Abstract
Whereas studies of somatotopic representation of touch have been useful to distinguish multiple somatosensory areas within primary (SI) and secondary (SII) somatosensory cortex regions, no such analysis exists for the representation of pain across nociceptive modalities. Here we investigated somatotopy in the operculo-insular cortex with noxious heat and pinprick stimuli in 11 healthy subjects using high-resolution (2 × 2 × 4 mm) 3T functional magnetic resonance imaging (fMRI). Heat stimuli (delivered using a laser) and pinprick stimuli (delivered using a punctate probe) were directed to the dorsum of the right hand and foot in a balanced design. Locations of the peak fMRI responses were compared between stimulation sites (hand vs. foot) and modalities (heat vs. pinprick) within four bilateral regions of interest: anterior and posterior insula and frontal and parietal operculum. Importantly, all analyses were performed on individual, non-normalized fMRI images. For heat stimuli, we found hand-foot somatotopy in the contralateral anterior and posterior insula [hand, 9 ± 10 (SD) mm anterior to foot, P < 0.05] and in the contralateral parietal operculum (SII; hand, 7 ±10 mm lateral to foot, P < 0.05). For pinprick stimuli, we also found somatotopy in the contralateral posterior insula (hand, 9 ±10 mm anterior to foot, P < 0.05). Furthermore, the response to heat stimulation of the hand was 11 ± 12 mm anterior to the response to pinprick stimulation of the hand in the contralateral (left) anterior insula (P < 0.05). These results indicate the existence of multiple somatotopic representations for pain within the operculo-insular region in humans, possibly reflecting its importance as a sensory-integration site that directs emotional responses and behavior appropriately depending on the body site being injured.
INTRODUCTION
The cortical representation of innocuous somatosensory stimuli has been the subject of investigations for many decades. Detailed electrophysiological studies of receptive field somatotopies revealed multiple representations of the body within the primary (SI) and secondary (SII) somatosensory cortex in monkeys (Fitzgerald et al. 2004; Kaas 1983; Krubitzer et al. 1995), and these somatosensory subdivisions exhibited different functional properties. Functional imaging studies with tactile stimuli in humans have supported these subdivisions within SI and SII (Disbrow et al. 2000; Eickhoff et al. 2006a,b; Gelnar et al. 1998; Young et al. 2004).
The cerebral representation and processing of nociceptive stimuli has been studied quite extensively with the evolution of neuroimaging techniques. An expansive set of regions, including S1, thalamus and distinct divisions of the insular, prefrontal and anterior cingulate cortices among other, has been described as relevant (for review, see Apkarian et al. 2005; Tracey and Mantyh 2007). Surprisingly few studies have investigated the somatotopy for nociceptive stimuli, probably because it was anticipated to be identical to that for touch. The somatotopic maps for pain and touch are similar in the thalamus, where the face is represented medially and the foot laterally (Lenz et al. 1988, 1994; Lenz and Dougherty 1997), and in SI, where the face is represented laterally and the foot medially (Andersson et al. 1997; Bingel et al. 2004; DaSilva et al. 2002; Tarkka and Treede 1993).
For cortical processing of painful stimuli, the operculo-insular cortex plays an important role (Treede et al. 2000). Nociceptive areas within this region include several parts of the insula deep inside the lateral sulcus, and those parts of the frontal and parietal lobes that cover the insula (called the opercula). This region receives nociceptive input as early as or even earlier than SI (Frot and Mauguière 2003; Ploner et al. 1999; Rios et al. 1999; Tarkka and Treede 1993). Its electrical stimulation elicits painful sensations (Afif et al. 2008; Mazzola et al. 2009; Ostrowsky et al. 2002), whereas lesions impair pain sensitivity (Greenspan et al. 1999). Furthermore its activity is enhanced during nociceptive discrimination tasks (Schlereth et al. 2003) and correlates reliably with perceived pain intensity (Iannetti et al. 2005), and opiate receptor density is comparable to that in the cingulate cortex (Baumgärtner et al. 2006a).
For the somatotopy in the operculo-insular cortex, there are two conflicting concepts: all tactile representations in the parietal operculum (including SII and parietal ventral area PV) are oriented similar to SI, i.e., the face laterally and the foot medially (Fitzgerald et al. 2004). In contrast, nociceptive input to the dorsal insula has been suggested to derive from the posterior part of the proposed ventral medial thalamic nucleus (Vmpo) (Craig and Dostrovsky 1997; Craig et al. 1994) with a completely different somatotopy: face anterior and foot posterior (Craig 1995). Some studies with painful stimuli confirmed the anterior-posterior somatotopy (Baumgärtner et al. 2006b; Brooks et al. 2005; Henderson 2007; Vogel et al. 2003), whereas others showed a mediolateral somatotopy (Bingel et al. 2004). As a combination of these two concepts, a parallel projection of spinal cord neurons to both the insula and the operculum (SII) has been demonstrated very recently by a viral tracing study in monkey (Dum et al. 2009).
A better understanding of the somatotopic represention of painful stimuli in the operculo-insular cortex may resolve conflicting concepts of its organization. Therefore we addressed these two questions: whether multiple somatotopical maps exist in the operculo-insular area and whether different types of cutaneous pain (heat and pinprick) share similar cortical representations.
METHODS
Eleven subjects (7 males and 4 females; mean age, 28 yr, range, 26–34 yr) participated in the study after giving fully informed consent, which conformed to the guidelines of the Declaration of Helsinki (1996) and had been approved by the local ethics committee.
Laser stimulation
Infrared laser pulses selectively activate heat-sensitive Aδ- and C-nociceptors in the skin (Treede et al. 1995). They evoke a very brief pinprick-like and/or burning sensation and the input transmitted via type II A-fiber mechano-heat nociceptors (type II AMH) rapidly activates the operculo-insular cortex (Iannetti et al. 2004; Tarkka and Treede 1993; Xu et al. 1997). In the present study, nociceptive heat stimuli were generated by an infrared neodymium yttrium aluminum perovskite (Nd:YAP) laser (El En, Florence, Italy, www.elengroup.com) with a wavelength of 1.34 μm. The laser beam was transmitted via optic fiber into the scanner room and directed to the skin area that was to be stimulated (hand or foot dorsum). The diameter of the laser beam was set at 6 mm (irradiated area: ∼28 mm2) by focusing lenses. Laser pulses produced by Nd:YAP stimulators do not induce damage to the irradiated skin that is sometimes produced by the widely used, high-intensity CO2-laser pulses (Cruccu et al. 2003; Iannetti et al. 2003).
Pinprick stimulation
Painful mechanical stimuli were applied using a hand-held 256 mN pinprick probe that has a flat cylindrical tip (diameter: 250 μm) and evokes a pinprick sensation primarily mediated by activation of a different type of Aδ-nociceptors (type I AMH) (Magerl et al. 2001; Slugg et al. 2000). These mechanical stimulators have been proven to be an adequate tool to induce pinprick pain in psychophysical and clinical investigations (Baumgärtner et al. 2002; Greenspan and McGillis 1994; Greenspan et al. 1997; Ziegler et al. 1999) and are commonly used as part of the protocol for quantitative sensory testing in the German Research Network on Neuropathic Pain (Rolke et al. 2006).
