Abstract
Recent studies have found that some forms of endocannabinoid-dependent synaptic plasticity in the hippocampus are mediated through activation of transient potential receptor vanilloid (TRPV) receptors instead of cannabinoid receptors CB1 or CB2. The potential role for synaptic localization of TRPV receptors during endocannabinoid modulation of nociceptive synapses was examined in the leech CNS where it is possible to record from the same pair of neurons from one preparation to the next. Long-term depression (LTD) in the monosynaptic connection between the nociceptive (N) sensory neuron and the longitudinal (L) motor neuron was found to be endocannabinoid-dependent given that this depression was blocked by RHC-80267, an inhibitor of DAG lipase that is required for 2-arachidonoyl glycerol (2AG) synthesis. Intracellular injection of a second DAG lipase inhibitor, tetrahyrdolipstatin (THL) was also able to block this endocannabinoid-dependent LTD (ecLTD) when injected postsynaptically but not presynaptically. N-to-L ecLTD was also inhibited by the TRPV1 antagonists capsazepine and SB 366791. Bath application of 2AG or the TRPV1 agonists capsaicin and resiniferatoxin mimicked LTD and both capsaicin- and 2AG-induced depression were blocked by capsazepine. In addition, pretreatment with 2AG or capsaicin occluded subsequent expression of LTD induced by repetitive activity. Presynaptic, but not postsynaptic, intracellular injection of capsazepine blocked both activity- and 2AG-induced ecLTD, suggesting that a presynaptic TRPV-like receptor in the leech mediated this form of synaptic plasticity. These findings potentially extend the role ecLTD to nociceptive synapses and suggest that invertebrate synapses, which are thought to lack CB1/CB2 receptor orthologues, utilize a TRPV-like protein as an endocannabinoid receptor.
INTRODUCTION
Endocannabinoids, such as anandamide and 2-arachidonoyl glycerol (2AG), mediate both short- and long-term forms of synaptic depression in the mammalian brain (Chevaleyre et al. 2006; Diana and Marty 2004; Gibson et al. 2008; Heifets and Castillo 2009) via activation CB1 receptors (Devane et al. 1988). Endocannabinoids are also known to bind to transient potential vanilloid (TRPV1) receptors (De Petrocellis et al. 2001, 2007), and these receptors have recently been found to mediate endocannabinoid-dependent long-term depression (ecLTD) in the hippocampus and superior colliculus (Di Marzo et al. 2001; Gibson et al. 2008; Maione et al. 2009; Toth et al. 2009). This TRPV-mediated ecLTD is thought to be the result of retrograde signaling of endocannabinoids onto presynaptic TRPV1 receptors (Gibson et al. 2008; Maione et al. 2009); however, no direct manipulation of presynaptic TRPV1 receptors during induction of ecLTD has been carried out. This is a critical element in understanding the cellular mechanisms of a potentially important and relatively novel form of neuroplasticity given that TRPV receptors are observed throughout the CNS and appear to have a variety of functional roles (see review by Kauer and Gibson 2009).
The potential contribution of a TRPV-like receptor during ecLTD was examined in an identified sensory-motor synapse in the leech in which it is possible to record from the same exact pair of synaptically-connected neurons throughout the study (Kristan et al. 2005; Muller and Scott 1981). The leech CNS utilizes the same endocannabinoids found in the vertebrate brain, including anandamide and 2AG (Salzet and Stefano 2002), and ecLTD has been observed in other synapses in the leech (Li and Burrell 2009). In addition, the TRPV1 receptor agonist capsaicin has been observed to activate nociceptive neurons (Pastor et al. 1996) and elicit nocifensive behaviors in the leech (Burrell, unpublished observation), suggesting the presence of a TRPV-like receptor in the leech CNS.
These studies were carried out in the monosynaptic connection between the nociceptive neurons and the longitudinal motor neuron (N-to-L synapse). Similar to mammals, the leech possesses three types of cutaneous mechanosensory neurons: low threshold touch (T), moderate threshold pressure (P), and high threshold nociceptive (N) neurons (Nicholls and Baylor 1968). All three mechanosensory cell types synapse onto the longitudinal motor neuron (L cell), which mediates symmetrical contraction of the leech such as during whole-body shortening (Nicholls and Purves 1970; Shaw and Kristan 1995). Low-frequency stimulation (LFS) of the touch mechanosensory neurons induced heterosynaptic LTD at the N-to-L synapse that was blocked by inhibitors of 2AG synthesis or by the TRPV1 receptor antagonists capsazepine and SB 366791. Exogenous application of 2AG mimicked ecLTD, and this 2AG-induced depression was also blocked by capsazepine. Presynaptic injection of capsazepine blocked ecLTD, whereas postsynaptic injection had no effect, indicating presynaptic localization of the putative TRPV-like receptor during ecLTD. These results demonstrate that repetitive activation of a nonnociceptive afferent can elicit persistent depression of a nociceptive synapse that is endocannabinoid-dependent and that this ecLTD may be mediated by an invertebrate TRPV-like receptor.
METHODS
Animal preparation
Leeches [Hirudo verbana (Siddall et al. 2007), 3 g] were obtained from a commercial supplier (Leeches USA, Westbury, NY and/or Niagara Medicinal Leeches, Cheyenne, WY) and maintained in artificial pond water (0.52 g/l H20 Hirudo salt) on a 12 h light/dark cycle at 18°C. Individual mid-body ganglia were dissected and placed in a recording chamber (2 ml) with constant perfusion (1.5 ml/min). Dissections and recordings were carried out in normal leech saline solution (containing, in mM: 114 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 5 NaOH, and 10 HEPES; pH = 7.4). For pharmacological experiments, drugs were dissolved in leech saline from frozen stock solutions. Final concentrations were made from stock solutions just prior to the individual experiments. The following drugs were obtained from Tocris (Ellisville, MO): capsazepine, 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-pyrazole-3-carboxamide (AM251), and 2-arachidonoyl glycerol (2AG), resiniferatoxin (RTX), and SB 366791. Drugs obtained from Sigma-Aldrich (St. Louis, MO) included: capsaicin, CNQX, Orlistat (tetrahyrdolipstatin, THL), and dimethyl sulfoxide (DMSO). RHC-80267 was purchased from Enzo (Plymouth Meeting, PA).
Electrophysiology
Current clamp (bridge balanced) intracellular recordings were made using sharp glass microelectrodes (35–40 MΩ) fabricated from borosilicate capillary tubing (1.0 mm OD, 0.75 mm ID; FHC, Bowdoinham, ME) using a horizontal puller (Sutter Instruments P-97; Novato, CA). Each microelectrode was filled with 3 M K+ acetate. Impalement of individual neurons was carried out using a manual micropositioner (Model 1480; Siskiyou, Grants Pass, OR). Current pulses were delivered to the electrodes using a multi-channel programmable stimulator (STG 1004; Multi-Channel Systems; Reutlingen, Germany). Signals were recorded using a bridge amplifier (BA-1S; NPI, Tamm, Germany) and then digitally converted (Digidata 1322A A/D converter) for observation and analysis (Axoscope; Molecular Devices, Sunnyvale, CA).
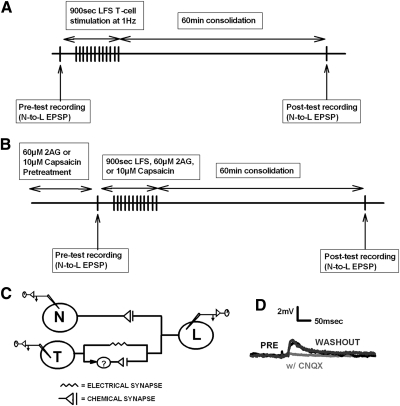
Touch (T), nociceptive (N), and longitudinal motor (L) cells were identified based on their size, position within the ganglion, and action potential shape. The T cells (3 bilateral pairs) and N cells (2 bilateral pairs) are located on the ventral side of the ganglion, while the L motor neurons (1 bilateral pairs) are found on the dorsal side. In these experiments, the ganglion was pinned dorsal side up in the recording chamber; this permits recordings of the L and the lateral most T and N cells. L cell identification was confirmed by recording from the electrically coupled contralateral homologue. In most experiments, LTD of the N-to-L synapse was induced by stimulating the T cell using a well-established low-frequency stimulation (LFS) protocol in which the presynaptic cell was stimulated 900 times at 1 Hz (Anwyl 2006). In some experiments, LTD was induced homosynaptically via LFS of the N cell. Pretest recordings of the N-to-L or T-to-L excitatory postsynaptic potentials (EPSPs) were made prior to LFS and a posttest recording was carried out 60 min after the LFS (Fig. 1A). It is not possible to continuously record from the L cell during the pre- and posttest time points because chronic recordings result in a progressive rundown of the EPSP, likely due to damage of the postsynaptic cell (Eliot et al. 1994). Therefore separate sharp electrode impalements were made for the pre- and posttest recordings. The neurons were impaled for pretest recordings and then withdrawn to preserve the cells. Impalement of the same T, N, and L cell were done for posttest recordings. Input resistance was recorded during the pre- and posttests, and only stable recordings were included in the data analysis. For all LFS experiments, mean pretest and posttest input resistance was 23 ± 1.3 and 23 ± 1.5 mΩ, respectively. The peak EPSP amplitude was determined by averaging of 5–10 separate EPSPs (recorded every 10 s). Drugs were applied via gravity-fed superfusion during the LFS or for 15 min when LFS was omitted. During occlusion experiments, capsaicin or 2AG was bath applied via perfusion for 15 min prior to the pretest recordings of the N-to-L EPSP. The synapses then underwent LFS or a second drug application (2AG or capsaicin) and then re-tested 60 min later (Fig. 1B). Capsazepine (500 μM) iontophoresis was applied for 5 min after the initial pretest recording. Injection of the drug was either applied on the presynaptic N cell or postsynaptic L cell, depending on the particular experiment. After capsazepine injection, LFS was induced (controls had no stimulation) followed by a 60 min consolidation period. A final posttest recording was taken between the N-to-L synapse.
