Abstract
Sphingosine 1-phosphate (S1P) through its interaction with a family of G protein–coupled receptors (S1PR) is proving to have a significant impact on the activation of a variety of cell types, most notably those cells mediating the inflammatory response. Previously, we showed that S1P enhanced the excitability of small diameter sensory neurons, and mRNA for S1PR1–4 was expressed in sensory neurons. These initial findings did not determine which S1PR subtype(s) mediated the increased excitability. Here, we report that exposure to the selective S1PR1 agonist, SEW2871, produced a significant increase in excitability of some, but not all, sensory neurons. To further examine the role of S1PR1, neurons were treated with siRNA targeted to S1PR1. siRNA reduced S1PR1 protein expression by 75% and blocked the sensitization produced by SEW2871, although some neurons remained responsive to subsequent exposure to S1P. Treatment with scramble siRNA did not alter S1PR1 expression. Recordings from siRNA- and scramble-treated neurons suggested three distinct populations based on their sensitivities to SEW2871 and S1P. Approximately 50% of the neurons exhibited a significant increase in excitability after exposure to SEW2871 and subsequent S1P produced no additional increase; ∼25% were not affected by SEW2871 but S1P significantly increased excitability; and ∼25% of the neurons were not sensitized by either SEW2871 or S1P. RT-PCR measurements obtained from single neurons showed that 50% of the small diameter neurons expressed the mRNA for S1PR1. These results indicate that S1PR1 plays a prominent, although not exclusive, role in mediating the enhancement of excitability produced by S1P.
INTRODUCTION
Seminal studies established that the lysophospholipid molecule, sphingosine 1-phosphate (S1P), is an important signaling molecule between cells but also plays an important role as an intracellular second messenger (Hannun and Obeid 2008; Hla 2004; Hla and Maciag 1990; Spiegel and Milstien 2003; Takabe et al. 2008; Van Brocklyn et al. 1998). S1P interacts with a family of five G protein–coupled receptors (S1PR1–5, previously known as Edg receptors) and has a significant impact on the development and activation of different cell types (Anliker and Chun 2004; Meyer zu Heringdorf and Jakobs 2007; Rosen et al. 2009; Sanchez and Hla 2004). On activation, a variety of immuno-competent cells (e.g., mast cells and platelets) release S1P, where it can function as either an autocrine or paracrine signaling molecule (Goetzl and Rosen 2004; Olivera and Rivera 2005; Rivera et al. 2008; Weigart et al. 2009). In particular, interaction between S1P and the S1P receptor S1PR1 plays a critical role in regulating many aspects of the inflammatory response. Our greatest understanding involves the relationship between circulating levels of S1P, S1PR1, and the trafficking of T lymphocytes in and out of lymph nodes (reviewed by Rivera et al. 2008; Rosen and Goetzl 2005). Reduction of S1P levels or agonist-induced/conditional deletions of S1PR1 results in T lymphocytes failing to leave the node and therefore removing these cells from circulation (Allende et al. 2004; Brinkmann et al. 2002; Chiba et al. 1998; Mandala et al. 2002; Matloubian et al. 2004; Pappu et al. 2007; Schwab et al. 2005). These findings resulted in the idea that removal of S1PR1 (e.g., with the S1PR agonist FTY720) can be a potent immunosuppressant and provides a mechanistic model for the development of selective S1PR1 agonists that produce immunosuppression. Interestingly, because of its immunosuppressive properties, FTY720 has exhibited therapeutic potential in treating multiple sclerosis (Brinkmann 2009; Cohen et al. 2010; Kappos et al. 2010).
On activation of immuno-competent cells, the release of multiple mediators can heighten the sensitivity of sensory neurons to a variety of stimuli (reviewed by DeLeo and Yezierski 2001; Miller et al. 2009; Milligan and Watkins 2009; Scholz and Woolf 2007; Thacker et al. 2007; White et al. 2005). However, the impact of these different mediators on neuronal sensitivity is poorly understood. Our previous work showed that S1P enhanced the excitability of small diameter sensory neurons and that sensory neurons expressed the mRNA for S1PR1–4 (Zhang et al. 2006). This work did not explore the idea as to which S1PR subtype(s) mediated increased excitability. In this report, we focus on S1PR1 because of the well-established S1P/S1PR1 interaction in regulating the inflammatory response, the mechanistic pathways are currently the best understood, and unlike other S1PRs, S1PR1 is activated by the selective agonist SEW2871. Using the combination of activation by S1P or SEW2871, siRNA targeted to S1PR1, and single-cell RT-PCR, we show that S1PR1 plays a prominent role in directly augmenting the excitability of small diameter capsaicin-sensitive sensory neurons, although other S1PRs also are involved.
METHODS
Isolation and maintenance of adult rat sensory neurons
Sensory neurons were isolated from young adult male Sprague-Dawley rats (100–150 g) using procedures developed by Lindsay (1988) with slight modifications (Chi and Nicol 2007). Briefly, the young rats were killed by placing them in a chamber filled with CO2. The dorsal root ganglia (DRGs) were collected in a culture dish filled with sterilized Puck's solution. For the experiments examining the acute effects of S1P and SEW2871, the ganglia were transferred to a conical tube with F-12 media containing papain (20 U/ml) and incubated for 15 min at 37°C, followed by incubation with 1 mg/ml collagenase IA and 2.5 mg/ml dispase for 10 min at 37°C. For the experiments involving siRNA, the ganglia were transferred to a conical tube with F-12 media containing 1 mg/ml collagenase IA and 2.5 mg/ml dispase and incubated for 35 min at 37°C. The suspension was centrifuged for 30 s (1,000g), after which the enzyme-containing supernatant was removed. The pellet was resuspended in F-12 media supplemented with nerve growth factor (30 ng/ml) and mechanically dissociated with fire-polished pipettes. Isolated cells were plated onto plastic coverslips or the bottom of the culture dish previously coated with poly-d-lysine (100 μg/ml) and laminin (5 μg/ml). Isolated cells were cultured at 37°C and 3% CO2. The cells were maintained in culture for either 24 h (acute electrophysiological recordings) or 7 days (siRNA and electrophysiological studies). All procedures were approved by the Animal Use and Care committee of Indiana University School of Medicine.
