Abstract
Left ventricular hypertrophy leads to heart failure and represents a high risk leading to premature death. Cyclic nucleotides (cAMP and cGMP) play a major role in heart contractility and cyclic nucleotide phosphodiesterases (PDEs) are involved in different stages of advanced cardiac diseases. We have investigated their contributions in the very initial stages of left ventricular hypertrophy development. Wistar male rats were treated over two weeks by chronic infusion of angiotensin II using osmotic mini-pumps. Left cardiac ventricles were used as total homogenates for analysis. PDE1 to PDE5 specific activities and protein and mRNA expressions were explored.
Rats developed arterial hypertension associated with a slight cardiac hypertrophy (+24%). cAMP-PDE4 activity was specifically increased while cGMP-PDE activities were broadly increased (+130% for PDE1; +76% for PDE2; +113% for PDE5) and associated with increased expressions for PDE1A, PDE1C and PDE5A. The cGMP-PDE1 activation by Ca2+/CaM was reduced. BNP expression was increased by 3.5-fold, while NOX2 expression was reduced by 66% and AMP kinase activation was increased by 64%. In early cardiac hypertrophy induced by angiotensin II, all specific PDE activities in left cardiac ventricles were increased, favoring an increase in cGMP hydrolysis by PDE1, PDE2 and PDE5. Increased cAMP hydrolysis was related to PDE4. We observed the establishment of two cardioprotective mechanisms and we suggest that these mechanisms could lead to increase intracellular cGMP: i) increased expression of BNP could increase “particulate” cGMP pool; ii) increased activation of AMPK, subsequent to increase in PDE4 activity and 5′AMP generation, could elevate “soluble” cGMP pool by enhancing NO bioavailability through NOX2 down-regulation. More studies are needed to support these assumptions. Nevertheless, our results suggest a potential link between PDE4 and AMPK/NOX2 and they point out that cGMP-PDEs, especially PDE1 and PDE2, may be interesting therapeutic targets in preventing cardiac hypertrophy.
Introduction
Left ventricular hypertrophy is a major risk factor for premature death [1]. There is a correlation between plasmatic angiotensin II, hypertension, development of cardiac hypertrophy [2] and remodeling leading to heart failure [3]. Molecular mechanisms altered by angiotensin II have been mainly studied in vessels (for review see [4]); only a few studies have been performed in heart [5]. Cyclic nucleotides play a major role in the activation of different intracellular signaling pathways. In the heart, cyclic nucleotide phosphodiesterases (PDEs), by hydrolyzing cAMP and/or cGMP, regulate contractility in response to different stimuli such as β-adrenergic receptors [6] and natriuretic peptide receptors [7] and participate to cardiac remodeling [8].
PDE superfamily is constituted of eleven families, PDE1 to PDE11, but only the five former have been well studied [9]. PDE1 is activated by calmodulin (CaM) in presence of Ca2+ (PDE1-Ca2+/CaM complex). PDE1A and PDE1B hydrolyze mainly cGMP, while PDE1C hydrolyzes equally cAMP and cGMP. The activations of PDE1A and PDE1C by Ca/CaM are decreased by PKA phosphorylation. PDE2 hydrolyzes cGMP and cAMP, and its cAMP hydrolysis is allosterically activated by cGMP [10]. PDE3 hydrolyzes both cAMP and cGMP. cGMP has a greater affinity for PDE3 while being hydrolyzed 10 times slower than cAMP, which makes it acts as a PDE3 inhibitor. PDE4 hydrolyzes specifically cAMP and could be activated subsequently through PKA-dependent phosphorylation while PDE5 hydrolyzes specifically cGMP and could be activated by cGMP and PKG-dependent phosphorylation [11].
Some studies have shown changes in the activity, expression and distribution of PDEs in various forms of cardiac hypertrophy [12], [13] and heart failure [14], [15]. Indeed, a decrease in cAMP-PDE activity, especially PDE3A activity, was observed in a2+dvanced cardiac hypertrophy induced by ligation of the aorta in mice as well as in human failing hearts [16]. In dog, heart failure was associated with a decrease in membrane expression of PDE3, without change in cytosolic PDE3 activity [17]. Furthermore, inactivation of the PDE4D gene in mouse was associated with progressive heart failure and arrhythmias, despite a normal overall cAMP level [14]. These results are consistent with the observation that, in spontaneously hypertensive rats subjected to a chronic β-adrenergic stimulation in vivo, pentoxyfilline (a non-selective PDE inhibitor) promotes the transition from left ventricular hypertrophy to left ventricular dilatation [18]. In cardiomyocytes, cGMP has opposite effects depending on its compartmentation. The elevation of cGMP due to the stimulation of particulate guanylyl cyclase (GC) by natriuretic peptides cause positive inotropic and chronotropic effects [19], mediated by cGMP/cAMP cross-talk [10]. In contrast, a permeable cGMP analogue [6] or the stimulation of GC by NO donors through activation of PKG causes a negative inotropic effect via the myofilaments [20]. It was interestingly shown that PDE5A was upregulated in different models of cardiac disease [15], [21] and that early correction of PDE5 alterations could restore heart function and prevent cardiac hypertrophy [22], [23].
Therefore, PDEs in the heart could be valuable therapeutic targets for the treatment and prevention of cardiac dysfunction. Since no studies were done investigating simultaneously the various cAMP- and cGMP-PDE isoform contributions in cardiac pathology, we aimed to explore the variations of PDE1 to PDE5 in left cardiac ventricle on a rat model of cardiac hypertrophy induced by angiotensin II, and especially in the initial stages of cardiac hypertrophy development.
Materials and Methods
In vivo Treatment of Rats
The study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No. 85-23, revised 1996) and has been approved by the local ethics committee of animal experimentation (CREMEAS). Experimental protocol and physiological measurements were conducted as previously described [24]. Male Wistar rats (10-week old) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.; Centravet). A 1-cm incision was made in the mid-scapular region and an osmotic mini-pump (Alzet 2004, Charles River Laboratories) was implanted. Pumps contained angiotensin II (Sigma-Aldrich, St. Louis, MO, USA), which was dissolved in saline solution, and the infusion rate was 0.1 mg.kg−1.d−1 (ANGII 0.1) or 0.4 mg.kg−1.d−1 (ANGII 0.4) for 14 days. Sham-operated rats underwent an identical surgical procedure without pump implantation. Systolic blood pressure and heart rate were measured using a tail-cuff plethysmograph connected to a computerized system (LE 5002, BIOSEB) in conscious rats before the infusion (D0) and at the end of treatment (D14). Body weight was also recorded at D0 and D14. Rats were then sacrificed, hearts were weighted and left cardiac ventricles (LCV) were quickly frozen in liquid nitrogen and stored at −80°C.
Preparation of Protein Extracts from Left Cardiac Ventricles
Frozen left ventricles were powdered in liquid nitrogen with a freezermill (Freezer/Mill 6750 from SPEX CERTIPREP INC). Grinding protocol included 5 cycles (1 min freezing, 2 min grinding with 1 min freezing between two grindings). Powders were suspended (100 mg of tissue/ml) in a protease inhibitor buffer (20 mM Tris, 2 mM Mg2 + acetate, 5 mM EGTA, pH 7.5, 1 mM dithiothreitol, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml soybean trypsin inhibitor, 0.33 mM Pefabloc). Homogenates were then mixed and treated by ultrasounds for 10 seconds. Total homogenates obtained were aliquoted and stored at −80°C until use. Protein levels were assessed by the Lowry method [25] using bovine serum albumin (BSA) as standard. DNA levels were determined by fluorometric measurement as previously described [26] using a DNA standard provided by Sigma.
Western Blot Analysis
Proteins (30 µg) from left cardiac ventricle homogenates were subjected to Western blot as described previously [27]. Immunodetection was carried out with: anti-PDE1A (PD1A-101AP; 1/2,500), anti-PDE1B (PD1B-201AP; 1/2,500), anti-PDE1C (PD1C-301AP; 1/2,500), anti-PDE2A (PD2A-101AP; 1/1,000), anti-PDE4C (PD4-301AP; 1/2,500), anti-PDE4D (PD4-401AP; 1/2,500), anti-PDE5A (PD5A-101AP; 1/2,500) and anti-phospho-specific PDE5A (PPD5-140AP; 1/1,000; labels PDE5A proteins when phosphorylated at Ser92 by protein kinase G (PKG)) from FabGennix Inc. (Frisco, TX, USA); anti-PDE4A (AC55;1/2,000) and anti-PDE4B (K118; 1/2,000) from Dr M. Conti [28]; anti-phospho-AMPKα (Thr172) subunit (2531; 1/1,000) from Cell Signaling Technology, Inc.; anti-p47-phox (sc-14015; 1/,1000) from Santa Cruz Biotechnology, Inc.; anti-NOS3 (610297; 1/2,000) from BD Transduction Laboratories; anti-GAPDH (1/60,000; Chemicon). Immobilized antigens were detected by chemiluminescence using horse radish peroxidase-conjugates as secondary antibodies (1/60,000; Promega), an ECL kit (GE Healthcare) and autoradiography films. Autoradiography signals were captured on a GeneGenius Bio Imaging System (Syngene) using the GeneSnap software and analysed using the GeneTool software. PDE signal values were normalized with GAPDH signal values.
