Abstract
Dopamine (DA) receptors are the principal targets of drugs used in the treatment of schizophrenia. Among the five DA receptor subtypes, the D4 subtype is of particular interest because of the relatively high affinity of the atypical neuropleptic clozapine for D4 compared with D2 receptors. GABA-containing neurons in the thalamic reticular nucleus (TRN) and globus pallidus (GP) express D4 receptors. TRN neurons receive GABAergic afferents from globus pallidus (GP), substantia nigra pars reticulata (SNr), and basal forebrain as well as neighboring TRN neuron collaterals. In addition, TRN receives dopaminergic innervations from substantia nigra pars compacta (SNc); however, the role of D4 receptors in neuronal signaling at inhibitory synapses is unknown. Using whole cell recordings from in vitro pallido-thalamic slices, we demonstrate that DA selectively suppresses GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs) evoked by GP stimulation. The D2-like receptor (D2,3,4) agonist, quinpirole, and selective D4 receptor agonist, PD168077, mimicked the actions of DA. The suppressive actions of DA and its agonists were associated with alterations in paired pulse ratio and a decrease in the frequency of miniature IPSCs, suggesting a presynaptic site of action. GABAA receptor agonist, muscimol, induced postsynaptic currents in TRN neurons were unaltered by DA or quinpirole, consistent with the presynaptic site of action. Finally, DA agonists did not alter intra-TRN inhibitory signaling. Our data demonstrate that the activation of presynaptic D4 receptors regulates GABA release from GP efferents but not TRN collaterals. This novel and selective action of D4 receptor activation on GP-mediated inhibition may provide insight to potential functional significance of atypical antipsychotic agents. These findings suggest a potential heightened TRN neuron activity in certain neurological conditions, such as schizophrenia and attention deficit hyperactive disorders.
INTRODUCTION
The thalamus relays sensory and motor information to the cerebral cortex and receives strong modulatory input back from the cortex. Both thalamocortical and corticothalamic projection neurons send collaterals to ventral thalamic nuclei (Jones 1975). GABA-containing thalamic reticular nucleus (TRN) neurons provide a major source of inhibitory synaptic input to thalamocortical (TC) neurons (Huguenard and McCormick 2007; Pinault 2004; Schofield et al. 2009). By modulating the flow of information through the thalamus, TRN has been hypothesized to play a major role in the control of attention and sensory processing (Guillery et al. 1998; Mayo 2009; McAlonan et al. 2008; Rees 2009; Yu et al. 2009). The TRN is also a key player in various types of rhythmic activity associated with certain arousal mechanisms and epileptiform activities (Hughes et al. 2002; Huguenard and McCormick 2007; Huntsman et al. 1999; McCormick and Bal 1997; Sohal et al. 2000; Steriade 1992, 1997; Steriade et al. 1993; vonKrosigk et al. 1993).
Dopamine (DA) is a major neuromodulator in the brain, and its dysfunction has been implicated in multiple human neurological and psychiatric disorders (Di Chiara 2002; Takahashi et al. 2006). Within thalamic circuitry, DA-dependent actions are thought to play a potentially significant role in emotion, attention, cognition, and complex somatosensory and visual processing (Takahashi et al. 2006). Alterations in thalamic DA receptors are also implicated in various neurological and psychiatric disorders (Behrendt 2006; Buchsbaum et al. 2006; Di Chiara 2002; Kane et al. 2009; Takahashi et al. 2006; Yasuno et al. 2004). Anatomical studies have shown that TRN receives a dopaminergic innervation from the substantia nigra pars compacta (SNc) (Anaya-Martinez et al. 2006; Gandia et al. 1993; Garcia-Cabezas et al. 2007, 2009; Sanchez-Gonzalez et al. 2005) and expresses DA receptors (Khan et al. 1998; Mrzljak et al. 1996). In addition, TRN receives GABAergic projections from globus pallidus (GP), substantia nigra pars reticulata (SNr), and basal forebrain (Anaya-Martinez et al. 2006; Asanuma and Porter 1990; Asanuma 1994; Bickford et al. 1994; Gandia et al. 1993; Hazrati and Parent 1991; Pare et al. 1990). Inhibitory innervations within TRN arise from local collaterals (Deleuze and Huguenard 2006; Lam et al. 2006; Shu and McCormick 2002). GABAergic terminals within TRN are hypothesized to express D4 receptors (Ariano et al. 1997; Defagot et al. 1997; Mrzljak et al. 1996); however, the action of DA on GABAergic inhibitory signaling in TRN has not been explored. In this study, we found that DA, via presynaptic D4 receptors, selectively suppresses GABAA-receptor mediated inhibition arising from GP efferents without altering intra-TRN inhibitory signaling.
METHODS
The present study was performed on Sprague Dawley rats (postnatal age 11–20 days). All experimental procedures were carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Illinois Animal Care and Use committee. Animals were maintained with 12 h on-off light/dark schedule in a temperature-controlled environment, and food and water were provided ad libitum.
Brain slices containing thalamus and GP were prepared as previously described with modifications (Lee et al. 2007). Rats were deeply anesthetized with sodium pentobarbital (50 mg/kg) and decapitated. The brains were quickly removed and placed into chilled (4°C), oxygenated (5% CO2-95% O2) slicing medium containing (in mM) 2.5 KCl, 1.25 NaH2PO4, 10.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose. Brain slices (250–300 μm thick) were cut in the horizontal plane using a vibrating tissue slicer (Leica, Germany). The slices were transferred to a holding chamber containing oxygenated, physiological solution and incubated (32°C) for ≥1 h prior to recording. The physiological solution contained (in mM) 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose. The solution was continuously gassed with 95% O2-5% CO2 to a final pH of 7.4. Individual slices were transferred to a submersion type recording chamber maintained at 30 ± 1°C (mean ± SD) and continuously superfused (3 ml/min) with oxygenated solution.
