Abstract
Sensorineural hearing loss during early childhood alters auditory cortical evoked potentials in humans and profoundly changes auditory processing in hearing-impaired animals. Multiple mechanisms underlie the early postnatal establishment of cortical circuits, but one important set of developmental mechanisms relies on the neuromodulator serotonin (5-hydroxytryptamine [5-HT]). On the other hand, early sensory activity may also regulate the establishment of adultlike 5-HT receptor expression and function. We examined the role of 5-HT in auditory cortex by first investigating how 5-HT neurotransmission and 5-HT2 receptors influence the intrinsic excitability of layer II/III pyramidal neurons in brain slices of primary auditory cortex (A1). A brief application of 5-HT (50 μM) transiently and reversibly decreased firing rates, input resistance, and spike rate adaptation in normal postnatal day 12 (P12) to P21 rats. Compared with sham-operated animals, cochlear ablation increased excitability at P12–P21, but all the effects of 5-HT, except for the decrease in adaptation, were eliminated in both sham-operated and cochlear-ablated rats. At P30–P35, cochlear ablation did not increase intrinsic excitability compared with shams, but it did prevent a pronounced decrease in excitability that appeared 10 min after 5-HT application. We also tested whether the effects on excitability were mediated by 5-HT2 receptors. In the presence of the 5-HT2-receptor antagonist, ketanserin, 5-HT significantly decreased excitability compared with 5-HT or ketanserin alone in both sham-operated and cochlear-ablated P12–P21 rats. However, at P30–P35, ketanserin had no effect in sham-operated and only a modest effect cochlear-ablated animals. The 5-HT2-specific agonist 5-methoxy-N,N-dimethyltryptamine also had no effect at P12–P21. These results suggest that 5-HT likely regulates pyramidal cell excitability via multiple receptor subtypes with opposing effects. These data also show that early sensorineural hearing loss affects the ability of 5-HT receptor activation to modulate A1 pyramidal cell excitability.
INTRODUCTION
Hearing impairment during development produces significant changes in the acquisition of speech, sound discrimination, and cognitive function that may permanently diminish auditory perceptual skills and compromise language acquisition (Emmorey et al. 2003; Kidd et al. 2002; Psarommatis et al. 2001; Sanes and Bao 2009). Although hearing loss in animal models of deafness has been associated with numerous alterations in the cell biology and physiology of brain stem and cortical neurons, the mechanisms that underlie the changes in the primary auditory cortex (A1) remain largely unexplored. Understanding which elements of neural plasticity are engaged or perturbed in the CNS following sensorineural hearing loss (SNHL) is important, since such mechanisms may represent sites for novel clinical intervention strategies to improve or restore perceptual skills.
Perinatal bilateral hearing loss has been shown to increase the intrinsic excitability of auditory cortical neurons, decrease the strength of inhibition, decrease adaptation, and increase the strength of excitation in young gerbils (Kotak et al. 2005, 2008; Xu et al. 2007). Although these results strongly indicate that hearing loss raises overall cortical excitability, they also raise questions as to what other aspects of cortical physiology are influenced by early hearing loss. In particular, the normal development of the auditory cortical circuit depends on both sensory stimulation and modulatory systems that gate synaptic and intrinsic plasticity. For example, it is well documented that the tuning of cortical neurons and the organization of the tonotopic map in A1 depend on cholinergic afferents that arise from the nucleus basalis in the basal forebrain (Bakin and Weinberger 1996; Kilgard and Merzenich 1998; Kilgard et al. 2001) and on dopaminergic afferents from the ventral tegmental area (Bao et al. 2001). Two other neuromodulatory systems known to innervate A1 neurons include serotonin (5-hydroxytryptamine [5-HT]) and noradrenaline (Campbell et al. 1987), but the role of these systems in regulating cortical plasticity, either in the normal or in the compromised auditory system, have not been actively explored. Ji et al. (2007) found that application of 5-HT in the bat auditory cortex can suppress or potentiate fear-induced plasticity of acoustic response areas, implicating serotonergic systems in the regulation of cortical plasticity according to behavioral context.
Clear evidence exists regarding the influential role of the serotonergic system during brain development, where it affects cellular proliferation, migration and differentiation, synaptogenesis, and apoptosis (Azmitia 2001; Lauder 1990). However, 5-HT also plays an important role in normal auditory processing in the adult brain. In humans, depletion of the 5-HT precursor tryptophan decreases the intensity dependence of auditory evoked magnetic N1/P2 dipole source activity (Kähkönen et al. 2002a,b) and modulates auditory selective attention (Ahveninen et al. 2003). Serotonin also modulates auditory cortical evoked potentials (Dierks et al. 1999; Hegerl et al. 1993; Juckel et al. 1997). Although 5-HT plays a crucial role in human auditory processing, the mechanisms by which 5-HT modulates auditory cortical activity at the cellular level are unknown. The effects of 5-HT are mediated by >14 receptor subtypes (Hoyer et al. 2002), many of which can be found in the developing cerebral cortex. The 5-HT2A and 5-HT3 receptors are highly expressed by postmitotic neurons of the cerebral cortex (Johnson and Heinemann 1995; Vitalis and Parnavelas 2003), whereas 5-HT1A, 5-HT2B, and 5-HT3 are localized to the ventricular zones (Johnson and Heinemann 1995). Serotonin has been shown to affect the electrical excitability of neurons in several cortical areas, although the effects seem to be region-specific. In prefrontal cortex, 5-HT2A and 5-HT7 receptors mediate depolarization during the first 2 postnatal weeks, whereas 5-HT1A receptors mediate hyperpolarization during the third week (Beique et al. 2004). In the lateral entorhinal cortex, 5-HT reduces input resistance and hyperpolarizes layer II/III neurons through potassium channels coupled to 5-HT1A receptors (Grünschlag et al. 1997). In the medial entorhinal cortex, 5-HT evokes a biphasic response, first hyperpolarizing neurons via 5-HT1A receptors and then depolarizing neurons by an Ih channel-dependent mechanism (Ma et al. 2007). The role of 5-HT in regulating cortical excitability therefore depends on the developmental trajectory and activity-dependent expression of both specific receptor subtypes and target ion channels.
Existing evidence cited earlier suggests that 5-HT can developmentally regulate cortical neuron excitability and that 5-HT may play a role in auditory plasticity in adults. On the other hand, sensory activity may also regulate the developmentally programmed establishment of adultlike 5-HT receptor expression and function. We tested this hypothesis by investigating how 5-HT neurotransmission and 5-HT2 receptors influence the intrinsic excitability of layer II/III pyramidal neurons in A1 and how the effects of 5-HT are modified by prehearing bilateral cochlear ablations at postnatal day 8 (P8) in rats.
METHODS
All protocols for cochlear ablation, sham surgeries, and brain slice preparation were reviewed and approved by the University of North Carolina, Chapel Hill Institutional Animal Care and Use Committee. These experiments report on results from 14 normal P12–P21 rat pups, 12 sham surgery P12–P21 pups, 8 cochlear-ablated P12–P21 pups, 4 sham surgery P30–P35 rats, and 4 cochlear-ablated P30–P35 rats.
