chen and Toney 2009), PVN-RVLM neurons lacked spontaneous discharge at resting Vm, but depolarizing current injections consistently evoked repetitive action potential firing. SK current regulation of excitability among neurons from NT and HT rats was investigated by comparing the relationship between the amplitude of injected current and the frequency of evoked discharge in the absence (control) and presence of the SK channel blocker apamin (100 nM).
Figure 2A shows repetitive action potential firing of neurons from NT rats in response to +200 pA current pulses in the absence (top) and presence (bottom) of apamin. Under control conditions, the frequency of spiking among neurons from NT rats (Fig. 2A, top) was significantly lower than that of neurons from HT rats (B, top, and C, left vs. right, P < 0.05–0.01). Blockade of SK channels with apamin significantly increase discharge frequency in the NT group (Fig. 2, A, bottom, and C, left, P < 0.05–0.01), but had no effect in the HT group (Fig. 2, B, bottom, and C, right). As a result, peak discharge frequency during SK channel blockade was similar across groups (Fig. 2, C, left vs. right).
Fig. 2.
Effect of SK channel blockade on excitability of PVN-RVLM neurons from NT and HT rats. A: voltage traces showing representative responses of PVN-RVLM neurons from NT rats to a 200 pA depolarizing current injection in the absence (top, control) and presence (bottom) of the SK channel blocker apamin (100 nM). Note that traces were recorded from 2 different neurons. B: representative responses of 2 different PVN-RVLM neurons from HT rats to depolarizing current injections in the absence (top, control) and presence (bottom) of apamin. C: graphs showing the relationship between the amplitude of injected current and the frequency of evoked discharge for cells from NT (left) and HT (right) rats in the absence (control) and presence of apamin. Note that the maximum discharge frequency achieved was lower in the NT (left) than HT (right) group under control conditions and that apamin increased discharge in the NT group (left) but not the HT group (right). D: the slope of the linear portion of current-discharge response (0–125 pA) curves is shown for neurons from NT and HT rats in the absence (control) and presence of apamin. Note that under control conditions the slope was significantly greater for neurons of the HT group than the NT group. Apamin significantly increased the slope of the response of the NT group but not the HT group (left). Interspike intervals (ISIs) plotted for trains of current-evoked action potentials revealed that average ISI and spike-frequency adaptation (ISI prolongation) were greater (right) among neurons from NT than HT rats. *P < 0.01, **P < 0.001 apamin vs. control groups from NT rats (2-way ANOVA). †P < 0.05, ††P < 0.01 HT vs. NT groups in the absence of apamin (2-way ANOVA). #P < 0.05 vs. NT groups in the absence of apamin (1-way Kruskall-Wallis ANOVA).
Excitability of PVN-RVLM neurons was also compared by examining the slope of stimulus-response curves for current pulses between 0 and +125 pA, which represented the linear portion of the relationship (Fig. 2D, left). The slope of the stimulus-response curve under control conditions was significantly greater for neurons from the HT than NT group (HT: 0.31 ± 0.04 Hz/pA; NT: 0.18 ± 0.03 Hz/pA; P < 0.05). Compared with control conditions, the slope was significantly greater (P < 0.05) in the NT group (0.35 ± 0.03 Hz/pA,) exposed to apamin. Apamin treatment was without affect in the HT group (0.39 ± 0.04 Hz/pA).
We next sought to determine if increased excitability was associated with a reduced rate of interspike interval (ISI) prolongation (i.e., spike frequency adaptation) in neurons from HT rats. Spike-frequency adaptation was compared by examining the slope of the ISI-ISI number curve (Fig. 2D, right). In the NT group, the slope of the liner fit of ISI-ISI number curve was significantly greater than that of the HT group (NT: 1.4 ± 0.2 ms/ISI; HT: 0.4 ± 0.2 ms/ISI; P < 0.05), suggesting that spike-frequency adaptation under control conditions was reduced in HT compared with NT neurons. The slope of ISI-ISI number curve in the NT group exposed to apamin was significantly less than that of the NT group under control conditions (0.7 ± 0.2 ms/ISI, P < 0.05 vs. NT-control). Compared with the control HT group, slope was unchanged in the HT group exposed to apamin (0.3 ± 0.1 ms/ISI, P > 0.05 vs. HT-control).