PDE Assay
PDE activity was determined with a radioenzymatic assay as described previously [29]. Total cAMP-PDE activity was assessed at 1 µM cAMP and the contribution of PDE isozymes was determined by using selective inhibitors: 1 µM cilostamide for PDE3 (Ki = 45 nM [30]) and 10 µM rolipram for PDE4 (Ki = 0.8 µM [30]), the residual cAMP-PDE activity representing essentially PDE2 [29]. Total cGMP-PDE activity was assessed at 1 µM cGMP and the contribution of PDE isozymes was determined by using the selective inhibitors: 10 µM nimodipine for PDE1 (Ki = 1.4 µM [30]), 20 µM EHNA for PDE2 (IC50 = 0.8–2 µM [31]) and 0.1 µM DMPPO for PDE5 (Ki = 3 nM [32]). The residual cGMP-PDE activity represented essentially PDE3, which is able to hydrolyze both cAMP and cGMP, but with a higher affinity and a 10-fold lower Vmax for cGMP [29]. As EHNA is effective only on cGMP-stimulated PDE2 and not on basal PDE2 [33], it was not usable to determine cAMP-PDE2 activity. Specific activities were expressed as pmol.min−1.mg−1 protein and for some experiments also as pmol.min−1.mg−1 DNA.
Quantitative real-time RT-PCR
Total RNA was isolated from left cardiac ventricle using the RNeasy® kit (Qiagen), including an RNase-free DNase treatment to prevent co-amplification of genomic DNA. The concentration of the purified RNA was determined spectrophotometrically at 260 nm. The integrity of purified RNA was checked by agarose gel electrophoresis in denaturing condition. The reverse transcription was performed using iScript® cDNA synthesis kit from Bio-Rad. The 18S rRNA house-keeping gene was used for normalization. Specific primers for PDEs and 18S rRNA were designed with Primer3 (v. 0.4.0) software using mRNA sequences retrieved from NCBI nucleotide database and are presented in Table 1. The transcript levels of PDEs and 18S rRNA were quantified by real-time PCR performed in the MyiQ® single-color detector system (Bio-Rad Laboratories, Inc.) using the iQ SYBR® Green Supermix (product no. 170-8884) containing the hot-start iTaq DNA polymerase. The amplification conditions were: 95°C for 5 min, 40 cycles at 94°C for 10 sec, 60°C for 10 sec and 72°C for 15 sec. All amplification efficiencies were 1.0±0.1. Specificity of amplification products was assessed by melting curve analysis and their size was checked by agarose gel electrophoresis. Samples were processed in triplicate according to the manufacturer's guidelines. Relative gene expression was calculated using the comparative threshold (Ct) method (2−ΔΔCt) [34].
Table 1. Primer sequences used for Real-Time PCR experiments.
| Sequence | Product size (pb) | GenBank accession no. | ||
| PDE1A | Forward | 5′-ACCATGATTGGGTTCCATGTT-3′ | 175 | NM_030871 |
| Reverse | 5′-CAGCCAACTCTTTCCACCT-3′ | |||
| PDE1B | Forward | 5′-TCCACATCCAGACCAAGTCA-3′ | 194 | NM_022710 |
| Reverse | 5′-GCAGGACATGTCTGTGGCT-3′ | |||
| PDE1C | Forward | 5′-CAGCCTACCGTCTTCTCCA-3′ | 179 | NM_031078 |
| Reverse | 5′-TTCAATTGCTTCTGGTTGCTG-3′ | |||
| PDE2A | Forward | 5′-AGTGCTGGGAGAAGAGGTC-3′ | 181 | NM_031079 |
| Reverse | 5′-GTCAGTGGCTCGACTGATG-3′ | |||
| PDE3A | Forward | 5′-GCCCCAGTGTTGATGACTCT-3′ | 161 | NM_017337 |
| Reverse | 5′-GGTGATCCTTGAGGAGGTGA-3′ | |||
| PDE3B | Forward | 5′-ATGGAATTCAAGCGTTTTCG-3′ | 169 | NM_017229 |
| Reverse | 5′-TGCACACCTGGCAGACTAAG-3′ | |||
| PDE4A | Forward | 5′-GAAGACAACCGGGACTGGT-3′ | 172 | NM_013101 |
| Reverse | 5′-CCTCAGTGGTAGGCAATCC-3′ | |||
| PDE4B | Forward | 5′-CCTCCGACACCTTCGTAAC-3′ | 153 | NM_017031 |
| Reverse | 5′-CCAGGTCTGTGAAGACAGC-3′ | |||
| PDE4C | Forward | 5′-GAAGGGCACTACCACTCCA-3′ | 153 | XM_001070301 |
| Reverse | 5′-GTGTATAGCGCACGCAAAGA-3′ | |||
| PDE4D | Forward | 5′-TGGGCAGACCTCGTACATC-3′ | 190 | NM_017032 |
| Reverse | 5′-CAGTGTCTGACTCGCCATC-3′ | |||
| PDE5A | Forward | 5′-GGGAAGAGGTCGTTGGTGT-3′ | 162 | NM_133584 |
| Reverse | 5′-TTTGTTCTCCAGCAGTGACG-3′ | |||
| NOX1 | Forward | 5′-CATGGATGGATCTCTTGCCT-3′ | 168 | NM_053683 |
| Reverse | 5′-ACCATGAGAACCAAAGCCAC-3′ | |||
| NOX2 | Forward | 5′-AACGTGGAGTGGTGTGTGAA-3′ | 222 | AF298656 |
| Reverse | 5′-TTTGGTGGAGGATGTGATGA-3′ | |||
| NOX3 | Forward | 5′-AGTGAACAAGGGAAGGCTCA-3′ | 176 | AY573239 |
| Reverse | 5′-GCAATCTGCTTGAATTCCTCA-3′ | |||
| NOX4 | Forward | 5′-TGTCTGCATGGTGGTGGTAT-3′ | 154 | NM_053524 |
| Reverse | 5′-ATACTTCAACAAGCCACCCG-3′ | |||
| p47phox | Forward | 5′-AGCTCCCAGGTGGTATGATG-3′ | 205 | AY029167 |
| Reverse | 5′-ATCTTTGGCCGTCAGGTATG-3′ | |||
| NOS1 | Forward | 5′-CTGCAAAGCCCTAAGTCCAG-3′ | 207 | NM_052799 |
| Reverse | 5′-AGTGTTCCTCTCCTCCAGCA-3′ | |||
| NOS2 | Forward | 5′-AGGGAGTGTTGTTCCAGGTG-3′ | 232 | NM_012611 |
| Reverse | 5′-TCCTCAACCTGCTCCTCACT-3′ | |||
| NOS3 | Forward | 5′-TGTGACCCTCACCGATACAA-3′ | 212 | NM_021838 |
| Reverse | 5′-CTGGCCTTCTGCTCATTTTC-3′ | |||
| ANP | Forward | 5′-AGCGAGCAGACCGATGAA-3′ | 161 | M27498 |
| Reverse | 5′-GCCCTCAGTTTGCTTTTCAA-3′ | |||
| BNP | Forward | 5′-CAGCTCTCAAAGGACCAAGG-3′ | 191 | M25297 |
| Reverse | 5′-TAAAACAACCTCAGCCCGTC-3′ | |||
| CNP | Forward | 5′-GGCAATCAGAAAAAGGGTGA-3′ | 185 | D90219 |
| Reverse | 5′-CCTTTGGACAAGCCCTTCTT-3′ | |||
| 18S rRNA | Forward | 5′-AAACGGCTACCACATCCAAG-3′ | 155 | M11188 |
| Reverse | 5′-CCTCCAATGGATCCTCGTTA-3′ |
Immunohistochemistry
Frozen left cardiac ventricles embedded in Tissue-Tek® O.C.T (Sakura Finetek) were cryosectioned (4 µm) and fixed with 4% paraformaldehyde. Fixed sections were incubated with antibodies directed against PDE5A (1/100; FabGennix Inc.). The corresponding IgG coupled to Alexa 488 (Invitrogen, Molecular Probes) was used as secondary antibody. Images were obtained with a Leica DM 400 inverted epifluorescence microscope (x63 objective).
Statistical Analysis
All data are expressed as mean ± S.E.M. Statistical analyses were performed with Student's t test for unpaired data excepted for PDE4 enzymatic activities measured with H89 (paired data), using GraphPad Prism software (San Diego, USA). For Real-Time PCR, statistical analyses were performed with Student's t test for unpaired data as described previously [35]. A value of P<0.05 was considered as significant.
