Abstract
A decrease in the excitability of CA1 pyramidal neurons contributes to the age related decrease in hippocampal function and memory decline. Decreased neuronal excitability in aged neurons can be observed as an increase in the Ca2+- activated K+- mediated post burst afterhyperpolarization (AHP). In this study, we demonstrate that the slow component of AHP (sAHP) in aged CA1 neurons (aged-sAHP) is decreased ∼50% by application of the reducing agent dithiothreitol (DTT). The DTT-mediated decrease in the sAHP was age specific, such that it was observed in CA1 pyramidal neurons of aged (20–25 mo), but not young (6–9 mo) F344 rats. The effect of DTT on the aged-sAHP was blocked following depletion of intracellular Ca2+ stores (ICS) by thapsigargin or blockade of ryanodine receptors (RyRs) by ryanodine, suggesting that the age-related increase in the sAHP was due to release of Ca2+ from ICS through redox sensitive RyRs. The DTT-mediated decrease in the aged-sAHP was not blocked by inhibition of L-type voltage gated Ca2+ channels (L-type VGCC), inhibition of Ser/Thr kinases, or inhibition of the large conductance BK potassium channels. The results add support to the idea that a shift in the intracellular redox state contributes to Ca2+ dysregulation during aging.
Keywords: afterhyperpolarization, aging, oxidative stress, redox state, neuronal excitability, ryanodine receptor, hippocampus
INTRODUCTION
An age-related decline in hippocampus-dependent memory is thought to result from dysregulation of Ca2+-dependent processes in CA1 pyramidal neurons including synaptic plasticity and neuronal excitability (Burke and Barnes 2010; Foster 2007, 1999; Kumar et al. 2009; Magnusson et al. 2010; Oh et al. 2010). One of the well characterized markers of aging in CA1 pyramidal neurons is an increase in the slow component of the Ca2+ activated, K+ - mediated afterhyperpolarization (sAHP) (Disterhoft et al. 1996; Kumar and Foster 2007; Kumar and Foster 2002, 2004; Landfield and Pitler 1984; Matthews et al. 2009; Moyer et al. 1992; Thibault et al. 2007; Tombaugh et al. 2005).
The exact mechanism that underlies the age-related increase in sAHP is unknown. The increase in sAHP may be due to altered Ca2+ regulation, including an increase in L-type voltage gated Ca2+ channels (L-type VGCC) (Thibault and Landfield 1996; Veng and Browning 2002), increased release of Ca2+ from intracellular Ca2+ stores (ICS) (Gant et al. 2006; Kumar and Foster 2004), or an increase in the function or density of K+ channels that mediate the sAHP (Power et al. 2001; Power et al. 2002). Importantly, aging is associated with increased oxidative stress that could influence the highly redox sensitive ryanodine receptor (RyR), which regulates Ca2+ release from the ICS (Bull et al. 2008; Eager and Dulhunty 1998; Hidalgo et al. 2004; Huddleston et al. 2008). Moreover, aged neurons are characterized by a decrease in their redox buffering capacity (Bodhinathan et al. 2010; Parihar et al. 2008) and recent work from our lab demonstrates that the shift in redox state contributes to decreased N-methyl d-aspartate receptor (NMDAR) function involving altered CaMKII activity in CA1 neurons from aged animals (Bodhinathan et al. 2010). Based on these observations, we tested the hypothesis that the redox state of the aged neuron contributes to the increase in sAHP (Foster 2007; Kumar et al. 2009).
The results reveal that the sAHP is decreased by the reducing agent dithiothreitol (DTT) in an age-dependent manner. Application of ryanodine, to block RyRs, prevented the DTT-mediated decrease of sAHP in the aged neurons. Depletion of ICS by the application of thapsigargin also blocked the DTT effect on aged-sAHP. The DTT-mediated decrease in the aged-sAHP was independent of the activity of L-type VGCC or Ser/Thr kinase activity. Finally, inhibition of large conductance potassium (BK) channel activity did not influence the DTT-mediated decrease in the aged-sAHP. The results point to an ICS-dependent and RyR-mediated mechanism that links altered redox state during aging with the enhanced sAHP in CA1 pyramidal neurons. Reversal of the redox state of aged hippocampal CA1 pyramidal neurons is a potential target to ameliorate Ca2+ dysregulation, decrease sAHP, and restore normal functionality in aged neurons.
METHODS
Animals
Procedures involving animals have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals. Male Fischer 344 rats, young (3–8 mo) and aged (20–25 mo), were obtained from National Institute on Aging colony at Harlan Sprague Dawley Inc (Indianapolis, IA). All animals were group housed (2 per cage), and maintained on a 12:12 h light schedule, and provided ad lib access to food and water.
Hippocampal slice preparation
The animals were deeply anesthetized using isoflurane (Webster, Sterling, MA) and decapitated with a guillotine (MyNeurolab, St Louis, MO). The brains were rapidly removed and hippocampi were dissected. Hippocampal slices (∼400 μm) were cut parallel to the alvear fibers using a tissue chopper (Mickle Laboratory Engineering Co, Surrey, UK). The slices were incubated in a holding chamber (at room temperature) with artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and d-glucose 10. ≥30 min before recording, slices were transferred to a standard interface recording chamber (Warner Instrument, Hamden, CT). In some experiments, the Ca2+ of the ACSF was raised to 4 mM. The chamber was continuously perfused with oxygenated ACSF (95%-O2-5%-CO2) at the rate of 2 mL/min. The pH and temperature were maintained at 7.4 and 30 ± 0.5°C (maintained using the automatic temperature controller TC-324B; Warner Instrument, Hamden, CT), respectively.