Recording procedures
Whole cell recordings were obtained using a microscope equipped with differential interference contrast (DIC) optics (Axioskop 2FS, Carl Zeiss) similar to that previously used (Govindaiah and Cox 2006a,b). Specific thalamic nuclei were distinguished using a low-power objective, and a high-power water-immersion objective was used to identify individual neurons. Recording pipettes were pulled from borosilicate glass tubing and had tip resistances of 4–7 MΩ. For voltage clamp recordings, the pipette solution contained (in mM) 117.0 Cs-gluconate, 13.0 CsCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, and 0.4 Na-GTP. For current clamp experiments, the pipette solution contained (in mM) 117.0 K-gluconate, 13.0 KCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP, and 0.3% biocytin. The pH and osmolarity of internal solution were adjusted to 7.3 and ∼290 mosM, respectively. The internal solution resulted in a 10 mV junction potential that has been corrected in the voltage measures. After forming whole cell configuration, the recording was allowed to stabilize for ≥5 min prior to data acquisition. Inhibitory postsynaptic currents (IPSCs) were optimized by using the cesium (Cs+)-based internal solution and a 0 mV holding potential. All signals were obtained using a Multiclamp 700 amplifier (Molecular Devices, Foster City, CA). For current-clamp recordings, an active bridge circuit was continuously monitored and, if necessary, adjusted to balance the drop in potential produced by passing current through the recording electrode. Recordings included in this study had initial access resistances ranging from 5 to 12 MΩ and typically remained stable throughout the recording. Data were omitted from the analyses if initial access resistance changed by >20%. Only neurons that exhibited stable baseline with resting membrane potentials greater than −55 mV and overshooting action potentials were included for data analyses.
Stimulation procedures
IPSCs were evoked in TRN neurons by electrically stimulating GP (>0.5 mm lateral to TRN border, Fig. 1A) using monopolar electrode (200–700 μA, 50 μs duration). The stimulus intensity typically ranged from 200 to 400 μA, but on occasions, ≤700 μA was used if no response was observed at the lower intensities. The actions of the dopaminergic agonists were reversible, thereby suggesting a lack of damage to afferent fibers from the stimulus paradigm. IPSCs were pharmacologically isolated using N-methyl-d-aspartate (NMDA) and non-NMDA receptor antagonists, ±3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10 μM) and 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM), respectively. In some neurons, focal application of glutamate via pressure ejection was used to evoke IPSCs in TRN neurons by neighboring neurons.
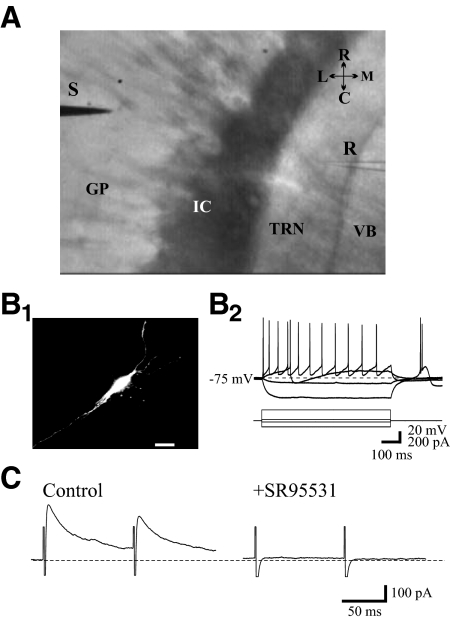
Fig. 1.
A: image of horizontal brain slice at the level of globus pallidus (GP) and thalamic reticular nucleus (TRN) illustrating electrode placement. IC, internal capsule; VB, ventrobasal; R, recording pipette, S, stimulation electrode. B1: photomicrograph of a representative TRN neuron. The morphology of neuron was recovered by including a fluorescent dye Alexa-594 in recording pipette. B2: characteristic responses of a TRN neuron to hyper- and depolarizing current steps. TRN neurons display characteristic low-threshold calcium spikes (LTS) in response to hyperpolarizing current steps. C: paired pulse stimulation of GP evokes inhibitory postsynaptic currents (IPSCs) recorded in TRN neuron. The IPSCs were isolated by using N-methyl-d-aspartate (NMDA) receptor antagonist ±3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10 μM) and non-NMDA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20 μM) and were blocked by GABAA receptor antagonist SR95531 (10 μM).
Drug administration
Concentrated stock solutions were originally prepared in appropriate solvents and diluted in physiological saline to final concentration just prior to use. Agonists were applied via a short bolus into an input line using a syringe pump (Govindaiah and Cox 2005). DA and SKF38393 were prepared fresh prior to application, and DA was prepared with 0.08% ascorbic acid to prevent oxidation. All antagonists were bath applied ≥5–10 min prior to subsequent agonist application. DA was purchased from Sigma (St. Louis, MO), whereas all remaining compounds were purchased from Tocris (Ellisville, MO).
Data acquisition and analyses
Quantification of evoked IPSC amplitude was performed on 5–10 consecutive responses in each experimental condition using pClamp software (Molecular Devices, Sunnyvale, CA). Miniature IPSCs (mIPSCs) were detected and analyzed using MiniAnalysis software (Synaptosoft, Leonia, NJ). The threshold for mIPSC detection was 10 pA and automatic detection was verified post hoc by visual analysis. The threshold for mIPSC detection was established from the baseline noise level recorded in the presence of GABAA receptor antagonist (SR95531, 10 μM) and glutamate receptor antagonists, CPP and DNQX. For quantification of mIPSCs, the average mIPSC frequency was calculated from 60-s time windows: 1 min prior to agonist application and 30 s following agonist application (Govindaiah and Cox 2006b). To analyze glutamate evoked IPSCs in TRN, the frequency and amplitude of IPSCs were quantified from 1-s windows for five consecutive sweeps for each condition. Due to lack of clear baseline in these cases, the events were manually detected and subsequently the frequency and amplitude of IPSCs quantified using Minianalysis software. Data are expressed as means ± SD unless otherwise noted. Most analyses consisted of Student's t-test, paired test if appropriate, unless otherwise noted. P values <0.05 were considered statistically significant.
Histology
To confirm that the recordings were obtained from TRN neurons, the morphology of recorded TRN neurons was recovered by filling neurons with a fluorescent dye (Alexa-594, 50 μM). Alexa-594 was included in the recording pipette and after recording, a z-stack of images was captured using custom-made two-photon laser scanning microscopy (Prairie Technologies, Middleton, WI) and collapsed using ImageJ software (National Institutes of Health).
RESULTS
The results presented in the present study were obtained from 80 TRN neurons. IPSCs in TRN neurons were evoked by GP stimulation (Fig. 1A) in the presence of NMDA and AMPA receptor glutamatergic antagonists, CPP (10 μM) and DNQX (20 μM), respectively. The eIPSCs were completely attenuated by the antagonist SR95531 (10 μM), indicating that these events are mediated by GABAA receptors (Fig. 1C).
