Abstract
Information on the fine-scale movement of predators and their prey is important to interpret foraging behaviours and activity patterns. An understanding of these behaviours will help determine predator-prey relationships and their effects on community dynamics. For instance understanding a predator's movement behaviour may alter pre determined expectations of prey behaviour, as almost any aspect of the prey's decisions from foraging to mating can be influenced by the risk of predation. Acoustic telemetry was used to study the fine-scale movement patterns of the Broadnose Sevengill shark Notorynchus cepedianus and its main prey, the Gummy shark Mustelus antarcticus, in a coastal bay of southeast Tasmania. Notorynchus cepedianus displayed distinct diel differences in activity patterns. During the day they stayed close to the substrate (sea floor) and were frequently inactive. At night, however, their swimming behaviour continually oscillated through the water column from the substrate to near surface. In contrast, M. antarcticus remained close to the substrate for the entire diel cycle, and showed similar movement patterns for day and night. For both species, the possibility that movement is related to foraging behaviour is discussed. For M. antarcticus, movement may possibly be linked to a diet of predominantly slow benthic prey. On several occasions, N. cepedianus carried out a sequence of burst speed events (increased rates of movement) that could be related to chasing prey. All burst speed events during the day were across the substrate, while at night these occurred in the water column. Overall, diel differences in water column use, along with the presence of oscillatory behaviour and burst speed events suggest that N. cepedianus are nocturnal foragers, but may opportunistically attack prey they happen to encounter during the day.
Introduction
Information on the foraging behaviour of large mobile predators provides additional information to methods such as dietary analysis to better understand predator-prey relationships and their effects on community dynamics [1], [2]. The foraging behaviour of a predator can determine anti-predatory behaviour of its prey such as increased vigilance, or it can influence habitat selection [3]–[5]. Increased vigilance or foregoing foraging opportunities in risky habitats can decrease the prey's foraging ability, consequently affecting its fitness [4]–[6]. Conversely, increased anti-predator behaviour may mean that the predator needs to be continually moving between foraging locations to keep the element of surprise in the predator-prey game [1], [5], [7], [8] and, as a result, the predator's fitness may also be affected. Determining fine-scale movement patterns of predators and their prey is an important component of studying predator-prey interactions and evaluating the likely consequences of these interactions for the predator, prey and overall community [1].
Large mobile marine predators are often elusive, have large home ranges and low population densities [9]. Obtaining information on fine-scale movement patterns and foraging behaviours can be difficult [9]. The advent of remote electronic data collection and monitoring techniques in recent decades has allowed for some of the complexities associated with studying large marine predators to be overcome. For example, active acoustic tracking, radio-acoustic positioning and animal-borne video, audio and data collection systems have been used to study fine-scale movements of a relatively small number of large shark species [9]–[12].
Active tracking can give high spatial resolution, and the pattern of a movement path can be measured and used to predict foraging behaviour. For example, the tortuosity of the path was used to estimate foraging strategy and patch use of blacktip reef sharks Carcharhinus melanopterus among various tropical reef habitats [12]. Animal-borne video, audio and data collection system (Crittercam) was used to provide information on foraging behaviour and fine-scale habitat preferences of tiger sharks Galeocerdo cuvier in shallow sea grass habitats in Shark Bay, Western Australia [9], [13]. In California, a radio-acoustic positioning system was used to describe the foraging behaviour and interactions between white sharks Carcharodon carcharias while hunting seals [10]. To date, this is the only shark species studied using this system.
Broadnose sevengill sharks N. cepedianus are large coastal predators with a wide temperate distribution [14]. Within their distributional range, they can be one of the most abundant apex predators in shallow coastal areas over summer [15]–[17]. N. cepedianus has similar dietary patterns globally, consuming a variety of prey including sharks, batoids, teleosts and marine mammals [16]–[20]. In particular, sharks of the genus Mustelus are one of the most common prey in all parts of their range [16]–[21].
In southeast Tasmania, high abundances of elasmobranchs including the Gummy shark, M. antarcticus, occur in coastal regions over summer [15], [18], [22], [23], with this shark being the main prey species of N. cepedianus [21]. The use of these coastal areas by juvenile M. antarcticus (mature at ∼95–110 cm TL) during summer implies these areas are beneficial for protection or that they contain abundant food to accelerate growth [24]. Although there might be other explanations, such as protection from exposure or socializing, it is more likely that Juvenile sharks use these habitats based on trade-offs between predation risk and food availability [24]. As such, the high abundance of N. cepedianus and the common occurrence of M. antarcticus in their diets [15], [18] suggests that M. antarcticus is exposed to high predation risk and therefore that these coastal areas may have little benefit for protection. So, foraging may be the primary reason for the continued use of these areas.
The large sizes (150–290 cm) of N. cepedianus consistently caught in coastal areas of Tasmania and the absence of neonates in the catches indicates that these areas are not used for shelter or as nursery areas and pupping grounds for this species [15], [18]. Furthermore, the large number of mature and immature individuals, and the low incidence of mature females containing mating scars (16%) also suggests that these areas do not have any specific reproductive relevance [15]. Therefore, foraging is probably the primary reason that N. cepedianus use these coastal areas. The fact that the peak in N. cepedianus abundance in summer coincides with the seasonal occurrence of its main elasmobranch prey [15], [18], [20]–[23] further indicates that N. cepedianus may be moving into coastal areas following prey.
The high abundances of N. cepedianus in coastal systems suggest that they have the potential to significantly influence community dynamics through both direct and indirect interactions, but to date no studies have addressed the movement patterns and possible foraging behaviour of N. cepedianus. The aim of this study was to use acoustic telemetry methods to examine the fine-scale movements of N. cepedianus and its primary prey species, M. antarcticus, in a coastal bay in southeastern Tasmania. In particular, we aimed to compare activity patterns over the diel cycle and describe potential foraging behaviours.
Methods
Ethics Statement
All research was conducted with approval from the University of Tasmania Animal Ethics Committee (#A0009120) and the Department of Primary Industries and Water (Permit # 8028).