Results
Effect of angiotensin II treatment on cardiovascular parameters
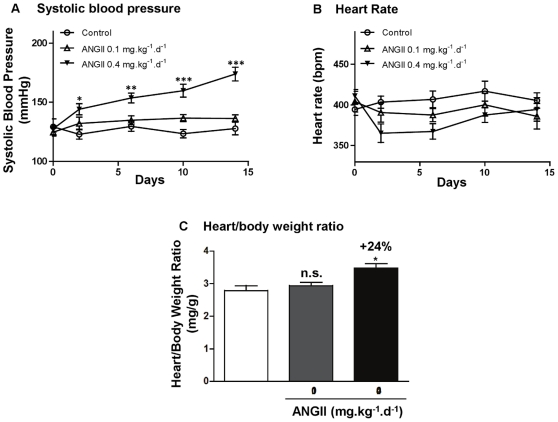
Before the implantation of mini-pumps, rats presented similar systolic blood pressures (129±7 mmHg for control rats, 125±3 mmHg for 0.1 mg.kg−1.d−1 and 128±3 mmHg for 0.4 mg.kg−1.d−1 angiotensin II treated rats). After two week-treatment, systolic blood pressure was significantly increased (+36%, P<0.001) only in rats treated with 0.4 mg ANGII (174±5 mm Hg) compared to control rats (128±5 mm Hg) ( Fig. 1A ). Heart rate did not change significantly among the three groups ( Fig. 1B ). The heart/body weight ratio was calculated as an indicator for estimating cardiac hypertrophy. The development of a slight cardiac hypertrophy (+24%, P = 0.02, Fig. 1C ) was observed only in rats treated with 0.4 mg ANGII. This dose was kept for the forthcoming analysis.
Figure 1. Effects of angiotensin II treatment on rat physiological parameters.
Systolic blood pressure (A), heart rate (B) and heart/body weight ratio (C). Rats were treated with angiotensin II at 0.1 mg.kg−1.d−1 (ANGII 0.1; n = 8) or 0.4 mg.kg−1.d−1 (ANGII 0.4; n = 13). Effects were expressed in comparison with control rats (n = 6). n.s.: not significant; *: P<0.05; **: P<0.01; ***: P<0.001.
PDE isozyme distribution in left cardiac ventricle
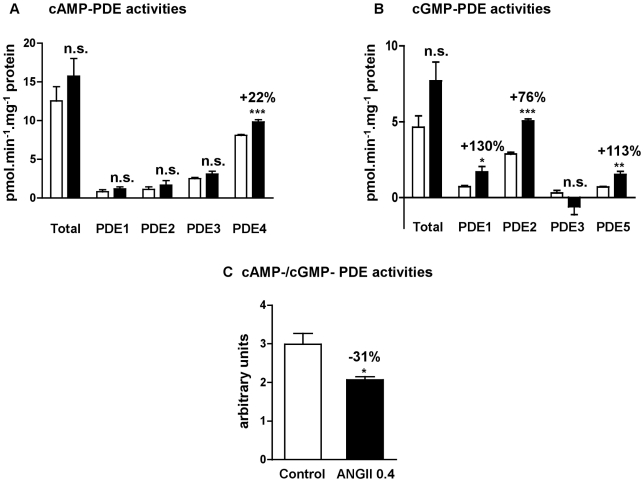
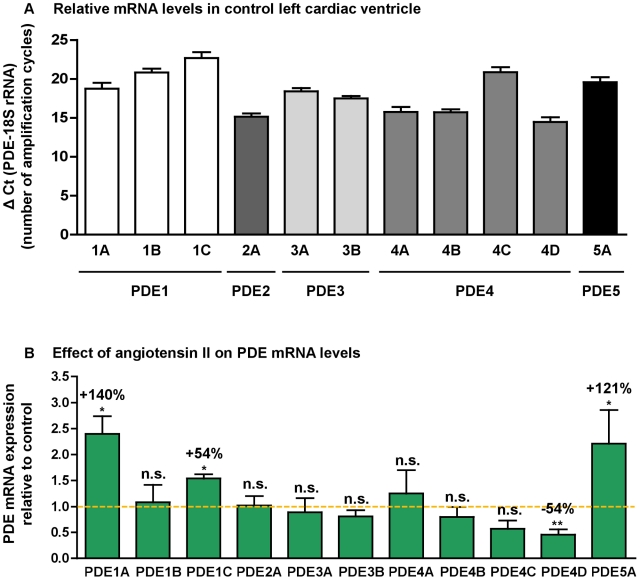
In left cardiac ventricle of control rats, PDE4 represented 64% of total cAMP-PDE activity whereas PDE1, PDE2 and PDE3 contributed respectively for 7%, 9% and 20% ( Fig. 2A ). PDE2 represented 62% of total cGMP-PDE activity, whereas PDE1, PDE3 and PDE5 contributed, respectively for 15%, 7% and 16% for total cGMP-PDE activity ( Fig. 2B ). This pattern is in accordance with the PDE mRNA distribution in control left cardiac ventricle, where PDE2A, PDE4A, PDE4B and PDE4D are the major PDE transcripts, followed by PDE3A, PDE3B, PDE5A and PDE1A at almost the same level, and finally PDE4C and PDE1C are the least expressed ( Fig. 3A ).
Figure 2. Effects of angiotensin II treatment on left cardiac ventricle PDE specific activities.
cAMP-PDE specific activities and contribution of PDE2, PDE3 and PDE4 (A). cGMP-PDE specific activities and contributions of PDE1, PDE2, PDE3 and PDE5 (B). cAMP-PDE over cGMP-PDE total activity ratio (C). Specific activities were determined on total homogenate and expressed as pmol.min−1.mg−1 of protein. Effects on treated rats (▪; n = 4) were compared with control rats (□; n = 6). n.s.: not significant; *: P<0.05; **: P<0.01; ***: P<0.001.
Figure 3. PDE mRNA distribution in control left cardiac ventricle and effect of angiotensin II treatment.
Relative mRNA levels in control left cardiac ventricle (A). Results were expressed as ΔCt = Ct of PDE – Ct of 18S rRNA, which corresponds to the number of amplification cycles needed to detect fluorescence signal, each cycle corresponding to a 2-fold amplification. ΔCt being inversely proportional to initial PDE mRNA level, the fewer is ΔCt, the greater is initial mRNA level. Effects of angiotensin II treatment on PDE mRNA expressions (B). Results were normalized with the 18S rRNA housekeeping gene and expressed as amplification folds relative to control. n.s.: not significant; *: P<0.05; **: P<0.01.
Effect of angiotensin II treatment on PDE activities
As seen in Figure 1 , rats treated with angiotensin II at 0.4 mg.kg−1.d−1 for two weeks showed arterial hypertension associated with cardiac hypertrophy, without developing tachycardia. Although this treatment did not significantly change total cAMP-PDE activity, it induced a specific 22%-increase in PDE4 activity (P<0.001) ( Fig. 2A ). On the other side, cGMP-PDE activities were broadly increased, for PDE1 (+130%, P = 0.02), PDE2 (+76%, P<0.001) and PDE5 (+113%, P = 0.002) ( Fig. 2B ). Moreover, the total cAMP-PDE/cGMP-PDE activity ratio was decreased by 31% in hypertrophic left cardiac ventricles, indicating a shift to a greater cGMP hydrolysis ( Fig. 2C ).
Because cardiac hypertrophy induces alterations in cellular protein content and could thus introduce a bias in the measurement of PDE activity expressed as pmol.min−1.mg−1 of protein, PDE activity was also expressed as pmol.min−1.mg−1 of DNA. We observed similar effects: cAMP-PDE4 activity was increased (+13%), as were cGMP-PDE1 (+105%), cGMP-PDE2 (+60%) and cGMP-PDE5 (+61%) (not illustrated).
Exploration of increased cAMP hydrolysis activity
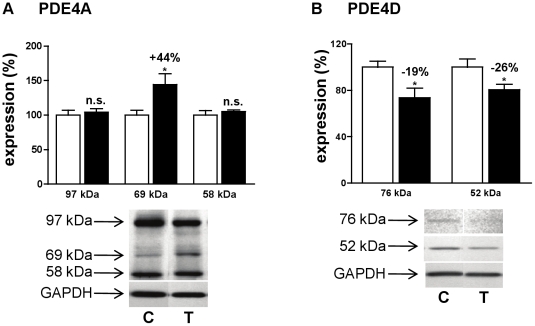
Considering the increased PDE4 activity, the expression of PDE4 subtypes has been explored. Although PDE4A mRNA was not significantly modified ( Fig. 3B ), Western blot revealed a specific 44%-increase in a 69 kDa-PDE4A variant ( Fig. 4A ) among the three variants expressed (97 kDa, 69 kDa and 58 kDa). No significant changes for PDE4B and PDE4C mRNA ( Fig. 3B ) and protein (not illustrated) expressions were observed. Oppositely, angiotensin II treatment induced significant decreases in PDE4D variant expressions (−19% for 76 kDa and −26% for 52 kDa, Fig. 4B ) and PDE4D transcript (−54%, Fig. 3B ). PDE3A and PDE3B mRNA expressions were not significantly changed ( Fig. 3 ).
Figure 4. Effect of angiotensin II treatment on cAMP hydrolysis activity.
Effects of angiotensin II treatment at 0.4 mg.kg−1.d−1, on protein expression of PDE4A (A), PDE4D (B). Effects on treated rats (T, ▪; n = 4) were compared with control rats (C, □; n = 4). Results were normalized with GAPDH signal and expressed in percentage of untreated rat. n.s.: not significant; *: P<0.05.