Cochlear ablations
Cochlear ablations were performed in P8 Sprague–Dawley rat pups. The pups were anesthetized with ketamine–xylazine (80 mg/kg–8 mg/kg, administered intraperitoneally [ip], respectively) and, after anesthesia was confirmed (no withdrawal induced by tail pinch), the surgical field was cleaned and made sterile. The incision site was cleaned with chlorhexidine soap scrub and a retroauricular incision was made in the skin and dissection carried down to the tympanic bulla. The middle ear was entered and ossicles were removed, after which a small hole was made in the cochlear wall and the contents were removed with small forceps. A small piece of Gelfoam was then placed in the cavity and the wound closed with 5–0 proline suture threads. Ablations were performed bilaterally. Following surgery, the pups were given ketoprofen analgesic (5 mg/kg) and warmed on a heating pad until they were ambulatory. They were then returned to their home cage. Pups were then reared with their mothers until they were used for experiments (P12–P21) or were weaned at P21 and raised in groups of four or fewer until they were tested at P30–P35. Sham surgeries were also performed, in which the anesthesia, skin incision, and wound closure were the same as those for cochlear ablations, although the bulla was not invaded, the ossicular chain was not removed, and the cochlea was not ablated.
Prior to each slice experiment, the animals were first tested for a Preyer's reflex (Jero 2001), anesthetized with ketamine–xylazine (80 mg/kg–8 mg/kg, ip, respectively), decapitated, and the brain removed. In a subset of experiments, the Preyer's test was supplemented with auditory brain stem evoked response measures to confirm hearing loss. In all experiments, the inner wall of the cochlea was also observed under a dissection microscope to confirm the absence of cochlear tissue and the persistence of the Gelfoam insert. Therefore the recordings were not performed blind. Rats without any surgery (normal), sham-surgery rats, and cochlear-ablated rats were recorded when they were P12–P21 or at P30–P35. Recordings from rats with cochlear ablations were then compared with aged-matched sham-operated controls. Following surgery, no vestibular complications were observed, as would be indicated by balance or overall motor activity, suggesting that the vestibular apparatus was not significantly affected by the surgical procedure.
Auditory cortex brain slice recordings
Following decapitation, the brain was immersed in ice-cold (4°C) cutting solution, blocked to a region containing A1, and 400 μm thick slices were cut with a tissue slicer (Leica VT1000-S; Leica Microsystems, Bannockburn, IL). All slices were cut along the plane of the auditory thalamocortical fibers (Cruikshank et al. 2002; Metherate and Cruikshank 1999). Two sections, starting ≥400 μm dorsal to the rhinal fissure, were selected for study. Slices were transferred to an incubation chamber, maintained at 34°C for 30 min, and thereafter incubated at room temperature (∼22°C) until recording. The standard slicing, incubation, and recording solution was an artificial cerebrospinal fluid (aCSF) that contained (in mM) 134 NaCl, 3.0 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 KH2PO4, 10 glucose, 20 NaHCO3, 0.4 ascorbic acid, 2 sodium pyruvate, and 3 myoinositol. Slicing in P12–P21 rats was performed in this standard solution. Slicing in P30–P35 rats was done in an N-methyl-d-glucamine–based solution to increase neuron survival (Tanaka et al. 2008); afterward, the slices were incubated and recorded in the standard aCSF. All solutions were continually equilibrated with 95% O2-5% CO2, setting the pH to 7.3–7.4. During recording, the slices were bathed at 34°C in a submersion-type recording chamber on the stage of an upright fixed-stage microscope (Zeiss FS-2). The preparation was viewed with a ×40, 0.75 NA water-immersion objective, using video-enhanced differential interference contrast illumination in infrared light. Whole cell current-clamp recordings were obtained from layer II/III pyramidal neurons in A1 using Multiclamp 700A and 700B amplifiers (MDS Analytical Technologies, Toronto). Recording electrodes were pulled (P-2000; Sutter Instrument, Novato, CA) from 1.5-mm diameter KG-33 glass (Garner Glass, Claremont, CA) to a tip diameter of about 1 μm, ends were fire polished, and tips were coated with Sylgard 184 (Dow Corning, Midland, MI). The pipettes were backfilled with a solution containing (in mM) 130 K-gluconate, 4 NaCl, 0.2 EGTA, 10 HEPES, 2 Mg2ATP, 0.3 Na3GTP, and 10 phosphocreatine (pH 7.2 with KOH). Membrane potentials were corrected for the measured junction potential (−12 mV for gluconate) between the electrode and bathing solutions. Data were acquired from neurons with a resting potential negative to −50 mV and with overshooting action potentials (APs). Three separate protocols were tested on each cell. First, complete current–response curves were obtained for 500-ms duration test pulses, to measure both the input resistance and the frequency–current (F–I) curve. Second, the AP threshold was measured in some experiments by injecting a 5-ms current pulse at multiple levels. The brief pulses were alternated with a 1/4 -amplitude hyperpolarizing pulse, which was then scaled and added to the AP during analysis to remove the passive component of the response. The AP threshold was measured as the point of voltage inflection on the rising phase of the AP that exhibited the maximum point of curvature (Erisir et al. 1999). In experiments in older animals, the brief pulse failed to consistently elicit APs during the drug treatment, in which case measurements were taken from trains of APs during longer current pulses (as in Francis and Manis 2000). Third, when drugs were washed onto the slice or washed out, a current pulse that produced an average of five APs in control conditions was presented every 20 s and changes in excitability were monitored. The current–voltage and current–firing relationships were also measured at the end of each solution wash period.
The primary auditory cortex (A1) was first located at low magnification (×4 or ×5) and the recording electrode position was established in layer II/III. Neurons were visually identified using infrared–differential contrast optics at ×40 magnification and whole cell current-clamp recordings were then obtained following formation of a tight seal. Layer II/III pyramidal neurons were selected for recording and identified by their electrophysiological characteristics. Fast-spiking neurons and bursting neurons were excluded from analysis.
5-HT and 5-HT2 receptor electrophysiology
To investigate the effects of 5-HT on intrinsic excitability, serotonin hydrochloride (Sigma-Aldrich, St. Louis, MO) was bath applied at 50 μM to each slice, using the protocol in Fig. 1. This concentration was chosen to be in the middle of the dose–response curve for modulation of synaptic currents in the brain slice (Tanaka and North 1993). To investigate the role of 5-HT2 receptors, the 5-HT2 receptor antagonist ketanserin tartrate (Sigma-Aldrich) was bath applied at 1 μM, a concentration high enough to ensure nearly complete receptor block (Beique et al. 2004; Shapiro et al. 2000). The excitability of each recorded cell was measured before and during a series of drug applications. After an initial period in control aCSF lasting 5 min, 50 μM 5-HT was bath applied to each slice for 5 min. Following the application of 5-HT, the drug was washed out for 10–15 min and ketanserin was then applied alone for 5 min and in combination with 5-HT for another 5 min. A final aCSF wash lasted 10 min. The same protocol was used for all age and surgery groups. In an independent set of experiments, the 5-HT2 receptor agonist 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT; Sigma-Aldrich) was bath applied at 5 μM, a concentration that is above the concentration required for half-maximal activation (Kact) for phosphotidyl inositol production from 5-HT2A receptors, but which is below saturation (Shapiro et al. 2000). Because 5-HT can initiate long-lasting signal transduction cascades, only one cell was studied from each slice.
