Abstract
Immunization with the Yersinia pestis F1 and LcrV proteins improves survival in mouse and non-human primate models of pneumonic plague. F1- and LcrV-specific antibodies contribute to protection, however, the mechanisms of antibody-mediated defense are incompletely understood and serum antibody titers do not suffice as quantitative correlates of protection. Previously we demonstrated roles for tumor necrosis factor-alpha (TNFα) and gamma-interferon (IFNγ) during defense against conditionally attenuated pigmentation (pgm) locus-negative Y. pestis. Here, using intranasal challenge with fully virulent pgm-positive Y. pestis strain CO92, we demonstrate that neutralizing TNFα and IFNγ interferes with the capacity of therapeutically administered F1- or LcrV-specific antibody to reduce bacterial burden and increase survival. Moreover, using Y. pestis strain CO92 in an aerosol challenge model, we demonstrate that neutralizing TNFα and IFNγ interferes with protection conferred by immunization with recombinant F1-LcrV fusion protein vaccine (p<0.0005). These findings establish that TNFα and IFNγ contribute to protection mediated by pneumonic plague countermeasures targeting F1 and LcrV, and suggest that an individual’s capacity to produce these cytokines in response to Y. pestis challenge will be an important co-determinant of antibody-mediated defense against pneumonic plague.
Keywords: Yersinia pestis, vaccine, antibody, cytokine
INTRODUCTION
Yersinia pestis causes plague, one of the world’s most deadly infectious diseases. Fleabite transmission of Y. pestis from infected rodents to humans causes bubonic and septicemic plague [1–5]. Occasionally humans develop secondary pulmonary infections. This pneumonic form of plague is nearly always fatal and can spread from person to person via infectious respiratory droplets [6, 7]. There is no licensed vaccine for pneumonic plague.
Natural outbreaks of pneumonic plague are uncommon today, but there is significant concern that Y. pestis could be used as an airborne bioweapon. Indeed, antibiotic-resistant strains of Y. pestis are known to exist, and Cold War scientists developed the technology to aerosolize large quantities of Y. pestis [5, 6, 8]. Accordingly, substantial research effort and financial investment have been devoted to the development of vaccines and other countermeasures for pneumonic plague.
Human clinical trials are underway for subunit vaccines comprised of the Y. pestis F1 and LcrV proteins [9–11]. These vaccines provide mice [12, 13] and cynomolgus macaques [14, 15] with robust protection from aerosolized Y. pestis. Purposefully challenging humans with Y. pestis is unethical. Thus, the licensure of these F1/LcrV-based vaccines, and other pneumonic plague countermeasures, will be based solely on safety data from human trials and efficacy data from animal models [16]. For products licensed in this manner, the prescribed doses and treatment regimens for humans must be extrapolated from data generated in the animal models [17, 18]. Confidence in the accuracy of such extrapolations should be bolstered by a comprehensive understanding of the mechanisms of protection in the animal models.
Antibodies play key roles in the protection mediated by F1/LcrV-based vaccines. Passively immunizing mice with F1- or LcrV-specific monoclonal antibodies (mAb) confers protection from pulmonary Y. pestis challenge [19–22], and serum titers of F1 and LcrV antibody generally correlate with protection in mouse and non-human primate models [11, 23]. However, serum antibody titers do not suffice to predict efficacy in all models [18, 23, 24]. For example, immunizing mice with live attenuated Salmonella expressing Y. pestis LcrV confers protection against plague that does not correlate with LcrV antibody titers [25]. Moreover, immunizing African green monkeys with recombinant F1-LcrV fusion protein (rF1V) confers incomplete protection against aerosolized Y. pestis and the level of protection does not reliably correlate with either F1 or LcrV antibody titers [14, 15]. Given that overall antibody titers do not always suffice as correlates of protection, a number of other correlate assays have been proposed: serum from immunized animals and humans can be titered based on its capacity to (i) passively transfer protection to naïve mice, (ii) compete with a protective LcrV-specific mAb in ELISA, (iii) suppress Yersinia-induced macrophage cytotoxicity in vitro, (iv) reduce translocation of virulence factors via the LcrV-dependent type III secretion system, and (v) promote phagocytosis [11, 15, 24, 26–30]. While each of these assays can predict vaccine efficacy in specific animal models, none have been shown to suffice as robust correlates of protection across the various rodent and non-human primate models.