Intracellular electrophysiological recordings
Intracellular recordings were performed on CA1 pyramidal neurons to record the sAHP as previously described (Kumar and Foster 2004). Sharp microelectrodes were pulled from thin walled (1 mm) borosilicate capillary glass using a Flaming/Brown horizontal micropipette puller (Sutter Instruments, San Rafael, CA). The microelectrode resistances ranged from 38 to 90 MΩ when filled with 3 M potassium acetate. Microelectrodes were visually positioned in the CA1 pyramidal cell layer using a dissecting microscope (SZH10, Optical Elements Corp, Washington, DC). The signals were amplified using an Axoclamp 2B amplifier (Axon Instruments, Union City, CA), and sampled in continuous bridge mode at 5 kHz, and stored on a computer disk for off-line analysis (Data Wave Technologies).
Only neurons with a resting membrane potential (RMP) more hyperpolarized than −57 mV, and an input resistance >20 MΩ, and action potential amplitude rising ≥70 mV from the point of spike initiation were included in the analysis as described earlier (Kumar and Foster 2004). On cell entry, positive or negative current was applied to hold the neuron at the holding membrane potential (HMP) of −63 mV for the rest of the experiment. Voltage deflection resulting from hyperpolarizing current (1.0 nA) was used to determine the input resistance. Depolarizing current pulses (0.1–1.0 nA, 100 ms duration) were delivered every 20 s through the microelectrode to elicit sodium spike bursts containing a train of 5 action potentials.
The AHPs in the control and in the experimental conditions were elicited at the constant HMP of −63 mV, by manually clamping the membrane potential with DC current injection not exceeding ±1 nA. The sAHP amplitude was measured as the difference between the average membrane potential recorded during the 100-ms period immediately preceding the onset of the depolarizing current, and the average membrane potential recorded over a 100 ms window spanning the 400–500 ms after the offset of the depolarizing current pulse. The amplitude of the sAHP was compared before and during drug administration in the same neuron.
All drugs were prepared according to the manufacturer's specifications and ultimately dissolved in ACSF prior to bath application on the hippocampal slices. Nifedipine (Sigma, St. Louis, MO) and paxilline (Tocris Bioscience, Ellisville, MO) were initially dissolved in a small amount of dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO) and diluted in ACSF to a final DMSO concentration of <0.01% and to a final nifedipine, paxilline concentration of 10 μM. Xanthine (20 μg/ml) (Calbiochem) was initially dissolved in a small amount of 0.1N NaOH and finally dissolved in ACSF. DTT (0.7 mM), thapsigargin (1 μM) (Sigma, St. Louis, MO), ryanodine (20 μM) (Calbiochem, San Diego, CA), (±)-1-(5-Isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride (H-7, 10 μM) (Tocris Bioscience, Ellisville, MO), and xanthine oxidase (0.25 U/mg xanthine, Roche Diagnostics, Indianapolis, IN) were directly dissolved in ACSF.
Statistical analysis
All statistical analyses were performed using Stat View 5.0 (SAS Institute Inc, NC). Student's t-test were used to examine for differences between treatments or groups with significance set at P < 0.05. Paired Student's t-test were used to analyze the effect of treatment before and after application of drugs and unpaired Student's t-test were used to examine the effect of treatments between age groups. Where stated, n represents the number of slices used in each experiment. All data are reported as group mean ± standard error of mean (SEM). In general only one or two slices per animal were employed for a given experimental condition; although, several conditions could be examined using slices from the same animal.
RESULTS
Age dependent decrease in the sAHP following DTT application
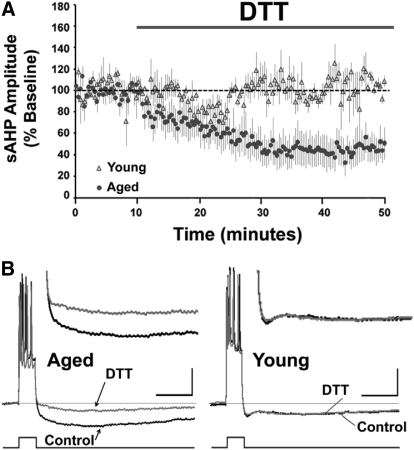
To study the effects of altered redox state on the aged-sAHP, the reducing agent DTT was applied to aged and young hippocampal CA1 pyramidal neurons while continuously recording the sAHP. In confirmation of previous reports (Kumar and Foster 2007; Kumar and Foster 2004; Landfield and Pitler 1984; Matthews et al. 2009; Moyer et al. 2000; Moyer et al. 1992; Power et al. 2002; Tombaugh et al. 2005) the sAHP was significantly (P < 0.05) increased in aged (6.44 ± 0.32 mV, n = 40) relative to young CA1 pyramidal neurons (4.23 ± 0.17 mV, n = 12). The properties of the CA1 pyramidal neurons recorded from the young and aged animals are indicated in Table 1. In a subset of these neurons, after a stable baseline recording for 10 min, DTT was applied for 40 min. Application of DTT significantly (P < 0.05) decreased the sAHP amplitude from the baseline levels in the aged (48 ± 14% of baseline, n = 5), but not in the young animals (105 ± 10%, n = 3) (Fig. 1, A and B).
Table 1.
Physiological properties of CA1 pyramidal neurons recorded from young and aged rats
| IR, MΩ | RMP, mV | Sp Amp, mV | |
|---|---|---|---|
| Young (n = 12) | 37.0 ± 2.5 | −62.8 ± 1.5 | 80.6 ± 1.9 |
| Aged (n = 40) | 37.8 ± 1.1 | −61.9 ± 0.6 | 82.8 ± 0.7 |
The values for input resistance (IR), resting membrane potential (RMP), and spike amplitude (Sp Amp) are indicated as mean ± SE. The number in parentheses indicates the number of cells from the young and aged animals.
Fig. 1.