Fig. 1.
Experimental protocol for measuring neuronal excitability and the actions of 5-HT. aCSF, artificial cerebrospinal fluid; 5-HT, serotonin; KTS, ketanserin. Each condition lasted 5 min, except aCSF wash1 and wash2, which lasted 15 and 10 min, respectively.
Data acquisition and analysis
Electrophysiological data were acquired using custom-written scripts in MATLAB (Version 7.0–7.6; The MathWorks, Natick, MA) with the Data Acquisition Toolbox and high-speed 12- or 16-bit A/D, D/A boards (National Instruments, Austin, TX). Analysis was performed with MATLAB using custom routines and statistical analysis was performed with Prism 5.0 (GraphPad, San Diego, CA). Frequency–current plots were collected in steps of 20 or 50 pA for younger and older rat experiments, respectively, and interpolated onto a common scale. Input resistance was measured as the maximum slope in the region of the current–voltage relationship just below resting potential. Spike-rate adaptation ratios were calculated as the mean of the last two interspike intervals divided by the first interspike interval, for firing rates between 8 and 20 Hz (4 to 10 spikes elicited by a 500-ms depolarizing pulse). The sag in the hyperpolarizing direction was measured as the steady-state voltage divided by the peak voltage, for peak voltages between −90 and −110 mV during hyperpolarizing currents, as described by Fujino and Oertel (2003). Action potential properties such as afterhyperpolarization depth, AP height, maximum rising slope, width at half-amplitude, and threshold were measured from isolated spikes elicited by a brief current pulse or from trains of spikes elicited by 500-ms depolarizing current steps, as discussed earlier. For calculations of significance of resting membrane potential and input resistance, paired (where required) and unpaired Student's two-tailed t-tests were used. For calculations of significance of F–I curves, a two-way ANOVA was used followed by a Bonferroni post hoc test (df, degrees of freedom; F, F ratio). Whenever possible, ANOVAs were computed using repeated measures across treatments in single cells. However, in a few cases cells could not be recorded for the entire duration of the protocol, and so there were unequal numbers of observations across experimental treatments. In these cases, the two-way ANOVAs were computed without matching and the number of observations for each treatment group is given. Results are presented as mean and SE.
RESULTS
Recordings were made from “normal” animals, in which the cochleae were not surgically manipulated; “sham” animals, which were subject to sham surgery; and “ablated” animals with bilateral cochlear ablations. Neurons were included in the final analysis only if they met criteria for input resistance (≥40 MΩ), resting potential (negative to −50 mV), and spike height (>80 mV, as measured from rest). In this section, we will first discuss the effects of 5-HT on normal A1 neurons. We then present the effects of the sham surgery and cochlear ablations. We conclude with an examination of the effects of cochlear ablations on the responses of A1 neurons to 5-HT.
5-HT modulation of intrinsic excitability in normal A1
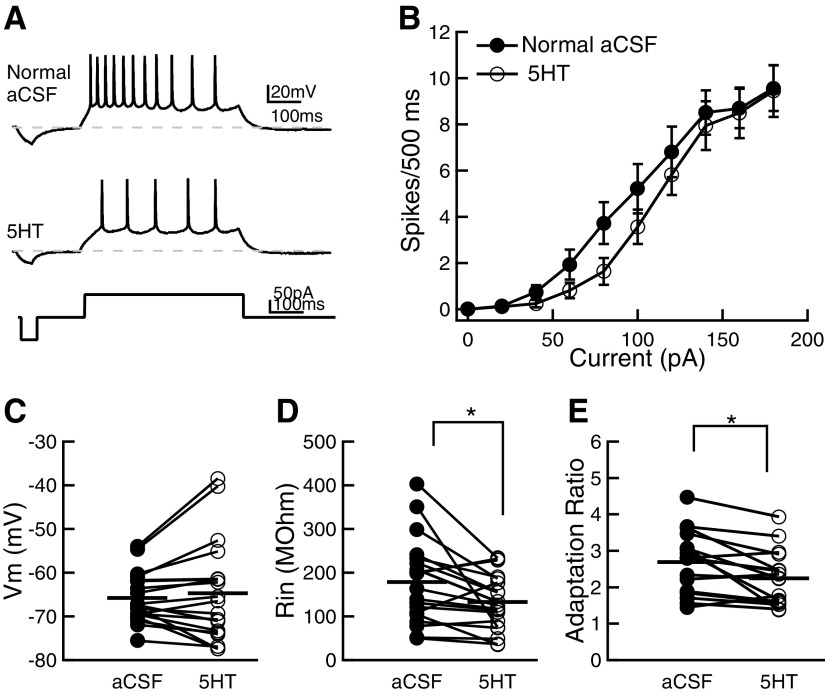
Serotonin has been shown to affect the intrinsic properties of neurons in several cortical areas, with specific regional effects (see introduction). To investigate how 5-HT affects intrinsic firing in A1, responses to intracellular current pulses were collected from neurons in normal A1. Application of 5-HT decreased the number of spikes evoked by depolarizing current injections (Fig. 2, A and B; two-way ANOVA, F–I in aCSF vs. F–I in 5-HT, df = 1, F = 12.25, P = 0.0006; n = 17). Serotonin produced a rightward shift in the F–I relationship that was largest for currents just above spike threshold and was statistically significant for the 80- and 100-pA steps (P < 0.05; Bonferroni posttest). Serotonin produced no difference in firing rates at the highest currents tested. Moreover, serotonin did not affect the resting membrane potential (Fig. 2C, aCSF: −65.8 ± 1.3 mV; 5-HT: −64.7 ± 2.6 mV, n = 19, P = 0.47, paired t-test) or the sag seen with hyperpolarizing pulses (see Supplemental Table S1).1 However, it did reduce input resistance (Fig. 2D). Immediately after establishing whole cell recordings, and prior to the application of 5-HT, the mean input resistance was 178.5 ± 22.6 MΩ. At the end of a 5-min application of 5-HT, the input resistance fell to 132.5 ± 14.7 MΩ (P = 0.020, paired t-test; n = 19). Serotonin also significantly decreased the adaptation ratio (Fig. 2E), from 2.7 ± 0.2 to 2.2 ± 0.2 (P = 0.004, paired t-test; n = 17). We conclude that 5-HT modulates the excitability of A1 layer II/III neurons in three ways: it decreases the firing rate, the input resistance, and firing rate adaption.
Fig. 2.
Serotonin decreases excitability of primary auditory cortex (A1) neurons in normal rats. A: voltage responses of an example neuron to 100 pA depolarizing current steps. The current protocol is shown below the voltage traces. Dashed gray lines indicate resting membrane potential. B: number of spikes evoked by depolarizing current injections for a population of neurons in aCSF (closed circles) and in presence of 50 μM 5-HT (open circles). Serotonin decreased the number of spikes for currents just above threshold. Error bars are 1SE. C: serotonin did not affect the mean resting membrane potential of neurons. D: serotonin significantly reduced the mean input resistance of neurons. E: serotonin significantly decreased the adaptation ratio of neurons. Horizontal lines in C, D, and E show the mean of each group. Statistical significance: *P < 0.05.
