Abstract
Background
Fieldwork has thoroughly established that most eggs are intensely predated. Among the few exceptions are the aerial egg clutches from the aquatic snail Pomacea canaliculata which have virtually no predators. Its defenses are advertised by the pigmented ovorubin perivitellin providing a conspicuous reddish coloration. The nature of the defense however, was not clear, except for a screening for defenses that identified a neurotoxic perivitellin with lethal effect on rodents.
Ovorubin is a proteinase inhibitor (PI) whose role to protect against pathogens was taken for granted, according to the prevailing assumption. Through biochemical, biophysical and feeding experiments we studied the proteinase inhibitor function of ovorubin in egg defenses.
Methodology/Principal Findings
Mass spectrometry sequencing indicated ovorubin belongs to the Kunitz-type serine proteinase inhibitor family. It specifically binds trypsin as determined by small angle X-ray scattering (SAXS) and cross-linking studies but, in contrast to the classical assumption, it does not prevent bacterial growth. Ovorubin was found extremely resistant to in vitro gastrointestinal proteolysis. Moreover feeding studies showed that ovorubin ingestion diminishes growth rate in rats indicating that this highly stable PI is capable of surviving passage through the gastrointestinal tract in a biologically active form.
Conclusions
To our knowledge, this is the first direct evidence of the interaction of an egg PI with a digestive protease of potential predators, limiting predator's ability to digest egg nutrients. This role has not been reported in the animal kingdom but it is similar to plant defenses against herbivory. Further, this would be the only defense model with no trade-offs between conspicuousness and noxiousness by encoding into the same molecule both the aposematic warning signal and an antinutritive/antidigestive defense. These defenses, combined with a neurotoxin and probably unpalatable factors would explain the near absence of predators, opening new perspectives in the study of the evolution and ecology of egg defensive strategies.
Introduction
Decades of fieldwork have thoroughly established that the eggs of most animals are subject to intense predation [1]–[3]. The reason is clear: Their high nutritional value offers to a pest or pathogen the best target for attack [4].
Among the few exceptions are the eggs from the freshwater apple snail Pomacea canaliculata which, though filled with large amounts of polysaccharides and proteins [5], have only one predator reported worldwide: the fire ant Solenopsis geminata [6]. P. canaliculata egg clutches are unusual in two respects: they are cemented outside the water and they are brightly coloured [7]–[9]. The strategy of laying eggs off the water allows eggs from aquatic organisms to avoid aquatic predators but at the same time they must face a variety of selective challenges, since they are exposed to stressful environmental conditions that may affect embryonic development and survival of offspring [10]; [11]. On the other hand, the conspicuously reddish coloration of the clutches (Figure 1) [12] advertises to visual-hunting predators the presence of egg defenses (aposematic warning). The message says: avoid me or pay the costs of a very unpleasant and/or unprofitable experience. However, the nature of these defenses remained a mystery until recently when, searching for defenses against predation, our group identified and characterized a proteinaceous neurotoxin (PV2) lethal to mice, the first genetically encoded toxin located inside an egg in the animal kingdom [13]; [14]. Eggs are toxic if orally administered to mice, but this slow-acting neurotoxin alone could not account for the virtual absence of predators, strongly suggesting the presence of other complementary noxious and/or unpalatable defensive factors, as the potential unpalatability reported for the eggs of a related species P. paludosa [15]. As in most gastropods, the female albumen gland provides eggs with the perivitellin fluid surrounding the fertilized oocyte to nourish and protect the embryos. Perivitellin fluid proteins, called perivitellins, have classically been considered merely storage proteins but recent work has shown that many of them serve other functions before being ingested by the embryos. For instance they provide eggs with lectins, proteinase inhibitors and other antimicrobial agents [16]–[20], growth factors for the developing embryo [21] and, in the case of P. canaliculata, a neurotoxin [13].
Figure 1. The conspicuous reddish egg clutches from P.canaliculata display a warning signal mostly provided by the perivitellin ovorubin.
Inset: Egg surface does not have any protective ornamentation.
In particular, the presence of proteinase inhibitors in eggs, has classically been assumed to play a role either to protect against microbial infection (inhibiting extracellular proteases secreted by microorganisms) [4] or to minimize degradation of important peptides and proteins from egg vitellus or perivitellus [22]. However, despite its intuitive appeal, the antimicrobial hypothesis has been proved only in egg PIs of very few species, such as the eggs of the amphibian, Odorrana grahami [23].
P. canaliculata eggs have a perivitellin called ovorubin which is a strong proteinase inhibitor [24], that is at the same time pigmented with a carotenoid, providing eggs with their aposematic coloration. This multifunctional protein is massively accumulated in the perivitellin fluid [25], providing protection against sun radiation [12], stabilizing and transporting antioxidant molecules in the perivitellin fluid [26] and helping to prevent egg dessication [27]. As in other eggs, ovorubin PI function was assumed to be antimicrobial based on its capacity to inhibit in vitro the bacterial proteinases subtilisin and fungal takadiastase but this hypothesis has never been tested [24].
Several structural features of this 300 kDa oligomeric perivitellin have been studied and relevant for the current work are its high stability in a wide range of pH and temperature and elevated glycosylation [28]–[30].
In the present study we investigated some structural and functional aspects of ovorubin as proteinase inhibitor in P. canaliculata egg defenses through a combination of biochemical, biophysical and feeding experiments. First we studied the primary structure of ovorubin and its interaction with trypspin. Then we tested if the proteinase inhibitor properties of ovorubin conform the “antimicrobial assumption” and provide evidence that it is an antinutritive factor with a role in egg biochemical defenses that would render them unprofitable for a predator.
Results
Mass spectrometry analysis and sequencing
As a first step, we conducted a structural analysis of ovorubin studying its primary structure by mass spectrometry and Edman degradation. This led to the inclusion of ovorubin into the family of Kunitz-type serine proteinase inhibitors.