Correlates based solely on measurement of antibody function may not suffice if antibody-independent mechanisms also contribute to vaccine-mediated defense against plague, particularly if the extent to which other mechanisms contribute is variable among animal models and humans [18, 23, 31]. In prior studies, we demonstrated roles for cytokine products of cellular immunity during antibody-mediated defense against plague [32, 33]. In a mouse model using intranasal inoculation with conditionally attenuated pigmentation locus (pgm)-negative Y. pestis as challenge, we demonstrated TNFα and IFNγ contribute to passive protection conferred by therapeutic administration of F1 and LcrV-specific mAb [33]. Here, we extend those findings to both passive and active immunization models employing fully virulent pgm-positive Y. pestis as challenge. Together, the data decisively demonstrate that cytokines play key roles during F1/LcrV-targeted defense in multiple models of fully virulent pneumonic plague.
MATERIALS AND METHODS
Mice
Wild type C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were cared for according to the Institutional Animal Care and Use Committee guidelines of the Public Health Research Institute (PHRI) and United States Army Medical Research Institute of Infectious Diseases (USAMRIID). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animal experiments and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996. The facilities where this research was conducted are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Bacteria
All studies used fully virulent pgm-positive Y. pestis strain CO92 for challenge. The intranasal challenge study was performed at PHRI and the aerosol challenge study was performed at USAMRIID. Detailed methods for challenging intranasally [34] and by whole-body aerosol [35] have been described previously.
Passive immunization
Hybridoma clones F1–04-A-G1 and 7.3 producing F1- and LcrV-specific mAb, respectively, were described previously [19, 33, 36]. The mAb produced by these hybridomas were purified using Protein G agarose. They contained less than 2.2 units per mg endotoxin as measured by Limulus Amebocyte Lysate assay. For passive therapy, mAb were diluted in phosphate buffered saline (PBS) and administered intraperitoneally on the day following infection at the doses listed in the figure legends.
Active immunization
The rF1V vaccine was purified by a two-step chromatographic process as described [35] and 2.9 ug rF1V was administered subcutaneously along with 500 ug of Alhydrogel (Brenntag Biosector, Denmark) in a total volume of 0.2 ml. Booster immunizations were administered 28 days later. Serum samples were collected just prior to the booster immunizations and again on day 49. Serum antibody titers were measured by ELISA as described previously [35]. On day 54, mice were challenged with 11 LD-50 aerosolized Y. pestis strain CO92 [35].
Cytokine neutralization
When indicated, animals were treated with 1 mg neutralizing mAb XT3.11 specific for TNFα and 600 µg neutralizing mAb XMG1.2 specific for murine IFNγ [33]. The mAb were diluted in PBS and administered intraperitoneally. Control mice received equal quantities of isotype-matched mAb of irrelevant specificity (rat immunoglobulin G1, clone HRPN). The purified mAb were supplied by Bio X Cell (West Lebanon, NH), who reported endotoxin levels less than 1.7 units per mg.
Survival endpoints and bacterial burden
In all survival studies, recumbent animals were considered moribund and euthanized. For measurement of bacterial burden, mice were euthanized by carbon dioxide narcosis on day 2 after initiating infection. Liver lobes and whole lungs were harvested aseptically and homogenized for one minute using an IKA® ULTRA-TURRAX® Disperser Workstation System (IKA Works, Inc., Wilmington NC) in 5 ml of sterile PBS. The tissue homogenates were serially diluted in sterile PBS and plated in duplicate on Congo Red Agar. Colony forming units (CFU) were counted after 72h of growth at 30°C.
