Abstract
Block of neurotransmission at the mammalian neuromuscular junction triggers an increase in the number of vesicles released (quantal content). The increase occurs whether nerve and muscle activity are both blocked by placement of a tetrodotoxin (TTX) containing cuff on the nerve or whether muscle activity is selectively blocked by injection of α-bungarotoxin (BTX). We used ANOVA to examine whether the mechanism underlying the increase in quantal content differed between the two types of activity blockade. We found that TTX-induced blockade increased the probability of release (p), whereas BTX-induced blockade increased the number of releasable vesicles (n). The lack of increase in p when postsynaptic activity was blocked with BTX suggests that block of presynaptic activity triggers the increase. To determine whether n is regulated by mismatch of pre- and postsynaptic activity introduced by BTX injection we combined BTX and TTX and still found an increase in n. We conclude that block of acetylcholine binding to acetylcholine receptors during spontaneous release triggers the increase in n.
INTRODUCTION
Chronic manipulations of activity trigger a series of changes in synaptic function that maintain firing rates of networks within certain boundaries and have thus been termed homeostatic regulation of synaptic function. In one commonly studied form, homeostatic synaptic plasticity is triggered by a decrease in activity and results in an increase in excitatory synaptic strength (Molder et al. 2006; Rich and Wenner 2007; Turrigiano 2008).
The mammalian neuromuscular junction (NMJ) is a classic excitatory synapse ideally suited to studies of homeostatic regulation of synaptic strength in vivo since there is only one presynaptic input and one neurotransmitter. The presence of only one input allows for studies of evoked release and quantal content that are not possible at central synapses. The first report of what would now be termed homeostatic regulation of synaptic strength was in 1971 at the neuromuscular junction. In the study, limb immobilization triggered an increase in postsynaptic acetylcholine receptors (AChRs) that was paralleled by an increase in quantal amplitude (Robbins and Fischbach 1971). Subsequent studies using tetrodotoxin (TTX) to block nerve activity in vivo found an increase in quantal content at the mouse NMJ (Snider and Harris 1979; Tsujimoto and Kuno 1988; Tsujimoto et al. 1990; Wang et al. 2004). It has also been found that block of AChRs with α-bungarotoxin (BTX) at the NMJ in vivo triggers an increase in quantal content (Molenaar et al. 1991; Plomp et al. 1992, 1994).
Application of TTX to the nerve and block of AChRs with BTX are fundamentally different ways of blocking synaptic transmission. TTX application blocks spiking of both nerve and muscle, whereas block of AChRs blocks only spiking of the muscle. Application of BTX blocks binding of acetylcholine to AChRs during spontaneous release of transmitter, whereas TTX does not affect this process. In the chick spinal cord block of transmitter receptors, but not block of spiking, triggers an increase in quantal amplitude (Wilhelm and Wenner 2008). Available evidence at the mammalian NMJ suggests that TTX and BTX increase quantal content via distinct mechanisms, but the two methods of blocking synaptic transmission have never been directly compared. The increase in quantal content triggered by TTX is evident only in solution containing low extracellular calcium (Wang et al. 2004), whereas the increase in quantal content following BTX is present at normal extracellular Ca2+ (Molenaar et al. 1991; Plomp et al. 1992, 1994). In the present study we demonstrate that TTX and BTX increase quantal content by distinct mechanisms. Block of distinct aspects of synaptic activity triggers the increases in quantal content triggered by TTX and BTX.
METHODS
Ethical approval
All procedures involving animals were approved by the Wright State LACUC committee.
Mice
For a previous study (Wang et al. 2005) we used ClCn1adr-mto2J mice obtained from The Jackson Laboratory (Bar Harbor, ME). Although no mutant mice were used in this study, to compare with our previous results we used unaffected litter mates of the strain. Unaffected littermates consisted of mice that were heterozygous for the ClC mutation and mice that carried no copy of the mutation. Since no unaffected siblings have myotonia by electromyogram it appears that heterozygous mice are unaffected (Wang et al. 2005).
TTX cuff application and BTX injection
Mice (2- to 4-mo-old) were anesthetized with intraperitoneal injection of chloral hydrate. Use of chloral hydrate for rodent anesthesia has been called into question (Baxter et al. 2009; Silverman and Muir 3rd 1993). Concerns that have been raised include respiratory depression (Flecknell 1996) and adynamic ileus (Fleischman et al. 1977). We have used chloral hydrate to anesthetize mice for the past 12 yr. If chloral hydrate caused significant respiratory depression in mice we would expect to have a high rate of overdose. We have found exactly the opposite to be true. In our hands chloral hydrate causes significantly less death due to overdose than injection of ketamine/xylazine. Although adynamic ileus was reported in rats this has not been our experience in mice; we have not seen poor appetite, abdominal distention, or lack of weight gain following administration. Finally, it has been suggested that chloral hydrate is not optimal because it is a hypnotic, rather than anesthetic, agent (Hall et al. 2001; however, see Flecknell 1996). We have not noted pain behavior during surgery, such as vocalization or movement in response to incision, to suggest that mice are experiencing inadequate anesthesia. We conclude that although choral hydrate may not be optimal for use in rats, it is safe and efficacious in our strain of mice. One reason for the difference between our experience and that of others may be the considerable strain variation in the response to chloral hydrate in rodents (Flecknell 1996). Because our surgeries involve toxins we work in a biosafety hood and thus find it easier to use injected anesthetic rather than inhaled anesthetic.