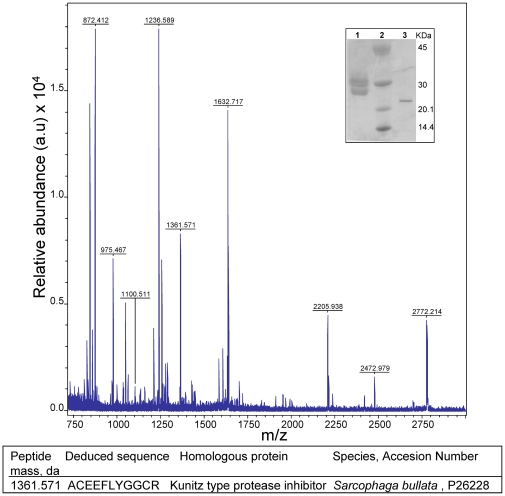
Before mass spectrometry analysis, ovorubin was chemically deglycosylated. This treatment reduced subunit heterogeneity to a single 24 kDa band in SDS-PAGE (Figure 2, inset). Mass spectrometry analysis of the tryptic products of this band resulted in a well resolved fingerprint (Figure 2). The analysis showed one peptide with m/z = 1361.57 which matched (MASCOT score 71) the serine proteinase inhibitor from the insect Sarcophaga bullata (SBPI) (Figure 2). This protein belongs to the small Kunitz-type inhibitors family that features identically spaced cysteines, along a peptide chain of varying length [31].
Figure 2. Tryptic digest fingerprint of deglycosilated ovorubin determined by quadrupole ion trap nanoelectrospray MS/MS (ESI ToF/ToF).
Inset: SDS-PAGE 8–20%. Lane 1: ovorubin; lane 2: MW markers; lane 3: Chemically-deglycosilated ovorubin. Bottom line, candidate sequence with homology to a Kunitz-type serine protease inhibitor.
Automated N-terminal Edman degradation identified 15 amino acid residues (Table 1). Interestingly, when the sequence was submitted to the NCBI non-redundant database without taxon restriction, no homology with known proteins was found.
Table 1. N-terminal amino acid sequence of deglycosilated ovorubin.
| 5 | 10 | 15 |
| N K E X L | L L D I (I) | D A T T S |
Ovorubin – trypsin interaction
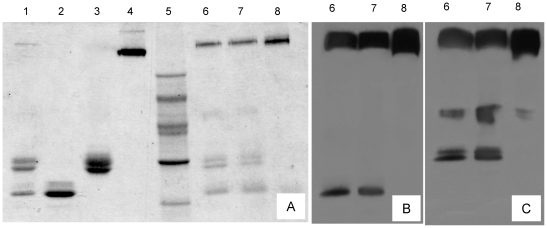
Incubation of the digestive serine protease trypsin and ovorubin in the presence of the cross-linker dithiobis[succinimidyl propionate] (DSP) allowed the study of the interaction between both proteins. It should be noted that the cross-linking reaction is irreversible under the experimental conditions. As shown by SDS–PAGE (Figure 3), the cross-linked products, represented by the high molecular weight (MW) band, increase with increasing DSP levels all free trypsin being cross-linked at 0.8 mM DSP. (Figure 3A lanes 6, 7 and 8). This band was immunoreactive to both anti-trypsin and anti-ovorubin antibodies as shown by Western blot assay, thus confirming specific interaction between both proteins (Figure 3 B, C). The absence of ovorubin-ovorubin cross-linking was confirmed by subjecting purified ovorubin to DSP cross-linking. This treatment rendered a high MW band which showed a lower Rf value than the ovorubin-trypsin complexes (Figure 3, lane 4), and was not immunoreactive to anti-trypsin IgG antibody (data not shown).
Figure 3. Analysis of ovorubin-trypsin cross-linked products by SDS-PAGE and immunobotting.
(A): SDS-PAGE 8–20%. Lane 1: Ovorubin and trypsin mix; lane 2: Trypsin; lane 3: ovorubin; lane 4: cross linked ovorubin; lane 5: Molecular mass standards; lanes 6–8: ovorubin-trypsin mix +0.05, 0.20 and 0.80 mM DSP, respectively. (B): Western blot analysis of lanes 6, 7 and 8 using anti-trypsin antibody. (C): Western blot analysis of lanes 6, 7 and 8 using anti-ovorubin antibody.
The interaction was then further characterized by Small angle X-ray scattering (SAXS) experiments on the complex, providing an indication of its size. From the Guinier plots of free ovorubin and ovorubin-trypsin complex it was possible to fit a gyration radius of 40.10±0.80 Å and 44.05±1.20 Å, respectively. The gyration radii obtained for the ovorubin-trypsin complex are compatible with a 1∶1 stoichometry, whereas the gyration radii for free ovorubin are compatible with previous reports [28], that is, a compact oligomer of about 300 kDa, the MW determined for ovorubin [32].
Trypsin inhibition
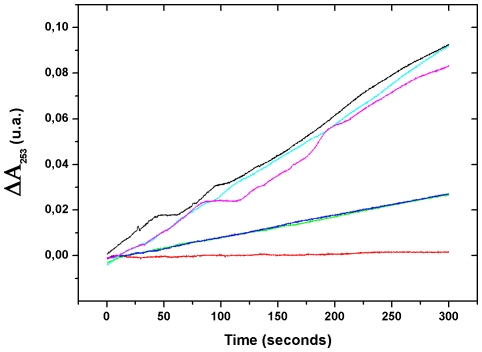
Trypsin inhibition properties of ovorubin were studied considering the effect of pH and temperature on this activity (Figure 4). The protein retained most of its inhibitory activity after heating at 100°C for 40 min at pH 7.4 (68.9±0.28% activity). In contrast, ovorubin lost almost all inhibitory activity by a combination of pre-incubation at pH = 2.0 for 48h followed by heating at 100°C for 40 min (3.4±0.07% activity) or by preincubation for 48 h at pH = 2.0 (3.0±0.20% activity).
Figure 4. Effect of pH and temperature on trypsin inhibition capacity of ovorubin.
Black line: negative control (no inhibitor); Red line: positive control (100% inhibition); Green line: pH = 7.4; Blue line: pH = 7.4+Ø; light blue line pH = 2.0; Violet line; pH = 2.0+Ø.
Antimicrobial activity of ovorubin
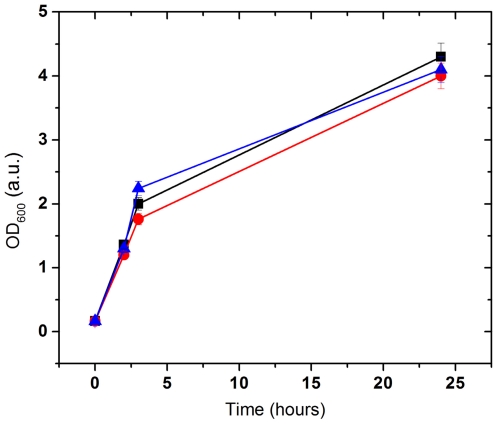
We tested the antimicrobial hypothesis adding ovorubin to bacterial cultures (Escherichia coli JM109, Salmonella typhimurium, Bacillus subtilis 168 and Lactobacillus casei) in liquid and solid media. Ovorubin showed no antibacterial activity against any of the strains tested, or the media employed in our experimental conditions (Figure 5).