SK current regulates Vt and depolarizing Rinput in PVN-RVLM neurons
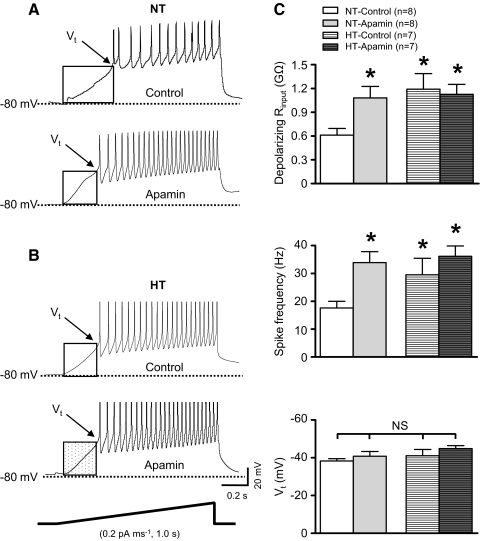
To determine whether action potential voltage threshold (Vt) and subthreshold depolarizing Rinput are altered in neurons from HT rats, ramp current injections (0.2 pA ms−1, 1 s duration) were performed. To evaluate regulation by SK channels, responses were compared across groups of control neurons and neurons exposed to apamin (100 nM). Prior to initiating each current ramp, Vm was set to −80 mV by negative current injection. Currents needed to hyperpolarize Vm were not different across NT (−17 ± 6 pA) and HT (−12 ± 3 pA) groups (P = 0.38) and were not different in groups exposed to apamin (NT-apamin: −17 ± 7 pA; P = 0.98 vs. NT-control; HT-apamin: −16 ± 5 pA; P = 0.38 vs. HT-control).
Figure 3A shows representative discharge responses of neurons from NT rats. Note that Vm depolarized gradually and action potentials were seen at Vt (Fig. 3A, top and bottom). Blockade of SK channels with apamin increased the rate of depolarization such that Vt was reached earlier during the current ramp (Fig. 3A, bottom). Thus apamin increased depolarizing Rinput measured below Vt. Figure 3B shows representative responses of a neuron from the control HT group (top) and the apamin exposed HT group (bottom). Note that the subthreshold depolarization rate of the control HT neuron (Fig. 3B, top) was greater than that of the control NT neuron (A, top). Figure 3C shows summary data of depolarizing Rinput (top), action potential firing rate (middle), and Vt (bottom) for separate groups of neurons from control and apamin treated NT and HT animals. Depolarizing Rinput was significantly greater among neurons of the control HT group compared with the control NT group (P < 0.05). Blockade of SK channels significantly increased depolarizing Rinput of neurons in the NT group but was without affect on neurons of the HT group (Fig. 3C, top, P < 0.05). Similar to discharge responses to depolarizing current pulses (Fig. 2C), action potential firing rate during current ramps was significantly greater for neurons in the HT group compared with NT controls. Apamin increased the firing rate of neurons in the NT group but was without affect in the HT group (Fig. 3C, middle, P < 0.05). Note that there was no difference in Vt between control NT and HT groups and separate groups exposed to apamin (Fig. 3C, bottom).
Fig. 3.
Effect of SK channel blockade on Vt and depolarizing Rinput of PVN-RVLM neurons from NT and HT rats. A: representative voltage traces from two different PVN-RVLM neurons from NT rats showing the response to ramp current injection in the absence (top, control) and presence (bottom) of the SK channel blocker apamin (100 nM). B: representative voltage traces from 2 different PVN-RVLM neurons from HT rats showing the response to ramp current injection in the absence (top, control) and presence (bottom) of apamin. In A and B, membrane potential depolarized gradually and action potential discharge began at a discrete voltage threshold (Vt). C: summary data showing that subthreshold depolarizing input resistance (Rinput, top) and spike frequency (middle) were greater under control conditions for neurons in the HT than NT group. Apamin increased both Rinput and spike frequency only in the NT group. Vt (bottom) was similar across groups under control conditions and was unchanged by apamin. *P < 0.05 vs. NT groups under control condition; NS, P > 0.05 (1-way Kruskall-Wallis ANOVA).