Following anesthesia the hip area was sterilized with betadine and a 1-cm incision was made over the sciatic nerve. TTX cuffs were fashioned from silastic tubing and were placed around the left sciatic nerve and attached to an Alzet osmotic pump placed subcutaneously in the back (model 1002, infusing at 0.25 μl/h; Durect, Cupertino, CA) containing 850 μM TTX dissolved in 0.9% NaCl. The tubing to the pump was filled with saline so that after surgery the foot muscles were not paralyzed. This allowed us to confirm that the nerve had not been injured during surgery. Following surgery mice were given one dose of subcutaneous buprenorphine for analgesia. By the next day mice were moving freely, eating and drinking, and exhibited no pain behavior. The next morning the mouse was checked for loss of ability to spread its toes to confirm that nerve block had developed. Prior to sacrifice at 8–9 days the nerve block was checked again. Mice were killed using CO2 inhalation and the tibialis anterior muscle was removed. Bungarotoxin injection was performed on anesthetized mice using a 31-gauge Hamilton syringe. After anesthesia the area over the tibialis anterior was shaved and washed with betadine prior to injection of 3 μl of 1 μg/μl α-bungarotoxin. Four to 5 days after injection, mice were killed and quantal content was measured.
Electrophysiologic recording
Mice were killed using CO2 inhalation and the tibialis anterior muscle was removed. For most experiments, the recording chamber was continuously perfused with Ringer solution containing (in millimoles per liter) NaCl, 118; KCl, 3.5; CaCl2, 2; MgSO4, 0.7; NaHCO3, 26.2; NaH2PO4, 1.7; and glucose, 5.5 (pH 7.3–7.4, 20–22°C) equilibrated with 95% O2-5% CO2. For lowering the probability of release for ANOVA, recordings were done in 1 mM Ca2+ solution in which the Mg2+ concentration was kept at 0.7 mM. Endplate recordings were performed as previously described (Wang et al. 2004, 2006, 2010). Briefly, the tibialis anterior muscle was pinned in a dish and stained with 10 μM 4-(4-diethylaminostyryl)-N-methylpyridinium iodide (4-Di-2ASP; Invitrogen, Carlsbad, CA). In most experiments muscle fibers were crushed away from the endplate band to eliminate contractions following nerve stimulation and two-electrode voltage clamp was used to set the holding potential to −45 mV (Wang et al. 2010). To detect miniature endplate currents (MEPCs) in muscle injected with BTX fibers were held at −100 mV. In these experiments contraction was prevented by 1 μM μ-conotoxin GIIIB (Peptide Institute, Osaka Japan). For all experiments, quantal content was calculated by dividing peak EPC current amplitude by peak MEPC current amplitude. For lowering the probability of release during 50-Hz repetitive stimulation, recordings were done in low (1 mM) Ca2+ solution in which the Mg2+ concentration was kept at 0.7 mM.
Determination of the role of P/Q Ca2+ channels in evoked release was performed by measuring mean EPC amplitude before and after application of ω-agatoxin-IVA (Peptide Institute) in both control and BTX-blocked muscles. After recording EPC amplitude from ≥15 endplates in a tibialis anterior muscle in the absence of ω-agatoxin-IVA, each muscle was placed in 2 ml of Ringer solution containing 1 μM ω-agatoxin-IVA for 1 h. The solution was continuously bubbled with 95% O2-5% CO2. After 1 h, the muscle was put into a recording chamber that was slowly perfused with Ringer solution containing no ω-agatoxin-IVA. EPCs were recorded from ≥15 endplates in each muscle. All endplate recordings following the application of ω-agatoxin-IVA were made within 40 min of placing the muscle in the recording chamber. During this 40-min period mean EPC amplitude did not increase because of washout of ω-agatoxin-IVA.
ANOVA
ANOVA was performed as previously described (Wang et al. 2010). For measurement of EPC variance, ≥20 stimulations of the nerve were performed at 0.5 Hz. However, even at this low rate of stimulation there was slight depression of the EPC during the first three pulses, especially after administration of BTX. This depression led to overestimation of EPC variance so the first three EPCs were discarded from measurement of EPC variance, leaving ≥17 values for analysis. The average number of EPCs measured to determine EPC variance was 22. Using a higher number of EPCs for ANOVA at individual endplates had little effect on estimates of mean variance for a given muscle. The mean variance and quantal content from each muscle was then averaged between muscles to get final values, which were plotted. Endplate recordings in which there was movement artifact or a change in EPC amplitude with time were discarded since such artifacts led to large increases in variance. For each muscle the average m and Var (m) were calculated. The average of m and Var (m) for each muscle were then averaged to generate plots.
Contribution of MEPC variance to EPC variance was ignored since MEPC variance is small relative to EPC variance (Wang et al. 2010). Interpretation of ANOVA is confounded by several assumptions made during the analysis. We have previously examined assumptions made in ANOVA and found that they were either valid or did not affect interpretation of data from the mouse NMJ (Wang et al. 2010).
