Abstract
In contrast to neurons of the lateral geniculate nucleus (LGN), neurons in the primary visual cortex (V1) are selective for the direction of visual motion. Cortical direction selectivity could emerge from the spatiotemporal configuration of inputs from thalamic cells, from intracortical inhibitory interactions, or from a combination of thalamic and intracortical interactions. To distinguish between these possibilities, we studied the effect of adaptation (prolonged visual stimulation) on the direction selectivity of intracellularly recorded cortical neurons. It is known that adaptation selectively reduces the responses of cortical neurons, while largely sparing the afferent LGN input. Adaptation can therefore be used as a tool to dissect the relative contribution of afferent and intracortical interactions to the generation of direction selectivity. In both simple and complex cells, adaptation caused a hyperpolarization of the resting membrane potential (−2.5 mV, simple cells, −0.95 mV complex cells). In simple cells, adaptation in either direction only slightly reduced the visually evoked depolarization; this reduction was similar for preferred and null directions. In complex cells, adaptation strongly reduced visual responses in a direction-dependent manner: the reduction was largest when the stimulus direction matched that of the adapting motion. As a result, adaptation caused changes in the direction selectivity of complex cells: direction selectivity was reduced after preferred direction adaptation and increased after null direction adaptation. Because adaptation in the null direction enhanced direction selectivity rather than reduced it, it seems unlikely that inhibition from the null direction is the primary mechanism for creating direction selectivity.
INTRODUCTION
One of the properties to emerge de novo in neurons of the primary visual cortex (V1) of cats and primates is direction selectivity. Many V1 neurons respond selectively to the direction of moving image elements (Hubel and Wiesel 1962; Movshon 1975), whereas lateral geniculate nucleus (LGN) neurons projecting to the cortex respond equally well to all directions of motion (Cleland and Levick 1974). To extract motion information, neurons must integrate information from their inputs across both space and time. As with orientation tuning, direction selectivity is not derived directly from retinal responses; instead its emergence is the result of specific computations made in V1 to transform the representation of the visual world.
In direction-selective simple cells of V1, spatiotemporal integration may be accomplished in part by a gradient in response timing to either bright or dark bars that is present in each of the on and off subregions of the receptive field (DeAngelis et al. 1993; Jagadeesh et al. 1997; McLean and Palmer 1989; McLean et al. 1994; Reid et al. 1987, 1991; Saul and Humphrey 1992). Direction selectivity, however, could also emerge, either in whole or in part, from intracortical mechanisms. For example, the timing changes within the simple cell subfield could be generated within the cortex rather than from differing geniculate inputs. Or if cortical neurons with different spatiotemporal receptive fields were to inhibit one another (Torre and Poggio 1978), direction selectivity could emerge from an inhibitory antagonism between cortical neurons selective for opposite motion directions.
To investigate the contribution of the cortical circuit to direction selectivity, we used adaptation to suppress the cortical circuit temporarily, while recording intracellularly to measure the effects of adaptation on synaptic inputs. By adapting a cortical neuron to a single visual stimulus and measuring the effects of this adaptation on the neuron's responses to various stimuli, it is possible to measure the functional interaction of processing channels (Blakemore and Campbell 1969). If cortically mediated inhibition evoked by the null direction were an important component of direction selectivity, adaptation to the null direction should reduce direction selectivity, as the inhibition would no longer be available to suppress the neuron's responses to motion in the null direction. On the other hand, if direction selectivity depends primarily on excitation from the LGN cells with different response timing, adaptation should have little effect on direction selectivity because it has little effect on LGN cells. Finally, if direction selectivity emerges from interactions between cortical cells that are not direction-selective, adaptation should reduce the responses of neurons equally for adaptation in the preferred or null direction.
Our intracellular recordings from V1 simple cells favor models of direction selectivity based on excitation from the LGN rather than cortical inhibition. In simple cells, the primary effect of adaptation was a hyperpolarization of the membrane potential (Carandini and Ferster 1997; Sanchez-Vives et al. 1997) rather than a change in the magnitude of the synaptic response or in direction selectivity. Therefore the primary effect of adaptation in simple cells is intrinsic to the neuron not to a change in the input. In contrast, for complex cells, adaptation caused a modest hyperpolarization of the membrane potential, reflecting a weak intrinsic change in conductance accompanied by a strong reduction in the visually evoked depolarization for stimuli in the same direction as the adapting stimulus. Adaptation in the preferred direction therefore decreased direction selectivity, whereas adaptation in the null direction increased direction selectivity. This result is the opposite of what would be expected if cortical inhibition from the nonpreferred direction was contributing significantly to direction selectivity.
METHODS
Physiological preparation
Intracellular sharp and patch electrode recordings were made in the primary visual cortex (V1) of anesthetized, paralyzed female cats (2–2.5 kg). Anesthesia was induced with ketamine (5–15 mg/kg) and acepromazine (0.7 mg/kg), and cannulae were inserted into the saphenous veins and the trachea. After intravenous administration of sodium thiopental (10–20 mg/kg), the animal's head then was fixed in a stereotaxic frame. Two additional measures were taken to increase the stability of recordings: the animal's thoracic vertebrae were suspended from the stereotaxic frame and a pneumothoracotomy was performed. The animal was maintained under anesthesia using an intravenous infusion of sodium thiopental (1–2 mg · kg−1 · h−1) for the duration of the experiment. To minimize drift in eye position, paralysis was maintained with an infusion of vecuronium bromide (Norcuron, 0.2 mg · kg−1 · h−1) or gallamine (10 mg · kg−1 · h−1). Body temperature was kept at 38.2°C with a thermostatically controlled heat lamp. The electrocardiogram, electroencephalogram, autonomic signs, and rectal temperature were continuously monitored to ensure the anesthetic and physiological state of the animal. The nictitating membranes were retracted with the application of phenylephrine hydrochloride, and the pupils were dilated using topical atropine. The corneas were protected by contact lenses with artificial pupils (4 mm diam). Supplementary lenses were selected by direct ophthalmoscopy to focus the display screen onto the retina.