SK current contributes to the mAHP and masks an ADP in PVN-RVLM neurons
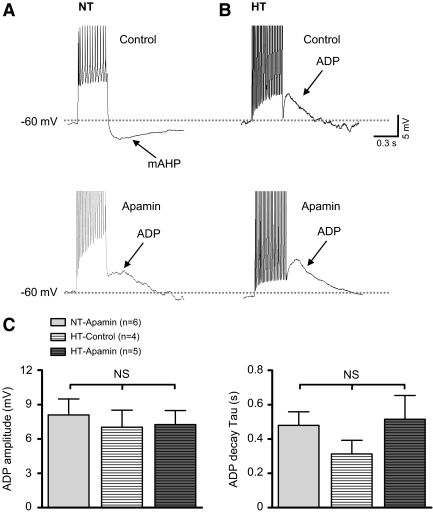
To explore a potential role for reduced SK current in the increased excitability observed among neurons from HT rats, we compared effects of SK channel blockade on evoked mAHPs and ADPs. Among neurons of the NT group under control conditions (Fig. 4A, top), a train of action potentials was induced by delivering a depolarizing current pulse (150 pA, 500 ms) from a potential of −60 mV. Note that a prominent mAHP was observed following termination of the action potential train in eight of eight neurons tested. In the presence of apamin (Fig. 4A, bottom), a shoulder-shaped ADP was observed following the spike train in six of eight neurons. The peak amplitude and decay time constant of ADPs averaged +8 ± 1 mV (Fig. 4C, left) and 479 ± 79 ms (C, right), respectively. The two neurons that did not show an obvious ADP during apamin treatment had smaller amplitude mAHPs (−4 ± 1 mV) compared with those recorded under control condition (n = 8; −7 ± 1 mV).
Fig. 4.
Effect of SK channel blockade on the mAHP and the ADP of PVN-RVLM neurons from NT and HT rats. A: representative voltage traces showing the effect of SK channel blockade with apamin (100 nM) on the mAHP recorded in neurons from NT rats. In the absence of apamin (top), a train of action potentials was induced by a depolarizing current pulse (150 pA, 500 ms). Following termination of action potential firing, a mAHP was observed. In the presence of apamin (bottom), the mAHP disappeared and ADP was revealed. B: representative voltage traces showing the effect of apamin (100 nM) on the mAHP and ADP of cells from HT rats. In the absence (top) and presence (bottom) of apamin, an ADP was observed. C: summary data showing the maximum ADP amplitude (left) and decay time constant (right) of cells from NT and HT rats. NS, P > 0.05 (1-way Kruskall-Wallis ANOVA).
Traces in Fig. 4B show representative ADPs recorded from neurons in the HT group. In the absence of apamin (top), an ADP was recorded in four of seven neurons. The peak amplitude and decay time constant of ADPs averaged +7 ± 1 mV (Fig. 4C, left) and 312 ± 80 ms (C, right), respectively. The remaining three cells in the HT group did not have an obvious ADP and showed a much smaller mAHP (−4 ± 2 mV) compared with that in the NT group under control conditions (P < 0.05). In the presence of apamin (Fig. 4B, bottom), an ADP was observed in five of seven neurons. The average peak amplitude of these ADPs was +8 ± 1 mV (Fig. 4C, left) and the decay time constant averaged 514 ± 139 ms (C, right). Similarly, cells in the HT group that did not have an obvious ADP in the presence of apamin had smaller amplitude mAHPs (−2.3 ± 1.4 mV) compared with cells from NT rats under control conditions. There was no difference in the peak amplitude or decay time constant of ADPs between control NT and HT groups and corresponding groups exposed to apamin.
It should be noted that an apamin-insensitive slow AHP was observed in eight of eight neurons from the NT group and seven of seven neurons from the HT group. Peak amplitude of slow AHPs did not differ across groups (NT: −2.8 ± 0.9 mV; HT: −2.4 ± 0.8 mV).