Morphological analysis of sprouting
After sacrifice by perfusion with 4% paraformaldehyde, the tibialis anterior muscle was removed and postfixed in 4% paraformaldehyde for 1 h, cryoprotected in 15% sucrose solution overnight, and frozen in liquid nitrogen. Muscles were sectioned (20 μm thickness) and acetylcholine receptors in motor endplates were labeled with rhodamine-conjugated α-bungarotoxin (Molecular Probes). Axons and motor terminals were labeled with a mouse monoclonal antibody against the phosphorylated heavy fragment of neurofilament protein (SMI31, 1:400; Sternberger Monoclonal). Synaptic vesicles were labeled using a mouse polyclonal antibody directed against the SV2 protein (1:40; Developmental Studies Hybridoma Bank, University of Iowa). Labeling of both primary monoclonal antibodies was visualized using a fluorescein-conjugated donkey anti-mouse secondary antibody (1:100; Jackson ImmunoResearch Laboratories). Z-axis stacks of images at sequential focal planes (0.5-μm separation) of NMJs were obtained using a Fluoview FV 1000 confocal microscope and a ×60 objective (Olympus Optical). Illustrated images are flat-plane in-focus projections obtained from z-series images using Fluoview software.
RESULTS
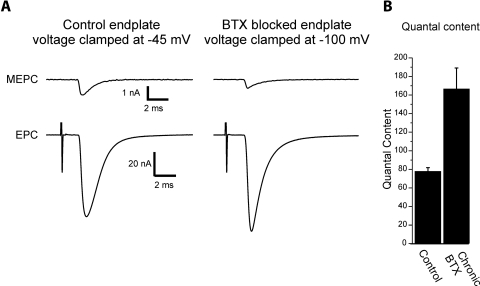
We sought to determine whether the mechanisms underlying increased quantal content differed when synaptic transmission was blocked by placement of a tetrodotoxin (TTX) cuff on the nerve or by injection of α-bungarotoxin (BTX). We first confirmed that injection of BTX triggered an increase in quantal content. One issue was that BTX blocked neurotransmission at the time of injection to such an extent that neither evoked endplate currents (EPCs) nor spontaneous miniature endplate currents (MEPCs) could be measured. Four to 5 days after a single injection of BTX it was possible to measure MEPCs and EPCs due to insertion of new acetylcholine receptors. Since MEPCs were still very small at the majority of endplates and could not be reliably detected at a holding potential of −45 mV, fibers were held at potentials of −100 mV and muscle twitch was blocked by adding 1 μM μ-conotoxin GIIIB to the perfusate to selectively block muscle sodium channels. Despite the use of hyperpolarized holding potentials, at some endplates MEPCs could still not be reliably detected and those fibers were not included in further analysis. In fibers in which both MEPCs and EPCs could be recorded, quantal content was significantly increased following block of acetylcholine receptors in vivo (Fig. 1).
Fig. 1.
Blockade of acetylcholine receptors in vivo with α-bungarotoxin (BTX) increases quantal content. A: shown are the average miniature endplate current (MEPC) and endplate current (EPC) recorded in 2 mM Ca2+ for a control endplate and an endplate 5 days following block of acetylcholine receptors with BTX. The control endplate recording was performed using the crushed fiber preparation and a holding potential of −45 mV. Quantal content was 70. The BTX EPC was recorded using μ-conotoxin to block contraction and a holding potential of −100 mV was used to allow detection of MEPCs. Quantal content was 145. If the same holding potential were used, the EPC from the BTX-blocked endplate would be smaller than that from the control endplate. A stimulus artifact precedes the EPCs by several milliseconds. B: mean quantal content of control and endplates from muscles injected with BTX 5 days earlier. There is a statistically significant increase in quantal content [P < 0.01, n = 4 muscles for control (65 fibers) and 4 muscles for BTX (67 fibers)].
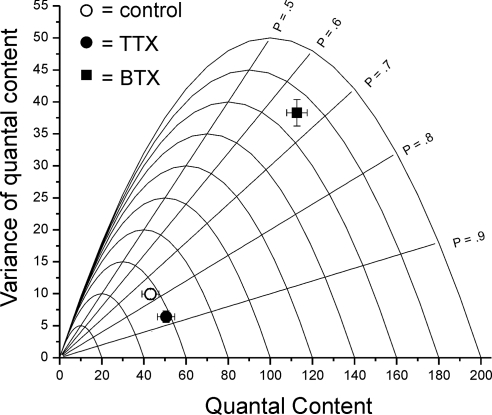
We used ANOVA to estimate probability of release (p) and number of releasable vesicles (n) following block of activity with either a TTX cuff or BTX injection. We have previously validated this analysis at the NMJ as well as the assumptions underlying the analysis (Wang et al. 2010). For the analysis we measured EPC amplitude, average MEPC amplitude, and variance of EPC amplitude to directly calculate variance of quantal content. This allowed us to estimate changes in p and n for individual NMJs. To lower probability of release so that increases in probability of release could be detected, measurements were performed in solution containing 1 mM extracellular Ca2+ (Wang et al. 2010). ANOVA indicated that block of both pre- and postsynaptic action potentials by application of TTX to the nerve increased quantal content by increasing p from 0.73 ± 0.04 to 0.85 ± 0.03 (P < 0.05 control vs. TTX) with no change in n (59.3 ± 2.9 vs. 59.1 ± 3.7). In contrast, 4 to 5 days following application of BTX, quantal content was increased by an increase in n from 59.3 ± 2.9 to 108.1 ± 15.4 (P < 0.01 control vs. BTX, Fig. 2) with no significant change in p (0.73 ± 0.04 vs. 0.66 ± 0.03, P = 0.22). This suggested the increases in quantal content following block of synaptic activity with TTX and BTX occur via distinct mechanisms.
