Abstract
Background
A major problem in Chagas disease donor screening is the high frequency of samples with inconclusive results. The objective of this study is to describe patterns of serological results among donors to the three Brazilian REDS-II blood centers and correlate with epidemiological characteristics.
Methods
The centers screened donor samples with one T cruzi lysate EIA. EIA-reactive samples were tested with a second lysate EIA, a recombinant-antigen based EIA, and an immunfluorescence assay (IFA). Based on the serological results, samples were classified as confirmed positive (CP), probable positive (PP), possible other parasitic infection (POPI) and (FP) false positive.
Results
In 2007–2008, 877 of 615,433 donations were discarded due to Chagas assay reactivity. The prevalence (95% CI) among first time donors for CP, PP, POPI and FP patterns were 114 (99 – 129), 26 (19–34), 10 (5–14) and 96 (82–110) per 100,000 donations, respectively. CP and PP had similar patterns of prevalence when analyzed by age, gender, education, and location, suggesting that PP cases represent true T cruzi infections; in contrast the demographics of donors with POPI were distinct and likely unrelated to Chagas disease. No CP cases were detected among 218,514 repeat donors followed for a total of 718,187 person-years.
Conclusion
We have proposed a classification algorithm that may have practical importance for donor counseling and epidemiological analyses of T cruzi seroreactive donors. Absence of incident T cruzi infections is reassuring with respect to risk of window phase infections within Brazil and travel related infections in non-endemic countries such as the US.
Keywords: Blood donors, Chagas disease, T cruzi, serology
INTRODUCTION
Chagas disease is a parasitic infection caused by Trypanosoma cruzi (T cruzi), which is transmitted by hematophagous triatomine insects. The parasite can also be transmitted vertically (through the placenta or peripartum) and by transfusions of blood products and organ transplantations1,2. Screening for Chagas disease has been mandatory in Brazil since 1969. Parallel testing with 2 different T cruzi antibody assays was mandatory in Brazil until 2004, since then screening was performed using a single high sensitivity EIA assay. Laboratory diagnosis of either acute or chronic T. cruzi infection, is challenging1,2 Diagnosis is generally based on serological assays because the direct detection of parasites is difficult even with modern molecular techniques such as PCR, due to very low levels or even absence of parasitemia3–5. Most of the commercially available antibody assays use crude parasite extracts or subcellular fractions of cultured parasites asantigen preparations2. More recently assays using immunodominant recombinant T cruzi antigens have been developed6,7. Recombinant antigens for T. cruzi are more specific than parasite extracts that cross-react with sera from patients with other diseases such as leishmaniasis8,9–10 and Trypanosomarangeli infection11.
Samples with low-level reactivity and inconclusive T cruzi antibody results are frequently found in large scale screening, especially when parallel testing with 2 or more assays is performed2,12,13. Such samples present challenges not only for donor counseling but also when evaluating the performance of new tests or estimating prevalence or incidence rates for national or regional epidemiological surveillance. These samples could represent cases of cross-reactivity with other parasitic infections, self-limited (resolved) infections with waning antibodies, or active Chagas infection with low antibody responses or with antibody not detected by currently employed commercial assays. In the latter cases, failure of robust detection of infected donors would imply that the blood supply could still be a route for T cruzi transmission.
Recently, a recombinant T cruzi antigen-based EIA was developed and commercialized that has eliminated cross reactivity with Leishmania seropositve samples while retaining sensitivity to T cruzi antibodies similar to other lysate-based EIAs and RIPA and other confirmatory test results 4,14. We hypothesized that if a subset of the low-level T cruzi EIA reactive donor samples are due to cross reactivity to other parasitic infections, the recombinant EIA could provide a useful tool to employ in a supplemental testing algorithm to differentiate these groups.
We describe here the Chagas serological patterns obtained by testing at 3 large blood centers during 2007 and 2008 as part of the REDS-II International study in Brazil14. Based on our findings we propose a new algorithm for classification of T cruzi seroreactive donor specimens. We also report the epidemiological characteristics of T cruzi confirmed seropositive Brazilian donors, and demonstrate virtual absence of seroconversions attributable to incident infections in these regions in Brazil.