Figure 5. Effect of ovorubin on E. coli and B. subtillis growth.
Bacteria were incubated in LB at 37°C, OD600 was measured at 2, 4 and 24 h. Black line: control; Red line: 100 µg ovorubin; Blue line: 20 µg ovorubin.
Simulated gastrointestinal digestion of ovorubin
The lack of antibacterial activity of ovorubin combined with a previous report indicating a high structural stability in a wide range of pH (pH 4.0–12.0) [28], suggested that the protein could be tailored to withstand the gastrointestinal tract of a predator. Therefore we tested this assumption in vitro, using a physiologically relevant digestion system, and then in vivo, by feeding studies (see below).
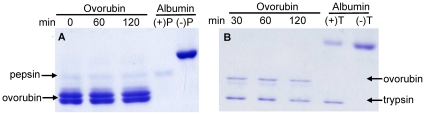
We found that ovorubin was resistant to simulated gastric digestion for 2 h, as shown by SDS-PAGE (Figure 6A). After this simulated gastric digestion, the pH was adjusted to duodenal conditions, trypsin was added and ovorubin simulated intestinal digestion performed for another 2 h. Again, ovorubin showed no significant alteration (Figure 6B).
Figure 6. In vitro digestibility of ovorubin analyzed by SDS-PAGGE.
(A) gastric digestion and (B) duodenal digestion. Lanes 1–3: 0, 60 and 120 min of incubation, lanes 4 and 5: positive (with enzyme) and negative control (without enzyme), respectively.
Effect of ovorubin-supplemented diet on rat growth rate
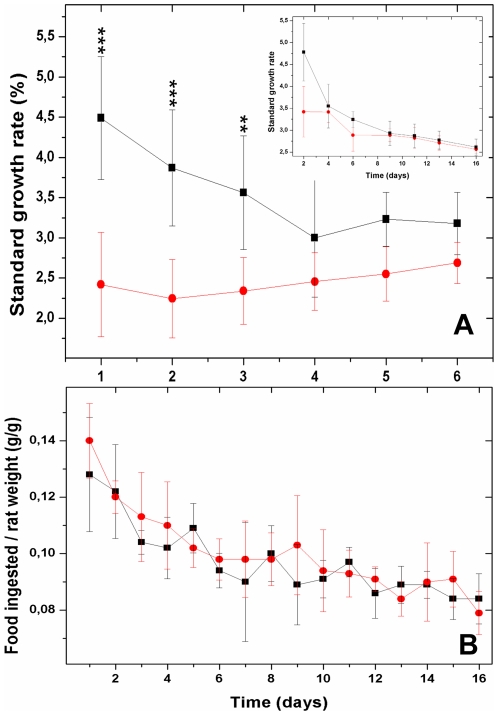
Finally, a bioassay to test the biological effect of ovorubin was performed using rats. During the first 3 days of ovorubin oral administration the animals showed a significantly lower standard growth rate than the control ones (Figure 7A and inset). This effect on growth rate disappeared after the fourth day of treatment. Daily food ingestion was similar in control and ovorubin-supplemented rats along the experimental period (Figure 7B).
Figure 7. Effect of ovorubin supplemented diets on Wistar rats' standard growth rate and food consumption.
(A) Standard growth rate during the first 6 days. Inset: Standard growth rate during 16 days showing rat adaptation to PI. Control (black square), treated (red circle). Values represent the mean ±1 SD (n = 12). *** p<0.001; ** p<0.01. (B) Food ingestion during a 16-day experiment.
Discussion
The principal functions so far attributed to egg proteins are: (i) provision of nutrients for the developing embryo; (ii) protection from microbial attack; and (iii) transport of nutrients into the developing embryo [33]. In the present study we provide evidence that ovorubin additionally functions as an antinutritive molecule, protecting the eggs against predation.
Ovorubin is a small Kunitz-type proteinase inhibitor with many of the structural features of the family
Partial sequencing allowed the inclusion of ovorubin among the small Kunitz-type inhibitors family. This family includes many very well studied plant inhibitors, most of them with only one active site and resistant to proteolysis [34]; [35]. These characteristics are present in ovorubin, and provided us with the first clue on the role that its proteinase inhibitor function might play in the egg (see below). However, unlike most animal and plant proteinase inhibitors [36]–[38], ovorubin is a high MW oligomeric protein composed of several glycoforms and isoelectric point isoforms [27]. This large size for a single-site PI can be understood considering that ovorubin is in fact a multifunctional perivitellin displaying several other key functions related to the reproductive strategy of this freshwater snail [12]. Interestingly, the majority of Kunitz-type inhibitors are proteins with a molecular weight of about 20 kDa, which is the approximate MW of the deglycosilated ovorubin subunits (Figure 2) [27].
Despite its large size and oligomeric nature, ovorubin and the other members of the Kunitz-type inhibitors family share stability properties, such as a high structural stability in a pH range of at least 4.0–12.0 and a high thermostability [28]; [29]; [38]–[40]. This high structural stability was also reflected in its PI activity. Heating ovorubin at 100°C caused a minor loss to its trypsin inhibition capacity. PI activity was retained after a short exposure to acidic pH values (not shown), but was lost if exposed for 48 h at pH 2.0. This inactivation is in agreement with the unfolding and disassembling of the particle described for this protein at pH<4.0 [28]. Although proteinase inhibitors have been reported in eggs of several species, the use of SAXS and cross-linking provided the first demonstration, to our knowledge, of the protein-protein interaction of an egg proteinase inhibitor with a digestive protease in a stoichiometric relationship.
The proteinase inhibitor role of ovorubin in eggs is not antimicrobial
Many serine proteinase inhibitors have been identified in egg-laying organisms such as arthropods, birds, and reptiles and invariably a role in either resistance to pathogens or the protection of critical peptides for embryo development has been ascribed to them [4]; [20]; [23]; [24]. In this line of thought a role to prevent microbial infection was assumed for ovorubin [24]. When we tested this assumption we found that ovorubin did not display antibacterial properties against Gram positive or Gram negative bacteria strains, at least in the experimental conditions used. In agreement with this lack of antimicrobial properties, a recent study reported that eggs of P. canaliculata can be experimentally infected by fungi [41]. Further, the absence of biochemical antimicrobial defenses in hard-shell eggs has been reported in other gastropods [2]; [16].