Fig. 2.
Block of neuromuscular transmission by tetrodotoxin (TTX) and BTX increase quantal content by increasing p and n, respectively. Plot of the mean value of variance of quantal content vs. mean quantal content for control, TTX-blocked, and BTX-blocked endplates in solution containing 1 mM Ca. Each parabola represents the theoretical plot for a given number of releasable vesicles (n) as probability of release (p) is increased from 0 to 1.0, assuming uniform and stationary p for all releasable vesicles. Included in the plot are the parabolas from n = 20 to n = 200. Intersecting the parabolas are straight lines representing the theoretical plot for a given p as n is increased. The lines for p = 0.5 to p = 0.9 are labeled on the graph. Following block of neuromuscular transmission with a TTX cuff on the nerve, there was a statistically significant increase in probability of release (0.73 ± 0.04 in control vs. 0.85 ± 0.03 following TTX, P < 0.05), but no change in n (59.3 ± 2.9 vs. 59.1 ± 3.7). Following block of neuromuscular transmission by block of acetylcholine receptors with BTX there was a statistically significant increase in number of releasable vesicles (59.3 ± 2.9 vs. 108.1 ± 15.4, P < 0.01), but no significant change in p (0.73 ± 0.04 vs. 0.66 ± 0.03, P = 0.22). Quantal content for control was 43.1 ± 4.0; following TTX it was 50.5 ± 4.1; and after BTX it was 112.6 ± 4.9. All recordings were made in 1 mM external Ca. Number of muscles studied: control = 8, TTX cuff = 8, BTX = 5.
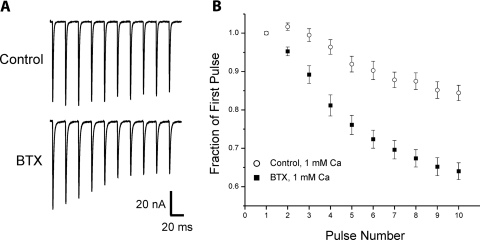
We previously reported that the increase in p following application of TTX was accompanied by increased depression during repetitive stimulation (Wang et al. 2004). We examined whether the increase in n following BTX was accompanied by a change in response to repetitive stimulation. Four to 5 days following application of BTX, depression was increased (Fig. 3). Thus despite the suggestion from ANOVA that TTX and BTX trigger distinct mechanisms to increase quantal content, both treatments had similar effects on short-term synaptic plasticity.
Fig. 3.
Treatment with BTX increases depression during repetitive stimulation. A: the average EPCs from 6 to 10 trains of 50-Hz pulses in solution containing 1 mM Ca2+ are shown for a representative control and a representative endplate blocked by BTX injection 5 days earlier. In the control endplate there is facilitation at the beginning of the train followed by mild depression. In the endplate exposed to BTX there is immediate depression. B: plot of the normalized average EPCs during trains of 50-Hz pulses for control endplates (n = 6 muscles) and endplates treated with BTX 4–5 days earlier (n = 5 muscles) in solution containing 1 mM Ca2+. Whereas control endplates had facilitation on the second pulse, BTX-treated endplates had depression (P < 0.01). By the tenth pulse there was substantially more depression in the BTX-treated endplates (P < 0.01).
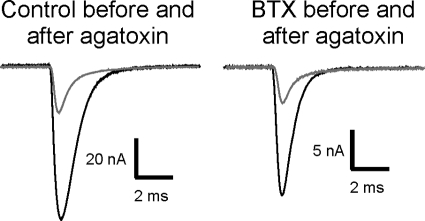
The finding that short-term plasticity was similar called into question the suggestion that TTX and BTX induce distinct mechanisms to increase quantal content. To determine whether the BTX-induced increase in n revealed by ANOVA reflected a mechanism distinct from that triggered by TTX cuff treatment, we examined sensitivity to block of P/Q channels with ω-agatoxin-IVA. We previously found that after TTX cuff treatment, block of P/Q channels with ω-agatoxin-IVA eliminated the increase in quantal content (Wang et al. 2004). Given the difficulty of measuring MEPCs to calculate quantal content in endplates from muscles injected earlier with BTX we examined the role of P/Q channels following BTX injection without measuring MEPC amplitude and calculating quantal content. If an increase in Ca2+ entry through P/Q channels was increasing EPC amplitude following BTX, EPC amplitude from BTX-blocked endplates would be more sensitive to block of P/Q channels. At least 15 EPC amplitudes were measured in each muscle before and after block of P/Q channels in control muscle and muscle that had been treated with BTX 4 days earlier. In control muscle the mean reduction in EPC amplitude following block of P/Q channels was 75 ± 5% (Fig. 4). In BTX-treated endplates ω-agatoxin-IVA inhibited the same percent of current (80 ± 4%, P = 0.47 vs. control, n = 4 muscles for control, 5 muscles for BTX). The similarity in sensitivity to block of P/Q channels indicates Ca2+ entry through P/Q channels is not increased following block of acetylcholine receptors with BTX. This is further evidence that the increase in quantal content induced by BTX occurs by a mechanism distinct from that induced by TTX.
Fig. 4.
