Abstract
Studies directed at the synthesis of (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones from (Z)-3-aryl-3-haloenoic acids are described. The successful strategy relies on the preparation of (Z)-3-aryl-3-haloenoic acids from acetophenones through the corresponding (Z)-3-aryl-3-haloenals and the conversion of the (Z)-3-aryl-3-haloenoic acids to (Z)-5-benzylidene-4-aryl-5H-furan-2-ones. The furanones were subsequently treated with primary amines and dehydrated to the corresponding (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones.
Keywords: Haloenals, Haloeneoic Acids, Furanones, Pyrrolones
1. Introduction
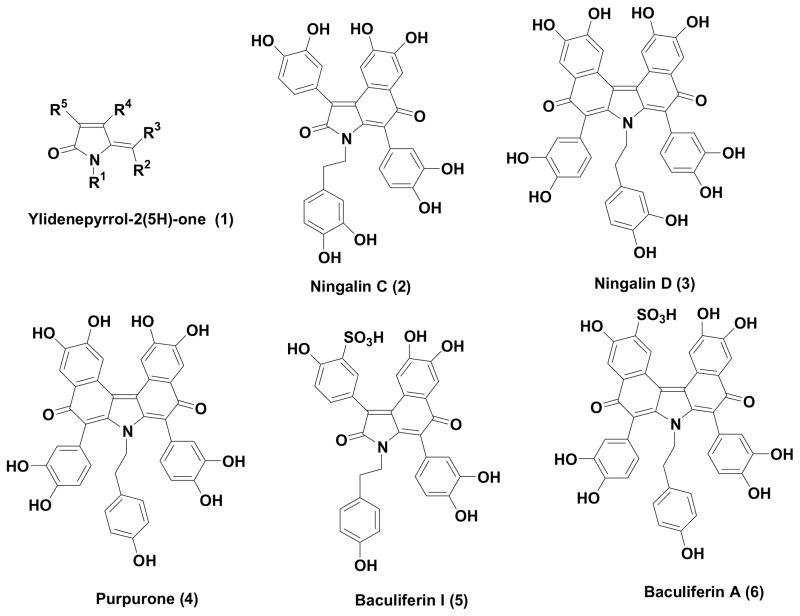
Ylidenepyrrol-2(5H)-ones (1) can be viewed (Fig. 1) as being present as a core structure in a variety of naturally occurring alkaloids and most recently are represented in the ningalin1 (2–4) and baculiferin2 (5–6) family of marine natural products. Several of the ningalins have been shown to possess very good cytotoxic activity3 against a variety of cancer cell lines and very significant multidrug resistance reversal4 activity. Other members of the family have exhibited ATP-citrate lyase inhibitory properties5 and the recently isolated baculiferins have been found to be significant inhibitors2 of the HIV-IIIB virus in MT4 and MAGI cells. Since the ylidenepyrrol-2(5H)-one motif is a key structural element of such bioactive alkaloids, efficient synthetic methods for construction of this heterocyclic framework is of some importance.
Figure 1.
Ylidenepyrrol-2(5H)-one containing natural products
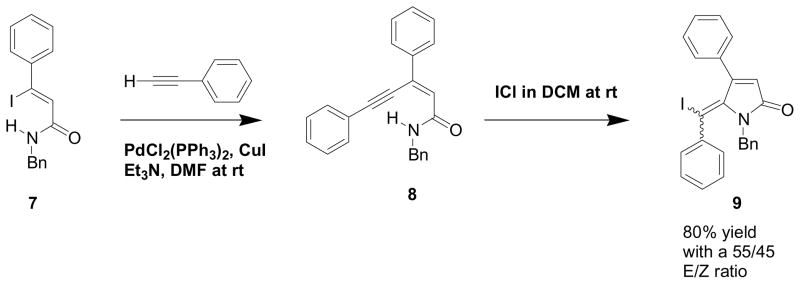
Abarbri and coworkers6 have recently indicated that a minimal number of synthetic methods exist for the construction of the ylidenepyrrol-2(5H)-ones (1) although more extensive strategies exist for the corresponding ylidenefuran-2(5H)-ones. The same workers6 have utilized a very efficient methodology (Scheme 1) involving (Z)-3-iodoalk-2-enamides (7) for the preparation of the 5-(iodoalkylidene)-pyrrol-2(5H)-ones (9). The Sonogashira coupling of the (Z)-3-iodoalk-2-enamides (7) produced good yields of the (Z)-alk-2-en-4-ynamides (8), which underwent cyclization with ICl to give an E/Z mixture of the 5-(iodoalkylidene)-pyrrol-2(5H)-ones (9) with the E isomer normally predominating.
Scheme 1.
Abarbri synthesis of 5-(iodoalkylidene)-pyrrol-2(5H)-ones
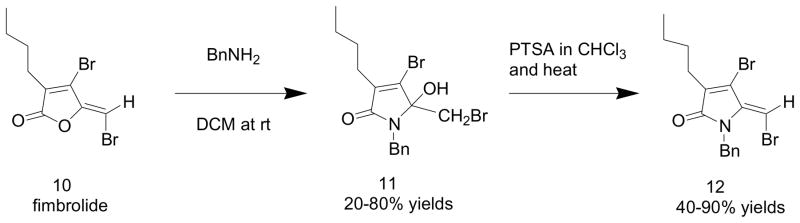
Since the availability of (Z)-ylidenefuran-2(5H)-ones has been previously established7, we decided to explore these heterocycles as potential building blocks for the (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones (1). This strategy became more intriguing in light of the recent work (Scheme 2) by Kumar, St. Black and coworkers8 who recently demonstrated the lactamization of the fimbrolides such as 10, which possess the ylidenefuran-2(5H)-one functionality and are marine derived natural products. The pyrrolones (12) resulting from the lactamization process exhibited the Z configuration for all of the examples reported. This work was carried out in order to develop a new class of antimicrobial agents based on targeting the N-acylated homoserine lactone system.
Scheme 2.
Kumar, St. Black lactamization of fimbrolides
The sequence presented in Scheme 2 utilizes a primary amine to open the fimbrolide (10) and recyclize to a hydroxylactam (11), which can then be dehydrated to the nitrogen analog (12) of the starting fimbrolide. The (Z) stereoisomer predominated in all of the examples, which were studied.
2. Results and Discussions
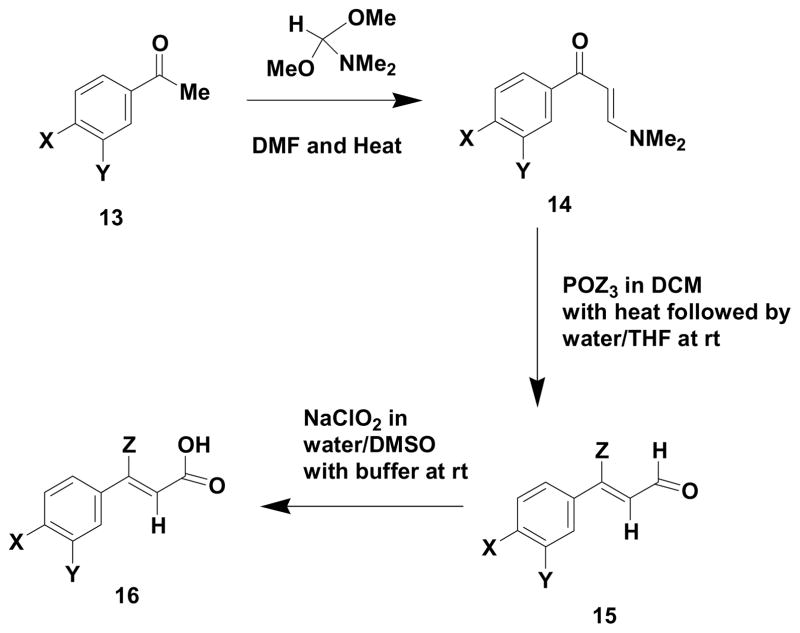
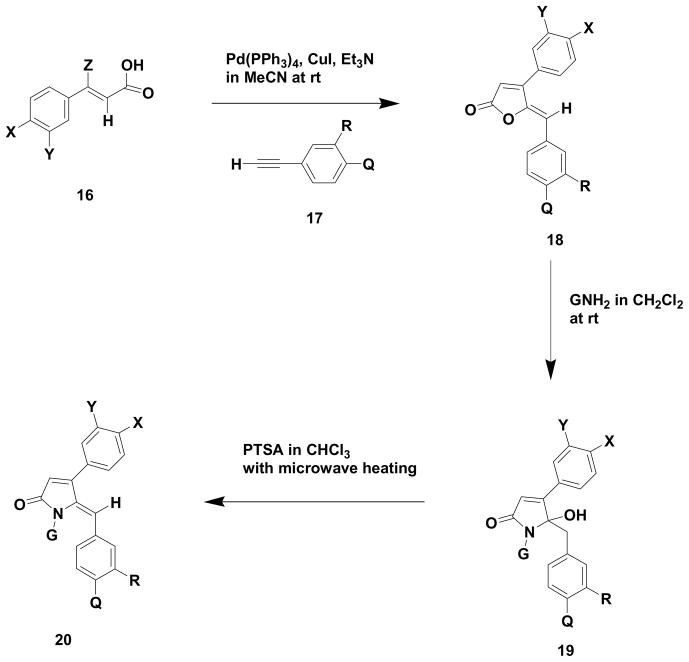
We have previously reported the use of (Z)-haloenals9 as important building blocks for the construction of pyrrole containing marine natural products. We anticipated that such compounds could serve as properly functionalized building blocks for a very general route to (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones via a (Z)-5-benzylidene-4-aryl-5H-furan-2-one based strategy. (Z)-3-aryl-3-haloeneoic acids are known to form (Z)-ylidenefuran-2(5H)-2-ones10 via Sonogashira type reactions with terminal alkynes. Our route to such (Z)-3-aryl-3-haloenoic acids (18) is presented in Scheme 3 and Table 1.
Scheme 3.
Route to (Z)-haloenoic acids
Table 1.