Experimental paradigm
Each experiment consisted of four different stimulation conditions: laser stimulation of the right hand, laser stimulation of the right foot, pinprick stimulation of the right hand, and pinprick stimulation of the right foot. In a psychophysical session prior to the fMRI experiment, the intensity of both laser and pinprick stimuli was adjusted to achieve a similarly perceived intensity of both heat and mechanically induced pain at both stimulated sites (hand, foot): For the hand, we used a 256-mN pinprick probe and 1.5-J laser pulses; for the foot, we used the same 256-mN pinprick probe and 2-J laser pulses. During this psychophysical session, subjects were asked to rate verbally the intensity of the perceived pricking pain on a numerical rating scale ranging from 0 to 10 (0 = no pricking pain, 10 worst pricking pain imaginable).
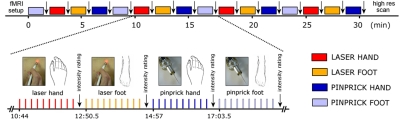
During the fMRI recording, stimuli were delivered in blocks of 10 repeats. Within each block, stimuli of the same modality were delivered to the same body region every 11.5 s, with a break of 23 s separating the last stimulus of one block from the first stimulus of the next block. Each of the four stimulation conditions was repeated four times (16 blocks) resulting in 40 stimuli of the same modality (laser or pinprick) to the same body region (hand or foot); in total, 160 stimuli were delivered to each subject. The order of the blocks was balanced across subjects. As an example, a whole stimulation sequence for one subject is shown in Fig. 1. Subjects were instructed to focus their attention on the stimuli without any specific discrimination task and to give an average intensity rating of each block of stimuli using their fingers 12.5 s after the last stimulus of that block, when the experimenter inside the scanner lightly touched their left ankle. To avoid nociceptor fatigue or sensitization, the laser beam or the pinprick stimulator was slightly moved after each stimulus.
Fig. 1.
Experimental design. The figure illustrates the timing of actions performed during the functional magnetic resonance imaging (fMRI) experiment. Laser heat and pinprick stimuli were delivered to the skin of the dorsum of the hand and of the foot by an experimenter inside the scanner room. Stimuli of the same modality and to the same body site were delivered in blocks of 10, with an interstimulus interval of 11.5 s. At the end of each block, the subject was asked to provide an average intensity rating for that block. The order of blocks was balanced across subjects.
MR image acquisition
Functional MRI scanning was performed continuously on a 3 Tesla Varian INOVA MRI system. A head-only gradient coil (Magnex SGRAD MKIII) was used with a birdcage radiofrequency head coil for pulse transmission and signal reception. A high resolution, gradient-echo, echo-planar imaging sequence was used for functional scans (TE = 45 ms, 12 contiguous 4-mm axial slices, flip angle 87°, in-plane field of view 256 × 256 mm, image matrix: 128 × 128) with a repetition time (TR) of 3 s over 680 volumes, corresponding to a total scan time of 34 min. By examining sagittal and coronal scout images, the 12 axial slices were adjusted to cover the maximum superior-inferior extent of the insula (Özcan et al. 2005). Furthermore, at the end of the functional scan, for each subject a T1-weighted, high-resolution structural image (70 contiguous 3-mm axial slices, in-plane field of view, 256 × 192 mm; matrix, 256 × 192) was collected for verification of anatomical structures.
Region of interest (ROI) analysis
As our intention was to analyze the somatotopy for the two types of nociceptive stimuli focusing on a confined area with high spatial resolution rather than the whole brain, we anatomically defined four ROIs within the operculo-insular cortex in each hemisphere (8 ROIs in total). Following the approach described by Bense et al. (2001) and Afif et al. (2009), the insular cortex was divided into an anterior part, containing the three short (anterior) gyri, and a posterior part, containing the two long (posterior) gyri. Similarly the opercular cortex was divided into a frontal and a parietal part, using the central sulcus as the separating structure. As the quality of the EPI scans was high enough to recognize necessary anatomical landmarks, such as the boundaries of the insular cortex and insular sulci, the sylvian fissure and the central sulcus, this ROI definition was performed on the functional, blood-oxygen-level-dependent (BOLD)-sensitive images of each subject.
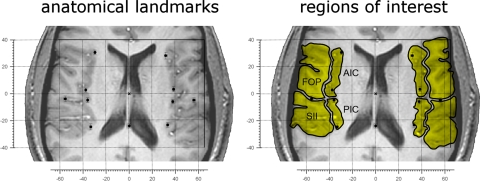
To obtain a measure of the structural brain variability of the subjects and to have anatomical landmarks to relate the activations to, we measured five landmarks in each hemisphere (Fig. 2) using the BOLD-sensitive images: the anterior and posterior poles of the insula (on the transversal slice where the insula appeared longest), the center of the curvature of the insula, the sulcus between the third and fourth insular sulcus (anatomical separation of anterior and posterior insula) and the location where the central sulcus ends (separation frontal versus parietal operculum). Locations of landmarks and of BOLD signal increases were measured relative to the anterior commissure (AC). Thus the origin of axes in our data are identical to that in the brain atlases of the Montreal Neurologial Institute and Talairach.
Fig. 2.
Variability in structural anatomy and definition of regions of interest on EPI scans. Left: the variability in structural anatomy of the population explored in this study. The individual brains were aligned to the anterior commissure (AC, origin of axes) to display variability of structural anatomy of the insular and opercular regions on a single brain. As AC is approximately located in the center of the brain as well as of the regions of interest (ROIs), this Talairach-like alignment was chosen to minimize the influence of interindividual anatomical differences. Each dot represents the average location of the landmarks used to define the eight ROIs. On each hemisphere, the medial dots indicate the anterior pole of the insula, the middle of the curvature of the insula, the sulcus between the 3rd and 4th insular gyri (the anatomical separation of anterior and posterior insula) (Bense et al. 2001) and the posterior pole of the insula. The lateral dot indicates the location where the central sulcus ends (separation between the frontal and the parietal operculum). The single dot on the midline represents the posterior commissure (PC). Axis scaling is in mm, error bars represent the standard error of the mean. Right: the manner in which 8 ROIs (in yellow) were determined in a representative subject. FOP, frontal operculum; AIC, anterior insular cortex; PIC, posterior insular cortex; SII, secondary somatosensory cortex.
Data analysis
Image analysis to reveal significant brain activity based on changes in BOLD signal was performed using FMRI Expert Analysis Tool (part of FSL, www.fmrib.ox.ac.uk/fsl). Prior to statistical analysis, the following preprocessing steps were applied to each subject's time series of fMRI volumes: motion correction (FMRIB's Linear Image Registration Tool) (Jenkinson et al. 2002), spatial smoothing using a Gaussian kernel of full width at half-maximum of 2 mm, subtraction of the mean of each voxel time course from that time course, and nonlinear high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with a high-pass filter cut-off of 50 s). The fMRI signal was then linearly modeled (Worsley and Friston 1995) on a voxel-by- voxel basis using a general linear model approach with local autocorrelation correction (Woolrich et al. 2001).