Study area
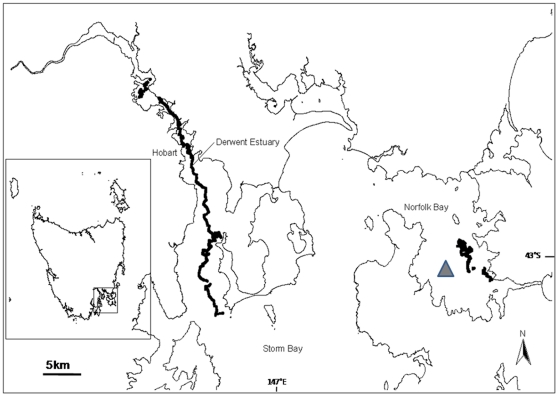
A VEMCO radio-acoustic positioning system (VRAP) was deployed in Norfolk Bay in southeast Tasmania, Australia (Fig. 1) to track 18 N. cepedianus and 10 M. antarcticus (see Table S1 for sex and size ranges) that were fitted with coded acoustic transmitters. Norfolk Bay is a relatively shallow (average depth 15 m, max depth 20 m), semi-enclosed bay with an area of approximately 180 km2. The substrate is mostly silt and silty sand with algae (predominately species from the family Caulerpaceae) and seagrass around the edges. The VRAP was positioned in the southwest corner of Norfolk Bay, in a depth of ∼16 m. This site was chosen due to previous records of high catch rates of N. cepedianus [15], [23]. Two individuals were also manually tracked using a VEMCO VR100 acoustic receiver coupled with a directional hydrophone, one in Norfolk Bay and the other in the Derwent Estuary (Fig. 1). The Derwent Estuary runs through the City of Hobart, before opening into Storm Bay (Fig. 1) and consistently reaches depths of 20–30 m, with a maximum depth of 44 m.
Figure 1. Study area, showing Norfolk Bay and the Derwent Estuary in southeast Tasmania.
Triangle represents the VRAP location. The two thick bold black lines (one in the Derwent Estuary and one in Norfolk Bay) are the movement paths of the two actively tracked N. cepedianus.
VRAP: animal capture and transmitter attachment
The VRAP was deployed from the 25th January to the 22nd April 2008. Sharks were tagged with coded transmitters (VEMCO Ltd., Halifax, Canada) during the period from the 26th January to 7th March 2008 (Table S1). For each species, half of the transmitters (nine for N. cepedianus and five for M. antarcticus) also recorded depth via a calibrated pressure sensor. Tags for N. cepedianus were V16 6H >3 year battery life and V16P 5H >2 year battery life programmed to transmit randomly every 50 to 130 seconds, while those for M. antarcticus were V13 1L >3 year battery life n = 1, V13P 1H >1 year battery life n = 5 and V9 2H ∼180 day battery life n = 4 programmed to transmit every 80 to 160 seconds. Transmitter sizes and program times were chosen to accommodate the different sizes of sharks. Sharks were caught on bottom-set longlines and brought on board the boat and restrained by two people holding them down on a foam mattress while a third person implanted the transmitter into the body cavity via a 1–2 cm incision in the abdominal wall. The incision was closed with a surgical suture and the shark released. Total length was measured in a straight line from snout to tip of tail to the nearest cm with the shark on its side and the caudal fin flexed, so the individual was as straight as possible (i.e. the longest longitudinal axis). The entire procedure was normally accomplished in 3–5 minutes during which time running water was pumped over the shark's gills. Due to the large size of the sharks and the rapid completion of surgery, the animals were not sedated.
VRAP (VEMCO radio-acoustic positioning system) (VEMCO Ltd., Halifax, Canada)
The VRAP consisted of three buoys aligned in an equilateral triangle array. Each buoy had a unidirectional hydrophone and receiver for detecting ultrasonic signals. The buoys triangulate the x, y and time coordinates of an animal fitted with an acoustic transmitter. This information was sent to a base station computer in near real time. For transmitters configured with a pressure sensor, the depth (the z coordinate) was also retrieved [10], [25]. The VRAP records two types of data: resolved and unresolved. Resolved data are available when all three buoys triangulate the coordinates of an individual, giving a precise location. Previous studies have indicated that the accuracy of such coordinates can be within two metres [10], [25]. The precision of calculated positions in this study was high, with the positions of each sentinel tag consistently being within 1 m of each other. Unresolved data are obtained when only one or two of the buoys detect a tagged animal, giving information only on whether the animal is in range, and its depth, if it has been fitted with a pressure tag, but no information on its precise location is available. In this study, the VRAP buoys were moored 400 m apart, giving the triangle an area of 0.069 km2. Resolved detections were recorded up to 1200 m outside the triangle.
Active tracking
Two N. cepedianus were equipped with a continuous acoustic transmitter with a pressure sensor that was configured to transmit every second at a set frequency. The tags were externally attached with a stainless steel tag head inserted into the muscle region near the base of the dorsal fin. In both cases, the procedure took less than a minute (see Table S1 for shark details). The sharks were tracked from a 6 m vessel using a VEMCO VR100 acoustic receiver and a directional hydrophone.
Data analysis
Frequency of occurrence
Detections from the day of tagging were excluded from all VRAP data analysis to eliminate any bias resulting from the tagging event. Both resolved and unresolved VRAP data was used to investigate differences in occurrence between night and day for each species. Chi-square (χ2) analysis was used to test for a departure from the expected 14.5∶9.5 ratio (daylight vs. night hours) in the number of hits between day and night hours for each individual shark. A t-test was also used to test for differences in number of hits between the day and night periods for each species. This was done by comparing the proportion of hits detected during the day to 60.4%, the proportion of time corresponding to the 14.5 h of daylight. Each individual was considered as a replicate.
Diel depth profiles
For each species, detection data from all individuals were pooled before analyses. This was done only after detecting no interaction between individual and depth (surface (0–5 m), mid (5–10 m), bottom (>10 m)) (F16, 589 = 0.682, p = 0.8130 for N. cepedianus; F6,143 = 0.468, p = 0.8310 for M. antarcticus); or individual and period of day (day vs. night) (F8, 598 = 1.5333, p = 0.1621 for N. cepedianus; F3 147 = 1.390, p = 0.2481 for M. antarcticus) with a two-way ANOVA. Here, the number of detections per day for each individual was considered as independent in the following analysis. Hence, data from each individual in each day can be considered as independent. Moreover, given the size of the animals, their movement rates and the area covered by the VRAP (∼1 km2), any individual could enter and exit the area many times in a day, and detections from each individual shark in any day can be considered as independent from detections in any other day. This means that data can be pooled for all individual sharks of each species.
For each species, the Rao's spacing test (Oriana v.3 software) was used to test if depth use was uniformly distributed over the 24 h period. Additionally, a three-dimensional contingency table was used to test for mutual independence between depth use, species and time of day (day vs. night). Here, depth was divided in three groups: 0–5 m (surface), 5–10 m (mid) and >10 m (bottom). This was followed by a three-dimensional chi-square partial independence test to determine if each of the three variables is independent of the other two. Rao's spacing tests were also used to test for uniformity in time of use of each depth range.
Active tracks of N. cepedianus (VR100 data) were overlayed on bathymetric maps of Norfolk Bay and the Derwent Estuary to observe the distance and area covered by the two sharks. To further examine diel depth profiles, the altitude of the shark in relation to the bottom was determined for the animal tracked in the Derwent Estuary using Eonfusion software (Myriax, Hobart, Australia) and segments representative of the day and night tracks presented graphically.