Borosilicate glass electrodes (A-M Systems, Carlsborg, WA) filled with 2 M potassium acetate (for sharp recordings) or K-gluconate (for patch recordings) were introduced by a motorized microdrive (Sutter Instruments, Novato, CA) into area 17 ∼2 mm lateral of the midline (Lampl et al. 2001; Priebe and Ferster 2005). After the electrode was in place, warm agarose solution was placed over the craniotomy to protect the surface of the cortex and reduce pulsations. The recording sessions lasted between 24 and 48 h.
All procedures were approved by the Northwestern University Animal Care and Use Committee.
Stimulus presentation and data acquisition
Visual stimuli were generated by a Macintosh computer using Psychophysics toolbox (Brainard 1997; Pelli 1997) for Matlab (Natick, MA) and presented on a ViewSonic video monitor. The video monitor had a noninterlaced refresh rate of 100 Hz and a spatial resolution of 1,024 × 768 pixels, which subtended 40 cm horizontally and 30 cm vertically. The monitor was placed 48 cm from the cat's eyes so that there were ≥22 pixel/° of visual angle. The video monitor had a mean luminance of 20 cd/cm2.
Stimuli consisted of drifting sinusoidal gratings presented monocularly at 2 Hz temporal frequency for 4 s, preceded and followed by 250 ms blank periods. Orientation and spatial-frequency tuning curves were made for each cell, and subsequent adaptation tests were performed at the optimal orientation and spatial frequency. We used an adaptation protocol similar to previous work (Carandini and Ferster 1997) in which adaptation was induced by presenting four consecutive 4-s trials in the same direction. Test stimuli were then interleaved one-for-one with additional presentations of the adapting stimulus. Eight test stimuli consisted of gratings drifting in the preferred and null directions at four different contrast levels (4, 8, 16, and 32%, using Michelson definition for contrast). Blocks of adaptation trials were presented to each cell with three different adapting stimuli: gratings drifting in the preferred direction at 64% contrast, a blank screen (at the mean luminance) and gratings drifting in the null direction at 64% contrast. The full sequence of 8 test stimuli embedded in the three different adaptation blocks was presented multiple times in a pseudorandom sequence in each adaptation block. To make sure that any change in membrane potential was caused by the adaptation state, and not by a degradation in the recording quality, the complete sequence of adaptation blocks (preferred adaptation, control adaptation, and null adaptation) was repeated between three and eight times for each neuron.
The voltage responses of neurons were recorded in current-clamp mode (Axoclamp 2B amplifier, Axon Instruments), low-passed filtered with a cutoff frequency of 10 kHz, sampled at 4,096 Hz and stored for subsequent analysis. Data were analyzed on-line to determine when enough trials had been performed to yield mean responses with low noise.
Simple and complex cells were identified on the basis of the F1/F0 ratio of the membrane potential response to optimal drifting gratings because not all of the cells in our database showed robust spiking responses. A previous study demonstrated that using a cutoff value of 0.5 for voltage F1/F0 matches the spiking classification for 95% of simple cells and 80% of complex cells (Priebe et al. 2004). We define the peak response to visual stimulation as sum of the modulated response (F1) and the mean depolarization (DC) from the resting potential over four second stimulus duration.
Specific analyses of the data are described in the Results section. The statistical significance of the effects of adaptation was determined using paired t-test. The confidence intervals for the fit variables were computed from the residuals and Jacobian matrix using the Matlab (Mathworks, Natick, MA) function nlparci.
RESULTS
Effects of adaptation on resting potential and visually evoked depolarization
Simple and complex cells are affected differently by contrast adaptation, and the effects of adaptation on direction selectivity can be largely understood in this context. Prolonged visual stimulation in a simple cell's preferred direction and orientation has been shown to induce a significant (2–10 mV) hyperpolarization of the resting membrane potential but with little effect on the peak amplitude of visually evoked depolarization, when measured relative to the adapted resting potential (Rabsadapt, Fig. 1) (Carandini and Ferster 1997; Sanchez-Vives et al. 2000). The cell in Fig. 2, for example, is a simple cell in which preferred- and null-direction stimuli at high contrast evoked sinusoidal responses with peak amplitudes of 17 and 7 mV when the cell was in the unadapted state (Fig. 2A), giving it a direction index, or DI, of 0.42. The peak response is defined as the sum of the modulated component of the response at the stimulus frequency (F1) and the mean depolarization (DC) from the resting potential over the stimulus duration. Here, the direction index is
| (1) |
where Rp and Rn are the amplitudes of the peak response to the preferred and null directions. The DI ranges from 0 for non direction selective cells to 1 for completely direction selective cells.
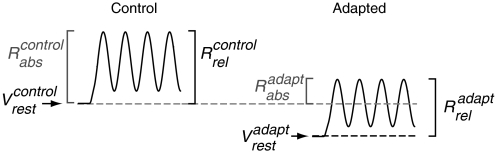
Fig. 1.
Measurements of the effects of visual adaptation. For both simple and complex cells, the response was defined by the peak response, which is equal to the sum of the membrane potential modulation (F1 component) and mean response (DC component). The measurement was made in 2 ways: relative to the resting potential (Vrest) in the control condition (Rabs), or the response relative to the resting potential in the adapted condition (Rrel). In the control, unadapted condition (left), Rabs and Rrel are equivalent. In the adapted state (right) Rabs and Rrel are potentially distinct if there are changes in the Vrest caused by adaptation.