Effects of sham surgery
Since the cochlear ablations were performed in neonatal P8 rat pups, we were concerned that the surgery alone might influence cortical development. Therefore before testing for the effects of 5-HT in cochlear-ablated animals, we first evaluated the effect of surgery on A1 neurons, by comparing the physiology of cells from normal and sham surgery controls. Surprisingly, the sham surgery significantly decreased the number of spikes (Fig. 3, A and B) evoked by depolarizing current injections at all levels (two-way ANOVA, F–I in normal vs. F–I in sham, df = 1, F = 15.5, P < 0.0001). However, the changes in spike rate were not accompanied by significant changes in the resting membrane potential, input resistance, or the spike adaptation ratio (Fig. 3, C–E; also see Supplemental Table S1). Our results suggest that removal of the pup from the nest, anesthesia, and recovery from anesthesia during surgery are sufficient to produce an effect on cortical physiology and, specifically, electrical excitability, in these young animals. Thus in subsequent experiments, comparisons were made between the sham and ablated surgery groups, which differed only in the surgical removal of the cochlea.
Fig. 3.
Sham surgery decreases, whereas cochlear ablation increases, excitability in postnatal day 12 (P12) to P21 rat A1. A: voltage responses of neurons in response to 100 pA depolarizing current steps. Dashed gray lines indicate resting membrane potential; the current protocol is shown below the voltage traces. B: spike count as a function of depolarizing current amplitude in neurons from normal (closed circles), sham (closed squares), and cochlear-ablated animals (closed triangles). Sham surgery significantly decreased the firing rate, compared with normal A1. Cochlear ablation increased the firing rate, compared with both shams and normals. C–E: sham surgery and cochlear ablation did not affect mean resting membrane potential (C), input resistance (D), or adaptation ratio (E). Horizontal lines in C, D, and E represent the mean of each group and vertical lines represent 1SE.
Effects of cochlear ablation on excitability in rat A1
Kotak et al. (2005) showed that layer II/III neurons from animals with SNHL had an increased intrinsic excitability when compared with normal animals. The changes in excitability seen in the animals with sham surgeries prompted us to reevaluate the changes due to cochlear ablations, by comparing effects of bilateral cochlear ablation on the excitability of layer II/III A1 neurons to sham surgery controls. Cochlear ablation resulted in significantly increased firing rate of neurons when compared with shams (Fig. 3, A and B, two-way ANOVA, F–I in sham n = 27 vs. F–I in ablated n = 20, df = 1, F = 49.1, P < 0.0001). The mean firing rate was elevated at all current levels and was significant for all currents ≥100 pA (P < 0.05, Bonferroni posttest). Consistent with the results of Kotak et al. (2005), the firing rate was also elevated, compared with that of normal hearing animals (Fig. 3, A and B, two-way ANOVA, F–I in 17 cochlear-ablated rats vs. F–I in 17 normal rats, df = 1, F = 6.44, P = 0.012). We conclude that the increase in excitability caused by cochlear ablation is still evident, compared with sham surgery, and thus is not solely the consequence of other aspects of the surgical procedure. However, paradoxically, the amplitude of the APs in the sham animals was significantly larger than that in the ablated animals (Supplemental Table S1; unpaired t-test, P = 0.034), whereas no other aspects of the AP shape were different.
Effects of cochlear ablation on 5-HT modulation of excitability
We next assessed the effects of SNHL on 5-HT modulation of excitability, by testing the effects of bath-applied 5-HT (50 μM) on cells from cochlear-ablated and sham-operated rats. In contrast to its effects in normal animals (Fig. 2), 5-HT did not alter the number of spikes produced by neurons from sham animals aged P12–P21 (Fig. 4, A and C, two-way ANOVA, F–I in aCSF vs. F–I in 5-HT, df = 1, F = 0.34, P = 0.56, n = 21). These results in sham animals were obtained in two separate experimental series performed over a year apart, on different setups, and by different individuals. Since both series showed the same lack of effect of 5-HT compared with contemporaneous controls and were not different from each other, the data from the two series have been combined. In addition, 5-HT had no effect on the intrinsic excitability of cells from animals with cochlear ablations (Fig. 4, B and D, two-way ANOVA, F–I in aCSF vs. F–I in 5-HT, df = 1, F = 2.23, P = 0.13; n = 15).
Fig. 4.
Cochlear ablation does not affect 5-HT modulation of excitability in P12–P21 rat A1. A: voltage traces from 2 example neurons from sham animals in response to a 100 pA depolarizing current step under different drug conditions (as in Fig. 1). Dashed gray lines indicate resting membrane potential; current injection is shown below the voltage traces. Traces in aCSF and 5-HT are taken from a neuron different from those in wash, KTS, and KTS + 5-HT. B: voltage traces as in A from 2 example neurons from cochlear-ablated animals. C: mean firing rates evoked by depolarizing current injections inform a population of sham rats: aCSF (squares), in the presence of 50 μM 5-HT (blue squares), after wash1 (open squares with dashed line), in 1 μM KTS (green squares), and in KTS + 5-HT (red squares). Neither serotonin nor ketanserin changed the firing rate. In the presence of ketanserin, serotonin significantly decreased firing rate. D: data for cochlear-ablated rats, in the same format as that in C.
Figure 5 summarizes the measurements of resting potential, input resistance, and adaptation ratio through the protocol shown in Fig. 1 for each of the experimental conditions for both age groups. Although the effects of 5-HT in the sham and ablated P12–P21 animals do not always reach statistical significance, the overall patterns of membrane potential and input resistance changes resemble those of the normal group, suggesting that there may be an attenuated response to 5-HT. Similar to its effect in normal hearing animals, 5-HT reduced the input resistance of neurons from both sham (P = 0.023, two-tailed t-test) and ablated animals (P = 0.020, two-tailed t-test; Fig. 5B, left). Serotonin significantly decreased the adaptation ratio of neurons in ablated animals (Fig. 5C, left; Supplemental Table S1, P = 0.0002, two-tailed t-test), but not in sham animals.
Fig. 5.
Summary of changes in resting potential, input resistance, and adaptation ratios for each experimental group. In each row, data from normal (unoperated) cells are shown in filled circles, data from shams with squares, and data from ablated animals with triangles. A: effects of 5-HT pharmacology on resting membrane potential. B: effects of 5-HT pharmacology on input resistance. C: effects of 5-HT pharmacology on adaptation ratio. Summary of 5-HT effects on intrinsic properties on normal, sham, and ablated neurons with development effects. Normals (black closed circles), P12–P21 sham (black closed squares), and ablated (black closed triangles). P30–P35 sham (black closed squares) and ablated (black closed triangles). B: effects of 5-HT pharmacology on input resistance. C: effects of 5-HT pharmacology on adaptation ratio. Paired t-test significant differences only where asterisks (*) are placed.