Ovorubin PI activity is part of egg defense against predation
The PI functional features of ovorubin were not concurring with the roles classically ascribed to egg proteinase inhibitors. Surveying the literature on the roles of PIs we found that, like ovorubin, plant storage proteins in seeds [42], tubers [43] and fruits [44] have at the same time PI activity providing defenses against embryo predation. Moreover, these plant storage proteins/PIs share other biological activities with ovorubin. They are synthesized only in organs of reproduction, propagation and dispersal; they are accumulated in large amounts, display antioxidant properties and exhibit activities consistent with a role in protecting embryos from abiotic stresses [45]. However, in contrast to plant storage protein-PI, ovorubin has several additional protective functions as mentioned in the background section and discussed below [12]; [26]; [27].
Plant PIs in seeds and tubers comprise a complex defense system against insects, nematodes, birds and mammals by the inhibition of their digestive proteases, thus preventing the predator from digesting and incorporating nutrients from the tissues consumed [22]; [46]; [47]. Similarly, simulated gastrointestinal digestion showed that ovorubin withstands the harsh condition of the digestive tract. The high stability against pH of plant PIs is explained by the need of maintaining the native (active) conformation within the digestive fluids of predators [42]; [48]; [49]. In this regard, ovorubin pH stability falls within the pH range of vertebrate and invertebrate digestive tract fluids [50]–[53]. Thus, ovorubin could reach the predator's intestine in a fully active form as it has been reported for soybean Kunitz-type and other plant PIs fed to rats [54]. Feeding experiments provided in vivo evidence that ovorubin was indeed capable of decreasing rat growth rate during the first 3 days. The effect disappeared after continuous ovorubin feeding, probably because the animal adapts to the PI as it has been reported for several plant PI-herbivorous interactions [46]
Taking into account that trypsin has a highly conserved structure in animals, ovorubin inhibitory activity could be directed against the digestive tracts of a wide variety of organisms, including vertebrates [50]; [51]; [53] and insects [52], though this assumption needs experimental validation. Moreover, since trypsin catalytically activates the other gut protease zymogens, if this key enzyme of the cascade is blocked, it would render most of the other proteases also inactive. The action of the snail egg trypsin inhibitor on rats may therefore involve both the inhibition of trypsin activity (antidigestive role) and the resistance of the inhibitor to digestion by gut enzymes (antinutritive) limiting the predator's capacity to digest egg nutrients.
Though plant proteinase inhibitors have long been recognized as components in their defenses against predation, this is, to our knowledge, the first report in the animal kingdom.
Ecological implications
Escaping predation is essential to survival for most animals and has resulted in the evolution of an amazing diversity of predator avoidance tactics. Among them, conspicuous coloration and unpalatability advertise chemical antipredator defense across many taxa. In this regard, there is a current debate regarding the allocation costs of avoiding predators: To effectively avoid predation, is it more advantageous to invest in increased conspicuousness or greater noxiousness, or to allocate equally to both signal modalities? [55]. In this study we present a novel alternative to the debate where there is no need of such trade-off, since noxiousness and conspicuousness are provided by the same molecule: ovorubin. In addition, by genetically encoding both the warning signal and the antinutritive/antidigestive defense, synthesis is even more cost-effective because females do not need to ingest toxic preys to endow eggs with chemical defenses. Furthermore, the “leftovers” of these defenses are in fact storage proteins consumed at a later time by developing embryos and hatchlings [5]. On the whole, apple snail egg defenses appears as a unique solution to allocation costs.
When considering the evolution of defenses, it is important to remember that something effective against one set of predators may be ineffectual against others. With only one reported predator worldwide, P. canaliculata eggs are an exception. It appears that their multifunctional perivitellins provide not only nutrients, but also a suite of defenses composed at least of antinutritive/antidigestive, neurotoxic and aposematic components (resumed in Table 2). These defenses acting simultaneously, and probably complemented by unpalatable factors, would impair the acquisition of nutrients and toxify the predator rendering P. canaliculata eggs unusually well defended. Regarding apple snail egg laying strategy to avoid predation it is important to note that there is neither ornamentation of the eggshell nor the use of external protection as oviposition on spiny vegetation or in protected areas (Figure 1, inset).
Table 2. Components of the biochemical defense system of P. canaliculata eggs.
| Perivitellin | Composition | Feature | Role in defense | Reference |
| Ovorubin | Glyco-lipo-caroteno protein | Red-coloured | Aposematic (warning coloration) | [12]; [26]; [27]; [30]; [66] |
| Ovorubin | Glyco-lipo-caroteno protein | Proteinase inhibitor | Antinutritive/antitrypsin | Present paper |
| PV3 | Lipo-caroteno protein | Orange-coloured | Aposematic | [12]; [32] |
| PV2 | Glyco- lipoprotein | Lethal to mice | Neurotoxic | [13]; [14]; [30]; [66] |
Considering that eggs with conspicuous coloration are very frequent across the Ampullariidae, this biochemical defense is probably not exclusive of P. canaliculata, and might be found more widely in other Pomacea with aerial oviposition, though more comparative work is needed to test this hypothesis.
Plant and apple snail embryos are sitting targets to predators, surrounded by highly nutritious compounds, and the evidence provided here suggests that both use proteinase inhibitors for protection. In plants, the loss of essential nutrients caused by these defensive proteins is predicted to be one of the most ecologically and evolutionally stable forms of defense against predation [56], this may very well be the case with apple snail eggs.
Conclusions
This study shows that, in contrast with the classical assumption, ovorubin would not function as an antimicrobial agent in the eggs of P. canaliculata. Instead, we provide evidence for a different function of this proteinase inhibitor as part of the biochemical defenses of snail eggs against predation
Its structural and functional properties are similar to plant storage proteins that play a dual role to nourish embryos and as a defense against predators by limiting predator's ability to digest egg nutrients. This function for an egg proteinase inhibitor is to our knowledge, the first description in the animal kingdom.
Unlike plant proteinase inhibitors, ovorubin is actively involved in the defense of the embryos not only by rendering them antinutritive, but also by providing them with a genetically encoded warning signal, comprising a new level of coordination and complementation of egg defenses. This strategy is a novel alternative solution to energy allocation costs to avoid predation by combining toxicity and conspicuousness in the same molecule, opening new perspectives in the study of aposematism and mimicry.