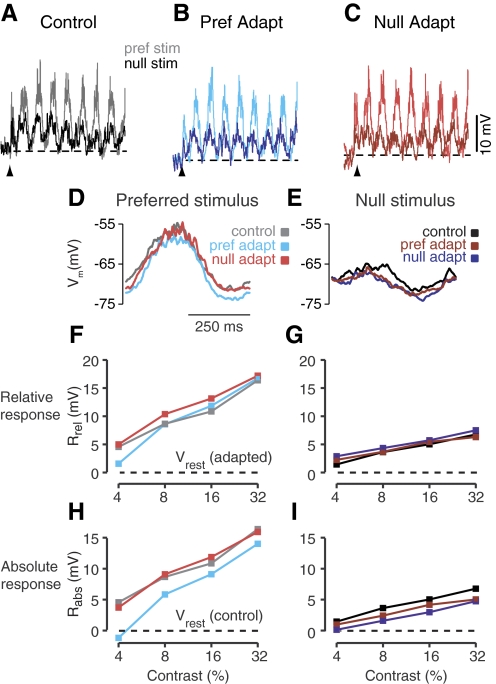
Fig. 2.
Effects of prolonged stimulation on a simple cell. Average responses to drifting gratings in the preferred (light traces) and opposite (dark traces) directions after no visual adaptation (A), prolonged visual stimulation in the preferred direction (B), or prolonged visual stimulation in the opposite (null) direction (C). The resting membrane potential for each adaptation condition is indicated by the black dashed lines. Upward pointing arrows indicate the beginning of visual stimulation. D and E: contrast-response curves for the six stimulus conditions: preferred and null direction stimuli after adaptation to a blank stimulus, to the preferred direction, or to the null direction. Responses are measured as the peak depolarization (F1+DC) relative to the adapted resting potential (Rrel). F and G: same as D and E for the peak depolarization measured relative to the unadapted resting potential (Rab).
After adaptation to high-contrast sinusoidal gratings in the preferred direction, the cell's resting membrane potential hyperpolarized by ∼3 mV (Fig. 2B, dotted line). The amplitudes of the responses to 32% contrast grating when measured relative to the hyperpolarized resting level (Rreladapt) were largely unchanged (17 and 7.5 mV). As a result, the DI was little changed (0.39).
In addition to changes in resting potential, we also observed small changes in response timing to high contrast gratings (32%). For the neuron in Fig. 2, adaptation caused a small phase delay in the response to the preferred direction (preferred adaptation, Fig. 2D, cyan: 6° = 8 ms; null adaptation, red: 9° = 13 ms). Across the simple cell population adaptation caused an average delay of 12.7 ± 3.9 ms following preferred adaptation and 13.0 ± 4.4 ms following null adaptation. The timing of the response to the null direction showed no consistent effect of adaptation (preferred adaptation, phase advance of 2.2 ± 8.5 ms; null adaptation, phase advance of 0.9 ± 10 ms following null adaptation).
Contrast response curves for Rrel are plotted in Fig. 2, F and G, for the six different stimulus conditions: preferred (F) and null (G) stimuli with no adaptation (black), preferred adaptation (blue), and null adaptation (red). Response curves are based on the sum of the membrane potential modulation (F1 component) and mean depolarization during the course of the visual stimulation. Note that the lack of change in the DI, referred to in the preceding text, is based on the amplitude of the depolarization relative to the resting potential, which changes with adaptation state. To gauge the impact of adaptation on the output (firing rate) of simple cells, it is important to measure the direction selectivity of the visually evoked depolarization on an absolute scale (Sanchez-Vives et al. 1997). For this measure, we use Rabsadapt (Fig. 1), measuring the peak depolarization relative to the unadapted resting potential. Because adaptation in the preferred direction hyperpolarized the resting potential but had little effect on the size of evoked depolarizations, Rabs was ∼3 mV smaller than in the unadapted state for each direction at the highest tested contrast: 15.5 mV for the preferred direction and 4.8 mV for the null direction. Adaptation in the null direction (Fig. 2C) also hyperpolarized the resting potential but only by about half as much as adaptation in the preferred direction (1.5 mV). Full contrast response curves for Rabs in the adapted and control states are shown in Fig. 2, H and I. Because a pure hyperpolarization reduces Rabsadapt proportionately less for the null response than for the preferred response, direction selectivity measured from Rabs rose somewhat in the adapted condition relative to the unadapted, control condition (preferred adaptation, DI = 0.47; null adaptation, DI = 0.52).