Movement rates
Arcview 3.2 Animal Extension v.2 program was used to determine the distances sharks moved between two successive points in the VRAP. This distance was then divided by the elapsed time to gain the minimum estimate of movement rate. To increase accuracy, only tracks where the time between the two detection points was less than the maximum transmission interval of the tags were used. This gives the best chance that the shark was swimming in a direct path, as longer times between points indicates that the shark left the detection range of the VRAP and later re-entered to be recorded again. Thus, the shark would be swimming in a non linear path. The accuracy of movement rates may also be influenced by variation in depth between the two points (i.e. up and down movement in the water column) since changes in depth would imply faster swimming rates than those calculated by considering exclusively horizontal distances. However, this confounding effect is probably minimal as the depth range in the study area is less than 15 m [10]. Considering these possible sources for bias, movement results should be considered as conservative estimates. Movement rates were pooled into day and night periods and examined for each species separately using a one-way ANOVA.
Path analysis
Circular statistics (Oriana v.3 software) were used to calculate angular changes in the movement paths of M. antarcticus. VRAP Data were pooled for night and day period and the angular changes were used to compare the linearity of movement and path structure between day and night. To enable the analysis of angular change, a shark must have been recorded by the VRAP for at least three consecutive resolved detections, when the detections were not separated by more than the maximum tag off time (i.e. <3 minutes for M. antarcticus). Watson-Williams F-test was used to compare angular changes between day and night. Rayleigh's Uniformity Test was used to determine if movements were uniformly distributed over the 360° or if they show some directionality. Due to the low number of three consecutive positional fixes for N. cepedianus, angular analysis could not be performed. Movement paths of M. antarcticus were also analysed in Fractal 5.0 software (V. O. Nams, Nova Scotia Agricultural College, Nova Scotia, Canada) to measure the fractal dimension. A fractal dimension is a measure of tortuosity of a movement path. The fractal mean function was used to estimate the overall fractal D value for movement paths at night and day. Fractal D movement paths range from 1 to 2, where 1 is a straight line and 2 is a path so tortuous that it completely covers a plane [26], [27].
Results
Frequency of occurrence
N. cepedianus were detected in the VRAP on 52% of days and M. antarcticus 75% of days. Daily detections ranged from 0–4 individuals ( = 0.9±1.1 (±SD)) for N. cepedianus and 0–5 individuals (
= 0.9±1.1 (±SD)) for N. cepedianus and 0–5 individuals ( = 1.8±1.4 (±SD)) for M. antarcticus. Multiple detections (individuals detected at the same time) of M. antarcticus occurred 40 times during the day compared to 11 times at night. Resolved detections constituted only 14% of the total number of detections for N. cepedianus and 9% for M. antarcticus. Resolved detections for N. cepedianus individuals ranged from 0 to 63, with a mean of 15, and unresolved ranged from 1 to 502, with a mean of 118. Resolved detections for M. antarcticus individuals ranged from 2 to 415 (mean = 60) and unresolved 52 to 3804 (mean = 730).
= 1.8±1.4 (±SD)) for M. antarcticus. Multiple detections (individuals detected at the same time) of M. antarcticus occurred 40 times during the day compared to 11 times at night. Resolved detections constituted only 14% of the total number of detections for N. cepedianus and 9% for M. antarcticus. Resolved detections for N. cepedianus individuals ranged from 0 to 63, with a mean of 15, and unresolved ranged from 1 to 502, with a mean of 118. Resolved detections for M. antarcticus individuals ranged from 2 to 415 (mean = 60) and unresolved 52 to 3804 (mean = 730).
There was no evidence of a diel pattern in detections between day and night period for N. cepedianus (n = 16) or M. antarcticus (n = 9), with some individuals being detected more during day and others more during the night (see Table S1 for χ2 results). Therefore, there was no significant effect of time of day on occurrence of N. cepedianus (t = −0.8799, df = 16, p = 0.3919) or M. antarcticus (t = 1.4855, df = 8, p = 0.1809). However, for four M. antarcticus, there were more detections during the day, with three of these individuals showing considerably higher number of detections (71–83%) during this period (Table S1).
Diel depth profiles – VRAP
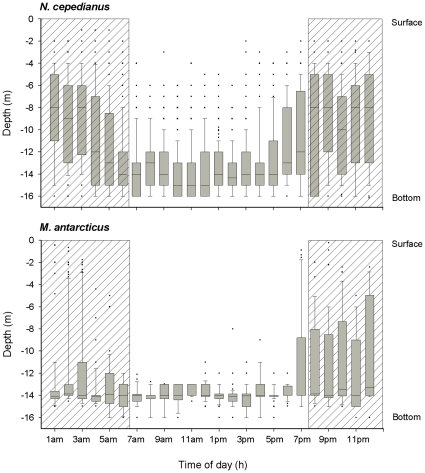
Rao's spacing test indicated that both N. cepedianus (U = 142.365, p<0.0001, n = 6241 depth records) and M. antarcticus (U = 149.442, p<0.0001, n = 1349) did not use the different depths evenly throughout the diel cycle. During night-time, N. cepedianus were consistently detected at all depths from the bottom to near surface, whereas during the day most detections where close to the substrate (Fig. 2). During the night, M. antarcticus was also detected in the water column, particularly during the first half of the night. However, most detections at night (79%) and all detections during the day were close to the substrate (Fig. 2).
Figure 2. Diel pattern in depth use for N. cepedianus and M. antarcticus in southeast Tasmania.
Box plots show the median (line within the boxes), interquartile ranges (boxes), 10th and 90th percentiles (whiskers) and outliers ( ) of depths detected during each 1 h interval. For each species, data from the whole sampling period was pooled. Dashed area indicates nocturnal period.
) of depths detected during each 1 h interval. For each species, data from the whole sampling period was pooled. Dashed area indicates nocturnal period.
The two species used various depths differently, and the depths used also differed between time of day (day vs. night). Depth use (surface, mid, bottom), time (day vs. night) and species (N. cepedianus vs. M. antarcticus) were not all mutually independent for the animals sampled (χ2 0.05,7 = 185.4721, p<0.0001). Partial independence tests also indicate that each of the three variables was not independent from the other two (species: χ2 0.05,5 = 140.5915, p<0.0001; depth use: χ2 0.05,6 = 4088.726, p<0.0001; and time: χ2 0.05,5 = 2133.895, p<0.0001), indicating an interaction between the three factors.