DA suppresses GABAA receptor- mediated inhibition in TRN neurons
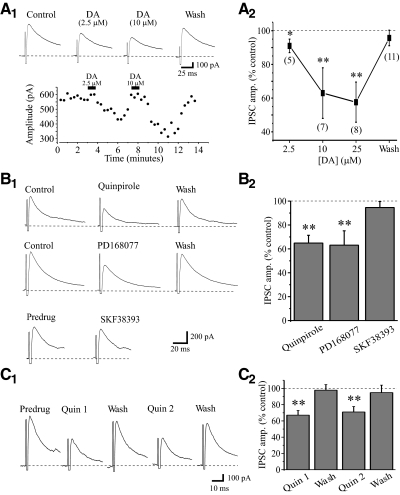
We initially tested the effects of DA on GABAA receptor- mediated IPSCs evoked by GP stimulation. Short-duration DA application (2.5–25 μM, 20–30 s) reversibly suppressed the eIPSCs amplitudes in a reversible manner (Fig. 2Ai). Overall DA produced a concentration-dependent suppression of the eIPSC amplitude ≤43 ± 11% with an IC50 of 5.1 μM (Boltzman fit). As illustrated in Fig. 2A2, DA reduced the eIPSC amplitude by 9 ± 4% (control: 496 ± 143 pA, DA: 455 ± 144 pA, n = 5, P < 0.001), 37 ± 14% (control: 481 ± 128 pA, DA: 306 ± 108 pA, n = 7, P < 0.0007), and 43 ± 11% (control: 469 ± 124 pA, DA: 275 ± 100 pA, n = 8, P < 0.0004) at 2.5, 10, and 25 μM, respectively. There was no significant differences between 10 and 25 μM DA.
Fig. 2.
Dopamine (DA) suppresses IPSCs in TRN neurons. A1: in a representative neuron, the IPSC is reversibly attenuated by short application of DA at different concentrations (2.5 and 10 μM) Top traces: representative IPSCs; bottom traces: the time course of DA-mediated actions. A2: population data indicating a significant concentration-dependent suppression of evoked IPSC (eIPSC) by DA. *P < 0.001, **P < 0.0001. The number of cells tested for each group is shown in parenthesis. B: D2-like receptor agonist quinpirole and D4 receptor agonist PD168077 mimic the actions of DA. B1: in a representative TRN neuron, quinpirole (10 μM) reversibly suppresses IPSC amplitude. In a different neuron, PD168077 (25 μM) also reversibly suppresses IPSC amplitude. B2: population data indicate a significant suppression of IPSC amplitude by quinpirole (n = 10) and PD168077 (n = 5). ** P < 0.0001.
Suppressive actions of DA are mediated via activation of D4 receptors
We next used selective receptor agonists and antagonists to determine the receptor subtype(s) mediating the DA effect. Similar to DA, the D2-like receptor (D2,3,4) agonist quinpirole (5–10 μM) and selective D4 receptor agonist PD168077 (25–50 μM) reversibly suppressed eIPSCs in TRN neurons (Fig. 2B). The suppressive action of quinpirole (10 μM) was reproducible to similar degree when applied at the interval of 5–10 min between each application (Fig. 2C). Short bath application (45 s) of quinpirole significantly reduced the eIPSC amplitude from 544 ± 245 to 347 ± 140 pA (35.1 ± 6.5%, n = 10, P < 0.0001, Fig. 2B). The magnitude of the quinpirole effects did not significantly differ from the DA-mediated effects (P > 0.2). The selective D4 receptor agonist PD168077 (25–50 μM) also suppressed the eIPSC by 37 ± 12% (control: 565 ± 179 pA, PD168077: 355 ± 122 pA, n = 5, P < 0.0001, Fig. 2B2). In contrast, the D1-like receptor agonist SKF38393 (10 μM) did not alter eIPSC amplitude in all cells tested (3.4 ± 5%, control: 506 ± 182 pA, SKF38393: 501 ± 174 pA, n = 4, P < 0.2, Fig. 2B). We further confirmed the suppressive affects of D2-like and selective D4 receptor agonists on eIPSCs using selective antagonists. As illustrated in Fig. 3A, quinpirole produced a significant reduction in the eIPSC amplitude from 366 ± 66 to 233 ± 33 pA (36.3 ± 5%, n = 5, P < 0.002), which recovered following washout (360 ± 70 pA). In the presence of D2-like receptor antagonist sulpiride (10 μM), subsequent quinpirole application significantly reduced eIPSC amplitude by 28.3 ± 8.8% (Fig. 3A, n = 5, P < 0.02). The quinpirole-mediated suppression of the eIPSC amplitude did not significantly differ between control and sulpiride conditions (Fig. 3A, P = 0.2). Considering the repeated quinpirole applications in this experiment, in a different population of TRN cells, we made repeated quinpirole applications (10 μM, 10 min interval) in control conditions and found that the eIPSC amplitude was suppressed to a similar degree with each quinpirole application, suggesting no desensitization with repeated applications (n = 4, P = 0.27, Fig. 2C). Of the D2-like receptors (D2, D3, D4), sulpiride has a higher affinity for D2 and D3 receptors (Werner et al. 1996), suggesting the suppression may be mediated via D4 receptor activation.
Fig. 3.
Suppressive actions of DA on IPSCs mediated via D4 receptor activation. A1: representative synaptic responses (top) and time course (bottom) illustrating that quinpirole10 μM) suppresses the IPSC in a reversible manner. Following washout, the D2-like receptor antagonist sulpiride (10 μM) was bath applied, and the subsequent application of quinpirole still significantly attenuates IPSC amplitude. A2: population data illustrating the suppressive actions of quinpirole (**P < 0.002, n = 5), and this effect is not completely antagonized by sulpiride (*P < 0.02, n = 5). B1: in a different TRN neuron, PD168077 (25 μM) reversibly suppresses the IPSC similar to quinpirole. Following recovery, the selective D4 receptor antagonist L745870 (50 μM) was bath applied, and the subsequent application of PD168077 does not alter IPSC amplitude. B2: population data illustrating reversible suppressive actions of PD168077 (*P < 0.001, n = 4). In the presence of L745870, PD168077 no longer alters the IPSC (P = 0.1, n = 4). C1: L745870 also blocks the DA-mediated suppression of the IPSC. In different TRN neurons, DA strongly attenuates the IPSC. In the presence of L745870 (50 μM), the DA-mediated suppression is greatly reduced. C2: population data illustrating the suppressive actions of DA alone (**P < 0.007, n = 5) and its reduced action in presence of L745870 (*P < 0.03, n = 5).