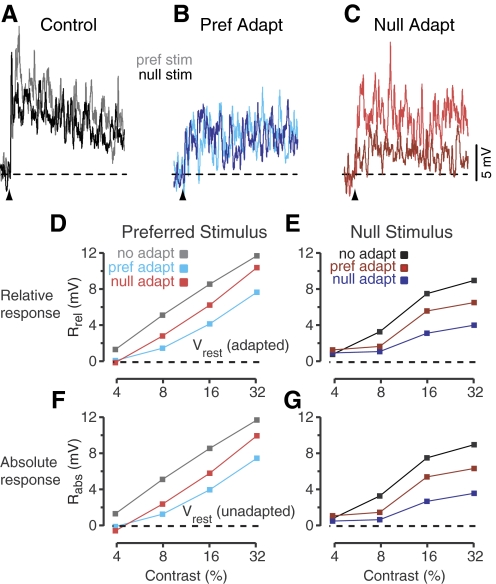
The effects of adaptation in complex cells were almost the exact opposite of the effects of adaptation in simple cells. The adaptation-induced hyperpolarization in complex cells was much smaller than in simple cells, and adaptation instead caused a significant decrease in the size of visually evoked depolarizations. An example is shown in Fig. 3 with the same format as in Fig. 2. The unadapted responses to high-contrast stimuli in the preferred and null directions (Rabscontrol) were 12 and 9 mV in amplitude (Fig. 3A) for a DI of 0.15. The DI for spike rate was higher (0.35) because of the nonlinearity of spike threshold (see Jagadeesh et al. 1993; Priebe and Ferster 2005). By definition, for complex cells, the responses are not strongly modulated at the stimulus frequency. They do show a steady decline over the course of the trial that is likely a result of rapid adaptation. When the complex cell was adapted in the preferred direction, the preferred and null responses were both reduced in amplitude (Fig. 3B). The steady decline in the response over the course of the trial disappeared, presumably because the cell was already adapted at the start of each trial. Note that preferred adaptation had differential effects on the cell's preferred and null responses: the preferred response was reduced more than the null response. Exactly the opposite happened for null adaptation (Fig. 3C): the amplitude of the null response was reduced more than the preferred response. In other words, adaptation in one direction most strongly affects the response to that same direction of motion. As a result, for these high-contrast stimuli, the cell became less direction selective after adaptation in the preferred direction (DI = 0.03) and more direction selective after adaptation in the null direction (DI = 0.45).
Fig. 3.
Effects of prolonged stimulation on a complex cell. Format follows Fig. 2 with the exception of cycle-averaged responses.
These effects of adaptation are quantified in the graphs of Rrel for the six adaptation and stimulus conditions (Fig. 3, D and E): at all contrasts, the peak response to preferred stimulation (measured relative to the adapted resting level) was depressed more by preferred adaptation (Fig. 3D, red) than by null adaptation (blue); conversely, the response to null stimulation (E) was depressed more by null adaptation than by preferred adaptation. The effects were almost identical when response amplitude was quantified as Rabs (peak response measured relative to the unadapted resting level) because adaptation had little effect on the resting potential in this cell.
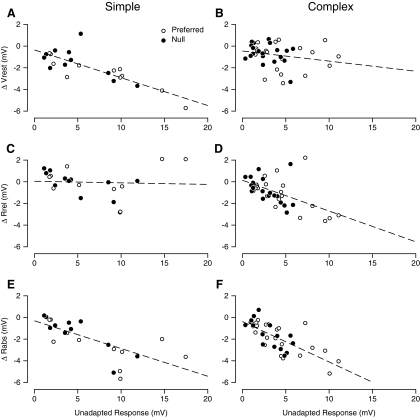
The two example neurons in Figs. 2 and 3 were representative of simple and complex cells across the population of recorded neurons. The resting membrane potential was hyperpolarized by the adapting stimulus far more in simple cells than in complex cells (Fig. 4A) (Carandini and Ferster 1997). In simple cells (n = 11), the degree of hyperpolarization was related to the amplitude of the response to the 32% contrast adapting stimulus (preferred direction, ○; null direction, ●) (Sanchez-Vives et al. 1997). The relationship between response to the adapting stimulus and hyperpolarization was fit by a line with slope of −0.26 ± 0.09 (95% confidence interval). In complex cells (Fig. 4B), the slope of the corresponding best-fit line was −0.09 ± 0.13, which, because the confidence interval overlapped 0, was not significantly different from 0. That is, across the population of complex cells (n = 20), the adaptation-induced change in resting potential was not related to the amplitude of the response to the adapting stimulus. On average, preferred direction adaptation caused simple cells to hyperpolarize by 2.5 mV (P < 0.01) and complex cells to hyperpolarize by 0.9 mV (P < 0.05).
Fig. 4.
Changes in resting potential and depolarization amplitude across the population. A and B: the relationship between depolarization evoked by the adapting stimulus and the change in resting membrane potential observed during adaptation. A: simple cells, B: complex cells. ○, response to the preferred direction; ●, response to the null direction. C and D: changes in Rrel, the peak visually evoked depolarization measured relative to the adapted Vrest. C: simple cells, D: complex cells. E and F: the changes Rabs, the depolarization measured relative to the unadapted Vrest, E: simple cells, F: complex cells.
The effects of adaptation on the peak amplitude of the visual response to high-contrast gratings showed the opposite trend when amplitude is measured relative to the adapted resting potential (Rreladapt). Figure 4, C and D, shows the relationship between adapted Rreladapt and the amplitude of the response to the adapting stimulus. The slope of the fitted line is not significantly different from 0 for simple cells (Fig. 4C, slope = −0.01 ± 0.13) but is significantly different from 0 for complex cells (D, slope = −0.28 ± 0.14, or a 1 mV reduction in adapted response amplitude for every 3.6 mV of response to the adapted stimulus).
Finally, we measured the effects of adaptation on the absolute response amplitude, measured relative to the unadapted resting potential (Rabsadapt), which reflects the summed effects on resting potential and on Rreladapt. In both simple and complex cells, there is a significant effect, with comparable slopes (Fig. 4E, slope = −0.27 ± 0.12; Fig. 4F, slope = −0.38 ± 0.11) (Sanchez-Vives et al. 1997). As is evident from Fig. 4, A–D, however, the underlying causes are different. In simple cells, the main effect is a change in resting potential with only a weak effect on the size of the visually evoked depolarization relative to rest. Conversely in complex cells, the change in resting potential is modest relative to the reduction in the visually evoked response amplitude.