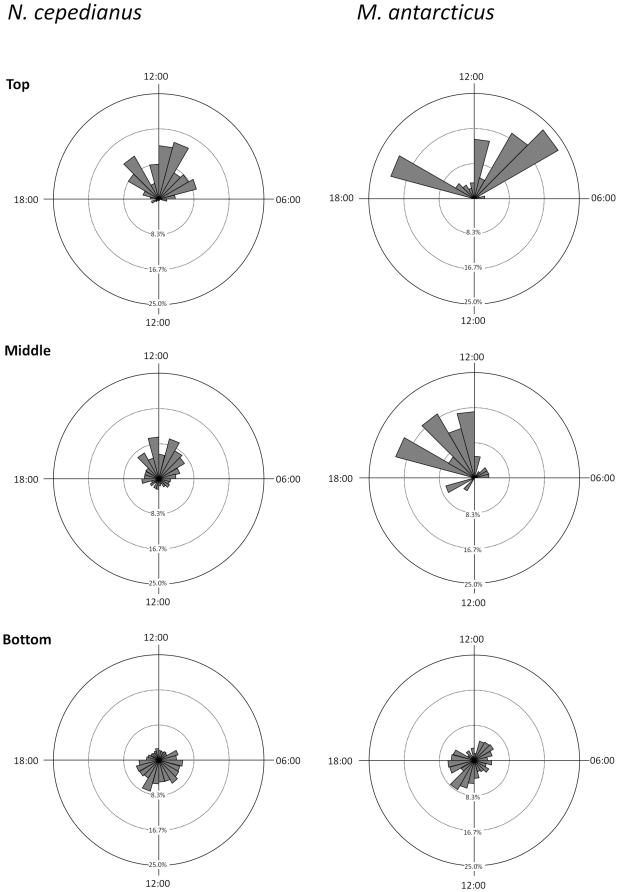
For N. cepedianus, Rao's spacing test indicated that the use of the three depth ranges was not uniformly distributed over time (p<0.01 in all cases). The top 0–5 m was mostly used during the night period, although there were some detections at this depth during the crepuscular periods (Fig. 3). Intermediate depths of 5–10 m were used throughout the 24 h period, but mostly during the night-time, while the bottom (>10 m depth) was mostly used during the day (Fig. 3).
Figure 3. Diel variations in depth use for N. cepedianus (in % time) for the top (0–5 m depth), middle (5–10 m depth) and bottom (>10 m depth) depth ranges.
As with N. cepedianus, Rao's test indicates that depth use for M. antarcticus was not uniformly distributed in time for any of the three depth ranges (p<0.01 in all cases). They occurred in the top 5 m during dusk (∼19:00–20:00 h), and again from around midnight to 04:00, but there were no detections at this depth during the day hours (Fig. 3). Intermediate depths were mainly used during the first few hours after sunset. Bottom depths were used throughout the diel cycle, although a greater proportion of detections was in the afternoons (Fig. 3). However, only 6% of the total detections for this species were in the top 0–5 m and 4% between 5 and 10 m, while the great majority (90%) was close to the bottom. Therefore, M. antarcticus is highly substrate associated throughout the diel cycle, although it occasionally moves up into the water column at night.
On several occasions an individual N. cepedianus was continually detected on or near the substrate for extended periods of time. The majority of these detections were unresolved, so an exact location could not be obtained. However, the constant recording of a particular depth for periods that ranged from 30 min to 4 hours indicates that the shark would have to be either stationary or milling (limited or slow movements in a centralised area) around the location. These periods of inactivity were only observed during the day.
Movement rates
Eleven N. cepedianus individuals recorded movement events that met the criteria of being less than 130 s between detection points (maximum tag transmit period). In total, there were 65 movement events that could be used to analyse rate of movement. The average swimming rate was 0.99±0.16 m.s−1 (± se) (range: 0.05 m.s−1 to 6.1 m. s−1). To gain a more accurate estimate of the animals' cruising speed, burst speed events were removed from the analysis. As the vast majority of movement rates were less than 1 ms−1 (82%), any movement rate greater than 1 ms−1 was considered to be faster than a possible cruising speed. The adjusted average movement rate was considerably lower, with a value of 0.48±0.03. There were no significant differences in movement rates between day (0.50±0.04 m.s−1) and night (0.43±0.04 m.s−1) (ANOVA: F (1, 52) = 1.2039, p = 0.2343).
Twelve burst speed events (5 individuals) were recognised for N. cepedianus. These events ranged from 1.4 m.s−1 up to 6.1 m. s−1. The seven burst speed events during the day were all close to the substrate (shallowest depth 11.3 m). In contrast, the five nocturnal burst speed events were closer to the surface, ranging from 2.5 to 7 m depth. Nocturnal burst speed events were associated with the shark entering the VRAP area with high speed. In contrast, day burst speed events were associated with long periods of inactivity, where the shark may have been stationary or milling around a restricted area, as the depth did not change during this period. On two occasions, burst speed events were followed by an extremely slow track (0.1 ms−1 and 0.3 ms−1).
Five M. antarcticus individuals recorded movement events that met the criteria of being less than 180 seconds between detections (maximum tag transmit period). In total, 245 events were recorded. M. antarcticus showed no difference in movement speed between day (0.33±0.01 m.s−1) and night (0.32±0.02 m.s−1). Movement rates ranged from 0.07 ms−1 to 1.02 ms−1.
Path analysis
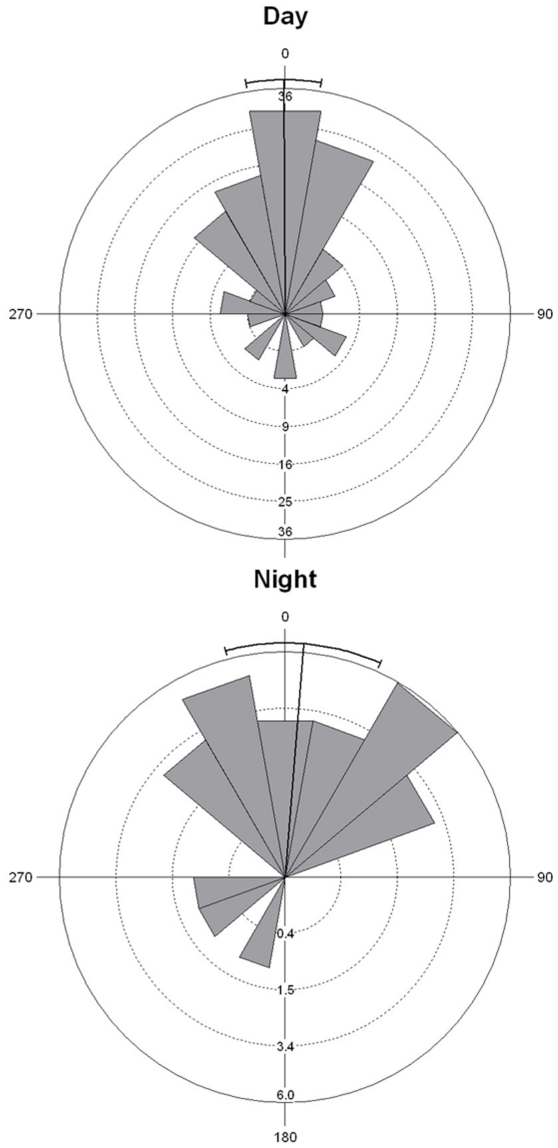
Mustelus antarcticus showed similar movement paths for day and night (Fig. 4). Turning angles were not significantly different between night and day (Watson-Williams F-test: F = 0.213, df = 1, p = 0.6450). For both periods, there were relatively small angular changes (Rayleigh test, p<0.0001 for both day and night), represented by the mean bearings: mean day: 350°±9°; night: 345°±24° (±sd) (Fig. 4). Fractal D showed that M. antarcticus did not move in a tortuous path for day or night (D = 1.09±0.02 in both cases).