METHODS
Blood screening and supplemental testing
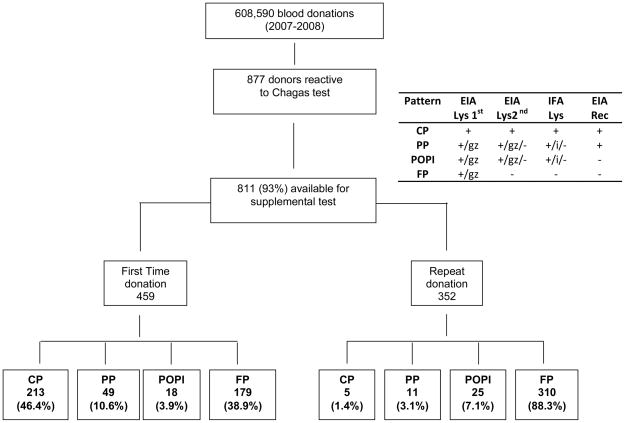
The three blood centers included in this study are: Hemope, Recife, Pernambuco; Hemominas, Belo Horizonte, Minas Gerais; and Fundacao Pro-Sangue, Sao Paulo. All centers screened for T cruzi antibodies using one EIA based on crude parasite extracts. Table 1 lists the manufacturers of the screening EIAs employed at each blood center during the 2 year analysis period (1/07–12/08). Independent of the kit used, the centers classified as “grey zone” all samples with signal to cutoff (S/C) ratios of 0.8 to 1.2. All positive or grey zone units were discarded, and the samples were sent to a central lab in Sao Paulo and tested with a second lysate EIA (Chagatek Biomérieux,, Argentina; or Elisa cruzi Biomérieux, Brazil), a recombinant T cruzi antigen EIA that does not react with Leishmania samples (Chagatest Wiener rec. V.3.0, Argentina), and a T cruzi immunfluorescence assay (IFA; Biolab Merieux, Brazil). In the central laboratory we used the manufactures’ criteria to consider samples positive, grey zone or negative: the Wiener and Chagatek kits classify samples as positive or negative, while Elisa cruzi classifies samples with S/C between 0.8 and 1 as grey zone. IFA testing was initially performed on 1/20 dilutions of EIA reactive donor sera and if reactive further dilutions were tested to determine IFA titers. Samples were considered IFA positive when the titer was higher than 1/20, and indeterminate when the titer was equal to 1/20 (see figure 1).
Table 1.
Description of the kit used in each site during the study period
| CENTERID | Period | Screening Test Kit | Number of Screened Units | % of Screened Units | False Positive | % of False Positive |
|---|---|---|---|---|---|---|
| Hemope (Recife) | 1. 01/01/2007 -- 21/07/2007 | C: Bioschile | 56132 | 9.2 | 10 | 0.02 |
| 2. 22/07/2007 -- 31/12/2008 | B: Elisa Cruzi - Biomerieux | 145363 | 23.9 | 64 | 0.04 | |
| Hemominas (Belo Horizonte) | 1. 01/01/2007 -- 11/02/2007 | C: Bioschile | 9056 | 1.5 | 0 | 0.00 |
| 2. 12/02/2007 -- 26/09/2007 | D: Gold Chagas REM | 42589 | 7.0 | 221 | 0.52 | |
| 3. 27/09/2007 -- 16/01/2008 | B: Elisa Cruzi - Biomerieux | 20653 | 3.4 | 4 | 0.02 | |
| 4. 17/01/2008 -- 31/12/2008 | D: Gold Chagas REM | 64589 | 10.6 | 25 | 0.04 | |
| FPS/HSP (São Paulo) | 1. 01/01/2007 -- 22/02/2007 | B: Elisa Cruzi - Biomerieux | 20352 | 3.3 | 7 | 0.03 |
| 2. 23/02/2007 -- 27/02/2007 | A:Chagateck - Biomerieux | 1666 | 0.3 | 1 | 0.06 | |
| 3. 28/02/2007 -- 31/12/2008 | B: Elisa Cruzi - Biomerieux | 248190 | 40.8 | 157 | 0.06 |
Figure 1.
Distribution of the samples according to serological algorithm (see methods). CP = Confirmed Positive; PP = Probably Positive; POPI = Probable other parasitic infection; FP = false positive; EIA Lys 1st –Enzyme linked immunoassay based on parasite lysate used for routine screening; EIA Lys 2nd –Enzyme linked immunoassay based on parasite lysate used as a supplemental test; EIA Rec – Enzyme linked immunoassay based on parasite recombinant DNA expressed proteins; IFA – Immunfluorescence assay; gz= grey zone; i= indeterminate by IFA (Titer=1:20)
Classification criteria
Samples were classified as Confirmed Positive (CP) when reactive by all 4 assays as Probable Positive (PP) when reactive on the screening EIA and recombinant antigen EIA, but inconclusive or negative by the supplemental lysate EIA or IFA as possible Other Parasitic Infection (POPI) when positive or grey-zone reactive by the lysate assays (EIAs and IFA) but negative in the recombinant antigen EIA, and as False Positive (FP) when reactive only by the screening assay.