Conversion of Acetophenones to (Z)-Haloenoic Acids
| Entry | X | Y | % Yield (14a–d) | Z | % Yield (15a–h) | % Yield (16a–h) |
|---|---|---|---|---|---|---|
| 1 | Me | H | 94 (14a)11 | Cl | 98 (15a)13 | 90 (16a)13 |
| 2 | Me | H | 94 (14a)11 | Br | 96 (15b)14 | 92 (16b) |
| 3 | OMe | H | 98 (14b)11 | Cl | 98 (15c)10 | 98 (16c)13 |
| 4 | OMe | H | 98 (14b)11 | Br | 94 (15d)10 | 83 (16d)10 |
| 5 | Cl | H | 98 (14c)11 | Cl | 90 (15e)10 | 84 (16e)15 |
| 6 | Cl | H | 98 (14c)11 | Br | 91 (15f)10 | 93 (16f)10 |
| 7 | OMe | OMe | 98 (14d)12 | Cl | 99 (15g)16 | 92 (16g) |
| 8 | OMe | OMe | 98 (14d)12 | Br | 88 (15h)14 | 79 (16h) |
Kirsch and coworkers10 have used a similar scheme to obtain (Z)-3-haloeneoic acids with the exception that Vilsmeier-Haack conditions were employed on ketone or alkyne starting materials. The stepwise preparation (Scheme 3) of the vinylogous amides (14) prior to formation of the (Z)-3-aryl-3-haloenals appears to give much better overall yields and does not seem to be sensitive to various types of substitution on the aromatic ring. It should also be noted that the (Z) stereochemistry of the 3-aryl-3-haloenals (15) is well established10 and can be easily determined by examination of proton NMR chemical shifts. The preparation of bromoenals as well as chloroenals was easily and cleanly accomplished by the indicated methodology.
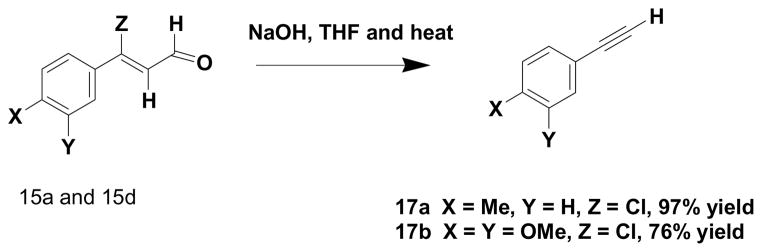
The other component required for the Sonogashira coupling was a terminal alkyne. Although a variety of such alkynes are commercially available, one can also use the (Z)-3-aryl-3-haloenals17 as an efficient source of this reaction component. Refluxing the (Z)-3-aryl-3-chloroenals in THF in the presence of sodium hydroxide produces (Scheme 4) very reasonable yields (76–97%) of the respective terminal alkynes and thereby allows a common intermediate to be used for construction of both components in the Sonogashira reaction.
Scheme 4.
Preparation of terminal alkynes from chloroenals
With the ability to access both (Z)-3-aryl-3-haloenoic acids (16) and terminal alkynes (17) from (Z)-3-aryl-3-haloenals (15), these components were subjected to standard Sonogashira reaction conditions, in which case good yields (50–96%) of a variety of (Z)-5-benzylidene-4-aryl-5H-furan-2-ones (18) were obtained (Scheme 5 and Table 2). Kirsch and coworkers10 have also used this strategy in a very straight forward preparation of the rubrolide skeleton, which represents (Z)-ylidenefuran-2(5H)-one type natural products.
Scheme 5.
Preparation of (Z)-5-benzylidene pyrrol-2(5H)-ones from (Z)-haloenoic acids and terminal alkynes
Table 2.
Preparation of (Z)-5-Benzylidenepyrrol-2(5H)-ones from (Z)-Haloenoic Acids
| Entry | X | Y | Q | R | %Yield (18) | G | %Yield (19) | %Yield (20) |
|---|---|---|---|---|---|---|---|---|
| 1 | Me | H | H | H | 96 (18a) | n-Butyl | 87 (19a) | 96 (20a) |
| 2 | OMe | H | H | H | 67 (18b)10 | n-Butyl | 88 (19b) | 73 (20b) |
| 3 | Cl | H | H | H | 67 (18c)10 | n-Butyl | 62 (19c) | 75 (20c) |
| 4 | OMe | OMe | H | H | 77 (18d) | n-Butyl | 70 (19d) | 46 (20d) |
| 5 | Me | H | H | H | 96 (18a) | n-Hexyl | 96 (19e) | 96 (20e) |
| 6 | Me | H | H | H | 96 (18a) | 2,4-Dimethoxy- benzyl | 95 (19f) | 96 (20f) |
| 7 | Me | H | Me | H | 93 (18e) | n-Butyl | 95 (19g) | 95 (20g) |
| 8 | Me | H | OMe | H | 88 (18f) | n-Butyl | 87 (19h) | 96 (20h) |
| 9 | OMe | OMe | OMe | OMe | 50 (18g)18 | 3,4-Dimethoxy- phenethyl | 57 (19i) | 50 (20i) |
Although it had been previously established that the (Z)-5-benzylidene-4-aryl-5H-furan-2-ones (18) were the thermodynamically preferred stereoisomer10, NOESY, HSQC and HMBC NMR experiments were conducted on one of our typical 5-benzylidene-4-aryl-5H-furan-2-one (18a) to further confirm the stereochemistry of our materials, prior to their conversion to (Z)-5-benzylidenepyrrol-2(5H)-ones (20). It should also be mentioned that the (Z)-5-benzylidene-4-aryl-5H-furan-2-ones (18) in general are not highly stable to chromatographic purification and should be rapidly purified to avoid low yields in spite of the presence of relatively clean crude reaction products. In all of the cases reported (Table 2) in this paper, (Z)-3-aryl-3-bromoenoic acids (16 b,d,f and h) were used in the cross-coupling reaction. However, (Z)-3-aryl-3-chloroenoic acids (eg. 16g) can be used in the Sonogashira reaction but such substrates required microwave heating in DMF at 150 °C for one hour in order to obtain reasonable conversion to the respective 5-benzylidene-4-aryl-5H-furan-2-one (18d).
The (Z)-5-benzylidene-4-aryl-5H-furan-2-ones (18) were then treated with primary amines in methylene chloride at room temperature to afford 5-benzyl-5-hydroxy-4-arylpyrrol-2(5H)-ones in the 46–96% yield range. It was necessary to use an excess of the primary amine in order to drive such reactions to completion. Several different primary amines were evaluated in this transformation and only 3,4-dimethoxyphenethylamine produced a yield below the 60% level. These crude hydroxylactams (19) were relatively pure and were amenable to chromatography and no degradation of these substances was noted during the purification process. Detailed NMR experiments, such as NOESY, HMBC, HSQC, APT and COSY, were subsequently carried out on several hydroxylactams (19a and 19i) to insure proper structural assignments.
The hydroxylactams (19) were then treated with PTSA and subjected to traditional or microwave heating to produce the desired (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones (20) with the microwave heated reaction conditions giving much improved yields ranging from 46–96%. Several (Z)-5-benzylidene-4-arylpyrrol-2(5H)-ones (20a and 20f) were subjected to NOESY, HMBC, HSQC and DQF-COSY NMR experiments to confirm the Z stereochemistry of the exocyclic double bond and to allow for proper structural assignment. It should be noted that examples 20a–c, 20e and 20i exhibited a greater than 9:1 preference for the Z isomer whereas examples 20d, 20f, 20g and 20h produced a 3:2 preference for the Z stereochemistry when the crude products were initially isolated. For the latter examples, when the crude products containing such mixtures of Z and E isomers were allowed to stir in chloroform at room temperature overnight, Z:E ratios in the range of 4:1 to 13:1 in favor of the Z isomer were normally obtained. The variations in such ratios appear to be related to the steric nature of the substituent attached at the nitrogen of the pyrrolones.
3. Conclusions
We believe that the six step sequence of reactions described in this paper offers a very efficient and stereoselective strategy for the preparation of N-substituted 5-benzylidene-4-arylpyrrol-2(5H)-ones (20). If for example one examines the six step transformation of aryl ketone 13a to (Z)-5-benzylidene-4-arylpyrrol-2(5H)-one 20a, a 71% overall yield is realized. The reaction sequence appears to have considerable generality for a variety of acetophenones (13), vinylogous amides (14), (Z)-haloenals (15), (Z)-haloeneoic acids (16), alkynes (17) and amines and could offer important precursors for construction of a number of nitrogen containing marine natural products. The utility of the (Z)-3-aryl-3-haloenals (15) to serve as a source for both the (Z)-3-aryl-3-haloeneoic acids (16) and the alkynes (17) is an additional attractive feature of this strategy.
4. Experimental
4.1 General
All chemicals were used as received from the manufacturer (Aldrich Chemicals). Some of the alkynes used in the Sonogashira reaction were purchased from Aldrich Chemicals. All solvents were dried over 4 angstrom molecular sieves prior to their use. NMR spectra were obtained on either a Bruker 300 MHz spectrometer or a Bruker 500 MHz spectrometer in either CDCl3, d6-DMSO or d6-acetone solutions. IR spectra were recorded on a Nicolet Avatar 320 FT-IR spectrometer with an HATR attachment. High resolution mass spectra were provided on a Biotof Q electrospray mass spectrometer at the University of Richmond. Low resolution GC-MS spectra were obtained on a Shimadzu QP 5050 instrument. Melting points and boiling points are uncorrected. Chromatographic separations were carried out on a Biotage SP-1 instrument (equipped with a silica cartridge) and ethyl acetate/hexane was used as the eluant. The reaction products were eluted within the range of 6–8 column volumes of eluant with a mix of 60–80% ethyl acetate: 20–40% hexane. TLC analyses were conducted on silica plates with hexane/ethyl acetate as the eluant.
All purified reaction products gave TLC results, MS spectra, and 13C NMR spectra consistent with a sample purity of >95%. When the preparation of an analytical sample is reported, the crude reaction product was of sufficient purity to be used in subsequent steps without further purification.