The information gathered here and in previous reports indicates that the acquisition of this complex defense system including aposematic, neurotoxic and antinutritive components provides the eggs with a protection that predators have not managed to overcome yet. It is to our knowledge the first study that unveils the nature of the defenses of a prey which has virtually no predators.
Apple snail eggs provide an exceptional model to study the evolution of biochemical and physiological adaptations, which may have profound implications for addressing questions on ecology and evolution heretofore not fully appreciated.
Methods
Ethics Statement
All the studies performed with rats were approved by the Directive Board of the INIBIOLP and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals [57]; (Instituto de Investigaciones Bioquimicas de La Plata's Animal Welfare Assurance No. A5647–01).
Ovorubin isolation and purification
Adults of P. canaliculata were collected in streams or ponds near La Plata, province of Buenos Aires, Argentina. Eggs were collected from females either raised in our laboratory or taken from the wild between November and April (reproductive season). Embryo development was checked in each egg mass microscopically [32], and only egg masses having embryos developed to no more than the morula stage were used.
Methods for ovorubin purification have been described previously [25]. In short, egg homogenate was centrifuged sequentially at 10,000 xg for 30 min, and then at 100,000 xg for 60 min and the supernatant stored at −70°C until analysis.
The soluble protein fraction obtained was purified in a Merck-Hitachi high performance liquid chromatograph (HPLC) (Hitachi Ltd., Tokyo, Japan) by a serial HPLC purification method. First, the sample was analyzed in a Mono Q HR 10/10 (Amersham-Pharmacia, Uppsala, Sweden) using a gradient of 0–1 M NaCl in a 20 mM Tris buffer. The ovorubin peak was then further purified by size exclusion chromatography (Superdex 200 HR 10/20, Amersham-Pharmacia, Uppsala, Sweden) using an isocratic gradient of sodium phosphate buffer 50 mM, 150 mM NaCl, pH 7.6. Purity of the single peak obtained was checked by native PAGE performed in a Mini-Protean III System (Bio Rad Laboratories, Inc.) following manufacturer directions, MW standards were obtained from GE Healthcare (Uppsala, Sweden). Protein content was determined by the method of Bradford [58].
Internal sequences determination by mass spectrometry
Ovorubin was first deglycosilated using trifluoromethansulfonic acid (TFMS, Sigma Chemical Co, St. Louis, USA) as described by Edge et al.[59] and the products were analyzed by SDS-PAGE. Peptide sequencing of tryptic digests of deglycosilated ovorubin was carried out by quadrupole ion trap nanoelectrospray MS/MS (ESI ToF/ToF) in an LCQ instrument (Finnigan TermoQuest, San Jose, CA), at the Proteomic Service, National Centre of Biotechnology, Madrid, Spain. The interpretation of MS/MS spectra was done manually, but assisted by various software packages, including Mascot (Matrix Science Ltd., London) and MSProduct, a facility of the Protein Prospector package [60].
N-Terminal amino acid sequence determination
Sequencing was performed by automatic Edman degradation at Laboratorio Nacional de Investigación y Servicios en Péptidos y Proteínas (LANAIS-PRO, Universidad de Buenos Aires - CONICET). The system used was an Applied Biosystems 477A Protein/Peptide Sequencer interfaced with a 120 HPLC for one-line phenylthiohydantoin amino acid analysis.
Trypsin inhibition assays
In order to test the effect of pH and temperature on ovorubin trypsin inhibition, ovorubin solutions (0.5 mg/ml) at pH 2.0 and 7.0 were heated at 100°C for 40 min. After this treatment, ovorubin preparations were incubated with a 10 fold molar excess of trypsin for 1 h and trypsin inhibition determined [61]. In short, N-benzoil-L-arginine ethyl ester (BAEE) is hydrolyzed by trypsin at the ester linkage causing an increase in absorbance at 253 nm at 25°C. Results were expressed as units of activity (the amount of enzyme that causes an absorbance increase of 0.003 per minute at 25°C).
Interaction between ovorubin and trypsin
The interaction was analyzed by cross-linking experiments as well as by small angle X-ray scattering (SAXS).
For the in vitro chemical cross-linking, purified ovorubin (5mg/ml) and trypsin (5 mg/ml) (Sigma) in a total volume of 200 µl were cross-linked for 30 min at room temperature using DSP (Pierce, IL, USA) at final concentrations of 0.05, 0.2 and 0.8 mM in a reaction buffer composed of 0.1M phosphate, 0.15M NaCl, pH 7.2. Ovorubin self cross-linking was checked at 0.8 mM DSP. Reaction was terminated by the addition of 1.0 M Tris, pH 7.5 to a final concentration of 50 mM. The complexes were analyzed by 8–20% SDS-PAGE, transferred onto nitrocellulose membranes and subjected to immunoblotting, as described previously [62]. For the complex detection, membranes were incubated for 2 h with an anti-trypsin polyclonal antibody (Santa Cruz Biotecnology, Inc.) (diluted 1∶5,000) and an anti-ovorubin polyclonal antibody in 10 mM Tris-HCl, pH 7.4, 0.15 M NaCl. Specific antigens were detected by goat anti-rabbit IgG horseradish peroxidase conjugate (Bio-Rad Laboratories) diluted (1∶3,000). Immunoreactivity was visualized by electrochemiluminescence.
The interaction between ovorubin and trypsin was also studied by SAXS experiments. Complexes obtained by chemical cross-linking were purified by size exclusion chromatography and purity checked by native electrophoresis, as described in the purification section. Experiments were performed at the D02A-SAXS2 line operating in the Laboratório Nacional de Luz Síncrotron, Campinas (SP, Brazil). The scattering pattern was detected using a MARCCD bidimensional charge-coupled device assisted by FIT2D v12.012 software [63]. The experiments were performed using a wavelength of 1.448 Å for the incident X-ray beam to minimize carbon absorption. The distance between the sample and the detector was kept at 1044 mm, allowing a Q-range between 0.012 and 0.25 Å−1 (nominal Dmax ≤260 Å). The temperature was controlled using a circulating water bath, and kept at 25°C. Each individual run was corrected for sample absorption, photon flux, buffer scattering, and detector homogeneity. At least three independent curves were averaged for each single experiment. SAXS experiments in a protein range of 2.4–0.20 mg/mL were performed to rule out a concentration effect in the data. The size of ovorubin-trypsin complex was determined using the gyration radii (R G) obtained by analysis of SAXS patterns as Guinier plots (ln(I) = ln(I0)−R GQ2/3, Q = 4πsin(θ)/λ, R GQ≤1).