Single-cell RT-PCR
The presence of gene transcripts for S1PR1 was detected using techniques described by Chi and Nicol (2007) with modification. Briefly, a small (≤25 μm) or large (>50 μm) diameter sensory neuron was aspirated into a sterilized micropipette (baked at 250°C for 2 h) containing diethylpyrocarbonate (DEPC)-treated water. The contents of the micropipette were forced into a 0.2-ml microtube with 5 μl DEPC water, and the RNA was reverse transcribed using the iScriptTM cDNA synthesis kit (Bio-Rad, Hercules, CA) for cDNA synthesis in 20-μl reactions according to the manufacturer's instructions. The cDNA was stored at –20°C before PCR detection. Amplification of S1PR1 was performed by using the forward primer (278–296 bp) ATGGTGTCCTCCACCAGCATCCC and the reverse primer (725–708 bp) TTAAGAAGAAGAATTGACCTTTCC (accession NM 017301.2, product size 448 bp) using the Platinum PCR Supermix (Invitrogen, Carlsbad, CA). These PCR reactions were run on a PTC-100 programmable thermal controller (MJ Research, Watertown, MA) for 45 cycles (94°C for 45 s, 46°C for 45 s, 72°C for 2 min). The primers for Kv1.1 are described in Chi and Nicol (2007). The PCR products were sequenced using an ABI Prism 3100 genetic analyzer at facilities in the Department of Biochemistry and Molecular Biology, Indiana University School of Medicine.
siRNA treatment
An siRNA sequence was selected according to the software provided by Dharmacon siDESIGN website (//www.dharmacon.com/designcenter/designcenterpage.aspx). siRNA labeled with the fluorescent tag, DY547, was synthesized by Dharmacon. The sense strand was 5′-GAAGGACCAUGGCAUUAAA-3′, and the antisense strand was 5′-AAUGCCAUGGUCCUUCUU DY547-3′. For the scrambled siRNA (SC), the sense sequence was 5′-GCGCGCUUUGUAGGAUUCG-3′ and antisense sequence was 5′-CGAAUCCUACAAAGCGCGC DY547-3′; SC was used as a negative control. Isolated sensory neurons were maintained for 2 days in normal media with 30 ng/ml NGF. Normal media was replaced with F-12 media lacking antibiotics and bathed the cells for ∼12 h. The cells were rinsed once with Optimem media and incubated at 37°C for ∼30 min. The metafectene-siRNA complex (100 μl, 200 nM siRNA) was added wherein the neurons were exposed to the siRNA targeted to S1PR1, SC, or metafectene alone for 48 h at 37°C. After 2 days, the metafectene −/+ siRNA was washed out, and the normal media containing antibiotics and NGF were added to the neurons and allowed to incubate for 24 h before electrophysiology recordings or Western blots were performed.
Western blotting
Isolated sensory neurons that were either untreated or exposed to siRNA targeted to S1PR1, SC, or metafectene were sonicated in fresh TNN-SDS buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 50 mM NaF, 20 mM EDTA, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 0.1% SDS, and 2 mM phenylmethylsulfonyl fluoride) and incubated at 4°C for 30 min, and samples were vortexed every 10 min, followed by centrifugation (5,000g for 10 min). The supernatant was stored at −80°C. Protein concentration was determined by the Bradford method (Bio-Rad protein assay, Bio-Rad Life Science Research, Hercules, CA). Equivalent amounts of protein (30–100 μg) were loaded in each well. Before loading, samples were kept at room temperature for 30 min and subjected to NuPAGETM 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) electrophoresis and transferred to a PVDF membrane (Invitrogen). After serial incubation with specific antibodies, immunoreactive bands on the membrane were developed using an ECL kit (Amersham Biosciences, Piscataway, NJ) and visualized by exposure to ISC Bio Express film. The density of bands was measured using a Kodak 1D 3.6 imaging system (New Haven, CT).
Electrophysiology
Recordings were made using the whole cell patch-clamp technique (Chi and Nicol 2007; Hamill et al. 1981). Briefly, a coverslip with sensory neurons was placed in a recording chamber where neurons were bathed in normal Ringers solution of the following composition (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH at 7.4 with NaOH. Recording pipettes were pulled from disposable borosilicate glass tubing and typically had resistances of 2–5 MΩ when filled with the following solution (in mM): 140 KCl, 5 MgCl2, 4 ATP, 0.3 GTP, 2.5 CaCl2, 5 EGTA (calculated free Ca2+ concentration of ∼100 nM), and 10 HEPES, at pH 7.3 with KOH. Whole cell currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The whole cell recording configuration was established in normal Ringers solution. After establishing the whole cell configuration, both the capacitance and resistance were compensated by ∼80%. To assess excitability in the current-clamp experiment, the neurons were held at their resting potentials (range between −45 and −65 mV), and ramps of depolarizing currents (900 ms in duration) were used to evoke two to four action potentials (APs) under control conditions. The same ramp was used throughout the recording period for each individual neuron. All drugs were applied by external superfusion of the recording chamber using a VC-8 bath perfusion system (Warner Instruments, Hamden, CT). The traces were filtered at 5 kHz and sampled at 1 kHz using pClamp 8.0 (Molecular Devices). At the end of each recording, the neuron was exposed to 100 nM capsaicin. This neurotoxin was used to distinguish capsaicin-sensitive sensory neurons because these neurons are believed to transmit nociceptive information (Holzer 1991). However, the correlation between the idea that a neuron is a nociceptor and capsaicin sensitivity is not absolute. Some nociceptive neurons are insensitive to capsaicin and some capsaicin-sensitive neurons are not nociceptors (Petruska et al. 2000). Therefore this agent was used to define a population of small diameter sensory neurons that could serve a nociceptive function. The results reported here were obtained from only capsaicin-sensitive neurons. All experiments were performed at room temperature (∼23°C).
Data analysis
Data are presented as the means ± SE. The excitability parameters described in Table 1 were determined, in part, by differentiating the voltage trace (dV/dt) in the current-clamp recordings. The voltage and time at which the first AP was fired were taken as the point that exceeded the baseline value of dV/dt by >20-fold. The baseline value of dV/dt was determined by averaging the points between the onset of the ramp and the next 100 ms (135–235 ms). The rheobase was measured as the amount of ramp current at the firing threshold. The resistance at threshold (RTh) was calculated as the difference between the firing threshold and the resting membrane potential divided by the rheobase current. Statistical differences between the control recordings and those obtained under various treatment conditions were determined by using either a paired t-test, ANOVA, or repeated-measures (RM) ANOVA. When a significant difference was obtained with an ANOVA, post hoc analyses were performed using a Tukey test. Values of P < 0.05 were judged to be statistically significant.