Statistics
Survival data were analyzed by Log-rank tests and CFU data were analyzed by ANOVA (Kruskal-Wallis with Dunn’s multiple comparison test) or Mann Whitney, as indicated (Prism 4.0, GraphPad Software). For presentation and assessment of statistical significance, CFU measurements that fell below the detection limit of our assays were assigned log10 values of 2.2 (lung) or 1.2 (liver). Log10 transformed antibody titers were compared using Students t-test.
RESULTS
Cytokines contribute to protection conferred by F1- and LcrV-specific mAb
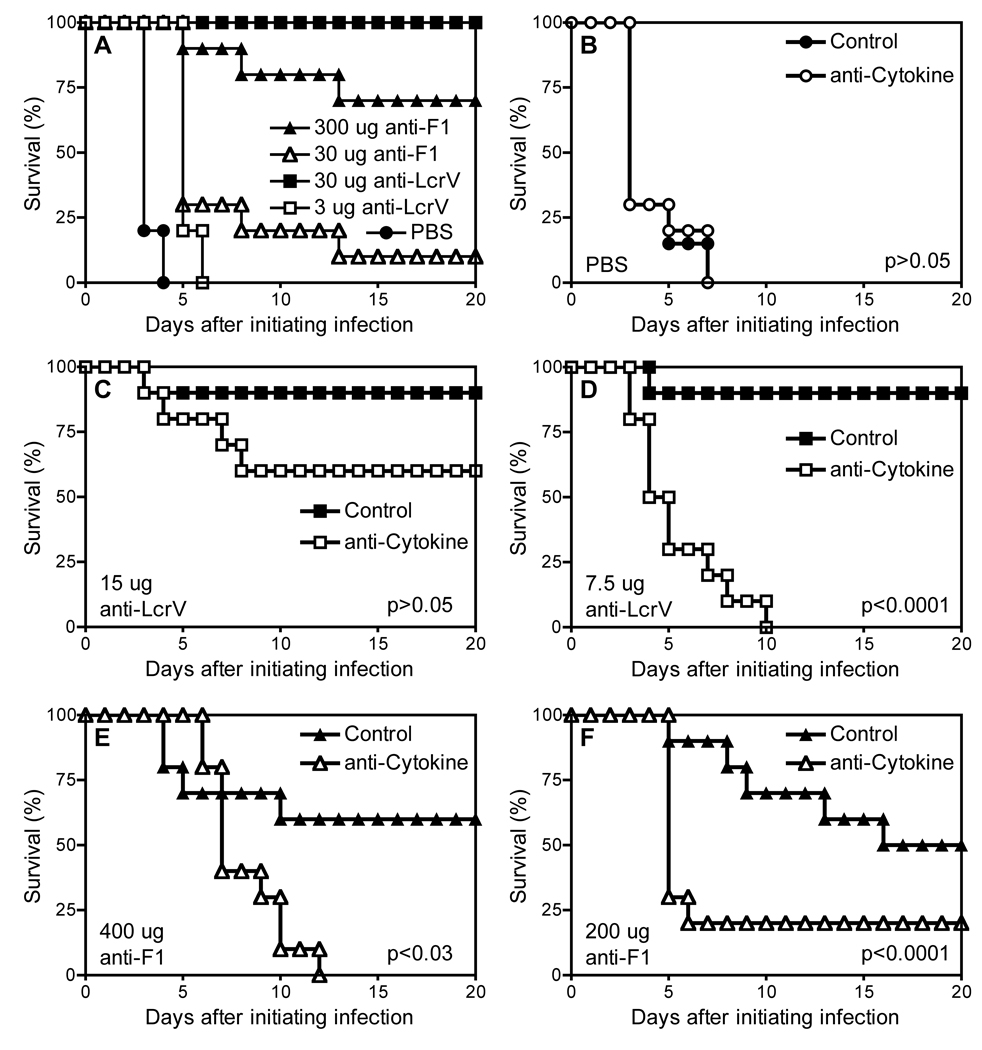
Prior studies established that passive transfer of mAb specific for F1 or LcrV confers mice with protection against pulmonary challenge with virulent Y. pestis [19–22]. Using a mouse model employing conditionally attenuated pgm-negative Y. pestis strain D27 as challenge we recently demonstrated that neutralizing the cytokines TNFα and IFNγ abrogates protection conferred by therapeutic administration of F1- or LcrV-specific mAb, particularly when the mAb are administered at suboptimal dosages [33]. Figure 1 and Figure 2 demonstrate that cytokines also contribute to the protection conferred by passive immunotherapy in a mouse model employing fully virulent pgm-positive Y. pestis as challenge. First, we identified doses of F1- and LcrV-specific mAb that confer measurable protection (Figure 1A). Wild type C57BL/6 mice were infected intranasally with 12 LD-50 pgm-positive Y. pestis strain CO92. The next day, cohorts of mice were treated with F1-specific mAb F1–04-A-G1, LcrV-specific mAb 7.3, or PBS as control. The highest treatment dose of F1-specific mAb (300 ug) was suboptimal, as it only protected 70% of the mice from mortality. In contrast, 30 ug LcrV-specific mAb conferred full protection. Even 3 ug of LcrV-specific mAb significantly increased the time to mortality, although it failed to save any mice from lethal disease.
Figure 1. TNFα and IFNγ contribute to the increased survival mediated by mAb specific for F1 or LcrV after pulmonary exposure to fully virulent pgm-positive Y. pestis.