Defining seroconversion status
The REDS-II donation dataset classifies donors as first time versus repeat and provides the date of previous donation for repeat donors. Since this computerized dataset does not include T cuzi antibody test results, we determined if seroconversion occurred in a positive repeat donor by manually verifying that a previous donation was T cruzi antibody negative. This step was performed in order to confirm that donors were not misclassified as seroconverters. We also reviewed if the donor had returned subsequent to a positive antibody test to provide samples for follow up testing. We computed the incidence rate of Chagas infection by dividing the number of new events by the number of person-years of follow-up. The follow-up time was measured in years, from the first available donation date to the last available donation date or date of confirmed or probable Chagas testing. The pre-REDS-II donation dates were used when available, and those dates could go back to 1988 for Minas, 1994 for Sao Paulo, and 1998 for Recife. Donors could be followed up to 2008 in all centers.
Statistical Analysis
We computed 2-year prevalence rates (2007 & 2008) per 100,000 donors and corresponding 95% confidence intervals (CI). The 95% CI was calculated using the normal approximation. Chi-square tests were used to assess differences in proportions according to the classification of infection types in first time donors. A p-value < 0.05 based on two-tailed alternatives was considered to be statistically significant. All statistical tests were performed using SAS™ 9.1 software (SAS Institute, 2004)15.
RESULTS
Study population
The population for this analysis consisted of 615,428 donations by 410,457 donors during 2007 and 2008; Chagas screening testing was not conducted on 6,775 (1.1%) donations and these donations were excluded from the analysis. Additionally, for 66 specimens (22 from Recife and 44 from Belo Horizonte) with reactive Chagas screening test results no residual samples were available for supplemental testing at the central lab in Sao Paolo; these cases were excluded from the analysis of interpretive algorithms Our primary analysis dataset therefore consisted of 608,590 donations, including donations from 186,970 first time donors and 421,620 donations from repeat donors.
With regard to demographic characteristics of the donor population, the majority were male (69.8%), the mean age was 33.3 years old (SD = 10.38), and 67.3% of donations were given by community donors and 32.7% by replacement donors. With respect to education status, 13% of donations were by donors who did not finish high school, 16.5% by donors who had a high school degree, 54.5% by donors who had completed at least 3 years of college, and 16% by donors who were college graduates. More donations were made in Sao Paulo (44%), followed by Recife (33%) and Minas (23%).
Reactivity Patterns in all donors
During the 2 year study period 877 samples were discarded due to reactive or grey zone primary T cruzi EIA reactivity, with 811 (93%) available for confirmatory testing. Figure 1 summarizes the samples classification according to first time and repeat donation status. The number of reactive donations and prevalence rates per 100,000 donations for each serological pattern are shown in Table 2, sorted by blood center. Of the 811 primary EIA-reactive samples evaluated by the 3 supplemental assays, 322 (40%) were reactive by at least one of these assays while 489 (60%) were negative by all 3 supplemental assays and were classified as FP. The FP rates were very different depending on the screening test used (Table 1), with one kit lot responsible for almost half of the FP cases. The overall prevalence of CP donations was 36 per 100,000 (95% CI: 31 – 41/100,000). Sao Paulo had the highest prevalence of CP donations followed by Belo Horizonte and Recife (Table 2). The distribution of reactive donations with the PP pattern was similar to that of the CP cases in terms of relative rates by blood center.
Table 2.
Serological Pattern by Location among All Donors
| Classification | Blood Center Region |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Recife | Belo Horizonte | Sao Paulo | Total | ||||||
| Total donations n = 204,124 | Total donations n = 139,429 | Total donations n = 271,880 | Total donations n = 615,433 | ||||||
| N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | p-value* | |
| CP | 31 | 15 (10 –21) | 46 | 34 (24 – 43 | 141 | 52 (44 – 61) | 218 | 36 (31 – 41) | < 0.001 |
| PP | 10 | 5 (2 – 8) | 13 | 9 (4 – 15) | 37 | 14 (9 – 18) | 60 | 10 (7 – 12) | 0.011 |
| POPI | 19 | 9 (5 – 14) | 12 | 9 (4 – 14) | 13 | 5 (2 – 7) | 44 | 7 (5 – 9) | 0.136 |
| FP | 74 | 37 (28 – 45) | 250 | 183 (160 – 205) | 165 | 61 (52– 70) | 489 | 80 (73 – 87) | < 0.001 |
| Total | 134 | N/A | 321 | N/A | 356 | N/A | 811 | N/A | N/A |
| PP/CP, ratio | 0.32 | 0.28 | 0.26 | 0.28 | =0.876 | ||||
| POPI/CP, ratio | 0.61 | 0.26 | 0.09 | 0.20 | <0.001 | ||||
95%CI: 95% Confidence Interval
p-value for chi-square statistics comparing the Recife, Belo Horizonte and Sao Paulo blood centers
N/A: Not Applicable
We calculated ratios of PP/CP and of POPI/CP donations to gain insights into the possible relationships of these patterns to confirmed T cruzi infection rates in each center. For all centers the PP/CP ratio was ~0.30, suggesting that this PP pattern is probably related to Chagas disease exposure. In contrast, the POPI/CP ratio was significantly different, being higher in Recife followed by Belo Horizonte and Sao Paulo (P<0.001), implying that POPI reactivity is unrelated to T cruzi exposure.