Age-dependent reduction in the sAHP by DTT. (A) Time course of the change in the normalized sAHP amplitude in the aged (filled circles) (n = 5) and young (open triangles) animals (n = 3), following application of DTT for 40 min. (B) Representative traces illustrating the change in the AHP of aged (left) and young (right) animals under control conditions and at the end of 40 min application of DTT. The line beneath the traces indicates the onset and offset of the step current used to elicit a train of 5 action potentials. In this and subsequent figures, the faint line within the traces represents the baseline. Calibration bars: 200 ms, 10 mV. Inset: Magnified representation of change in the aged and young AHP under control condition, and 40 min after DTT application.
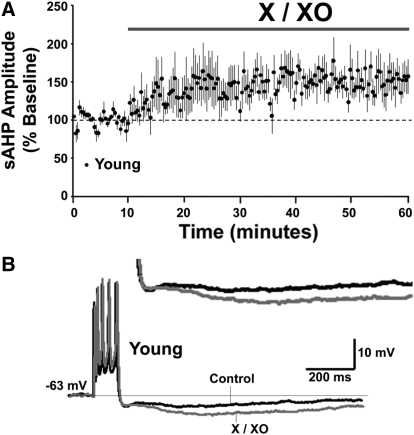
As such, the sAHP amplitude in aged following DTT application was similar to that observed in young animals (Table 2). The DC holding current required to maintain the membrane potential at −63 mV was similar for baseline recording and 45 min after DTT application (Table 2) indicating that the DTT-mediated reduction in sAHP was not associated with altered membrane properties. Finally, application of xanthine/xanthine oxidase (X/XO) to hippocampal slices from young animals increased the AHP in 4 out of 5 cells resulting in an overall significant increase in the sAHP amplitude (150 ± 21% of the baseline, n = 5) (Fig. 2, A and B). The results point to a link between altered redox state and the increased sAHP amplitude in aged neurons.
Table 2.
DTT effect on absolute sAHP amplitude from young and aged rats
| Holding current, nA |
|||
|---|---|---|---|
| sAHP amplitude, mV | Range | Mean ± SE | |
| Young Control (n = 12) | 4.23 ± 0.17 | −0.8 to +0.4 | −0.183 ± 0.114 |
| Young DTT (n = 3) | 4.38 ± 0.15 | −0.2 to +0.1 | −0.067 ± 0.088 |
| Aged Control (n = 40) | 6.44 ± 0.32 | −0.7 to +0.7 | −0.083 ± 0.051 |
| Aged DTT (n = 5) | 3.70 ± 0.78 | −0.2 to +0.2 | 0.032 ± 0.078 |
The values for sAHP amplitude are indicated as mean ± S.E. The number in parentheses indicates the number of cells from the young and aged animals. The values of the holding. currents are presented as range and mean ± S.E.
Fig. 2.
Oxidizing agent decreases sAHP in young animals. (A) Time course of the change in the normalized sAHP amplitude in young animals (n = 5), following application of X/XO for 50 min. (B) Representative traces illustrating the change in the AHP for a young animal under control conditions (black trace) and at the end of 50 min application of X/XO (gray trace). Calibration bars: 200 ms, 10 mV. Inset: Magnified representation of change in the young AHP under control condition, and 50 min after X/XO application.
DTT-mediated decrease in aged-sAHP involves intracellular Ca2+ stores and ryanodine receptors
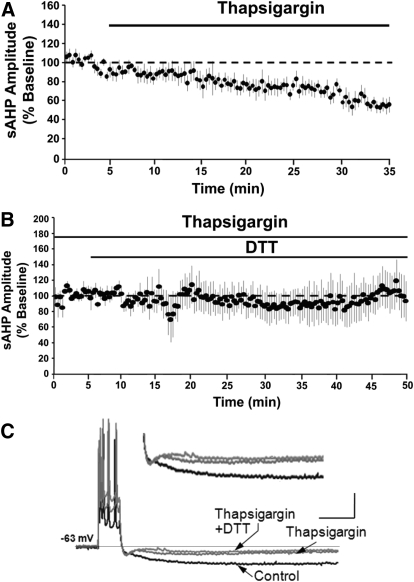
To test the hypothesis that the DTT-mediated decrease in the aged-sAHP was due to decreased Ca2+ mobilization from ICS, thapsigargin was applied prior to and during the application of DTT to aged hippocampal slices. Application of thapsigargin for 30 min significantly decreased (P < 0.05) the amplitude of aged-sAHP to 58 ± 8% (n = 7) of the baseline levels (Fig. 3A). In a subset of these cells (n = 4), a new baseline was established and DTT was applied in the presence of thapsigargin. Application of DTT for 50 min failed to decrease the aged-sAHP amplitude (104 ± 23%) (Fig. 3, B and C). The results suggest that ICS provide a redox sensitive Ca2+ source that contributes to the age-related increase in the sAHP.
Fig. 3.
Intracellular Ca2+ stores underlie DTT-mediated decrease in aged-sAHP. (A) Time course of the change in the normalized sAHP amplitude in the aged animals on application of thapsigargin (n = 7). (B) Time course of change in the normalized sAHP amplitude in cells (n = 4) incubated with thapsigargin prior to and during DTT application. (C) Representative traces illustrating the AHP of aged animals under control condition (black trace), and at the end of 40 min application of thapsigargin (gray trace) and at the end of 50 min application of thapsigargin+DTT (gray trace). Calibration bars: 200 ms, 10 mV. Inset: Magnified representation of change in the aged AHP under control condition, thapsigargin, and thapsigargin + DTT.