DISCUSSION
This study investigated the role of SK channels in regulating excitability of PVN-RVLM neurons from NT control and AngII-Salt HT rats. We found that the amplitude of whole cell SK current was reduced and that neuronal excitability was increased in the HT group compared with NT controls. Excitability of neurons from NT but not HT rats was significantly increased by SK channel blockade. The rate of subthreshold depolarization during ramp current injections was greater among neurons from HT than NT rats, indicating that depolarizing Rinput was greater in the HT group. During SK channel blockade, however, a greater increase in subthreshold depolarizing Rinput was observed in the NT group than the HT group, suggesting that SK current slows sub-threshold depolarization considerably more in the NT group. The latter is consistent with loss of SK current among neurons from the HT group. To explore potential mechanisms underlying greater excitability of neurons from HT rats, the SK channel-mediated mAHP was analyzed and its amplitude was found to be significantly reduced in the HT compared with the NT group. Moreover, most neurons from HT rats had a prominent ADP. Blockade of SK channels not only inhibited the mAHP in neurons from NT rats but also uncovered an ADP resembling that observed in the HT group. A striking observation was that SK channel blockade in the HT group had no obvious effect on either the mAHP or the ADP. Taken together, these findings indicate that diminished SK current likely contributes to increased in vitro excitability of PVN-RVLM neurons from rats with AngII-Salt hypertension. This appears to be accomplished, at least in part, by a blunting of the mAHP that opposes an ADP in these neurons and by enhancing subthreshold depolarizing Rinput.
We recently demonstrated that PVN-RVLM neurons express a prominent SK current (Chen and Toney 2009). The present study extended this observation by determining that neurons from rats with AngII-Salt hypertension have a significantly diminished SK current. It should be noted that Sonner et al. (2008) recently reported that PVN-RVLM neurons from rats with renal-vascular hypertension have smaller amplitude transient outward current (IA) compared with neurons from normotensive controls. Interestingly, they reported that the density of IA was not different between neurons from HT and NT rats, suggesting that the reduction of IA in renal-vascular hypertension could reflect a smaller membrane surface area rather than a reduction in channel expression or gating. Reduced membrane surface area of PVN-RVLM neurons does not appear to be a common feature of hypertension as the present study found that both the amplitude and density of SK current were significantly reduced in the AngII-Salt HT group compared with NT controls (Fig. 1C). Mechanisms that contribute to differential PVN-RVLM neuronal adaptive responses to renal-vascular and AngII-Salt hypertension are not presently known, but in the renal-wrap model of hypertension used by Sonner et al., Haywood et al. reported reduced GABAA receptor mediated inhibition of the PVN. Mover, gabaergic synapses have been reported to redistribute on dendrites and soma of PVN-RVLM neurons in renal-vascular hypertensive rats (Biancardi et al. 2010). Thus it appears that the function of both ligand- and voltage-gated inhibitory channels is reduced in the PVN in the renal-wrap model of renal-vascular hypertension. Interestingly, the reduction of GABAergic inhibition does not appear to reflect a reduction of GABAA receptor expression in proportion to reduced membrane surface area as GABAA receptor density in the PVN was reported to be unaltered in hypertensive rats (Haywood et al. 2001).
Although increased in vitro spontaneous activity of PVN-RVLM neurons from spontaneously hypertensive (Li and Pan 2006) and renal-vascular hypertensive (Sonner et al. 2008) rats involves both synaptic and intrinsic mechanisms, effects of AngII and a high salt diet on the discharge behavior of these neurons has not been previously investigated. We found that in vitro excitability is significantly greater among PVN-RVLM neurons from HT than NT rats. Blocking SK channels caused a significantly blunted increase in excitability of neurons from HT rats compared with controls with maximum firing rates during exposure to apamin being nearly the same in both groups. These finding indicate that diminished SK channel function (or expression) among PVN-RVLM neurons of HT animals could contribute to their greater in vitro excitability and perhaps to greater in vivo discharge as well.
During a train of action potentials, AHPs can develop and increase the rate of ISI prolongation, i.e., spike-frequency adaptation (Bond et al. 2005; Stocker 2004). SK channels can mediate a mAHP that leads to spike-frequency adaptation (Greffrath et al. 2004; Liu and Herbison 2008; Teshima et al. 2003), thereby regulating excitability. Studies have shown that PVN-RVLM neurons not only display a prominent mAHP but also undergo significant spike-frequency adaptation in response to depolarizing current injections (Chen and Toney 2009; Stern 2001) and the neuropeptide AngII (Cato and Toney 2005). To determine whether a reduction of spike-frequency adaptation underlies increased excitability of PVN-RVLM neurons from our AngII-Salt HT rats, we analyzed the ISI distributions of current-evoked action potential trains. We determined the slope of the linear portion of the ISI-ISI number curve (Fig. 2D, right) and found that it was significantly greater in the NT than HT group under control conditions. Furthermore, we observed that blockade of SK channels with apamin significantly increased the slope only in the NT group. These data suggest that SK current normally contributes to spike-frequency adaptation and that reduced SK current among PVN-RVLM neurons from AngII-Salt HT likely contributes to reduced spike-frequency adaptation and greater excitability of these neurons.