Fig. 1.
Experimental protocols and synaptic circuitry. A: a pretest recording of the N-to-L or T-to-L synapse was made prior to low-frequency stimulation (LFS; 900 s, 1 Hz) of the T cell [or N cell if homosynaptic long-term depression (LTD) was being elicited]. In some experiments, the LFS was replaced by superfusion of 2-arachidonoyl glycerol (2AG), capsaicin, or resiniferatoxin for 900 s. Following a 60 min consolidation period, a posttest recording of the N-to-L or T-to-L synapse was carried out. B: during occlusion experiments, pretreatment with either 60 μM 2AG or 10 μM capsaicin was carried out prior to the pretest recordings of the N-to-L synapse. This was followed by LFS, 2AG (60 μM), or capsaicin (10 μM), depending on the experiment. Following a 60 min consolidation period, the posttest recordings of the N-to-L synapse were carried out. C: the nociceptive (N cell) sensory neuron has a monosynaptic chemical connection onto the longitudinal (L) motor neuron. The touch (T cell) sensory neuron has a monosynaptic electrical synapse and a polysynaptic chemical connection onto the L motor neuron; the interneuron(s) mediating the polysynaptic connection is unknown (?). D: 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) abolished the N-to-L excitatory postsynaptic potential (EPSP), indicating a glutamatergic synapse. Representative traces of the N-to-L synapse in normal saline (black line), during application of 20 μM of CNQX (gray line) and after 30 min washout in normal saline (dark gray line). Similar results were observed in CNQX experiments performed on the T-to-L synapse (data not shown) indicating that the polysynaptic chemical component of this circuit is glutamatergic.
Statistics
EPSP amplitude measurements were normalized based on their initial values and presented as means ± SE [ = 100 · (posttest/pretest)]. Statistical analyses to determine main effects were performed using a one-way ANOVA and Newman-Keuls post hoc tests with Statistica analysis software (Statsoft). All significance was at an alpha level of at least P < 0.05.
Coefficient of variation analyses were done to determine pre- or postsynaptic mechanisms (Faber and Korn 1991). The inverse square of the coefficient of variation (CV-2) is proportional to the probability of release determined by
, where SD = standard deviation of the raw pre-or post − test EPSP amplitudes and ¯X the mean of the individual raw EPSP amplitudes of the pretest or posttest
The inverse CV2 was normalized between the posttest and pretest, which was compared with the normalized change in mean pre- and posttest EPSP amplitudes and graphed (Sjostrom et al. 2003).
RESULTS
Homosynaptic and heterosynaptic LTD at the N-to-L synapse
The N-to-L connection is mediated by a monosynaptic, chemical synapse (Nicholls and Purves 1970) (Fig. 1C). To determine whether the N-to-L synapse is glutamatergic, ganglia were treated with CNQX (20 μM). In three of three synapses tested, CNQX reduced the N-to-L EPSP amplitude by 99.8 ± 0.1%, effectively eliminating synaptic transmission with synaptic signaling returning to pre-CNQX levels after 30 min of washout (Fig. 1D). The T-to-L synapse consists of both an electrical component and a polysynaptic chemical component (Fig. 1C). Treatment with CNQX also indicated that the T-to-L chemical synapse is glutamatergic (data not shown), consistent with experiments from other synapses in which the T cell is the presynaptic neuron (Li and Burrell 2008).
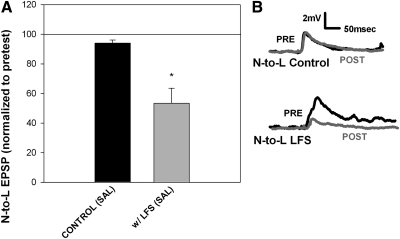
Low-frequency stimulation (1 Hz for 900 s) has previously been shown to induce a LTD in leech synapses (Li and Burrell 2008, 2009). LFS of the N cell induced a homosynaptic LTD in the N-to-L synapse (Supplemental Fig. S1A),1 whereas LFS of the T cell induced homosynaptic LTD in the T-to-L synapse (Supplemental Fig. S1B). Interestingly, LFS of the T cell also induced heterosynaptic LTD in the inactive N-to-L pathway (Fig. 2A). This finding is significant given that stimulation of non-nociceptive sensory neurons is known to attenuate nociceptive signaling, a phenomenon often referred to as the gate control (Melzack and Wall 1965). This theory proposes a convergence of nociceptive and non-nociceptive neurons onto common postsynaptic targets in the spinal cord, an arrangement that is also observed in the leech CNS (Fig. 1C). Normally, gate control only lasts for the duration of the nonnociceptive stimulation, but in the present set of experiments, depression of the N-to-L EPSP persisted ≥1 h after T cell stimulation has ceased. No changes in EPSP amplitude were observed in control experiments in which the T-to-L and N-to-L synapses were tested, but no LFS was applied (Fig. 2 and Supplementary Fig. S1B). These results indicate that repetitive activity in the T-to-L pathway can modify synaptic transmission in the non-activated nociceptive pathway that shares the same postsynaptic target. This modulation of a nociceptive synapse by a non-nociceptive pathway was examined in more detail to better understand this important mechanism for modifying nociceptive signaling.
Fig. 2.
Heterosynaptic LTD at the N-to-L synapse. A: bar graph showing that 15 min of LFS (1 Hz) of the T cell synapse produced a heterosynaptic LTD in the N-to-L synapse (n = 6) compared with controls tested without LFS (n = 9). Data were analyzed using a 1-way ANOVA [F(1,13) = 10.18; P < 0.01] with a post hoc Newman-Keuls test (*P < 0.01). B: sample traces showing no change in N-to-L EPSP amplitude in the control experiments (top) and a decrease in EPSP amplitude in synapses following T cell LFS (bottom). Pretest traces are indicated by a black line and posttest traces are represented by a gray line.
N-to-L heterosynaptic LTD is blocked by inhibitors of 2AG synthesis
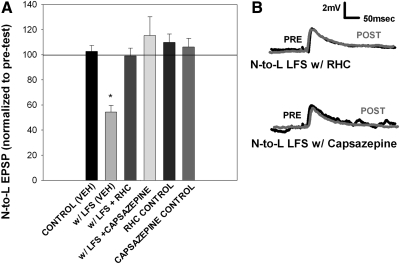
In an earlier study, 900 s LFS of the T cell induced ecLTD in the T-to-S synapse (Li and Burrell 2009). This ecLTD was mediated by 2AG, which is the most abundant endocannabinoid in the CNS in both vertebrates and the leech (Matias et al. 2001; Stella et al. 1997). Diacylglycerol (DAG) lipase is a necessary enzyme for the synthesis of 2AG (Bisogno et al. 2003) and is known to be present in invertebrates (Adams et al. 2000; Bisogno et al. 2003; Elphick and Egertova 2005; Leung et al. 2008). Therefore to determine whether homosynaptic LTD in the T-to-L and heterosynaptic LTD in the N-to-L synapses are also endocannabinoid-dependent, T cell LFS was carried out in the presence of RHC-80267 (100 μM; RHC), an inhibitor of DAG lipase. When applied during LFS of the T cell, RHC prevented both heterosynaptic LTD in the N-to-L synapse and homosynaptic LTD of the T-to-L synapse (Fig. 3; Supplementary Fig. S2), consistent with earlier findings in the T-to-S synapse (Li and Burrell 2009). Application of RHC alone did not induce any significant change in either the T-to-L or N-to-L synapses.
Fig. 3.