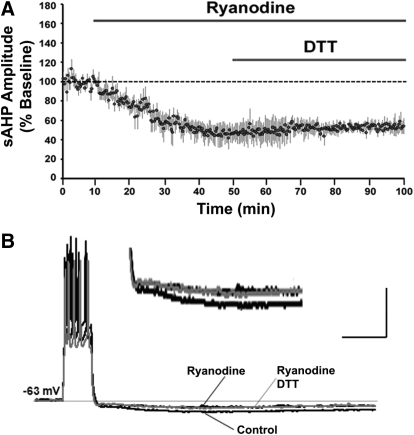
RyRs mobilize Ca2+ from the ICS and are highly redox sensitive. To test whether RyRs were involved in the DTT-mediated decrease in aged-sAHP, RyRs were blocked by ryanodine prior to and during the application of DTT. Application of ryanodine for 40 min, significantly (P < 0.05) decreased the aged-sAHP amplitude to 47 ± 10% (n = 4). Application of DTT for 50 min failed to further decrease the aged-sAHP amplitude such that it was 54 ± 7% (n = 4) of the original baseline (Fig. 4, A and B).
Fig. 4.
RyR blockade inhibits the DTT-mediated decrease in aged-sAHP. (A) Time course of the change in the normalized sAHP amplitude recorded from aged animals that were incubated with the ryanodine receptor antagonist ryanodine (n = 4) followed by the application of DTT. (B) Representative traces illustrating the change in the AHP of aged animals under control condition (black trace), at the end of a 40 min application of ryanodine (black trace), and at the end of 50 min application of ryanodine+DTT (gray trace). Calibration bars: 200 ms, 20 mV. Inset: Magnified representation of change in the aged AHP under control condition, ryanodine, and ryanodine + DTT.
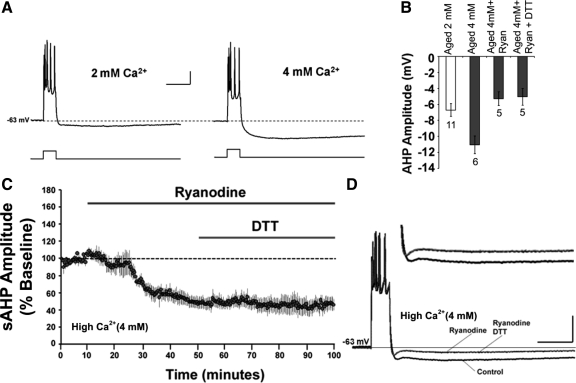
Both DTT (Fig. 1) and ryanodine (Fig. 4) decreased the aged-sAHP to ∼50% of baseline. The similar magnitude of the response for the two conditions raises the possibility of a “floor effect” of ryanodine, which may have masked DTT influences on the sAHP. To address this issue, the sAHP of aged neurons was enhanced by increasing the extracellular Ca2+ concentration from 2 mM to 4 mM. Increasing the extracellular Ca2+ to 4 mM increased the sAHP almost two fold, from 6.71 ± 0.79 mV (n = 11) to 11.06 ± 1.09 mV (n = 6) (Fig. 5, A and B). In five cells, a baseline was recorded in 4 mM Ca2+, followed by application of ryanodine for 40 min, which was then followed by the application of DTT for 50 min (Fig. 5C). Application of ryanodine decreased (P < 0.05) the aged-sAHP amplitude to 53 ± 6% (5.31 ± 0.86 mV) and application of DTT for 50 min failed to further decrease the aged-sAHP amplitude (51 ± 7%, 5.06 ± 1.05 mV) of the original baseline (Fig. 5, B–D). Thus DTT failed to reduce the sAHP amplitude under high Ca2+ and ryanodine application, despite the fact that sAHP amplitude was similar to the baseline under normal 2 mM Ca2+ conditions (Fig. 5B). The results indicate that the ryanodine blockade of the DTT-mediated decrease in the sAHP was not due to a floor effect of the sAHP during ryanodine application. Rather, these data suggest that the DTT effect on sAHP in aged animals is mediated by RyRs.
Fig. 5.
RyR blockade inhibits the DTT-mediated decrease in aged-sAHP when the AHP is increased by increasing Ca2+ in the recording medium. (A) Representative traces illustrating the AHP from aged neurons recorded under conditions of 2 mM or 4 mM Ca2+ in the ACSF. Calibration bars: 200 ms, 10 mV. (B) Quantification of the mean sAHP amplitude in aged neurons recorded under 2 mM Ca2+ (n = 11, open bar), under 4 mM Ca2+ (n = 6), under 4 mM Ca2+ with ryanodine (n = 5), and under 4 mM Ca2+ with ryanodine + DTT (n = 5); all values under 4 mM Ca2+ are represented as filled bars. (C) Time course of the change in the normalized sAHP amplitude recorded in 4 mM ACSF from aged animals and incubated with ryanodine (n = 5) prior to and during DTT application. (D) Representative traces illustrating the AHP of aged animals under control condition, and at the end of a 40 min application of ryanodine and at the end of 50 min application of ryanodine + DTT. Calibration bars: 200 ms, 20 mV. Inset: Magnified representation of change in the aged AHP (4 mM Ca2+) under control condition, ryanodine, ryanodine + DTT.
Finally, application of DTT for 40 min, decreased the aged-sAHP amplitude to 44 ± 7% (n = 4) of the baseline (similar to that observed in Fig. 1). In two of these cells, application of ryanodine for 40 min failed to further decrease the aged-sAHP amplitude such that it was 55 ± 6% (n = 2) of the original baseline. In the other two cells, application of thapsigargin also failed to further decrease the aged-sAHP amplitude such that it was 41 ± 8% (n = 2) of the original baseline. This suggests that application of DTT occluded the effect of ryanodine and thapsigargin on the aged-sAHP amplitude.