4.1.1 3-(Dimethylamino)-1-(p-tolyl)prop-2-en-1-one (14a)
To a round bottom flask equipped with a magnetic stir bar and reflux condensor was added 4-methylacetophenone (6.00 g, 0.045 mmol), N,N-dimethylformamide dimethyl acetal (21.3 g, 0.179 mmol) in 100 mL of DMF. The reaction mixture was refluxed for 24 hours and solvent was removed in vacuo to give a light brown solid (8.0 g, 94%). The crude product was sufficiently pure for subsequent reactions and exhibited the following physical properties: mp 112–114°C; 1H NMR (CDCl3) δ2.25 (s, 3H), 2.73 (broad s, 6H), 5.68 (d, J = 12.6 Hz, 1H), 7.08 (d, J = 7.8 Hz, 2H), 7.63 (d, J = 12.6 Hz, 1H) and 7.71 (d, J = 7.8 Hz, 2H); 13C NMR (CDCl3) δ 187.9, 153.9, 141.0, 137.8, 128.7, 127.5, 91.8, 45.0, 37.0, 21.3; IR (neat) 1639 cm−1; HRMS (ES) m/z calcd for C12H16NO 190.1226, found 190.1168. This compound had NMR spectral properties which were consistent with those previously reported.11
4.1.2 3-(Dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one (14b)
This compound was prepared by the above procedure with the exception that 4-methoxyacetophenone was used in the reaction in which case a 98 % yield of a solid was obtained. This material exhibited the following physical properties: mp 59–63 °C; 1H NMR (CDCl3) δ 2.99 (broad s, 6H), 3.82 (s, 3H), 5.69 (d, J = 12.6 Hz, 1H), 6.89 (d, J = 7.8 Hz, 2H), 7.76 (d, J = 12.6 Hz, 1H) and 7.89 (d, J = 8.7 Hz, 2H); 13C NMR (CDCl3) δ 187.4, 161.9, 153.8, 133.2, 129.4, 113.3, 91.7, 55.3, 45.0, 37.0; IR (neat) 1664 cm−1; HRMS (ES) m/z calcd for C12H16NO2 206.1176, found 206.1189. This compound had NMR spectral properties which were consistent with those previously reported.11
4.1.3 1-(4-Chlorophenyl)-3-(dimethylamino)prop-2-en-1-one (14c)
This compound was prepared by the above procedure with the exception that 4-chloroacetophenone was used in the reaction in which case a 98 % yield of a solid was obtained. This material exhibited the following physical properties: mp 76–77 °C; 1H NMR (CDCl3) δ 2.57 (broad s, 3H), 2.78 (broad s, 3H), 5.39 (d, J = 12.6 Hz, 1H), 7.09 (d, J = 8.1 Hz, 2H), 7.49 (d, J = 12.6 Hz, 2H) and 7.60 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3) δ 186.2, 154.2, 138.8, 136.4, 128.8, 128.1, 91.3, 44.7, 37.0; IR (neat) 1635 cm−1; HRMS (ES) m/z calcd for C11H13ClNO2 210.0680, found 210.0727. This compound had NMR spectral properties which were consistent with those previously reported.11
4.1.4 1-(3,4-Dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one (14d)
This compound was prepared by the above procedure with the exception that 3,4-dimethoxycetophenone was used in the reaction in which case a 98 % yield of a solid was obtained. This material exhibited the following physical properties: mp 112–114 °C; 1H NMR (CDCl3) δ 2.88 (broad s, 6H), 3.79 (s, 3H), 3.83 (s, 3H), 5.61 (d, J = 12.3 Hz, 1H), 6.75 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 7.66 (d, J = 12.3 Hz, 1H); 13C NMR (CDCl3) δ 187.1, 153.7, 151.5, 148.7, 133.4, 121.0, 110.5, 110.0, 91.5, 55.9, 45.0, 37.0; IR (neat) 1634 cm−1; HRMS (ES) m/z calcd for C13H18NO3 236.1281, found 236.1300. This compound had NMR spectral properties which were consistent with those previously reported.12
4.1.5 (Z)-3-Chloro-3-(p-tolyl)acrylaldehyde (15a)
To a round bottom flask equipped with a magnetic stir bar and reflux condensor was added 3-(dimethylamino)-1-(p-tolyl)prop-2-en-1-one (2.00 g, 0.105 mol), phosphorus oxychloride (2 mL, 0.021 mol) in 25 mL of dichloromethane. The reaction mixture was refluxed for 2 hours and the solvent was removed in vacuo. The residue was dissolved in 50 ml of a 1:1 mixture of water:THF and was allowed to stir at room temperature for 24 hours. The mixture was diluted with water and extracted with ethyl acetate (3 × 30 mL). The organic extract was washed with brine (3 × 15 mL), dried over anhydrous sodium sulfate and concentrated in vacuo to yield a brown solid (1.87 g, 98 % yield). This material was sufficiently pure to be used in subsequent reactions and exhibited the following physical properties: mp 75–77 °C; 1H NMR (CDCl3) δ 2.39 (s, 3H), 6.65 (d, J = 6.5 Hz, 1H), 7.26 (d, J = 8.0 Hz, 2H), 7.65 (d, J = 8.0 Hz, 2H) and 10.21 (d, J = 6.5 Hz, 1H); 13C NMR (CDCl3) δ 191.1, 152.2, 142.6, 132.5, 129.5, 127.1, 123.4, 21.3; IR (neat) 1668 cm−1; HRMS (ES) m/z calcd for C10H10ClO 181.0415, found 181.0420. NMR spectral properties were consistent with those previously reported.13
4.1.6 (Z)-3-Chloro-3-(4-methoxyphenyl)acrylaldehyde (15c)
This compound was prepared by the above procedure with the exception that 3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one was used in the reaction in which case a 98 % yield of a solid was obtained. This material exhibited the following physical properties: mp 35–37 °C; 1H NMR (CDCl3) δ 3.88 (s, 3H), 6.63 (d, J = 7.0 Hz, 1H), 6.98 (d, J = 9.0 Hz, 2H), 7.75 (d, J = 9.0 Hz, 2H) and 10.21 (d, J = 7.0 Hz, 1H); 13C NMR (CDCl3) δ 191.5, 162.8, 152.1, 129.0, 127.7, 122.6, 114.3 and 55.5; IR (neat) 1647 cm−1; HRMS (ES) m/z calcd for C10H10ClO2 197.0364, found 197.0444. NMR spectral properties were consistent with those previously reported.10
4.1.7 (Z)-3-Chloro-3-(4-chlorophenyl)acrylaldehyde (15e)
This compound was prepared by the above procedure with the exception that 1-(4-chlorophenyl)-3-(dimethylamino)prop-2-en-1-one was used in the reaction in which case a 90 % yield of a solid was obtained. This material exhibited the following physical properties: mp 98–100 °C; 1H NMR (CDCl3) δ 6.66 (d, J = 6.9 Hz, 1H), 7.46 (d, J = 9.3 Hz, 2H), 7.71 (d, J = 9.3 Hz, 2H) and 10.23 (d, J = 6.9 Hz, 1H); 13C NMR (CDCl3) δ 191.5, 150.8, 138.2, 134.0, 129.2, 128.4 and 124.6; IR (neat) 1663 cm−1; HRMS (ES) m/z calcd for C9H10Cl2O 200.9869, found 200.9898. NMR spectral properties were consistent with those previously reported.10
4.1.8 (Z)-3-Chloro-3-(3,4-dimethoxyphenyl)acrylaldehyde (15g)
This compound was prepared by the above procedure with the exception that 1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one was used in the reaction in which case a 99 % yield of a solid was obtained. This material exhibited the following physical properties: mp 102–105 °C; 1H NMR (CDCl3) δ 3.96 (s, 3H), 3.97 (s, 3H), 6.64 (d, J = 6.5 Hz, 1H), 6.94 (d, J = 8.5 Hz, 1H), 7.26 (d, J = 2.0 Hz, 1H), 7.45 (dd, J = 2.0 Hz, J = 8.5 Hz, 1H) and 10.21 (d, J = 6.5 Hz, 1H); 13C NMR (CDCl3) δ 191.5, 152.4, 152.1, 149.0, 128.0, 122.8, 121.2, 110.9, 109.8 and 56.1; IR (neat) 1658 cm−1; HRMS (ES) m/z calcd for C11H12ClO3 227.0470, found 227.0473. NMR spectral properties were consistent with those previously reported.16
4.1.9 (Z)-3-Bromo-3-(p-tolyl)acrylaldehyde (15b)
To a round bottom flask equipped with a magnetic stir bar and reflux condensor was added 3-(dimethylamino)-1-(p-tolyl)prop-2-en-1-one (2.00 g, 0.105 mol), phosphorus oxybromide (6.31g, 0.0217 mol) in 25 mL of dichloromethane. The reaction mixture was refluxed for 2 h and the solvent was removed in vacuo. The residue was dissolved in 50 ml of a 1:1 mixture of water:THF and was allowed to stir at room temperature overnight. The mixture was diluted with water and extracted with ethyl acetate (3 × 30 mL). The organic extract was washed with brine (3 × 15 mL), dried over anhydrous sodium sulfate and concentrated in vacuo to yield a solid (2.29g, 96 %). This material exhibited the following physical properties: mp 155–158 °C; 1H NMR (CDCl3) δ 2.40 (s, 3H), 6.76 (d, J = 6.6 Hz, 1H), 7.21 (d, J = 8.1 Hz, 2H), 7.59 (d, J = 8.1 Hz, 2H) and 10.05 (d, J = 6.6 Hz, 1H); 13C NMR (CDCl3) δ 193.5, 145.1, 142.6, 134.5, 129.5, 128.0, 126.6, and 21.4; IR (neat) 1659 cm−1; HRMS (ES) m/z calcd for C10H10BrO 224.9910, found 224.9970. NMR spectral properties were consistent with those previously reported.14
4.1.10 (Z)-3-Bromo-3-(4-methoxyphenyl)acrylaldehyde (15d)
This compound was prepared by the above procedure with the exception that 3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one was used in the reaction in which case a 94 % yield of a solid was obtained. This material exhibited the following physical properties: mp 43–45 °C; 1H NMR (CDCl3) δ 3.89 (s, 3H), 6.76 (d, J = 6.5 Hz, 1H), 6.96 (d, J = 9.0 Hz, 2H), 7.71 (d, J = 9.0 Hz, 2H), and 10.06 (d, J = 6.5 Hz, 1H); 13C NMR (CDCl3) δ 193.8, 162.6, 145.0, 129.9, 129.5, 125.6, 114.2, and 55.6; IR (neat) 1639 cm−1; HRMS (ES) m/z calcd for C10H10BrO2 240.9859, found 240.9868. NMR spectral properties were consistent with those previously reported.10
4.1.11 (Z)-3-Bromo-3-(4-chlorophenyl)acrylaldehyde(15f)