Heterosynaptic LTD is mediated by endocannabinoids and a transient potential receptor vanilloid (TRPV)-like receptor. A: bar graph showing the inhibition of heterosynaptic LTD through application of RHC-80267, a 2AG synthesis blocker, and capsazepine, a selective antagonist of TRPV1 receptors. Data were analyzed through a 1-way ANOVA [F(5,20) = 13.39; P < 0.01]. Bath application of 60 μM RHC (n = 4) or 10 μM capsazepine (n = 4) significantly blocked the heterosynaptic LTD observed with vehicle LFS [w/LFS (VEH)]. Post hoc Newman-Keuls tests detected a significant difference between veh LFS vs. veh control (*P < 0.05), vehicle LFS vs. LFS with RHC (*P < 0.05), vehicle LFS vs. LFS with capsazepine (*P < 0.05). There was no change in EPSP amplitude with RHC application alone (n = 3) or capsazepine application alone (n = 3), indicating that the drug itself did not have an effect. B, top: traces of the N-to-L synapse with RHC during LFS. Pretest traces (black line) show no changes compared with posttest traces (gray line) taken after stimulation. Bottom: traces of the N-to-L synapse with capsazepine during LFS. Pretest traces (black line) show no changes compared with posttest traces (gray line), indicating that capsazepine blocked heterosynaptic LTD.
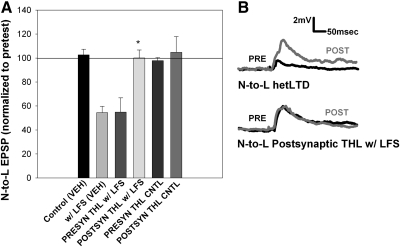
To provide additional support that the N-to-L synaptic depression was 2AG-dependent, LFS-induced depression was carried out in the presence of tetrahydrolipstatin (THL) a DAG lipase inhibitor that exhibits greater specificity than RHC (Min et al. 2010; Ortar et al. 2008). Injection of THL (10 μM) into the presynaptic nociceptive neuron did not inhibit LFS-induced heterosynaptic LTD (Fig. 4A). However, THL injection into the postsynaptic L motor neuron blocked the LTD normally observed following LFS (Fig. 4, A and B). Control studies of both pre- and postsynaptic THL injections without LFS did not affect the N-to-L synapse. Together the results from the RHC and THL experiments support a role for 2AG during LTD in the N-to-L synapse and the THL experiments are consistent with numerous ecLTD studies in which synaptic depression is mediated by endocannabinoids synthesized in the postsynaptic neuron (Chevaleyre et al. 2006).
Fig. 4.
LFS-induced LTD is mediated by postsynaptic endocannabinoid synthesis. A: bar graph showing tetrahyrdolipstatin (THL) iontophoresis in the pre- or postsynaptic neuron. Data were analyzed through a 1-way ANOVA [F(3,21) = 7.09; P < 0.01]. Newman-Keuls post hoc analyses indicated that iontophoretic injection of 10 μM THL into the postsynaptic L motor neuron (POSTSYN THL w/ LFS; n = 5) significantly blocked LFS-induced LTD compared with both presynaptic THL injection (PRESYN THL w/ LFS; n = 5; *P < 0.05) and LFS-induced LTD in saline [w/ LFS (VEH); *P < 0.05]. B, top: traces of the N-to-L synapse in saline during LFS. Posttest traces (gray lines) showing a significant depression in EPSP amplitude after LFS in saline compared with pretest traces (black lines). Bottom: traces of the N-to-L synapse after postsynaptic THL injection and LFS. Posttest traces (gray lines) show no change from pretest traces (black lines) after THL iontophoresis, even with LFS.
N-to-L ecLTD is blocked by TRPV1 antagonists and mimicked by TRPV1 agonists
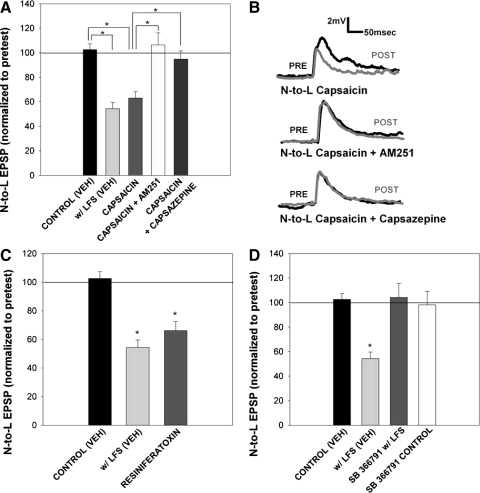
To test whether endocannabinoid-dependent LTD requires activation of TRPV-like receptors, capsazepine, a selective antagonist of the TRPV1 receptor (Bevan et al. 1992), was applied during LFS. Capsazepine (10 μM) blocked heterosynaptic LTD at the N-to-L synapse (Fig. 3, A and B) and homosynaptic LTD in the T-to-L synapse (Supplementary Fig. S2). Capsazepine alone did not have any effect on either N-to-L or T-to-L synaptic transmission. To test whether direct activation of TRPV-like receptors can elicit LTD, capsaicin, a TRPV1 receptor agonist (Jung et al. 1999) was applied in place of LFS. Application of 10 μM capsaicin for 900 s (identical to the LFS duration) induced significant depression of the N-to-L EPSP (Fig. 5A). Co-application of capsazepine blocked this capsaicin-induced depression (Fig. 5A).
Fig. 5.
Capsaicin and resiniferatoxin mimicked LFS-induced LTD and was blocked by inhibitors of endocannabinoid and TRPV1 receptors. A: bar graph comparing the effects on synaptic transmission following treatment with either vehicle [control (VEH)], LFS in vehicle [w/LFS (VEH)], capsaicin alone (capsaicin, n = 12), capsaicin with 10 μM AM251 (capsaicin + AM251, n = 4), and capsaicin with 10 μM capsazepine (capsaicin + capsazepine, n = 5). One-way ANOVA [F(4,29) = 13.16; P < 0.01] detected a significant effect of treatment group. Newman-Keuls post hoc analysis detected a significant difference between the control (VEH) and w/LFS (VEH) groups (P < 0.05*) and between the capsaicin and control (VEH; P < 0.05*), capsaicin + AM251 (P < 0.05*), and capsaicin + capsazepine (P < 0.05*) groups. B, top: traces of the N-to-L synapse in the presence of 10 μM capsaicin. Baseline pretest traces (black line) show the EPSP amplitude before capsaicin application, while posttest traces (gray line) show a EPSP amplitude after treatment. Middle: traces of the N-to-L synapse in the presence of 10 μM capsaicin with 10 μM AM251. Posttest EPSP traces show no change from pretest EPSP traces if AM251 is applied with capsaicin, indicating that AM251 binds to TRPV receptors. Bottom: traces of the N-to-L synapse in the presence of 10 μM capsaicin and 10 μM capsazepine. Similar to AM251 traces, posttest EPSP amplitude did not differ from pretest EPSP amplitude if capsazepine is applied with capsaicin. C: bar graph comparing the effects on synaptic transmission following treatment with either vehicle [control (VEH)], LFS in vehicle [w/LFS (VEH)], and resiniferatoxin (n = 5). One-way ANOVA detected a significant effect of the resiniferatoxin treatment [F(2,14) = 20.73; P < 0.01]. Post hoc analysis using Newman-Keuls detected a significant difference between the control (VEH) group and w/LFS (VEH) group (*P < 0.05). Post hoc analysis also detected a significant difference between the control (VEH) group and the resiniferatoxin group (*P < 0.05). D: bar graph representing the effect of SB 366791 treatment on heterosynaptic LTD with vehicle [control (VEH)], LFS in vehicle [w/LFS (VEH)], LFS in the presence of SB 366791 (SB 366791 w/LFS; n = 5), and drug treatment control (SB 366791 control, n = 5). One-way ANOVA analysis indicated a significant main effect of the treatment group [F(3,18) = 9.68, P < 0.01]. Newman-Keuls post hoc analyses detected a significance difference between the vehicle LFS group with control (VEH; *P < 0.05) group and SB 366791 w/ LFS (*P < 0.05) group. Drug treatment alone (SB 366791 control) did not have any effect.
Although both leeches and mollusks have been reported to be sensitive to capsaicin and capsazepine (Kalil-Gasper 2007; Pastor et al. 1996), both Drosophila and Caenorhabditis elegans are capsaicin insensitive (but see Wittenberg and Baumeister 1999). Given that both capsaicin and capsazepine can act on non-TRPV targets, the ability of more potent/selective TRPV1 agonists and antagonists to alter N-to-L synaptic transmission was tested. Resiniferatoxin (0.5 μM), an extremely potent TRPV1 agonist, induced LTD as effectively as capsaicin and 2AG (Fig. 5C). In addition SB 366791 (10 μM), the highly selective TRPV1 antagonist (Gunthorpe and Szallasi 2008), blocked LFS-induced LTD as effectively as capsazepine (Fig. 5D). The ability of these various TRPV1 agents to consistently mimic (in the case of capsaicin and resiniferatoxin) or block (in the case of capsazepine and SB 366791) N-to-L LTD suggests the presence of a TRPV-like receptor in the leech.
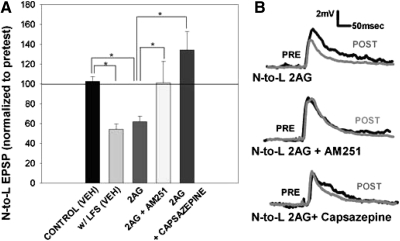
If ecLTD requires TRPV activation, then application of an endocannabinoid should elicit an LTD that can be blocked by co-application of a TRPV1 antagonist. In previous studies in the leech (Li and Burrell 2009), bath application of 2AG induced a persistent synaptic depression similar to that induced by LFS. Consistent with this earlier finding, bath application of 2AG (60 μM) for 900 s elicited significant depression of the N-to-L EPSP that was indistinguishable from LTD following either capsaicin treatment or LFS (Fig. 6A). This 2AG-induced depression was blocked by capsazepine, consistent with the hypothesis that endocannabinoid-dependent depression is mediated by the activation of a TRPV-like receptor in the leech (Fig. 6).