Group analysis
Before the analysis of single subject data, a group analysis was carried out using a mixed-effect approach, thus generating group-representative statistical maps of brain responses to laser and pinprick stimulation. For the purpose of the group-level analysis, registration of low-resolution functional images to the corresponding high-resolution structural images was performed for each subject (Jenkinson and Smith 2001) and followed by registration to a standard brain (MNI template) (Collins et al. 1994). The raw Z statistic images from the group analysis were thresholded at Z scores >2.3. A cluster-based approach (threshold P < 0.05) was used to correct for multiple comparisons (Worsley et al. 1992).
Single-subject analysis
As the quality of the EPI scans was high enough to recognize necessary anatomical landmarks, no image coregistration procedures were applied in this analysis, not even the alignment of functional to structural scans was necessary to assign activations to the ROIs chosen.
Single subject data were initially thresholded at Z = 2.3 and cluster corrected (minimum number of contiguous voxels constituting a cluster >4). For each subject, the coordinates of the voxel with the highest Z score (i.e., most significantly activated) in each of the eight ROIs (directly identified on the non-normalized functional images) were noted. This way, significant activations were found in 344/352 ROI analyses (11 subjects × 8 regions of interest × 4 conditions = 352 analyses). Lowering the statistical threshold to Z = 2 0.0 yielded two additional activations. The whole procedure of identification of the peak voxel within each of the ROIs was done separately by two experimenters (U. Baumgärtner and G. D. Iannetti) to double-check the results. The few cases (11 of 346) where different locations had been selected by these experimenters were re-examined until agreement regarding the location of the peak voxel was reached.
To check for the presence of hand-foot somatotopy, differences in coordinates (x, y, z) between hand and foot peak Z-score activation were calculated for each subject. Significance was tested using paired Student's t-test separately for x, y, and z coordinates (in mm) for all subjects; presence of somatotopy was assumed in cases where the difference between hand and foot peak activations within an ROI was significantly larger than 0 mm for at least one axis. Differences in location <2 mm in the horizontal plane and 4 mm in vertical direction were not considered as valid even in case they were significant, because this was below the scanning resolution (2 × 2 × 4 mm). Differences in the location of activations induced by the two stimulation modalities (heat or pinprick) within the same stimulation area (hand or foot) were tested using the same approach.
RESULTS
Pain ratings
During the fMRI experiment the average pricking pain intensity ratings (on 0–10 scale) were as follows: laser hand = 2.8 ± 1.1; laser foot = 3.2 ± 1.3; pinprick hand = 1.7 ± 0.7; pinprick foot = 2.1 ± 0.7. Two-way ANOVA revealed a significant main effect of the modality (higher pain ratings for laser stimulation compared with pinprick stimulation; P < 0.01), no significant main effect for stimulus site, and no significant interaction. Average pain ratings remained stable throughout the experiment (Kruskall-Wallis test, P > 0.3).
fMRI activations
GROUP ANALYSIS.
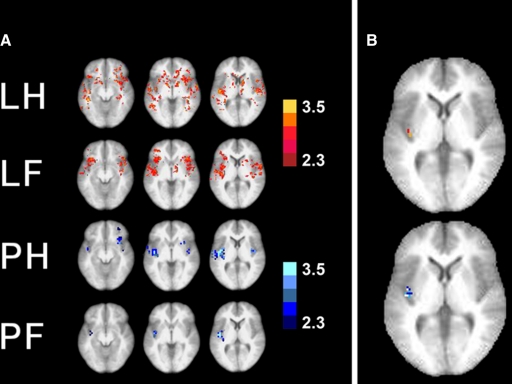
The initial group analysis using a significance threshold of 2.3 (cluster threshold of 4 voxels) yielded significant activations in various brain areas. In Fig. 3A, three transversal slices 8 mm apart from each other in superior-inferior (z-coordinate) direction show voxels with significant activations (Z-score >2.3, see color bars) following stimulation of the hand or foot with laser (LH, laser hand; LF, laser foot) or pinprick stimuli (PH, pinprick hand; PF, pinprick foot). At a glance, there is a scattering of voxels visible without clear hand-foot somatototopy within the operculo-insular and frontal regions with the widest distribution for laser hand stimulation (top) and the smallest number of significant voxels for pinprick foot stimulation (bottom). The area that is active most reliably in all of the four modalities (laser hand, laser foot, pinprick hand, pinprick foot) is the posterior insula of the left (contralateral) hemisphere. In Fig. 3B, right, hand and foot stimulation is shown on the same brain slice for laser (top) and pinprick stimulation (bottom). The significance threshold was raised from 2.3 to 3.8; this unmasks a different representation for hand and foot in the posterior insula. The hand representation (red voxels for laser, dark blue voxels for pinprick stimuli) is shown to be anterior to the foot representation (orange voxels for laser, light blue voxels for pinprick stimuli; white voxels: overlap).
Fig. 3.
Group analysis. A: at the significance threshold of Z = 2.3, 3 transversal slices through the operculo-insular region are shown (left to right; 4 mm below AC level, 4 mm above AC level, 12 mm above AC level) in 4 lines for the 4 modalities (laser hand, laser foot, pinprick hand, pinprick foot). Although especially for the laser stimuli, the brain activations are scattered throughout the operculo-insular and frontal cortices, the brain area that is activated most consistently across the 4 different stimulation modalities is the left posterior insula. B: brain activation of both hand and foot stimulation is shown on the same slice (laser: top; pinprick: bottom). At an elevated threshold of Z = 3.8, a differential somatotopic representation within the posterior insula in anterior-posterior direction can be seen: the hand representation is anterior to the foot representation. Top: laser hand (red pixels) vs. foot (orange pixels). Bottom: pinprick hand (dark blue) vs. foot (light blue), overlapping pixels in white. Note that on group analysis level, the only somatotopic representation that can be identified is in the posterior insula. Left: the left side of the brains.
SINGLE SUBJECT ROI ANALYSIS.
The quality of the functional EPI scans was high enough to localize peak activations within each of the eight ROIs in almost every case (346 of 352 analyses). The peak Z-scores ranged from 2.2 to 8.5. Differences in location and Z-scores of peak voxels for all ROIs between locations of stimulation (hand and foot) in each of the two modalities are shown in Table 1. Results for each for the single dimensions are shown separately (x: medial-lateral, y: anterior-posterior, z: superior-inferior) as well as in three-dimensional distance (3D). An asterisk marks significant differences.
Table 1.