This compound was prepared by the above procedure with the exception that 1-(4-chlorophenyl)-3-(dimethylamino)prop-2-en-1-one was used in the reaction which case a 91 % yield of a solid was obtained. This material exhibited the following physical properties: mp 97–99 °C; 1H NMR (CDCl3) δ 6.78 (d, J = 6.3 Hz, 1H), 7.43 (d, J = 8.7 Hz, 2H), 7.67 (d, J = 8.7 Hz, 2H) and 10.07 (d, J = 6.3 Hz, 1H); 13C NMR (CDCl3) δ 193.3, 143.3, 138.2, 135.9, 129.3, 129.1, 127.7; IR (neat) 1664 cm−1; HRMS (ES) m/z calcd for C9H7BrClO 244.9363, found 244.9429. NMR spectral properties were consistent with those previously reported.10
4.1.12 (Z)-3-Bromo-3-(3,4-dimethoxyphenyl)acrylaldehyde (15h)
This compound was prepared by the above procedure with the exception that 1-(3,4-dimethoxyphenyl)-3-(dimethylamino)prop-2-en-1-one was used in the reaction in which case an 88 % yield of a solid was obtained. This material exhibited the following physical properties: mp 93–95 °C; 1H NMR (CDCl3) δ 3.96 (s, 3H), 3.97 (s, 3H), 6.78 (d, J = 6.5 Hz, 1H), 6.93 (d, J = 8.5 Hz, 1H), 7.24 (s, 1H), 7.40 (d, J = 8.5 Hz, 1H) and 10.07 (d, J = 6.5 Hz, 1H); 13C NMR (CDCl3) δ 193.7, 152.3, 148.9, 144.9, 129.8, 125.8, 122.1, 110.8 and 56.1; IR (neat) 1658 cm−1; HRMS (ES) m/z calcd for C11H12BrO3 270.9964, found 270.9949. NMR spectral properties were consistent with those previously reported.14
4.1.13 4-Ethynltoluene (17a)
Into a round bottom flask was equipped with a magnetic stir bar and reflux condensor was added (Z)-3-chloro-3-(p-tolyl)acrylaldehyde (1.00 g, 5.54 mmol), sodium hydroxide (0.900 g, 22.15 mmol) and THF (30 mL). The resulting mixture was refluxed overnight, cooled to rt, diluted with water (40 mL) and extracted with ethyl acetate (3 × 20 mL). The combined organic phases were washed with brine (2 × 15 mL), dried over anhydrous sodium sulfate and concentrated in vacuo to give a yellow liquid (0.625 g, 97 % yield), which exhibited the following properties: 25–26 °C @ 1.30 Torr; 1H NMR (CDCl3) δ 2.44 (s, 3H), 3.13 (s, 1H), 7.20 (d, J = 8.1 Hz, 2H) and 7.49 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3) δ 139.0, 132.1, 129.2, 119.3, 84.0, 76.6 and 21.5; IR (neat) 3294 and 2107 cm−1; HRMS (ES) m/z calcd for C9H8Na 139.0518, found 139.1117. NMR spectral properties were consistent with an authentic Aldrich Chemicals sample.
4.1.14 4-Ethynyl-1,2-dimethoxybenzene (17b)
This compound was prepared by the above procedure with the exception that (Z)-3-chloro-3-(3,4-dimethoxyphenyl)acrylaldehyde was used in the reaction in which case a yellow solid (76 % yield) was obtained, which exhibited the following properties: 48–51 °C; 1H NMR (CDCl3) δ 3.02 (s, 1H), 3.90 (s, 3H), 3.91 (s, 3H), 6.82 (d, J = 8.5 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H) and 7.13 (dd, J = 2.0 Hz, J = 8.5 Hz, 1H); 13C NMR (CDCl3) δ 149.9, 148.7, 125.5, 114.8, 114.3, 111.0, 83.8, 75.6 and 55.9; IR (neat) 3242 cm−1; HRMS (ES) m/z calcd for C10H9NaO2 185.0529, found 185.1220. NMR spectral properties were consistent with those previously reported.17
4.1.15 (Z)-3-Chloro-3-(p-tolyl)acrylic acid (16a)
To a round bottom flask equipped with a magnetic stir bar, was added (Z)-3-chloro-3-(p-tolyl)acrylaldehyde (1.00 g, 5.50 mmol) which was previously dissolved in 35 mL of DMSO. Sodium hydrogen phosphate monohydrate (0.759g, 5.50 mmol), dissolved in 10 mL of water was added and the reaction mixture was cooled in ice bath at 0° C. Sodium chlorite (1.502 g, 16.6 mmol) was dissolved in 10 mL of water and added dropwise to the reaction mixture. After the addition was complete, the ice bath was removed and the reaction mixture was stirred for 24 h at room temperature. The reaction mixture was cooled in an ice bath and acidified to pH of 2, diluted with 20 mL of brine and extracted with ethyl acetate (3 × 30 mL). The organic phase was dried over anhydrous sodium sulfate and concentrated in vacuo to give a yellow solid (0.970 g, 90%). This material exhibited the following physical properties: mp 139–141 °C; 1H NMR (CDCl3) δ 2.43 (s, 3H), 6.60 (s, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.64 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3) δ 169.1, 141.7, 134.2, 129.4, 128.7, 127.3, 114.5, 21.3; IR (neat) 1687, 2918 cm−1; HRMS (ES) m/z calcd for C10H10ClO2 197.0364, found 197.0409. NMR spectral properties were consistent with those previously reported.13
4.1.16 (Z)-3-Chloro-3-(4-methoxyphenyl)acrylic acid (16c)
This compound was prepared by the above procedure with the exception that (Z)-3-chloro-3-(4-methoxyphenyl)acrylaldehyde was used in the reaction in which case a 98 % yield of a solid was obtained. This material exhibited the following physical properties: mp 144–146 °C; 1H NMR (CDCl3) δ 3.88 (s, 3H), 6.55 (s, 1H), 6.95 (d, J = 8.5 Hz, 2H), 7.71 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3) δ 167.6, 162.1, 148.7, 129.3, 129.1, 114.1, 55.5; IR (neat) 1670, 2909 cm−1; MS (DI) m/z 214 (M+). NMR spectral properties were consistent with those previously reported.13
4.1.17 (Z)-3-Chloro-3-(4-chlorophenyl)acrylic acid (16e)
This compound was prepared by the above procedure with the exception that (Z)-3-chloro-3-(4-chlorophenyl)acrylaldehyde was used in the reaction in which case an 84 % yield of a solid was obtained. This material exhibited the following physical properties: mp 149–151 °C; 1H NMR (CDCl3) δ 6.60 (s, 1H), 7.44 (d, J = 7.0 Hz, 2H), 7.68 (d, J = 7.0 Hz, 2H); 13C NMR (CDCl3) δ 167.9, 147.7, 137.4, 129.0, 128.7, 115.6; IR (neat) 1676, 2897 cm−1; MS (DI) m/z 216 (M+). NMR spectral properties were consistent with those previously reported.15
4.1.18 (Z)-3-Chloro-3-(3,4-dimethoxyphenyl)acrylic acid (16g)
This compound was prepared by the above procedure with the exception that (Z)-3-chloro-3-(3,4-dimethoxyphenyl)acrylaldehyde was used in the reaction in which case a 92 % yield of a solid was obtained. This material exhibited the following physical properties: mp 148–152 °C; 1H NMR (CDCl3) δ 3.96 (s, 3H), 3.97 (s, 3H), 6.56 (s, 1H), 6.92 (d, J = 8.0 Hz, 1H), 7.24 (d, J = 2.5 Hz, 1H), 7.38 (dd, J = 2.5 Hz, J = 8.0 Hz, 1H); 13C NMR (CDCl3) δ 168.8, 151.5, 148.8, 140.5, 131.7, 121.7, 117.5, 111.3, 110.7, 56.1; IR (neat) 1690, 3014 cm−1; MS (DI) m/z 242 (M+).
4.1.19 (Z)-3-Bromo-3-(p-tolyl)acrylic acid (16b)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(p-tolyl)acrylaldehyde was used in the reaction in which case a 92 % yield of a yellow solid was obtained. This material exhibited the following physical properties: mp 143–145 °C; 1H NMR (CDCl3) δ 2.42 (s, 3H), 6.79 (s, 1H), 7.24 (d, J = 8.3 Hz, 2H), 7.59 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3) δ 169.7, 141.4, 140.8, 136.4, 129.3, 128.2, 118.6, 21.3; IR (neat) 1683, 2913 cm−1; MS (DI) m/z 240 (M+).
4.1.20 (Z)-3-Bromo-3-(4-methoxyphenyl)acrylic acid (16d)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(4-methoxyphenyl)acrylaldehyde was used in the reaction in which case an 87% yield of a solid was obtained. This material exhibited the following physical properties: mp 114–115 °C; 1H NMR (CDCl3) δ 3.88 (s, 3H), 6.74 (s, 1H), 6.94 (d, J = 8.5 Hz, 2H), 7.66 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3) δ 168.1, 161.9, 140.5, 131.4, 129.9, 117.0, 113.9, 55.9; IR (neat) 1682, 2897 cm−1; MS (DI) m/z 258 (M+). NMR spectral properties were consistent with those previously reported.10
4.1.21 (Z)-3-Bromo-3-(4-chlorophenyl)acrylic acid (16f)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(4-chlorophenyl)acrylaldehyde was used in the reaction in which case a 93 % yield of a solid was obtained. This material exhibited the following physical properties: mp 136–139 °C; 1H NMR (CDCl3) δ 6.78 (s, 1H), 7.40 (d, J = 8.4 Hz, 2H), 7.62 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3) δ 169.1, 139.1, 137.6, 129.5, 128.9, 119.8; IR (neat) 1681, 2806 cm−1; MS (DI) m/z 260 (M+). NMR spectral properties were consistent with those previously reported.10
4.1.22 (Z)-3-Bromo-3-(3,4-dimethoxyphenyl)acrylic acid (16h)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(3,4-dimethoxyphenyl)acrylaldehyde was used in the reaction in which case a 79 % yield of a solid was obtained. This material exhibited the following physical properties: mp 105–107 °C; 1H NMR (CDCl3) δ 3.95 (s, 3H), 3.96 (s, 3H), 6.75 (s, 1H), 6.90 (d, J = 8.0 Hz, 1H), 7.21 (d, J = 2.5 Hz, 1H), 7.32 (dd, J = 2.5 Hz, J = 8.0 Hz, 1H); 13C NMR (CDCl3) δ 169.3, 151.5, 148.8, 140.5, 131.7, 121.8, 117.6, 111.4, 110.7, 56.1; IR (neat) 1689, 3005 cm−1; MS (DI) m/z 288 (M+).