Effect of angiotensin II treatment on calmodulin-dependent activation of PDE1
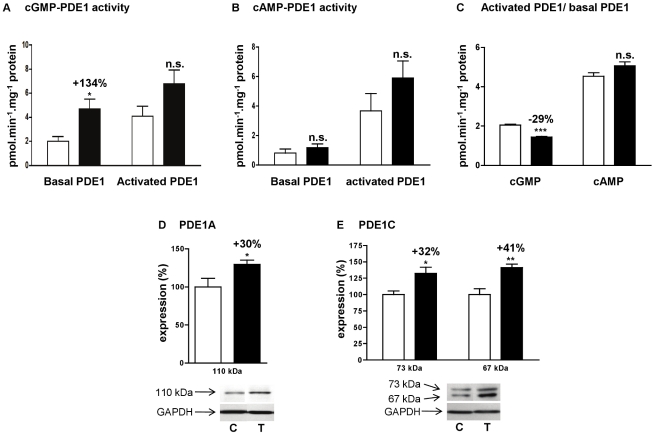
The main characteristic of PDE1 is to be activated by intracellular calcium concentration increases through association with Ca2+/calmodulin (CaM) complexes. To explore the increase in cGMP-PDE1 activity reported in Figure 2B , we compared basal PDE1 activity (assessed in presence of EGTA) and CaM-activated PDE1 (assessed in presence of Ca2+ and CaM). As seen above, basal cGMP-PDE1 activity was significantly increased by 134% in ANGII 0.4 rats. But this increase was no longer significant when PDE1 was activated, by Ca2+/CaM complexes, indicating a loss in CaM-dependent activation ( Fig. 5A ). The ratio between activated and basal cGMP-PDE1 activities was significantly decreased by 29% ( Fig. 5C ), suggesting that PDE1 activation capacity was more important in control rats that in ANGII 0.4 rats. PDE1C hydrolyzing both cAMP and cGMP, the same approach was done for cAMP-PDE1 activity ( Fig. 5B ). No significant change in the CaM-dependent activation was observed between control and ANGII 0.4 rats ( Fig. 5C ), suggesting that different PDE1 families contribute to PDE1 activity.
Figure 5. Effect of angiotensin II treatment on PDE1 activity in left cardiac ventricle.
cGMP-PDE1 specific activity (A), cAMP-PDE1 specific activity (B), and PDE1 activated over basal activity ratio (C). Basal PDE1 activity was assessed in presence of EGTA and CaM-activated PDE1 was assessed in presence of Ca2+ and CaM. Effects on treated rats (T, ▪; n = 4) were compared with control rats (C, □□; n = 6). Specific activities were determined on total homogenate and expressed as pmol.min−1.mg−1 of protein. Effects of angiotensin II treatment on protein expression of PDE1A (D) and PDE1C (E). Results were normalized with GAPDH signal and expressed in percentage of untreated rat. n.s.: not significant; *: P<0.05; **: P<0.01; ***: P<0.001.
Besides, angiotensin II treatment induced strong increases of PDE1 expressions. PDE1A mRNA was increased by 140% ( Fig. 3B ), associated with a 30% increase of 110 kDa-PDE1A protein expression ( Fig. 5D ). PDE1C mRNA, that was increased by 54% ( Fig. 3B ), was associated with an increased expression of the two detected variants (+41% for the 67 kDa signal and +32% for the 73 kDa signal) ( Fig. 5E ). PDE1B mRNA was detected by real-time PCR without being affected by angiotensin treatment ( Fig. 3B ) but PDE1B protein was not detected by Western blot.
Effect of angiotensin II treatment on PDE5
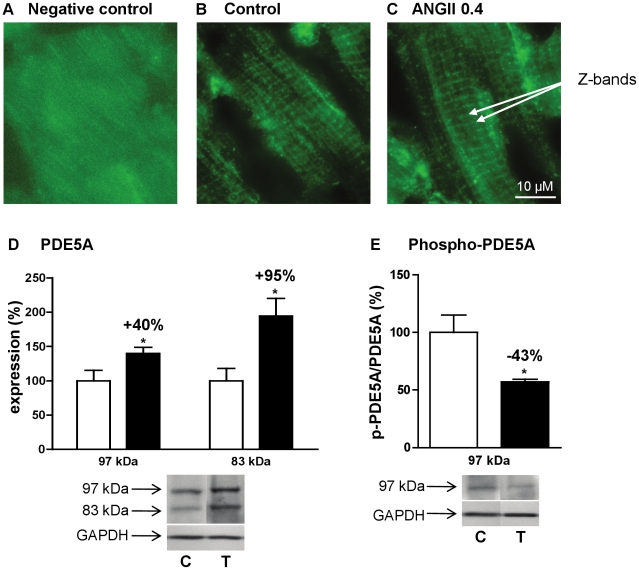
PDE5 was subjected to the most important changes in expression after angiotensin II treatment. PDE5A mRNA increased by 121% ( Fig. 3B ) and was associated with an increase of the two detected PDE5 variants: + 95% for 97 kDa-signal and +40% for 83 kDa-signal ( Fig. 6D ). Furthermore, Ser92-phophosphorylated 97 kDa-PDE5A/PDE5A 97 kDa-protein ratio was reduced by 43%, indicating a decrease in PDE5A activation by PKG ( Fig. 6E ). We also looked at the intracellular localization of PDE5A. Previous work reported that PDE5A was localized to Z-bands and that this localization was lost in advanced cardiac hypertrophy [36]. In our model of early cardiac hypertrophy, PDE5A localization to Z-bands ( Fig. 6B ) was not affected by angiotensin II treatment ( Fig. 6C ).
Figure 6. Effects of angiotensin II treatment on PDE5 expression and intracellular localization.
Immunostaining of PDE5A cellular distribution in left cardiac ventricle: negative control (A), control rats (B) and angiotensin II treated rats (C). PDE5A is localized to Z-bands. Effects of angiotensin II treatment at 0.4 mg.kg−1.d−1 on protein expression of PDE5A (D) and phospho-PDE5A over 97 kDa PDE5A (E). Effects on treated rats (T, ▪; n = 4) were compared with control rats (C, □; n = 4). Results were normalized with GAPDH signal and expressed in percentage of untreated rat. *: P<0.05.
PDE2 and natriuretic peptides
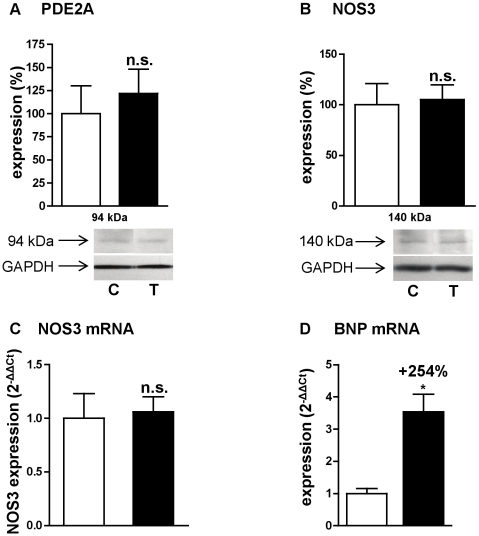
cGMP is produced in part by natriuretic peptide receptors. Natriuretic peptides (ANP, BNP and CNP) are synthesized in the heart and can exert their effect through an autocrine/paracrine pathway. Moreover, natriuretic peptide production in heart is increased in cardiac hypertrophy [7]. We therefore studied the expression of the natriuretic peptide transcripts in early cardiac hypertrophy induced by angiotensin II. While ANP and CNP transcripts were not significantly modified (not illustrated), BNP transcript was significantly increased by 254% ( Fig. 7D ).
Figure 7. Effects of angiotensin II treatment on PDE2, BNP and NOS.
Expression of PDE2A protein (A), NOS3 protein (B), NOS3 mRNA (C) and BNP mRNA (D). Effects on treated rats (T, ▪; n = 4) were compared with control rats (C, □; n = 4). Western blot results were normalized with GAPDH signal and expressed in percentage of untreated rat. Q-RT-PCR results were normalized with the 18S rRNA housekeeping gene and expressed as amplification folds relative to control. n.s.: not significant; *: P<0.05.
On the other hand, despite an important increase in cGMP-PDE2 hydrolysis activity ( Fig. 2B ), angiotensin II treatment did not induce any change either in PDE2A mRNA expression ( Fig. 3B ) or in PDE2A protein expression ( Fig. 7A ).
Effect on AMPK
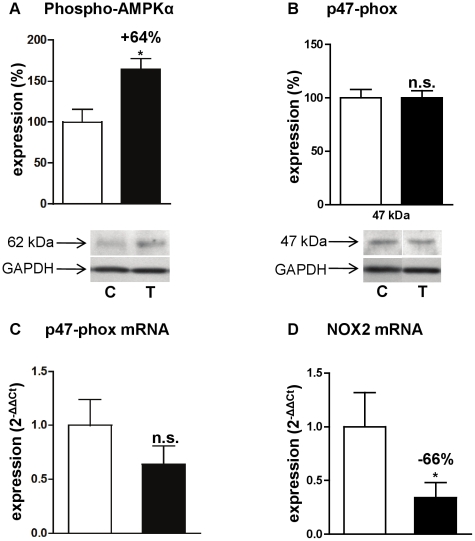
5′AMP kinase (AMPK) plays a very important role in heart energy metabolism and protein synthesis. It produces ATP from AMP and is activated when the AMP/ATP ratio increases, i.e. under conditions of stress [37]. As we observed an increased cAMP hydrolysis into 5′AMP, we looked for AMPK activation. Consistently, an increase of 64% of the Thr172-phosphorylated AMPKα catalytic subunit was observed ( Fig. 8A ).