To confirm the selective D4 receptor activation by agonist PD168077, we next tested the actions of PD168077 in the presence of selective D4 receptor antagonist L745870 (Patel et al. 1997). PD168077 (25 μM) reduced the eIPSC amplitude by 39.0 ± 13.1% in control conditions (Fig. 3B, control: 563 ± 207 pA, PD168077: 341 ± 137 pA, n = 4, P < 0.001). In the presence of L745870 (50 μM), the subsequent application of PD168077 did not alter the eIPSC amplitude (Fig. 3B, L7458706: 485 ± 260 pA, PD168077 + L7458706: 493 ± 254 pA, n = 4, P = 0.1). We further examined the sensitivity of the DA-mediated suppression of eIPSCs to the D4 receptor antagonist. In these neurons, DA (10 μM) significantly suppressed the eIPSC amplitude (Fig. 3C, control: 522 ± 127 pA, DA: 314 ± 191 pA, n = 5, P < 0.007). Following recovery, the D4 receptor antagonist L745870 (25 μM) was bath applied for 7–10 min. In the presence of L745870, the subsequent DA application did not alter the eIPSC amplitude significantly (Fig. 3C, control: 519 ± 126 pA, L745870 + DA: 483 ± 120 pA, n = 5, P < 0.03). Overall, these data indicate that activation of D4 receptors attenuates evoked inhibitory synaptic transmission arising from GP stimulation.
Presynaptic D4 receptors modulates GABAergic signaling in TRN
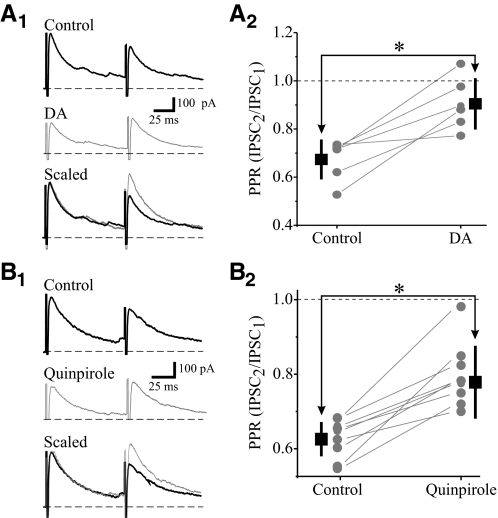
To examine whether the inhibitory actions of DA on IPSCs is mediated by a pre- or postsynaptic action, we studied the effect of DA and quinpirole on eIPSCs produced by paired pulse stimulation as well as their effect on miniature IPSCs (mIPSCs). Paired pulse stimulation within GP (75–125 ms interstimulus intervals, ISI) resulted in paired pulse depression of the eIPSCs in TRN neurons (Fig. 4). The paired pulse ratio (PPR: IPSC2/ IPSC1) was calculated prior to and after agonist application. DA (25 μM) attenuated the eIPSC and the PPR was significantly increased from 0.67 ± 0.08 to 0.91 ± 0.10 (Fig. 4A2; n = 6, P < 0.002), indicating a decrease in paired pulse depression. Similar to DA, quinpirole (10 μM) also significantly increased the PPR from 0.63 ± 0.04 to 0.79 ± 0.09 (Fig. 4B; n = 8, P < 0.002). The alteration in PPR is consistent with a presynaptic site of action such as a change in release properties (Zucker and Regehr 2002).
Fig. 4.
DA reduces paired pulse depression of IPSC in TRN neurons. A1: representative IPSCs recorded from TRN neuron using paired-pulse stimulation [100 ms interspike interval (ISI)] of GP. DA (25 μM) reduces the amplitude of the 1st IPSC (IPSC1) with little alteration in the 2nd IPSC (IPSC2), leading to an increase in paired pulse ratio (PPR). Scaling of the 1st IPSC clearly illustrates the reduction in paired pulse depression. A2: population data illustrate a significant increase in PPR by DA (*P < 0.002, n = 6), indicating a reduction in paired pulse depression. B1: in a different neuron, quinpirole (10 μM) produces a similar alteration in PPR as DA. B2: population data illustrate a significant increase in PPR by quinpirole (*P < 0.002, n = 8).
We next tested the effects of DA and dopaminergic agonists on mIPSC activity in TRN neurons. The mIPSCs were pharmacologically isolated using 1 μM TTX, 10 μM CPP, and 10 μM DNQX, and recorded using a holding potential of 0 mV. Under these conditions, quinpirole (10 μM, n = 9) and DA (25 μM, n = 3) reduced mIPSC frequency but not mIPSC amplitude. Cumulative probability analyses indicate that quinpirole significantly increased the interevent interval (Fig. 5, B and C without significant alterations in mIPSC amplitude (D and E). Overall quinpirole decreased the frequency of mIPSCs by 37.0 ± 12.5% (control: 1.0 ± 0.4 Hz, quinpirole: 0.6 ± 0.2 Hz, n = 9, P < 0.001, Fig. 5C). In contrast, quinpirole did not significantly alter mISPC amplitude (control: 15.6 ± 1.0 pA; quinpirole: 14.7 ± 3.2 pA, n = 9; P = 0.5, Fig. 5E). The selective alteration in mIPSC frequency, but not mIPSC amplitude, is consistent with a presynaptic site of action.
Fig. 5.
Activation of D2 receptors increases miniature IPSC (mIPSC) frequency but not mIPSC amplitude in TRN neurons. mIPSCs were recorded in presence of TTX (1 μM). A: representative current traces reveal mIPSCs in control and following quinpirole (10 μM) application. B: cumulative probability plots for neuron in A illustrating the increase in interevent intervals by quinpirole. C: population data revealing that quinpirole significantly reduces mIPSC frequency (*P < 0.001, n = 9). D: cumulative probability plot for neuron in A illustrating that mIPSC amplitude is unaltered by quinpirole. E: population data reveal quinpirole does not alter mIPSC amplitude (P = 0.5, n = 9).