Table 1.
Effects of SEW2871 on excitability properties
| Untreated Neurons | S1PR1 siRNA Treated | Scramble | |
|---|---|---|---|
| Resting membrane potential, mV | |||
| Control | −56.5 ± 1.7 | −61.2 ± 1.3 | −59.9 ± 1.4 |
| 2 min | −52.1 ± 2.2 | −62.6 ± 1.8 | −58.8 ± 1.5 |
| 4 min | −49.3 ± 4.2* | −63.0 ± 1.8 | −56.9 ± 1.8* |
| 6 min | −47.8 ± 4.4* | −63.2 ± 1.9 | −55.1 ± 2.2* |
| 10 min | −48.8 ± 4.5* | −62.5 ± 2.0 | −51.8 ± 2.6* |
| Firing threshold, mV | |||
| Control | −15.7 ± 1.7 | −16.5 ± 3.5 | −18.6 ± 9.9 |
| 2 min | −18.2 ± 1.6 | −14.8 ± 4.8 | −19.3 ± 10.7 |
| 4 min | −17.1 ± 1.8 | −12.8 ± 3.8 | −19.7 ± 10.4 |
| 6 min | −14.3 ± 2.6 | −14.1 ± 3.4 | −18.4 ± 11.1 |
| 10 min | −16.1 ± 2.8 | −15.1 ± 3.2 | −22.7 ± 11.3 |
| Rheobase, pA | |||
| Control | 225 ± 67 | 493 ± 206 | 341 ± 166 |
| 2 min | 120 ± 23 | 504 ± 210 | 280 ± 124 |
| 4 min | 123 ± 27 | 525 ± 202 | 268 ± 135 |
| 6 min | 111 ± 25 | 528 ± 198 | 259 ± 140 |
| 10 min | 98 ± 18* | 527 ± 226 | 196 ± 114* |
| Normalized rheobase | |||
| Control | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 |
| 2 min | 0.63 ± 0.11* | 0.99 ± 0.04 | 0.76 ± 0.10 |
| 4 min | 0.61 ± 0.14* | 1.17 ± 0.08 | 0.75 ± 0.05 |
| 6 min | 0.55 ± 0.10* | 1.17 ± 0.05 | 0.72 ± 0.11* |
| 10 min | 0.53 ± 0.11* | 1.12 ± 0.07 | 0.47 ± 0.13* |
| Resistance at threshold, MΩ | |||
| Control | 268 ± 78 | 223 ± 55 | 296 ± 82 |
| 2 min | 336 ± 71 | 227 ± 42 | 335 ± 107 |
| 4 min | 354 ± 110 | 209 ± 43 | 372 ± 124 |
| 6 min | 366 ± 75 | 206 ± 47 | 378 ± 105 |
| 10 min | 379 ± 62 | 226 ± 55 | 406 ± 118 |
| Normalized resistance at threshold | |||
| Control | 1.0 ± 0 | 1.0 ± 0 | 1.0 ± 0 |
| 2 min | 1.48 ± 0.26 | 1.09 ± 0.07 | 1.14 ± 0.09 |
| 4 min | 1.45 ± 0.22 | 1.01 ± 0.06 | 1.24 ± 0.13 |
| 6 min | 1.62 ± 0.26 | 0.96 ± 0.04 | 1.35 ± 0.17 |
| 10 min | 1.85 ± 0.51 | 0.99 ± 0.04 | 1.65 ± 0.38 |
Values are means ± SE. Untreated neurons, n = 5; S1PR1 siRNA treated, n = 11; Scramble, n = 7.
P < 0.05, repeated-measures ANOVA.
Chemicals
S1P was obtained from Avanti Polar Lipids (Alabaster, AL) and dissolved according to instructions provided by the supplier (www.avantilipids.com/SyntheticSphingosine-1-phosphate.asp). SEW2871 was purchased from Cayman Chemical (Ann Arbor, MI). The S1PR1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA); the actin antibody was from Thermo Scientific (Waltham, MA). Metafectene was purchased from Biontex-USA (San Diego, CA). Tissue culture supplies were purchased from Fisher (Pittsburgh, PA). All other chemicals were obtained from Sigma Chemical (St. Louis, MO). Capsaicin and SEW2871 were dissolved in 1-methyl-2-pyrrolidinone (HPLC grade). These stock solutions were diluted with Ringers solution to yield the appropriate concentration. We previously showed that the vehicle 1-methyl-2-pyrrolidinone has no effect on AP firing or the activation of either TTX-R INa or IK (Zhang et al. 2002).
RESULTS
S1P enhances the excitability of small diameter sensory neurons
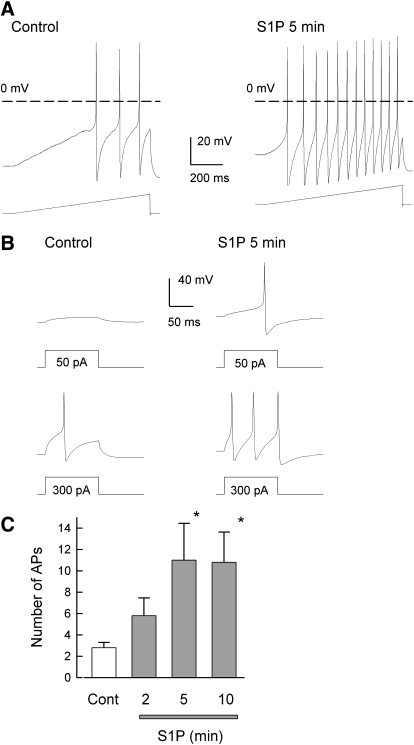
Exposure of small diameter (≤25 μm) sensory neurons to externally applied S1P produced a time-dependent increase in the number of APs evoked by a ramp of depolarizing current. A representative recording is shown in Fig. 1A, where under control conditions (left), this neuron had a resting membrane potential of −49 mV and the ramp elicited three APs. After a 5-min exposure to 1 μM S1P (right), the membrane depolarized to −42 mV and now the same ramp evoked 12 APs. Similar results were obtained in this same neuron when a series of current steps was used (see Fig. 1B). Under control conditions, a 50-pA step elicited a small depolarization without generating an AP, whereas a step to 300 pA was required to elicit one AP. However, after S1P, the 50- and 300-pA steps evoked one and three APs, respectively. The ability of S1P to increase the number of APs evoked by the ramp is summarized in Fig. 1C. Both 5- and 10-min exposures to S1P significantly increased the number of evoked APs by about fourfold. Thus these results indicate that S1P quickly augments the excitability of sensory neurons and are consistent with our previous observations (Zhang et al. 2006).