Differences in location and Z-scores of fMRI responses to hand and foot stimulation
| Left SII | Right SII | Left PI | Right PI | Left AI | Right AI | Left FOP | Right FOP | |
|---|---|---|---|---|---|---|---|---|
| Laser stimulation | ||||||||
| x, mm | 6.9* | −5.0 | 2.2* | −2.0 | 0.9 | 0.4 | 3.0 | 2.5 |
| y, mm | −2.4 | −5.2 | 8.5* | 4.6 | 9.3* | 4.0 | −8.2 | 2.9 |
| z, mm | 1.1 | 0.0 | −2.2 | −2.4* | −3.6 | −3.3 | 1.2 | 2.5 |
| 3D, mm | 7.4 | 7.2 | 9.1 | 5.6 | 10.0 | 5.2 | 8.8 | 4.6 |
| Z-score | 0.2 | −0.6 | −1.2 | −0.8 | −0.4 | −0.3 | −0.5 | −0.4 |
| Pinprick stimulation | ||||||||
| x, mm | 1.3 | −4.2 | 0.9 | −0.2 | 0.4 | 1.1 | −0.9 | 3.2 |
| y, mm | −0.5 | −5.8 | 8.9* | 5.6 | −2.6 | 4.7 | 0.4 | −2.2 |
| z, mm | −0.4 | 2.5 | −1.5 | −1.8 | −1.6 | −2.9 | −4.0 | −0.8 |
| 3D, mm | 1.4 | 7.6 | 9.1 | 5.9 | 3.1 | 5.7 | 4.1 | 4.0 |
| Z-score | 0.5 | 0.1 | −0.4 | −0.4 | 0.5 | 0.1 | 0.8 | 0.7 |
All differences are obtained by subtracting the foot values from the hand values; stimuli were delivered to the right side of the body;
P < 0.05 (paired t-test).
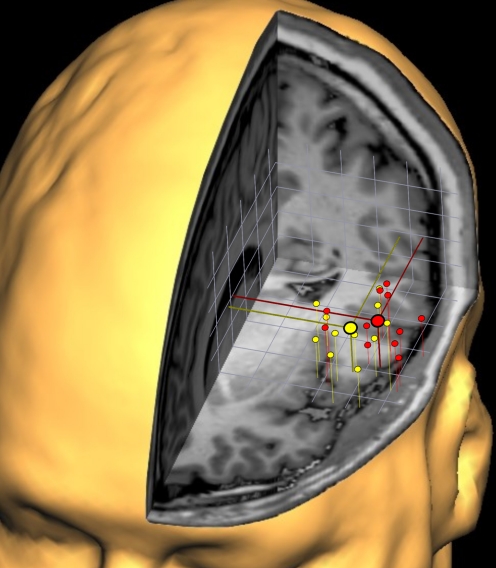
As an example of the spatial scattering of individual cortical activations in 3D space, Fig. 4 illustrates the individual peak voxel activations of laser hand (red dots) and laser foot (yellow dots) stimulation within the ROI “left SII” projected into the brain of one of the participants of this study. The average localizations (large symbols) show a shift of hand and foot representation in medial-lateral direction with the hand representation further lateral. The overlap of the distributions of red and yellow symbols demonstrates why the individual analysis with pairwise comparisons within subjects is likely to yield sharper separations with a better spatial resolution than the resolution that can be achieved by means of a group analysis.
Fig. 4.
Three-dimensional clustering of the individual cortical responses obtained within SII following laser hand (red) and foot (yellow) stimulation. To gain an impression of the variability of cortical representations within SII, the individual activations of all subjects (n = 11) were projected onto 1 subject's 3-dimensional reconstruction of the brain. The individual coordinate systems were aligned to the anterior commissure and the AC-PC plane. Despite the overlap between the scatterings of the red and yellow symbols, a systematic difference in medial-lateral direction is visible, and the average localizations for hand and foot representation are illustrated by the large symbols. Grid width: 1 cm.
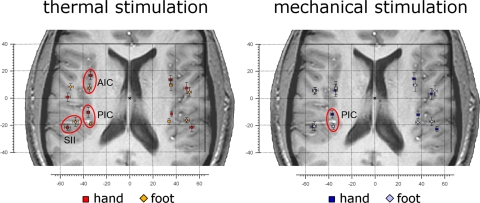
For heat stimulation (Table 1A), we found a significant somatotopy in contralateral (left) SII/PV, with the hand 6.9 mm lateral to the foot, and a 3D distance of the x, y, and z coordinates [square root (x2 + y2 + z2)] of 7.4 mm; contralateral (left) posterior insula, with hand 8.5 mm anterior to the foot and a 3D distance of 9.1 mm; and contralateral (left) anterior insula, with hand 9.3 mm anterior to the foot, and a 3D distance of 10.0 mm (Fig. 5, left).
Fig. 5.
Somatotopic representation of hand and foot following noxious stimulation. Left: the average location of the activations in response to heat (laser) stimulation of the hand (red squares) and foot dorsum (orange diamonds) in each ROI. As indicated by red ellipsoids, the location of the activations was significantly different (circled in red) in the contralateral anterior insula, posterior insula, and parietal operculum (SII; for a quantitative description of location differences see Table 1A). Right: the average location of the activations in response to pinprick (punctate probe) stimulation of the hand (dark blue squares) and foot dorsum (pale blue diamonds), in each ROI. The location of the activations was significantly different in the contralateral posterior insula (for a quantitative description of location differences see Table 1B). In all panels, error bars represent SE. The left side of the brains is shown on the left. AIC, anterior insular cortex; PIC, posterior insular cortex; SII, secondary somatosensory cortex.
For pinprick stimulation (Table 1B), we found a significant somatotopy only in the contralateral (left) posterior insula, with hand 8.9 mm anterior to the foot, and a 3D distance of 9.1 mm (Fig. 5, right).
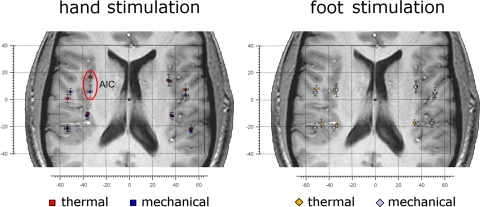
For hand stimulation (Table 2A), we found a significant difference between the location of the responses to laser and pinprick stimulation in the contralateral (left) anterior insula, with the response to laser stimulation 11.0 mm anterior to the response to pinprick stimulation, and a 3D distance of 11.4 mm (Fig. 6, left). For foot stimulation (Table 2B), we could not identify significant differences between stimulation modalities. Differences in location (in mm) and Z-scores of peak voxels for all ROIs between modalities of stimulation (heat and pinprick) in each ROI are shown in Table 2.
Table 2.
Differences in location and Z-scores of fMRI responses to laser and pinprick stimulation
| Left SII | Right SII | Left PI | Right PI | Left AI | Right AI | Left FOP | Right FOP | |
|---|---|---|---|---|---|---|---|---|
| Hand stimulation | ||||||||
| x, mm | 0.2 | −0.2 | −0.9 | 1.3 | −0.4 | −1.5 | 1.4 | −0.6 |
| y, mm | 0.5 | −1.5 | −1.5 | −0.5 | −11.0* | 0.7 | 5.2 | −3.6 |
| z, mm | 1.5 | −1.8 | 0.4 | 1.1 | −2.8 | −0.7 | 0.4 | −1.2 |
| 3D, mm | 1.6 | 2.3 | 1.8 | 1.8 | 11.4 | 1.8 | 5.4 | 3.8 |
| Z-score | −0.4 | −0.2 | 0.0 | 0.1 | −0.3 | −0.2 | 0.3 | 0.4 |
| Foot stimulation | ||||||||
| x, mm | −5.5§ | −0.8 | −2.2 | 2.2 | 1.3 | 0.7 | −1.1 | 0.0 |
| y, mm | 1.3 | −0.4 | −1.8 | −1.0 | 0.0 | 0.0 | −2.9 | −1.0 |
| z, mm | 0.0 | 0.4 | 1.1 | 2.4 | −1.8 | 0.4 | 1.8 | −3.2 |
| 3D, mm | 5.6 | 1.0 | 3.0 | 3.4 | 2.2 | 0.8 | 3.6 | 3.4 |
| Z-score | 0.0 | 0.2 | 0.8 | 0.5 | 0.5 | 0.7 | 0.9 | 0.7 |
All differences are obtained by subtracting the laser values from the pinprick values; stimuli were delivered to the right side of the body;
P < 0.05 (paired t-test). §P = 0.0508.