Sensitivity to block of P/Q channels with ω-agatoxin-IVA is similar in control endplates and endplates from muscle injected 4–5 days earlier with BTX. Black traces represent EPCs from a control and a BTX-blocked endplate prior to application of ω-agatoxin-IVA. Gray traces represent EPCs following block of P/Q channels by application of ω-agatoxin-IVA. In control muscle the mean reduction in EPC amplitude following block of P/Q channels was 75 ± 5%. In BTX-treated endplates the mean reduction in EPC amplitude was similar (80 ± 4%, P = 0.47, n = 4 muscles for control, 5 muscles for BTX). All EPCs were measured at a holding potential of −45 mV. Due to use of the same holding potential the EPCs following application of BTX were smaller and are shown at a higher gain.
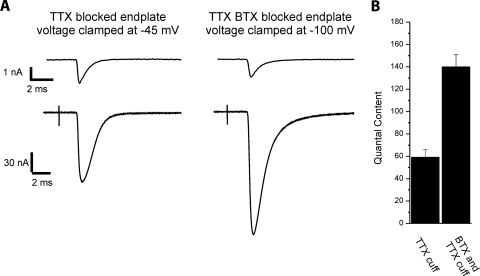
Following BTX injection there is selective block of postsynaptic action potentials, whereas presynaptic action potential frequency may actually increase in response to reduction in efficacy of neuromuscular transmission (Kimura 2001; Miyata et al. 1995). This introduces a mismatch in pre- and postsynaptic activity. To determine whether mismatch in activity triggers the increase in n, BTX was injected into muscle already paralyzed by application of a TTX cuff to the nerve. In this setting BTX does not introduce a mismatch in activity, since both pre- and postsynaptic activity are already blocked. BTX injection in the presence of TTX-induced block activity triggered an increase in quantal content in solution containing normal calcium (59.1 ± 6.8 in TTX-blocked endplates vs. 140.0 ± 10.8 in TTX- and BTX-blocked endplates, n = 7 muscles for TTX and 4 muscles for TTX/BTX, P < 0.01, Fig. 5). Since p is near one in solution containing normal calcium (Wang et al. 2010) the increase in quantal content must be due to increased n. We conclude that block of acetylcholine receptors rather than mismatch of activity triggers the increase in n.
Fig. 5.
Block of acetylcholine binding to acetylcholine receptors during spontaneous acetylcholine release triggers the increase in n. A: average MEPCs and EPCs for 2 endplates 5 days following block of synaptic activity by placement of a TTX on the nerve. The endplate on the right is from a muscle that was injected with BTX immediately after TTX cuff placement. Quantal contents calculated from the traces shown were 65 and 148, respectively. B: bar graph of the mean quantal content of endplates following block of activity by a TTX cuff and endplates following both TTX cuff and BTX injection. Quantal content averaged 59.1 ± 6.8 in TTX blocked endplates (n = 7 muscles) and 140.0 ± 10.8 in TTX- and BTX-blocked muscles (n = 4 muscles, P < 0.01).
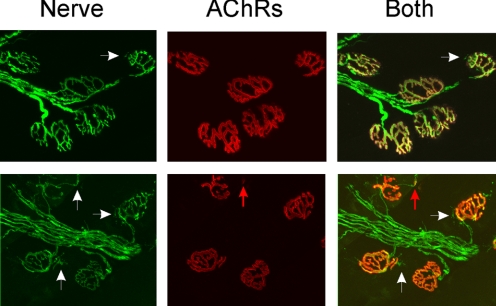
One mechanism that could be responsible for an increase in n is sprouting of nerve terminals and the formation of new synaptic sites. We examined whether the block of acetylcholine receptors for 4 days in vivo triggered formation of new synaptic sites that might account for the increase in n. We found little evidence of sprouts extending beyond the endplate border and very few endplates had what appeared to be new patches of acetylcholine receptors. Of 2,797 endplates from three BTX-injected muscles, only 188 (6.7%) had sprouts extending beyond the endplate (Fig. 6). In the small number of endplates with sprouts, most of the sprouts lacked associated clusters of postsynaptic acetylcholine receptors below them and thus could not account for an increase in number of functional synaptic sites. In fact, we found greater sprouting in TTX-blocked endplates, where n does not increase. Of 2,809 endplates from three muscles, 724 (25.8%) had sprouts extending beyond the endplate. Thus the presence of sprouts did not correlate with changes in n. This finding agrees well with a previous study that found that BTX treatment did not enlarge endplates (Molenaar et al. 1991).
Fig. 6.
Increase in n following block of acetylcholine receptors is not due to new synaptic sites. Shown in each row is a set of endplates from muscle injected with BTX 4 days earlier. Nerve staining is shown in the first column in green, acetylcholine receptors stained with rhodamine-conjugated BTX are shown in red in the second column, and the superimposed images are shown in the third column. In both sets of endplates small sprouts can be seen (white arrows). However, only one of the sprouts is associated with a small region of acetylcholine receptors (red arrow).
DISCUSSION
We determined probability of release (p) and number of releasable vesicles (n) using ANOVA following block of synaptic transmission at the mouse neuromuscular junction in vivo, either by placement of a TTX cuff on the nerve or by injection of BTX into the tibialis anterior muscle. Our analysis indicates that increases in p and n are differentially triggered depending on the way that synaptic activity is blocked. Placement of a TTX cuff on the nerve increased p, but had no effect on n, whereas injection of BTX had no effect on p, but increased n. By comparing aspects of synaptic transmission blocked by TTX and BTX it is possible to deduce what triggers the increases in p (Table 1). BTX blocks binding of acetylcholine to acetylcholine receptors during evoked release and postsynaptic action potentials, but does not trigger an increase in p. This leaves presynaptic action potentials as the step that is sensed to modulate p. We previously reported that block of P/Q channels could reverse in the increase in p (Wang et al. 2004). Combining our new and old work we propose that block of presynaptic spiking triggers an increase in p due to increased Ca2+ entry through P/Q channels.