Fig. 6.
2AG mimicked LFS-induced LTD and was blocked by inhibitors of endocannabinoid and TRPV1 receptors. A: bar graph comparing the effects on synaptic transmission following treatment with either vehicle [control (VEH)], LFS in vehicle [w/LFS (VEH)], 2AG alone (2AG, n = 7), 2AG with 10 μM AM251 (2AG + AM251, n = 5), and 2AG with 10 μM capsazepine (2AG + capsazepine, n = 7). One-way ANOVA detected a significant effect of treatment group [F(4,24) = 8.08; P < 0.01]. Newman-Keuls post hoc analysis detected a significant difference between the w/LFS (VEH) group and the control (VEH; P < 0.05*) group and between the 2AG group and the control (VEH; P < 0.05*), 2AG + AM251 (P < 0.05*) and 2AG + capsazepine (P < 0.05*) groups. B, top: traces of the N-to-L synapse in the presence of 60 μM 2AG. Baseline pretest traces (black line) show the EPSP amplitude before 2AG application, while posttest traces (gray line) show EPSP amplitude after treatment. Middle: traces of the N-to-L synapse in the presence of 60 μM 2AG with 10 μM AM251. Posttest EPSP traces show no change from pretest EPSP traces if AM251 is applied with 2AG. Bottom: traces of the N-to-L synapse in the presence of 60 μM 2AG and 10 μM capsazepine. Similar to AM251 traces, posttest EPSP amplitude did not differ from pretest EPSP amplitude if capsazepine is applied with 2AG.
Many studies of endocannabinoids in invertebrates have reported the effectiveness of the vertebrate CB receptor agonists and antagonists (Jimenez-Del-Rio et al. 2008; Lemak et al. 2007; Li and Burrell 2009; McPartland et al. 2006; Rawls et al. 2007; Salzet and Stefano 2002). In the leech, ecLTD at the T-to-S synapse was blocked by the CB1 receptor antagonist AM251 and mimicked by the synthetic cannabinoid agonist CP55,940 (Li and Burrell 2009). However, analyses of protosomal invertebrates (Drosophila and C. elegans) and at least one deuterstomal invertebrate (the echinoderm Strongylocentritus) with fully sequenced genomes have failed to find any orthologues of the vertebrate CB1/CB2 receptors (Burke et al. 2006; Elphick and Egertova 2005). These findings suggest that whatever the identity of the invertebrate cannabinoid receptor, it is sensitive to drugs that act on vertebrate CB1 and CB2 receptors. Therefore, it is possible that the effectiveness of CB receptor agonists and antagonists in the leech is due to their action on TRPV-like receptors. To test this possibility, the ability of AM251, the CB1 receptor antagonist, to block capsaicin-induced synaptic depression was assessed. Pretreatment of ganglia with AM251 (10 μM) prevented capsaicin-induced LTD as effectively as capsazepine (Fig. 5A). AM251 also blocked 2AG-induced LTD (Fig. 6A), consistent with previous studies of ecLTD in the leech (Li and Burrell 2009). These results are consistent with the idea that AM251's capacity to block endocannabinoid-dependent processes in invertebrates is due to its ability to antagonize the interaction of endocannabinoids (e.g., 2AG) with the TRPV-like receptor.
Capsaicin or 2AG treatment occludes subsequent depression of the N-to-L synapse following LFS or drug application
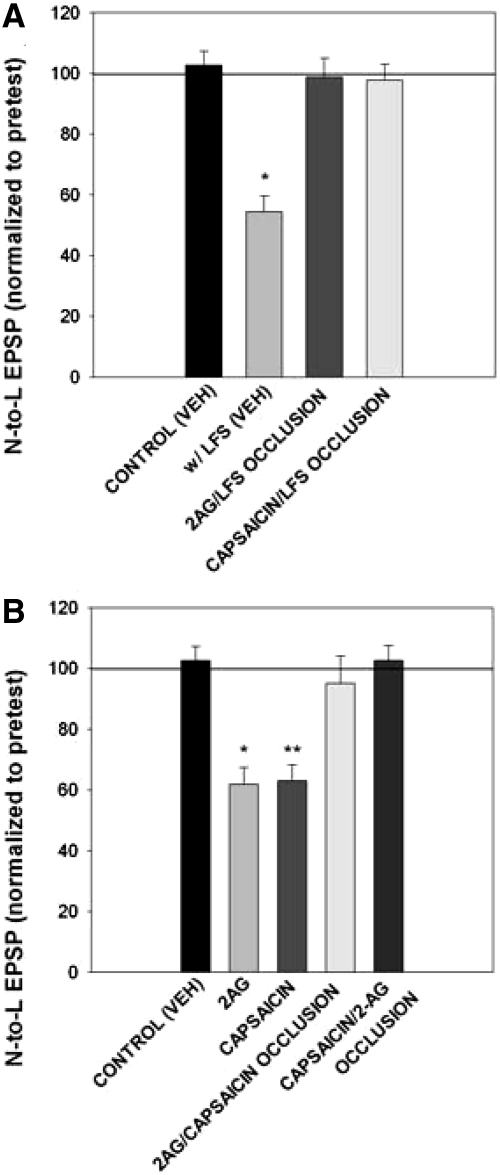
To provide additional support for the hypothesis that 2AG and capsaicin acted on the same signaling pathway activated by LFS, the ability of prior application of 2AG or capsaicin to occlude subsequent activity-induced LTD was tested. In ganglia pretreated with 2AG, LFS failed to induce N-to-L LTD (Fig. 7A), indicating that the exogenously applied 2AG had already engaged the signaling processes required for LTD in such a way that the subsequent LFS could not induce further depression. No LFS-induced LTD was observed in ganglia pretreated with capsaicin (Fig. 7A), again indicating that signaling processes responsible for synaptic depression had already been maximally engaged by the pretreatment. A comparison of the raw EPSP amplitudes demonstrates that the initial (pretest) EPSP levels in the 2AG or capsaicin pretreated groups were substantially reduced compared with the EPSPs recorded in normal saline (Supplementary Fig. S3A) indicating that the N-to-L synapse was depressed prior to delivery of the LFS. These data also show that occlusion of LFS-induced depression by 2AG or capsaicin was not due to a “basement effect” and that the N-to-L EPSP amplitude had the capacity to be further reduced.
Fig. 7.
Pretreatment with 2AG or capsaicin occluded further depression. A: bar graph representing the effects of synaptic transmission with and without LFS [control (VEH); w/LFS (VEH)] compared with occlusions with 2AG pretreatment (2AG/LFS occlusion, n = 7) and capsaicin pretreatment (capsaicin/LFS occlusion, n = 5). Data were analyzed through a 1-way ANOVA [F(3,20) = 18.16; P < 0.01] for main treatment effect. Control groups had no change between pre- and posttest values, while LFS groups had a decrease in EPSP posttest amplitude. Pre- and posttest normalized values in the 2AG/LFS (n = 7) and capsaicin/LFS (n = 5) occlusion groups did not have an overall change. Pretest values were depressed due to 2AG or capsaicin pretreatment and subsequent LFS did not induce further depression, resulting in no change in posttest values. Newman-Keuls post hoc analysis detected a significant difference between the w/LFS (VEH) group and the control (VEH; P < 0.05*) group and between the LFS (veh) group with the 2AG/LFS occlusion (P < 0.05*) group and capsaicin/LFS occlusion (P < 0.05*) group. B: bar graph comparing occlusion experiments in control groups, control (VEH), 2AG alone (2AG), capsaicin alone (capsaicin), 2AG pretreatment with subsequent capsaicin application (2AG/capsaicin occlusion, n = 4), or capsaicin pretreatment with subsequent 2AG application (capsaicin/2AG occlusion, n = 5). Data were analyzed through a one-way ANOVA [F(4,29) = 11.99; P < 0.01] for main treatment effect. Control groups had no change between pretest and posttest values, while 2AG and capsaicin groups had a decrease in EPSP posttest amplitude. Pre- and posttest normalized values in the 2AG/capsaicin and capsaicin/2AG occlusion groups did not have an overall change leading to no change in normalized values. In the occlusion groups, pretest EPSPs were depressed after capsaicin or 2AG pretreatment and subsequent drug applications did not induce further depression, resulting in no change in posttest values. Newman-Keuls posthoc analysis detected a significant difference between the control (VEH) and 2AG (P < 0.05*) group and between the 2AG group with the 2AG/capsaicin occlusion (P < 0.05*) group and capsaicin/2AG occlusion (P < 0.05*) group. There was also a significant difference between the control (VEH) group and the capsaicin alone (P < 0.05**) groups and between the capsaicin group with the 2AG/capsaicin occlusion (P < 0.05**) group and capsaicin/2AG occlusion (P < 0.05**) group.