Antimicrobial Activity Assays
The antimicrobial activity of ovorubin was tested on Gram (+) (E. coli JM109 and S. typhimurium) and Gram (-) strains (B. subtilis 168 and L. casei), both in solid and liquid media. For all the tests the microorganisms were grown overnight to mid-logarithmic phase in Luria-Bertani broth (LB) for E. coli, S. typhimurium and B. subtilis and de Man, Rogosa and Sharpe (MRS) broth for L. casei. For the solid medium assay, 50 µl of each culture were spread onto LB/agar or RMS/agar plates, and 20 min later 10 µl drops containing 20 µg, 10 µg and 2 µg of ovorubin were dispensed on each plate; sterile phosphate buffer was used as negative control. The plates were incubated for 18 h at 37°C and the formation of inhibition rings was observed. The liquid media assays were performed using one E. coli (JM109) and B. subtilis (168) strains, grown as indicated above. Aliquots of culture were diluted with fresh medium in glass test tubes to obtain an OD600 = 0.19, and supplemented with 100, 20, 10, or 2 µg of ovorubin, respectively; sterile buffer was used as control. The tubes were then incubated at 37°C with vigorous shaking and changes in OD600 recorded.
In vitro ovorubin digestibility
The simulated gastrointestinal digestion of ovorubin was performed in vitro following the method previously described by Moreno [64] with slight modifications. Briefly, gastric digestion was performed at 37°C for 120 min at pH 2.5 in the presence of porcine pepsin (Sigma, Dorset, UK; product No. P 6887) at a ratio of enzyme: substrate 1∶20 (w/w). Aliquots were taken at 0, 60 and 120 min and analyzed by SDS-PAGE as described above. The digestion was stopped by raising the pH to 7.5 using 50 mM phosphate buffer. For in vitro duodenal digestion the 120 min gastric digest was used as starting material. The duodenal digestion was performed using trypsin from bovine pancreas (Sigma, product No. T 9935) at a ratio of enzyme: substrate 1∶400 (w/w), at 37°C taking aliquotes at 0, 60 and 120 min for SDS-PAGE analysis. Albumin was used as positive (with enzyme) and negative control (without enzyme) in both gastric and duodenal digestion.
Effect of ovorubin supplemented diet on rat growth
Male Wistar rats 6 weeks old (weighing approximately 180 g at the start of the experiments) were separated into two groups (control and treated) of 12 animals each and fed ad libitum with a commercial diet for 16 days. The treated group was orally administered 100 µl of purified ovorubin (4 mg/ml) in 50mM phosphate buffer pH 7.4 on a daily basis, while the control group received 100 µl of buffer. Food consumption as well as body weight was determined daily for each animal. The standard growth rate (SGR) was calculated as follows:
Where Wto is the initial weight, Wt is the final weight, and t is the time in days [65].
The experiment was replicated twice. Data were analyzed by one-way ANOVA using Instat, v. 2.0 (GraphPad, San Diego, CA) and considered significant at a level of 5%.
Acknowledgments
MSD is member of Carrera del Investigador CICBA, Argentina. HH is member of Carrera del Investigador CONICET, Argentina. SI is a postdoctoral fellow CONICET, Argentina.
We thank LNLS - Brazilian Synchrotron Light Laboratory for access to their facilities (Projects D11A-SAXS1-7586/06 and 8583). We also thank Dr. Paradela from CNB, Spain; his help in the mass spectrometry analysis went far beyond the service provided. We are indebted to Carmen Sanchez-Rivas for her invaluable help in the microbiology experiments and Marcelo Ceolin for his help in the SAXS data analysis.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was partially supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas PIP No. 5888 to HH and Comisión de Investigaciones Científicas 2009 to MSD. The authors also thank LNLS/MCT-Brazilian Synchrotron Light Laboratory/Ministério da Ciência e Tecnologia for access to their facilities and partial financial support (Projects D11A-SAXS1-7586/06 and 8583). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kamler E. Parent-egg ontogeny Relationships in Teleost Fishes: An Energetics Perspective. Rev Fish Biol Fisheries. 2005;15:399–421. [Google Scholar]
- 2.Pechenik JA. The encapsulation of eggs and embryos by Molluscs: An overview. Am Malacological Bull. 1986;4:165–172. [Google Scholar]
- 3.Ricklefs R. An analysis of nesting mortality in birds. Smithsonian Contr Zool. 1969;9:1–48. [Google Scholar]
- 4.Christeller JT. Evolutionary mechanisms acting on proteinase inhibitor variability. FEBS J. 2005;272:5710–5722. doi: 10.1111/j.1742-4658.2005.04975.x. [DOI] [PubMed] [Google Scholar]
- 5.Heras H, Garín CF, Pollero RJ. Biochemical composition and energy sources during embryo development and in early juveniles of the snail Pomacea canaliculata (Mollusca: Gastropoda). J Exp Zool. 1998;280:375–383. [Google Scholar]
- 6.Yusa Y. Predation on eggs of the apple snail Pomacea canaliculata (Gastropoda: Ampullariidae) by the fire ant Solenopsis geminata. J Mollus Stud. 2001;67:275–279. [Google Scholar]
- 7.Estebenet AL, Martín PR. Pomacea canaliculata (Gastropoda: Ampullariidae): life-history traits and their plasticity. Biocell. 2002;26:83–89. [PubMed] [Google Scholar]
- 8.Albrecht EA, Carreño NB, Castro-Vazquez A. A quantitative study of copulation and spawning in the South American apple-snail, Pomacea canaliculata (Prosobranchia: Ampullariidae). Veliger. 1996;39:142–147. [Google Scholar]
- 9.Estebenet AL, Cazzaniga NJ. Egg variability and the reproductive strategy of Pomacea canaliculata (Gastropoda: Ampullariidae). APEX. 1993;8:129–138. [Google Scholar]
- 10.Przeslawski R. A review of the effects of environmental stress on embryonic development within intertidal gastropod egg masses. Moll Res. 2004;24:43–63. [Google Scholar]
- 11.Przeslawski R, Davis AR, Benkendorff K. Effects of ultraviolet radiation and visible light on the development of encapsulated molluscan embryos. Mar Ecol Prog Ser. 2004;268:151–160. [Google Scholar]