Prevalence in first time donors
Of the 811 reactive samples 459 were from first time donors and 352 were from repeat donors. The prevalence per 100,000 donations for each serological pattern among the first time donors is shown in Table 3. The prevalence of CP donations was 114 per 100,000 (95% CI: 99 – 129/100,000). Significantly different rates of CP infections were found by donor age, education status, and blood center location variables. As expected there was a marked relationship between donor age and CP results, ranging from a prevalence of 15/100,000 (95% CI: 6 – 24) in the <25 years old group to 756/100,000 (95% CI: 499 –1013) in the ≥55 years old group (P-value < 0.001). On the other hand, an inverse relationship was observed for education with CP prevalence higher among those who did not graduate high school versus those with a college degree or higher level educations (P-value < 0.001). With respect to blood center location, we found a higher prevalence in Sao Paulo, followed by Belo Horizonte, and Recife (P-value < 0.001). CP prevalence rates did not significantly differ by gender or donor type (replacement versus community donors). For the PP cases, the overall prevalence in FT donors was 26 per 100,000 donations (95% CI: 19 – 34/100,000). As with the overall donor analysis, we found similar patterns of PP and CP prevalence rates when first time donors were analyzed by age, gender, education, donation history and location.
Table 3.
Serological Pattern by Demographic Characteristics among 1st time Donors
| Characteristics | Serological pattern |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Confirmed Positive Chagas (CP) | Probable Positive Chagas (PP) | Possible Other Parasitic Infection (POPI) | False Positives (FP) | ||||||
| N (Donations) | N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | N (Positive) | Prevalence/100,000 donations (95%CI) | |
| Overall | 186970 | 213 | 114 (99 – 129) | 49 | 26 (19 – 34) | 18 | 10 (5 – 14) | 179 | 96 (82 – 110) |
| Age | |||||||||
| age <25 | 73596 | 11 | 15 (6 –24)* | 6 | 8 (2 – 15)* | 4 | 5 (0 – 11) | 64 | 87 (66 – 108) |
| age ≥ 25 – <35 | 61857 | 45 | 73 (52 –94) | 15 | 24 (12 – 37) | 8 | 13 (4 – 22) | 53 | 86 (63 – 109) |
| age ≥ 35 – <45 | 31329 | 67 | 214 (163 – 265) | 15 | 48 (24 – 72) | 3 | 10 (0 – 20) | 38 | 121 (83 – 160) |
| age ≥ 45 – <55 | 15761 | 57 | 362 (268 –455) | 10 | 63 (24 – 103) | 2 | 13 (0 – 30) | 19 | 121 (66 – 175) |
| age ≥ 55 | 4363 | 33 | 756 (499 – 1013) | 3 | 69 (0 – 147) | 1 | 23 (0 – 68) | 5 | 115 (14 – 215) |
| Gender | |||||||||
| Female | 73630 | 87 | 118 (93 – 143) | 21 | 29 (16 – 41) | 3 | 4 (0 – 9)* | 56 | 76 (56 – 96) |
| Male | 113340 | 126 | 111 (92 – 131) | 28 | 25 (16 – 34) | 15 | 13 (7 – 20) | 123 | 109 (89 – 128) |
| Education | |||||||||
| < High School | 12817 | 60 | 468 (350 – 586)* | 11 | 86 (35 – 137)* | 2 | 16 (0 – 37) | 9 | 70 (24 – 116) |
| High School | 16913 | 39 | 231 (158– 303) | 6 | 35 (7 – 64) | 2 | 12 (0 – 28) | 15 | 89 (44 – 134) |
| Complete 3 of College | 62486 | 29 | 46 (30 – 63) | 6 | 10 (2 – 17) | 2 | 3 (0 – 8) | 33 | 53 (35 – 71) |
| College + | 15566 | 3 | 19 (0 – 41) | -- | -- | 1 | 6 (0 – 19) | 11 | 71 (29 – 112) |
| Type of Donation | |||||||||
| Community | 100092 | 105 | 105 (85 – 125) | 22 | 22 (13 – 31) | 10 | 10 (4 – 16) | 85 | 85 (67 – 103) |
| Replacement | 86843 | 108 | 124 (101 – 148) | 27 | 31 (19 – 43) | 8 | 9 (3 – 16) | 94 | 108 (86 – 130) |
| Location | |||||||||
| Recife | 57921 | 27 | 47 (29 – 64)* | 8 | 14 (4 – 23)* | 8 | 14 (4 – 23) | 26 | 45 (28 – 62) |
| Belo Horizonte | 43871 | 45 | 103 (73 – 133) | 7 | 16 (4 – 28) | 5 | 11 (1 – 21) | 87 | 198 (157 – 240) |
| Sao Paulo | 85178 | 141 | 166 (138 – 193) | 34 | 40 (27 – 53) | 5 | 6 (1 – 11) | 66 | 77 (59 – 96) |
95%CI: 95% Confidence Interval
p-value < 0.05
The prevalence of POPI was not significantly different in the 3 regions, but the demographic correlates of POPI were different than CP and PP. For examples, the prevalence of POPI was higher among males and demonstrated an opposite trend to that noted for PP and CP rates with respect to center location, with the highest rate of POPI in Recife and lowest rate in Sao Paolo (Table 3).