Effects of contrast adaptation on direction selectivity
Because adaptation affects the responses of simple and complex cells in fundamentally different ways, it also affects direction selectivity in different ways. We began by measuring the DI for 32% contrast gratings using Rreladapt, which takes into account only the effects of adaptation on the size of the visually evoked depolarization. In simple cells, adaptation reduced the preferred and null responses on average by similar, relatively small amounts (16 and 21% for adaptation in the preferred direction; 13 and 8% for adaptation in the null direction). As a result, the DI calculated from Rreladapt was relatively unaffected by adaptation in either direction (Fig. 5A). The mean DI of the simple cell population was 0.23 in the unadapted state, 0.22 after adaptation in the preferred direction, and 0.25 after adaptation in the null direction. These are not significant changes (t-test, P > 0.05).
Fig. 5.
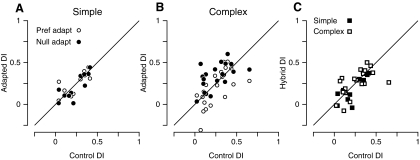
Adaptation-induced changes in the direction index. For each panel, the postadaptation direction index (DI) is plotted relative to the control (unadapted) DI. Indices were calculated from Rrel. Each symbol indicates the preand postadaptation DI for a single cell. A: for simple cells, neither preferred direction adaptation (○) nor opposite (null) direction adaptation (●) caused significant changes in the direction indices. B: for complex cells, preferred direction adaptation decreased the DI whereas opposite direction adaptation increases DI. C: the hybrid direction index (HDI) for simple (●) and complex (○) cells shows little change in direction selectivity after adaptation of the cortical network.
To take into account the effects of the adaptation-induced hyperpolarization on the direction selectivity of simple cells, we also calculated the DI from Rabsadapt. Note that the hyperpolarization reduces the size of Rabsadapt for the preferred and null absolute responses by the same amount relative to the unadapted response; this leads to a proportionately larger effect on the null response than on the preferred response. The expectation, then, is that the hyperpolarization should raise the DI derived from Rabsadapt. Indeed adaptation in the preferred direction significantly raised the DI from 0.23 to 0.45 (t-test, P < 0.05); adaptation in the null direction had a smaller effect, raising the DI to 0.34 (not significant, t-test, P = 0.25).
In complex cells, adaptation in the preferred direction tended to reduce the size of the preferred response more than it reduced the null response (preferred, 35%; null, 17%). On average, DI changed from 0.26 to 0.20 after adaptation (P < 0.05; Fig. 5B, ○), with seven cells showing decreases in direction selectivity of >0.12. Conversely, adaptation in the null direction decreased the response to the null stimulus more than it decreased the response to the preferred stimulus (null, 29%; preferred 17%), so on average direction selectivity increased relative to the control 0.33 versus 0.26 (P < 0.01; Fig. 5B, ●) with eight cells showing increases of >0.12. Note that here we have used Rrel to measure the size and direction selectivity of the responses, but because adaptation has little effect on the resting potential in complex cells, Rabs gave comparable results (not shown).
Overall changes in direction selectivity: the hybrid index
The direction indices we have calculated thus far show how direction selectivity is affected when cells are adapted to gratings moving in one direction or the other. We also wished to know how direction selectivity is affected when the cells are adapted to both directions of motion at once. Is direction selectivity affected when all the cortical presynaptic cells that can be adapted, are adapted? This measurement is difficult to make directly because it is difficult to maintain adaptation to both directions of motion for the duration that is required to test direction selectivity. Alternatively, a single counterphasing grating would not adapt all the inputs if some of them were sensitive to spatial phase. Multiple stimuli of different phases would need to be interleaved and so would not maintain strong adaptation. We therefore approximated the effects of adaptation in both directions by calculating a hybrid direction selectivity index (HDI) from the response to the preferred direction after preferred adaptation (Rrelpp) and the response to the null direction after null adaptation (Rrelnn). Together they can be combined to calculate the HDI as follow
| (2) |
Our reasoning behind the HDI is that for each test stimulus, all the neurons that respond preferentially to the stimulus and therefore all the neurons that contribute synaptic inputs to the response of the recorded neuron have been strongly adapted by that same stimulus.
The HDI is plotted against the unadapted DI in Fig. 5C. In simple cells (■), the adaptation-induced changes in the visual response were small and similar to one another in amplitude for the preferred and null directions (Fig. 5C). As a result, the average HDI (0.25 ± 0.24) was very similar to the control DI (0.23 ± 0.14). In complex cells (☐), even though the adaptation to one or the other direction significantly changed the DI (Fig. 5B), the HDI (0.25 ± 0.16) was not significantly changed (P = 0.8). That is, the reduction in response to preferred stimulation after preferred adaptation is similar to the reduction in response to null stimulation after null adaptation, and when all the terms of Eq. 2 are reduced proportionately the same amount, the HDI is similar to the control DI. Thus the HDI suggests that while simple and complex cells may receive inputs from cortical cells that adapt differentially to stimuli in the preferred and null direction, in aggregate these inputs have similar direction selectivity to the nonadapting inputs, and therefore do not significantly change the direction selectivity of the cells.
DISCUSSION
To assess the cortical contribution to direction selectivity, we have exploited the effect of prolonged visual stimulation (adaptation) on cortical responses: exposure to visual motion for tens of seconds causes a sustained reduction in the firing-rate responses of cortical neurons (Maffei et al. 1973), while largely sparing the afferent input from the LGN (Solomon et al. 2004). In combination with intracellular recording, this functional inactivation allows us to assay the direction selectivity of cortical and noncortical synaptic inputs as reported by the membrane potential responses that are spared or suppressed by the prolonged stimulation. The effects of an adapting stimulus on the responses to a test stimulus indicate the degree of common processing of the two stimuli (Blakemore and Campbell 1969).