- 12.Heras H, Dreon MS, Ituarte S, Pollero RJ. Egg carotenoproteins in neotropical Ampullariidae (Gastropoda: Arquitaenioglossa). Comp Biochem Physiol C. 2007;146:158–167. doi: 10.1016/j.cbpc.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Heras H, Frassa MV, Fernández PE, Galosi CM, Gimeno EJ, et al. First egg protein with a neurotoxic effect on mice. Toxicon. 2008;52:481–488. doi: 10.1016/j.toxicon.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Frassa MV, Ceolin M, Dreon MS, Heras H. Structure and stability of the neurotoxin PV2 from the eggs of the apple snail Pomacea canaliculata. Biochim Biophys Acta. 2010;1804:1492–1499. doi: 10.1016/j.bbapap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Snyder NFR, Snyder HA. Defenses of the Florida apple snail Pomacea paludosa. Behaviour. 1971;40:175–215. [Google Scholar]
- 16.Benkendorff K, Davis AR, Bremner JB. Chemical Defense in the Egg Masses of Benthic Invertebrates: An Assessment of Antibacterial Activity in 39 Mollusks and 4 Polychaetes. J Invert Path. 2001;78:109–118. doi: 10.1006/jipa.2001.5047. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya H, Sakai R, Jimbo M. Bioactive Molecules from Sea Hares. In: Cimino G, Gavagnin M, editors. Subseries Marine Molecular Biotechnology. Berlin: Springer-Verlag; 2006. Molluscs From Chemo-ecological Study to Biotechnological Application. [DOI] [PubMed] [Google Scholar]
- 18.Mukai ST, Hoque T, Morishita F, Saleuddin AS. Cloning and characterization of a candidate nutritive glycoprotein from the albumen gland of the freshwater snail, Helisoma duryi (Mollusca: Pulmonata). Invertebr Biol. 2004;123:83–92. [Google Scholar]
- 19.Sanchez JF, Lescar J, Chazalet V, Audfray A, Gagnon J, et al. Biochemical and structural analysis of Helix pomatia agglutinin. A hexameric lectin with a novel fold. J Biol Chem. 2006;281:20171–20180. doi: 10.1074/jbc.M603452200. [DOI] [PubMed] [Google Scholar]
- 20.Nagle GT, de Jong-Brink M, Painter SD, Li KW. Structure, localization and potential role of a novel molluscan trypsin inhibitor in Lymnaea. Eur J Biochem. 2001;268:1213–1221. doi: 10.1046/j.1432-1327.2001.01972.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagle GT, Akalal DBG, Painter SD. Maternal impact on egg development in Lymnaea stagnalis: A growth factor is produced by the albumen gland in the reproductive tract. Invert Reprod Dev. 1999;36:171–174. [Google Scholar]
- 22.Chye ML, Sin SF, Xu ZF, Yeung EC. Serine proteinase inhibitor proteins: Exogenous and endogenous functions. In Vitro Cell Dev Biol - Plant. 2006;42:100–108. [Google Scholar]
- 23.Han YP, Yu HN, Yang XB, Rees HH, Liu JZ, et al. A serine proteinase inhibitor from frog eggs with bacteriostatic activity. Comp Biochem Physiol B. 2008;149:58–62. doi: 10.1016/j.cbpb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Norden DA. The inhibition of trypsin and some other proteases by ovorubin, a protein from the eggs of Pomacea canaliculata. Comp Biochem Physiol B. 1972;42:569–576. doi: 10.1016/0305-0491(72)90319-7. [DOI] [PubMed] [Google Scholar]
- 25.Dreon MS, Heras H, Pollero RJ. Metabolism of ovorubin, the major egg lipoprotein from the apple snail. Mol Cell Biochem. 2003;243:9–14. doi: 10.1023/a:1021616610241. [DOI] [PubMed] [Google Scholar]
- 26.Dreon MS, Schinella G, Heras H, Pollero RJ. Antioxidant defense system in the apple snail eggs, the role of ovorubin. Arch Biochem Biophys. 2004;422:1–8. doi: 10.1016/j.abb.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Ituarte S, Dreon MS, Pasquevich MY, Fernandez PE, Heras H. Carbohydrates and glycoforms of the major egg perivitellins from Pomacea apple snails (Architaenioglossa: Ampullariidae). Comp Biochem Physiol B. 2010;157:66–72. doi: 10.1016/j.cbpb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Dreon MS, Ituarte S, Ceolin M, Heras H. Global shape and pH stability of ovorubin, an oligomeric protein from the eggs of Pomacea canaliculata. FEBS J. 2008;275:4530. doi: 10.1111/j.1742-4658.2008.06595.x. [DOI] [PubMed] [Google Scholar]
- 29.Dreon MS, Ceolín M, Heras H. Astaxanthin binding and structural stability of the apple snail carotenoprotein ovorubin. Arch Biochem Biophys. 2007;460:107–112. doi: 10.1016/j.abb.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Dreon MS, Heras H, Pollero RJ. Characterization of the major egg glycolipoproteins from the perivitellin fluid of the apple snail Pomacea canaliculata. Mol Reprod Dev. 2004;68:359–364. doi: 10.1002/mrd.20078. [DOI] [PubMed] [Google Scholar]
- 31.Papayannopoulos IA, Biemann K. Amino acid sequence of a protease inhibitor isolated from Sarcophaga bullata determined by mass spectrometry. Protein Sci. 1992;1:278–288. doi: 10.1002/pro.5560010210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garín CF, Heras H, Pollero RJ. Lipoproteins of the egg perivitellin fluid of Pomacea canaliculata snails (Mollusca: Gastropoda). J Exp Zool. 1996;276:307–314. doi: 10.1002/(SICI)1097-010X(19961201)276:5<307::AID-JEZ1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.Stevens L. Egg proteins: what are their functions? Sci Prog. 1996;79(Pt 1):65–87. [PubMed] [Google Scholar]
- 34.Moreno FJ. Gastrointestinal digestion of food allergens: effect on their allergenicity. Biomed Pharmacother. 2007;61:50–60. doi: 10.1016/j.biopha.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Mosolov VV, Valueva TA. Proteinase Inhibitors and Their Function in Plants: A Review. Appl Biochem Microbiol. 2005;41:227–246. [PubMed] [Google Scholar]
- 36.Polya GM. Protein and non-protein protease inhibitors from plants. In: Atta-ur-Rahman (ed) Studies in Natural Products Chemistry. Elsevier. 2003:567–641. [Google Scholar]
- 37.Sasaki SD, Cotrin SS, Carmona AK, Tanaka AS. An unexpected inhibitory activity of Kunitz-type serine proteinase inhibitor derived from Boophilus microplus trypsin inhibitor on cathepsin L. Biochem Biophys Res Commun. 2006;341:266–272. doi: 10.1016/j.bbrc.2005.12.178. [DOI] [PubMed] [Google Scholar]