Assuming that PP and CP cases both represent true T cruzi seropositivity, these groups were combined and we calculated a total seroprevalence rate among FT donors of 140 (123 – 157)/100,000. Demographic correlates of the combined CP and PP groups are summarized in Table 4.
Table 4.
Confirmed or Probable Positive Chagas infection by Demographic Characteristics among 1st time Donors.
| Characteristics | Serological pattern |
||
|---|---|---|---|
| Confirmed Positive Chagas (CP) or Probable Positive Chagas (PP) | |||
| N (Donations) | N (Positive) | Prevalence/100,000 donations (95%CI) | |
| Overall | 186970 | 262 | 140 (123 – 157) |
| Age | |||
| age <25 | 73596 | 17 | 23 (12 – 34)* |
| age ≥ 25 - <35 | 61857 | 60 | 97 (72 – 125) |
| age ≥ 35 - <45 | 31329 | 82 | 262 (205 – 318) |
| age ≥ 45 - <55 | 15761 | 67 | 425 (323 – 527) |
| age ≥ 55 | 4363 | 36 | 825 (557 – 1094) |
| Gender | |||
| Female | 73630 | 108 | 147 (119 – 174) |
| Male | 113340 | 154 | 136 (114 – 157) |
| Education | |||
| < High School | 12817 | 71 | 554 (425 – 682)* |
| High School | 16913 | 45 | 266 (188 – 344) |
| Complete 3 of College | 62486 | 35 | 56 (37 – 75) |
| College + | 15566 | 3 | 19 (0 – 41) |
| Type of Donation | |||
| Community | 100092 | 127 | 127 (105 – 149) |
| Replacement | 86843 | 135 | 155 (129 – 182) |
| Location | |||
| Recife | 57921 | 35 | 60 (40 – 80)* |
| Belo Horizonte | 43871 | 52 | 119 (86 – 151) |
| Sao Paulo | 85178 | 175 | 206 (175 – 236) |
95%CI: 95% Confidence Interval
p-value < 0.05
Incidence in repeat donors
Of 426,166 repeat donations collected in 2007–2008, there were 352 T cruzi EIA repeat reactive samples at screening. Of those 310 (88.3%) did not react to any of the supplemental assays and were considered false positive, while 42 (11.7%) samples were reactive by at least one supplemental assay with 5 classified as CP, 11 as PP and 26 as POPI. However after reviewing the test result history of these donors (Table 5) using all available blood center records, we established that none of the 5 CP cases were true seroconverters: 4 were T cruzi antibody repeat reactive on their previous donation and donated again by mistake (i.e., due to failure of the notification and deferral process), and one tested T cruzi antibody negative by EIA on follow-up samples and hence the CP result probably resulted from a specimen mix-up or contamination problem at the time of donation or initial sample processing. Of the 11 PP cases, 10 were negative on previous donations, 7 of whom returned for a follow-up counseling and repeat sampling and testing; 5 of the follow-up samples tested negative and 2 were grey zone. Our expectation was that if cases of true seroconversion were observed, most of them should be classified CP cases because the inter-dontaion interval among possible seroconverters was large (the median is 15.13 months, minimum = 1.17 and maximum = 145.67). The window phase for Chagas is expected to be less than 60 days. It would not be reasonable to think that all seroconverters would come to donate soon after getting infected and during the transient window phase when the serological pattern could be inconclusive based on differential detection by the different assays. The fact that at follow-up many of these cases did not fully seroconverted corroborate our conclusion that these were not true incident infections. Hence, although not all PP cases could be investigated, there were no observed cases of confirmed T cruzi antibody seroconversion among repeat donors with preliminary CP or PP results. Overall 218,514 repeat donors were followed for a total of 718,187 person-years. The median follow-up time per donor was 2.32 years (range, 0.03 to 20.57 years). Thus, no incident case of confirmed T cruzi infection was identified during our follow-up period which yield an upper 95% confidence interval for possible T cruzi incidence of ~1: 240,000 person-years.
Table 5.
Summary of the results obtained among the 42 cases initially considered as seroconverters.
| Serological pattern | Total | Excluded based on previous donation tested positive | Previous donation negative | Returned for follow-up* | Results at follow up |
|---|---|---|---|---|---|
| CP | 5 | 4 | 1 | 1 | Negative |
| PP | 11 | 1 | 10 | 7 | 5 negative 2 grey-zone |
| POPI | 26 | 7 | 19 | 14 | 8 negative 3 grey-zone 3 EIA reactive |
Subsets of Previous donation negative group.