Prolonged visual stimulation of simple cells causes a sustained hyperpolarization in their resting potential (Carandini and Ferster 1997; Sanchez-Vives et al. 2000). In our experiments, the size of this hyperpolarization depended on the size of the response evoked by the adapting stimulus, consistent with the proposal that the hyperpolarization arises from an activity-dependent K+ conductance (Sanchez-Vives et al. 2000). In addition, we found that the hyperpolarization was larger in simple cells than in complex cells for a given evoked peak depolarization (F1+DC), suggesting that simple and complex cells differ in their intrinsic properties. Alternatively, the stronger activation of the postulated K+-conductance in simple cells could arise from the visually evoked modulation of the membrane potential, which is absent in complex cells. Adaptation to motion in the preferred direction generated greater hyperpolarization than adaptation to motion in the null direction, which directly follows from the hyperpolarization being proportional to response amplitude (see Fig. 4). While simple cells were hyperpolarized by prolonged stimulation, the amplitude of their responses to visual stimuli—when measured relative to the adapted resting potential—was largely unaffected. Thus the effects of adaptation on simple cell responses appear to be largely intrinsic to the cell and depend less on extrinsic changes in synaptic inputs. Direction selectivity in these cells is therefore not likely to be cortically mediated; instead direction selectivity in simple cells of V1 arises from some nonadapting mechanism, such as input from LGN relay cells.
We also observed small phase delays in simple cell responses after adaptation. The effect was significant only in responses to the preferred direction and was largest for adaptation in the preferred direction, averaging 2.5% of a stimulus cycle (12 ms). This lag could originate either from adaptation-induced changes in the intrinsic properties of cortical neurons or from changes in the timing of subcortical and cortical synaptic inputs. For example, adaptation-induced hyperpolarization in a simple cell should create a lag in the time to the first spike on the rising phase of the response. If, because of spike-adaptation effects, the hyperpolarization did not equally advance the time of the last spike on the falling phase of the depolarization, a net shift in the phase of the spike response could result. In an alternative model, adaptation shifts the time course of the response by increasing and temporally shifting the time course of inhibition tuned to the preferred direction (Saul 1995; Saul and Cynader 1989a). Prolonged activation, however, appears to decrease, rather than increase the responses of inhibitory interneurons (Descalzo et al. 2005). Further experiments are necessary to resolve this open question in simple cells.
In complex cells, prolonged stimulation hyperpolarized the membrane potential much less than in simple cells, but it did alter direction selectivity of the synaptic inputs. In particular, the response to the adapting stimulus was selectively reduced, whereas the response to the opposite direction of motion was relatively unaltered. Thus adaptation in the preferred direction decreased direction selectivity, whereas adaptation in the opposite direction increased direction selectivity.
When measured from spike rate (Giaschi et al. 1993; Marlin et al. 1988) or from peak (absolute) membrane potential (Fig. 4, E and F), adaptation has similar effects across the entire population of cortical cells: adaptation in the preferred direction decreases direction selectivity; adaptation in the nonpreferred direction increases selectivity. And yet these effects appear to arise from very different subthreshold mechanisms in simple and complex cells: an activity-dependent hyperpolarization in simple cells and visually specific adaptation of synaptic inputs in complex cells. These effects can be incorporated into a simple model (Fig. 6A) in which a direction selective complex cell receives excitatory input from a set of simple cells, most of which are selective for the complex cell's preferred direction, and a few of which are selective for the opposite direction. The overall direction selectivity of the complex cell's inputs need not be very large because the threshold nonlinearity greatly amplifies the direction selectivity of the spike output relative to the synaptic inputs (Jagadeesh et al. 1993; Priebe and Ferster 2005). Adaptation in the preferred direction reduces direction selectivity in the complex cell by preferentially hyperpolarizing and therefore reducing the firing rate of the presynaptic simple cells tuned to the preferred direction (Fig. 6B, gray cells). Conversely, adaptation in the null direction increases direction selectivity in the complex cell by more strongly reducing activity in the presynaptic simple cells tuned to the null direction (Fig. 6C, gray cells). Adaptation in both directions (HDI, Fig. 5C) suppresses the responses of all neurons providing synaptic input but leaves the overall direction selectivity of the inputs unchanged relative to the control condition. We note here, however, that some complex cells, such as those in the lower parts of layer 3, likely receive input directly from the LGN (Ferster and Lindström 1983; LeVay and Gilbert 1976; Singer et al. 1975). These cells might be among the population in Fig. 5B for which direction selectivity is more resistant to the effects of adaptation.
Fig. 6.
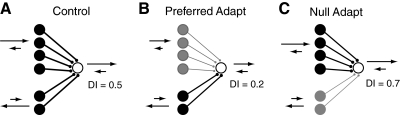
A schematic model that describes the changes in direction selectivity caused by prolonged visual stimulation in complex cells. A: a diagram of the input to the complex cell coming from simple cells tuned to preferred direction (top 4 inputs) and simple cells tuned to the null direction (bottom 2 inputs). B: after preferred direction adaptation, those simple cells tuned to the preferred direction (top 4 inputs, gray) have reduced responses relative to those inputs tuned to the opposite direction the responses of which are less affected by the adapting stimulus. The target complex cell's direction selectivity is therefore reduced. C: in the case of null direction adaptation, the simple cells tuned to the opposite direction have reduced responses, causing the direction selectivity of the complex cell to increase.
One assumption underlying our model is that our adaptation protocol was effective in reducing the responses of cortical inhibitory neurons as well as cortical excitatory neurons. Extracellular studies of adaptation have found that all cortical neurons are affected by prolonged visual stimulation (Giaschi et al. 1993; Marlin et al. 1988; Ohzawa et al. 1985; Saul and Cynader 1989b). In addition, slow adaptation accompanied by prolonged hyperpolarization has been observed directly in fast spiking cells of the ferret visual cortex (Descalzo et al. 2005). One could still hypothesize, however, that adaptation is weaker in inhibitory neurons than in excitatory neurons and not require an alteration to our model. Adaptation in inhibitory interneurons tuned to the opposite direction would give rise to effects opposite from what we have observed. The experimental results therefore require adaptation in excitatory circuit components similar to what we have proposed.