Previous work has shown that activation of different 5-HT receptor subtypes can trigger depolarizing or hyperpolarizing membrane potential responses in various CNS neurons (Andrade et al. 1987, 1991; Araneda et al. 1991; Chapin et al. 2001; Tanaka et al. 1993). Previously, we found that 5-HT2 receptors are highly expressed in layer II/III neurons of the auditory cortex (Basura et al. 2008). We therefore used ketanserin (a 5-HT2 receptor antagonist, 1 μM) to block the effects of 5-HT on 5-HT2 receptors. In the absence of exogenous 5-HT, blocking 5-HT2 receptors with ketanserin did not affect firing of neurons from sham (Fig. 4, A and C, two-way ANOVA, F–I in aCSF vs. F–I in Ket, df = 1, F = 0.58, P = 0.45; n = 11) or cochlear-ablated animals (Fig. 4, B and D, two-way ANOVA, F–I in aCSF vs. F–I in Ket, df = 1, F = 0.67, P = 0.42; n = 8). This suggests that the basal tone of 5-HT in the slice is not sufficient to drive changes in excitability or firing. Interestingly, when ketanserin was applied to the bath concurrently with 5-HT (50 μM), the number of spikes in neurons from sham animals decreased relative to ketanserin (Fig. 4, A and C, two-way ANOVA, F–I in Ket vs. F–I in Ket + 5-HT, df = 1, F = 45.3, P < 0.0001; n = 11) and was significantly reduced for currents between 100 and 140 pA (P < 0.05, Bonferroni posttest). Moreover, in the presence of ketanserin, 5-HT markedly reduced the firing rates in cochlear-ablated animals below the rates evoked in ketanserin alone (Fig. 4, B and D, two-way ANOVA, F–I in Ket vs. F–I in Ket + 5-HT, df = 1, F = 30.0, P = 0.0001; n = 8). The rate reduction was significant for currents between 80 and 120 pA (P < 0.05, Bonferroni posttest). We conclude that blocking 5-HT2 receptors with ketanserin unmasks an action of 5-HT on other 5-HT receptor subtypes and that this effect is not changed by cochlear ablation in animals tested at P12–P21.
To test the hypothesis that specific activation of 5-HT2 receptors affects excitability, we bath applied a 5-HT2 receptor agonist 5-MeO-DMT (5 μM) in 0.1% DMSO. DMSO alone had no effect on the firing rate. Similarly, 5-MeO-DMT did not affect the firing rate in normal neurons (P16–P18) (Fig. 6, two-way ANOVA, F–I in DMSO vs. F–I in 5-MeO-DMT, df = 1, F = 0.023, P = 0.88; n = 5 cells). Although the spike rate was not changed by 5-MeO-DMT, the input resistance decreased (97.5 ± 11.0 MΩ in DMSO vs. 79.1 ± 11.6 MΩ in 5-MeO-DMT, two-tailed t-test, P = 0.0007). However, the adaptation ratio did not significantly change. These results suggest that stimulation of 5-HT2 receptors does not influence the firing rate of A1 neurons.
Fig. 6.
Activation of 5-HT2 receptors does not affect the firing rate to current pulses. A: firing rate as a function of current level from cells in normal P16–P18 rats measured in 0.1% DMSO (filled circles) and subsequently in the presence of 5 μM 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT; open circles); n = 5 cells.
Developmental effects of hearing loss on 5-HT2 modulation of excitability
Neurons in the auditory cortex exhibit several prominent changes during development. In rat, the tonotopic map undergoes significant refinement between P14 and P22 (Chang et al. 2003; de Villers-Sidani et al. 2007; Zhang et al. 2001). The bandwidths of excitatory receptive fields continue to decrease and the ability of neurons to follow repetitive stimuli improves until at least P35 (Chang et al. 2003, 2005). At the cellular level, there is an overall decrease in neural excitability and a rightward shift in the F–I curves of auditory cortical neurons at P19–P29, compared with P10–P18 (Oswald and Reyes 2008). The data we have presented so far (up to P21) were collected prior to an important developmental turning point. Consequently, we next examined an older group of P8 sham-operated and cochlear-ablated animals using the same paradigm as that used for the P12–P21 group.
First, we tested whether the increased firing rate of neurons following hearing loss persists to P30–P35. However, we could find no difference in excitability due to hearing loss between sham and cochlear-ablated animals at P30–P35 (Supplemental Fig. S1; two-way ANOVA, F–I in sham vs. ablated, df = 1, F = 0.83, P = 0.37; n = 3 sham and 6 ablated cells). Furthermore, the difference in AP height seen in P12–P21 animals was absent in the older animals (Supplemental Table S1; unpaired t-test, P = 0.49). We next asked whether 5-HT2 modulation of excitability is present only transiently during P12–P21 or persists to P30. Serotonin did decrease the firing rates of neurons from sham animals 5 min after application (Fig. 7C, two-way ANOVA, F–I in aCSF vs. F–I in 5-HT, df = 1, F = 8.56, P = 0.0062; n = 4). However, in contrast to younger animals, there was an even stronger suppression of firing that appeared 10–15 min after 5-HT was washed out (Fig. 7C, two-way ANOVA, F–I in aCSF vs. F–I in Wash1, df = 1, F = 7.00, P = 0.011; n = 4, aCSF and n = 3, Wash1). In cochlear-ablated P30–P35 animals, however, 5-HT did not suppress firing either during application or 10–15 min later (Fig. 7, B and D, 5-min: two-way ANOVA, F–I in aCSF vs. F–I in 5-HT, df = 1, F = 0.5, P = 0.48; 10–15 min: F–I in aCSF vs. F–I in Wash1, df = 1, F = 1.87, P = 0.17). In contrast to these effects on the F–I relationship, the effects of 5-HT on resting potential, input resistance, and the adaptation ratio were small and not significant (Fig. 5, right). Thus at this later time point, 5-HT was ineffective in depressing the excitability of cells after cochlear ablation.
Fig. 7.
Cochlear ablation decreases the ability of 5-HT to modulate excitability in P30–P35 rat A1. A: voltage traces from an example neuron from a sham animal in response to a 100 pA depolarizing current step under different drug conditions (as in Fig. 1). Dashed gray lines indicate resting membrane potential; current injection is shown below the voltage traces. B: voltage traces as in A from an example neuron from a cochlear-ablated animal. C: mean firing rates evoked by depolarizing current injections inform a population of sham rats: aCSF (squares), in the presence of 50 μM 5-HT (blue squares), after wash1 (open squares with dashed line), in 1 μM KTS (green squares), and in KTS + 5-HT (red squares). Serotonin acutely decreased the firing rate, but also had a strong delayed effect after 15 min of aCSF wash, greatly raising threshold and decreasing the firing rage. Subsequent challenges with KTS and 5-HT had no further effect. D: data for cochlear-ablated rats, in the same format as that for C. 5-HT had no acute or delayed effect. KTS decreased firing slightly, whereas the subsequent addition of 5-HT decreased firing further.
In these same cells, we also tested whether age affected 5-HT2 receptor modulation of excitability following cochlear ablation using the paradigm shown in Fig. 1, with simultaneous application of ketanserin (1 μM) and 5-HT (50 μM). Application of ketanserin alone did not alter firing in sham animals (Fig. 7, A and C, two-way ANOVA, F–I in Wash1 vs. F–I in Ket, df = 1, F = 1.75, P = 0.19). Simultaneous application of ketanserin and 5-HT also did not alter firing (Fig. 7, A and C, two-way ANOVA, F–I in Wash1 vs. F–I in Ket, df = 1, F = 0.14, P = 0.71). In contrast to our findings in younger neurons (Fig. 4), application of ketanserin alone significantly decreased neuronal firing in cochlear-ablated animals (Fig. 7, B and D, two-way ANOVA, F–I in Wash1 vs. F–I in Ket, df = 1, F = 5.75, P = 0.021). This result is surprising, given that 5-HT alone had no effect in these animals. A subsequent 5-min application of 5-HT in the presence of ketanserin, however, produced an additional small but significant decrease in firing (Fig. 7, B and D, two-way ANOVA, F–I in Ket vs. F–I in Ket + 5-HT, df = 1, F = 5.19, P = 0.028). These results suggest that non-5-HT2 receptors are present and can regulate the intrinsic excitability. They also suggest, compared with the P12–P21 cells, that there is a developmental shift in the expression of 5-HT receptor subtypes or their signaling mechanisms that is further affected by cochlear ablation.