Fig. 1.
Sphingosine 1-phosphate (S1P) enhances the excitability of small diameter sensory neurons. A: representative voltage traces recorded under control conditions (left) and after a 5-min exposure to 1 μM S1P (right). Under control conditions, this small diameter sensory neuron had a resting membrane potential of −49 mV, and 3 action action potentials (APs) were evoked by the ramp of depolarizing current (450 pA, bottom trace). After S1P, the resting potential was depolarized to −42 mV and now the ramp evoked 12 APs. The dashed line indicates the 0 mV level. B: representative voltage recordings obtained from the same neuron as in A for steps of depolarizing current. Left: response to a 50- (top) and 300-pA (bottom) step of current. Right: response to the same current steps after a 5-min exposure to 1 μM S1P. C: the time-dependent increase in excitability produced by S1P in 5 sensory neurons for APs elicited by the ramps of depolarizing currents. The asterisks indicate a significant difference compared with the control condition with P < 0.05, RM ANOVA.
SEW2871 enhances the excitability of some, but not all, sensory neurons
We previously reported that both the isolated intact DRG and isolated sensory neurons maintained in culture expressed the mRNA for four of the five S1P receptor subtypes (S1PR1–4, but not S1P5; Zhang et al. 2006). These observations raise the question as to which receptor subtype(s) mediates the enhanced excitability produced by S1P. To address this question, sensory neurons were treated with SEW2871, a selective agonist for S1PR1. SEW2871 has no activity at S1PR2–5 (EC50 S1PR1 ∼13 nM; Jo et al. 2005; Sanna et al. 2004). As shown in Fig. 2, external exposure to 100 nM SEW2871 produced a rapid increase in the number of APs evoked by the ramp. The increased excitability produced by SEW2871 was associated with a significant time-dependent depolarization of the resting membrane potential (∼8 mV) and a reduction in the rheobase (∼50%); however, the firing threshold was unaffected (summarized in Table 1). These effects were similar to that observed for S1P. Despite the reduction in rheobase, SEW2871 did not significantly alter RTh (P = 0.22; Table 1), although there was a trend to increased values over time. Normalization of RTh to their respective control values indicate a trend toward increased RTh, for example, after a 10-min exposure, the value was 1.85 ± 0.51 compared with the controls. The lack of significance may result from the variance in these measurements. Interestingly, of the nine neurons treated with SEW2871, only five exhibited increased excitability; the other four remained unaffected by the S1PR1 agonist. These results suggest that S1P activation of S1PR1 is sufficient to produce sensitization and that about one half of the neurons are functionally sensitive to activation of S1PR1. However, this finding does not eliminate the possibility that other S1P receptors may contribute to the increased excitability.
Fig. 2.
The S1P receptor (S1PR1) selective agonist, SEW2871, increases the number of evoked APs in some, but not all, sensory neurons. In 5 of 9 neurons, 100 nM SEW2871 produced a significant increase in the number of APs evoked by the ramp of current, whereas in 4 of the 9, SEW2871 had no effect. We define sensitivity to SEW2871 as a >2-fold increase in the number of evoked APs after exposure. The asterisks indicate a significant difference compared with the control condition with P < 0.05, RM ANOVA.
siRNA targeted to S1PR1 blocks the sensitization produced by SEW2871 but not S1P
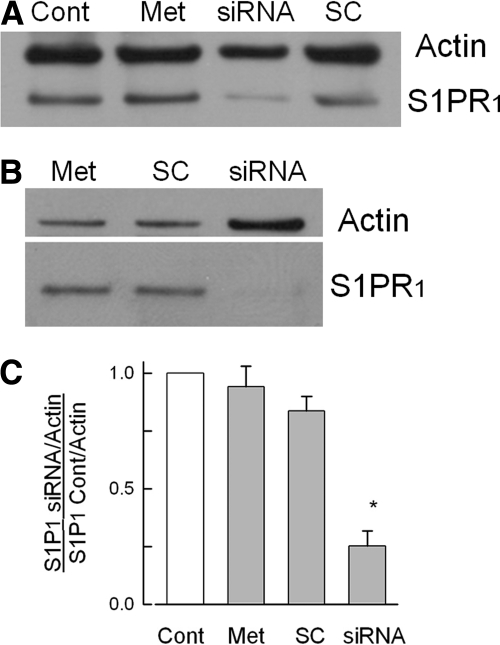
To further examine the role of S1PR1 in augmenting neuronal excitability, siRNA targeted to S1PR1 was designed and applied to the sensory neurons maintained in culture (see methods). Two representative Western blots are shown in Fig. 3. Figure 3A shows that 200 nM siRNA targeted to S1PR1 produced a dramatic reduction in the expression of S1PR1 compared with the untreated control neurons. Neither treatment with metafectene nor the scramble siRNA had any effect on the levels of S1PR1. Figure 3B shows another tissue harvest and siRNA treatment, wherein S1PR1 was greatly reduced even when the protein content for that particular lane was doubled as indicated by the larger actin band. The extent of knockdown for S1PR1 from five different tissue harvests (1 tissue harvest corresponds to 1 siRNA treatment) is summarized in Fig. 3C. The level of S1PR1 expression was reduced significantly compared with the untreated control levels (decreased by 75 ± 7%, n = 5, P < 0.05, ANOVA), whereas metafectene or scramble siRNA had no effect on S1PR1 expression (reductions of 6 ± 9 and 16 ± 6%, respectively).