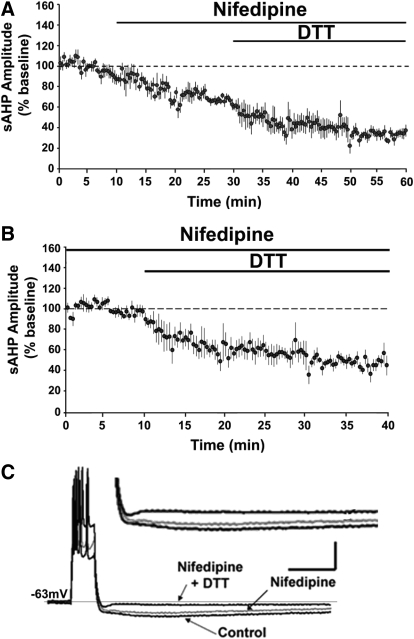
DTT-mediated reduction in the aged-sAHP is independent of L-type VGCCs
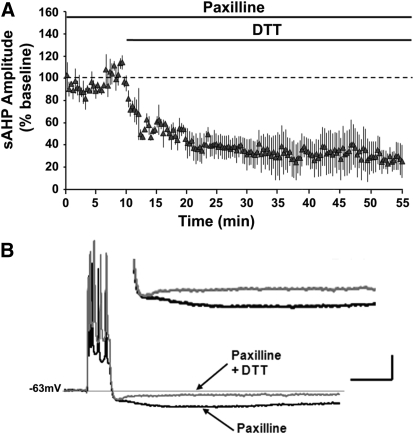
L-type VGCCs are another major source of Ca2+ for the sAHP. To test the hypothesis the DTT-mediated decrease in the aged-sAHP involves the L-type VGCC, nifedipine was applied prior to and during the application of DTT to aged hippocampal slices (Fig. 6A). Application of nifedipine for 20 min decreased the sAHP to 68 ± 4% (n = 5) of the baseline. Subsequent application of DTT for 30 min further decreased the amplitude of aged-sAHP to 34 ± 4% (n = 5) of the original baseline. The results suggest that the effects of nifedipine and DTT may be independent. In fact, using the sAHP responses recorded in nifedipine (bath application for ≥20 min) as the baseline, application of DTT decreased the amplitude of the aged-sAHP to 48 ± 6% (P < 0.05; n = 6) (Fig. 6B), a decrease comparable to that observed following DTT application in the absence of nifedipine (Fig. 6B). The activity of BK channels is sensitive to oxidation (DiChiara and Reinhart 1997). Moreover, an increase in BK channel activity can reduce the sAHP amplitude by decreasing the action potential spike width (Giese et al. 1998; Murphy et al. 2004; Shao et al. 1999). To test the hypothesis that the DTT-mediated decrease in aged-sAHP involves the BK channels, paxilline was first applied to inhibit BK channel activity (Sanchez and McManus 1996). Aged hippocampal slices were incubated in paxilline for ≥60 min prior to recording the sAHP and applying DTT. Paxilline failed to block the DTT-mediated decrease in aged-sAHP, such that DTT application was still able to decrease the amplitude of the aged-sAHP to 28 ± 11% (n = 3) of the baseline (Fig. 7, A and B). Furthermore, the DTT-mediated decrease in the presence of paxilline was not significantly (P > 0.05) different from the decrease observed in the presence of DTT alone.
Fig. 6.
DTT-mediated decrease is independent of L-type Ca2+ channel function. (A) Time course of the change in the normalized sAHP amplitude recorded from aged animals that were incubated with nifedipine (n = 4), followed by application of DTT. (B) Time course of the change in the normalized sAHP amplitude in the aged animals that were incubated with nifedipine (n = 6) for ≥45 min prior to the application of DTT for 30 min. Calibration bars: 200 ms, 10 mV. (C) Representative traces illustrating the change in the AHP of aged animals under control condition (black trace), and at the end of a 20 min application of nifedipine (gray trace) and at the end of 30 min application of nifedipine+DTT (black trace). Inset: Magnified representation of change in the aged AHP under control condition, nifedipine, and nifedipine + DTT.
Fig. 7.
DTT effects on aged-sAHP are independent of BK channel function. (A) Time course of the change in the normalized sAHP amplitude in the aged animals (filled triangles) that were incubated with paxilline (n = 3) for ≥60 min prior to DTT application. (B) Representative traces illustrating the change in the AHP of aged animals under paxilline (black trace), and at the end of 45 min under paxilline+DTT (gray trace). Calibration bars: 200 ms, 10 mV. Inset: Magnified representation of change in the aged AHP under paxilline, and paxilline + DTT.
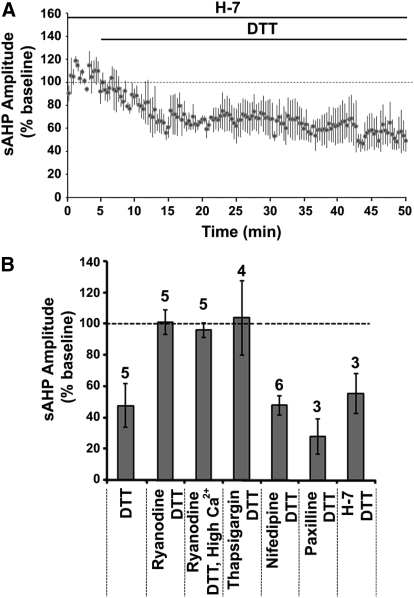
Serine/threonine kinases provide another potential mechanism for regulating RyRs and the K+ channels that mediate the sAHP. Protein kinase A increases the activity of cardiac RyRs (RyR subtype 2) (Danila and Hamilton 2004; Morimoto et al. 2009; Xiao et al. 2007; Yoshida et al. 1992), and kinase activity inhibits the sAHP (Madison and Nicoll 1986; Malenka et al. 1986; Melyan et al. 2002; Muller et al. 1992; Pedarzani and Storm 1993). To test whether the changes in kinase activity underlie the decrease in sAHP of aged neurons on application of DTT, the broad spectrum serine/threonine kinases inhibitor H-7 was applied prior to and during the application of DTT. Aged hippocampal slices were incubated with H-7 for ≥60 min before recording the sAHP. In the presence of H-7, application of DTT significantly (P < 0.05) decreased the aged-sAHP to 53 ± 14% (n = 3) of the baseline (Fig. 8A). The results suggest that DTT is not altering the sAHP through modulation of kinase activity.