Figure 4. Rose diagrams showing the angular changes of N. cepedianus for both day and night periods.
Note differences in scale between the two periods.
Active tracking
The Norfolk Bay individual (female N. cepedianus) was tracked for 20 daylight hours over 3 days. Due to bad weather, tracking was only conducted during the day. The shark only moved within an area of 9,722 m2 (Fig. 1) and remained milling or stationary on the substrate for the entire tracking period.
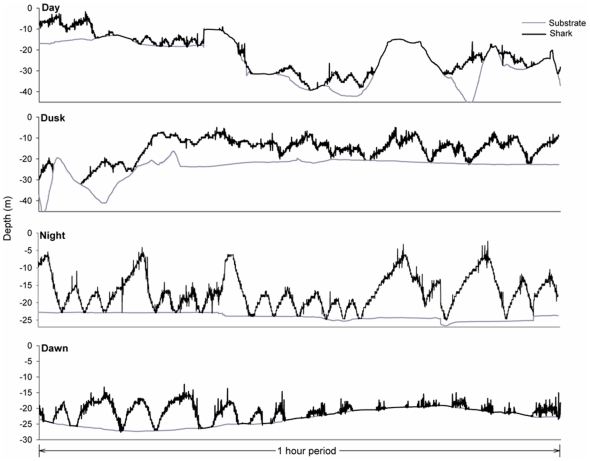
The Derwent Estuary individual was tracked continuously for 22 hours, from 1100 h to 0900 h the following morning. The shark moved constantly and when tracking ceased it had moved over 37 km (straight line measurement) (Fig. 1). During the day, the shark swam at constant depths and was often moving just above the substrate. In contrast, at night, it continually moved up and down in the water column (Fig. 5). This oscillating behaviour was consistent throughout the nocturnal period (Fig. 5) and was characterised by slow ascents ( = 0.07 m.s−1) off the bottom followed by faster descents (
= 0.07 m.s−1) off the bottom followed by faster descents ( = 0.16 m.s−1) back to the substrate. The depth to which the shark ascended to varied, but almost all descents concluded with the shark swimming along the substrate (Fig. 5). Crepuscular periods showed marked changes between bottom or constant depth swimming and oscillating behaviour (dusk: ∼20:00 to 20:30; dawn: ∼05:30 to 06:00) (Fig. 5).
= 0.16 m.s−1) back to the substrate. The depth to which the shark ascended to varied, but almost all descents concluded with the shark swimming along the substrate (Fig. 5). Crepuscular periods showed marked changes between bottom or constant depth swimming and oscillating behaviour (dusk: ∼20:00 to 20:30; dawn: ∼05:30 to 06:00) (Fig. 5).
Figure 5. Typical depth profiles for each of the different periods of the diel cycle.
Data illustrates the profile over representative 1 h periods during the day (16:00–17:00 h), dusk (20:00–21:00 h), night (01:00–02:00 h) and dawn (05:00–06:00 h) periods for the N. cepedianus individual tracked in the Derwent Estuary on the 16th March 2007.
Discussion
The distinct difference in depth use between day and night periods detected for N. cepedianus in the VRAP indicates different activity patterns for day and night. The use of multiple depths during the night suggests similar movements to that observed in the active track, where nocturnal movement was characterised by an oscillatory, or yo-yo, swimming motion, in which they repeatedly ascended into the water column and dived back to the substrate.
Oscillatory swimming motion has previously been reported for other shark species in offshore or deeper waters [28]–[32], but has not been commonly observed in shallow coastal areas. However, tiger sharks G. cuvier also display similar oscillating movements to N. cepedianus in shallow inshore habitats (<10 m depth) in Shark Bay, Western Australia [9]. Both N. cepedianus and G. cuvier move slower on the ascent stage ( = 0.07 m.s−1 and
= 0.07 m.s−1 and  = 0.10 m.s−1 respectively) than on descents (
= 0.10 m.s−1 respectively) than on descents ( = 0.16 m.s−1 for both species). However, N. cepedianus is more bottom oriented and initiates the oscillations from the substrate, ascending into the water column before returning to the substrate. G. cuvier appears to be more of a surface swimmer, initiating the oscillations from the surface and descending before returning to near surface waters [9]. In contrast to N. cepedianus, the yo-yo behaviour in G. cuvier was observed during the day [9].
= 0.16 m.s−1 for both species). However, N. cepedianus is more bottom oriented and initiates the oscillations from the substrate, ascending into the water column before returning to the substrate. G. cuvier appears to be more of a surface swimmer, initiating the oscillations from the surface and descending before returning to near surface waters [9]. In contrast to N. cepedianus, the yo-yo behaviour in G. cuvier was observed during the day [9].
Although Heithaus et al. [9] suggested a number of possible explanations for the oscillatory movement of G. cuvier, they believed that foraging behaviour was the most likely cause. They proposed that an oscillating foraging strategy allowed G. cuvier to ambush benthic prey from above, and air breathing prey such as mammals and turtles from below. For instance, Crittercam showed that G. cuvier descending from the surface were able to get close to benthic prey before evoking a flight response [9]. Since bottom associated prey such as M. antarcticus, skates and urolophids are the most common prey in the locations of the present study [18], N. cepedianus may similarly use the yo-yo behaviour to attack benthic prey from above. This could also explain the faster decent rates. Alternative hypotheses for this yo-yo behaviour include searching through the water column for olfactory cues [33], or minimization of energy consumption by swimming on the ascent and gliding (resting) on the descent. Gliding behavior on descents has been noted in pinnipeds and is possibly used to gain energetic benefits during foraging dives [34], [35].
Diel differences in depth use have been observed for a number of shark species, but have normally been associated with nocturnal migrations from deep water to forage in shallow waters [36]–[39]. For example, vertical oscillations at night by pacific sleeper sharks Somniosus pacificus have been related to foraging [32]. This behaviour is believed to be consistent with predators that use olfactory cues to search through the water column for prey [28], [32]. Both N. cepedianus and S. pacificus are large sluggish looking predators that consume fast moving animals such as marine mammals and teleosts [40]–[44]. The similarities in movement patterns, body size and diets suggest that the two species employ similar hunting strategies, where they use vertical oscillations and possibly olfactory cues, during no or low light conditions to ambush fast moving prey.