Fig. 3.
siRNA targeted to S1PR1 greatly reduces the protein expression of this receptor. A: a representative Western blot where the expression of actin and S1PR1 were measured for 4 different conditions. The untreated control is indicated by the lane labeled Cont, exposure to only the transfecting detergent metafectene is labeled Met, treatment with 200 nM siRNA targeted to S1PR1 is labeled siRNA, and treatment with 200 nM scramble siRNA is labeled SC. The antibody for actin was used at a dilution of 1:1,000; the antibody to S1PR1 was used at a dilution of 1:200. B: another representative Western blot where the amount of protein added to the lane labeled siRNA was doubled for detection purposes. C: levels of expression of S1PR1 for the 4 treatment conditions obtained from 5 different tissue harvests: untreated control condition (Cont), exposure to only metafectene (Met), treatment with 200 nM scramble siRNA (SC), and treatment with 200 nM siRNA targeted to S1PR1 (siRNA). The densities for the protein bands for S1PR1 under these different treatment conditions have been normalized to their respective levels for actin densities, and all values have been normalized to their respective untreated controls. The asterisk indicates a significant difference compared with the control condition with P < 0.05, ANOVA.
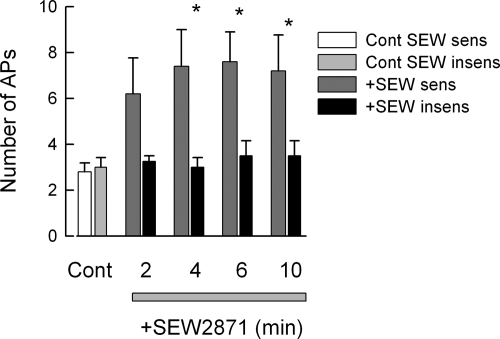
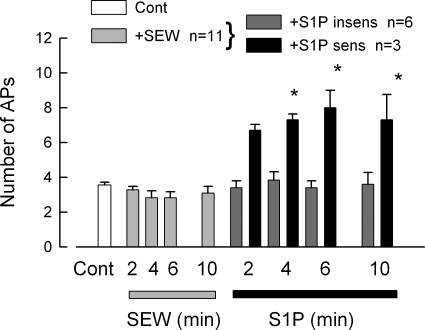
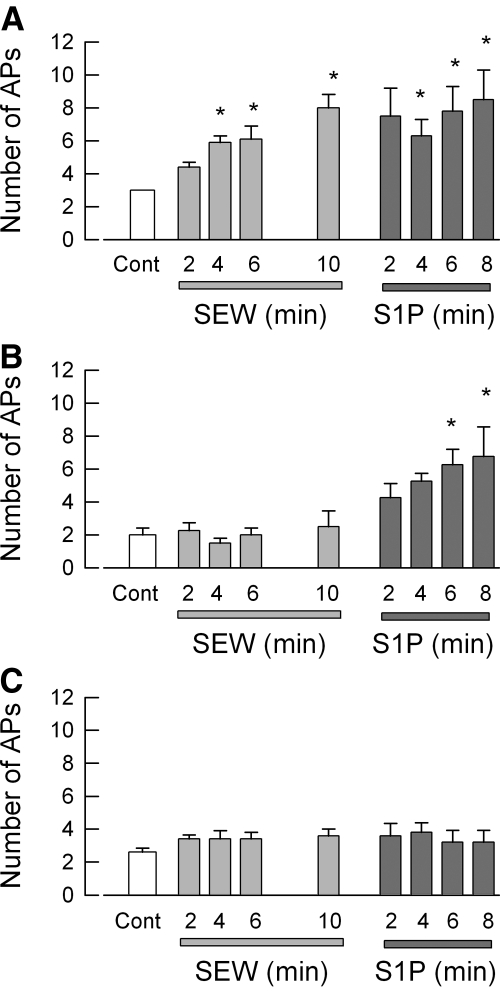
Having established that treatment with siRNA targeted to S1PR1 effectively reduced expression of this receptor, further studies determined the specific role of S1PR1 in the enhancement of excitability. Because the siRNA targeted to S1PR1 was labeled with the fluorescent tag DY547, only neurons that took up siRNA were used for electrophysiology experiments. siRNA-treated neurons were exposed sequentially to 100 nM SEW2871 for 10 min and then 1 μM S1P for 10 min. The results obtained from eleven sensory neurons are summarized in Fig. 4. After treatment with siRNA targeted to S1PR1, none of the 11 neurons exhibited increased AP firing during the 10-min exposure to the specific S1PR1 agonist, SEW2871. Additionally, after siRNA treatment, SEW2871 failed to depolarize the resting membrane potential and reduce the rheobase compared with the effects of SEW2871 on untreated control or the scramble siRNA condition (Table 1). The SEW2871-induced trend toward increased RTh over time that was exhibited for both untreated and scramble siRNA-treated sensory neurons was not observed after siRNA treatment, wherein the values remained quite similar to those obtained under control conditions (Table 1). These results indicate that the S1PR1 siRNA effectively blocked the response to SEW2871 or that none of these neurons expressed S1PR1 (however, see single-cell results described below). Additionally, 9 of these 11 neurons were exposed to S1P, wherein 6 were not sensitized but 3 neurons exhibited a significant two- to threefold increase in the number of evoked APs after S1P. Assuming that all nine neurons expressed S1PR1, which was functionally blocked by siRNA, and then the six that were not sensitized by S1P did not express other S1P receptors, whereas the three that were sensitized expressed additional S1P receptors. As a negative control, the effect of labeled scramble siRNA on the modulation of excitability by SEW2871 and S1P was examined. The results from 16 sensory neurons are summarized in Fig. 5. It appeared that there were three distinct populations of sensory neurons based on their sensitivities to these agents. Figure 5A shows that 7 of the 16 neurons exhibited a significant increase in AP firing after exposure to 100 nM SEW2871 and that exposure to S1P produced no additional increase in AP firing (P = 0.87 compared with the 10-min SEW2871 results). Figure 5B shows that 4 of the 16 neurons were not affected by SEW2871 but that S1P significantly increased AP firing after 6 and 8 min. Last, Fig. 5C indicates that 5 of the 16 neurons were not sensitized by either SEW2871 or S1P. Taken together, these results suggest that ∼50% of these neurons express S1PR1, 25% do not express S1PR1 but do express other functional S1P receptors, and ∼25% do not express S1P receptors.