Fig. 8.
Ser/Thr kinase activity does not mediate DTT effects on aged-sAHP. (A) Time course of the change in the normalized sAHP amplitude in the aged animals that were incubated with the broad spectrum Ser/Thr kinase inhibitor H-7 (n = 3) for ≥60 min prior to DTT application. (B) Summary diagram representing the mean percent change in the sAHP amplitude of aged neurons following DTT application under various conditions (filled bars). In each case, the response represents the percent change relative to the preDTT application baseline (dashed line). The numbers above each bar represents the number of neurons recorded in each condition.
Figure 8B summarizes the change in the sAHP amplitude of aged CA1 neurons following DTT application in the presence of various inhibitors. In each case, the response was normalized to the preDTT baseline. Treatments that blocked Ca2+ release from ICS (thapsigargin, ryanodine) blocked the DTT-mediated reduction in the sAHP. However the DTT-mediated reduction in sAHP was not blocked in the presence of nifedipine, paxilline, or H-7. Consistent with previous reports, we observed ∼50% and ∼30% reduction in sAHP amplitude on application of ryanodine (Gant et al. 2006; Kumar and Foster 2004) and nifedipine (Disterhoft et al. 2004; Power et al. 2002) respectively.
DISCUSSION
The results demonstrate a link between the age-related increase in the sAHP and redox state, through the release of Ca2+ from ICS. A shift in Ca2+ regulation and altered Ca2+ channel function is a characteristic of aging CA1 neurons (Burke and Barnes 2010; Foster 2007, 1999; Gant et al. 2006; Hemond and Jaffe 2005; Kumar et al. 2009; Landfield and Pitler 1984; Magnusson et al. 2010; Oh et al. 2010; Thibault et al. 2001; Thibault and Landfield 1996). Recently, we demonstrated that DTT could reverse an age-related decrease in NMDA receptor function in region CA1 (Bodhinathan et al. 2010). In the current study, the reducing agent, DTT, decreased the sAHP in aged, but not in young CA1 neurons. The data are consistent with the altered redox buffering in aged animals as a mechanism contributing to Ca2+ dysregulation and electrophysiological changes observed in aged neurons.
Redox modulation has been observed for several ion channels including K+ and Ca2+ channels (Chiamvimonvat et al. 1995; DiChiara and Reinhart 1997; Hidalgo et al. 2004; Ruppersberg et al. 1991; Stephens et al. 1996), which could contribute to the sAHP. The identity of the K+ channel that underlies the sAHP is unknown (Furuichi et al. 1994; Sah and Faber 2002); however, the amplitude to the sAHP is reduced by activation of Ser/Thr kinases, including PKA (Madison and Nicoll 1986; Pedarzani and Storm 1993), CaMKII (Muller et al. 1992), and PKC (Malenka et al. 1986). In the current study, the broad spectrum Ser/Thr kinase inhibitor H-7 had no influence on the DTT-mediated decrease in aged-sAHP, indicating that the reduction was not mediated through kinase activity.
In the case of K+ channels, previous reports indicate that cysteine specific oxidation decreases BK channel activity (Tang et al. 2001; Tang et al. 2004), and that the reducing agent DTT increases BK channel activity (DiChiara and Reinhart 1997). The BK channels are involved in repolarization of action potential, and an increase in BK channel activity will reduce the width of the action potential (Shao et al. 1999). Moreover, a decrease in the spike width can decrease the sAHP, by limiting the duration of depolarization-induced Ca2+ entry through L-type VGCCs (Giese et al. 1998; Murphy et al. 2004). Thus DTT could be acting on the BK channels to decrease L-type VGCC activity and the sAHP amplitude. However, several pieces of evidence suggest that this might not be the case. First, blockade of BK channels with paxilline did not block the DTT-mediated decrease in aged-sAHP. Second, blockade of VGCC's with nifedipine did not influence the DTT-mediated decrease in aged-sAHP. Finally, the fast AHP, which is mediated by the BK channel, is not altered with age (Matthews et al. 2009).
The amplitude of the sAHP is dependent on the level of cytosolic Ca2+. L-type VGCCs play a role in determining the amplitude of the sAHP (Landfield and Pitler 1984; Moyer et al. 1992; Norris et al. 1998) and contribute to the increase in the sAHP during aging (Thibault and Landfield 1996; Veng and Browning 2002). However, it does not appear that the DTT-mediated reduction in the aged-sAHP is acting through L-channels. The DTT-mediated reduction in the sAHP was larger than that observed for L-channel blockade, and was specific to aged animals. Previous research indicates that the decrease in the sAHP following blockade of L-channels is quantitatively larger in aged animals; however, the percent decrease (∼30%) is similar across ages, suggesting other mechanisms contribute to the age-related increase in the sAHP amplitude in aged animals (Disterhoft et al. 2004; Power et al. 2002). In the present study, blockade of the L-channel reduced the aged-sAHP ∼30%, consistent with previous reports (Norris et al. 1998; Power et al. 2002). Regardless of L-channel function, DTT reduced the aged-sAHP by ∼50% and the effect of DTT was specific to aged animals. The results suggest that DTT is acting on mechanisms other than the L-channel, which may mediate the age-related increase in the sAHP.
RyR and redox state dependent effects on sAHP
Release of Ca2+ from ICS through RyR activation, plays a role in determining the sAHP amplitude (Borde et al. 2000; Davies et al. 1996; Sah and McLachlan 1991; Usachev et al. 1993; van de Vrede et al. 2007) and Ca2+ from ICS contributes to altered physiology during aging (Gant et al. 2006; Kumar and Foster 2004, 2005). In addition, the RyRs are highly redox sensitive (Bull et al. 2008), such that oxidation of the cysteine residues increases the Ca2+ sensitivity and activity of RyR (Eager and Dulhunty 1998; Hidalgo et al. 2004; Huddleston et al. 2008). In the present study, the DTT-mediated decrease in the sAHP was blocked on depletion of ICS by thapsigargin or blockade of RyRs by ryanodine, indicating the involvement of ICS and RyRs in the decreased Ca2+ mobilization by DTT application. Together, the results suggest that the increase in the sAHP in aged neurons is related to redox sensitive Ca2+ mobilization from the ICS through the RyRs.