Fig. 6.
Different location of activations in response to heat and pinprick stimulation. Left: the average location of the activations in response to heat (laser) and pinprick (punctate probe) stimulation of the hand dorsum in each ROI. Red squares represent heat stimulation, blue squares represent pinprick stimulation. The location of the activations was significantly different in the contralateral anterior insula (circled in red; for a quantitative description of location differences see Table 2A). Right: the average location of the activations in response to heat (laser) and pinprick (punctuate probe) stimulation of the foot dorsum. Orange diamonds represent heat stimulation, pale blue diamonds represent pinprick stimulation. For a quantitative description of location differences see Table 2B. In all panels error bars represent SE. The left side of the brains is shown on the left. AIC, anterior insular cortex.
In all comparisons (both between body sites and between modalities), the Z-scores were constant across subjects within the conditions tested, and the differences in Z-scores were not significantly different from zero (Tables 1 and 2).
DISCUSSION
Activation of the operculo-insular cortex has been demonstrated in the vast majority of studies investigating the brain responses to nociceptive stimuli in humans (Apkarian et al. 2005; Peyron et al. 2000). Using high-resolution fMRI based on individual, untransformed anatomical and functional data, we now demonstrate a somatotopic representation of nociceptive stimulation in three operculo-insular ROIs. There were spatially separate cortical responses to hand and foot stimulation in the contralateral posterior insula following both heat and pinprick stimulation and in the contralateral anterior insula and parietal operculum following heat stimulation only. The mediolateral somatotopy in the parietal operculum was consistent with that of tactile representation in SII or PV (Disbrow et al. 2000). The posterior-anterior somatotopy in the insula was consistent with the predicted somatotopy for cortical projection areas of the proposed thalamic nucleus, VMpo (Craig 1995). Furthermore, we found differences in location of cortical responses to heat stimulation versus pinprick stimulation in the contralateral anterior insula following hand stimulation.
Specificity of nociceptive stimuli and specificity of the areas activated
We can assume with enough certainty that the stimuli applied in this study were specific to activate nociceptive fibers. This is clearly the case for the laser stimulus, which has been shown to activate Aδ- and C-nociceptors, only a tiny fraction of C-warm-fibers (∼10%), and no Aβ-fibers as demonstrated in single fiber recordings (Bromm and Treede 1984; Bromm et al. 1984; Treede et al. 1995). The contribution of the C-warm fibers to the centrally conducted signal seems negligible because 80% of this fraction stops firing at all at temperatures in the higher noxious range (45–50°C) (Darian-Smith et al. 1979; LaMotte and Campbell 1978).
The pinprick stimulus, on the other hand, is less specific for nociception, because it co-activates Aβ-, Aδ-, and C-fibers. Whereas the surface of the mechanical probe seems to be hardly relevant to single unit responses of low threshold mechanoreceptors (Aβ-fibers), the prickliness is differentially coded by a population of Aδ- and polymodal C-nociceptors (Garnsworthy et al. 1988). Among these, probe size (diameter) and force are better reflected in activity of Aδ- than in activity of C-nociceptors with one subpopulation having high discharge rates at the threshold where mechanical stimuli are just recognized as sharp and another subpopulation with high discharge rates at higher intensities (Garell et al. 1996; Greenspan and McGillis 1994).
Taken together, the stimulation used in our study was nociceptive, and thus the brain areas activated, like the frontal operculum, SII/PV, and anterior and posterior insula (and other regions outside our ROIs) were responsive to noxious stimuli. Because we did not compare brain responses following noxious versus nonnoxious stimulation in this study, we cannot conclude directly that these brain areas are, in turn, specific for nociceptive processing. The fact that for tactile stimuli so far only a lateral-medial representation has been identified in the area of SII/PV suggests that the double anterior-posterior somatotopy in the insula might be specific for nociception. On the other hand, there is evidence for polymodality of regions within the operculo-insular cortex coming from a number of studies (Davis et al. 1998; Downar et al. 2000; Matsuhashi et al. 2004).
Somatotopy in the opercular cortex
In functional imaging studies of the human brain, usually the entire parietal operculum and sometimes even parts of the frontal operculum are labeled SII. According to electrophysiological and imaging studies in monkeys and humans of the parietal operculum (Disbrow et al. 2000; Fitzgerald et al. 2004; Krubitzer et al. 1995), this large area can be separated into two or three functionally distinct regions with separate representations of the body: PV (anterior part, facing the posterior insula), SII proper (more posterior than PV), and possibly a third area further posterior. Cytoarchitectonically, the human parietal opercular region has recently been divided into four areas (OP1–OP4) (Eickhoff et al. 2006b), where OP1 corresponds to SII and OP4 to PV. A meta-analysis of imaging studies investigating the responses to nociceptive and nonnociceptive stimuli found the clusters responding to nociceptive stimuli within OP1, the posterior part of the parietal operculum (Eickhoff et al. 2006a), and the clusters responding to nonnociceptive stimuli more anterior, at the border of OP1 and OP4. The fMRI peaks within the posterior operculum (SII/PV) as identified in the single subject analysis of the present study (Table 1, Fig. 5) were located mostly in OP1 or at the border of OP1 and OP4, and thus are consistent with being in SII proper.
The somatotopy that we found in SII is consistent with that known for tactile representation in SII (Disbrow et al. 2000). A similar somatotopy, with hand representation lateral to foot representation in SII, had previously been reported in a fMRI study using laser stimulation (Bingel et al. 2004). However, possibly due to the limited spatial resolution, they were not able to ascertain whether the peak of activity following foot stimulation was located in SII or posterior insula. We found distinct locations for foot representation both in SII proper and posterior insula and hence demonstrated the presence of a nociceptive homunculus for thermal stimuli in SII. Ferretti et al. (2004) found two activation areas for hand and foot in SII/PV; within each activation area, nociceptive stimuli were represented more posteriorly than nonnociceptive stimuli. These authors reported a significant somatotopy for the nonnociceptive stimuli only; however, they used two different intensities of electrical stimulation, and brain responses were detected using a 1.5 T scanner, thus raising the questions of specificity of the afferent neuronal populations activated and spatial resolution of the imaging technique. Our data indicate that apart from tactile information, as has been demonstrated by other investigators (Disbrow et al. 2000; Ferretti et al. 2004), also nociceptive information is projecting to SII as part of the “classical” lateral pain pathway and is somatotopically organized.