Fig. 4.
siRNA targeted to S1PR1 blocks the sensitization to SEW2871, although S1P can still sensitize some neurons. After neurons were treated with 200 nM siRNA targeted to S1PR1 (fluorescently labeled) using our standard protocol, they were used for recordings. The 11 small diameter sensory neurons that were exposed to 100 nM SEW2871 failed to exhibit an increase in AP firing by the ramp over a 10-min period. Of the 11 neurons, 9 were exposed to 1 μM S1P for another 10 min. Six of the 9 neurons remained unaffected after exposure to S1P, whereas 3 of the 9 exhibited increased firing after S1P. The asterisks indicate a significant difference compared with the 10-min SEW2871 condition with P < 0.05, ANOVA.
Fig. 5.
In scramble siRNA-treated sensory neurons, SEW2871 and S1P have varying capacities to augment neuronal excitability. Sensory neurons were treated with 200 nM scramble siRNA that was fluorescently labeled using our standard protocol. Recordings were obtained from a total of 16 small diameter neurons during exposure to 100 nM SEW2871 for 10 min and then to 1 μM S1P for an additional 10 min. A: results obtained from 7 of the 16 neurons wherein SEW2871 significantly increased AP firing and that S1P produced no additional effect. B: results obtained from 4 of the 16 neurons wherein SEW2871 was ineffective, but S1P did produce a significant increase in AP firing. C: 5 of the 16 neurons were not affected by either SEW2871 or S1P. The asterisks indicate a significant difference compared with the control condition with P < 0.05, ANOVA.
Some, but not all, small diameter sensory neurons express the mRNA for S1PR1
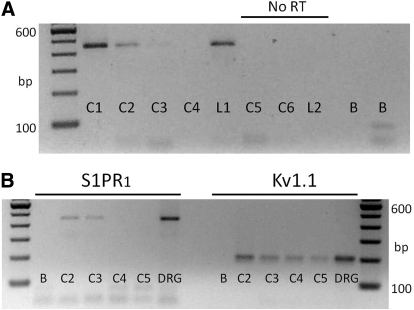
The sensitivities to SEW2871 and S1P would suggest that some, but not all, sensory neurons express S1PR1. To determine the frequency of S1PR1 expression, individual isolated small diameter sensory neurons were aspirated into a large diameter pipette (∼25 μm) from which the contents were deposited into an RT-PCR tube. The mRNA from individual cells was probed for S1PR1. Two representative gels are presented in Fig. 6. Figure 6A shows that the mRNA for S1PR1 was detected in two of four small diameter (<25 μm) sensory neurons (although there is rather faint band for C3). S1PR1 was also detected in a large diameter neuron (>50 μm diam). For three individual neurons (two small C5 and C6 and one large diameter L2), no amplicons were detected in the absence of RT (no RT). In addition, for two samples lacking template (labeled B, blank), no amplicons were detected. In another preparation, the cDNA products from individual neurons were split into two samples, wherein one (5-μl sample) was probed for S1PR1 and the other (2-μl sample) was probed for the potassium channel Kv1.1 as a positive control. As shown in Fig. 6B, two of the four neurons expressed the mRNA for S1PR1, whereas all four neurons expressed the mRNA for Kv1.1. Both amplicons were detected in mRNA isolated from intact DRGs, whereas no products were detected in no-template reactions (labeled B). A number of these single-cell RT-PCR reactions were run, wherein from 10 separate preparations, 46 small diameter neurons were processed for S1PR1; of those 46 neurons, 23 exhibited the mRNA for S1PR1. Consistent with the electrophysiological results described above, the single-cell RT-PCR studies indicate that ∼50% of the small diameter sensory neurons express S1PR1. These results indicate that individual sensory neurons can express multiple S1PRs that may have differing functional effects on the capacity of the cell to fire APs.
Fig. 6.
Single-cell RT-PCR analysis shows that some, but not all, small diameter sensory neurons express the mRNA for S1PR1. A: representative gel for the detection of S1PR1 in 5 individual sensory neurons. The lanes labeled C1–C6 (cell1–cell6) are the mRNAs from individual small diameter neurons, whereas those labeled L1–L2 (large cell1 and cell2) are mRNAs obtained from individual large diameter neurons. Lanes C1–L1 represent the detection of S1PR1 amplicons with a product size of 448 bp. Lanes C5–L2 underwent PCR in the absence of RT (No RT). Lanes labeled B (blank) represent reactions performed in the absence of any template. B: in a different tissue harvest than shown in A, the detection of S1PR1 and the potassium channel, Kv1.1 from the same 4 individual sensory neurons. For small diameter sensory neurons C2–C5, Kv1.1 amplicons were detected, whereas S1PR1 was detected in only 2 of the 4 neurons. The lanes labeled DRG were amplicons obtained from mRNA isolated from 10 ganglia.
DISCUSSION
We showed that S1P via activation of S1PR1 has the capacity to enhance the excitability of small diameter sensory neurons. This idea is supported by our findings that the S1PR1 selective agonist, SEW2871, increased the excitability in a manner that was very similar to S1P, and treatment with siRNA targeted to S1PR1 completely blocked the increase in AP firing produced by SEW2871. However, other observations indicate that S1PR1 is not the only S1PR capable of modulating neuronal excitability. This notion is consistent with the results obtained in untreated neurons where SEW2871 produced increased AP firing in ∼50% of the recorded neurons as well as in neurons treated siRNA targeted to S1PR1, where S1P increased firing in only ∼25% of the neurons. Furthermore, experiments involving scramble siRNA as a negative control showed that SEW2871, but not S1P, sensitized ∼50% of the neurons, whereas S1P but not SEW2871 sensitized ∼25% of the neurons. These studies also showed that ∼25% of the recorded neurons were insensitive to both SEW2871 and S1P. Previously, we detected the mRNA for S1PR1–4 in both the intact DRG and isolated sensory neurons maintained in culture (Zhang et al. 2006). In that study, internal perfusion with GDP-β-S blocked the sensitization produced by external S1P, showing that these effects were mediated by activation of a G protein-coupled receptor(s). The detection of mRNA expression for S1PRs other than S1PR1 suggests that S1PRs can have a significant impact on excitability. This will be an area for future study to establish the role of other S1PRs in regulating excitability. However, in contrast to S1PR1, establishing the specific role of other S1PRs will be more elusive because S1PR2–4 currently lack any selective agonists and receptor antagonists have overlapping specificities (e.g., see VPC44116 below) and in some cases act as partial agonists (Lynch and Macdonald 2008). Using the approach taken in these studies, siRNA targeted to individual S1PRs and their use in specific combinations may elucidate the role of each S1PR in regulating neuronal excitability.