DISCUSSION
Results of these experiments may be summarized as follows. First, we have shown that a sham surgery has an effect on the excitability of auditory cortical neurons. Second, we have confirmed that rat auditory cortical layer II/III pyramidal cells show increased excitability with hearing loss, even when compared with sham controls. Third, we show that 5-HT decreases excitability in P12–P21 normal auditory cortex. Fourth, we found that both sham surgery and cochlear ablation occlude the ability of 5-HT to decrease excitability. However, in the presence of ketanserin to block 5-HT2 receptors, 5-HT can still further decrease excitability, suggesting that 5-HT likely operates through two receptor systems with opposing actions on excitability. Finally, electrical excitability is the same in sham and ablated animals at P30–P35, or 21–27 days after the cochlear ablation. However, the modulation of excitability by 5-HT is blunted in the animals with hearing loss. Overall, our results suggest that 5-HT plays a functional role in regulating cellular excitability in A1 and that this role is developmentally regulated. In addition, the ability of 5-HT to modulate the intrinsic excitability of auditory cortical neurons depends on the hearing status of the animal. These experiments also raise a cautionary note regarding comparisons between cochlear-ablated and nonoperated experimental groups.
Effects of SNHL on intrinsic properties
The present data demonstrate increased pyramidal cell excitability in A1 layer II/III neurons following bilateral cochlear ablation (Fig. 3), as a model of early-onset SNHL, confirming similar findings in gerbils (Kotak et al. 2005). Kotak et al. (2005) reported cochlear ablation resulted in a decrease in adapting-type neurons and an increase in sustained-type neurons. Our results indicate that cochlear ablation increased the excitability of adapting-type neurons.
The underlying mechanisms contributing to increased excitability after cochlear ablation are unclear. In cochlear-ablated gerbils, A1 neurons display a depolarized resting potential, increased input resistance, and a higher incidence of sustained firing (Kotak et al. 2005). However, in the present study, resting potential, input resistance, and spike rate adaptation were not affected either by sham surgery or by cochlear ablations; only the relationship between injected current and the firing rate was altered. This is consistent with previous reports that deprivation of afferent input can result in changes in intrinsic excitability in cerebral cortex (Desai et al. 1999; Maffei et al. 2004; but see Maravall et al. 2004) and in the cochlear nucleus (Francis and Manis 2000; Wang and Manis 2006). Such changes likely reflect sensory activity-dependent homeostatic mechanisms, perhaps driven by down-regulation of brain-derived neurotrophic factor and phosphorylated cAMP response element binding after hearing loss (Tan et al. 2008). Tan et al. (2008) also observed a reduction in sodium channel immunoreactivity, which should reflect channel availability and might indicate reduced excitability. Our finding that cochlear ablation caused a significant decrease in the AP height (and a trend toward a decrease in the rising slope; Supplemental Table S1), which is primarily controlled by sodium channel density, is consistent with a reduction in sodium channel availability. The increased excitability, measured as the number of spikes for a given injection current, which we and Kotak et al. (2005) have both observed, could in turn be due to a decrease in the activation of conductances that control the interspike interval of cortical pyramidal cells. Our data in older animals are also consistent with this overall argument, in that there was no change in AP amplitude or in the firing rate for a given current between sham and ablated animals. We would expect that smaller APs could lead to less calcium influx, could decrease the engagement of calcium-activated potassium currents that regulate the slow afterhyperpolarization (Lorenzon and Foehring 1993) in cortical pyramidal neurons, and could lead to higher firing rates. Interestingly, and consistent with this argument, calcium-activated potassium currents have also been shown to be modulated by sensory experience (Maravall et al. 2004) in the somatosensory cortex during the early critical period between P12 and P17.
The role of homeostatic mechanisms in regulating excitability may be more complex than that with simple deprivation because it is unlikely that A1 is completely electrically silent after cochlear ablation. In the absence of auditory inputs, A1 has been shown to become responsive over time to both somatosensory and visual stimuli (Hunt et al. 2006; Kral 2007). This raises the possibility that part of the difference between the P12–P21 neurons and the P30–P35 neurons, where the F–I curves were not different between sham and ablated animals, is that the latter are engaged in a crossmodal sensory processing. This sensory activity may be sufficient to return the excitability of the neurons to their “normal” operating point.
On the other hand, the coupling between 5-HT receptors and modulation of neuronal firing is decreased in the ablated animals at P30–P35, suggesting that the receptors failed to become engaged, due either to inadequate expression, dysfunctional coupling to their second messengers, or to a mislocalization with respect to their target proteins. Thus it appears that early hearing loss disrupts the serotonergic signaling system in auditory cortex and, in turn, likely limits the serotonergic modulation of cortical function later in life. The manner in which these changes in the 5-HT systems affect subsequent cortical function and plasticity, for example with reintroduction of auditory activity with cochlear implants, remains to be studied.
Role of 5-HT and 5-HT2 receptors in A1 neurons
Our observation that serotonin decreased neuronal firing in the normal auditory cortex suggests that one role of 5-HT is to suppress neural activity under conditions when serotonergic neurons are activated (Ji et al. 2007). In contrast to the clear effects in normal animals, activation of 5-HT receptors did not alter firing rate or AP shape in sham-operated P12–P21 animals, although it did affect firing rate adaptation in cochlear-ablated animals. In both shams and cochlear-ablated animals, 5-HT reduced firing even in the presence of the 5-HT2 receptor antagonist ketanserin, compared with the effects of ketanserin alone. This suggests that the reduction in firing depends on non-5-HT2 receptors and is consistent with our observation that the specific 5-HT2 agonist 5MeO-DMT did not affect the firing rate in normal cortex. It would appear that 5-HT2 receptors do not directly couple to ion channels in auditory cortex. One interpretation of these results is that there are two different serotonergic receptor systems that can regulate excitability in A1. This idea is supported by observations in pyramidal cells of the adult prefrontal cortex, which coexpress 5-HT1A and 5-HT2A receptors (Martin-Ruiz et al. 2001). For example, parallel electrophysiological studies have shown that 5-HT1A receptors can mediate hyperpolarization, whereas 5-HT2A receptors can cause depolarization (Araneda and Andrade 1991; Davies et al. 1987; Tanaka and North 1993). These systems affect excitability in opposite directions, such that when the 5-HT2 receptors are blocked by ketanserin, a separate class of 5-HT receptors drives a decrease in excitability. A similar regulatory interaction of 5-HT2A/C receptors on modulation by 5-HT1A receptors has been shown for N-methyl-d-aspartate receptors in prefrontal cortex (Yuen et al. 2008).