It is important to emphasize that the present conclusion that SK current normally regulates spike-frequency adaptation in PVN-RVLM neurons is contrary to the conclusion we reached in a recent report (Chen and Toney 2009). We previously concluded that SK channels do not participate in spike-frequency adaptation in PVN-RVLM neurons, but this was based on our observation that the ratio of the 13th and 1st ISI under control conditions was not significantly changed during SK channel blockade with apamin. In the present study, linear slope of ISI-ISI number curve was used to quantify spike-frequency adaptation. Because of its apparently greater sensitivity relative to comparing the ratio of th 13th and 1st ISI, a significant role for SK channels in spike-frequency adaptation was detected.
To further explore mechanisms contributing to reduced spike-frequency adaptation among neurons from HT rats, we analyzed mAHPs and found their amplitudes to be significantly smaller in neurons from HT than NT rats. Moreover, most PVN-RVLM neurons from HT rats had a prominent ADP under control conditions that was not present in the NT group. Interestingly, blockade of SK channels in the NT group not only reduced the mAHP but revealed an ADP resembling that recorded in the HT group. Thus diminished SK current appears to contribute to increased excitability of PVN-RVLM neurons from HT rats by reducing the mAHP amplitude and uncovering an ADP.
The present study confirmed our earlier report that blocking SK channels increases sub-threshold depolarizing Rinput of PVN-RVLM neurons (Chen and Toney 2009). Here we found that neurons from AngII-Salt HT rats had significantly greater subthreshold depolarizing Rinput compared with those from control rats. Blocking SK channels with apamin caused depolarizing Rinput to increase significantly more in neurons from NT than HT rats. Collectively, these findings indicate that apamin-sensitive depolarizing Rinput might also contribute to increased excitability of PVN-RVLM neurons from rats with AngII-Salt hypertension, at least during the subthreshold phase of depolarization.
It should be mentioned that SK conductance does not appear to be active at rest and therefore does not influence resting Vm (approximately −63 mV) or spontaneous discharge of PVN-RVLM neurons from NT (Chen and Toney 2009) or HT rats (present study). Based on our ramp current tests, however, it appears that SK channels become active when depolarization starts from a relatively hyperpolarized potential of −80 mV. This observation may be explained by hyperpolarization-induced de-inactivation of T-type Ca2+ channels (Lee et al. 2003; Shin et al. 2008). Accordingly, Ca2+ influx could then activate SK channels as neurons depolarize from a hyperpolarized potential. This possibility is worthy of further study given a recent report that PVN-RVLM neurons do indeed express a low voltage activated T-type Ca2+ current (Lee et al. 2008). Another, perhaps less likely, possibility is that hyperpolarization of Vm in our ramp current injection tests could have activated hyperpolarization-activated cation channels (HCN). Although parvocellular PVN neurons have been reported to express HCN (Qiu et al. 2005), it is not known if these HCN are Ca2+ permeable like those expressed in dorsal root ganglion neurons (Yu et al. 2004) and ventricular myocytes (Yu et al. 2007). An argument against a role for HCN in the present study is that currents needed to adjust Vm to −80 mV prior to initiation of current ramps was not different between NT and HT groups and were unaltered by bath application of apamin. Thus at hyperpolarized potentials, neurons from NT and HT rats have similar input resistance and may therefore have similar profiles of active ionic currents, including those mediated by HCN.