The ability of 2AG and capsaicin to occlude each other was also examined. As shown in Fig. 7B, pretreatment with 2AG prevented subsequent capsaicin-induced N-to-L depression. When the order of the drug treatments was reversed, capsaicin pretreatment prevented subsequent 2AG-induced depression (Fig. 7B). Again a comparison of the raw EPSP amplitudes indicates that synapses pretreated with 2AG or capsaicin were depressed relative to synapses tested in normal saline (Supplementary Fig. S3B). The results from these occlusion studies are consistent with the hypothesis that endocannabinoid signaling and activation of a TRPV-like receptor are part of the same signaling pathway that is responsible for LFS-induced LTD.
N-to-L ecLTD involves changes at the presynaptic locus
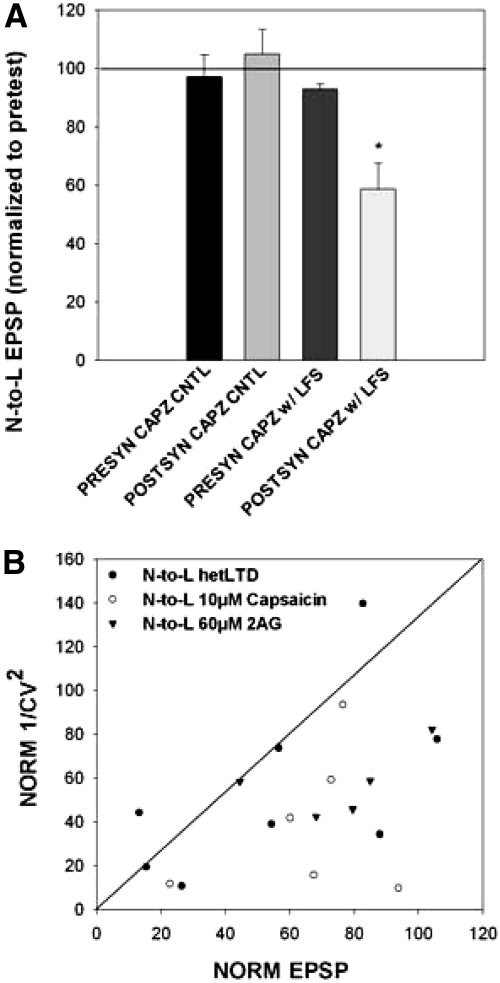
To assess the location of the putative TRPV-like receptors during ecLTD in the leech, iontophoretic injection of capsazepine into either the presynaptic N cell or postsynaptic L cell was carried out prior to LFS. Because capsazepine binding site is located on the intracellular side of the TRPV1 receptor (Jordt and Julius 2002), it has been proposed that intracellular injection of capsazepine can be used to selectively block TRPV receptors in the injected cell (Gibson et al. 2008). Presynaptic injection of capsazepine blocked ecLTD normally observed following LFS (Fig. 8A) but did not affect the N-to-L synapse when LFS was omitted. Postsynaptic injection of capsazepine had no effect on ecLTD following LFS and did not affect N-to-L synaptic transmission when the LFS was omitted. These experiments were repeated using bath-applied 2AG to induce synaptic depression. Presynaptic intracellular capsazepine injection blocked 2AG-induced LTD, while postsynaptic injection had no effect (Supplementary Fig. S4). These results indicate that the TRPV-like receptor that mediates N-to-L ecLTD is located on the presynaptic neuron.
Fig. 8.
TPRV-like receptor-mediated endocannabinoid-dependent LTD (ecLTD) has a presynaptic locus. A: bar graph representing iontophoresis of 500 μM capsazepine pre- and postsynaptically without LFS (presyn capz cntl, n = 5; postsyn capz cntl, n = 3) and with LFS (presyn capz w/ LFS, n = 5; postsyn capz cntl, n = 8). One-way ANOVA revealed a main effect between the groups [F(3,17) = 7.23; P < 0.01]. Newman-Keuls post hoc analysis detected a significant difference between postsynaptic capsazepine iontophoresis with LFS with the controls (P < 0.05*) and presynaptic capsazepine iontophoresis with LFS (P < 0.05*). B: the normalized inverse CV2 was plotted in the y axis in relation to normalized EPSP amplitude in the x axis. N-to-L heterosynaptic LTD (●) mainly fell below the regression line, although 2 data points fell above the regression. Coefficient of variation analyses of capsaicin (○)- or 2AG (▾)-induced depression also fell below the regression line, indicating presynaptic expression.
Coefficient of variation analyses was also carried out to further support ecLTD changes at the pre- or postsynaptic level. Data were plotted as the inverse CV2 relative to the normalized EPSP in such a way that data points to the right of the linear regression line indicate a presynaptic locus and points that fall to the left indicate a postsynaptic mechanism (Faber and Korn 1991; Sjostrom et al. 2003). The majority of the data points from the LFS-induced LTD experiments were to the right of the linear regression line, suggesting that synaptic depression was mediated via a presynaptic mechanism (Fig. 8B). This was also observed for synapses in which depression was induced by bath application of either 2AG or capsaicin. Although not conclusive, these results suggest that TRPV receptor dependent ecLTD is mediated presynaptically.
DISCUSSION
LFS of the non-nociceptive T cell induced heterosynaptic LTD in the monosynaptic, nociceptive N-to-L connection as well as homosynaptic LTD in the T-to-L pathway. LTD could also be induced homosynaptically in the N-to-L synapse following LFS of the N cell. The heterosynaptic LTD is endocannabinoid-dependent given that depression was prevented by RHC or THL, inhibitors of DAG lipase, which is required for synthesis of the endocannabinoid, 2AG. THL inhibited LTD when injected into the postsynaptic but not the presynaptic cell, indicating that LFS initiates 2AG synthesis in the postsynaptic L motor neuron. In addition, exogenous application of 2AG mimicked LTD and occluded subsequent induction of LTD by LFS. N-to-L ecLTD was also inhibited by treatment with the selective TRPV1 antagonists capsazepine or SB 366791 and capsazepine blocked homosynaptic LTD of the polysynaptic T-to-L connection as well. Bath application of the TRPV1 agonists, capsaicin or resiniferatoxin, mimicked LTD, and this synaptic depression was also blocked by capsazepine. Most important, capsazepine blocked synaptic depression induced by 2AG bath application, indicating that these TRPV1 antagonists were acting on an endocannabinoid-sensitive receptor. Additional support for this conclusion comes from occlusion experiments in which both 2AG and capsaicin treatments occluded subsequent LFS-induced LTD. 2AG and capsaicin were also capable of occluding each other's capacity to induce synaptic depression.
The putative TRPV-like receptor mediating ecLTD was found to be presynaptic given that presynaptic, but not postsynaptic, injection of capsazepine prevented both LFS- and 2AG-induced synaptic depression. This supports evidence from an earlier study by Gibson et al. (2008) indicating that ecLTD requires activation of presynaptic TRPV receptors. These findings, combined with the THL experiments in the present study, suggest that LFS elicits postsynaptic 2AG synthesis and that the newly synthesized 2AG travels in a retrograde manner to the presynaptic neurons where it binds to a TRPV-like receptor. Further support for a presynaptic locus during ecLTD comes from coefficient of variation analysis, which indicated a decrease in probability of release following depression elicited by LFS, 2AG treatment, and capsaicin treatment. Given that TRPV receptors gate Ca2+ (as well as other cations), it might be surprising that activation of presynaptic TRPV receptors induces depression because increases in presynaptic Ca2+ typically enhance synaptic transmission. In fact, activation of presynaptic TRPV receptors has been shown to potentiate neurotransmitter release (Medvedeva et al. 2008; Sikand and Premkumar 2007); however, these are short-term effects on synaptic transmission. In the case of persistent depression of synaptic transmission, it is possible that Ca2+ influx through presynaptic TRPV receptors initiates a biochemical signaling cascade that ultimately results in a decrease in neurotransmitter release (see review by Kauer and Gibson 2009). These findings do not preclude the possibility of postsynaptic TRPV receptors modulating synaptic transmission at different synapses in the brain or under different conditions at the same synapse.
Although invertebrates possess an active endocannabinoid system that utilizes many of the same transmitters and transmitter synthesizing and metabolizing enzymes found in vertebrates (Elphick and Egertova 2005; Leung et al. 2008; Matias et al. 2001; McPartland et al. 2006; Salzet and Stefano 2002), invertebrates lack orthologues of the vertebrate CB1/CB2 receptors. The evidence for a lack of invertebrate CB1/CB2 is explained in detail by Elphick and Egertova (2005) and Burke et al. (2006). In brief, a family of related receptors, including the CB1 and CB2 receptors, is absent from the several fully sequenced genomes from protostomal (Drosophila and C. elegans) and deuterostomal invertebrates (Ciona). Despite this lack of CB1/CB2 receptor orthologues, many CB receptor-specific agonists and antagonists, surprisingly, have cannabinoid-specific activity in the invertebrate CNS (Buznikov et al. 2009; Li and Burrell 2009; McPartland et al. 2006; Salzet and Stefano 2002; Schuel et al. 1994). In the leech, the CB1 receptor antagonist, AM251, prevents LFS- and 2AG-induced LTD, and the CB1 receptor agonist CP55,940 mimics ecLTD in the leech (Li and Burrell 2009). Other invertebrates that have demonstrated sensitivity to CB1 receptor agonists and antagonists include flatworms, mollusks, segmented worms, and arthropods (Jimenez-Del-Rio et al. 2008; Lemak et al. 2007; McPartland et al. 2006; Rawls et al. 2007).