The model is based on the additional assumption that adaptation acts only on cortical cells and not on LGN relay cells. Recent work has demonstrated that LGN relay cell responses can be reduced by prolonged exposure to rapidly moving gratings but far less by the slowly moving gratings we have employed here (Solomon et al. 2004). Furthermore because LGN relay cells are not tuned for direction, it is unlikely that adaptation in their responses could account for the direction-specific effects observed here.
Methods other than adaptation have been used to inactivate all or part of the cortical circuit. When a portion of cortex surrounding a cortical neuron is inactivated by cooling (Ferster et al. 1996), direction selectivity is unaffected or slightly increased. When inhibition is blocked by intracellular application of Cl–-channel blockers, however, direction selectivity in some cells is decreased (Nelson et al. 1994) in support of the inhibition-based models. Blocking cortical inhibition by the external application of GABAA antagonists also reduced direction selectivity by disproportionately increasing the spiking responses to the null direction (Sillito 1975, 1977; Tsumoto et al. 1979). While this effect could reflect the interruption of a specific cortical inhibitory mechanism that suppresses the null response, we think it more likely that it reflects a nonspecific change in neuronal excitability. Threshold is normally responsible for greatly amplifying direction selectivity of the spike output relative to synaptic inputs. That is, the DI measured from firing rate is often 3 to 4 times larger than the DI of synaptic inputs (Jagadeesh et al. 1993; Priebe and Ferster 2005) largely because spike threshold disproportionately amplifies the larger inputs evoked by preferred stimuli relative to the smaller null-evoked inputs. When inhibition is removed, neurons likely depolarize, decreasing the distance between rest and threshold. This nonspecific depolarization would in turn reduce the difference in the amplification between the preferred and null inputs and thereby decrease the direction selectivity of the spike output of cortical cells.
Conclusions
The effects of adaptation on the subthreshold inputs to cortical cells, then, do not support models of cortical function that rely on intracortical mechanisms to create or sharpen direction selectivity. These include models in which timing changes within cortical receptive fields is generated by synaptic or axonal delays in a cortical relay, or models in which the timing changes are constructed by inhibitory circuits, as it is in some retinal ganglion cells (Barlow and Levick 1965; Borg-Graham 2001; Taylor et al. 2000). Instead our results, together with those from previous experiments using cortical inactivation and adaptation, favor a hierarchical model of direction selectivity in which simple cell direction selectivity originates in the diversity of timing built directly into the monosynaptic thalamic input Mclean and Palmer 1988, 1989; Priebe and Ferster 2005; Reid et al. 1987, 1991; Saul and Humphrey 1992). Adaptation does not significantly alter this input but instead hyperpolarizes the simple cells in a response-dependent, and therefore direction selective, manner (Carandini and Ferster 1997; Sanchez-Vives et al. 1997). Spike threshold then amplifies these effects as outlined in the preceding text. In down-stream complex cells, the direction-selective adaptation of the simple cells' output is then reflected in the direction selective adaptation of synaptic input. As with orientation tuning then (Priebe and Ferster 2008), it appears that cortical direction selectivity is generated largely at the thalamocortical synapse and then relayed, and perhaps shaped, in subsequent stages.
GRANTS
This work was supported by National Eye Institute Grants EY-019288 to N. J. Priebe and EY-04726 to D. Ferster.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are thankful to J. Hanover for helpful discussions and comments.
REFERENCES
- Barlow and Levick, 1965.Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol 178: 477–504, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore and Campbell, 1969.Blakemore C, Campbell FW. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol 203: 237–260, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg-Graham, 2001.Borg-Graham LJ. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nat Neurosci 4: 176–183, 2001 [DOI] [PubMed] [Google Scholar]
- Brainard, 1997.Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 443–446, 1997 [PubMed] [Google Scholar]
- Carandini and Ferster, 1997.Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex [see comments]. Science 276: 949–952, 1997 [DOI] [PubMed] [Google Scholar]
- Cleland and Levick, 1974.Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol 240: 457–492, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis et al., 1993.DeAngelis GC, Ohzawa I, Freeman RD. Spatiotemporal organization of simple-cell receptive fields in the cat's striate cortex. II. Linearity of temporal and spatial summation. J Neurophysiol 69: 1118–1135, 1993 [DOI] [PubMed] [Google Scholar]
- Descalzo et al., 2005.Descalzo VF, Nowak LG, Brumberg JC, McCormick DA, Sanchez-Vives MV. Slow adaptation in fast-spiking neurons of visual cortex. J Neurophysiol 93: 1111–1118, 2005 [DOI] [PubMed] [Google Scholar]
- Ferster et al., 1996.Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380: 249–252, 1996 [DOI] [PubMed] [Google Scholar]
- Ferster and Lindström, 1983.Ferster D, Lindström S. An intracellular analysis of geniculocortical connectivity in area 17 of the cat. J Physiol 342: 181–215, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaschi et al., 1993.Giaschi D, Douglas R, Marlin S, Cynader M. The time course of direction-selective adaptation in simple and complex cells in cat striate cortex. J Neurophysiol 70: 2024–2034, 1993 [DOI] [PubMed] [Google Scholar]