Only few nociceptive neurons have been identified in SII proper (Robinson and Burton 1980), and the largest published series of nociceptive neurons in that region is from a more posterior area (Dong et al. 1989). The scarcity of monkey electrophysiological data may be due to inadequate search stimuli: both type I and II AMH are not activated by gentle mechanical search stimuli, and electrical stimulation is necessary to study these primary afferents (Treede et al. 1998).
Although in the frontal operculum we observed significant responses to the nociceptive stimulation, we did not find significant hand-foot somatotopy in this area. We could thus not reproduce the posterior-anterior somatotopy in the frontal operculum observed by Vogel and coworkers (Vogel et al. 2003). One possible explanation could be that in that their source analysis study, the somatotopy of the dorsal insula was projected into the operculum. On the other hand, invasive depth recordings have confirmed a generator source within the frontal operculum, so this generator may be nonsomatotopically organized.
Somatotopy in the insular cortex
As there is not yet an established standard on how to segregate the insula into functionally different ROIs, we chose to use gross anatomical features. The separation into an anterior part, containing the three short gyri, and a posterior part, containing the two long gyri, separated by the central insular sulcus, has been used previously by Ostrowsky et al. (2000) during invasive mapping and by Bense et al. (2001) in an fMRI study. Efforts to establish a stereotactic template of the insula have also been made (Afif et al. 2009). According to Brodmann (1909), the human insula contains a dorsogranular field and a ventrorostral agranular field; however, he did not assign any area numbers to it. Furthermore, monkey data show different cytoarchitecture between anterior and posterior insula, possibly with three distinct subregions (Augustine 1985). Very recently, first data on cytoarchitectonic mapping of the human posterior insula obtained from analysis of post mortem brains have become available (Kurth et al. 2009). These data suggest an even finer separation of the insula than previously supposed, but this work is currently still in progress.
We found two separate representations in the insula, both with the anterior-posterior somatotopy predicted for VMpo projection areas. A similar somatotopy with hand representation anterior to foot representation in dorsal posterior insula had previously been reported in a fMRI study using contact heat stimulation (Brooks et al. 2005) and was very recently demonstrated by an intra-operative stimulation study using laser stimuli (Mazzola et al. 2009). Another study with painful stimulation of muscles also reported hand representation anterior and lateral of foot in dorsal posterior insula (Henderson et al. 2007).
These data underline the importance of the (posterior) insular cortex as a projection target for the spinothalamic pathway through lamina 1 and the proposed VMpo thalamic nucleus (Craig and Zhang 2006). In addition to the previous studies, we found the same anterior-posterior gradient of hand and foot representation in both parts (anterior and posterior) of the contralateral (left) insula. In response to pinprick stimuli, a similar somatotopical organization was found in the same direction within the posterior insula. If one follows the suggestion by Craig (2009b), this somatotopy in the posterior insula could be a VMpo projection with re-representation in the anterior part of the insula.
The findings of enhanced activity of the left frontal operculo-insular cortex during a discrimination task using electroencphalographic (EEG) source analysis (Schlereth et al. 2003) and increased activity of the anterior insula (bilaterally) during a memory task of intensity discrimination using fMRI (Albanese et al. 2007) following hand stimulation, supports the finding of hand-foot somatotopy in this region since stimulus discrimination requires an anatomical map of the body.
Separate representation of noxious heat and pinprick stimuli in the anterior insula?
An unexpected finding was the significantly different representation of noxious heat and pinprick stimulation of the hand in the anterior insular cortex with heat pain representation located on average 11 mm more anterior compared with the representation of mechanical pain. We did not observe the same phenomenon for stimulation of the foot. One possible explanation could be that a larger hand representation yields stronger fMRI activations, making it easier to detect differences. However this seems implausible because Z-scores between hand and foot activations differed <0.5 and were nonsignificant (Tables 1, A and B), and pain ratings were even slightly lower following hand stimulation.
The anterior part of the insula has been assigned to the processing of emotion as well as pain anticipation and human awareness (Craig 2009a; Damasio et al. 2000; Ploghaus et al. 1999). It has been further characterized as center for interoception that integrates somato-visceral input from the body together with autonomic input and emotional awareness to drive adequate behavioral and motor responses (Craig 2009b; Critchley 2005). Different self-induced emotions, mostly negative, lead to different activations within the anterior insulas (left and right) (Damasio et al. 2000). Although the subjects in our study had to rate the pricking aspect of the evoked pain sensation that better emphasizes the sensory than the affective dimension, because sensory and affective pain dimensions are closely interrelated, one may speculate that the interoceptive input is different with the result of different representations within the anterior insula for noxious heat and mechanical stimuli. The different representation of heat pain could be due to different levels of attention, emotion, or anticipation induced by a higher level of potential threat or negative affect associated with the laser stimulus. Because sensory inputs from the hands are more important than from the feet in everyday-life, it appears reasonable why we found this segregation for the hands only.
Conclusion
By evaluating four subregions of the operculo-insular cortex separately, we identified at least three representations of nociceptive inputs within this region. We have therefore contributed to a resolution of a controversy in the previously published literature that tried to establish the single representation of pain in that region. Our data indicate that both the classical lateral pain pathway from VPI to SII, as well as the pathway from VMpo to dorsal posterior insula, have cortical projections that are somatotopically organized but at 90° angle from each other. Due to the limited temporal resolution of fMRI, it was not possible to ascertain whether the projection target in the anterior insula is processing information in parallel to the posterior insula or is activated following the posterior part in a sequential way.