One potential explanation for these observations is that vertebrates and invertebrates share a non-CB1/CB2 endocannabinoid receptor that is nevertheless sensitive to CB1/CB2 pharmacological agents. Endocannabinoids have been found to bind to TRPs including TRPV receptors, and TRPV1 has been found to mediate some forms of ecLTD (De Petrocellis et al. 2001; Gibson et al. 2008) Significantly, agonists and antagonists supposedly selective for CB receptors are also able to bind to TRPV receptors (see review by De Petrocellis and Di Marzo 2010). This may explain how vertebrate CB receptor drugs are able to have an effect on endocannabinoid-dependent processes in invertebrates. That is, they are binding to invertebrate TRPV-like receptors or other TRPs that are acting as endocannabinoid receptors in invertebrates. TRPs, including the TRPV family, are present throughout the animal kingdom (Buznikov et al. 2009; Damann et al. 2008; Montell 2003; Montell and Rubin 1989; Montell et al. 1985; Tobin and Bargmann 2004; Wittenburg and Baumeister 1999), making these proteins attractive candidates for the invertebrate endocannabinoid receptor. Evidence in support of the hypothesis that TRP receptors may mediate endocannabinoid-dependent modulation in invertebrates comes from the present study in which TRPV1 antagonists blocked both LFS- and 2AG-induced synaptic depression, the CB1 receptor antagonist, AM251, blocked both LFS- and capsaicin-induced synaptic depression, and 2AG and capsaicin were observed to occlude each other's effects on the N-to-L synapse. It should be noted that TRPV1-mediated ecLTD in the rat was not affected by AM251, although a different cannabinoid receptor antagonist, SR141716A, did inhibit this form of synaptic depression. The reasons for this discrepancy in the effects of AM251 versus SR141716A on mammalian TRPV1 (and the potential effects of SR141716A on ecLTD in the leech) are not known at this time.
How does one resolve the apparent effectiveness of TRPV1 drugs in a protostomal invertebrate when capsaicin sensitivity is supposed to be restricted to mammalian forms of TRPV1? First, the distinction in capsaicin sensitivity between mammals and nonmammals is not absolute. Avian ortholgues of TRPV1 do in fact exhibit a weak response to capsaicin, albeit under conditions in which capsaicin is co-applied with low pH (Jordt and Julius 2002). Capsaicin also elicits nocifensive responses in mollusks (Kalil-Gaspar et al. 2007) and hypersensitivity to thermal stimuli in C. elegans (Wittenburg and Baumeister 1999); in both cases, these capsaicin-induced effects were inhibited by capsazepine. Similar capsaicin-induced nocifensive behavior and behavioral sensitization has also been observed in the leech, and these capsaicin-induced effects were sensitive to inhibition by SB366791 (Burrell, unpublished data). Capsaicin also elicits activity in leech N cells, although once again the sensitivity to capsaicin is lower compared with mammals (Pastor et al 1996). Second, differences in capsaicin sensitivity should not preclude TRPV1-like functions in nonmammalian species. Avian TRPV1 receptors respond to nociceptive thermal stimuli and low pH, and capsazepine inhibits heat-induced currents in both avian and mammalian neurons with equal effectiveness (Marin-Burgin et al. 1996). Other aspects of nonmammalian forms of TRPV receptors, e.g., sensitivity to endocannabinoids or to other TRPV1 agonists and antagonists, have not been widely studied, and therefore it is difficult to reach firm conclusions about their functional and pharmacological properties.
In some respects, the situation resembles past controversies regarding the presence of NMDA receptors in invertebrates. Invertebrates were once thought to lack NMDA receptors due to their insensitivity to the agonist NMDA, but subsequent molecular and physiological experiments have shown that these receptors are present in invertebrates. Furthermore, while invertebrate NMDA receptors are relatively insensitive to NMDA itself, they are sensitive to other NMDA receptor-specific pharmacological agents and play a similar functional role as vertebrate receptors, e.g., LTP and LTD (Brockie et al. 2001; Glantz and Pfeiffer-Linn 1992; Glanzman 2010; Grey and Burrell 2010; Grey et al. 2009). The presence of a TRPV-like receptor in the leech cannot be definitively claimed based solely on pharmacological evidence, but it seems unlikely that all four of TRPV1 drugs used in the present study (capsaicin, resiniferatoxin, capsazepine, and SB366791) can produce such consistent results due to a fortuitous convergence of non-TRPV effects. A final resolution as to whether a TRPV-like receptor mediates endocannabinoid-dependent neuromodulation in the leech will require cloning of the putative leech TRPV-like receptor, determination of its physiological properties (including its responsiveness to endocannabinoids such as 2AG and anandamide) and genetic knockdown of this putative TRPV-like receptor to confirm its responsiveness to TRPV agonists and endocannabinoids. A putative TRPV-encoding sequence is present in the genome database of the leech Helobdella robusta (ID # 77785), which is closely related to Hirudo.
Anandamide is the endocannabinoid most often identified as a TRPV1 ligand (De Petrocellis et al. 2000). Although TRPV1 has been reported as being insensitive to 2AG (De Petrocellis et al. 2000), a recent study by Qin et al. (2008) stated that 2AG did bind to TRPV1 receptors. In the present study, the ability of RHC and THL to prevent synaptic depression indicates that 2AG is necessary for N-to-L LTD. In addition, 2AG-induced depression was blocked by capsazepine treatment, and 2AG and capsaicin were able to occlude each other's effects on the N-to-L synapse. Nevertheless anandamide is present in the leech CNS (Salzet and Stefano 2002) and may also contribute ecLTD in the leech. These issues of anadamide sensitivity and differences in sensitivity to 2AG and AM251 illustrate the need for further characterization of the putative leech TRPV-like receptor, and it is possible that the pharmacological properties the leech TRPV-like receptor and the mammalian TRPV1 will differ significantly.
It is interesting that N-to-L ecLTD could be induced heterosynaptically by activation of the touch cell. Stimulation of non-nociceptive sensory fibers has been known to attenuate nociceptive signaling, referred to as the gate control theory of pain (Melzack and Wall 1965). This is typically thought to be a short-term process, although there is evidence that inhibition of nociceptive signaling can outlast the duration of non-nociceptive stimulation (Sluka and Walsh 2003). The findings in the current study show that activity in non-nociceptive neurons can elicit a persistent and substantial decrease in nociceptive synaptic signaling and provides a cellular mechanism to explain this modulatory process. This form of neuroplasticity may represent a fundamental mechanism for modifying nociceptive synaptic transmission that has applications for the control of physiological and pathological pain conditions. Endogenous and synthetic cannabinoids are thought to have analgesic properties, although the mechanisms of these effects are not well understood (Nyilas et al. 2009; Toth et al. 2009). Similarly, activation of central TRPV1 receptors has also been proposed to represent a potential therapeutic approach for the treatment of pain (Gunthorpe and Szallasi 2008; Knotkova et al. 2008; Toth et al. 2009).
That nociceptive synaptic transmission can be modulated by endocannabinoids via the activation of a TRPV-like receptor in the leech represents a potentially significant finding with applications for the control of pain from a clinical perspective. Nociception is a fundamental sensory process that is highly conserved across the animal phyla (Tobin and Bargmann 2004), and invertebrates have been widely used to study the basic mechanisms of nociception and its modulation (Kalil-Gaspar et al. 2007; Smith and Lewin 2009; Tracey et al. 2003; Walters and Moroz 2009). The leech CNS provides an especially useful model system to understand these processes given that it is possible to reliably record from identifiable touch, pressure, and nociceptive sensory neurons as well as the synaptic targets of all three types of afferents. The leech N cell is very similar to vertebrate nociceptive neurons in terms of its sensitivity to strong mechanosensory stimuli, tissue damage, heat, acid, and high salt concentrations (Pastor et al. 1996). Furthermore, the leech N cell is known to have synaptic input to both motor neurons (e.g., the L motor neuron) and modulatory interneurons that contribute to whole-body shortening, a defensive withdrawal reflex initiated by noxious stimuli (Shaw and Kristan 1995). These latter synaptic targets include the serotonergic Retzius cells and the S interneuron, which contributes to sensitization of this reflex (Burrell et al. 2003; Modney et al. 1997; Nicholls and Purves 1970; Sahley et al. 1994; Velazquez-Ulloa et al. 2003; S. A. Baccus, B. D. Burrell, and K. J. Muller, unpublished observation). As a result, it is possible in the leech to study the potential anti-nociceptive properties of endocannabinoid and TRPV-like receptors from the synaptic to the behavioral levels. Although examined in the context of nociceptive synaptic transmission, TRPV receptors have been shown to contribute to synaptic plasticity in a variety of other regions in the brain and play a wide range of functional roles including anxiety, learning and memory, neurodevelopment, and homeostasis (Gibson et al. 2008; Kauer and Gibson 2009; Maione et al. 2009; Marsch et al. 2007; Peters et al. 2010). Understanding the cellular and molecular details of this emerging form of neuromodulation will generate important insights to the preceding processes and will also provide possible therapeutic approaches for treating dysfunction in these processes.