Wild-type C57BL/6 mice were infected intranasally with 12 LD-50 Y. pestis strain CO92. (A) The following day, they received intraperitoneal injections of PBS or the indicated doses of LcrV- or F1-specific mAb (n= 10 mice/group). In a subsequent study, the infected mice received (B) PBS vehicle control, (C) 15 ug LcrV-specific mAb, (D) 7.5 ug LcrV-specific mAb, (E) 400 µg F1-specific mAb, or (F) 200 µg F1-specific mAb. This time, the injections also included neutralizing mAb specific for the cytokines TNFα and IFNγ (anti-Cytokine; open symbols) or isotype-matched control mAb (Control; closed symbols). In comparison with mice treated with control mAb, mice treated with suboptimal LcrV or F1-specific mAb along with cytokine-neutralizing mAb (D and F, respectively) exhibited significantly reduced survival (both p<0.0001 by Log rank test; n = 10 mice per group).
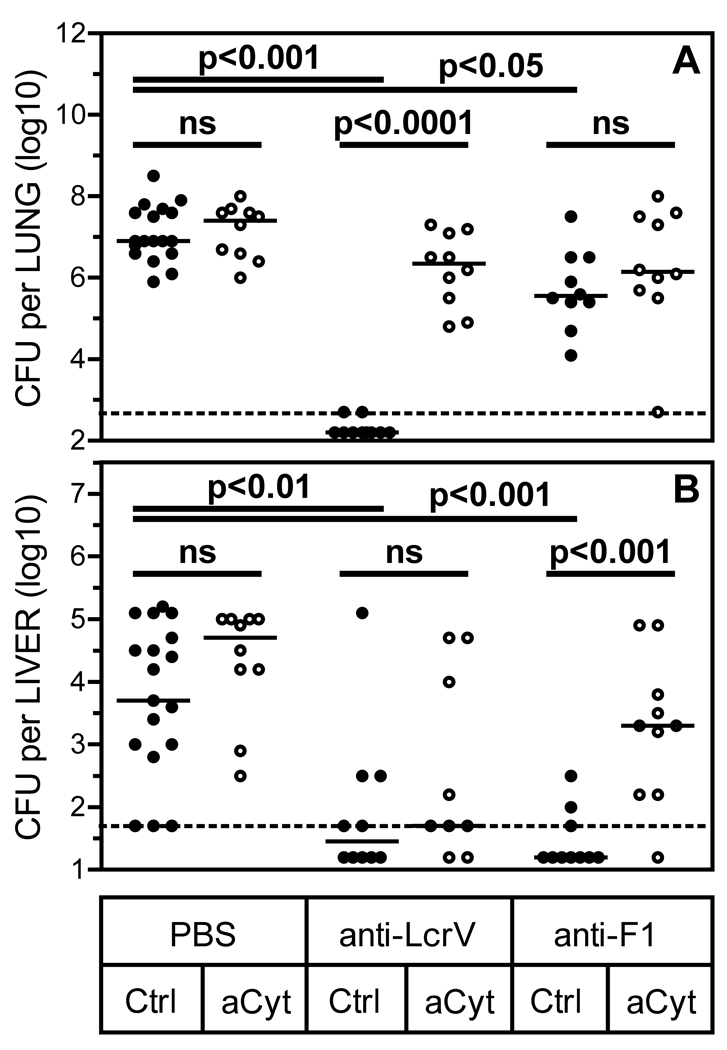
Figure 2. TNFα and IFNγ contribute to the reduced bacterial burden mediated by mAb specific for F1 or LcrV after pulmonary exposure to fully virulent pgm-positive Y. pestis.
Bacterial burden in lung (A) and liver (B) tissue were measured in cohorts of mice (n=10 per group) that received treatments in parallel with those analyzed for survival in Figure 1. The measurements were made one day after injection of mAb (i.e. on day 2 after infection with Y. pestis). In comparison with mice treated only with control (Ctrl) mAb, mice treated with control mAb and 200 ug F1-specific mAb or 7.5 ug LcrV-specific mAb showed significantly reduced pulmonary and hepatic burden (all p<0.05 by ANOVA). Administration of neutralizing mAb specific for TNFα and IFNγ (aCyt) increased pulmonary burden in mice treated with LcrV-specific mAb and increased hepatic burden in mice treated with F1-specific mAb (p<0.0001 and p<0.001, respectively by Mann-Whitney tests). ns; not significant (i.e. p>0.05).