The decrease in input resistance and firing rate in auditory cortex might be driven by 5-HT1A receptors (Gurevich et al. 1990), which have been shown to activate outward currents mediated by G protein-activated inwardly rectifying K+ channels (Lüscher et al. 1997). 5-HT1A receptors are poorly expressed in the cerebral cortex immediately after birth, but increase in expression during the early postnatal period (Daval et al. 1987; Miquel et al. 1994). Although perinatal layer V prefrontal cortical neurons can be depolarized through the activation of 5-HT2A and 5-HT7 receptors, by the beginning of the third week of age in rats, the depolarization is replaced by a hyperpolarization mediated by 5-HT1A receptors (Beique et al. 2004). In our experiments, changes in membrane potential in the normal cells (Fig. 5A, left) are somewhat consistent with this pattern in that before the third week of life, 5-HT produces a depolarization. In this respect, it is interesting that in the P30–P35 sham group, there is a very strong effect of 5-HT alone, although this effect is delayed by many minutes as if it might be mediated through a slow second messenger cascade. However, the changes in firing with current injection, which were independent of membrane potential, suggest that an additional set of target mechanisms is involved in auditory cortex.
The acute decrease in firing rate adaptation in normal A1 (Fig. 5C, left) suggests that 5-HT receptors can modulate cortical information processing by modifying spike timing patterns. In particular, we observed a decrease in spike rate adaptation acutely in the presence of 5-HT in normal and cochlear-ablated animals at P12–P21 and a similar (but not significant) trend in ablated animals at P30–P35. A decrease in the adaptation ratio implies that the neuron may be signaling the steady-state component of its response to stimuli more than its transient response to the onset of the stimulus. That this occurs acutely, appears to be reversible (Fig. 5C) and is dissociated from the mean firing rate patterns would suggest that adaptation may be regulated by a separate mechanism that is coupled to the 5-HT receptors. Modification of rate adaption could be related to attentive or aroused states, where the presence of sustained discharge patterns, as opposed to rapidly adapting firing, might be critical for auditory signal detection or discrimination (Ahveninen et al. 2003; Oranje et al. 2008).
Serotonin has been shown to play an important role in the developing mammalian brain (Hoyer et al. 2002; Lauder et al. 2000). Consequently, a shift in the expression of these receptors during critical periods of development, as we have shown to be produced by both the sham surgery and by cochlear ablation in neonatal rats, could have long-lasting effects on cortical wiring and sensory processing. Although many 5-HT receptor subtypes may also be present and play roles in regulating excitability and synaptic strength (e.g., 5-HT1A, 5-HT3, and 5-HT7), the current data provide a foundation for focused pharmacological experiments that specifically isolate 5-HT receptor pathways and their contributions to A1 pyramidal cell activity in this model of SNHL. Such studies could provide understanding of the electrophysiological changes observed following bilateral cochlear ablation and provide important pharmacological clues for methods to help restore auditory function and plasticity in hearing-impaired children.
GRANTS
This work was supported by a Deafness Research Foundation grant to G. Basura, National Institute on Deafness and Other Communication Disorders Grants R03 DC-009893 to G. Basura, T32 DC-005360 to S. Daniels and J. Roche, and R01 DC-000425 to D. Rao, J. G. Mancilla, and P. B. Manis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Ahveninen et al., 2003. Ahveninen J, Jääskeläinen IP, Pennanen S, Liesivuori J, Ilmoniemi RJ, Kähkönen S. Auditory selective attention modulated by tryptophan depletion in humans. Neurosci Lett 340: 181–184, 2003. [DOI] [PubMed] [Google Scholar]
- Andrade and Chaput, 1991. Andrade R, Chaput Y. 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257: 930–937, 1991. [PubMed] [Google Scholar]
- Andrade and Nicoll, 1987. Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol 394: 99–124, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda and Andrade, 1991. Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412, 1991. [DOI] [PubMed] [Google Scholar]
- Azmitia, 2001. Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56: 413–424, 2001. [DOI] [PubMed] [Google Scholar]
- Bakin and Weinberger, 1996. Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA 93: 11219–11224, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao et al., 2001. Bao S, Chan VT, Merzenich MM. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature 412: 79–83, 2001. [DOI] [PubMed] [Google Scholar]
- Basura et al., 2008. Basura GJ, Abbas AI, O'Donohue H, Lauder JM, Roth BL, Walker PD, Manis PB. Ontogeny of serotonin and serotonin2A receptors in rat auditory cortex. Hear Res 244: 45–50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique et al., 2004. Beique JC, Campbell BM, Perring P, Hamblin MW, Walker PD, Mladenovic L, Andrade R. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24: 4807–4817, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell et al., 1987. Campbell MJ, Lewis DA, Foote SL, Morrison JH. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta- hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol 261: 209–220, 1987. [DOI] [PubMed] [Google Scholar]
- Chang and Merzenich, 2003. Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science 300: 498–502, 2003. [DOI] [PubMed] [Google Scholar]
- Chang and Merzenich, 2005. Chang EF, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA 102: 16460–16465, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin and Andrade, 2001. Chapin EM, Andrade R. A 5-HT(7) receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h). J Pharmacol Exp Ther 297: 403–409, 2001. [PubMed] [Google Scholar]
- Cruikshank et al., 2002. Cruikshank SI, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro, J Neurophysiol 87: 361–384, 2002. [DOI] [PubMed] [Google Scholar]
- Daval et al., 1987. Daval G, Verge D, Becerril A, Gozlan H, Spampinato U, Hamon M. Transient expression of 5-HT1A receptor binding sites in some areas of the rat CNS during postnatal development. Int J Dev Neurosci 5: 171–180, 1987. [DOI] [PubMed] [Google Scholar]
- Davies et al., 1987. Davies MF, Deisz RA, Prince DA, Peroutka SJ. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res 423: 347–352, 1987. [DOI] [PubMed] [Google Scholar]
- Desai et al., 1999. Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999. [DOI] [PubMed] [Google Scholar]
- De Villers-Sidani et al., 2007. De Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci 27: 180–189, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks et al., 1999. Dierks T, Barta S, Demisch L, Schmeck K, Englert E, Kewitz A, Maurer K, Poustka F. Intensity dependence of auditory evoked potentials (AEPs) as biological marker for cerebral serotonin levels: effects of tryptophan depletion in healthy subjects. Psychopharmacology 146: 101–107, 1999. [DOI] [PubMed] [Google Scholar]
- Emmorey et al., 2003. Emmorey K, Allen JS, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci USA 100: 10049–10054, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir et al., 1999. Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999. [DOI] [PubMed] [Google Scholar]
- Francis and Manis, 2000. Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons. Hear Res 149: 91–105, 2000. [DOI] [PubMed] [Google Scholar]