GRANTS
This work was supported by a subproject of the National Institutes of Health grant (P20 RR-015567), which is designated as a Center of Biomedical Research Excellence (COBRE).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Drs. Brenda Moss and Joyce Keifer for helpful comments during the preparation of this manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Adams et al., 2000.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, Gabor GL, Abril JF, Agbayani A, An HJ, Andrews-Pfannkoch C, Baldwin D, Ballew RM, Basu A, Baxendale J, Bayraktaroglu L, Beasley EM, Beeson KY, Benos PV, Berman BP, Bhandari D, Bolshakov S, Borkova D, Botchan MR, Bouck J, Brokstein P, Brottier P, Burtis KC, Busam DA, Butler H, Cadieu E, Center A, Chandra I, Cherry JM, Cawley S, Dahlke C, Davenport LB, Davies P, de Pablos B, Delcher A, Deng Z, Mays AD, Dew I, Dietz SM, Dodson K, Doup LE, Downes M, Dugan-Rocha S, Dunkov BC, Dunn P, Durbin KJ, Evangelista CC, Ferraz C, Ferriera S, Fleischmann W, Fosler C, Gabrielian AE, Garg NS, Gelbart WM, Glasser K, Glodek A, Gong F, Gorrell JH, Gu Z, Guan P, Harris M, Harris NL, Harvey D, Heiman TJ, Hernandez JR, Houck J, Hostin D, Houston KA, Howland TJ, Wei MH, Ibegwam C, Jalali M, Kalush F, Karpen GH, Ke Z, Kennison JA, Ketchum KA, Kimmel BE, Kodira CD, Kraft C, Kravitz S, Kulp D, Lai Z, Lasko P, Lei Y, Levitsky AA, Li J, Li Z, Liang Y, Lin X, Liu X, Mattei B, McIntosh TC, McLeod MP, McPherson D, Merkulov G, Milshina NV, Mobarry C, Morris J, Moshrefi A, Mount SM, Moy M, Murphy B, Murphy L, Muzny DM, Nelson DL, Nelson DR, Nelson KA, Nixon K, Nusskern DR, Pacleb JM, Palazzolo M, Pittman GS, Pan S, Pollard J, Puri V, Reese MG, Reinert K, Remington K, Saunders RD, Scheeler F, Shen H, Shue BC, Siden-Kiamos I, Simpson M, Skupski MP, Smith T, Spier E, Spradling AC, Stapleton M, Strong R, Sun E, Svirskas R, Tector C, Turner R, Venter E, Wang AH, Wang X, Wang ZY, Wassarman DA, Weinstock GM, Weissenbach J, Williams SM, Woodage T, Worley KC, Wu D, Yang S, Yao QA, Ye J, Yeh RF, Zaveri JS, Zhan M, Zhang G, Zhao Q, Zheng L, Zheng XH, Zhong FN, Zhong W, Zhou X, Zhu S, Zhu X, Smith HO, Gibbs RA, Myers EW, Rubin GM, Venter JC. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195, 2000 [DOI] [PubMed] [Google Scholar]
- Anwyl, 2006.Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 78: 17–37, 2006 [DOI] [PubMed] [Google Scholar]
- Bevan et al., 1992.Bevan J, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CSJ, Yeats JC. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 107: 544–552, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno et al., 2003.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol 163: 463–468, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie et al., 2001.Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31: 617–630, 2001 [DOI] [PubMed] [Google Scholar]
- Burrell et al., 2003.Burrell BD, Sahley CL, Muller KJ. Progressive recovery of learning during regeneration of a single synapse in the medicinal leech. J Comp Neurol 457: 67–74, 2003 [DOI] [PubMed] [Google Scholar]
- Burke et al., 2006.Burke RD, Angerer LM, Elphick MR, Humphrey GW, Yaguchi S, Kiyama T, Liang S, Mu X, Agca C, Klein WH, Brandhorst BP, Rowe M, Wilson K, Churcher AM, Taylor JS, Chen N, Murray G, Wang D, Mellott D, Olinski R, Hallbook F, Thorndyke MC. A genomic view of the sea urchin nervous system. Dev Biol 300: 434–460, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buznikov et al., 2009.Buznikov GA, Nikitina LA, Bezuglov VV, Francisco ME, Boysen G, Obispo-Peak IN, Peterson RE, Weiss ER, Schuel H, Temple BR, Morrow AL, Lauder JM. A putative ‘pre-nervous’ endocannabinoid system in early echinoderm development. Dev Neurosci 32: 1–18, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre et al., 2006.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76, 2006 [DOI] [PubMed] [Google Scholar]
- Damann et al., 2008.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol 18: R880–R889, 2008 [DOI] [PubMed] [Google Scholar]
- De Petrocellis et al., 2000.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett 483: 52–56, 2000 [DOI] [PubMed] [Google Scholar]
- De Petrocellis et al., 2001.De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem 276: 12856–12863, 2001 [DOI] [PubMed] [Google Scholar]
- De Petrocellis and Di Marzo, 2005.De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 77: 1651–1666, 2005 [DOI] [PubMed] [Google Scholar]
- De Petrocellis and Di Marzo, 2010.De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol 5: 103–121, 2010 [DOI] [PubMed] [Google Scholar]
- De Petrocellis et al., 2007.De Petrocellis L, Starowicz K, Moriello AS, Vivese M, Orlando P, Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp Cell Res 313: 1911–1920, 2007 [DOI] [PubMed] [Google Scholar]
- Devane et al., 1988.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34: 605–613, 1988 [PubMed] [Google Scholar]
- Di Marzo et al., 2001.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol Sci 22: 346–349, 2001 [DOI] [PubMed] [Google Scholar]
- Diana and Marty, 2004.Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 142: 9–19, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot et al., 1994.Eliot LS, Kandel ER, Hawkins RD. Modulation of spontaneous transmitter release during depression and posttetanic potentiation of Aplysia sensory-motor neuron synapses isolated in culture. J Neurosci 14: 3280–3292, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick and Egertova, 2001.Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 356: 381–408, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick and Egertova, 2005.Elphick MR, Egertova M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb Exp Pharmacol 283–297, 2005 [DOI] [PubMed] [Google Scholar]
- Elphick et al., 2003.Elphick MR, Satou Y, Satoh N. The invertebrate ancestry of endocannabinoid signalling: an orthologue of vertebrate cannabinoid receptors in the urochordate Ciona intestinalis. Gene 302: 95–101, 2003 [DOI] [PubMed] [Google Scholar]
- Faber and Korn, 1991.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J 60: 1288–1294, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson et al., 2008.Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57: 746–759, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz and Pfeiffer-Linn, 1992.Glantz RM, Pfeiffer-Linn C. NMDA receptors in invertebrates. Comp Biochem Physiol C 103: 243–248, 1992 [Google Scholar]
- Glanzman, 2010.Glanzman DL. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr Biol 20: R31–R36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey and Burrell, 2010.Grey KB, Burrell BD. Co-induction of LTP and LTD and its regulation by protein kinases and phosphatases. J Neurophysiol 103: 2737–2746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey et al., 2009.Grey KB, Moss BL, Burrell BD. Molecular identification and expression of the NMDA receptor NR1 subunit in the leech. Invert Neurosci 9: 11–20, 2009 [DOI] [PubMed] [Google Scholar]
- Gunthorpe and Szallasi, 2008.Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharmacol Design 14: 32–41, 2008 [DOI] [PubMed] [Google Scholar]
- Heifets and Castillo, 2009.Heifets BD, Castillo PE. Endocannabinoid-signaling and long-term synaptic plasticity. Annu Rev Physiol 71: 283–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Del-Rio et al., 2008.Jimenez-Del-Rio M, Daza-Restrepo A, Velez-Pardo C. The cannabinoid CP55,940 prolongs survival and improves locomotor activity in Drosophila melanogaster against paraquat: implications in Parkinson's disease. Neurosci Res 61: 404–411, 2008 [DOI] [PubMed] [Google Scholar]
- Jordt and Julius, 2002.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108: 421–430, 2002 [DOI] [PubMed] [Google Scholar]
- Jung et al., 1999.Jung J, Hwang SW, Kwak J, Lee S-Y, Kang C-J, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci 19: 529–538, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil-Gaspar et al., 2007.Kalil-Gaspar P, Marcuzzo S, Rigon P, Molina CG, Achaval M. Capsaicin-induced avoidance behavior in the terrestrial Gastropoda megalobulimus abbreviatus: evidence for TRPV-1 signaling and opioid modulation in response to chemical noxious stimuli. Comp Biochem Physiol A Mol Integr Physiol 148: 286–291, 2007 [DOI] [PubMed] [Google Scholar]
- Kauer and Gibson, 2009.Kauer J, Gibson HE. Hot flash: TRPV channels in the brain. Trends Neurosci 32: 215–223, 2009 [DOI] [PubMed] [Google Scholar]
- Kirifides et al., 2004.Kirifides ML, Kurnellas MP, Clark L, Bryant BP. Calcium responses of chicken trigeminal ganglion neurons to methyl anthranilate and capsaicin. J Exp Biol 207: 715–722, 2004 [DOI] [PubMed] [Google Scholar]