It is well established that SK channels are gated by even small increases of intracellular Ca2+ (Bond et al. 2005; Fakler and Adelman 2008; Sah and Faber 2002; Stocker 2004). Given that Ca2+ influx through voltage-dependent Ca2+ channels can activate SK channels, it is possible that a reduction of voltage-dependent Ca2+ influx could explain diminished SK current observed among PVN-RVLM neurons from HT rats. This appears plausible since, as mentioned in the preceding text, PVN-RVLM neurons express a low voltage activated T-type Ca2+ current (Lee et al. 2008). Although activation of SK channels by Ca2+ through T-type Ca2+ channels is known to occur in dopaminergic midbrain neurons (Wolfart and Roeper 2002) and thalamic dendrites (Cueni et al. 2008), similar information is not presently available for PVN-RVLM neurons. It is also possible that diminished SK current among neurons from HT rats could involve reduced expression of SK channel proteins and/or reduced trafficking of SK channels to the plasma membrane. In this regard, studies have reported that expression of SK channel mRNA and protein is reduced in rat mesenteric arteries during development of hypertension induced by chronic infusion of AngII (Hilgers and Webb 2007). No study of hypertension has yet compared SK channel mRNA/protein expression levels in the CNS, and additional studies are clearly needed to address this question.
It should be mentioned that SK channels can function at presynaptic sites (Obermair et al. 2003; Roncarati et al. 2001) to influence neurotransmission and excitability. In specific regard to PVN-RVLM neurons, it is noteworthy that glutamatergic activity is dominant under conventional slice recording conditions, i.e., Vm held at −80 mV (Chen and Toney 2009; Li and Pan 2005). Whether enhanced excitability of PVN-RVLM neurons from rats with AngII-Salt hypertension is due to augmentation of glutamatergic excitation resulting from loss of presynaptic SK current has not been investigated. This seems unlikely, however, because available evidence indicates that SK channel blockade with apamin does not affect glutamatergic miniature excitatory postsynaptic current activity among PVN-RVLM neurons from NT rats (Chen and Toney 2009). Therefore elevated PVN-RVLM neuronal excitability in AngII-Salt hypertension appears more likely to be caused by diminished postsynaptic SK current (Fig. 1).
In summary, the present study revealed that SK current is diminished among PVN-RVLM neurons from rats with AngII-Salt hypertension compared with NT controls. Diminished SK current appears to underlie the reduced mAHP in these neurons and likely allows detection of a prominent ADP following a train of action potentials. Reduced SK current in neurons from HT rats may also contribute to greater depolarizing Rinput at potentials below spike threshold. Collectively, these functional alterations appear to contribute to the observed increase in excitability of PVN-RVLM neurons from rats with AngII-Salt hypertension. We speculate that increased excitability could play an important role in the development and/or maintenance of sympathetic activation in AngII-Salt hypertension. Future studies will be needed to fully determine the contribution of neuronal SK channel dysfunction and/or down-regulation in the pathogenesis of hypertension.
GRANTS
This study was funded by National Heart, Lung, and Blood Institute Grants HL-088052 and HL-076312 to G. M. Toney and American Heart Association Grants 0865107F and SDG2640130 (Q. H. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank L. P. LaGrange for critically reviewing the manuscript.
REFERENCES
- Barth and Gerstberger, 1999.Barth SW, Gerstberger R. Differential regulation of angiotensinogen and AT1A receptor mRNA within the rat subfornical organ during dehydration. Brain Res Mol Brain Res 64: 151–164, 1999 [DOI] [PubMed] [Google Scholar]
- Biancardi et al., 2010.Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 518: 567–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond et al., 2005.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol 15: 305–311, 2005 [DOI] [PubMed] [Google Scholar]
- Bourque et al., 2007.Bourque CW, Ciura S, Trudel E, Stachniak TJ, Sharif-Naeini R. Neurophysiological characterization of mammalian osmosensitive neurons. Exp Physiol 92: 499–505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner et al., 2005.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- Brooks et al., 2001.Brooks VL, Scrogin KE, McKeogh DF. The interaction of angiotensin II and osmolality in the generation of sympathetic tone during changes in dietary salt intake. An hypothesis. Ann NY Acad Sci 940: 380–394, 2001 [DOI] [PubMed] [Google Scholar]