- 38.Roch P, Ville P, Cooper EL. Characterization of a 14 kDa plant-related serine protease inhibitor and regulation of cytotoxic activity in earthworm coelomic fluid. Dev Comp Immunol. 1998;22:1–12. doi: 10.1016/s0145-305x(97)00047-5. [DOI] [PubMed] [Google Scholar]
- 39.Azarkan M, Dibiani R, Goormaghtigh E, Raussens V, Baeyens-Volant D. The papaya Kunitz-type trypsin inhibitor is a highly stable β-sheet glycoprotein. Biochim Biophys Acta. 2006;1764:1063–1072. doi: 10.1016/j.bbapap.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues Macedo ML, Machado Freire MG, Cabrini EC, Toyama MH, Novello JC, et al. A trypsin inhibitor from Peltophorum dubium seeds active against pest proteases and its effect on the survival of Anagasta kuehniella (Lepidoptera: Pyralidae). Biochim Biophys Acta. 2003;1621:170–182. doi: 10.1016/s0304-4165(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 41.Maketon M, Suttichart K, Domhom J. Effective control of invasive apple snail (Pomacea canaliculata Lamarck) using Paecilomyces lilacinus (Thom) Samson. Malacologia. 2009;51:181–190. [Google Scholar]
- 42.Alves García V, Machado Freire MdG, Novello JC, Rodrigues Macedo ML. Trypsin inhibitor from Poecilanthe parviflora seeds: purification, characterization, and activity against pest proteases. The Prot J. 2004;23:343. doi: 10.1023/b:jopc.0000032654.67733.d5. [DOI] [PubMed] [Google Scholar]
- 43.Walsh TA, Twitchell WP. Two Kunitz-type proteinase inhibitors from potato tubers. Plant Physiol. 1991;97:15–18. doi: 10.1104/pp.97.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wingate VP, Broadway RM, Ryan CA. Isolation and characterization of a novel, developmentally regulated proteinase inhibitor I protein and cDNA from the fruit of a wild species of tomato. J Biol Chem. 1989;264:17734–17738. [PubMed] [Google Scholar]
- 45.Shewry PR. Tuber storage proteins. Ann Bot. 2003;91:755–769. doi: 10.1093/aob/mcg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jongsma MA, Bolter C. The adaptation of insects to plant protease inhibitors. J Insect Physiol. 1997;43:885–895. doi: 10.1016/s0022-1910(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 47.Johnson R, Narvaez J, An G, Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989;86:9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno FJ, Maldonado BM, Wellner N, Mills EN. Thermostability and in vitro digestibility of a purified major allergen 2S albumin (Ses i 1) from white sesame seeds (Sesamum indicum L.). Biochim Biophys Acta. 2005;1752:142–153. doi: 10.1016/j.bbapap.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 49.Teles RC, Calderon LA, Medrano FJ, Barbosa JA, Guimaraes BG, et al. pH dependence thermal stability of a chymotrypsin inhibitor from Schizolobium parahyba seeds. Biophys J. 2005;88:3509–3517. doi: 10.1529/biophysj.104.045682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randall D, Burggren W, French K. Energy acquisition: Feeding, digestion and metabolism. In: Randall D, Burggren W and French K (eds) Eckert Animal Physiology. Mechanisms and Adaptations. Freeman, New York, 1997:683–724. [Google Scholar]
- 51.Denbow DM. Gastrointestinal Anatomy and Physiology. In: Whittow GC, editor. Sturkie's Avian Physiology. Boston: Academic Press; 2000. pp. 299–325. [Google Scholar]
- 52.Nation JL. Digestion. In: Nation JL, editor. Insect physiology and biochemistry. Boca Raton: CRC press; 2002. pp. 27–64. [Google Scholar]
- 53.Birk Y. Protein proteinase inhibitors in legume seeds. Overview. Arch Latinoam Nutr. 1996;44:26S–30S. [PubMed] [Google Scholar]
- 54.Hajos G, Gelencser E, Pusztai A, Grant G, Sakhri M, et al. Biological effects and survival of trypsin inhibitors and the agglutinin from soybean in the small intestine of the rat. J Agric Food Chem. 1995;43:165–170. [Google Scholar]
- 55.Darst CR, Cummings ME, Cannatella DC. A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proc Natl Acad Sci USA. 2006;103:5852–5857. doi: 10.1073/pnas.0600625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felton GW. Indigestion is a plant's best defense. Proc Natl Acad Sci USA. 2005;102:18771–18772. doi: 10.1073/pnas.0509895102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Research Council. Washington, USA: Academic Press; 1996. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 58.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–274. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 59.Edge AS, Faltynek CR, Hof L, Reichert LE, Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981;118:131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- 60.Clauser KR, Baker P, Burlingame AL. Role of accurate mass measurement (+/- 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem. 1999;71:2871–2882. doi: 10.1021/ac9810516. [DOI] [PubMed] [Google Scholar]
- 61.Schwert GW, Takenaka Y. A spectrophotometric determination of trypsin and chymotrypsin. Biochim Biophys Acta. 1955;16:570–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- 62.Dreon MS, Lavarías S, Garín CF, Heras H, Pollero RJ. Synthesis, distribution, and levels of an egg lipoprotein from the apple snail Pomacea canaliculata (Mollusca: Gastropoda). J Exp Zool. 2002;292:323–330. doi: 10.1002/jez.10043. [DOI] [PubMed] [Google Scholar]
- 63.Hammersley AP. Fit2D. 1997. An Introduciton and overview ESRF Internal Report ESRF97HAO2T. Grenoble, France, European Synchrotron Radiation Facility.
- 64.Moreno FJ, Mellon FA, Wickham MS, Bottrill AR, Mills EN. Stability of the major allergen Brazil nut 2S albumin (Ber e 1) to physiologically relevant in vitro gastrointestinal digestion. FEBS J. 2005;272:341–352. doi: 10.1111/j.1742-4658.2004.04472.x. [DOI] [PubMed] [Google Scholar]
- 65.Burrells C, Williams PD, Southgate PJ, Crampton VO. Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Vet Immunol Immunopathol. 1999;72:277–288. doi: 10.1016/s0165-2427(99)00143-9. [DOI] [PubMed] [Google Scholar]
- 66.Dreon MS, Heras H, Pollero RJ. Biochemical composition, tissue origin and functional properties of egg perivitellins from Pomacea canaliculata. Biocell. 2006;30:359–365. [PubMed] [Google Scholar]