Of the 26 possible POPI cases in repeat donors, 19 were previously negative on all donations, and of these 14 returned for a follow up sample: 8 were T cruzi EIA negative, 3 had borderline reactivity on EIA and were classified as inconclusive, and 3 were again EIA reactive with similar levels of reactivity on plasma from the index donation and at follow-up (probable POPI).
Sensitivity of assays and classification algorithm
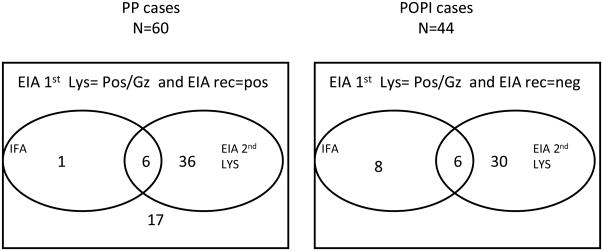
Figure 2 summarizes the test results of the samples classified as POPI or PP. Of the 60 PP samples, only 7 were positive or indeterminate by IFA, so if we assume that all CP and PP cases represent true T cruzi exposure, the sensitivity of IFA for confirmation was 81.1% (218+7/218+60). Of the 60 PP cases, 18 were negative by the second EIA based on crude antigen (and by IFA), so those 18 cases would have been missed if the second lysate-based EIA were it to have been used as the primary screening assay. Of the 44 POPI samples, only six were reactive to the second EIA based on crude antigen and to IFA, 30 samples were only reactive to EIA and 8 only to IFA. Hence detection of donors with POPI reactivity was inconsistent among the supplemental assays, presumably reflecting variable cross-reactivity to antibodies directed against Leishmania or other parasites endemic to Brazil.
Figure 2.
Summary of test results obtained on cases classified as PP and POPI. The samples positive or indeterminate by IFA and positive or grey zone for 2nd EIA test are summed inside the corresponded circles. PP = Probably Positive (Pos/gz for screening EIA and Pos for Recombinant EIA); POPI = Probable other parasitic infection (Pos/gz for screening EIA Neg for Recombinant EIA and pos/gz for EIA 2nd Lyz)
DISCUSSION
This report describes the serological patterns of Chagas disease serological markers among 877 T cruzi EIA reactive donations detected following screening of over 600,000 allogeneic donations given to 3 large blood centers in Brazil over a 2 year period. Diagnostic confirmation of Chagas disease is difficult due to the lack of widely available and well validated confirmatory assays (e.g., RIPA) or accepted gold standard supplemental testing algorithms4,14,15,18. Inconclusive results are commonly reported and have been systematically described and analyzed by several groups with different conclusions regarding the interpretation of these reactivity patterns4,14,15,18. Remesar and coauthors12, analyzing data from a highly endemic area for Chagas disease in Argentina, recently showed that of the 20% of the units discarded due to Chagas disease screening test reactivity, one third (6%of donations overall) had an inconclusive result based on combinations of results from different assays. In our study, inconclusive results were also common, with 104 PP or POPI results relative to 218 CP results, a similar ratio (~1/3) to that documented in the highly endemic donor population in Argentina.
We have developed a classification scheme for inconclusive samples based primarily on reactivity to a widely available recombinant T cruzi antigen-based EIA assay, which sorts these samples into PP cases that we hypothesized represent remote T cruzi exposure and POPI cases (samples non-reactive by the recombinant EIA assay) that we suspect are attributable to cross reactivity resulting from past Leishmania infections11. We then evaluated if these 2 categories differ in terms of epidemiological characteristics, relative to the characteristics of donors with CP and FP reactivity patterns. The PP and CP samples had very similar age, educational level and geographical distributions (PP/CP ratio), supporting our hypothesis that PP are predominantly related to T cruzi infection, and perhaps reflect remote infections which had resolved and in which antibody titers are waning. In contrast, the POPI/CP ratio was significantly different in each region suggestion that they are not related to the same biological phenomenon. The gender distribution of POPI cases (male predominance) was also different from that of the PP and CP equal male and female rates). Leishmania is endemic in Belo Horizonte and is increasing in incidence in Pernambuco state (where Recife is located), but is not common in Sao Paolo city. Acute or chronic Leishmania infections are well known to induce antibodies that cross react with Chagas lysate EIAs, but not with recombinant antigen EIAs. Unfortunately, there are no commercially available Leishmania-specific antibody assays to allow us to confirm the proportion of donations with POPI reactivity that represent Leishmania antibodies versus other parasite exposures or non-specific reactivity to 2 lystate EIAs with or without T cruzi IFA reactivity.