Figure 8. Effects of angiotensin II treatment on AMPK and NADPH oxidase.
Expressions of the phosphorylated α-catalytic subunit of AMPK (A), p47-phox protein (B), p47-phox mRNA (C) and NOX2 mRNA (D). Effects on treated rats (T, ▪; n = 4) were compared with control rats (C, □; n = 4). Western blot results are expressed in percentage after correcting PDE signal with GAPDH signal. Q-RT-PCR results were normalized with the 18S rRNA housekeeping gene and expressed as amplification folds relative to control. n.s.: not significant; *: P<0.05.
Effect on nitric oxide synthase (NOS) expression
Another source of cGMP is that produced by soluble guanylyl cyclases in response to NO. Therefore, NOS1, NOS2 and NOS3 expressions were examined in left cardiac ventricle. Early cardiac hypertrophy induced by angiotensin II did not change either NOS1 or NOS2 mRNA expressions (not illustrated), NOS3 mRNA expression ( Fig. 7C ) or NOS3 protein expression ( Fig. 7B ).
Effect on NADPH oxidases
Reactive oxygen species (ROS) have been shown to be implicated in the development of cardiac hypertrophy [38]. Angiotensin II modulates ROS production through NADPH oxidase (NOX) activity and expression in heart [39]. We studied therefore the effect of angiotensin II-induced early cardiac hypertrophy on the three major NOX enzymes present in the heart, NOX1, NOX2 (or gp91-phox) and NOX4 [40]. We found a significant 66% decrease in the mRNA expression of NOX2 ( Fig. 8D ), while NOX1 and NOX4 mRNA expressions were unchanged (not illustrated). Activation of NOX2 is known to need different subunits, and notably p47-phox subunit [38], but we found no change either in mRNA expression ( Fig. 8C ) or in protein expression ( Fig. 8B ) of p47-phox.
Discussion
cAMP- and cGMP-PDE activities, previously determined in our laboratory on isolated cardiac myocytes from left cardiac ventricle of adult rats, showed that cAMP hydrolysis activity was almost 3 times greater than that of cGMP and that PDE2 and PDE4 were the major enzymes implicated [41]. The present results in left cardiac ventricles of control rats are consistent with those data, and they highlight the importance of cardiac cAMP catabolism in regulating cardiac contractility. PDE4 is associated with many intracellular complexes involved in β-adrenergic signaling, calcium signaling with the ryanodine receptors and L-type calcium channels (LTCCs), contraction associated to myofilaments [42] and lastly IKs potassium channel [43]. PDE2 is also associated with β-adrenergic signaling complexes and LTCCs. Although the activity of PDE2 toward the hydrolysis of cAMP is relatively low, its presence in plasma membrane contributes to regulate the cardiac LTCC activity when the level of cGMP is increased [44], since PDE2 inhibits the activation of LTCCs by reducing cAMP concentration after being activated by cGMP. By this mechanism, PDE2 establishes a link in the heart between cGMP generated by guanylyl cyclases and β-adrenergic cAMP signal [45].
Only rats undergoing a two week-treatment with angiotensin II at 0.4 mg.kg−1.d−1 developed hypertension associated with cardiac hypertrophy. However, this cardiac hypertrophy was weak (+24%) indicating that cardiac hypertrophy is in its early stage.
We observed a specific increase in PDE4 activity, which results of opposite changes. Indeed, while all detected isoforms of the PDE4D subfamily were down-regulated, we observed a specific increase in a short 69 kDa-PDE4A isoform by angiotensin II treatment, without increase in PDE4A mRNA. This apparent discrepancy is due to the fact that the set of primers used were designed to detect all PDE4A mRNAs while three variants were separated by Western blot. PDE4A seems to be the major subfamily responsible for cAMP hydrolysis in rat left cardiac ventricle. It has been shown in isolated rat ventricular myocytes that PDE4A contribution was one third of total PDE4 activity, while PDE4D accounted only for 15% [46]. This explains in part why, despite a decreased PDE4D expression, global cAMP-PDE4 activity is increased. Little is known about the distribution and role of PDE4A in heart. It was shown that PDE4A1 could be associated to the Golgi membrane [47]. On the other hand, PDE4D has already been linked to cardiac diseases and its role in heart was intensively investigated. Several epidemiologic studies found an association between PDE4D gene and ischemic stroke [48], [49]. PDE4D deficiency in the ryanodine-receptor complex (RyR2) in mice was also involved in heart failure. Accumulation of cAMP contributes to PKA-hyperphosphorylated RyR2 channels leaking calcium that promotes cardiac dysfunction and arrhythmias [14]. PDE4D3, which is the isoform supposed to be in the ryanodine-receptor complex, is also associated to sarcoplasmic [50] and perinuclear [51] membranes. Furthermore, PDE4D was shown to be associated to isolated cardiac nuclei [52]. Moreover, PDE4D5 is implicated in β2-adrenergic receptors signaling through β-arrestin interactions [53]. Our data are consistent with those results and show moreover that this PDE4D decrease occurs at a very early stage in the development of cardiac hypertrophy.
The highest cGMP-PDE effect of angiotensin II treatment at 0.4 mg.kg−1.d−1 was on PDE1 activity in its basal state, with a 130% increase. The expressions of 110 kDa-PDE1A, and 67 kDa- and 73 kDa-PDE1C proteins were similarly increased by angiotensin II treatment. But in the same time, PDE1 activation by Ca2+/CaM complexes was less important than in control rats. This could be the result of a PKA-dependent phosphorylation. Indeed, PKA-dependent phosphorylation of PDE1A and PDE1C has been shown to reduce their ability to get activated by Ca2+/CaM complexes [54]. However, considering cAMP hydrolysis activity of PDE1 which concerned PDE1C ( Fig. 5C ), no difference in PDE1 ratio was observed, suggesting that during cardiac hypertrophy, PDE1A was specifically PKA-phosphorylated. An increase in PDE1 activity and PDE1A expression has been reported to be associated with cardiac hypertrophy induced by abdominal aortic banding in Sprague-Dawley rats [12], and more recently by neurohumoral stimuli such as angiotensin II and isoproterenol [55]. Interestingly, it was shown in cultured rat aortic vascular smooth muscle cells that angiotensin II treatment upregulates PDE1A [56], suggesting that such mechanism could participate to PDE1A upregulation described herein. PDE1A hydrolyzes mainly cGMP and PDE1C hydrolyzes cGMP and cAMP equally. PDE1C is expressed in rat heart [57] and PDE1C1 is expressed at high levels in human cardiac myocytes [58]. PDE1C has been associated with arterial smooth muscle cell proliferation in several studies [59], [60]. Interestingly, an increase in PDE1 activity and PDE1C protein was proposed to explain in part the cardioprotective effect of prostacyclin in rat heart [57]. Thus, to our knowledge, it is the first time that, additively to PDE1A, PDE1C is shown to be associated with cardiac hypertrophy in the heart.
Angiotensin II treatment induced a 113% increase in PDE5 activity, corresponding to strong increases in both 83 kDa- and 97 kDa-PDE5A isoforms and a 121% increase in PDE5A-mRNA. This is in agreement with the reported increase of PDE5A expression induced by angiotensin II in vascular smooth muscle cells representing a new mechanism by which angiotensin II antagonizes cGMP signaling [61]. In that way, PDE5 activity and expression have been reported to be elevated in right ventricular hypertrophy in rats as well as in humans [21], and cardiac hypertrophy induced by aortic banding in C57Bl/6 mice [22]. On one hand, cardiac over-expression of PDE5A in mice predisposed to left ventricle remodeling [15], and interestingly PDE5A inactivation by gene silencing blunted phenylephrine-induced hypertrophy [62]. On the other hand, PDE5 inhibition improved cardiac contractility [21], [63], and prevented and reversed cardiac hypertrophy [22]. This effect is mediated by one member of the Regulator of G-protein Signaling family RGS2 [64]. All these results brought increasing interest in using PDE5 inhibitors in the treatment of heart failure [65]. Recently, it was shown in the heart that: i) PDE5A is coupled to NOS3; ii) this coupling targets PDE5A to a specific intracellular location (Z-bands) enabling PDE5A to regulate cGMP generated through NOS3 activation in response to β-adrenergic stimulation; and iii) chronic inhibition of NOS3 abolishes this localization [36]. Herein, we show that NOS3 expression is not affected in early cardiac hypertrophy induced by angiotensin II and that PDE5A keeps a Z-band localization pattern. Taken together, our data additively show that PDE5A alterations begin at a very early stage in cardiac hypertrophy leading to heart failure, and that the reported increase in PDE5 activity occurs before the loss of its intracellular localization to Z-bands and alteration of NOS3 expression.