Next we investigated roles for the cytokines at suboptimal doses of F1- and LcrV-specific mAb. Mice were infected intranasally with 12 LD-50 Y. pestis strain CO92. The next day cohorts were treated with PBS, F1-specific mAb or LcrV-specific mAb. Each cohort was further subdivided; half of the animals received a mixture of mAb that neutralize the activities of TNFα and IFNγ, and the other animals received isotype-matched control mAb. Mice treated with PBS, rather than F1- or LcrV-specific mAb, all succumbed to plague within 7 days of infection, regardless of whether or not they received mAb neutralizing TNFα and IFNγ (Figure 1B). In comparison with PBS-treated animals, mice that received suboptimal doses of F1- or LcrV-specific mAb exhibited markedly improved survival (Figure 1C–F). Neutralizing TNFα and IFNγ significantly reduced the survival conferred by the suboptimal doses F1- or LcrV-specific mAb (Figure 1D/F; both p<0.0001). Neutralizing TNFα and IFNγ also reduced protection conferred by higher doses of F1- or LcrV-specific mAb (Figure 1C/E), but the impact was less dramatic than that observed with lower doses. We conclude that TNFα and IFNγ contribute to antibody-mediated protection against fully virulent pneumonic plague, especially at suboptimal levels of protective antibody.
In tandem with the study described above, parallel cohorts of identically treated mice were euthanized on day 2 after infection so that bacterial burden could be assessed. As shown in Figure 2, therapeutic administration of suboptimal doses of F1- or LcrV-specific mAb significantly reduced the day 2 bacterial burden in both lung and liver tissues. In mice treated with LcrV-specific mAb, neutralization of TNFα and IFNγ significantly increased bacterial burden in the lung, but not the liver. In contrast, cytokine neutralization significantly increased bacterial burden in the liver, but not the lung, when mice were treated with F1-specific mAb. These data highlight the complexity of the mechanisms underlying the contributions of cytokines to antibody-mediated protection, and suggest the mechanisms of protection differ for F1- and LcrV-specific antibody.