A previous study from our group has shown that donors with low reactive Chagas results had epidemiologic evidence of higher rates of exposure to the Chagas’ disease vector as compared to negative controls13. Levy and colleagues studying Chagas reactive samples in Peru also concluded that samples with discrepant results are geographically associated with confirmed Chagas cases19. A more recent study from an Argentinean group in the Chaco region has shown that among low reactive samples, those reactive in a recombinant assay had epidemiological evidence of Chagas exposure, where as those who tested non-reactive on that test did not. 16
If all 60 PP samples represent true infection it is important to note that there were 36 cases that were negative by IFA and 17 cases that were negative by the second lysate-based EIA and by IFA. Hence it is important to recognize that there are true low reactive T cruzi seropositive cases that are missed by both current screening and confirmatory assays. Indeed, studies based on a well characterized panel that include low reactive samples, shows that most EIAs and confirmatory assays do not achieve 100% sensitivity2,12,17. Low serorectivity may correlate with spontaneous parasite clearance and represent evolving seroreversion, and hence such cases may not present serious concerns for blood safety, although this issue will require further study.
One consequence of the lack of sensitivity of existing screening assays is the detection of apparent seroconversions (i.e., detection of reactivity in subsequent donations by donors who previously tested negative) when a different screening assay with a similar or higher sensitivity is implemented. However, despite the change in screening assays, we did not detect any CP seroconversions when such cases were carefully analyzed. Instead, we found that the apparent “seroconversions” in the 3 centers in this study showed only the PP or POPI pattern. We believe that even these cases do not represent new T cruzi infections, but rather long-standing (possibly reverting) T cruzi or Leishmania seroreactivity that had been missed by a previous screening kit
Overall the results on demographics of T cruzi infection in donors document a dramatic decreasing prevalence by age, consistent with the findings of other studies. The prevalence of CP among donors below 25 years old was 75-fold lower than that observed in the oldest donor strata (15/100,000 versus 756/100,000), and there was clear trend with age across all strata. Since the 1970s, Brazil has implemented a program to eradicate T cruzi vectorial transmission in houses18. The decline in donor seroprevalence rates may be explained by a combination of successful vector control along with increasing urbanization of the population in Brazil, such that younger donors have not lived in rural settings with an increased risk of exposure.
The lack of observed seroconversions in our repeat donors, combined with the dramatic decline in prevalence in younger first-time donors, suggests that the acquisition of new T cruzi infection in Brazil is rare. The data also imply that travel to Brazil is very unlikely to result in T cruzi infection, and therefore indicate that there is no justification for other countries to defer potential donors who travel to urban areas in Brazil. Furthermore, in countries such as the US with a selective T cruzi antibody testing policy, there is no reason to retest donors following travel or even prolonged residence in urban areas of Brazil.
Our data also indicate a very low risk of T cruzi window phase transmission within Brazil. However, residual risk could exist if low-level reactive samples that are not consistently detected by the primary screening EIAs represent cases of active parasitemic infection with weak antibody responses. However, we believe that most such cases represent resolved infections and evolving seroreversion, as has been reported by others among the control group arm of benzonidazole clinical trials 19,20. Cases of transfusion transmitted T cruzi infection in Brazil have been exceedingly rare since screening procedures were made mandatory in the 70s. Recently, Souza et al described one case of transmission of Chagas disease to a liver transplant recipient.21 The donor tested negative for Chagas antibodies by IFA. However, a sample from the organ donor was not available for further testing to be determine whether it was from a low level antibody-reactive individual or was due to an error in performing the IFA (the only test used for screening in this case). Further studies including performance of sensitive PCR on follow-up samples, are warranted to address these issues. Recently, Cooley and coauthors have cloned and screened 400 new proteins for diagnostic purposes, and it is possible that these new antigens may help in improving serodiagnosis of Chagas disease and will allow discrimination of active from resolved infections22.
In conclusion, we have proposed a classification algorithm that may have practical importance for donor counseling and for epidemiological analyses of Chagas disease. Importantly, we believe the sensitivity of T cruzi assays should be defined based on their capacity to detect both low- and high-level reactive true T cruzi seroreactive samples, and not samples that are cross-reacting to other parasitic infections. Such borderline-reactive samples are frequent, and were responsible for all our of the apparent incident donor cases in our study. It is important to continue to study donors with borderline reactivity, including determination if this group includes cases that are parasitemic for T cruzi by PCR analysis, and thus represent active infections that would drive the development of new screening assays to consistently interdict these donations.
Acknowledgments
This work was supported by NHLBI contract HHSN268200417175C
Footnotes
Conflict of Interest: no conflict of interest.