4.1.23 (Z)-5-Benzylidene-4-p-tolyl-5H-furan-2-one (18a)
A round bottom flask was equipped with a magnetic stir bar and (Z)-3-bromo-3-p-tolylacrylic acid (4.00g, 0.0167 mol) and phenyl acetylene (2,42 g, 0.0183 mol) were placed in the flask along with 100 mL of acetonitrile. Triethylamine (6.72 g, 0.066 mol), copper (I) iodide (0.158 g, 0.83 mmol) and tetrakis-(triphenylphosphine)palladium (0) (0.960 g, 0.83 mmol) were added to the flask and the resulting reaction mixture was capped and stirred at rt for 24 h. The reaction mixture was diluted with 50 mL of ethyl acetate, passed through a short plug of silica gel and concentrated in vacuo to yield a brown solid (4.18 g, 96% yield). The resulting product was sufficiently pure for further transformations and was somewhat unstable to chromatography. However, an analytical sample was prepared by flash chromatography on a Biotage SP-1 instrument (equipped with a silica cartridge) and utilizing an ethyl acetate/hexane gradient. The product was eluted with 5 column volumes of eluant and the resulting solid exhibited the following physical properties: mp 159–161° C; 1H NMR (CDCl3) δ 2.48 (s, 3H), 6.21 (s, 1H), 6.22 (s, 1H), 7.36 (m, 3H), 7.43 (m, 4H) and 7.38 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3) δ 168.9, 158.9, 148.1, 140.9, 133.1, 130.8, 129.8, 129.2, 128.8, 128.5, 127.6, 114.0, 113.7 and 21.4; IR (neat) 1752 cm−1; HRMS (ES) m/z calcd for C18H15O2 263.1067, found 263.1075.
4.1.24 (Z)-5-Benzylidene-4-(4-methoxyphenyl)-5H-furan-2-one (18b)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(4-methoxyphenyl)acrylic acid was used for the cross-coupling reaction in which case a solid (67% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 92–95 ° C; 1H NMR (CDCl3) δ 3.92 (s, 3H), 6.17 (s, 1H), 6.22 (s, 1H), 7.06 (d, J = 7.0 Hz, 2H), 7.36 ( t, J = 7.0 Hz, 1H), 7.42 (t, J = 7.0 Hz, 2H), 7.49 (d, J = 7.5 Hz, 2H) and 7.83 (d, J = 7.5 Hz, 2H); 13C NMR (CDCl3) δ 169.0, 161.5, 158.5, 148.2, 133.1, 130.8, 130.0, 129.2, 128.8, 122.8, 114.6, 113.7, 113.3 and 55.5; IR (neat) 1755 cm−1; HRMS (ES) m/z calcd for C18H15O3 279.1016, found 279. 1088. NMR spectral properties were consistent with those previously reported.10
4.1.25 (Z)-5-Benzylidene-4-(4-chlorophenyl)-5H-furan-2-one (18c)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(4-chlorophenyl)acrylic acid was used for the cross-coupling reaction in which case a solid (67% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 147–148 ° C; 1H NMR (CDCl3) δ 6.15 (s, 1H), 6.24 (s, 1H), 7.37 (t, J = 7.0 Hz, 1H), 7.43 (t, J = 7.0 Hz, 2H), 7.47 (d, J = 8.5 Hz, 2H), 7.54 (d, J = 8.5 Hz, 2H) and 7.83 (d, J = 7.0 Hz, 2H); 13C NMR (CDCl3) δ 168.5, 157.5, 147.8, 136.8, 132.8, 130.8, 129.8, 129.5, 129.4, 128.9, 128.8, 114.9 and 113.9; IR (neat) 1755 cm−1; HRMS (ES) m/z calcd for C17H12ClO2 283.0520, found 283.0515. NMR spectral properties were consistent with those previously reported.10
4.1.26 (Z)-5-Benzylidene-4-(3,4-dimethoxyphenyl)-5H-furan-2-one (18d)
This compound was prepared by the above procedure with the exception that Z-3-bromo-3-(3,4-dimethoxyphenyl)-acrylic acid was used for the cross-coupling reaction in which case a solid (77% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 132–134 ° C; 1H NMR (CDCl3) δ 3.97 (s, 3H), 3.99 (s, 3H), 6.19 (s, 1H), 6.26 (s, 1H), 7.02 (m, 2H), 7.14 (dd, J = 2.0 Hz, J = 8.5 Hz, 1H), 7.36 (t, J = 7.0 Hz, 1H), 7.43 (t, J = 7.0 Hz, 2H) and 7.84 (d, J = 7.0 Hz, 2H); 13C NMR (CDCl3) δ 168.9, 158.6, 151.1, 149.5, 148.2, 133.1, 130.8, 129.3, 128.8, 123.0, 121.6, 113.7, 113.5, 111.6, 111.5, 56.2 and 56.1; IR (neat) 1754 cm−1; HRMS (ES) m/z calcd for C19H17O4 309.1121, found 309.1118. In addition to the routine 1H and 13C NMR spectra obtained for this solid, NOESY, HSQC and HMBC experiments were performed, all H and C assignments were made and the Z-stereochemistry of the exocyclic double bond of the furanone was established.
4.1.27 (Z)-5-(4-Methylbenzylidene)-4-p-tolyl-5H-furan-2-one (18e)
This compound was prepared by the above procedure with the exception that 4-ethynyltoluene was used for the cross-coupling reaction in which case a solid (93% yield) was obtained. An analytical sample was obtained after flash chromatography and this material exhibited the following physical properties: mp 74–76 ° C; 1H NMR (CDCl3) δ 2.40 (s, 3H), 2,47 (s, 3H), 6.17 (s, 1H), 6.19 (s, 1H), 7.22 (d, J = 8.1 Hz, 2H), 7.34 (d, J = 8.1 Hz, 2H), 7.42 (d, J = 8.1 Hz, 2H) and 7.72 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3) δ 169.1, 158.8, 147.6, 140.8, 139.7, 130.8, 130.3, 129.8, 129.6, 128.5, 127.7, 113.9, 113.6, and 21.5; IR (neat) 1754 cm−1; HRMS (ES) m/z calcd for C19H17O2 277.1223, found 277.1212.
4.1.28 5-(Z)-(4-Methoxybenzylidene)-4-p-tolyl-5H-furan-2-one (18f)
This compound was prepared by the above procedure with the exception that 4-ethynylanisole was used for the cross-coupling reaction in which case a solid (88% yield) was obtained. An analytical sample was obtained after flash chromatography and this material exhibited the following physical properties: mp 115–118 ° C; 1H NMR (CDCl3) δ 2.47 (s, 3H), 3.87 (s, 3H), 6.14 (s, 1H), 6.17 (s, 1H), 6.94 (d, J = 9.0 Hz, 2H), 7.34 (d, J = 8.5 Hz, 2H), 7.42 (d, J = 8.5 Hz, 2H) and 7.79 (d, J = 9.0 Hz, 2H); 13C NMR (CDCl3) δ 169.3, 160.5, 158.8, 146.6, 140.7, 132.6, 129.8, 128.5, 127.8, 126.0, 114.4, 113.8, 112.9, 55.3 and 21.4; IR (neat) 1750 cm−1; HRMS (ES) m/z calcd for C19H17O3 293.1172, found 293.1117.
4.1.29 (Z)-5-(3,4-Dimethoxybenzylidene)-4-(3,4-dimethoxyphenyl)-5H-furan-2-one (18g)
This compound was prepared by the above procedure with the exception that (Z)-3-bromo-3-(3,4-dimethoxyphenyl)acrylic acid and 4-ethynyl-1,2-dimethoxybenzene were used for the cross-coupling reaction in which case a solid (50% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 154–156 ° C; 1H NMR (CDCl3) δ 3.94 (s, 3H), 3.96 (s, 3H), 3.97 (s, 3H), 3.98 (s, 3H), 6.13 (s, 1H), 6.19 (s, 1H), 6.90 (d, J = 8.5 Hz, 1H), 7.01 (m, 2H), 7.13 (dd, J = 1.5 Hz, J = 8.0 Hz, 1H), 7.33 (dd, J = 1.5 Hz, J = 8.0 Hz, 1H) and 7.49 (d, J = 1.5 Hz, 1H); 13C NMR (CDCl3) δ 169.1, 158.5, 151.0, 150.3, 149.4, 149.2, 146.8, 126.2, 124.9, 123.2, 121.6, 113.9, 113.0, 112.6, 111.6, 111.4, 111.1, 56.2, 56.1, 56.0 and 55.9; IR (neat) 1744 cm−1; HRMS (ES) m/z calcd for C21H20O6 369.1332, found 369.1285.
4.1.30 5-Benzyl-1-butyl-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (19a)
Into a round bottom flask equipped with a magnetic stir bar was placed methylene chloride (210 mL) and 5-benzylidene-4-p-tolyl-5H-furan-2-one (3.50 g, 0.0133 mol). The mixture was cooled in an ice water bath and n-butylamine (4.88 g, 0.067 mol) was added to the reaction mixture in a dropwise fashion and the resulting solution was stirred for 3 h in the cold. The ice water bath was removed and the reaction mixture was stirred overnight at rt and the solvent was removed in vacuo to give a solid (4.10 g, 87% yield). Although the crude material was sufficiently pure for subsequent transformations, an analytical sample was prepared by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product was eluted with 5 column volumes of eluant. The resulting dark solid exhibited the following physical properties: mp 165–167 ° C; 1H NMR (CDCl3) δ 0.95 (t, J = 7.2 Hz, 3H), 1.36 (sextet, J = 7.2 Hz, 2H), 1.71 (m, 2H), 2.43 (s, 3H), 3.27 (m, 3H), 3.56 (m, 1H), 4.37 (s, 1H), 5.78 (s, 1H), 6.68 (d, J = 7.8 Hz, 2H), 7.06 (m, 3H), 7.24 (d, J = 8.4 Hz, 2H) and 7.55 (d, J = 8.4 Hz, 2H); 1H NMR (CDCl3) δ 169.1, 157.3, 140.1, 134.2, 129.5, 129.4, 128.9, 127.9, 127.6, 126.9, 120.6, 94.1, 41.4, 39.7, 31.4, 21.4, 20.7 and 13.8; IR (neat) 3400 and 1652 cm−1; HRMS (ES) m/z calcd for C22H26NO2 336.1958, found 336.1953. Additional NMR experiments including NOESY, HMBC, HSQC and DQF-COSY were carried out in order to allow for complete assignment of all proton and carbon absorptions.