Table 1.
Deducing the location of activity sensors that trigger synaptic plasticity at the NMJ
| Step | TTX Cuff | BTX |
|---|---|---|
| Presynaptic action potentials | Blocked | Unchanged |
| Acetylcholine binding to acetylcholine receptors during evoked release | Blocked | Blocked |
| Acetylcholine binding to acetylcholine receptors during spontaneous release | Unchanged | Blocked |
| Postsynaptic action potentials | Blocked | Blocked |
| Probability of release (p) | Increased | Unchanged |
| Number of releasable vesicles (n) | Unchanged | Increased |
Steps in synaptic transmission that are blocked by placement of a tetrodotoxin (TTX) cuff on the nerve (TTX cuff) or injection of α-bungarotoxin (BTX) into the muscle to block acetylcholine receptors. In the last two rows are the changes in the synaptic parameters p and n triggered by TTX cuff or BTX injection.
One hope in studies of homeostatic synaptic plasticity is that mechanisms discovered in model systems (such as central neurons in vitro or the NMJ in vivo) will be broadly applicable to central synapses in vivo. Activity-dependent changes in transmitter release suggested to be due to changes in p have been reported in frog, crayfish, and Drosophila NMJs (Belair et al. 2005; Frank et al. 2006; Hong and Lnenicka 1993; Lnenicka and Atwood 1985, 1989; Lnenicka and Hong 1997) and may occur at central synapses in vitro (Molder et al. 2003). The current study adds mouse NMJ to this list, which suggests regulation of p is common to NMJs of many species. However, the study in frog suggested regulation of quantal content occurs via a Ca2+-independent mechanism (Belair et al. 2005), whereas at the NMJ in the other species, modulation of Ca2+ channels is involved (Frank et al. 2006; Lnenicka and Hong 1997; Wang et al. 2004). This suggests mechanisms governing p may diverge between synapses. The current study also adds to evidence that block of neurotransmitter receptors is an important trigger for homeostatic synaptic plasticity. In the developing chick spinal cord, block of neurotransmitter receptors triggers an increase in quantal amplitude (Wilhelm and Wenner 2008). At the Drosophila NMJ block of neurotransmitter receptors triggers increased probability of release (Frank et al. 2006). We found that block of acetylcholine receptors triggers an increase in n. Both the divergence of mechanisms triggered by neurotransmitter receptor block and the divergence of mechanisms governing p suggest caution must be used when generalizing between synapses about homeostatic synaptic plasticity.
Our study suggests block of receptor activation during spontaneous neurotransmitter release can trigger homeostatic synaptic plasticity. We were able to separate the effects of receptor activation during evoked release from activation during spontaneous release by comparing synapses blocked by both TTX and BTX to synapses blocked by TTX alone. At synapses silenced by TTX the only additional aspect of synaptic signaling that is blocked by BTX is activation of acetylcholine receptors during MEPCs or nonquantal release of acetylcholine (Edwards et al. 1985; Katz and Miledi 1977). Because the addition of BTX to TTX triggered the increase in n, we conclude that block of receptor activation during spontaneous release is the trigger. There is evidence that activation of receptors during spontaneous release is sufficient to regulate motor neuron electrical properties (Bichler et al. 2007; Nakanishi et al. 2005). This suggests spontaneous release of acetylcholine from motor nerve terminals is part of a signaling pathway that regulates both synaptic properties and intrinsic excitability of motor neurons. It remains possible that block of presynaptic acetylcholine receptors triggers the increase in n. There is evidence that acetylcholine receptors of the α7 type, which are blocked by BTX, are on the presynaptic terminals of some motor axons (for review see MacDermott et al. 1999). Alternatively, block of postsynaptic acetylcholine receptors might trigger the increase via a retrograde signal from muscle.
One difficulty in determining the mechanism underlying the increase in n is that the physical correlate of n is not known (for discussion see Wang et al. 2010). Current data limit the possibilities. We found block of P/Q channels had the same effect at BTX-blocked and control endplates. This suggests the increase in n is not mediated by increased Ca2+ entry through P/Q channels. A previous study found no selective effect of N- or L-type blockers after treatment with BTX (Plomp et al. 1994), so it appears unlikely that the increase in n is due to increased Ca2+ entry through any HVA Ca channel subtype. We also ruled out that formation of new synaptic accounts for the increase in n. It appears that the mechanism underlying the increase in n is either an ultrastructural change in the NMJ or has no morphological correlate. At central synapses in vitro there is activity-dependent regulation of the number of functional synaptic sites that is independent of probability of release and is not accompanied by morphologic changes in docked or total synaptic vesicle number (Molder et al. 2004, 2006, 2008).