There are relatively few studies that have examined the neurophysiological actions of S1P and the role of S1PRs in either the peripheral or central nervous systems (see recent review by Okada et al. 2009). Using S1P-induced 35S-GTPγS labeling as a measure of receptor localization, different brain regions have quite variable levels of expression (Sim-Selley et al. 2009; Waeber and Chiu 1999). For example, labeling in the cerebellum is high, whereas that in the thalamus is relatively low. Detecting S1PRs in the CNS raises interesting questions as to what is the origin of S1P and what role these sphingolipids may serve in neuronal function. An early study showed that depolarization by high potassium caused the release of S1P from rat cerebellar granule cells and that the transcripts for S1PR1–3 were detected in these neurons (Anelli et al. 2005). Similarly, in isolated rat hippocampal neurons, exposure to high potassium or S1P caused the release of glutamate as measured by either fluorescence detection or enhanced secretion as detected by FM4-64 loading of synaptic vesicles (Kajimoto et al. 2007). These authors also reported that both S1P and high potassium produced a rapid FRET response after transfection with tagged S1PR1 and β-arrestin. Interestingly, the high potassium effect was suppressed by pretreatment with dimethylsphingosine, which is an inhibitor of sphingosine kinase, whereas the S1P response was not altered. Such observations may reflect the capacity of S1P to serve as an intracellular second messenger or as an extracellular primary messenger. This dual character has been referred to as the inside-outside nature of S1P signaling (Hla et al. 1999; Takabe et al. 2008). In these hippocampal cells, siRNA targeted to S1PR1 or S1PR3 partially suppressed the effects of S1P on secretion; however, both siRNAs together produced complete inhibition. These results suggest that depolarization, via an undefined mechanism, results in the production of S1P via activation of sphingosine kinase, and somehow causes the release of glutamate. Subsequently, this S1P could be released from the neuron wherein autocrine and/or paracrine activation of S1PR1/S1PR3 can also release glutamate from hippocampal neurons. In a recent contrasting study, exposure to S1P or SEW2871 decreased the frequency (by ∼27%) as well as the amplitude (by ∼13%) of spontaneous glutamate-mediated excitatory postsynaptic currents (EPSCs) recorded from isolated and cultured cortical pyramidal neurons (Sim-Selley et al. 2009). The inhibitory actions of S1P were partially reversed by pretreatment with VPC44116, which is an S1PR1/S1PR3 antagonist as well as a partial agonist at S1PR4/S1PR5, suggesting that both receptors may be involved. It is curious that in hippocampal neurons S1P promotes glutamate release whereas in cortical pyramidal neurons S1P appears to suppress glutamate release. The physiological significance of these different observations are yet to be fully understood and may reflect the varied nature of S1P signaling in the brain.
Our findings in isolated small diameter sensory neurons suggest that elevated levels of S1P associated with peripheral inflammatory conditions can result in the increased sensitivity of these neurons through the modulation of G protein–coupled signaling cascades. The idea that peripheral S1P can be proinflammatory is supported by the observation that injection of S1P into the paw of a rat produced a dose-dependent edema that was associated with a significant infiltration of eosinophils (Roviezzo et al. 2004). Synovial tissue isolated from patients with rheumatoid arthritis exhibited increased expression S1PR1 compared with those with osteoarthritis. In addition, treatment with S1P significantly increased the proliferation of synoviocytes isolated from patients with rheumatoid arthritis and human MH7A cells, which is a cell line established from intraarticular tissues of the knee joint from rheumatoid arthritis patients (Kitano et al. 2006).
In contrast to the peripheral actions of S1P, two recent studies examined the central actions of S1P on nociceptive behavioral responses wherein S1P seems to diminish the sensitivity of neurons. In contrast to peripheral inflammatory conditions that are associated with increased levels of S1P, in either zymosan- or formalin-induced animal models of inflammatory pain the levels of cerebrospinal S1P were reduced (Coste et al. 2008). Intrathecal injection of S1P transiently reduced the secondary phase of nociceptive behaviors (i.e., number of flinches) produced by injection of formalin into the rat's paw. It is difficult to know which S1PR mediated these antinociceptive effects as their RT-PCR measurements from adult rat spinal cord indicated all five S1PRs were expressed. However, these authors showed that S1P reduced the forskolin-stimulated increase in cyclic AMP levels and this reduction was prevented by pertussis toxin; these results suggest that the actions of S1P were mediated by the inhibitory G protein, Gi. In another study, similar observations were obtained wherein 20 min after intracerebroventricular injection of S1P, the latency of the tail-flick in response to a thermal stimulus was significantly increased, indicating that S1P was antinociceptive (Sim-Selley et al. 2009). Interestingly, injection of SEW2871 was only ∼50% as effective as S1P and these effects were not reversed by pretreatment with VPC44116. Both results suggested that S1PRs other than S1PR1 were involved in this response. It is difficult to assess the specificity of the response to S1P since intracerebroventricular injection also elicited catalepsy, hypothermia, and locomotor inhibition.
In conclusion, these results suggest that enhancement of sensory neuron excitability associated with peripheral inflammation can result through the activation of S1PR1, although other S1PRs likely have an important role in this sensitization. S1P may prove to be a significant inflammatory signaling mediator that communicates the status of the external environment between immuno-competent cells and sensory neurons.
GRANTS
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant C06 RR015481-01 from the National Center for Research Resources. This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-046084 and R01 NS-060853.