- Cato and Toney, 2005.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurones that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Toney, 2001.Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathetic nerve activity. Am J Physiol Regulatory Integrative Comp Physiol 281: R1844–1853, 2001 [DOI] [PubMed] [Google Scholar]
- Chen and Toney, 2003.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003 [DOI] [PubMed] [Google Scholar]
- Chen and Toney, 2009.Chen QH, Toney GM. Excitability of PVN neurons that project to the rostral ventrolateral medulla is regulated by small-conductance Ca2+-activated K+ channels. J Physiol 587: 4235–4247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Toney, 2010.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueni et al., 2008.Cueni L, Canepari M, Luján R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Lüthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci 11: 683–692, 2008 [DOI] [PubMed] [Google Scholar]
- Fakler and Adelman, 2008.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- Ferguson and Bains, 1997.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997 [DOI] [PubMed] [Google Scholar]
- Greffrath et al., 2004.Greffrath W, Magerl W, Disque-Kaiser U, Martin E, Reuss S, Boehmer G. Contribution of Ca2+-activated K+ channels to hyperpolarizing after-potentials and discharge pattern in rat supraoptic neurons. J Neuroendocrinol 16: 577–588, 2004 [DOI] [PubMed] [Google Scholar]
- Gutman et al., 1998.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurones. Am J Physiol Regulatory Integrative Comp Physiol 254: R746–754, 1998 [DOI] [PubMed] [Google Scholar]
- Guyenet et al., 2001.Guyenet PG, Schreihofer AM, Stornetta RL. Regulation of sympathetic tone and arterial pressure by the rostral ventrolateral medulla after depletion of C1 cells in rats. Ann NY Acad Sci 940: 259–269, 2001 [DOI] [PubMed] [Google Scholar]
- Haywood et al., 2001.Haywood JR, Mifflin SW, Craig T, Calderon A, Hensler JG, Hinojosa-Laborde C. Gamma-aminobutyric acid (GABA)–a function and binding in the paraventricular nucleus of the hypothalamus in chronic renal-wrap hypertension. Hypertension 37: 614–618, 2001 [DOI] [PubMed] [Google Scholar]
- Hilgers and Webb, 2007.Hilgers RH, Webb RC. Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2275–2284, 2007 [DOI] [PubMed] [Google Scholar]
- Kannan and Yamashita, 1983.Kannan H, Yamashita H. Electrophysiological study of paraventricular nucleus neurones projecting to the dorsomedial medulla and their response to baroreceptor stimulation in rats. Brain Res 279: 31–40, 1983 [DOI] [PubMed] [Google Scholar]
- King and Fink, 2006.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension 48: 927–933, 2006 [DOI] [PubMed] [Google Scholar]
- King et al., 2008.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Reglatory Integrative Comp Physiol 294: R1262–1267, 2008 [DOI] [PubMed] [Google Scholar]
- Kline et al., 1990.Kline RL, Chow KY, Mercer PF. Does enhanced sympathetic tone contribute to angiotensin II hypertension in rats? Eur J Pharmacol 184: 109–118, 1990 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2008.Lee S, Han TH, Sonner PM, Stern JE, Ryu PD, Lee SY. Molecular characterization of T-type Ca(2+) channels responsible for low threshold spikes in hypothalamic paraventricular nucleus neurones. Neuroscience 155: 1195–1203, 2008 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2003.Lee SC, Hayashida Y, Ishida AT. Availability of low-threshold Ca2+ current in retinal ganglion cells. J Neurophysiol 90: 3888–3901, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Pan, 2005.Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther 313: 1035–1045, 2005 [DOI] [PubMed] [Google Scholar]
- Li and Pan, 2006.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006 [DOI] [PubMed] [Google Scholar]
- Li et al., 2008.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu and Herbison, 2008.Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology 149: 3598–3604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin and Haywood, 1992.Martin DS, Haywood JR. Sympathetic nervous system activation by glutamate injections into the paraventricular nucleus. Brain Res 577: 261–267, 1992 [DOI] [PubMed] [Google Scholar]
- Obermair et al., 2003.Obermair GJ, Kaufmann WA, Knaus HG, Flucher BE. The small conductance Ca2+-activated K+ channel SK3 is localized in nerve terminals of excitatory synapses of cultured mouse hippocampal neurons. Eur J Neurosci 17: 721–731, 2003 [DOI] [PubMed] [Google Scholar]
- Osborn et al., 2007.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007 [DOI] [PubMed] [Google Scholar]