In contrast to night-time behaviour, N. cepedianus appears to be less active during the day, and may have periods of resting and reduced foraging. The bottom associated movements of N. cepedianus during the day could also be linked to hunting marine mammals, an important part of their diet [18], using vision as their primary sense. If this hypothesis is the case, then N. cepedianus may remain close to the substrate during the day so that they can attack marine mammals at the surface from below. This stalking approach is also characteristic of white sharks hunting pinnipeds, where vision is thought to be the primary sense used to detect prey [45], [46]. Overall, the regular occurrence of both benthic prey and marine mammals in N. cepedianus diets [18] suggests that the foraging strategy proposed by Heithaus et al. [9], where G. cuvier attacks benthic prey from above and surface prey from below may also be applicable to N. cepedianus. In addition, if this hypothesis is correct, N. cepedianus may be using different senses to hunt at different diel periods.
Due to the short duration of the two active tracks in this study, the observed behaviours could be associated with adverse reactions to the tagging event. For instance, the long period of inactivity observed in the shark tracked in Norfolk Bay could have been a result of stress or injury caused by its capture and tagging. The individual actively tracked in the Derwent Estuary travelled more than 37 km in 22 hours, and this could have been a flight reaction after tagging. Increased swimming rates, heightened activity levels, deep dives and depth holding behaviour (little or no movement to other depths) in relatively shallow water have all been observed immediately after release in blue Prionace glauca and shortfin mako Isurus oxyrinchus sharks [28], [47], [48] and it may take hours [13], [48] to days [47], [49]–[51] for sharks to return to their natural behaviour. However, as both the VRAP and active tracking produced similar results, the tagging event appeared to have minimal influence on behaviour.
Movement rates and path analysis of M. antarcticus suggest similar activity patterns for day and night periods. The lack of directional changes or burst speed events may be connected with a diet of predominately slow or stationary prey such as crabs, sipunculids and polychaete worms. However, as most of the water column detections were at night, it is possible that this was a result of M. antarcticus chasing nocturnally active prey such as cephalopods off the substrate. The diet of M. antarcticus in coastal Tasmania supports this hypothesis. Crabs and worms dominate their diets with teleosts and cephalopods less frequent [23], [52].
Given the likely high rate of natural mortality inflicted by N. cepedianus on M. antarcticus in Norfolk Bay [21], movement into the water column at night could also be anti-predator behaviour, as fine-scale movement may mitigate predation risk [53]–[56]. Additionally, the higher occurrence of M. antarcticus individuals detected together in the VRAP during the day could be a result of temporary group formation. Group formation by juvenile shark species during the day and dispersal at night has been previously described, and associated to anti-predator behaviour [57], [58]. As Norfolk Bay does not have much structure to provide shelter, increased movement (including vertical) at night, when N. cepedianus is more active, and group formation during the day may be a tactic to avoid predation in a relatively featureless landscape. Landscape features are important factors influencing foraging locations and escape tactics for a range of prey and predator species [2], [59].
The reason for the burst speed events recorded for N. cepedianus is unclear. However, we speculate that they were predation attempts. Due to the size of these animals and the absence of likely predators, it is unlikely that these burst speed events were escape behaviour. Although these could be a result of social interaction, there is a lack of evidence that these areas are used for mating or any other reproductive purpose [15]. If we consider these burst speed events to be predation attempts, for the two occasions that these events were followed by an extremely slow track (0.1 ms−1 and 0.3 ms−1), we could speculate that the shark was either resting after exerting energy on an unsuccessful chase, or that it may have secured the prey and was eating it. White sharks showed similar behaviour in which speeds of 7 m s−1 were followed by a period of limited movement indicated that the shark may have caught prey [10].
Burst speeds for N. cepedianus during the day took place on the substrate and were associated with periods of inactivity. Conversely, burst speeds at night were all in the water column and had no association with inactivity periods. These results further suggest different foraging behaviours for day and night. One hypothesis is that during the day N. cepedianus cruise about on the substrate and may opportunistically attack prey they happen to encounter, while during the night they move throughout the water column actively searching for prey. Alternatively, N. cepedianus may hunt close to the substrate during periods of maximum light using mainly visual cues, and forage throughout the water column during periods of low light using mainly olfactory senses.
Conclusion
Although Norfolk Bay supports large seasonal aggregations of both N. cepedianus and M. antarcticus, the bay is large and the radio-acoustic positioning system only detects animals over a limited spatial range (∼1 km2 diameter [10]). Therefore, the low percentage of resolved detections and the low number of individuals detected per day in the current study suggests that movements recorded in the VRAP area are only a small representation of the total movement in the Norfolk Bay. However, despite only covering a small spatial scale, radio-acoustic positioning systems provide the triangulated position of tracked animals, which can elucidate fine-scale behaviours, compared to the presence/absence data provided over larger spatial scales by passive receivers [60]. Regardless, for large mobile predators such as sharks, this system will be more useful for species that have a focal point, i.e. a discreet area of intense activity to centre the study around. For example, white sharks congregating seasonally around seal colonies to hunt [10]. However, despite the inherent difficulties of obtaining fine-scale movement behaviour of large predators, empirical data is needed to complement other data sources such as dietary information and prey abundance, and to supplement predator-prey modelling studies [61].
Supporting Information
N. cepedianus and M. antarcticus. Details of sharks tagged; date ‐ date of tagging, TL ‐ total length in cm, Days‐ number of days detected in VRAP, DP‐ detection period representing the time in days between first detection until last detection. P‐values in bold are significant and % ratio indicates if they occurred more during the day (above 60%) or the night (below 60%). The 60% expected is based on 14.5 hours of daylight being 60% of the hours in the day. * denotes animals omitted from Chi‐square χ2 and t‐test because they were not detected on more than one day.