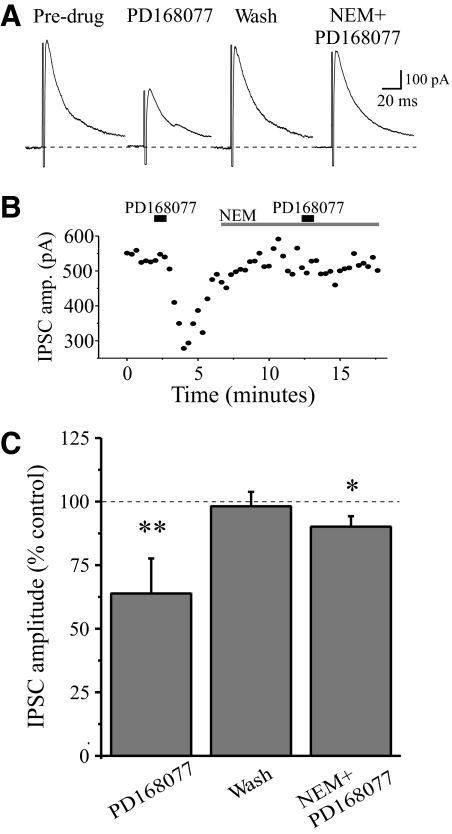
Previous studies have demonstrated that activation of D2-like receptors inhibits Ca2+ currents through a pertussis toxin (PTX)-sensitive G protein (Gi/o) (Yan et al. 1997). To determine whether the presynaptic D4 receptor-mediated effect involves this G protein subtype, we examined the effect of the sulfhydryl alkylating agent N-ethylmaleimide (NEM), which disrupts coupling of PTX-sensitive Gi/o type G proteins to Ca2+ channels (Shapiro et al. 1994). The suppressive action of D4 receptor agonist PD168077 on eIPSC was blocked by NEM (Fig. 6). In control conditions, PD168077 (25 μM) reduced the eIPSC amplitude by 36.2 ± 13.7% (n = 5, P < 0.01). Bath application of NEM (50 μM) alone had no effect on the evoked IPSCs. In the presence of NEM, PD168077 produced a smaller suppression of the eIPSC (9.9 ± 4.1%); this is significantly smaller than that in the absence of NEM (P < 0.02), indicating that NEM-sensitive Gi proteins are coupled to presynaptic D4 receptors.
Fig. 6.
The D4-mediated suppression of inhibition in TRN involves presynaptic inhibitory G proteins (Gi). A: in a representative TRN neuron, eIPSCs are reversibly attenuated by PD168077 (25 μM); however, the suppressive action of PD168077 is blocked in the presence of Gi-protein inhibitor N-ethylmaleimide (NEM) (50 μM). B: time course of the experiment illustrated in A. C: population data summarizing the effects of PD with and without NEM. n = 5, *P < 0.01, **P < 0.02.
As a measure of postsynaptic sensitivity, we tested if the DA and its agonists could alter GABAA receptor-mediated currents in TRN neurons. GABAA receptor-mediated currents were evoked by brief, focal application of the GABAA receptor agonist muscimol at 20-s intervals. Muscimol (50 μM, 0.5 s duration) elicited repeatable outward currents (Fig. 7A) that were completely attenuated by SR95531 (10 μM, not shown). The muscimol-induced currents were unaltered by 25 μM DA (Fig. 7B, control: 691 ± 167 pA, DA: 667 ± 170, n = 3; P = 0.2,) or 10 μM quinpirole (Fig. 7B, control: 766 ± 222, quinpirole: 729 ± 227, n = 6; P < 0.05). These data suggest that DA does not regulate postsynaptic GABAA receptors in TRN neurons. Overall these data indicate that the dopamine-mediated suppression of eIPSCs is due to a presynaptic action, thereby reducing GABA release from their terminals originating from GP.
Fig. 7.
DA does not alter postsynaptic GABAA receptor mediated currents in TRN neurons. A: current recording from a TRN neuron showing repeatable outward response to focal pressure application of GABAA receptor agonist muscimol (50 μM, 50 ms). Bath application of either DA (25 μM) or quinpirole (10 μM) did not alter the muscimol-mediated outward currents. Representative examples are shown below. B: population data illustrating amplitude of the muscimol-induced currents following DA (n = 3) or quinpirole (n = 6) exposure.
DA-mediated suppression of inhibition is restricted to GP-TRN pathway
Our results clearly demonstrate that the activation of DA D4 receptors suppress inhibitory synaptic transmission resulting from electrical stimulation of presumed GP efferents, but it is unclear if this is a general action affecting all inhibitory inputs onto TRN neurons. We next tested if DA could modulate intra-TRN inhibition considering these neurons form chemical synapses with each other (Deleuze and Huguenard 2006; Lam et al. 2006; Shu and McCormick 2002). To evoke intra-TRN inhibitory responses, we focally applied glutamate via pressure ejection within TRN near our recording. As shown in Fig. 8A, glutamate (500 μM, 50 ms) produced an inward current (likely via direct depolarization and/or electrical coupling) along with an increase in spontaneous IPSCs (sIPSCs). These sIPSCs were completely antagonized by a GABAA receptor antagonist SR95531 (Fig. 8A, SR95531). The glutamate-evoked IPSCs had an average frequency of 23.7 ± 11.4 Hz (Fig. 8B, n = 6). After obtaining a consistent IPSCs resulting from glutamate application, quinpirole (10 μM, 45–60 s) was applied via bolus. Quinpirole did not alter the frequency of glutamate-evoked sIPSCs (Fig. 8B, control: 25.8 ± 11.3 Hz, quinpirole: 24.3 ± 10.9 Hz, n = 5, P = 0.3). Similarly, D4 receptor agonist PD168077 (25–50 μM) did not alter sIPSC frequency (Fig. 8B, control: 23.3 ± 12.7 Hz, PD168077: 20.6 ± 11.5 Hz, n = 5, P = 0.1). Likewise sIPSC amplitudes were also unaltered by either quinpirole (control: 37.7 ± 11.8 pA, quinpirole: 39.7 ± 12.8 pA, n = 5, P = 0.9) or PD168077 (Fig. 8C, control: 35.9 ± 12.3 pA, PD168077: 33.9 ± 11.7 pA, n = 5, P = 0.4). The data suggests that the DA-mediated reduction in inhibitory synaptic transmission in TRN neurons is limited to the GP-TRN inhibition and not intra-TRN inhibition.
Fig. 8.