4.1.31 5-Benzyl-1-butyl-5-hydroxy-4-methoxyphenylpyrrol-2(5H)-one (19b)
This compound was prepared by the above procedure with the exception that 5-benzylidene-4-(4-methoxyphenyl)-5H-furan-2-one was used in the reaction in which case a solid (88% yield) was obtained. An analytical sample was produced after flash chromatography and this material exhibited the following physical properties: mp 99–102 ° C; 1H NMR (CDCl3) δ 1.00 (t, J = 7.5 Hz, 3H), 1.43 (sextet, J = 7.5 Hz, 2H), 1.72 (m, 1H), 1.82 (m, 1H), 3.29 (d, J = 14.0 Hz, 1H), 3.34 (m, 1H), 3.38 (d, J = 14.0 Hz, 1H), 3.70 (m, 1H), 3.90 (s, 3H), 5.97 (s, 1H), 6.71 (d, J = 7.0 Hz, 2H), 6.98 (d, J = 7.5 Hz, 2H), 7.10 (m, 3H) and 7.73 (d, J = 7.5 Hz, 2H); 13C NMR (CDCl3) δ 169.1, 161.0, 156.7, 134.0, 129.4, 129.2, 128.0, 127.0, 124.1, 119.8, 114.3, 94.2, 55.4, 41.1, 39.6, 31.8, 20.7 and 13.8; IR (neat) 3080 and 1658 cm−1; HRMS (ES) m/z calcd for C22H26NO3 352.1907, found 352.1919.
4.1.32 5-Benzyl-1-butyl-5-hydroxy-4-chlorophenylpyrrol-2(5H)-one (19c)
This compound was prepared by the above procedure with the exception that 5-benzylidene-4-(4-chlorophenyl)-5H-furan-2-one was used in the reaction in which case a solid (62% yield) was obtained after flash chromatographic purification as previously described. This material exhibited the following physical properties: mp 112–115 ° C; 1H NMR (CDCl3) δ 0.98 (t, J = 7.5 Hz, 3H), 1.45 (sextet, J = 7.5 Hz, 2H), 1.74 (m, 3H), 3.33 (m, 3H), 3.69 (m, 1H), 5.98 (s, 1H), 6.69 (d, J = 7.5 Hz, 2H), 7.10 (m, 3H), 7.40 (d, J = 8.4 Hz, 2H), and 7.66 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3) δ 168.6, 156.2, 136.0, 133.8, 130.1, 129.4, 129.1, 128.8, 128.0, 127.1, 122.0, 94.1, 41.1, 39.7, 31.5, 20.7 and 13.8; IR (neat) 3200 and 1667 cm−1; HRMS (ES) m/z calcd for C21H23ClNO2 356.1412, found 356.1406.
4.1.33 5-Benzyl-1-butyl-5-hydroxy-3,4-dimethoxyphenylpyrrol-2(5H)-one (19d)
This compound was prepared by the above procedure with the exception that 5-benzylidene-4-(3,4-dimethoxyphenyl)-5H-furan-2-one was used in the reaction in which case a solid (70% yield) was obtained after flash chromatographic purification as previously described. This material exhibited the following physical properties: mp 124–127 ° C; 1H NMR (CDCl3) δ 1.00 (t, J = 7.5 Hz, 3H), 1.44 (sextet, J = 7.5 Hz, 2H), 1.74 (m, 1H), 1.84 (m, 1H), 3.37 (m, 3H), 3.74 (m, 1H), 3.91 (s, 3H), 3.98 (s, 3H), 5.99 (s, 1H), 6.72 (d, J = 7.0 Hz, 2H), 6.95 (d, J = 8.0 Hz, 1H), 7.11 (m, 3H), 7.24 (d, J = 2.0 Hz, 1H) and 7.45 (dd, J = 2.0 Hz, J = 8.0 Hz 1H); 13C NMR (CDCl3) δ 169.0, 157.0, 150.7, 149.1, 134.0, 129.5, 127.9, 127.0, 124.5, 121.1, 120.1, 111.1, 110.5, 94.2, 56.0, 55.9, 41.3, 39.6, 31.7, 20.7 and 13.8; IR (neat) 3400 and 1662 cm−1; HRMS (ES) m/z calcd for C23H28NO4, 382.2013, found 382.2022.
4.1.34 5-Benzyl-1-hexyl-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (19e)
This compound was prepared by the above procedure with the exception that n-hexylamine was used in the reaction in which case a solid (96% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 107–110 ° C; 1H NMR (CDCl3) δ 0.92 (t, J = 6.6 Hz, 3H),1.35 (broad s, 6H), 1.72 (m, 2H), 2.52 (s, 3H), 3.31 (m, 3H), 3.65 (m, 1H), 5.95 (s, 1H), 6.70 (d, J = 7.2 Hz, 2H), 7.10 (m, 3H), 7.25 (d, J = 8.1 Hz, 2H) and 7.63 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3) δ 169.2, 157.4, 140.0, 134.2, 129.5, 129.0, 128.5, 127.9, 127.6, 126.9, 120.6, 94.1, 41.5, 39.9, 31.6, 29.3, 27.3, 22.7, 21.4 and 14.1; IR (neat) 3300 and 1662 cm−1; HRMS (ES) m/z calcd for C24H30NO2 364.2271, found 364.2282.
4.1.35 5-Benzyl-1-(2,4-dimethoxybenzyl)-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (19f)
This compound was prepared by the above procedure with the exception that 2,4-dimethoxybenzylamine was used in the reaction in which case a solid (95% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 136–138 ° C; 1H NMR (CDCl3) δ 2.43 (s, 3H), 3.32 (d, J = 14.5 Hz, 1H), 3.48 (d, J = 14.5 Hz, 1H), 3.81 (s, 3H), 3.94 (s, 3H), 4.60 (d, J = 15.0 Hz, 1H), 4.82 (d, J = 15.0 Hz, 1H), 6.04 (s, 1H), 6.50 (m, 2H), 6.72 (d, J = 7.0 Hz, 2H), 7.10 (m, 3H), 7.24 (d, J = 8.0 Hz, 2H),7.47 (d, J = 9.0 Hz, 1H) and 7.69 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3) δ 169.5, 160.4, 157.7, 157.4, 140.1, 134.3, 132.0, 129.6, 129.5, 129.0, 127.9, 127.8, 126.9, 120.6, 118.8, 105.0, 98.8, 94.0, 55.7, 55.4, 42.4, 36.7 and 21.4; IR (neat) 1669 cm−1; HRMS (ES) m/z calcd for C27H28NO4 430.2013, found 430.1997.
4.1.36 5-(4-Methylbenzyl)-1-butyl-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (19g)
This compound was prepared by the above procedure with the exception that 5-(4-methylbenzylidene)-4-p-tolyl-5H-furan-2-one was used in the reaction in which case a solid (95% yield) was obtained after flash chromatography and this material exhibited the following physical properties: mp 144–146 ° C; 1H NMR (CDCl3) δ 0.98 (t, J = 7.5 Hz, 3H), 1.41 (sextet, J = 7.5 Hz, 2H), 1.71 (m, 1H), 1.80 (m, 1H), 2.23 (s, 3H), 2.44 (s, 3H), 2.96 (s, 1H), 3.28 (m, 3H), 3.68 (m, 1H), 6.00 (s, 1H), 6.58 (d, J = 8.0 Hz, 2H), 6.88 (d, J = 8.0 Hz, 2H), 7.25 (d, J = 8.0 Hz, 2H), and 7.66 (d, J = 8.0 Hz, 2H); 13C NMR (CDCl3) δ 168.9, 157.0, 140.2, 136.5, 130.9, 129.6, 129.3, 128.7, 127.6, 120.9, 94.3, 40.7, 39.6, 31.7, 21.5, 21.0, 20.7 and 13.8; IR (neat) 3027 and 1651 cm−1; HRMS (ES) m/z calcd for C23H28NO2 350.2115, found 350.2129.
4.1.37 5-(4-Methoxybenzyl)-1-butyl-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (19h)
This compound was prepared by the above procedure with the exception that 5-(4-methylbenzylidene)-4-p-tolyl-5H-furan-2-one was used in the reaction in which case a solid (87% yield) was obtained after flash chromatography and this material exhibited the following physical properties: bp 79–80 ° C @ 0.41 Torr; 1H NMR (CDCl3) δ 0.97 (t, J = 7.2 Hz, 3H), 1.39 (sextet, J = 7.2 Hz, 2H), 1.75 (m, 2H), 2.42 (s, 3H), 3.19 (d, J = 14.1 Hz, 1H), 3.29 (d, J = 14.1 Hz, 1H), 3.32 (m, 1H), 3.64 (m, 1H), 3.71 (s, 3H), 5.92 (s. 1H), 6.60 (broad s, 4H), 7.22 (d, J = 8.1 Hz, 2H) and 7.62 (d, J = 8.1 Hz, 2H); 13C NMR (CDCl3) δ 169.1, 158.4, 157.3, 140.1, 130.5, 129.5, 128.9, 127.6, 126.2, 120.7, 113.3, 94.2, 55.1, 40.5, 39.6, 31.5, 21.4, 20.7 and 13.8; IR (neat) 3500 and 1671 cm−1; HRMS (ES) m/z calcd for C23H28NO3 366.2064, found 366.2138.