The increase in n triggered by BTX was accompanied by an increase in depression during repetitive stimulation. We previously found that the increase in p triggered by TTX was also accompanied by increased depression (Wang et al. 2004). One interpretation of these data is that ANOVA is not useful and the increases in quantal content following TTX and BTX are due to the same mechanism—an increase in p. However, the selective sensitivity of the TTX-induced increase in quantal content to ω-agatoxin-IVA suggests that the difference in mechanisms suggested by ANOVA is valid. This leads to the conclusion that, as previously suggested (Zucker 1989), an increase in depression is not useful in differentiating between increases in p versus increases in n. The data further suggest that the increase in n is not due to an increase in the readily releasable pool because this mechanism would either have no effect or would tend to decrease depression. Instead, the increase in depression suggests the increase in n is not accompanied by a parallel increase in the readily releasable pool, such that increased depletion increases depression (Zucker and Regehr 2002).
We report that at the mouse NMJ in vivo, block of acetylcholine receptors triggers a mechanism of synaptic plasticity that is distinct from that triggered by block of pre- and postsynaptic spiking. Since both forms of synaptic plasticity are triggered by block of activity it is tempting to speculate that calcium signaling is the general trigger involved in the plasticity in both cases. It is known that activation of nicotinic acetylcholine receptors allows for significant calcium entry (Elenes et al. 2009; Vernino et al. 1994) and muscle fiber action potentials generate a large calcium signal. However, the finding that the two forms of synaptic plasticity reported here can be independently triggered requires that the calcium signals generated be independently sensed. The calcium signal generated by opening of AChRs in response to spontaneous release of acetylcholine is generated at the surface membrane, where it might remain localized (Etter et al. 1996), whereas the calcium signal generated by a muscle action potential might be restricted to the interior of the fiber (Gomez et al. 2006). Thus distinct locations of sensors would allow the two Ca2+ signals to independently trigger synaptic plasticity.
Homeostatic synaptic plasticity is likely to be important in maintaining appropriate network activity during development and in disease states with altered excitability or synaptic function. Most studies of homeostatic synaptic plasticity have been performed using central synapses where evoked release is difficult to study. We used the mouse NMJ to study homeostatic regulation of evoked release and found that probability of release and number of releasable vesicles are independently regulated. Homeostatic regulation of the number of releasable vesicles is a novel mechanism of homeostatic plasticity and may play an important role in modulating network activity.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant P01-NS-057228 to M. M. Rich.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Baxter et al., 2009.Baxter MG, Murphy KL, Taylor PM, Wolfensohn SE. Chloral hydrate is not acceptable for anesthesia or euthanasia of small animals. Anesthesiology 111: 209; author reply 209–210, 2009 [DOI] [PubMed] [Google Scholar]
- Belair et al., 2005.Belair EL, Vallee J, Robitaille R. Long-term in vivo modulation of synaptic efficacy at the neuromuscular junction of Rana pipiens frogs. J Physiol 569: 163–178, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichler et al., 2007.Bichler EK, Carrasco DI, Rich MM, Cope TC, Pinter MJ. Rat motoneuron properties recover following reinnervation in the absence of muscle activity and evoked acetylcholine release. J Physiol 585: 47–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards et al., 1985.Edwards C, Dolezal V, Tucek S, Zemkova H, Vyskocil F. Is an acetylcholine transport system responsible for nonquantal release of acetylcholine at the rodent myoneural junction? Proc Natl Acad Sci USA 82: 3514–3518, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenes et al., 2009.Elenes S, Decker M, Cymes GD, Grosman C. Decremental response to high-frequency trains of acetylcholine pulses but unaltered fractional Ca2+ currents in a panel of “slow-channel syndrome” nicotinic receptor mutants. J Gen Physiol 133: 151–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter et al., 1996.Etter EF, Minta A, Poenie M, Fay FS. Near-membrane [Ca2+] transients resolved using the Ca2+ indicator FFP18. Proc Natl Acad Sci USA 93: 5368–5373, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknell, 1996.Flecknell P. Laboratory Animal Anaesthesia. San Diego, CA: Academic Press, 1996 [Google Scholar]
- Fleischman et al., 1977.Fleischman RW, McCracken D, Forbes W. Adynamic ileus in the rat induced by chloral hydrate. Lab Anim Sci 27: 238–243, 1977 [PubMed] [Google Scholar]
- Frank et al., 2006.Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron 52: 663–677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez et al., 2006.Gomez J, Neco P, DiFranco M, Vergara JL. Calcium release domains in mammalian skeletal muscle studied with two-photon imaging and spot detection techniques. J Gen Physiol 127: 623–637, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall et al., 2001.Hall LW, Clarke KW, Trim CM. Less rapidly acting intravenous agents. In: Veterinary Anaesthesia (10th ed.). Oxford, UK: Elsevier/Sanders, 2001, p. 125–127 [Google Scholar]
- Hong and Lnenicka, 1993.Hong SJ, Lnenicka GA. Long-term changes in the neuromuscular synapses of a crayfish motoneuron produced by calcium influx. Brain Res 605: 121–127, 1993 [DOI] [PubMed] [Google Scholar]