- Knotkova et al., 2008.Knotkova H, Pappagallo M, Szallasi A. Capsaicin (TRPV1 agonist) therapy for pain relief: farewell or revival? Clin J Pain 24: 142–154, 2008 [DOI] [PubMed] [Google Scholar]
- Kortschak et al., 2003.Kortschak RD, Samuel G, Saint R, Miller DJ. EST analysis of the cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Curr Biol 13: 2190–2195, 2003 [DOI] [PubMed] [Google Scholar]
- Kristan et al., 2005.Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol 76: 279–327, 2005 [DOI] [PubMed] [Google Scholar]
- Lemak et al., 2007.Lemak MS, Bravarenko NI, Bobrov MY, Bezuglov VV, Ierusalimsky VN, Storozhuk MV, Malyshev AY, Balaban PM. Cannabinoid regulation in identified synapse of terrestrial snail. Eur J Neurosci 26: 3207–3214, 2007 [DOI] [PubMed] [Google Scholar]
- Leung et al., 2008.Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL. DAG lipase activity is necessary for TRP channel regulation in drosophila photoreceptors. Neuron 58: 884–896, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Burrell, 2008.Li Q, Burrell BD. CNQX and AMPA inhibit electrical synaptic transmission: a potential interaction between electrical and glutamatergic synapses. Brain Res 1228: 43–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Burrell, 2009.Li Q, Burrell BD. Two forms of long-term depression in a polysynaptic pathway in the leech CNS: one NMDA receptor-dependent and the other cannabinoid dependent. J Comp Physiol [A] 195: 831–841, 2009 [DOI] [PubMed] [Google Scholar]
- Maione et al., 2009.Maione S, Cristino L, Migliozzi AL, Georgiou AL, Starowicz K, Salt TE, Di Marzo V. TRPV1 channels control synaptic plasticity in the developing superior colliculus. J Physiol 587: 2521–2535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin et al., 2000.Marin-Burgin A, Reppenhagen S, Klusch A, Wendland JR, Petersen M. Low-threshold heat response antagonized by capsazepine in chick sensory neurons, which are capsaicin-insensitive. Eur J Neurosci 12: 3560–3566, 2000 [DOI] [PubMed] [Google Scholar]
- Marsch et al., 2007.Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci 27: 832–839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias et al., 2001.Matias I, Bisogno T, Melck D, Vandenbulcke F, Verger-Bocquetz M, De Petrocellis L, Sergheraert C, Breton C, Di Marzo V, Salzet M. Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Brain Res Mol Brain Res 87: 145–159, 2001 [DOI] [PubMed] [Google Scholar]
- McPartland et al., 2006.McPartland JM, Agraval J, Gleeson D, Heasman K, Glass M. Cannabinoid receptors in invertebrates. J Evol Biol 19: 366–373, 2006 [DOI] [PubMed] [Google Scholar]
- Medvedeva et al., 2008.Medvedeva YV, Kim MS, Usachev YM. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci 28: 5295–5311, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack and Wall, 1965.Melzack R, Wall PD. Pain mechanisms: a new theory. Science 150: 971–979, 1965 [DOI] [PubMed] [Google Scholar]
- Min et al., 2010.Min R, Testa-Silva G, Heistek TS, Canto CB, Lodder JC, Bisogno T, Di Marzo V, Brussaard AB, Burnashev N, Mansvelder HD. Diacylglycerol lipase is not involved in depolarization-induced suppression of inhibition at unitary inhibitory connections in mouse hippocampus. J Neurosci 30: 2710–2715, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modney et al., 1997.Modney BK, Sahley CL, Muller KJ. Regeneration of a central synapse restores nonassociative learning. J Neurosci 17: 6478–6482, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell, 2003.Montell C. The venerable inveterate invertebrate TRP channels. Cell Calcium 33: 409–417, 2003 [DOI] [PubMed] [Google Scholar]
- Montell et al., 1985.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230: 1040–1043, 1985 [DOI] [PubMed] [Google Scholar]
- Montell and Rubin, 1989.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 2: 1313–1323, 1989 [DOI] [PubMed] [Google Scholar]
- Muller and Scott, 1981.Muller KJ, Scott SA. Transmission at a “direct” electrical connexion mediated by an interneuron in the leech. J Physiol 311: 565–583, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls and Baylor, 1968.Nicholls JG, Baylor DA. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol 31: 740–756, 1968 [DOI] [PubMed] [Google Scholar]
- Nicholls and Purves, 1970.Nicholls JG, Purves D. Monosynaptic chemical and electrical connexions between sensory and motor cells in the central nervous system of the leech. J Physiol 209: 647–667, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyilas et al., 2009.Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci 29: 1964–1978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortar et al., 2008.Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, Di Marzo V. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. J Med Chem 51: 6970–6979, 2008 [DOI] [PubMed] [Google Scholar]
- Pastor et al., 1996.Pastor J, Soria B, Belmonte C. Properties of the nociceptive neurons of the leech segmental ganglion. J Neurophysiol 75: 2268–2279, 1996 [DOI] [PubMed] [Google Scholar]
- Peters et al., 2010.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin et al., 2008.Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28: 6231–6238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible et al., 2005.Raible F, Tessmar-Raible K, Osoegawa K, Wincker P, Jubin C, Balavoine G, Ferrier D, Benes V, de Jong P, Weissenbach J, Bork P, Arendt D. Vertebrate-type intron-rich genes in the marine annelid Platynereis dumerilii. Science 310: 1325–1326, 2005 [DOI] [PubMed] [Google Scholar]
- Rawls et al., 2007.Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 52212-2). Pharmacol Biochem Be 86: 499–504, 2007 [DOI] [PubMed] [Google Scholar]
- Romanovsky et al., 2009.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61: 228–261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahley et al., 1994.Sahley CL, Modney BK, Boulis NM, Muller KJ. The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J Neurosci 14: 6715–6721, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzet and Stefano, 2002.Salzet M, Stefano GB. The endocannabinoid system in invertebrates. Prostaglandins Leukot Essent Fatty Acids 66: 353–361, 2002 [DOI] [PubMed] [Google Scholar]
- Schuel et al., 1994.Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (Arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing-capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci USA 91: 7678–7682, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw and Kristan, 1995.Shaw BK, Kristan WB., Jr The whole-body shortening reflex of the medicinal leech: motor pattern, sensory basis, and interneuronal pathways. J Comp Physiol [A] 177: 667–681, 1995 [DOI] [PubMed] [Google Scholar]
- Siddall et al., 2007.Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc Biol Sci 274: 1481–1487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand and Premkumar, 2007.Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol 581: 631–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom et al., 2003.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocorticol LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641–654, 2003 [DOI] [PubMed] [Google Scholar]
- Sluka and Walsh, 2003.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain 4: 109–121, 2003 [DOI] [PubMed] [Google Scholar]
- Smith and Lewin, 2009.Smith ES, Lewin GR. Nociceptors: a phylogenetic view. J Comp Physiol [A] 195: 1089–1106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella et al., 1997.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 388: 773–778, 1997 [DOI] [PubMed] [Google Scholar]
- Tobin and Bargmann, 2004.Tobin DM, Bargmann CI. Invertebrate nociception: behaviors, neurons and molecules. J Neurobiol 61: 161–174, 2004 [DOI] [PubMed] [Google Scholar]
- Toth et al., 2009.Toth A, Blumberg P, Boczan J. Anandamide and the vanilloid receptor (TRPV1). Vitamins Hormones 81: 389–419, 2009 [DOI] [PubMed] [Google Scholar]
- Tracey et al., 2003.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell 113: 261–273, 2003 [DOI] [PubMed] [Google Scholar]
- Velazquez-Ulloa et al., 2003.Velazquez-Ulloa N, Blackshaw SE, Szczupak L, Trueta C, Garcia E, De-Miguel FF. Convergence of mechanosensory inputs onto neuromodulatory serotonergic neurons in the leech. J Neurobiol 54: 604–617, 2003 [DOI] [PubMed] [Google Scholar]
- Walters and Moroz, 2009.Walters ET, Moroz LL. Molluscan memory of injury: evolutionary insights into chronic pain and neurological disorders. Brain Behav Evol 74: 206–218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenburg and Baumeister, 1999.Wittenburg N, Baumeister R. Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci USA 96: 10477–10482, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.