Cytokines contribute to protection conferred by immunization with rF1V
The data in Figure 1 and Figure 2 demonstrate important roles for cytokines during protection conferred by mAb specific for F1 or LcrV. Given that F1/LcrV-based subunit vaccines induce production of antibodies that confer protection [19–22], we hypothesized cytokines may contribute to protection conferred by these vaccines. To test this hypothesis, we immunized mice with rF1V, an F1-LcrV fusion protein vaccine that recently entered human trials [10]. Specifically, we immunized C57BL/6 mice subcutaneously with 2.9 ug rF1V in alhydrogel adjuvant, and then administered a booster immunization on day 28. Control mice received adjuvant alone. Serum collected on day 49 revealed rF1V antibody titers averaging 1,700,000 in rF1V-immunized mice and less than 50 in mice that received adjuvant alone (Table 1). On day 53, half of the mice were treated with mAb that neutralize TNFα and IFNγ. The remaining mice received isotype matched control mAb. The next day, all animals were aerosol challenged with 11 LD-50 pgm-positive Y. pestis strain CO92, and then monitored for morbidity for 21 days. As shown in Figure 3, mice that received alhydrogel alone succumbed by day 5 after challenge. In contrast, all mice that received rF1V vaccine survived to at least day 5. Most of the mice that received rF1V and control mAb survived until the end of the experiment (80%), whereas only 10% of the mice that received rF1V and mAb neutralizing TNFα and IFNγ survived until day 21. The observed decrease in survival of rF1V–immunized mice treated with cytokine-neutralizing mAb, as compared with those treated with control mAb, was highly significant (p=0.0004).
Table 1.
Antibody titers of rF1V immunized mice prior to aerosol challenge.
| Groupa | Immunization | rF1V IgG Antibody Titers | Treatment | |
|---|---|---|---|---|
| (Day 0 and 28) | (Day 28) | (Day 49) | (Day 53) | |
| 1. | Alhydrogel only | <50 | <50 | Control mAb |
| 2. | Alhydrogel only | <50 | <50 | Anti-cytokine |
| 3. | Alhydrogel + rF1V | 85,742 | 1,492,853 | Control mAb |
| 4. | Alhydrogel + rF1V | 69,644b | 1,714,838c | Anti-cytokine |
N is equal to 10 mice per group.
The titers of Groups 3 and 4 did not differ significantly after priming (p=0.39).
The titers of Groups 3 and 4 did not differ significantly after boosting (p=0.40).
Figure 3. TNFα and IFNγ contribute to the increased survival mediated by immunization with recombinant F1-V fusion protein after pulmonary exposure to fully virulent pgm-positive Y. pestis.
Wild-type C57BL/6 mice were immunized by subcutaneous administration of 2.9 ug rF1V fusion protein in alhydrogel adjuvant (alum) and boosted 28 days later. Control mice received only alum. On day 53, mice received either intraperitoneal injections of neutralizing mAb specific for TNFα and IFNγ (aCyt; open symbols) or isotype-matched control mAb (Ctrl; closed symbols). The following day all mice were challenged by whole-body aerosol with 11 LD-50 Y. pestis strain CO92. In comparison with rF1V-immunized mice treated with control mAb, rF1V–immunized mice treated with cytokine-neutralizing mAb exhibited significantly reduced survival (p<0.0004 by Log rank test; n = 10 mice per group).