Activation of DA receptors does not alter intra-TRN inhibition. A: current recording from a TRN neuron reveals that focal glutamate application via pressure ejection within TRN (500 μM, 50 ms, 30s interval) elicits short outward current and long-lasting inward current along with increase in spontaneous IPSCs (sIPSCs, *). After repeating this several times, either quinpirole (10 μM) or PD168077 (50 μM) was bath applied (45 s). The sIPSCs evoked by glutamate application are not altered by the DA agonists. Subsequent application of GABAA receptor antagonist SR95531 (10 μM) completely blocked sIPSCs. B and C: population data indicating no significant change in sIPSC frequency (B) or sIPSC amplitude (C) by quinpirole or PD168077.
DISCUSSION
We demonstrate that the activation of presynaptic D4 receptors selectively suppresses GABAA receptor-mediated inhibition at pallido-thalamic innervation without altering intra-TRN inhibition (Fig. 7). The reduction in GP-thalamic inhibition is by reducing GABA release at presynaptic terminals of GP neurons via D4 receptor activation. Despite the recognized association of D4 receptors with schizophrenia, attention deficit hyperactivity disorder, and other mental disorders (Oak et al. 2000; Seeman et al. 1993), the cellular mechanisms by which D4 receptors modulate neuronal functions remain elusive. Anatomical studies have shown that D2-like dopamine receptors are expressed by GABAergic neurons including TRN and GP (Khan et al. 1998; Mrzljak et al. 1996); however, their functional role on inhibitory synaptic transmission has not been explored.
TRN receives GABAergic projections from GP, SNr, and basal forebrain (Anaya-Martinez et al. 2006; Asanuma 1994; Asanuma and Porter 1990; Bickford et al. 1994). In addition, TRN neurons form intra-TRN connections via axon collaterals (Huntsman et al. 1999; Shu and McCormick 2002). Although the precise origin of GABA terminals within the TRN containing the D4 receptors remains unclear, GP neurons have been shown to express D4 receptor and its mRNA (Ariano et al. 1997; Defagot et al. 1997; Mrzljak et al. 1996). Anatomical evidence suggests that TRN neurons receive dopaminergic innervations from substantia nigra pars compacta (Anaya-Martinez et al. 2006; Garcia-Cabezas et al. 2007, 2009; Sanchez-Gonzalez et al. 2005) and expresses DA receptors (Ariano et al. 1997; Defagot et al. 1997; Khan et al. 1998; Mrzljak et al. 1996). In this study, we have demonstrated a functional role of D4 receptors exclusively found on GABAergic neurons (Mrzljak et al. 1996). Our present findings are supported by a evidence suggesting that the activation of D4 receptors can modulate GABA release in TRN (Floran et al. 2004).
The dopaminergic system in TRN is thought to play crucial role in sensory gating and has been postulated that some of the manifestations of disorders of dopaminergic transmission may be caused by abnormal TRN function. For example, the TRN plays a central role in the control of attention (Guillery et al. 1998; Mayo 2009; McAlonan et al. 2008; Rees 2009; Yu et al. 2009), and attention deficit hyperactive disorder (ADHD) is associated with genetic abnormalities of dopamine D4 receptors (Castellanos and Tannock 2002; LaHoste et al. 1996). Moreover, TRN lies at the interface of thalamocortical circuits between prefrontal cortex and associated thalamic relay nuclei, thus alterations in TRN signaling will influence the gating properties between these two structures, which could underlie the hypothesized role of TRN in attention mechanisms (Zikopoulos and Barbas 2006). Additionally, abnormal dopaminergic function in the TRN may also contribute to some of the manifestations of Parkinson's disease, such as the sleep disorders (Rye and Jankovic 2002) and the abnormal processing of proprioceptive signals (Dietz 2002). By its connections with motor-related structures, TRN is thought to play integrative role in motor functions (Anaya-Martinez et al. 2006; Kane et al. 2009; Obeso et al. 2008; Piggott et al. 2007). Thus alterations in dopaminergic system in TRN may lead to abnormal motor functions found in Parkinson's disease. In fact, the D4 receptor-containing GABAergic neurons of the SNr and GP are thought to constitute major links in the basal ganglia loop circuits that regulate the motor thalamus and the cortex in sequence. We propose that activation of SNc neurons lads to release of DA in TRN, and this in turn activates D4 receptor on GP terminals leading to reduced GABA release. The reduced inhibition by activation of SNc neurons may lead to increased excitability of TRN neurons and decrease in output of thalamocortical neurons. Thus activation of D4 receptor can influence the thalamocortical circuit activity.
Thalamic inhibitory mechanisms have been shown to play crucial role in thalamocortical oscillations associated with arousal and sleep mechanisms (Huguenard and McCormick 2007; McCormick and Bal 1997; Sohal et al. 2000, 2003; Steriade 1992, 1997; vonKrosigk et al. 1993). Schizophrenic patients have been reported to show abnormalities in slow-wave sleep that are correlated with the state of spindle activity and synchronization of cortical and thalamic activity (Keshavan et al. 1995). Thus thalamic D2–like receptors are thought to play key roles in pathophysiology of schizophrenia (Buchsbaum et al. 2006). Elevated levels of D4 receptors have been reported in schizophrenics (Seeman et al. 1993). In addition, alterations in thalamic D2 receptors have been reported in schizophrenia (Takahashi et al. 2006). Clearly there are a number of different alterations in thalamocortical activities that may result in alterations in sensory processing and attentional modulation, the underlying mechanisms leading to such alterations and further connection to the manifestation of specific schizophrenic behaviors remains speculative (Behrendt 2006). Nonetheless malfunctioning of DA receptors in TRN is one mechanism that would lead to abnormalities in thalamocortical rhythms and thalamic gating that could contribute to some symptoms of schizophrenia. Additional studies are required to further unravel the functional significance of DA in the thalamus under normal and pathological conditions such as schizophrenia.
GRANTS
This work was supported by funding from the National Eye Institute to C. L. Cox and from the National Alliance for Research on Schizophrenia and Depression to G. Govindaiah.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank R. and L. Peterson (Sunshine from Darkness Gala) for their generous donation to the National Alliance for Research on Schizophrenia and Depression, which helped in carrying out this work.