In agreement with PKG activity suppression reported in hypertrophied human right ventricle [21], a reduction in PDE5A Ser92 phosphorylation was observed herein indicating a decrease in PKG activity, which might result from increased cGMP-PDE1, -PDE2, and -PDE5 activities. Indeed, it was proposed that PDE5 phosphorylation could be used as an in vivo indicator for PKG activation [66]. Furthermore, PDE5A up-regulation might decrease the cGMP binding to PDE5A GAF domain and consequently its phosphorylation [67]. These data support a decrease in the availability of cGMP in the vicinity of PDE5, most likely to Z-bands.
PDE regulation is complex and one transduction signal may affect several PDEs. For example, cAMP differentially regulates PDEs through PKA by activating PDE4 and decreasing PDE1 activation [68]. Regarding cGMP, we could think that PDE1 and PDE5 may have a common pattern of regulation in cardiomyocytes. Indeed, they share some common intracellular localization like the Z-bands [36], [58], [69] and participate to contractile events [70], [71]. They are implicated also in cell growth and survival with nuclear localizations for PDE1A [71] and PDE1C [69] and centrosomal localization for PDE5A [69]. Their activities were simultaneously increased in vascular smooth muscle cell proliferation [60], [70] and herein in cardiac hypertrophy. Their increases might participate together to nitrate tolerance, since combination of nitrates with PDE5 inhibitors [72] or PDE1 inhibitors [73] improved pulmonary vasodilator response in pulmonary arterial hypertension. It was also suggested that sildenafil improved vasodilatation in pulmonary hypertension through PDE5 and PDE1 inhibitions [74]. Very few data are available in cardiac myocytes due to lack of simultaneous studies on PDE1 and PDE5.
Angiotensin II treatment at 0.4 mg.kg−1.d−1 induced a 76% increase in cGMP-PDE2 activity in cardiac ventricles without any change in expression. This suggests that increased PDE2 activity was the result of a post-translational regulation. Such increased PDE2 activity, without increased expression, has previously been reported in another rat cardiac hypertrophy model induced by abdominal aortic banding [12]. It was shown that PDE2 could be activated by protein kinase C (PKC)-dependent phosphorylation [75]. Since PKC is part of the AT1 receptor signaling pathway and is likely to be activated after angiotensin II stimulation, the increase in PDE2 activity subsequent to angiotensin treatment might be PKC-mediated [5]. In rat ventricular cardiomyocytes, stimulation of soluble or particulate GC to the production of cGMP in different functional compartments, which access is restricted to different cGMP-PDEs. For example, cGMP produced by particulate GC would be exclusively under the control of PDE2, whereas that produced by soluble GC would be under the control of PDE2 and PDE5 [76]. We observed an up-regulation of the brain natriuretic peptide which stimulates its receptor, through an autocrine/paracrine action, increasing the “particulate” cGMP pool [7]. It was reported that natriuretic peptides could inhibit angiotensin II-induced proliferation of rat cardiac fibroblasts through cGMP-dependent mechanism [77]. The increased PDE2 activity observed might then oppose the BNP-induced cGMP increase, which could blunt its cardioprotective effect. Moreover, it was shown that natriuretic peptide desensitization in heart failure relates, in part, to increased PDE5 activity, supporting a therapeutic role for PDE5 inhibition [78]. Furthermore, stimulation of isolated cardiomyocytes by phenylephrine induced hypertrophy, and PDE5A over-expression amplified natriuretic peptide gene expression from phenylephrine stimulation [62]. It is the first time that such PDE2 activation is reported in angiotensin II induced hypertrophy, strengthening previous data in aortic banding-induced cardiac hypertrophy in Sprague-Dawley rats [12].
As a direct consequence of increased PDE4 activity which, degrades cAMP, the increase of 5′AMP could lead to AMPK activation through an increase in AMP/ATP ratio. This hypothesis is supported by a recent work showing that IL-6 activates AMPK in skeletal muscle by transiently increasing the concentration of cAMP and, secondarily, the AMP/ATP ratio [79]. AMPK is a heterotrimeric protein consisting of a catalytic α subunit and β and γ regulatory subunits and is a serine/threonine protein kinase [80]. It is a key metabolic regulator that is activated under stress conditions. Its exact role in cardiac diseases is until now not well defined, but in recent studies an overall cardioprotective role of AMPK was suggested [37], [81]. Indeed, AMPK activation with AICAR (5-amino-4-imidazolecarboxamide riboside, an AMPK activator) inhibited growth and proliferation in rat neonatal cardiac fibroblasts [82]. AMPKα2 deficiency was shown to enhance myocardial ischemia/reperfusion injury in a mouse model with cardiomyocyte-specific overexpression of a mutant AMPKα2 catalytic subunit [83], and to exacerbate pressure overload induced left ventricular hypertrophy and dysfunction in AMPKα2 knock-out mice [84]. Direct and indirect activation of AMPK was also shown to have cardioprotective properties. Metformin was able to induce AMPK phosphorylation and to improve left ventricular function and survival in heart failure [85]. Resveratrol was also shown to inhibit cardiac hypertrophy in part through AMPK activation [86]. In rat neonatal cardiomyocytes, angiotensin II-induced hypertrophy was accompanied by a decreased activation of AMPK, and stimulation with the AMPK-activator AICAR inhibited this hypertrophy [87]. Long-term activation of AMPK with AICAR was also shown to attenuate cardiac hypertrophy induced by pressure overload [88]. Moreover, a recent study showed that the anesthetic sevoflurane induced AMPK activation protects the heart against ischemia and reperfusion injury, and relies on upstream production of ROS [89]. In our cardiac hypertrophy model, AMPK phosphorylation was increased by 64%. This activation might be the result of increased generation of 5′AMP by PDE4 activity, and therefore might be a physiological mechanism to oppose left ventricular hypertrophy induced by angiotensin II.
The implication of NOX and ROS in cardiac remodeling has been well documented (for review see [90], [91]). For example, it was shown in rat neonatal cardiomyocytes that NOX2 [92] is implicated in the cardiac hypertrophic effect of angiotensin II. Surprisingly, we observed a 66% decrease in the NOX2 mRNA expression. However, it was recently shown that natriuretic peptides such as ANP have antihypertrophic effect on cardiomyocytes, in part by counteracting increased ROS generation and NOX2 expression induced by angiotensin II [93]. Moreover, a recent study showed that AMPKα2 subunit functions as a physiological suppressor of NAD(P)H oxidase (NOX) and ROS production in endothelial cells [94]. It was also suggested that AMPK may enhance NO bioavailability indirectly through antioxidative mechanisms by limiting superoxide-triggered NO consumption and consecutive peroxynitrite (ONOO-) production, e.g., by inhibiting NOX [95], [96]. We could then make the assumption that the increased BNP and AMPK in our model may have a negative effect on NOX2 expression, at least in the early stages of cardiac hypertrophy development.
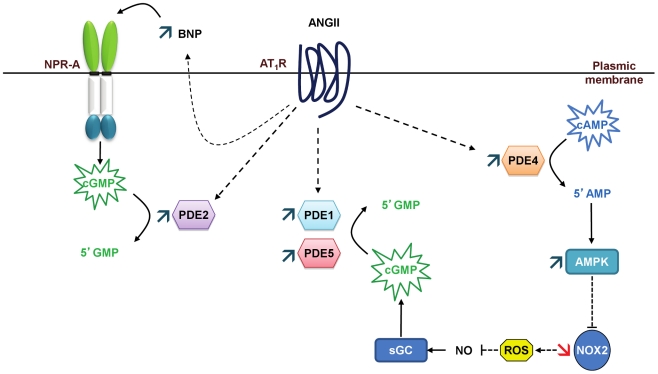
Cardiac hypertrophy and its progression to heart failure represent a major risk of sudden death. We show in this work that the early stages of cardiac hypertrophy induced by angiotensin II are associated with deep alterations in cAMP- and cGMP-PDE activities. While a specific rise in cAMP-PDE4 activity is observed, cGMP-PDEs activities (PDE1, PDE2 and PDE5) are broadly increased favoring a greater cGMP hydrolysis. This result is of particular interest knowing that previous works on other models focused either on cAMP- or cGMP-PDEs. PDE4D is strongly down-regulated, while PDE1A and PDE5A are highly up-regulated. Each of these alterations has already been reported in advanced cardiac hypertrophy and heart failure. Herein we show that these changes occur and vary in an opposing manner from the very beginning of the development of cardiac hypertrophy, confirming their potential role as targets in cardiac hypertrophy prevention. Moreover, we highlight for the first time that PDE1C is present in left cardiac ventricle and increased in early stages of cardiac hypertrophy. The role of PDE1 family in the heart has only very recently raised interest and is still not well known. Furthermore, we report that PDE2 accounts for the main contribution in cGMP-PDE activity in rat left cardiac ventricle and that PDE2 activity increases in the early stages of cardiac hypertrophy through a post-translational regulation. Finally, associated with the global increased cGMP hydrolysis in hypertrophic left cardiac ventricle, we observe the establishment at the PDE levels of two cardioprotective mechanisms and we suggest that these mechanisms could lead to increase cGMP: i) expression of natriuretic peptides could increase “particulate” cGMP pool; ii) activation of AMPK, subsequent to increase in PDE4 activity, could increase “soluble” cGMP pool through enhanced NO bioavailability ( Fig. 9 ). More studies are needed to support these assumptions. Nevertheless, our results suggest a potential link between PDE4 and AMPK/NOX2 and that cGMP-PDEs, especially PDE1 and PDE2, may be interesting therapeutic targets in preventing cardiac hypertrophy.