- Porter and Brody, 1985.Porter JP, Brody MJ. Neural projections from paraventricular nucleus that subserve vasomotor functions. Am J Physiol Regul Integr Comp Physiol 248: R271–281, 1985 [DOI] [PubMed] [Google Scholar]
- Qiu et al., 2005.Qiu DL, Chu CP, Shirasaka T, Tsukino H, Nakao H, Kato K, Kunitake T, Katoh T, Kannan H. Corticotrophin-releasing factor augments the IH in rat hypothalamic paraventricular nucleus parvocellular neurons in vitro. J Neurophysiol 94: 226–234, 2005 [DOI] [PubMed] [Google Scholar]
- Roncarati et al., 2001.Roncarati R, Di Chio M, Sava A, Terstappen GC, Fumagalli G. Presynaptic localization of the small conductance calcium-activated potassium channel SK3 at the neuromuscular junction. Neuroscience 104: 253–262, 2001 [DOI] [PubMed] [Google Scholar]
- Sah and Faber, 2002.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- Schad and Seller, 1975.Schad H, Seller H. Influence of intracranial osmotic stimuli on renal nerve activity in anesthetized cats. Pfluegers 353: 107–121, 1975 [DOI] [PubMed] [Google Scholar]
- Shi et al., 2008.Shi P, Martinez MA, Calderon AS, Chen QH, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurons that project to the hypothalamic paraventricular nucleus. J Physiol 586: 5231–5245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al., 2007.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis (OVLT) contributes to increased sympathetic nerve Activity induced by central hyperosmolality. Am J Physiol Regulatory Integrative Comp Physiol 293: R2279–R2289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin et al., 2008.Shin HS, Cheong EJ, Choi S, Lee J, Na HS. T-type Ca2+ channels as therapeutic targets in the nervous system. Curr Opin Pharmacol 8: 33–41, 2008 [DOI] [PubMed] [Google Scholar]
- Sonner et al., 2008.Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurons in renovascular hypertensive rats. J Physiol 586: 1605–1622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, 2001.Stern JE. Electrophysiological and morphological properties of pre-autonomic neurons in the rat hypothalamic paraventricular nucleus. J Physiol 537: 161–177, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, 2004.Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker et al., 1999.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurones. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker et al., 2004.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regulatory Integrative Comp Physiol 286: R719–R725, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker et al., 2006.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker and Toney, 2005.Stocker SD, Toney GM. Median preoptic neurons projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima et al., 2003.Teshima K, Kim SH, Allen CN. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience 120: 65–73, 2003 [DOI] [PubMed] [Google Scholar]
- Toney et al., 2003.Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand 177: 43–55, 2003 [DOI] [PubMed] [Google Scholar]
- Toney et al., 2010.Toney GM, Pedrino GR, Fink GD, Osborn JW. Does enhanced respiratory-sympathetic coupling contribute to peripheral neural mechanisms of AngII-salt hypertension? Exp Physiol 95: 587–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart and Roeper, 2002.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci 22: 3404–3413, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang and Coote, 1998.Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurons in the rostral ventrolateral medulla of the rat. J Physiol 513: 521–530, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al., 2001.Yang Z, Bertram D, Coote JH. The role of glutamate and vasopressin in the excitation of RVL neurones by paraventricular neurons. Brain Res 908: 99–103, 2001 [DOI] [PubMed] [Google Scholar]
- Yasuda et al., 2000.Yasuda Y, Honda K, Negoro H, Higuchi T, Goto Y, Fukuda S. The contribution of the median preoptic nucleus to renal sympathetic nerve activity increased by intracerebroventricular injection of hypertonic saline in the rat. Brain Res 867: 107–114, 2000 [DOI] [PubMed] [Google Scholar]
- Yu et al., 2007.Yu X, Chen XW, Zhou P, Yao L, Liu T, Zhang B, Li Y, Zheng H, Zheng LH, Zhang CX, Bruce I, Ge JB, Wang SQ, Hu ZA, Yu HG, Zhou Z. Calcium influx through If channels in rat ventricular myocytes. Am J Physiol Cell Physiol 292: C1147–C1155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al., 2004.Yu X, Duan KL, Shang CF, Yu HG, Zhou Z. Calcium influx through hyperpolarization-activated cation channels (Ih channels) contributes to activity-evoked neuronal secretion. Proc Natl Acad Sci USA 101: 1051–1056, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]