- Katz and Miledi, 1977.Katz B, Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci 196: 59–72, 1977 [DOI] [PubMed] [Google Scholar]
- Kimura, 2001.Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. New York: Oxford Univ. Press, 2001 [Google Scholar]
- Lnenicka and Atwood, 1985.Lnenicka GA, Atwood HL. Age-dependent long-term adaptation of crayfish phasic motor axon synapses to altered activity. J Neurosci 5: 459–467, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lnenicka and Atwood, 1989.Lnenicka GA, Atwood HL. Impulse activity of a crayfish motoneuron regulated its neuromuscular synaptic properties. J Neurophysiol 61: 91–96, 1989 [DOI] [PubMed] [Google Scholar]
- Lnenicka and Hong, 1997.Lnenicka GA, Hong SJ. Activity-dependent changes in voltage-dependent calcium currents and transmitter release. Mol Neurobiol 14: 37–66, 1997 [DOI] [PubMed] [Google Scholar]
- MacDermott et al., 1999.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci 22: 443–485, 1999 [DOI] [PubMed] [Google Scholar]
- Miyata et al., 1995.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995 [DOI] [PubMed] [Google Scholar]
- Molenaar et al., 1991.Molenaar PC, Oen BS, Plomp JJ, Van Kempen GT, Jennekens FG, Hesselmans LF. A non-immunogenic myasthenia gravis model and its application in a study of transsynaptic regulation at the neuromuscular junction. Eur J Pharmacol 196: 93–101, 1991 [DOI] [PubMed] [Google Scholar]
- Moulder et al., 2003.Moulder KL, Cormier RJ, Shute AA, Zorumski CF, Mennerick S. Homeostatic effects of depolarization on Ca2+ influx, synaptic signaling, and survival. J Neurosci 23: 1825–1831, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder et al., 2008.Moulder KL, Jiang X, Chang C, Taylor AA, Benz AM, Conti AC, Muglia LJ, Mennerick S. A specific role for Ca2+-dependent adenylyl cyclases in recovery from adaptive presynaptic silencing. J Neurosci 28: 5159–5168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder et al., 2006.Moulder KL, Meeks JP, Mennerick S. Homeostatic regulation of glutamate release in response to depolarization. Mol Neurobiol 33: 133–153, 2006 [DOI] [PubMed] [Google Scholar]
- Moulder et al., 2004.Moulder KL, Meeks JP, Shute AA, Hamilton CK, de Erausquin G, Mennerick S. Plastic elimination of functional glutamate release sites by depolarization. Neuron 42: 423–435, 2004 [DOI] [PubMed] [Google Scholar]
- Nakanishi et al., 2005.Nakanishi ST, Cope TC, Rich MM, Carrasco DI, Pinter MJ. Regulation of motoneuron excitability via motor endplate acetylcholine receptor activation. J Neurosci 25: 2226–2232, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp et al., 1992.Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol 458: 487–499, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp et al., 1994.Plomp JJ, van Kempen GT, Molenaar PC. The upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats: its dependency on calcium. J Physiol 478: 125–136, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich and Wenner, 2007.Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci 30: 119–125, 2007 [DOI] [PubMed] [Google Scholar]
- Robbins and Fischbach, 1971.Robbins N, Fischbach GD. Effect of chronic disuse of rat soleus neuromuscular junctions on presynaptic function. J Neurophysiol 34: 570–578, 1971 [DOI] [PubMed] [Google Scholar]
- Silverman and Muir, 1993.Silverman J, Muir WW., 3rd A review of laboratory animal anesthesia with chloral hydrate and chloralose. Lab Anim Sci 43: 210–216, 1993 [PubMed] [Google Scholar]
- Snider and Harris, 1979.Snider WD, Harris GL. A physiological correlate of disuse-induced sprouting at the neuromuscular junction. Nature 281: 69–71, 1979 [DOI] [PubMed] [Google Scholar]
- Tsujimoto and Kuno, 1988.Tsujimoto T, Kuno M. Calcitonin gene-related peptide prevents disuse-induced sprouting of rat motor nerve terminals. J Neurosci 8: 3951–3957, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto et al., 1990.Tsujimoto T, Umemiya M, Kuno M. Terminal sprouting is not responsible for enhanced transmitter release at disused neuromuscular junctions of the rat. J Neurosci 10: 2059–2065, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano, 2008.Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135: 422–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernino et al., 1994.Vernino S, Rogers M, Radcliffe KA, Dani JA. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J Neurosci 14: 5514–5524, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2004.Wang X, Engisch KL, Li Y, Pinter MJ, Cope TC, Rich MM. Decreased synaptic activity shifts the calcium dependence of release at the mammalian neuromuscular junction in vivo. J Neurosci 24: 10687–10692, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2006.Wang X, Engisch KL, Teichert RW, Olivera BM, Pinter MJ, Rich MM. Prolongation of evoked and spontaneous synaptic currents at the neuromuscular junction after activity blockade is caused by the upregulation of fetal acetylcholine receptors. J Neurosci 26: 8983–8987, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2005.Wang X, Li Y, Engisch KL, Nakanishi ST, Dodson SE, Miller GW, Cope TC, Pinter MJ, Rich MM. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci 25: 343–351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2010.Wang X, Pinter MJ, Rich MM. Ca2+ dependence of the binomial parameters p and n at the mouse neuromuscular junction. J Neurophysiol 103: 659–666, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm and Wenner, 2008.Wilhelm JC, Wenner P. GABAA transmission is a critical step in the process of triggering homeostatic increases in quantal amplitude. Proc Natl Acad Sci USA 105: 11412–11417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker, 1989.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci 12: 13–31, 1989 [DOI] [PubMed] [Google Scholar]
- Zucker and Regehr, 2002.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002 [DOI] [PubMed] [Google Scholar]