- Fujino and Oertel, 2003. Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci USA 100: 265–270, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünschlag et al., 1997. Grünschlag CR, Haas HL, Stevens DR. 5-HT inhibits lateral entorhinal cortical neurons of the rat in vitro by activation of potassium channel-coupled 5-HT1A receptors. Brain Res 770: 10–17, 1997. [DOI] [PubMed] [Google Scholar]
- Gurevich et al., 1990. Gurevich N, Wu PH, Carlen PL. Serotonin agonist and antagonist actions in hippocampal CA1 neurons. Can J Physiol Pharmacol 68: 586–595, 1990. [DOI] [PubMed] [Google Scholar]
- Hegerl and Juckel, 1993. Hegerl U, Juckel G. Intensity dependence of auditory evoked potentials as an indicator of central serotonergic neurotransmission: a new hypothesis. Biol Psychiatry 33: 173–187, 1993. [DOI] [PubMed] [Google Scholar]
- Hoyer et al., 2002. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554, 2002. [DOI] [PubMed] [Google Scholar]
- Hunt et al., 2006. Hunt DL, Yamoah EN, Krubitzer L. Multisensory plasticity in congenitally deaf mice: how are cortical areas functionally specified? Neuroscience 134: 1507–1524, 2006. [DOI] [PubMed] [Google Scholar]
- Jero et al., 2001. Jero J, Coling DE, Lalwani AK. The use of Preyer's reflex in evaluation of hearing in mice. Acta Otolaryngol 121: 585–589, 2001. [PubMed] [Google Scholar]
- Ji and Suga, 2007. Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. J Neurosci 27: 4910–4918, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson and Heineman, 1995. Johnson DS, Heineman SF. Embryonic expression of the 5-HT3 receptor subunit, 5-HT3R-A, in the rat: an in situ hybridization study. Mol Cell Neurosci 6: 122–138, 1995. [DOI] [PubMed] [Google Scholar]
- Juckel et al., 1997. Juckel G, Molnár M, Hegerl U, Csépe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity: first evidence in behaving cats. Biol Psychiatry 41: 1181–1195, 1997. [DOI] [PubMed] [Google Scholar]
- Kähkönen et al., 2002b. Kähkönen S, Ahveninen J, Pennanen S, Liesivuori J, Ilmoniemi RJ, Jääskeläinen IP. Serotonin modulates early cortical auditory processing in healthy. subjects: evidence from MEG with acute tryptophan depletion. Neuropsychopharmacology 27: 862–868, 2002b. [DOI] [PubMed] [Google Scholar]
- Kähkönen et al., 2002a. Kähkönen S, Jääskeläinen IP, Pennanen S, Liesivuori J, Ahveninen J. Acute tryptophan depletion decreases intensity dependence of auditory evoked magnetic N1/P2 dipole source activity. Psychopharmacology 164: 221–227, 2002a. [DOI] [PubMed] [Google Scholar]
- Kidd et al., 2002. Kidd G, Arbogast TL, Mason CR, Walsh M. Informational masking in listeners with sensorineural hearing loss. J Assoc Res Otolaryngol 3: 107–119, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard and Merzenich, 1998. Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science 279: 1714–1718, 1998. [DOI] [PubMed] [Google Scholar]
- Kilgard et al., 2001. Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol 86: 326–338, 2001. [DOI] [PubMed] [Google Scholar]
- Kotak et al., 2005. Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci 25: 3908–3918, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak et al., 2008. Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex 18: 2098–2108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral, 2007. Kral A. Unimodal and cross-modal plasticity in the “deaf” auditory cortex. Int J Audiol 46: 479–493, 2007. [DOI] [PubMed] [Google Scholar]
- Lauder, 1990. Lauder JM. Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann NY Acad Sci 600: 297–313, 1990. [DOI] [PubMed] [Google Scholar]
- Lauder et al., 2000. Lauder JM, Wilkie MB, Wu C, Singh S. Expression of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors in the mouse embryo. Int J Dev Neurosci 18: 653–662, 2000. [DOI] [PubMed] [Google Scholar]
- Lorenzon and Foehring, 1993. Lorenzon NM, Foehring RC. The ontogeny of repetitive firing and its modulation by norepinephrine in rat neocortical neurons. Brain Res Dev Brain Res 73: 213–223, 1993. [DOI] [PubMed] [Google Scholar]
- Lüscher et al., 1997. Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997. [DOI] [PubMed] [Google Scholar]
- Ma et al., 2007. Ma L, Shalinsky MH, Alonso A, Dickson CT. Effects of serotonin on the intrinsic membrane properties of layer II medial entorhinal cortex neurons. Hippocampus 17: 114–129, 2007. [DOI] [PubMed] [Google Scholar]
- Maffei et al., 2004. Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7: 1353–1359, 2004. [DOI] [PubMed] [Google Scholar]
- Maravall et al., 2004. Maravall M, Stern EA, Svoboda K. Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol 92: 144–156, 2004. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz et al., 2001. Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 21: 9856–9866, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate and Cruikshank, 1999. Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Exp Brain Res 126: 160–174, 1999. [DOI] [PubMed] [Google Scholar]
- Miquel et al., 1994. Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Matthiessen L, Hamon M, Verge D. Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Dev Brain Res 80: 149–157, 1994. [DOI] [PubMed] [Google Scholar]
- Oranje et al., 2008. Oranje B, Jensen K, Wienberg M, Glenthøj BY. Divergent effects of increased serotonergic activity on psychophysiological parameters of human attention. Int J Neuropsychopharmacol 11: 453–456, 2008. [DOI] [PubMed] [Google Scholar]
- Oswald and Reyes, 2008. Oswald A-MM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol 99: 2998–3008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarommatis et al., 2001. Psarommatis IM, Goritsa E, Douniadakis D, Tsakanikos M, Kontrogianni AD, Apostolopoulos N. Hearing loss in speech-language delayed children. Int J Ped Otorhinolaryngol 58: 205–210, 2001. [DOI] [PubMed] [Google Scholar]
- Sanes and Bao, 2009. Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol 19: 188–199, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro et al., 2000. Shapiro DA, Kristiansen K, Kroeze WK, Roth BL. Differential modes of agonist binding to 5-hyrdroxytraptamine2A serotonin receptors revealed by mutation and molecular modeling of conserved residues in transmembrane region 5. Mol Pharmacol 58: 877–886, 2000. [DOI] [PubMed] [Google Scholar]
- Tan et al., 2008. Tan J, Widjaja S, Xu J, Shepherd RK. Cochlear implants stimulate activity-dependent CREB pathway in the deaf auditory cortex: implications for molecular plasticity induced by neural prosthetic devices. Cereb Cortex 18: 1799–1813, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka and North, 1993. Tanaka E, North RA. Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol 69: 1749–1757, 1993. [DOI] [PubMed] [Google Scholar]
- Tanaka et al., 2008. Tanaka Y, Tanaky Y, Furuta T, Yanagawa Y, Kaneko T. The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices adult mice. J Neurosci Methods 171: 118–125, 2008. [DOI] [PubMed] [Google Scholar]
- Vitalis and Parnavelas, 2003. Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci 25: 245–256, 2003. [DOI] [PubMed] [Google Scholar]
- Wang and Manis, 2006. Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol 7: 412–424, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al., 2007. Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci 27: 9417–9426, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen et al., 2008. Yuen EY, Jiang Q, Chen P, Feng J, Yan Z. Activation of 5-HT2A/C receptors counteracts 5-HT1A regulation of N-methyl-D-asparate receptor channels in pyramidal neurons of prefrontal cortex. J Biol Chem 283: 17194–17204, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2001. Zhang LI, Bao S, Merzeinch MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci 4: 1123–1130, 2001. [DOI] [PubMed] [Google Scholar]
- Zhang, 2003. Zhang Z-W. Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci 23: 3373–3384, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.