(DOCX)
Acknowledgments
We would like to thank E. Forbes and D. Jones for field and technical assistance with the VRAP, the active tracking crew J. Hulls, E. Butal, T. Alexander, J. Yick and A. Pender, the TAFI habitat mapping section for supplying mapping data, H. Pederson for plotting the active track in Eonfusion and Dr. J. Seymour for statistical advice. We would also like to thank V. Namms and his wife for designing the Fractal analysis package and Dr. C. Simpfendorfer for advice for improving the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by grants from the Save Our Seas Foundation (www.saveourseas.com), Winifred Violet Scott Foundation (no website available) and the Holsworth Wildlife Research Endowment (http://www.anz.com/personal/private-bank-trustees/trustees/apply-grant/named-charitable-trusts/). No grant numbers are available at present due to finance department restructuring. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lima SL. Putting predators back into behavioural predator-prey interactions. Trends Ecol Evol. 2002;17:70–75. [Google Scholar]
- 2.Heithaus MR, Wirsing AJ, Burkholder D, Thomson J, Dill LM. Towards a predictive framework for predator risk effects: the interaction of landscape features and prey escape tactics. J Anim Ecol. 2009;78:556–562. doi: 10.1111/j.1365-2656.2008.01512.x. [DOI] [PubMed] [Google Scholar]
- 3.Sih A. Optimal behavior - can foragers balance two conflicting demands. Science. 1980;210:1041–1043. doi: 10.1126/science.210.4473.1041. [DOI] [PubMed] [Google Scholar]
- 4.Lima SL, Dill LM. Behavioral decisions made under the risk of predation - a review and prospectus. Can J Zool. 1990;68:619–640. [Google Scholar]
- 5.Brown JS, Laundre JW, Gurung M. The ecology of fear: Optimal foraging, game theory, and trophic interactions. J Mammal. 1999;80:385–399. [Google Scholar]
- 6.Wirsing AJ, Heithaus MR, Dill LM. Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators. Anim Behav. 2007;74:93–101. [Google Scholar]
- 7.Mitchell WA, Lima SL. Predator-prey shell games: large-scale movement and its implications for decision-making by prey. Oikos. 2002;99:249–259. [Google Scholar]
- 8.Meyer CG, Clark TB, Papastamatiou YP, Whitney NM, Holland KN. Long-term movement patterns of tiger sharks Galeocerdo cuvier in Hawaii. Mar Ecol Prog Ser. 2009;381:223–235. [Google Scholar]
- 9.Heithaus MR, Dill LM, Marshall GJ, Buhleier B. Habitat use and foraging behaviour of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar Biol. 2002;140:37–248. [Google Scholar]
- 10.Klimley AP, Le Boeuf BJ, Cantara KM, Richert JE, Davis SF, et al. Radio acoustic positioning as a tool for studying site-specific behavior of the white shark and other large marine species. Mar Biol. 2001;138:429–446. [Google Scholar]
- 11.Klimley AP, Le Boeuf BJ, Cantara KM, Richert JE, Davis SF, et al. The hunting strategy of white sharks (Carcharodon carcharias) near a seal colony. Mar Biol. 2001;138:617–636. [Google Scholar]
- 12.Papastamatiou YP, Lowe CG, Caselle JE, Friedlander AM. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology. 2009;90:996–1008. doi: 10.1890/08-0491.1. [DOI] [PubMed] [Google Scholar]
- 13.Heithaus MR, Marshall GJ, Buhleier B, Dill LM. Employing crittercam to study habitat use and behaviour of large sharks. Mar Ecol Prog Ser. 2001;209:307–310. [Google Scholar]
- 14.Last PR, Stevens JD. Melbourne: CSIRO Publishing; 2009. Sharks and rays of Australia 2nd edn.656 [Google Scholar]
- 15.Barnett A, Stevens JD, Frusher SD, Semmens JM. Seasonal occurrence and population structure of the broadnose sevengill shark (Notorynchus cepedianus) in coastal habitats of south east Tasmania. J Fish Biol. 2010;77(7):1688–1701. doi: 10.1111/j.1095-8649.2010.02810.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebert DA. Life History of the Sevengill Shark, N. cepedianus (Peron 1807), in two Northern California Bays. Calif Fish and Game. 1989;75:102–112. [Google Scholar]
- 17.Lucifora LO, Menni RC, Escalante AH. Reproduction, abundance and feeding habits of the broadnose sevengill shark N. cepedianus in north Patagonia, Argentina. Mar Ecol Prog Ser. 2005;289:237–244. [Google Scholar]
- 18.Barnett A, Abrantes K, Stevens JD, Yick J, Frusher SD, Semmens JM. Predator-prey relationships and foraging ecology of a marine apex predator with a wide temperate distribution. Mar Ecol Prog Ser. 2010;416:189–200. [Google Scholar]
- 19.Ebert DA. Diet of the sevengill shark N. cepedianus in the temperate coastal waters of Southern Africa. S Afr J Mar Sci. 1991;11:565–572. [Google Scholar]
- 20.Braccini JM. Feeding ecology of two high-order predators from south-eastern Australia: the coastal broadnose and the deepwater sharpnose sevengill sharks. Mar Ecol Prog Ser. 2008;371:273–284. [Google Scholar]
- 21.Barnett A, Redd KS, Frusher SD, Stevens JD, Semmens JM. Non-lethal method to obtain stomach samples from a large marine predator and the use of DNA analysis to improve dietary information. J Exp Mar Biol Ecol. 2010;393:188–192. [Google Scholar]
- 22.Williams H, Schaap AH. Preliminary results of a study into the incidental mortality of sharks in gill-nets in two Tasmanian shark nursery areas. Aust J Mar Freshwat Res. 1992;43:237–250. [Google Scholar]
- 23.Stevens JD, West GJ. Australia: Fisheries Research and Development Corporation Project 93/061 final report, CSIRO Marine Research; 1997. Investigation of school and Gummy shark nursery areas in southeastern Australia.76 [Google Scholar]
- 24.Heupel MR, Carlson JK, Simpfendorfer CA. Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser. 2007;337:287–297. [Google Scholar]
- 25.Jadot C, Donnay A, Acolas ML, Cornet Y, Anras MLB. Activity patterns, home-range size, and habitat utilization of Sarpa salpa (Teleostei: Sparidae) in the Mediterranean Sea. ICES J Mar Sci. 2006;63:128–139. [Google Scholar]
- 26.Nams VO. The VFractal: A new estimator for fractal dimension of animal movement paths. Landscape Ecol. 1996;11:289–297. [Google Scholar]
- 27.Nams VO. Using animal movement paths to measure response to spatial scale. Oecologia. 2005;143:179–188. doi: 10.1007/s00442-004-1804-z. [DOI] [PubMed] [Google Scholar]
- 28.Carey FG, Scharold JV. Movements of blue sharks (Prionace glauca) in depth and course. Mar Biol. 1990;106:329–342. [Google Scholar]
- 29.Klimley AP. Highly directional swimming by scalloped hammerhead sharks, Sphyrna lewini, and subsurface irradiance, temperature, bathymetry, and geomagnetic field. Mar Biol. 1993;117:1–22. [Google Scholar]
- 30.Gunn JS, Stevens JD, Davis TLO, Norman BM. Observations on the short-term movements and behaviour of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia. Mar Biol. 1999;135:553–559. [Google Scholar]