REFERENCES
- Anaya-Martinez et al., 2006.Anaya-Martinez V, Martinez-Marcos A, Martinez-Fong D, Aceves J, Erlij D. Substantia nigra compacta neurons that innervate the reticular thalamic nucleus in the rat also project to striatum or globus pallidus: implications for abnormal motor behavior. Neuroscience 143: 477–486, 2006 [DOI] [PubMed] [Google Scholar]
- Ariano et al., 1997.Ariano MA, Wang J, Noblett KL, Larson ER, Sibley DR. Cellular distribution of the rat D-4 dopamine receptor protein in the CNS using anti-receptor antisera. Brain Res 752: 26–34, 1997 [DOI] [PubMed] [Google Scholar]
- Asanuma, 1994.Asanuma C. Gabaergic and pallidal terminals in the thalamic reticular nucleus of squirrel monkeys. Exp Brain Res 101: 439–451, 1994 [DOI] [PubMed] [Google Scholar]
- Asanuma and Porter, 1990.Asanuma C, Porter LL. Light and electron-microscopic evidence for a GABAergic projection from the caudal basal forebrain to the thalamic reticular nucleus in rats. J Comp Neurol 302: 159–172, 1990 [DOI] [PubMed] [Google Scholar]
- Behrendt, 2006.Behrendt RP. Dysregulation of thalamic sensory “transmission” in schizophrenia: neurochemical vulnerability to hallucinations. J Psychopharmacol 20: 356–372, 2006 [DOI] [PubMed] [Google Scholar]
- Bickford et al., 1994.Bickford ME, Gunluk AE, Vanhorn SC, Sherman SM. Gabaergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J Comp Neurol 348: 481–510, 1994 [DOI] [PubMed] [Google Scholar]
- Buchsbaum et al., 2006.Buchsbaum M, Christian B, Lehrer D, Narayanan T, Shi B, Mantil J, Kemether E, Oakes T, Mukherjee J. D2/D3 dopamine receptor binding with [F-18]fallypride in thalamus and cortex of patients with schizophrenia. Schizophrenia Res 85: 232–244, 2006 [DOI] [PubMed] [Google Scholar]
- Castellanos and Tannock, 2002.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci 3: 617–628, 2002 [DOI] [PubMed] [Google Scholar]
- Defagot et al., 1997.Defagot MC, Malchiodi EL, Villar MJ, Antonelli MC. Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Mol Brain Res 45: 1–12, 1997 [DOI] [PubMed] [Google Scholar]
- Deleuze and Huguenard, 2006.Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci 26: 8633–8645, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara, 2002.Di Chiara G.Dopamine in the CNS. Berlin: Springer, 2002 [Google Scholar]
- Dietz, 2002.Dietz V. Proprioception and locomotor disorders. Nat Rev Neurosci 3: 781–790, 2002 [DOI] [PubMed] [Google Scholar]
- Floran et al., 2004.Floran B, Floran L, Erlij D, Aceves J. Activation of dopamine D4 receptors modulates [H-3]GABA release in slices of the rat thalamic reticular nucleus. Neuropharmacology 46: 497–503, 2004 [DOI] [PubMed] [Google Scholar]
- Gandia et al., 1993.Gandia JA, Delasheras S, Garcia M, Gimenezamaya JM.Afferent projections to the reticular thalamic nucleus from the globus pallidus and the substantia nigra in the rat. Brain Res Bull 32: 351–358, 1993 [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas et al., 2009.Garcia-Cabezas MA, Martinez-Sanchez P, Sanchez-Gonzalez MA, Garzon M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex 19: 424–434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cabezas et al., 2007.Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 34: 965–984, 2007 [DOI] [PubMed] [Google Scholar]
- Glase et al., 1997.Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD. Substituted [(4-phenylpiperazinyl)methyl]benzamides: selective dopamine D-4 agonists. J Med Chem 40: 1771–1772, 1997 [DOI] [PubMed] [Google Scholar]
- Govindaiah and Cox, 2005.Govindaiah G, Cox CL. Excitatory actions of dopamine via D1-like receptors in the rat lateral geniculate nucleus. J Neurophysiol 94: 3708–3718, 2005 [DOI] [PubMed] [Google Scholar]
- Govindaiah and Cox, 2006a.Govindaiah G, Cox CL. Depression of retinogeniculate synaptic transmission by presynaptic D-2-like dopamine receptors in rat lateral geniculate nucleus. Eur J Neurosci 23: 423–434, 2006a [DOI] [PubMed] [Google Scholar]
- Govindaiah and Cox, 2006b.Govindaiah G, Cox CL. Metabotropic glutamate receptors differentially regulate GABAergic inhibition in thalamus. J Neurosci 26: 13443–13453, 2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery et al., 1998.Guillery RW, Feig SL, Lozsadi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci 21: 28–32, 1998 [DOI] [PubMed] [Google Scholar]
- Hazrati and Parent, 1991.Hazrati LN, Parent A. Projection from the external pallidum to the reticular thalamic nucleus in the squirrel monkey. Brain Res 550: 142–146, 1991 [DOI] [PubMed] [Google Scholar]
- Hughes et al., 2002.Hughes SW, Cope DW, Blethyn KL, Crunelli V. Cellular mechanisms of the slow (< 1 Hz) oscillation in thalamocortical neurons in vitro. Neuron 33: 947–958, 2002 [DOI] [PubMed] [Google Scholar]
- Huguenard and McCormick, 2007.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci 56, 2007 [DOI] [PubMed] [Google Scholar]
- Huntsman et al., 1999.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science 283: 541–543, 1999 [DOI] [PubMed] [Google Scholar]
- Jones, 1975.Jones EG. Some aspects of organization of thalamic reticular complex. J Comp Neurol 162: 285–308, 1975 [DOI] [PubMed] [Google Scholar]
- Kane et al., 2009.Kane A, Hutchison WD, Hodaie M, Lozano AM, Dostrovsky JO. Dopamine-dependent high-frequency oscillatory activity in thalamus and subthalamic nucleus of patients with Parkinson's disease. Neuroreport 20: 1549–1553, 2009 [DOI] [PubMed] [Google Scholar]
- Keshavan et al., 1995.Keshavan MS, Miewald J, Haas G, Sweeney J, Ganguli R, Reynolds CF. Slow-wave sleep and symptomatology in schizophrenia and related psychotic disorders. J Psychiatric Res 29: 303–314, 1995 [DOI] [PubMed] [Google Scholar]
- Khan et al., 1998.Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: An immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol 402: 353–371, 1998 [DOI] [PubMed] [Google Scholar]
- LaHoste et al., 1996.LaHoste GJ, Swanson JM, Wigal SB, Glabe C, Wigal T, King N, Kennedy JL. Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry 1: 121–124, 1996 [PubMed] [Google Scholar]