GRANTS
This study was supported by Deutsche Forschungsgemeinschaft Grant DFG Tr 236/13-3. G. D. Iannetti is University Research Fellow of The Royal Society. FMRIB Centre is supported by the Medical Research Council of Great Britain.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Afif et al., 2009.Afif A, Hoffmann D, Becq G, Guenot M, Magnin M, Mertens P. MRI-based definition of a stereotactic two-dimensional template of the human insula. Stereotact Funct Neurosurg 87: 385–394, 2009 [DOI] [PubMed] [Google Scholar]
- Afif et al., 2008.Afif A, Hoffmann D, Minotti L, Benabid AL, Kahane P. Middle short gyrus of the insula implicated in pain processing. Pain 138: 546–555, 2008 [DOI] [PubMed] [Google Scholar]
- Albanese et al., 2007.Albanese MC, Duerden EG, Rainville P, Duncan GH. Memory traces of pain in human cortex. J Neurosci 27: 4612–4620, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson et al., 1997.Andersson JLR, Lilja A, Hartvig P, Långström B, Gordh T, Handwerker H, Torebjörk E. Somatotopic organization along the central sulcus, for pain localization in humans, as revealed by positron emission tomography. Exp Brain Res 117: 192–199, 1997 [DOI] [PubMed] [Google Scholar]
- Apkarian et al., 2005.Apkarian AV, Bushnell C, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005 [DOI] [PubMed] [Google Scholar]
- Augustine, 1985.Augustine JR. The insular lobe in primates including humans. Neurol Res 7: 2–10, 1985 [DOI] [PubMed] [Google Scholar]
- Baumgärtner et al., 2006a.Baumgärtner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeyer T, Rolke R, Höhnemann S, Piel M, Rösch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, Schreckenberger M. High opiate receptor binding potential in the human lateral pain system. Neuroimage 30: 692–699, 2006a [DOI] [PubMed] [Google Scholar]
- Baumgärtner et al., 2002.Baumgärtner U, Magerl W, Klein T, Hopf HC, Treede R-D. Neurogenic hyperalgesia versus painful hypoalgesia: two distinct mechanisms of neuropathic pain. Pain 96: 141–151, 2002 [DOI] [PubMed] [Google Scholar]
- Baumgärtner et al., 2006b.Baumgärtner U, Tiede W, Treede R-D, Craig AD. Laser-evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol 96: 2802–2808, 2006b [DOI] [PubMed] [Google Scholar]
- Bense et al., 2001.Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stuimulation (fMRI). J Neurophysiol 85: 886–899, 2001 [DOI] [PubMed] [Google Scholar]
- Bingel et al., 2004.Bingel U, Lorenz J, Glauche V, Knab R, Gläscher J, Weiller C, Büchel C. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. Neuroimage 23: 224–232, 2004 [DOI] [PubMed] [Google Scholar]
- Brodmann, 1909.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth, 1909 [Google Scholar]
- Bromm et al., 1984.Bromm B, Jahnke MT, Treede RD. Responses of human cutaneous afferents to CO2 laser stimuli causing pain. Exp Brain Res 55: 158–166, 1984 [DOI] [PubMed] [Google Scholar]
- Bromm and Treede, 1984.Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol 3: 33–40, 1984 [PubMed] [Google Scholar]
- Brooks et al., 2005.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27: 201–209, 2005 [DOI] [PubMed] [Google Scholar]
- Collins et al., 1994.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205, 1994 [PubMed] [Google Scholar]
- Craig, 2009a.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci 364: 1933–1942, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, 2009b.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70, 2009b [DOI] [PubMed] [Google Scholar]
- Craig, 1995.Craig AD. Supraspinal projections of lamina I neurons. In: Forebrain Areas Involved in Pain Processing, edited by Besson JM, Guilbaud GOH. Paris: John Libbey, 1995, p. 13–26 [Google Scholar]
- Craig et al., 1994.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770–773, 1994 [DOI] [PubMed] [Google Scholar]
- Craig and Dostrovsky, 1997.Craig AD, Dostrovsky JO. Processing of nociceptive information at supraspinal levels. In: Anesthesia: Biologic Foundations, edited by Yaksh TL. Philadelphia, PA: Lippincott-Raven, 1997, p. 625–642 [Google Scholar]
- Craig and Zhang, 2006.Craig AD, Zhang ET. Retrograde analyses of spinothalamic projections in the macaque monkey: input to posterolateral thalamus. J Comp Neurol 499: 953–964, 2006 [DOI] [PubMed] [Google Scholar]
- Critchley, 2005.Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol 493: 154–166, 2005 [DOI] [PubMed] [Google Scholar]
- Cruccu et al., 2003.Cruccu G, Pennisi E, Truini A, Iannetti GD, Romaniello A, Le Pera D, De Armas L, Leandri M, Manfredi M, Valeriani M. Unmyelinated trigeminal pathways as assessed by laser stimuli in humans. Brain 126: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- Damasio et al., 2000.Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci 3: 1049–1056, 2000 [DOI] [PubMed] [Google Scholar]
- Darian-Smith et al., 1979.Darian-Smith I, Johnson KO, LaMotte C, Shigenaga Y, Kenins P, Champness P. Warm fibers innervating palmar and digital skin of the monkey: responses to thermal stimuli. J Neurophysiol 42: 1297–1315, 1979 [DOI] [PubMed] [Google Scholar]
- DaSilva et al., 2002.DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci 22: 8183–8192, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al., 1998.Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80: 1533–1546, 1998 [DOI] [PubMed] [Google Scholar]
- Disbrow et al., 2000.Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of homo sapiens: evidence for SII and PV. J Comp Neurol 418: 1–21, 2000 [DOI] [PubMed] [Google Scholar]
- Dong et al., 1989.Dong WK, Salonen LD, Kawakami Y, Shiwaku T, Kaukoranta EM, Martin RF. Nociceptive responses of trigeminal neurons in SII-7b cortex of awake monkeys. Brain Res 484: 314–324, 1989 [DOI] [PubMed] [Google Scholar]
- Downar et al., 2000.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283, 2000 [DOI] [PubMed] [Google Scholar]
- Dum et al., 2009.Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29: 14223–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff et al., 2006a.Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlation with functional imaging results. Cereb Cortex 16: 268–279, 2006a [DOI] [PubMed] [Google Scholar]
- Eickhoff et al., 2006b.Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb Cortex 16: 254–267, 2006b [DOI] [PubMed] [Google Scholar]
- Ferretti et al., 2004.Ferretti A, Del Gratta C, Babiloni C, Caulo M, Arienzo D, Tartaro A, Rossini PM, Romani GL. Functional topography of the secondary somatosensory cortex for nonpainful and painful stimulation of median and tibial nerve: an fMRI study. Neuroimage 23: 1217–1225, 2004 [DOI] [PubMed] [Google Scholar]
- Fitzgerald et al., 2004.Fitzgerald PJ, Lane JW, Thakur PH, Hsiao SS. Receptive field properties of the macaque second somatosensory cortex: evidence for multiple functional representations. J Neurosci 24: 11193–11204, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frot and Mauguière, 2003.Frot M, Mauguière F. Dual representation of pain in the operculo-insular cortex in humans. Brain 126: 438–450, 2003 [DOI] [PubMed] [Google Scholar]
- Garell et al., 1996.Garell PC, McGillis SL, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. J Neurophysiol 75: 1177–1189, 1996 [DOI] [PubMed] [Google Scholar]
- Garnsworthy et al., 1988.Garnsworthy RK, Gully RL, Kenins P, Mayfield RJ, Westerman RA. Identification of the physical stimulus and the neural basis of fabric-evoked prickle. J Neurophysiol 59: 1083–1097, 1988 [DOI] [PubMed] [Google Scholar]
- Gelnar et al., 1998.Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV. Fingertip representation in the human somatosensory cortex: an fMRI study. Neuroimage 7: 261–283, 1998 [DOI] [PubMed] [Google Scholar]