- Hubel and Wiesel, 1962.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol 160: 106–154, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesh et al., 1993.Jagadeesh B, Wheat HS, Ferster D. Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science 262: 1901–1904, 1993 [DOI] [PubMed] [Google Scholar]
- Jagadeesh et al., 1997.Jagadeesh B, Wheat HS, Kontsevich L, Tyler CW, Ferster D. Direction selectivity of synaptic potentials in simple cells of the cat visual cortex. J Neurophysiol 78: 2772–2789, 1997 [DOI] [PubMed] [Google Scholar]
- Lampl et al., 2001.Lampl L, Anderson JS, Gillespie D, Ferster D. Prediction of orientation selectivity from receptive field architecture in simple cells of cat visual cortex. Neuron 30: 263–274, 2001 [DOI] [PubMed] [Google Scholar]
- LeVay and Gilbert, 1976.LeVay S, Gilbert CD. Laminar patterns of geniculocortical projection in the cat. Brain Res 113: 1–19, 1976 [DOI] [PubMed] [Google Scholar]
- Maffei et al., 1973.Maffei L, Fiorentini A, Bisti S. Neural correlate of perceptual adaptation to gratings. Science 182: 1036–1038, 1973 [DOI] [PubMed] [Google Scholar]
- Marlin et al., 1988.Marlin SG, Hasan SJ, Cynader MS. Direction-selective adaptation in simple complex cells in cat striate cortex. J Neurophysiol 59: 1314–1330, 1988 [DOI] [PubMed] [Google Scholar]
- McLean and Palmer, 1989.McLean J, Palmer LA. Contribution of linear spatiotemporal receptive field structure to velocity selectivity of simple cells in area 17 of the cat. Vision Res 29: 675–679, 1989 [DOI] [PubMed] [Google Scholar]
- McLean et al., 1994.McLean J, Raab S, Palmer LA. Contribution of linear mechanisms to the specification of local motion by simple cells in areas 17 and 18 of the cat. Vis Neurosci 11: 295–306, 1994 [DOI] [PubMed] [Google Scholar]
- Movshon, 1975.Movshon JA. The velocity tuning of single units in cat striate cortex. J Physiol 249: 445–468, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson et al., 1994.Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science 265: 774–777, 1994 [DOI] [PubMed] [Google Scholar]
- Ohzawa et al., 1985.Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat's visual system. J Neurophysiol 54: 651–667, 1985 [DOI] [PubMed] [Google Scholar]
- Pelli, 1997.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997 [PubMed] [Google Scholar]
- Priebe and Ferster, 2005.Priebe NJ, Ferster D. Direction selectivity of excitation and inhibition in simple cells of the cat primary visual cortex. Neuron 45: 133–145, 2005 [DOI] [PubMed] [Google Scholar]
- Priebe and Ferster, 2008.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron 57: 482–497, 2008 [DOI] [PubMed] [Google Scholar]
- Priebe et al., 2004.Priebe NJ, Mechler F, Carandini M, Ferster D. The contribution of spike threshold to the dichotomy of cortical simple and complex cells. Nat Neurosci 7: 1113–1122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid et al., 1987.Reid RC, Soodak RE, Shapley RM. Linear mechanisms of directional selectivity in simple cells of cat striate cortex. Proc Natl Acad Sci USA 84: 8740–8744, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid et al., 1991.Reid RC, Soodak RE, Shapley RM. Directional selectivity and spatiotemporal structure of receptive fields of simple cells in cat striate cortex. J Neurophysiol 66: 505–529, 1991 [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives et al., 1997.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular and network mechanisms generating adaptation to contrast in the visual cortex: an in vivo and in vitro study. Soc Neurosci Abstr 23: 1944, 1997 [Google Scholar]
- Sanchez-Vives et al., 2000.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci 20: 4286–4299, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul, 1995.Saul AB. Adaptation aftereffects in single neurons of cat visual cortex: response timing is retarded by adapting. Vis Neurosci 12: 191–205, 1995 [DOI] [PubMed] [Google Scholar]
- Saul and Cynader, 1989a.Saul AB, Cynader MS. Adaptation in single units in visual cortex: the tuning of aftereffects in the spatial domain. Vis Neurosci 2: 593–607, 1989a [DOI] [PubMed] [Google Scholar]
- Saul and Cynader, 1989b.Saul AB, Cynader MS. Adaptation in single units in visual cortex: the tuning of aftereffects in the temporal domain. Visual Neurosci 2: 609–620, 1989b [DOI] [PubMed] [Google Scholar]
- Saul and Humphrey, 1992.Saul AB, Humphrey AL. Evidence of input from lagged cells in the lateral geniculate nucleus to simple cells in cortical area 17 of the cat. J Neurophysiol 68: 1190–1208, 1992 [DOI] [PubMed] [Google Scholar]
- Sillito, 1975.Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol 250: 305–329, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito, 1977.Sillito AM. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol 271: 699–720, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer et al., 1975.Singer W, Tretter F, Cynader M. Organization of cat striate cortex: a correlation of receptive field properties with afferent and efferent connections. J Neurophysiol 38: 1080–1098, 1975 [DOI] [PubMed] [Google Scholar]
- Solomon et al., 2004.Solomon SG, Peirce JW, Dhruv NT, Lennie P. Profound contrast adaptation early in the visual pathway. Neuron 42: 155–162, 2004 [DOI] [PubMed] [Google Scholar]
- Taylor et al., 2000.Taylor WR, He S, Levick WR, Vaney DI. Dendritic computation of direction selectivity by retinal ganglion cells. Science 289: 2347–2350, 2000 [DOI] [PubMed] [Google Scholar]
- Torre and Poggio, 1978.Torre V, Poggio T. A synaptic mechanism possibly underlying direction selectivity to motion. Proc R Soc Lond B Biol Sci 202: 409–416, 1978 [Google Scholar]
- Tsumoto et al., 1979.Tsumoto T, Eckart W, Creutzfeldt OD. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp Brain Res 34: 351–363, 1979 [DOI] [PubMed] [Google Scholar]