REFERENCES
- Allende et al., 2004.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem 279: 15396–15401, 2004 [DOI] [PubMed] [Google Scholar]
- Anelli et al., 2005.Anelli V, Bassi R, Tettamanti G, Viani P, Riboni L. Extracellular release of newly synthesized sphingosine-1-phosphate by cerebellar granule cells and astrocytes. J Neurochem 92: 1204–1215, 2005 [DOI] [PubMed] [Google Scholar]
- Anliker and Chun, 2004.Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol 15: 457–465, 2004 [DOI] [PubMed] [Google Scholar]
- Brinkmann, 2009.Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 158: 1173–1182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann et al., 2002.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 277: 21453–21457, 2002 [DOI] [PubMed] [Google Scholar]
- Chi and Nicol, 2007.Chi XX, Nicol GD. Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J Neurophysiol 98: 2683–2692, 2007 [DOI] [PubMed] [Google Scholar]
- Chiba et al., 1998.Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats.I.FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J Immunol 160: 5037–5044, 1998 [PubMed] [Google Scholar]
- Cohen et al., 2010.Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, Pelletier J, Capra R, Gallo P, Izquierdo G, Tiel-Wilck K, de Vera A, Jin J, Stites T, Wu S, Aradhye S, Kappos L. TRANSFORMS Study Group. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 362: 402–415, 2010 [DOI] [PubMed] [Google Scholar]
- Coste et al., 2008.Coste O, Brenneis C, Linke B, Pierre S, Maeurer C, Becker W, Schmidt H, Gao W, Geisslinger G, Scholich K. Sphingosine 1-phosphate modulates spinal nociceptive processing. J Biol Chem 283: 32442–32451, 2008 [DOI] [PubMed] [Google Scholar]
- DeLeo and Yezierski, 2001.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90: 1–6, 2001 [DOI] [PubMed] [Google Scholar]
- Goetzl and Rosen, 2004.Goetzl EJ, Rosen H. Regulation of immunity by lysosphingolipids and their G protein-coupled receptors. J Clin Invest 114: 1531–1537, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill et al., 1981.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high-resolution current recordings from cells and cell free membrane patches. Pfluegers 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- Hannun and Obeid, 2008.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- Hla, 2004.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol 15: 513–520, 2004 [DOI] [PubMed] [Google Scholar]
- Hla et al., 1999.Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol 58: 201–207, 1999 [DOI] [PubMed] [Google Scholar]
- Hla and Maciag, 1990.Hla T, Maciag T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J Biol Chem 265: 9308–9313, 1990 [PubMed] [Google Scholar]
- Holzer, 1991.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43: 143–201, 1991 [PubMed] [Google Scholar]
- Jo et al., 2005.Jo E, Sanna MG, Gonzalez-Cabrera PJ, Thangada S, Tigyi G, Osborne DA, Hla T, Parrill AL, Rosen H. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol 12: 703–715, 2005 [DOI] [PubMed] [Google Scholar]
- Kajimoto et al., 2007.Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol 27: 3429–3440, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos et al., 2010.Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P. FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362: 387–401, 2010 [DOI] [PubMed] [Google Scholar]
- Kitano et al., 2006.Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, Iwasaki T, Sano H, Saba JD, Tam YY. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum 54: 742–753, 2006 [DOI] [PubMed] [Google Scholar]
- Lindsay, 1988.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci 8: 2394–2405, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch and Macdonald, 2008.Lynch KR, Macdonald TL. Sphingosine 1-phosphate chemical biology. Biochim Biophys Acta 1781: 508–512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala et al., 2002.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296: 346–349, 2002 [DOI] [PubMed] [Google Scholar]
- Matloubian et al., 2004.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360, 2004 [DOI] [PubMed] [Google Scholar]
- Meyer zu Heringdorf and Jakobs, 2007.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta 1768: 923–940, 2007 [DOI] [PubMed] [Google Scholar]
- Miller et al., 2009.Miller RJ, Jung H, Bhangoo SK, White FA. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 194: 417–449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan and Watkins, 2009.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10: 23–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada et al., 2009.Okada T, Kajimoto T, Jahangeer S, Nakamura S. Sphingosine kinase/sphingosine 1-phosphate signalling in central nervous system. Cell Signal 21: 7–13, 2009 [DOI] [PubMed] [Google Scholar]
- Olivera and Rivera, 2005.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol 174: 1153–1158, 2005 [DOI] [PubMed] [Google Scholar]
- Pappu et al., 2007.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316: 295–298, 2007 [DOI] [PubMed] [Google Scholar]
- Petruska et al., 2000.Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol 84: 2365–2379, 2000 [DOI] [PubMed] [Google Scholar]
- Rivera et al., 2008.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8: 753–763, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen and Goetzl, 2005.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5: 560–570, 2005 [DOI] [PubMed] [Google Scholar]
- Rosen et al., 2009.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 78: 743–768, 2009 [DOI] [PubMed] [Google Scholar]
- Roviezzo et al., 2004.Roviezzo F, Del Galdo F, Abbate G, Bucci M, D'Agostino B, Antunes E, De Dominicis G, Parente L, Rossi F, Cirino G, De Palma R. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA 101: 11170–11175, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez and Hla, 2004.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem 92: 913–922, 2004 [DOI] [PubMed] [Google Scholar]
- Sanna et al., 2004.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1PR1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem 279: 13839–13848, 2004 [DOI] [PubMed] [Google Scholar]
- Scholz and Woolf, 2007.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10: 1361–1368, 2007 [DOI] [PubMed] [Google Scholar]
- Schwab et al., 2005.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309: 1735–1739, 2005 [DOI] [PubMed] [Google Scholar]
- Sim-Selley et al., 2009.Sim-Selley LJ, Goforth PB, Mba MU, Macdonald TL, Lynch KR, Milstien S, Spiegel S, Satin LS, Welch SP, Selley DE. Sphingosine-1-phosphate receptors mediate neuromodulatory functions in the CNS. J Neurochem 110: 1191–1202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel and Milstien, 2003.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4: 397–407, 2003 [DOI] [PubMed] [Google Scholar]
- Takabe et al., 2008.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60: 181–195, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacker et al., 2007.Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg 105: 838–847, 2007 [DOI] [PubMed] [Google Scholar]
- Van Brocklyn et al., 1998.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol 142: 229–240, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waeber and Chiu, 1999.Waeber C, Chiu ML. In vitro autoradiographic visualization of guanosine-5′-O-(3-[35S]thio)triphosphate binding stimulated by sphingosine 1-phosphate and lysophosphatidic acid. J Neurochem 73: 1212–1221, 1999 [DOI] [PubMed] [Google Scholar]
- Weigert et al., 2009.Weigert A, Weis N, Brüne B. Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 214: 748–760, 2009 [DOI] [PubMed] [Google Scholar]
- White et al., 2005.White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4: 834–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2006.Zhang YH, Fehrenbacher JC, Vasko MR, Nicol GD. Sphingosine-1-phosphate via activation of a G-protein-coupled receptor(s) enhances the excitability of rat sensory neurons. J Neurophysiol 96: 1042–1052, 2006 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2002.Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol 544: 385–402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]