- Greenspan and McGillis, 1994.Greenspan JD, McGillis SLB. Thresholds for the perception of pressure, sharpness, and mechanically evoked cutaneous pain: effects of laterality and repeated testing. Somatosens Motor Res 11: 311–317, 1994 [DOI] [PubMed] [Google Scholar]
- Greenspan et al., 1999.Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 81: 273–282, 1999 [DOI] [PubMed] [Google Scholar]
- Greenspan et al., 1997.Greenspan JD, Thomadaki M, McGillis SLB. Spatial summation of perceived pressure, sharpness and mechanically evoked cutaneous pain. Somatosens Motor Res 14: 107–112, 1997 [DOI] [PubMed] [Google Scholar]
- Head and Holmes, 1911.Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain 34: 102–254, 1911 [Google Scholar]
- Henderson et al., 2007.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 128: 20–30, 2007 [DOI] [PubMed] [Google Scholar]
- Iannetti et al., 2004.Iannetti GD, Leandri M, Truini A, Zambreanu L, Cruccu G, Tracey I. A-delta nociceptor response to laser stimuli: selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin Neurophysiol 115: 2629–2637, 2004 [DOI] [PubMed] [Google Scholar]
- Iannetti et al., 2003.Iannetti GD, Truini A, Romaniello A, Galeotti F, Rizzo C, Manfredi M, Cruccu G. Evidence of a specific spinal pathway for the sense of warmth in humans. J Neurophysiol 89: 562–570, 2003 [DOI] [PubMed] [Google Scholar]
- Iannetti et al., 2005.Iannetti GD, Zambreanu L, Cruccu G, Tracey I. Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing: a study with laser evoked potentials in humans. Neuroscience 131: 199–208, 2005 [DOI] [PubMed] [Google Scholar]
- Jenkinson et al., 2002.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841, 2002 [DOI] [PubMed] [Google Scholar]
- Jenkinson and Smith, 2001.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156, 2001 [DOI] [PubMed] [Google Scholar]
- Kaas, 1983.Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–231, 1983 [DOI] [PubMed] [Google Scholar]
- Krubitzer et al., 1995.Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci 15: 3821–3839, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth et al., 2010.Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex 20: 1448–1461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte and Campbell, 1978.LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol 41: 509–528, 1978 [DOI] [PubMed] [Google Scholar]
- Lenz et al., 1988.Lenz FA, Dostrovsky JO, Tasker RR, Yamashiro K, Kwan HC, Murphy JT. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 59: 299–316, 1988 [DOI] [PubMed] [Google Scholar]
- Lenz and Dougherty, 1997.Lenz FA, Dougherty PM. Pain processing in the human thalamus. In: Thalamus. Experimental/Clinical Aspects, edited by Steriade M, Jones EG, McCormick DA. Oxford, UK: Elsevier, 1997, vol. II, p. 617–651 [Google Scholar]
- Lenz et al., 1994.Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO. Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol 72: 1570–1587, 1994 [DOI] [PubMed] [Google Scholar]
- Magerl et al., 2001.Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in pain and secondary hyperalgesia. Brain 124: 1754–1764, 2001 [DOI] [PubMed] [Google Scholar]
- Matsuhashi et al., 2004.Matsuhashi M, Ikeda A, Ohara S, Matsumoto R, Yamamoto J, Takayama M, Satow T, Begum T, Usui K, Nagamine T, Mikuni N, Takahashi J, Miyamoto S, Fukuyama H, Shibasaki H. Multisensory convergence at human temporo-parietal junction—epicortical recording of evoked responses. Clin Neurophysiol 115: 1145–1160, 2004 [DOI] [PubMed] [Google Scholar]
- Mazzola et al., 2009.Mazzola L, Isnard J, Peyron R, Guénot M, Mauguière F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain 146: 99–104, 2009 [DOI] [PubMed] [Google Scholar]
- Özcan et al., 2005.Özcan M, Baumgärtner U, Vucurevic G, Stoeter P, Treede RD. Spatial resolution of fMRI in the human parasylvian cortex: comparison of somatosensory and auditory activation. Neuroimage 25: 877–887, 2005 [DOI] [PubMed] [Google Scholar]
- Ostrowsky et al., 2000.Ostrowsky K, Isnard J, Ryvlin P, Guenot M, Fischer C, Mauguiere F. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia 41: 681–686, 2000 [DOI] [PubMed] [Google Scholar]
- Ostrowsky et al., 2002.Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguière F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex 12: 376–385, 2002 [DOI] [PubMed] [Google Scholar]
- Peyron et al., 2000.Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 30: 263–288, 2000 [DOI] [PubMed] [Google Scholar]
- Ploghaus et al., 1999.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science 284: 1979–1981, 1999 [DOI] [PubMed] [Google Scholar]
- Ploner et al., 1999.Ploner M, Schmitz F, Freund HJ, Schnitzler A. Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol 81: 3100–3104, 1999 [DOI] [PubMed] [Google Scholar]
- Rios et al., 1999.Rios M, Treede RD, Lee JI, Lenz FA. Direct evidence of nociceptive input to human anterior cingulate gyrus and parasylvian cortex. Curr Rev Pain 3: 256–264, 1999 [DOI] [PubMed] [Google Scholar]
- Robinson and Burton, 1980.Robinson CJ, Burton H. Somatic submodality distribution within the second somatosensory (SII), 7b, retroinsular, postauditory, and granular insular cortical areas of M. fascicularis. J Comp Neurol 192: 93–108, 1980 [DOI] [PubMed] [Google Scholar]
- Rolke et al., 2006.Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihöfner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the german research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain 123: 231–243, 2006 [DOI] [PubMed] [Google Scholar]
- Schlereth et al., 2003.Schlereth T, Baumgärtner U, Magerl W, Stoeter P, Treede R-D. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. Neuroimage 20: 441–454, 2003 [DOI] [PubMed] [Google Scholar]
- Slugg et al., 2000.Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. J Neurophysiol 83: 2179–2191, 2000 [DOI] [PubMed] [Google Scholar]
- Tarkka and Treede, 1993.Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol 10: 513–519, 1993 [DOI] [PubMed] [Google Scholar]
- Tracey and Mantyh, 2007.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 55: 377–391, 2007 [DOI] [PubMed] [Google Scholar]
- Treede et al., 2000.Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain 87: 113–119, 2000 [DOI] [PubMed] [Google Scholar]
- Treede et al., 1998.Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 80: 1082–1093, 1998 [DOI] [PubMed] [Google Scholar]
- Treede et al., 1995.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 483: 747–758, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel et al., 2003.Vogel H, Port JD, Lenz FA, Solaiyappan M, Krauss G, Treede RD. Dipole source analysis of laser-evoked subdural potentials recorded from parasylvian cortex in humans. J Neurophysiol 89: 3051–3060, 2003 [DOI] [PubMed] [Google Scholar]
- Woolrich et al., 2001.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage 14: 1370–1386, 2001 [DOI] [PubMed] [Google Scholar]
- Worsley et al., 1992.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12: 900–918, 1992 [DOI] [PubMed] [Google Scholar]
- Worsley and Friston, 1995.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited - again. Neuroimage 2: 173–181, 1995 [DOI] [PubMed] [Google Scholar]
- Xu et al., 1997.Xu X, Fukuyama H, Yazawa S, Mima T, Hanakawa T, Magata Y, Kanda M, Fujiwara N, Shindo K, Nagamine T, Shibasaki H. Functional localization of pain perception in the human brain studied by PET. Neuroreport 8: 555–559, 1997 [DOI] [PubMed] [Google Scholar]
- Young et al., 2004.Young JP, Herath P, Eickhoff S, Choi J, Grefkes C, Zilles K, Roland PE. Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci 24: 5391–5399, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler et al., 1999.Ziegler EA, Magerl W, Meyer RA, Treede R-D. Secondary hyperalgesia to punctate mechanical stimuli: central sensitization to A-fibre nociceptor input. Brain 122: 2245–2257, 1999 [DOI] [PubMed] [Google Scholar]