DISCUSSION
Previously, we reported that TNFα and IFNγ contribute to protection against lethal pulmonary Y. pestis infection [32, 33, 37]. Perhaps most importantly, we demonstrated those cytokines contribute to protection mediated by mAb specific for the Y. pestis F1 and LcrV proteins [33]. However, in those prior studies we challenged mice with Y. pestis strain D27, a conditionally attenuated pgm-negative strain. While the intranasal LD-50 for D27 is only 10-fold higher than that reported for fully virulent pgm-positive strains [37], the time to lethality after inoculation with pgm-negative strains is longer than that reported for pgm-positive strains [37, 38]. Moreover, recent studies suggest the disease caused by intranasal inoculation of pgm-negative strains results primarily from sepsis, whereas pgm-positive strains cause both severe pneumonia and sepsis [38].
While our prior studies had clearly documented roles for TNFα and IFNγ during immune defense against pgm-negative Y. pestis [32, 33, 37], this report is the first to extend such findings to challenge models employing fully virulent pgm-positive Y. pestis. Specifically, this report shows TNFα and IFNγ contribute to F1 and LcrV mAb-mediated defense against intranasal challenge with Y. pestis strain CO92 (Figure 1 and Figure 2). In addition, this report demonstrates roles for these cytokines in mice challenged with aerosolized CO92 after immunization with rF1V, a leading F1/LcrV-based vaccine candidate (Figure 3). Notably, CO92 is a member of biovar Orientalis, whereas the strain used in our prior studies (D27) is a derivative of Y. pestis strain KIM, a member of biovar Mediaevalis. Thus, in combination with our prior studies, this report establishes that TNFα and IFNγ participate in defense against multiple Y. pestis biovar, multiple routes of pulmonary infection, studies performed by multiple sets of researchers at distinct institutions using models of both passive and active immunization. Together, the accumulated data decisively demonstrate contributory roles for TNFα and IFNγ in state-of-the-art mouse models of F1/LcrV-targeted defense against pneumonic plague.
Neutralizing TNFα and IFNγ in naïve mice infected with pgm-negative Y. pestis strain D27 was previously reported to increase lethality and bacterial burden [33]. In contrast, this study failed to reveal such impacts in naïve mice infected with pgm-positive Y. pestis strain CO92 (Figure 1, Figure 2, and Figure 3). Thus, despite clear evidence that TNFα and IFNγ play significant roles during F1/LcrV-targeted defense in models employing either D27 or CO92 as challenge, significant roles for cytokines during basal defense against plague are only observed in the D27 model. We speculate this distinction may result from the delayed time to lethality observed after challenge with pgm-negative strains, which presumably provides a greater opportunity for cytokines to participate in basal levels of defense. The pgm locus contains multiple genes that impact virulence [39], as well as ripA, a gene that promotes the survival of Y. pestis with IFNγ activated macrophages in vitro [40]. Moreover, the suppressed early inflammatory response that accompanies infection with pgm-positive Y. pestis [41–43] may delay production of TNFα and IFNγ, thus limiting their opportunity to participate in basal defense against fully virulent strains. Presumably, therapeutics and vaccines that facilitate survival beyond the early anti-inflammatory phase of infection create an opportunity for cytokine production, in turn allowing cytokines to participate in basal defense.
Measurements of bacterial burden revealed another distinction between challenge models employing pgm-negative and positive strains. In a prior study using a pgm-negative strain as challenge, neutralization of TNFα and IFNγ significantly suppressed the F1 and LcrV-specific mAb-mediated reduction in bacterial burden in both lung and liver tissues [33]. In contrast, we observed that cytokine neutralization differentially impacted the lung and liver burden when using a pgm-positive strain as challenge, with cytokines more prominently contributing either to the reduction of pulmonary burden by LcrV-specific mAb or to the reduction of hepatic burden by F1-specific mAb (Figure 2). One notable difference between this report and the prior study using D27 challenge is the time chosen for measuring bacterial burden, which had to be shortened from day 3 for D27 challenge to day 2 for CO92 challenge. This change was necessary since pgm-positive CO92 causes mortality by day 3. Detailed studies of the kinetics of bacterial growth in these models may shed further light on distinctions in the mechanisms underlying F1 and LcrV-specific mAb-mediated protection from pneumonic plague and the means by which cytokines participate in that defense.