Figure 9. Proposed cardioprotective mechanisms that could lead to increase cGMP.
The expression of natriuretic peptides could increase “particulate” cGMP pool, and the activation of AMPK, subsequent to increased PDE4 activity, could increase “soluble” cGMP pool through enhanced NO bioavailability. These increases in cGMP could also have effects on PDE activities: “particulate” cGMP may contribute to further increase PDE2 activation and “soluble” cGMP may further activate PDE5, enhancing even more cGMP hydrolysis.
Acknowledgments
We are thankful to Jean-François Poirier from the animal facility for assistance with animals, Stéphanie Dal-Ros for helping with in vivo experiments, and Dr Marco Conti (Standford University, USA) for kindly providing PDE4A and PDE4B antibodies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding from patent sales have supported this work. There was no grant nor specific funder. Claire Lugnier was academic/CNRS co-author to patent deposits made by Neuro3D/CNRS/Université Louis Pasteur. Following the cessation of Neuro 3D, patents and molecules have been sold by Neuro3D on March 2007 to Via Pharmaceuticals Inc, San Francisco, California, United States of America. The CNRS/Université de Strasbourg, after renouncing their property rights, got money from this sale and the funding for the research team is part of it. Following are the patents: WO 2004/041258 - UTILISATION D'INHIBITEURS DES PHOSPHODIESTERASES DES NUCLEOTIDES CYCLIQUES, AYANT UNE STRUCTURE BENZODIAZEPINIQUE EN THERAPIE Copropriété UNISTRA/CNRS/Neuro3D/FORENAP. Date de priorité 30/10/2002; phase nationale FR 02/13607; Licence + cession Neuro3D. WO 2004/072078 - DERIVES DE PYRAZOLOTRIAZINE, PROCEDES DE PREPARATION ET UTILISATIONS Copropriété UNISTRA/CNRS/FORENAP Date de priorité 04/02/2003; FR 03/01256; Licence + cession Neuro3D. WO 2002/098865 - INHIBITEURS DES PHOSPHODIESTERASES DES NUCLEOTIDES CYCLIQUES, PREPARATION ET UTILISATIONS DE CES INHIBITEURS Déposant Neuro3D Date de priorité 07/06/2001; FR 01/07458 Licence + cession Neuro3D. This funding was used freely by the research team. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bayes-Genis A, Guindo J, Vinolas X, Tomas L, Elosua R, et al. Cardiac arrhythmias and left ventricular hypertrophy in systemic hypertension and their influences on prognosis. Am J Cardiol. 1995;76:54D–59D. doi: 10.1016/s0002-9149(99)80493-7. [DOI] [PubMed] [Google Scholar]
- 2.Weber KT, Brilla CG. Structural basis for pathologic left ventricular hypertrophy. Clin Cardiol. 1993;16:II10–14. doi: 10.1002/clc.4960161404. [DOI] [PubMed] [Google Scholar]
- 3.Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001;88:961–968. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 6.Muller B, Lugnier C, Stoclet JC. Implication of cyclic AMP in the positive inotropic effects of cyclic GMP-inhibited cyclic AMP phosphodiesterase inhibitors on guinea pig isolated left atria. J Cardiovasc Pharmacol. 1990;15:444–451. doi: 10.1097/00005344-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–328. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Lugnier C, Stoclet JC. Age related changes in cardiac and aortic phosphodiesterase activities in normotensive and hypertensive rats. Biochem Pharmacol. 1979;28:3581–3587. doi: 10.1016/0006-2952(79)90403-9. [DOI] [PubMed] [Google Scholar]
- 9.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 10.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 12.Yanaka N, Kurosawa Y, Minami K, Kawai E, Omori K. cGMP-phosphodiesterase activity is up-regulated in response to pressure overload of rat ventricles. Biosci Biotechnol Biochem. 2003;67:973–979. doi: 10.1271/bbb.67.973. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Osanai T, Nakano T, Wakui M, Okumura K. Enhanced activities and gene expression of phosphodiesterase types 3 and 4 in pressure-induced congestive heart failure. Heart Vessels. 2002;16:249–256. doi: 10.1007/s003800200032. [DOI] [PubMed] [Google Scholar]
- 14.Lehnart SE, Wehrens XH, Reiken S, Warrier S, Belevych AE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, et al. Ventricular Phosphodiesterase-5 Expression Is Increased in Patients With Advanced Heart Failure and Contributes to Adverse Ventricular Remodeling After Myocardial Infarction in Mice. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding B, Abe J, Wei H, Huang Q, Walsh RA, et al. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CJ, Huang R, Sun D, Ricketts S, Hoegler C, et al. Development of decompensated dilated cardiomyopathy is associated with decreased gene expression and activity of the milrinone-sensitive cAMP phosphodiesterase PDE3A. Circulation. 1997;96:3116–3123. doi: 10.1161/01.cir.96.9.3116. [DOI] [PubMed] [Google Scholar]
- 18.Badenhorst D, Anamourlis C, Gibbs M, Maseko M, Osadchii O, et al. A phosphodiesterase inhibitor promotes the premature development of adverse cardiac remodelling mediated by beta-adrenergic activation in hypertension. S Afr Med J. 2004;94:833–834. [PubMed] [Google Scholar]
- 19.Wollert KC, Yurukova S, Kilic A, Begrow F, Fiedler B, et al. Increased effects of C-type natriuretic peptide on contractility and calcium regulation in murine hearts overexpressing cyclic GMP-dependent protein kinase I. Br J Pharmacol. 2003;140:1227–1236. doi: 10.1038/sj.bjp.0705567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegener JW, Nawrath H, Wolfsgruber W, Kuhbandner S, Werner C, et al. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 21.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 22.Takimoto E, Champion HC, Li M, Belardi D, Ren S, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 23.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, et al. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol. 2008;294:H1398–1406. doi: 10.1152/ajpheart.91438.2007. [DOI] [PubMed] [Google Scholar]
- 24.Walter A, Etienne-Selloum N, Sarr M, Kane MO, Beretz A, et al. Angiotensin II Induces the Vascular Expression of VEGF and MMP-2 in vivo: Preventive Effect of Red Wine Polyphenols. J Vasc Res. 2008;45:386–394. doi: 10.1159/000121408. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Kissane JM, Robins E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958;233:184–188. [PubMed] [Google Scholar]
- 27.Campos-Toimil M, Keravis T, Orallo F, Takeda K, Lugnier C. Short-term or long-term treatments with a phosphodiesterase-4 (PDE4) inhibitor result in opposing agonist-induced Ca(2+) responses in endothelial cells. Br J Pharmacol. 2008;154:82–92. doi: 10.1038/bjp.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richter W, Jin SL, Conti M. Splice variants of the cyclic nucleotide phosphodiesterase PDE4D are differentially expressed and regulated in rat tissue. Biochem J. 2005;388:803–811. doi: 10.1042/BJ20050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keravis T, Thaseldar-Roumie R, Lugnier C. Assessment of phosphodiesterase isozyme contribution in cell and tissue extracts. Methods Mol Biol. 2005;307:63–74. doi: 10.1385/1-59259-839-0:063. [DOI] [PubMed] [Google Scholar]
- 30.Lugnier C, Komas N. Modulation of vascular cyclic nucleotide phosphodiesterases by cyclic GMP: role in vasodilatation. Eur Heart J. 1993;14(Suppl I):141–148. [PubMed] [Google Scholar]
- 31.Podzuweit T, Nennstiel P, Muller A. Isozyme selective inhibition of cGMP-stimulated cyclic nucleotide phosphodiesterases by erythro-9-(2-hydroxy-3-nonyl) adenine. Cell Signal. 1995;7:733–738. doi: 10.1016/0898-6568(95)00042-n. [DOI] [PubMed] [Google Scholar]
- 32.Coste H, Grondin P. Characterization of a novel potent and specific inhibitor of type V phosphodiesterase. Biochem Pharmacol. 1995;50:1577–1585. doi: 10.1016/0006-2952(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 33.Mery PF, Pavoine C, Pecker F, Fischmeister R. EHNA as an Inhibitor of PDE2: A Pharmacological and Biochemical Study in Cardiac Myocytes. In: Schudt C, Dent G, Rabe KF, editors. Phosphodiesterase Inhibitors. London: Academic Press Limited; 1996. pp. 81–88. [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 37.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112. doi: 10.1113/jphysiol.2006.109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirker A, Zhang M, Murdoch C, Shah AM. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol. 2007;27:649–660. doi: 10.1159/000109148. [DOI] [PubMed] [Google Scholar]
- 39.Das DK, Maulik N, Engelman RM. Redox regulation of angiotensin II signaling in the heart. J Cell Mol Med. 2004;8:144–152. doi: 10.1111/j.1582-4934.2004.tb00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 41.Keravis T, Vandecasteele G, Mokni W, Fischmeister R, Lugnier C. Identification of PDE1 et PDE5 in adult rat left ventricular cardiomyocytes. J Mol Cell Cardiol. 2007;42:S49–S50. [Google Scholar]
- 42.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, et al. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 43.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The Cardiac IKs Potassium Channel Macromolecular Complex Includes the Phosphodiesterase PDE4D3. J Biol Chem. 2009;284:9140–9146. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischmeister R, Castro L, Abi-Gerges A, Rochais F, Vandecasteele G. Species- and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Mongillo M, Tocchetti CG, Terrin A, Lissandron V, Cheung YF, et al. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 46.Abi-Gerges A, Richter W, Lefebvre F, Mateo P, Varin A, et al. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on beta-adrenergic cAMP signals. Circ Res. 2009;105:784–792. doi: 10.1161/CIRCRESAHA.109.197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baillie GS, Huston E, Scotland G, Hodgkin M, Gall I, et al. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–28309. doi: 10.1074/jbc.M108353200. [DOI] [PubMed] [Google Scholar]