- Lam et al., 2006.Lam YW, Nelson CS, Sherman SM. Mapping of the functional interconnections between thalamic reticular neurons using photostimulation. J Neurophysiol 96: 2593–2600, 2006 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2007.Lee SH, Govindaiah G, Cox CL. Heterogeneity of firing properties among rat thalamic reticular nucleus neurons. J Physiol 582: 195–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo, 2009.Mayo JP. Intrathalamic mechanisms of visual attention. J Neurophysiol 101: 1123–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan et al., 2008.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature 456: 391–394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick and Bal, 1997.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci 20: 185–215, 1997 [DOI] [PubMed] [Google Scholar]
- Mrzljak et al., 1996.Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, GoldmanRakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381: 245–248, 1996 [DOI] [PubMed] [Google Scholar]
- Oak et al., 2000.Oak JN, Oldenhof J, Van Tol HHM. The dopamine D-4 receptor: one decade of research. Eur J Pharmacol 405: 303–327, 2000 [DOI] [PubMed] [Google Scholar]
- Obeso et al., 2008.Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Move Disorders 23: S548–S559, 2008 [DOI] [PubMed] [Google Scholar]
- Pare et al., 1990.Pare D, Hazrati LN, Parent A, Steriade M. Substantia-nigra pars reticulata projects to the reticular thalamic nucleus of the cat—a morphological and electrophysiological study. Brain Res 535: 139–146, 1990 [DOI] [PubMed] [Google Scholar]
- Patel et al., 1997.Patel S, Freedman S, Chapman KL, Emms F, Fletcher AE, Knowles M, Marwood R, Mcallister G, Myers J, Curtis N, Kulagowski JJ, Leeson PD, Ridgill M, Graham M, Matheson S, Rathbone D, Watt AP, Bristow LJ, Rupniak NM, Baskin E, Lynch JJ, Ragan CI. Biological profile of L-745,870, a selective antagonist with high affinity for the dopamine D4 receptor. J Pharmacol Exp Therap 283: 636–647, 1997 [PubMed] [Google Scholar]
- Phillipson et al., 1993.Phillipson OT, Cornwall J, Jones MW, Kilpatrick IC. Thalamic control of dopamine function—a role for the reticular nucleus. Biogenic Amines 9: 395–402, 1993 [Google Scholar]
- Piggott et al., 2007.Piggott MA, Ballard CG, Dickinson HO, McKeith IG, Perry RH, Perry EK. Thalamic D-2 receptors in dementia with Lewy bodies, Parkinson's disease, and Parkinson's disease dementia. Int J Neuropsychopharmacol 10: 231–244, 2007 [DOI] [PubMed] [Google Scholar]
- Pinault, 2004.Pinault D. The thalamic reticular nucleus: structure, function and concept. Brain Res Rev 46: 1–31, 2004 [DOI] [PubMed] [Google Scholar]
- Rees, 2009.Rees G. Visual attention: the thalamus at the centre? Curr Biol 19:R213–R214, 2009 [DOI] [PubMed] [Google Scholar]
- Rye and Jankovic, 2002.Rye DB, Jankovic J. Emerging views of dopamine in modulating sleep/wake state from an unlikely source: PD. Neurology 58: 341–346, 2002 [DOI] [PubMed] [Google Scholar]
- Sanchez-Gonzalez et al., 2005.Sanchez-Gonzalez MA, Garcia-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci 25: 6076–6083, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield et al., 2009.Schofield CM, Kleiman-Weiner M, Rudolph U, Huguenard JR. A gain in GABA(A) receptor synaptic strength in thalamus reduces oscillatory activity and absence seizures. Proc Natl Acad Sci USA 106: 7630–7635, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman et al., 1993.Seeman P, Guan HC, Vantol HHM. Dopamine D4 receptors elevated in schizophrenia. Nature 365: 441–445, 1993 [DOI] [PubMed] [Google Scholar]
- Shu and McCormick, 2002.Shu YS, McCormick DA. Inhibitory interactions between ferret thalamic reticular neurons. J Neurophysiol 87: 2571–2576, 2002 [DOI] [PubMed] [Google Scholar]
- Shapiro et al., 1994.Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci 14: 7109–7116, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman et al., 1998.Silverman BD, Pitman MC, Platt DE, Rigoutsos I. Molecular moment similarity between clozapine and substituted [(4-phenylpiperazinyl)-methyl] benzamides: selective dopamine D4 agonists. J Comput Aided Mol Design 12: 525–532, 1998 [DOI] [PubMed] [Google Scholar]
- Sohal et al., 2000.Sohal VS, Huntsman MM, Huguenard JR. Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J Neurosci 20: 1735–1745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal et al., 2003.Sohal VS, Keist R, Rudolph U, Huguenard JR. Dynamic GABA(A) receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci 23: 3649–3657, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade, 1992.Steriade M. Basic mechanisms of sleep generation. Neurology 42: 9–18, 1992 [PubMed] [Google Scholar]
- Steriade, 1997.Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 7: 583–604, 1997 [DOI] [PubMed] [Google Scholar]
- Steriade et al., 1993.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993 [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 2006.Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry 59: 919–928, 2006 [DOI] [PubMed] [Google Scholar]
- vonKrosigk et al., 1993.vonKrosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261: 361–364, 1993 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2002.Wang X, Zhong P, Yan Z. Dopamine D-4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci 22: 9185–9193, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner et al., 1996.Werner P, Hussy N, Buell G, Jones KA, North RA. D-2, D-3, and D-4 dopamine receptors couple to G protein-regulated potassium channels in Xenopus oocytes. Mol Pharmacol 49: 656–661, 1996 [PubMed] [Google Scholar]
- Yan et al., 1997.Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol 77: 1003–1015, 1997 [DOI] [PubMed] [Google Scholar]
- Yasuno et al., 2004.Yasuno F, Suhara T, Okubo Y, Sudo Y, Inoue M, Ichimiya T, Takano A, Nakayama K, Haildin C, Farde L. Low dopamine D-2 receptor binding in subregions of the thalamus in schizophrenia. Am J Psychiatry 161: 1016–1022, 2004 [DOI] [PubMed] [Google Scholar]
- Yu et al., 2009.Yu XJ, Xu XX, He SG, He JF. Change detection by thalamic reticular neurons. Nat Neurosci 12: 1165–1171, 2009 [DOI] [PubMed] [Google Scholar]
- Zikopoulos and Barbas, 2006.Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci 26: 7348–7361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker and Regehr, 2002.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 64: 355–405, 2002 [DOI] [PubMed] [Google Scholar]