Treatments that modify intracellular redox state may provide a novel therapeutic strategy to restore Ca2+ homeostasis in the aged neurons. Interestingly, a learning mediated decrease in the sAHP is observed in aged memory-unimpaired rats (Matthews et al. 2009; Moyer et al. 2000; Murphy et al. 2006; Tombaugh et al. 2005). Future research should include an examination of a learning induced shift in redox state (Shvets-Teneta-Gurii et al. 2007) as a possible mechanism mediating the decrease in the sAHP of memory unimpaired animals.
GRANTS
This work was supported by, the Evelyn F. McKnight Brain Research Foundation, National Institutes of Health Grant AG014979 and MH059891 to TCF, a UF Alumni fellowship to K. Bodhinathan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Bodhinathan et al., 2010.Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30: 1914–1924, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borde et al., 2000.Borde M, Bonansco C, Fernandez de Sevilla D, Le Ray D, Buno W. Voltage-clamp analysis of the potentiation of the slow Ca2+-activated K+ current in hippocampal pyramidal neurons. Hippocampus 10: 198–206, 2000 [DOI] [PubMed] [Google Scholar]
- Bull et al., 2008.Bull R, Finkelstein JP, Galvez J, Sanchez G, Donoso P, Behrens MI, Hidalgo C. Ischemia enhances activation by Ca2+ and redox modification of ryanodine receptor channels from rat brain cortex. J Neurosci 28: 9463–9472, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke and Barnes, 2010.Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci 33: 153–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat et al., 1995.Chiamvimonvat N, O'Rourke B, Kamp TJ, Kallen RG, Hofmann F, Flockerzi V, Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res 76: 325–334, 1995 [DOI] [PubMed] [Google Scholar]
- Danila and Hamilton, 2004.Danila CI, Hamilton SL. Phosphorylation of ryanodine receptors. Biol Res 37: 521–525, 2004 [DOI] [PubMed] [Google Scholar]
- Davies et al., 1996.Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca(2+)-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol 495(Pt 2):353–366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiChiara and Reinhart, 1997.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci 17: 4942–4955, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft et al., 1996.Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci 59: 413–420, 1996 [DOI] [PubMed] [Google Scholar]
- Disterhoft et al., 2004.Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res Rev 3: 383–406, 2004 [DOI] [PubMed] [Google Scholar]
- Eager and Dulhunty, 1998.Eager KR, Dulhunty AF. Activation of the cardiac ryanodine receptor by sulfhydryl oxidation is modified by Mg2+ and ATP. J Membr Biol 163: 9–18, 1998 [DOI] [PubMed] [Google Scholar]
- Foster, 2007.Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell 6: 319–325, 2007 [DOI] [PubMed] [Google Scholar]
- Foster, 1999.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Brain Res Rev 30: 236–249, 1999 [DOI] [PubMed] [Google Scholar]
- Furuichi et al., 1994.Furuichi T, Kohda K, Miyawaki A, Mikoshiba K. Intracellular channels. Curr Opin Neurobiol 4: 294–303, 1994 [DOI] [PubMed] [Google Scholar]
- Gant et al., 2006.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci 26: 3482–3490, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese et al., 1998.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta1.1-deficient mice with impaired learning. Learn Mem 5: 257–273, 1998 [PMC free article] [PubMed] [Google Scholar]
- Hemond and Jaffe, 2005.Hemond P, Jaffe DB. Caloric restriction prevents aging-associated changes in spike-mediated Ca2+ accumulation and the slow afterhyperpolarization in hippocampal CA1 pyramidal neurons. Neuroscience 135: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- Hidalgo et al., 2004.Hidalgo C, Bull R, Behrens MI, Donoso P. Redox regulation of RyR-mediated Ca2+ release in muscle and neurons. Biol Res 37: 539–552, 2004 [DOI] [PubMed] [Google Scholar]
- Huddleston et al., 2008.Huddleston AT, Tang W, Takeshima H, Hamilton SL, Klann E. Superoxide-induced potentiation in the hippocampus requires activation of ryanodine receptor type 3 and ERK. J Neurophysiol 99: 1565–1571, 2008 [DOI] [PubMed] [Google Scholar]
- Kumar et al., 2009.Kumar A, Bodhinathan K, Foster TC. Susceptibility to calcium dysregulation during brain aging. Frontiers in Aging Neuroscience 1: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar and Foster, 2007.Kumar A, Foster T. Environmental enrichment decreases the afterhyperpolarization in senescent rats. Brain Res 1130: 103–107, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar and Foster, 2002.Kumar A, Foster TC. 17beta-estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol 88: 621–626, 2002 [DOI] [PubMed] [Google Scholar]
- Kumar and Foster, 2004.Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol 91: 2437–2444, 2004 [DOI] [PubMed] [Google Scholar]
- Kumar and Foster, 2005.Kumar A, Foster TC. Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain Res 1031: 125–128, 2005 [DOI] [PubMed] [Google Scholar]
- Landfield and Pitler, 1984.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science 226: 1089–1092, 1984 [DOI] [PubMed] [Google Scholar]
- Madison and Nicoll, 1986.Madison DV, Nicoll RA. Actions of noradrenaline recorded intracellularly in rat hippocampal CA1 pyramidal neurones, in vitro. J Physiol 372: 221–244, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson et al., 2010.Magnusson KR, Brim BL, Das SR. Selective vulnerabilities of N-methyl-D-aspartate (NMDA) receptors during brain aging. Frontiers in Aging Neuroscience 2: 1–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka et al., 1986.Malenka RC, Madison DV, Andrade R, Nicoll RA. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J Neurosci 6: 475–480, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews et al., 2009.Matthews EA, Linardakis JM, Disterhoft JF. The fast and slow afterhyperpolarizations are differentially modulated in hippocampal neurons by aging and learning. J Neurosci 29: 4750–4755, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan et al., 2002.Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34: 107–114, 2002 [DOI] [PubMed] [Google Scholar]