References
- 1.Gomes YM, Lorena VM, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):115–21. doi: 10.1590/s0074-02762009000900017. [DOI] [PubMed] [Google Scholar]
- 2.Otani MM, Vinelli E, Kirchhoff LV, et al. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49:1076–82. doi: 10.1111/j.1537-2995.2009.02107.x. [DOI] [PubMed] [Google Scholar]
- 3.Leiby DA, Herron RM, Jr, Garratty G, Herwaldt BL. Trypanosoma cruzi parasitemia in US blood donors with serologic evidence of infection. J Infect Dis. 2008;198:609–13. doi: 10.1086/590159. [DOI] [PubMed] [Google Scholar]
- 4.Britto CC. Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations. Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):122–35. doi: 10.1590/s0074-02762009000900018. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez JD, Guhl F, Umezawa ES, et al. Evaluation of adult chronic Chagas’ heart disease diagnosis by molecular and serological methods. J Clin Microbiol. 2009;47:3945–51. doi: 10.1128/JCM.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umezawa ES, Luquetti AO, Levitus G, et al. Serodiagnosis of chronic and acute Chagas’ disease with Trypanosoma cruzi recombinant proteins: results of a collaborative study in six Latin American countries. J Clin Microbiol. 2004;42:449–52. doi: 10.1128/JCM.42.1.449-452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CD, Cheng KY, Jiang LX, et al. Evaluation of a prototype Trypanosoma cruzi antibody assay with recombinant antigens on a fully automated chemiluminescence analyzer for blood donor screening. Transfusion. 2006;46:1737–44. doi: 10.1111/j.1537-2995.2006.00965.x. [DOI] [PubMed] [Google Scholar]
- 8.Umezawa ES, Bastos SF, Camargo ME, et al. Evaluation of recombinant antigens for serodiagnosis of Chagas’ disease in South and Central America. J Clin Microbiol. 1999;37:1554–60. doi: 10.1128/jcm.37.5.1554-1560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caballero ZC, Sousa OE, Marques WP, et al. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin Vaccine Immunol. 2007;14:1045–9. doi: 10.1128/CVI.00127-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho MR, Krieger MA, Almeida E, et al. Chagas’ disease diagnosis: evaluation of several tests in blood bank screening. Transfusion. 1993;33:830–4. doi: 10.1046/j.1537-2995.1993.331094054620.x. [DOI] [PubMed] [Google Scholar]
- 11.Coura JR, Fernandes O, Arboleda M, et al. Human infection by Trypanosoma rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 1996;90:278–9. doi: 10.1016/s0035-9203(96)90247-3. [DOI] [PubMed] [Google Scholar]
- 12.Remesar MC, Gamba C, Colaianni IF, et al. Estimation of sensitivity and specificity of several Trypanosoma cruzi antibody assays in blood donors in Argentina. Transfusion. 2009;49:2352–8. doi: 10.1111/j.1537-2995.2009.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salles NA, Sabino EC, Cliquet MG, et al. Risk of exposure to Chagas’ disease among seroreactive Brazilian blood donors. Transfusion. 1996;36:969–73. doi: 10.1046/j.1537-2995.1996.36111297091740.x. [DOI] [PubMed] [Google Scholar]
- 14.Carneiro-Proietti AB, Sabino EC, Sampaio D, et al. Demographic profile of blood donors at three major Brazilian blood centers: results from the International REDS-II study, 2007 to 2008. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAS Institute I. SAS/STAT Cary North Carolina. 2004. [Google Scholar]
- 16.Remessar MC, Sabino EC, Gamba C, Busch MP, Tobler LH, Puppo M, Ridolfi MA, Kupperman S, Del Pozo AE. Significance of Inconclusive Results on T. Cruzi Antibody Assays in Blood Donors from a Highly Endemic Region of Argentina. Transfusion. 2009;49 (Suppl):231a–2a. [Google Scholar]
- 17.Leiby DA, Wendel S, Takaoka DT, et al. Serologic testing for Trypanosoma cruzi: comparison of radioimmunoprecipitation assay with commercially available indirect immunofluorescence assay, indirect hemagglutination assay, and enzyme-linked immunosorbent assay kits. J Clin Microbiol. 2000;38:639–42. doi: 10.1128/jcm.38.2.639-642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104 (Suppl 1):31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- 19.de Andrade AL, Zicker F, de Oliveira RM, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–13. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 20.Viotti R, Vigliano C, Lococo B, et al. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144:724–34. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- 21.Souza FF, Castro ESO, Marin Neto JA, et al. Acute chagasic myocardiopathy after orthotopic liver transplantation with donor and recipient serologically negative for Trypanosoma cruzi: a case report. Transplant Proc. 2008;40:875–8. doi: 10.1016/j.transproceed.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Cooley G, Etheridge RD, Boehlke C, et al. High throughput selection of effective serodiagnostics for Trypanosoma cruzi infection. PLoS Negl Trop Dis. 2008;2:e316. doi: 10.1371/journal.pntd.0000316. [DOI] [PMC free article] [PubMed] [Google Scholar]