4.1.38 5-(3,4-Dimethoxybenzyl)-1-(3,4-dimethoxyphenethyl)-5-hydroxy-4-(3,4-dimethoxyphen-yl)-pyrrol-2(5H)-one (19i)
This compound was prepared by the above procedure with the exception that 5-(3,4-dimethoxybenzylidene)-4-(3,4-dimethoxyphenyl)-5H-furan-2-one and 3,4-dimethoxyphenethylamine were used in the reaction in which case a solid (57 % yield) was obtained after flash chromatographic purification as previously described. This material exhibited the following physical properties: mp 170–172 ° C; 1H NMR (CDCl3) δ 2.97 (m, 1H), 3.12 (d, J = 13.8 Hz, 1H), 3.19 (d, J = 13.8 Hz, 1H), 3.22 (m, 1H), 3.47 (m, 1H), 3.56 (s, 3H), 3.78 (s, 3H), 3.85 (s, 3H), 3.86 (s, 3H), 3.87 (s, 3H), 3.94 (s, 3H), 3.98 (m, 1H), 5.93 (s, 1H), 6.08 (d, J = 2.1 Hz, 1H), 6.28 (dd, J = 2.1 Hz, J = 8.4 Hz, 1H), 6.58 (d, J = 8.4 Hz, 1H), 6.80 (m, 3H), 6.90 (d, J = 8.7 Hz, 1H), 7.18 (d, J = 2.1 Hz, 1H) and 7.37 (dd, J = 2.1 Hz, J = 8.4 Hz 1H); 13C NMR (CDCl3) δ 169.1, 157.3, 150.7, 149.1, 149.0, 148.1, 148.0, 147.7, 132.1, 126.3, 124.3, 121.6, 121.2, 120.9, 119.5, 112.7, 112.3, 111.4, 111.0, 110.6, 110.3, 93.7, 56.0, 55.9, 55.8, 55.7, 55.5, 41.9, 41.0 and 34.0; IR (neat) 3200 and 1658 cm−1; HRMS (ES) m/z calcd for C31H36NO8 550.2441, found 550.2452. Additional NMR experiments including NOESY, HMBC, HSQC, APT and COSY were carried out in order to allow for complete assignment of all proton and carbon absorptions.
4.1.39 (Z)-5-Benzylidene-1-butyl-4-p-tolylpyrrol-2(5H)-one (20a). Method A
Into a round bottom flask containing a magnetic stir bar was placed 15 mL of chloroform, 5-benzyl-1-butyl-5-hydroxy-4-p-tolylpyrrol-2(5H)-one (0.200g, 0.600 mmol), and p-toluene sulfonic acid (0.017 g, 0.09 mmol) and the reaction mixture was stirred at rt for 3 h. The reaction mixture was diluted with additional chloroform (20 mL) and the resulting chloroform phase was extracted with saturated aqueous bicarbonate solution (3 × 20 mL) and brine (3 × 10 mL) and dried over anhydrous magnesium sulfate. After removing the drying agent by filtration, the filtrate was concentrated in vacuo to give a solid, which was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (0.181 g, 96% yield) was eluted with 6 column volumes of eluant. The resulting solid exhibited the following physical properties: mp 132–135 ° C; 1H NMR (CDCl3) δ 0.64 (t, J = 7.4 Hz, 3H), 0.88 (sextet, J = 7.4 Hz, 2H), 1.19 (pentet, J = 7.7 Hz, 2H), 2.43 (s, 3H), 3.56 (t, J = 7.5 Hz, 2H), 6.19 (s, 1H), 6.40 (s, 1H), 7.28 (d, J = 6.0 Hz, 2H), 7.32 (m, 3H) and 7.38 (m, 4H); 13C NMR (CDCl3) δ 171.2, 153.1, 139.3, 139.2, 135.0, 129.8, 129.3, 129.0, 128.0, 127.8, 120.0, 115.1, 41.1, 30.3, 21.3, 19.6 and 13.5; IR (neat) 1689 cm−1; HRMS (ES) m/z calcd for C22H24NO 318.1852, found 318.1868. Additional NMR experiments including NOESY, HMBC, HSQC and DQF-COSY were carried out in order to allow for complete assignment of all proton and carbon absorptions and to establish the Z stereochemistry.
4.1.40 (Z)-5-Benzylidene-1-butyl-4-(4-methoxyphenyl)pyrrol-2(5H)-one (20b). Method B
5-Benzyl-1-butyl-5-hydroxy-4-methoxyphenylpyrrol-2(5H)-one (0.200 g, 0.569 mmol) was placed in a 10 mL microwave reaction tube along with a stir bar, p-toluene sulfonic acid (0.016 g, 0.085 mmol) and 6 mL of chloroform. The microwave vial was sealed and heated with microwaves at 65 ° C for 1 h. The resulting reaction mixture was diluted with additional chloroform (15 mL), extracted with saturated aqueous bicarbonate solution (3 × 10 mL) and brine (2 × 10 mL) and dried over anhydrous magnesium sulfate. After removing the drying agent by filtration, the filtrate was concentrated in vacuo to give an orange oil, which was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (0.139 g, 73% yield) was eluted with 6 column volumes of eluant. The resulting solid exhibited the following physical properties: bp 72–72 ° C @ 0.67 Torr; 1H NMR (CDCl3) δ 0.64 (t, J = 7.0 Hz, 3H), 0.86 (sextet, J = 7.0 Hz, 2H), 1.19 (pentet, J = 7.0 Hz, 2H), 3.55 (t, J = 7.0 Hz, 2H), 3.88 (s, 3H), 6.16 (s, 1H), 6.39 (s, 1H), 7.00 (d, J = 8.5 Hz, 2H), 7.33 (m. 3H), 7.38 (d, J = 7.0 Hz, 2H), and 7.41 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3) δ 171.3, 160.5, 152.7, 139.3, 135.1, 130.4, 129.3, 128.0, 127.8, 125.0, 119.6, 114.9, 114.1, 55.4, 41.0, 30.3, 19.6 and 13.4; IR (neat) 1682 cm−1; HRMS (ES) m/z calcd for C22H24NO2 334.1802, found 334.1828.
4.1.41 (Z)-5-Benzylidene-1-butyl-4-(4-chlorophenyl)pyrrol-2(5H)-one (20c)
This compound was prepared by the above procedure (Method B) with the exception that 5-benzyl-1-butyl-5-hydroxy-4-chlorophenylpyrrol-2(5H)-one was used in the reaction. The crude product was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product was eluted with 6 column volumes of eluant. The resulting yellow oil (75% yield) exhibited the following physical properties; bp 71–72 ° C @ 0.68 Torr; 1H NMR (CDCl3) δ 0.63 (t, J = 7.5 Hz, 3H), 0.87 (sextet, J = 7.5 Hz, 2H), 1.18 (pentet, J = 7.5 Hz, 2H), 3.55 (t, J = 7.5 Hz, 2H), 6.20 (s, 1H), 6.31 (s, 1H), 7.32 (m, 3H), 7.37 (d, J = 6.0 Hz, 2H), 7.38 (d, J = 7.0 Hz, 2H) and 7.43 (d, J = 7.0 Hz, 2H); 13C NMR (CDCl3) δ 170.7, 151.7, 138.9, 135.4, 134.7, 131.0, 130.4, 129.3, 128.9, 128.1, 128.0, 120.8, 115.2, 41.1, 30.3, 19.6 and 13.5; IR (neat) 1685 cm−1; HRMS (ES) m/z calcd for C21H21NOCl 338.1306, found 338.1324.
4.1.42 (Z)-5-Benzylidene-1-butyl-4-(3,4-dimethoxyhenyl)pyrrol-2(5H)-one (20d)
This compound was prepared by the above procedure (Method B) with the exception that 5-benzyl-1-butyl-5-hydroxy-4-(3,4-dimethoxyphenyl)pyrrol-2(5H)-one was used in the reaction. The crude product was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product was eluted with 6 column volumes of eluant. The resulting yellow oil (46 % yield) was found to be a mixture of Z:E isomers (6:1 ratio, respectively as determined by NMR) and when this material was allowed to stir in chloroform overnight the Z:E isomeric ratio changed to 13:1 in favor of the Z isomer. The resulting material (Z isomer) exhibited the following physical properties; bp 86–87 ° C @ 1.10 Torr; 1H NMR (CDCl3) δ 0.64 (t, J = 7.5 Hz, 3H), 0.87 (sextet, J = 7.5 Hz, 2H), 1.19 (pentet, J = 7.5 Hz, 2H), 3.55 (t, J = 7.5 Hz, 2H), 3.94 (s, 3H), 3.95 (s, 3H), 6.18 (s, 1H), 6.43 (s, 1H), 6.97 (m, 2H), 7.05 (dd, J = 2.5 Hz, J = 7.5 Hz 1H), 7.34 (m, 3H) and 7.38 (m, 2H); 13C NMR (CDCl3) δ 171.1, 152.8, 150.1, 149.1, 139.2, 135.0, 129.3, 128.1, 127.8, 125.2, 121.9, 119.7, 112.3, 111.2, 115.0, 56.1, 56.0, 41.1, 30.3, 19.6 and 13.4; IR (neat) 1674 cm−1; HRMS (ES) m/z calcd for C23H26NO3 364.1907, found 364.1894.
4.1.43 (Z)-5-Benzylidene-1-hexyl-4-p-tolylpyrrol-2(5H)-one (20e)
This compound was prepared by the above procedure (Method B) with the exception that n-hexylamine was used in the reaction. The crude product was an orange solid and was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (96% yield) was eluted with 6 column volumes of eluant. The resulting solid exhibited the following physical properties; mp 142–144° C; 1H NMR (CDCl3) δ 0.80 (t, J = 7.5 Hz, 3H), 0.97–1.28 (m, 8 H), 2.43 (s, 3H), 3.55 (t, J = 7.5 Hz, 2H), 6.19 (s, 1H), 6.39 (s, 1H), and 7.27–7.38 (m, 9H); 13C NMR (CDCl3) δ 171.1, 153.0, 139.3, 139.2, 135.0, 129.7, 129.3, 129.0, 128.1, 127.8, 120.0, 115.1, 41.3, 31.2, 28.2, 26.0, 22.4, 21.3 and 13.9; IR (neat) 1694 cm−1; HRMS (ES) m/z calcd for C24H28NO 346.2165, found 346.2161.