- 48.Staton JM, Sayer MS, Hankey GJ, Attia J, Thakkinstian A, et al. Association between phosphodiesterase 4D gene and ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77:1067–1069. doi: 10.1136/jnnp.2006.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 50.Verde I, Pahlke G, Salanova M, Zhang G, Wang S, et al. Myomegalin is a novel protein of the golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J Biol Chem. 2001;276:11189–11198. doi: 10.1074/jbc.M006546200. [DOI] [PubMed] [Google Scholar]
- 51.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lugnier C, Keravis T, Le Bec A, Pauvert O, Proteau S, et al. Characterization of cyclic nucleotide phosphodiesterase isoforms associated to isolated cardiac nuclei. Biochim Biophys Acta. 1999;1472:431–446. doi: 10.1016/s0304-4165(99)00145-2. [DOI] [PubMed] [Google Scholar]
- 53.Bolger GB, McCahill A, Huston E, Cheung YF, McSorley T, et al. The unique amino-terminal region of the PDE4D5 cAMP phosphodiesterase isoform confers preferential interaction with beta-arrestins. J Biol Chem. 2003;278:49230–49238. doi: 10.1074/jbc.M303772200. [DOI] [PubMed] [Google Scholar]
- 54.Hashimoto Y, Sharma RK, Soderling TR. Regulation of Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase by the autophosphorylated form of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1989;264:10884–10887. [PubMed] [Google Scholar]
- 55.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 57.Kostic MM, Erdogan S, Rena G, Borchert G, Hoch B, et al. Altered expression of PDE1 and PDE4 cyclic nucleotide phosphodiesterase isoforms in 7-oxo-prostacyclin-preconditioned rat heart. J Mol Cell Cardiol. 1997;29:3135–3146. doi: 10.1006/jmcc.1997.0544. [DOI] [PubMed] [Google Scholar]
- 58.Vandeput F, Wolda SL, Krall J, Hambleton R, Uher L, et al. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem. 2007;282:32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- 59.Rybalkin SD, Rybalkina I, Beavo JA, Bornfeldt KE. Cyclic nucleotide phosphodiesterase 1C promotes human arterial smooth muscle cell proliferation. Circ Res. 2002;90:151–157. doi: 10.1161/hh0202.104108. [DOI] [PubMed] [Google Scholar]
- 60.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, et al. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292:L294–303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- 61.Kim D, Aizawa T, Wei H, Pi X, Rybalkin SD, et al. Angiotensin II increases phosphodiesterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signaling. J Mol Cell Cardiol. 2005;38:175–184. doi: 10.1016/j.yjmcc.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–2236. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guazzi M, Arena R, Pinkstaff S, Guazzi MD. Six months of Sildenafil therapy improves heart rate recovery in patients with heart failure. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 64.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lewis GD, Semigran MJ. The emerging role for type 5 phosphodiesterase inhibition in heart failure. Curr Heart Fail Rep. 2006;3:123–128. doi: 10.1007/s11897-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 66.Shimizu-Albergine M, Rybalkin SD, Rybalkina IG, Feil R, Wolfsgruber W, et al. Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J Neurosci. 2003;23:6452–6459. doi: 10.1523/JNEUROSCI.23-16-06452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Francis SH, Corbin JD, Bischoff E. Cyclic GMP-hydrolyzing phosphodiesterases. Handb Exp Pharmacol. 2009:367–408. doi: 10.1007/978-3-540-68964-5_16. [DOI] [PubMed] [Google Scholar]
- 68.Ang KL, Antoni FA. Reciprocal regulation of calcium dependent and calcium independent cyclic AMP hydrolysis by protein phosphorylation. J Neurochem. 2002;81:422–433. doi: 10.1046/j.1471-4159.2002.00903.x. [DOI] [PubMed] [Google Scholar]
- 69.Dolci S, Belmonte A, Santone R, Giorgi M, Pellegrini M, et al. Subcellular localization and regulation of type-1C and type-5 phosphodiesterases. Biochem Biophys Res Commun. 2006;341:837–846. doi: 10.1016/j.bbrc.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 70.Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 71.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, et al. Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res. 2006;98:777–784. doi: 10.1161/01.RES.0000215576.27615.fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stehlik J, Movsesian MA. Combined use of PDE5 inhibitors and nitrates in the treatment of pulmonary arterial hypertension in patients with heart failure. J Card Fail. 2009;15:31–34. doi: 10.1016/j.cardfail.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Evgenov OV, Busch CJ, Evgenov NV, Liu R, Petersen B, et al. Inhibition of phosphodiesterase 1 augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs with acute pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L723–L729. doi: 10.1152/ajplung.00485.2004. [DOI] [PubMed] [Google Scholar]
- 74.Schermuly RT, Inholte C, Ghofrani HA, Gall H, Weissmann N, et al. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: amplification by different type phosphodiesterase inhibitors. Respir Res. 2005;6:76. doi: 10.1186/1465-9921-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geoffroy V, Fouque F, Nivet V, Clot JP, Lugnier C, et al. Activation of a cGMP-stimulated cAMP phosphodiesterase by protein kinase C in a liver Golgi-endosomal fraction. Eur J Biochem. 1999;259:892–900. doi: 10.1046/j.1432-1327.1999.00123.x. [DOI] [PubMed] [Google Scholar]
- 76.Castro LR, Verde I, Cooper DM, Fischmeister R. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujisaki H, Ito H, Hirata Y, Tanaka M, Hata M, et al. Natriuretic peptides inhibit angiotensin II-induced proliferation of rat cardiac fibroblasts by blocking endothelin-1 gene expression. J Clin Invest. 1995;96:1059–1065. doi: 10.1172/JCI118092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, et al. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 79.Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58:1953–1960. doi: 10.2337/db08-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hutchinson DS, Summers RJ, Bengtsson T. Regulation of AMP-activated protein kinase activity by G-protein coupled receptors: potential utility in treatment of diabetes and heart disease. Pharmacol Ther. 2008;119:291–310. doi: 10.1016/j.pharmthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 81.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 82.Du J, Guan T, Zhang H, Xia Y, Liu F, et al. Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun. 2008;368:402–407. doi: 10.1016/j.bbrc.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, et al. AMP-Activated Protein Kinase Deficiency Enhances Myocardial Ischemia/Reperfusion Injury but Has Minimal Effect on the Antioxidant/Antinitrative Protection of Adiponectin. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang P, Hu X, Xu X, Fassett J, Zhu G, et al. AMP activated protein kinase-alpha2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Yong Ji S, et al. Activation of AMP-Activated Protein Kinase by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan AY, Dolinsky VW, Soltys CL, Viollet B, Baksh S, et al. Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt. J Biol Chem. 2008;283:24194–24201. doi: 10.1074/jbc.M802869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stuck BJ, Lenski M, Bohm M, Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem. 2008;283:32562–32569. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 88.Li HL, Yin R, Chen D, Liu D, Wang D, et al. Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem. 2007;100:1086–1099. doi: 10.1002/jcb.21197. [DOI] [PubMed] [Google Scholar]
- 89.Lamberts RR, Onderwater G, Hamdani N, Vreden MJ, Steenhuisen J, et al. Reactive oxygen species-induced stimulation of 5′AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation. 2009;120:S10–15. doi: 10.1161/CIRCULATIONAHA.108.828426. [DOI] [PubMed] [Google Scholar]
- 90.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009;47:15–22. doi: 10.1016/j.yjmcc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Anilkumar N, Sirker A, Shah AM. Redox sensitive signaling pathways in cardiac remodeling, hypertrophy and failure. Front Biosci. 2009;14:3168–3187. doi: 10.2741/3443. [DOI] [PubMed] [Google Scholar]
- 92.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, et al. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–191. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- 93.Laskowski A, Woodman OL, Cao AH, Drummond GR, Marshall T, et al. Antioxidant actions contribute to the antihypertrophic effects of atrial natriuretic peptide in neonatal rat cardiomyocytes. Cardiovasc Res. 2006;72:112–123. doi: 10.1016/j.cardiores.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Wang S, Zhang M, Liang B, Xu J, Xie Z, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulz E, Schuhmacher S, Munzel T. When metabolism rules perfusion: AMPK-mediated endothelial nitric oxide synthase activation. Circ Res. 2009;104:422–424. doi: 10.1161/CIRCRESAHA.109.194274. [DOI] [PubMed] [Google Scholar]