- 31.Klimley PA, Beavers SC, Curtis TH, Jorgensen SJ. Movements and swimming behavior of three species of sharks in La Jolla Canyon, California. Environ Biol Fishes. 2002;63:117–135. [Google Scholar]
- 32.Hulbert LB, Sigler MF, Lunsford CR. Depth and movement behaviour of the Pacific sleeper shark in the north-east Pacific Ocean. J Fish Biol. 2006;69:406–425. [Google Scholar]
- 33.Gardiner JM, Atema J. The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr Biol. 2010;20:1187–1191. doi: 10.1016/j.cub.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 34.Crocker GE, Gales NJ, Costa DP. Swimming speed and foraging strategies of New Zealand sea lions (Phocarctos hookeri). J Zool Lond. 2001;254:267–277. [Google Scholar]
- 35.Davis RW, Weihs D. Locomotion in diving elephant seals: physical and physiological constraints. Phil Trans R Soc B. 2007;362:2141–2150. doi: 10.1098/rstb.2007.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West GJ, Stevens JD. Archival tagging of school shark, Galeorhinus galeus, in Australia: Initial results. Environ Biol Fish. 2001;60:283–298. [Google Scholar]
- 37.Nakano H, Matsunaga H, Okamoto H, Okazaki M. Acoustic tracking of bigeye thresher shark Alopias supercilius in the eastern Pacific Ocean. Mar Ecol Prog Ser. 2003;265:255–261. [Google Scholar]
- 38.Stokesbury MJW, Harvey-Clark C, Gallant J, Block BA, Myers RA. Movement and environmental preferences of Greenland sharks (Somniosus microcephalus) electronically tagged in the St. Lawrence Estuary, Canada. Mar Biol. 2005;148:159–165. [Google Scholar]
- 39.Andrews KS, Williams GD, Farrer D, Tolimieri N, Harvey CJ, et al. Diel activity patterns of sixgill sharks, Hexanchus griseus: the ups and downs of an apex predator. Anim Behav. 2009;78:525–536. [Google Scholar]
- 40.Ebert DA. Observations on the predatory behaviour of the sevengill sharks N. cepedianus. S Afr J Mar Sci. 1991;11:455–465. [Google Scholar]
- 41.Yang MS, Page BN. Diet of Pacific sleeper shark, Somniosus pacificus, in the Gulf of Alaska. Fish Bull. 1999;97:406–409. [Google Scholar]
- 42.Ebert DA. Ontogenetic changes in the diet of the sevengill shark (N. cepedianus). Mar Freshwat Res. 2002;53:517–52. [Google Scholar]
- 43.Sigler MF, Hulbert LB, Lunsford CR, Thompson NH, Burek K, et al. Diet of Pacific sleeper shark, a potential Steller sea lion predator, in the north-east Pacific Ocean. J Fish Biol. 2006;69:392–405. [Google Scholar]
- 44.Yano K, Stevens JD, Compagno LJV. Distribution, reproduction and feeding of the Greenland shark Somniosus (Somniosus) microcephalus, with notes on two other sleeper sharks, Somniosus (Somniosus) pacificus and Somniosus (Somniosus) antarcticus. J Fish Biol. 2007;70:374–390. [Google Scholar]
- 45.Strong WR. Shape discrimination and visual predatory tactics in white sharks. In: Klimley PA, Ainley DG, editors. Great white sharks the biology of Carcharodon carcharias. San Diego: Academic press; 1996. pp. 229–249. [Google Scholar]
- 46.Laroche RK, Kock AA, Dill LM, Oosthuizen WH. Running the gauntlet: a predator-prey game between sharks and two age classes of seals. Anim Behav. 2008;76:1901–1917. [Google Scholar]
- 47.Campana SE, Joyce W, Manning MJ. Bycatch and discard mortality in commercially caught blue sharks Prionace glauca assessed using archival satellite pop-up tags. Mar Ecol Prog Ser. 2009;387:241–253. [Google Scholar]
- 48.Holts DB, Bedford DW. Horizontal and vertical movements of the shortfin Mako shark, Isurus oxyrinchus, in the southern California Bight. Aust J Mar Freshw Res. 1993;44:901–909. [Google Scholar]
- 49.Tricas TC, Taylor LR, Naftel G. Diel behaviour of the tiger shark, Galeocerdo Cuvier, at French Frigate Shoals, Hawaiian Islands. Copeia. 1981;4:904–908. [Google Scholar]
- 50.Sundstrom LF, Gruber SH, Clermont SM, Correia JPS, de Marignac JRC, et al. Review of elasmobranch behavioral studies using ultrasonic telemetry with special reference to the lemon shark, Negaprion brevirostris, around Bimini Islands, Bahamas. Environ Biol Fish. 2001;60:225–250. [Google Scholar]
- 51.Sundstrom LF, Gruber SH. Effects of capture and transmitter attachments on the swimming speed of large juvenile lemon sharks in the wild. J Fish Biol. 2002;61:834–838. [Google Scholar]
- 52.Yick J. 2008. The foraging and feeding ecology of Tasmanian coastal water elasmobranchs, Honours thesis, University of Tasmania.
- 53.Johnson CJ, Parker KL, Heard DC, Gillingham MP. A multiscale behavioral approach to understanding the movements of woodland caribou. Ecol Appl. 2002;12:1840–1860. [Google Scholar]
- 54.Johnson CJ, Parker KL, Heard DC, Gillingham MP. Movement parameters of ungulates and scale-specific responses to the environment. J Anim Ecol. 2002;71:225–235. [Google Scholar]
- 55.Anderson DP, Turner MG, Forester JD, Zhu J, Boyce MS, et al. Scale-dependent summer resource selection by reintroduced elk in Wisconsin, USA. J Wildlife Manage. 2005;60:298–310. [Google Scholar]
- 56.Hebblewhite M, Merrill EH. Multiscale wolf predation risk for elk: does migration reduce risk? Oecologia. 2007;152:377–387. doi: 10.1007/s00442-007-0661-y. [DOI] [PubMed] [Google Scholar]
- 57.Holland KN, Wetherbee BM, Peterson JD, Lowe CG. Movements and distribution of hammerhead shark pups on their natal grounds. Copeia. 1993;2:495–502. [Google Scholar]
- 58.Heupel MR, Simpfendorfer CA. Quantitative analysis of aggregation behaviour in juvenile blacktip sharks. Mar Biol. 2005;147:1239–1249. [Google Scholar]
- 59.Macia A, Abrantes KGS, Paula J. Thorn fish Terapon jarbua (Forskål) predation on juvenile white shrimp Penaeus indicus H. Milne Edwards and brown shrimp Metapenaeus monoceros (Fabricius): the effect of turbidity, prey density, substrate type and pneumatophore density. J Exp Mar Biol Ecol. 2003;291:29–56. [Google Scholar]
- 60.Heupel MR, Semmens JM, Hobday AJ. Automated acoustic tracking of aquatic animals: scales, design and deployment of listening station arrays. Mar Freshwat Res. 2006;57:1–13. [Google Scholar]
- 61.Frid A, Burns J, Baker GG, Thorne RE. Predicting synergistic effects of resources and predators on foraging decisions by juvenile Steller sea lions. Oecologia. 2009;158:775–786. doi: 10.1007/s00442-008-1189-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N. cepedianus and M. antarcticus. Details of sharks tagged; date ‐ date of tagging, TL ‐ total length in cm, Days‐ number of days detected in VRAP, DP‐ detection period representing the time in days between first detection until last detection. P‐values in bold are significant and % ratio indicates if they occurred more during the day (above 60%) or the night (below 60%). The 60% expected is based on 14.5 hours of daylight being 60% of the hours in the day. * denotes animals omitted from Chi‐square χ2 and t‐test because they were not detected on more than one day.
(DOCX)