4.1.44 (Z)-5-Benzylidene-1-(2,4-dimethoxybenzyl)-4-p-tolylpyrrol-2(5H)-one (20f)
This compound was prepared by the above procedure (Method B) with the exception that 2,4-dimethoxybenzylamine was used in the reaction. The crude product was an orange liquid was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (96% yield) was eluted with 6 column volumes of eluant and was found to be a mixture of Z:E isomers (as determined by NMR). When this material was allowed to stir in chloroform for 3 days, the Z:E isomeric ratio was 6:1 in favor of the Z isomer. The resulting material (Z isomer) exhibited the following physical properties; bp 85–86 ° C @1.3 Torr; 1H NMR (CDCl3) δ 2.43 (s, 3H), 3.50 (s, 3H), 3.77 (s, 3H), 4.69 (s, 2H), 6.22 (d, J = 2.4 Hz, 1H), 6.29 (s, 1H), 6.30 (s, 1H), 6.31 (dd, J = 2.4 Hz, J = 8.0 Hz, 1H), 6.51 (d, J = 8.4 Hz, 2H), 6.99 (d, J = 7.4 Hz, 2H), 7.15 (t, J = 7.4 Hz, 2H), 7.21 (t, J = 7.4 Hz, 1H, 7.29 (d, J = 7.9 Hz, 2H) and 7.39 (d, J = 7.9 Hz, 2H); 13C NMR (CDCl3) δ 171.7, 159.7, 157.5, 153.4, 139.4, 138.9, 134.5, 129.7, 129.3, 129.0, 128.9, 127.6, 127.2, 127.1, 119.6, 117.9, , 116.1, 103.5, 97.8, 55.3, 54.8, 40.5 and 21.3; IR (neat) 1694 cm−1; HRMS (ES) m/z calcd for C27H26NO3 412.1907, found 412.1882. Additional NMR experiments including NOESY, HMBC, HSQC and DQF-COSY were carried out in order to allow for complete assignment of all proton and carbon absorptions and to establish the Z stereochemistry.
4.1.45 (Z)-5-(4-Methylbenzylidene)-1-butyl-4-p-tolylpyrrol-2(5H)-one (20g)
This compound was prepared by the above procedure (Method B) with the exception that 4-ethynyltoluene was used in the reaction. The crude product was an orange liquid was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (95 % yield) was eluted with 6 column volumes of eluant and was found to be a mixture of Z:E isomers (as determined by NMR). When this material was allowed to stir in chloroform for 3 days the Z:E isomeric ratio was 4:1 in favor of the Z isomer. The resulting material (Z isomer) exhibited the following physical properties; bp 70–71 ° C @ 1.15 Torr; 1H NMR (CDCl3) δ 0.66 (t, J = 7.5 Hz, 3H), 0.91 (m, 2H), 1.20 (pentet, J = 7.5 Hz, 2H), 2.39 (s, 3H), 2.43 (s, 3H), 3.59 (t, J = 7.5 Hz, 2H), 6.18 (s, 1H), 6.37 (s, 1H), 7.18 (d, J = 8.5 Hz, 2H), 7.21 (d, J = 8.5 Hz, 2H), 7.28 (d, J = 8.5 Hz, 2H) and 7.35 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3) δ 171.3, 153.1, 139.2, 138.9, 137.8, 131.9, 129.8, 129.3, 129.2, 129.0, 128.7, 119.8, 115.5, 41.1, 30.3, 21.3, 21.2, 19.6 and 13.5; IR (neat) 1683 cm−1; HRMS (ES) m/z calcd for C23H26NO 332.2009, found 332.2029.
4.1.46 (Z)-5-(4-Methoxybenzylidene)-1-butyl-4-p-tolylpyrrol-2(5H)-one (20h)
This compound was prepared by the above procedure (Method B) with the exception that 4-ethynylanisole was used in the reaction. After isolation, the crude product was stirred overnight in chloroform at rt to insure that optimum conversion to the Z-isomer had taken place. The crude product was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product (96 % yield) was eluted with 6 column volumes of eluant and was found to be a mixture of Z:E isomers (as determined by NMR) in a 7:1 ratio in favor of the Z isomer. The resulting material (Z isomer) exhibited the following physical properties; bp 68–69 ° C @ 1.45 Torr; 1H NMR (CDCl3) δ 0.67 (t, J = 7.5 Hz, 3H), 0.90 (m, 2H), 1.24 (m, 2H), 2.70 (s, 3H), 3.62 (t, J = 7.5 Hz, 2H), 3.83 (s, 3H), 6.15 (s, 1H), 6.34 (s, 1H), 6.90 (d, J = 7.8 Hz, 2H), 7.26 (m, 4H) and 7.34 (d, J = 7.8 Hz, 2H); 13C NMR (CDCl3) δ 171.4, 159.4, 153.1, 139.2, 138.6, 130.7, 129.8, 129.3, 129.0, 127.1, 119.6, 115.4, 113.6, 55.3, 41.1, 30.3, 21.3, 19.7 and 13.5; IR (neat) 1686 cm−1; HRMS (ES) m/z calcd for C23H26NO2 348.1958, found 348. 2014.
4.1.47 (Z)-5-(3,4-Dimethoxybenzylidene)-1-(3,4-dimethoxyphenethyl)-4-(3,4-dimethoxyphenyl) -pyrrol-2(5H)-one (20i)
This compound was prepared by the above procedure (Method B) with the exception that 4-ethynyl-1,2-dimethoxybenzene, 3,4-dimethoxyphenethylamine and 5-(3,4-dimethoxybenzylidene)-4-(3,4-dimethoxyphenyl)-5H-furan-2-one were used in the reaction. After workup and isolation, the crude product was purified by flash chromatography on a Biotage SP-1 instrument, equipped with a silica cartridge, and employing an ethyl acetate/hexane gradient. The product was eluted with 6 column volumes of eluant and the resulting solid (50 % yield) exhibited the following physical properties: mp 112–115 ° C; 1H NMR (CDCl3) δ 2.50 (t, J = 8.1 Hz, 2H), 3,74 (s, 3H), 3.82 (s, 3H), 3.89 (m, 2H), 3.90 (s, 3H), 3.93 (s, 3H), 3.94 (s, 3H), 3.95 (s, 3H), 6.17 (s, 1H), 6.22 (d, J = 2.0 Hz, 1H), 6.33 (dd, J = 2.0 Hz, J = 8.5 Hz, 1H), 6.35 (s, 1H), 6.67 (d, J = 8.5 Hz, 1H), 6.86 (s, 1H), 6.93 (m, 3H), 6.95 (d, J = 8.0 Hz, 1H), and 7.01 (dd, J = 1.8 Hz, J = 8.0 Hz, 1H); 13C NMR (CDCl3) δ 171.4, 153.3, 150.1, 149.1, 148.7, 147.5, 138.7, 131.0, 127.4, 125.2, 122.7, 121.8, 120.9, 119.3, 115.3, 112.7, 112.3, 111.9, 111.2, 110.9, 56.1, 56.0, 55.9, 55.8, 55.7, 43.3 and 34.3; IR (neat) 1683 cm−1; HRMS (ES) m/z calcd for C31H34NO7 532.2330, found 532.2324.
Acknowledgments
We thank the National Institutes of Health (grant no. R15-CA67236) for support of this research. We are exceedingly grateful to Mr. Dave Patteson formerly of Biotage Inc. for the generous donation of a Horizon HFC and an SP-1 flash chromatography systems, which were used in the majority of sample purifications, and also for the generous donation of a Personal Chemistry Emrys Liberator US microwave reaction system, which was utilized for microwave accelerated reactions. Recent grants from the MRI program of the National Science Foundation for the purchase of a 500 MHz NMR spectrometer (CHE-0116492) and an electrospray mass spectrometer (CHE-0320669) are also gratefully acknowledged. We would also like to thank the Arnold and Mabel Beckman Foundation for fellowship support to Benjamin C. Giglio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Kang H, Fenical W. J Org Chem. 1997;62:3254. doi: 10.1021/jo962132+. [DOI] [PubMed] [Google Scholar]
- 2.Fan G, Li Z, Shen S, Yang Y, Xu M, Brihn T, Bruhn H, Morschhauser J, Bringmann G, Lin W. Bioorg Med Chem. 2010;18:5466. doi: 10.1016/j.bmc.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 3.Hamasaki A, Zimpleman J, Hwang I, Boger D. J Am Chem Soc. 2005;127:10767. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soenen D, Hwang I, Hedrick M, Boger D. Bioorg Med Chem Lett. 2003;13:1777. doi: 10.1016/s0960-894x(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 5.Peschko C, Steglich W. Tetrahedron Lett. 2000;41:9477. [Google Scholar]
- 6.Cherry K, Thibonnet J, Duchene A, Parrain J, Abarbri M. Tetrahedron Lett. 2004;45:2063. [Google Scholar]
- 7.Negishi E, Kotora M. Tetrahedron. 1997;20:6707. [Google Scholar]
- 8.Goh W, Iskander G, St Black D, Kumar N. Tetrahedron Lett. 2007;48:2287. [Google Scholar]
- 9.Gupton J, Giglio B, Eaton J, Rieck E, Smith K, Keough M, Barelli P, Firich L, Hempel J, Smith T, Kanters R. Tetrahedron. 2009;65:4283. doi: 10.1016/j.tet.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prim D, Fuss A, Kirsch G, Silva A. J Chem Soc Perkin Trans. 1999;2:1175. [Google Scholar]
- 11.Lin Y, Lang S. J Org Chem. 1980;45:4857. [Google Scholar]
- 12.Hernandez S, Moreno I, SanMartin R, Gomez G, Herrero T, Dominguez E. J Org Chem. 2010;75:434. doi: 10.1021/jo902257j. [DOI] [PubMed] [Google Scholar]
- 13.Vo-Quang Y. Ann Chim. 1962;7:785. [Google Scholar]
- 14.Ray D, Paul S, Brahma S, Ray J. Tetrahedron Lett. 2007;48:8005. [Google Scholar]
- 15.Abdel-Hamid Y, Hamdy A. J Chem Soc Chem Commun. 1974;8:288. [Google Scholar]
- 16.Volkmar D, Bodendorf K. Arch Pharm Pharm Med Chem. 1970;303:481. doi: 10.1002/ardp.19703030603. [DOI] [PubMed] [Google Scholar]
- 17.Bodendorf K, Mayer R. Chemische Berichte. 1965;98:3554. [Google Scholar]
- 18.Estelle M, Uriac P, Brunel Y, Poigny S. 2936244 A1 20100326. French Patent. 2010