- Morimoto et al., 2009.Morimoto S, J OU, Kawai M, Hoshina T, Kusakari Y, Komukai K, Sasaki H, Hongo K, Kurihara S. Protein kinase A-dependent phosphorylation of ryanodine receptors increases Ca2+ leak in mouse heart. Biochem Biophys Res Commun 390: 87–92, 2009 [DOI] [PubMed] [Google Scholar]
- Moyer et al., 2000.Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci 20: 5476–5482, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer et al., 1992.Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992 [DOI] [PubMed] [Google Scholar]
- Muller et al., 1992.Muller W, Petrozzino JJ, Griffith LC, Danho W, Connor JA. Specific involvement of Ca(2+)-calmodulin kinase II in cholinergic modulation of neuronal responsiveness. J Neurophysiol 68: 2264–2269, 1992 [DOI] [PubMed] [Google Scholar]
- Murphy et al., 2004.Murphy GG, Fedorov NB, Giese KP, Ohno M, Friedman E, Chen R, Silva AJ. Increased neuronal excitability, synaptic plasticity, and learning in aged Kvbeta1.1 knockout mice. Curr Biol 14: 1907–1915, 2004 [DOI] [PubMed] [Google Scholar]
- Murphy et al., 2006.Murphy GG, Rahnama NP, Silva AJ. Investigation of age-related cognitive decline using mice as a model system: behavioral correlates. Am J Geriatr Psychiatry 14: 1004–1011, 2006 [DOI] [PubMed] [Google Scholar]
- Norris et al., 1998.Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci 18: 3171–3179, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh et al., 2010.Oh MM, Oliveira FA, Disterhoft JF. Learning and aging related changes in intrinsic neuronal excitability. Frontiers in Aging Neuroscience 2: 1–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar et al., 2008.Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res 86: 2339–2352, 2008 [DOI] [PubMed] [Google Scholar]
- Pedarzani and Storm, 1993.Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11: 1023–1035, 1993 [DOI] [PubMed] [Google Scholar]
- Power et al., 2001.Power JM, Oh MM, Disterhoft JF. Metrifonate decreases sI(AHP) in CA1 pyramidal neurons in vitro. J Neurophysiol 85: 319–322, 2001 [DOI] [PubMed] [Google Scholar]
- Power et al., 2002.Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci 22: 7234–7243, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg et al., 1991.Ruppersberg JP, Stocker M, Pongs O, Heinemann SH, Frank R, Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature 352: 711–714, 1991 [DOI] [PubMed] [Google Scholar]
- Sah and Faber, 2002.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- Sah and McLachlan, 1991.Sah P, McLachlan EM. Ca(2+)-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurons: a role for Ca(2+)-activated Ca2+ release. Neuron 7: 257–264, 1991 [DOI] [PubMed] [Google Scholar]
- Sanchez and McManus, 1996.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- Shao et al., 1999.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521 Pt 1: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvets-Teneta-Gurii et al., 2007.Shvets-Teneta-Gurii TB, Troshin GI, Dubinin AG. Local changes in the redox potential in the rabbit cerebral cortex accompanying the acquisition of a conditioned defensive reflex. Neurosci Behav Physiol 37: 481–487, 2007 [DOI] [PubMed] [Google Scholar]
- Stephens et al., 1996.Stephens GJ, Owen DG, Robertson B. Cysteine-modifying reagents alter the gating of the rat cloned potassium channel Kv1.4. Pfluegers 431: 435–442, 1996 [DOI] [PubMed] [Google Scholar]
- Tang et al., 2001.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol 117: 253–274, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al., 2004.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol 11: 171–178, 2004 [DOI] [PubMed] [Google Scholar]
- Thibault et al., 2007.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell 6: 307–317, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault et al., 2001.Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci 21: 9744–9756, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault and Landfield, 1996.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272: 1017–1020, 1996 [DOI] [PubMed] [Google Scholar]
- Tombaugh et al., 2005.Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25: 2609–2616, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev et al., 1993.Usachev Y, Shmigol A, Pronchuk N, Kostyuk P, Verkhratsky A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 57: 845–859, 1993 [DOI] [PubMed] [Google Scholar]
- van de Vrede et al., 2007.van de Vrede Y, Fossier P, Baux G, Joels M, Chameau P. Control of IsAHP in mouse hippocampus CA1 pyramidal neurons by RyR3-mediated calcium-induced calcium release. Pfluegers 455: 297–308, 2007 [DOI] [PubMed] [Google Scholar]
- Veng and Browning, 2002.Veng LM, Browning MD. Regionally selective alterations in expression of the alpha(1D) subunit (Ca(v)1.3) of L-type calcium channels in the hippocampus of aged rats. Brain Res Mol Brain Res 107: 120–127, 2002 [DOI] [PubMed] [Google Scholar]
- Xiao et al., 2007.Xiao B, Tian X, Xie W, Jones PP, Cai S, Wang X, Jiang D, Kong H, Zhang L, Chen K, Walsh MP, Cheng H, Chen SR. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J Biol Chem 282: 30256–30264, 2007 [DOI] [PubMed] [Google Scholar]
- Yoshida et al., 1992.Yoshida A, Ogura A, Imagawa T, Shigekawa M, Takahashi M. Cyclic AMP-dependent phosphorylation of the rat brain ryanodine receptor. J Neurosci 12: 1094–1100, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]