A number of groups are working to develop F1/LcrV-targeted vaccines and therapeutics. Clinical trials cannot ethically challenge humans with virulent Y. pestis. Thus, one major hurdle for the licensure of pneumonic plague countermeasures is confidently establishing that any specific immunization or treatment regimen is likely to confer humans with protection. Presumably, licensure of such countermeasures will need to rely upon assays of protection correlates in animal models as tools to suggest prescribing regimen that should be efficacious in humans. To date, correlate assays have focused on antibodies, which clearly contribute to defense mediated by immunization with F1 and/or LcrV. However, serum antibody titers, at least as measured by ELISA, have not sufficed to accurately predict efficacy in primate models [14, 15, 24]. Improved correlate assays of antibody-mediated protection have been suggested [11, 15, 18, 24, 28, 29], but relatively little attention has focused on defining antibody-independent co-correlates of protection for F1/LcrV-targeted vaccines and therapeutics.
Over 50 years ago, Meyer, Jawetz and colleagues reported that immune serum is unable to kill Y. pestis in the absence of phagocytic cells [44, 45]. Nakajima and Brubaker subsequently reported that pretreatment of naïve mice with TNFα and IFNγ, confers protection from the lethal sepsis caused by intravenous inoculation of pgm-negative Y. pestis [46]. Subsequently, Nakajima et al showed that active immunization with recombinant LcrV improves the production of TNFα and IFNγ in mice challenged with pgm-negative Y. pestis [47]. TNFα and IFNγ are known to activate phagocytes, and Oyston and colleagues recently demonstrated that pretreating macrophages with these cytokines restricts the intracellular survival of pgm-positive Y. pestis [48]. In addition, we reported these same cytokines participate in both cellular and humoral defense against pgm-negative Y. pestis, including defense mediated by F1 and LcrV-specific mAb. In combination with studies demonstrating F1 and LcrV-specific antibodies can opsonize Y. pestis bacilli and neutralize its virulence mechanisms [15, 24, 26–30], the accumulating data suggest antibodies and cytokines work together to help phagocytes internalize and destroy plague-causing bacteria.
If cytokines and antibodies co-operate to provide optimal defense against plague, then correlate assays of efficacy for antibody-based countermeasures may need to consider both antibody titers and an individual’s capacity to produce cytokines upon exposure to Y. pestis. However, until this report, cytokines had not been shown to play significant roles during protection conferred by F1/LcrV-based vaccines or therapeutics in models of fully virulent pneumonic plague. Elvin and Williamson had reported that F1/LcrV-immunized STAT4-deficient mice were poorly protected from subcutaneous challenge with virulent Y. pestis [49]. The STAT4-deficient mice produced robust antibody responses to F1 and LcrV, but their splenocytes exhibited a reduced capacity to secrete antigen-specific IFNγ in vitro [49]. These findings suggested type 1 immunity, typically characterized by production of TNFα and IFNγ, may be critical for optimal vaccine-mediated defense against Y. pestis. However, STAT4-deficiency disrupts signaling from the IL-12 receptor, which participates in myriad immune mechanisms [50], so it was not clear that impairments in TNFα and IFNγ production per se contributed to the poor protection observed in that study.
The data reported here indicate that TNFα and IFNγ contribute to F1 and LcrV-specific antibody-mediated defense against pneumonic plague, as well as defense mediated by immunization with an F1/LcrV-based vaccine. As such, attempts to predict the efficacy of antibody-based plague countermeasures in any given animal or human may benefit from consideration of both the titers of antibody and the individuals’ capacities to produce cytokines upon exposure to Y. pestis. We anticipate that algorithms incorporating both the titer of serum antibody and the response of peripheral blood mononuclear cells to Y. pestis may better predict the survival of mammals challenged with plague.
ACKNOWLEDGEMENTS
We wish to acknowledge the technical assistance and expertise of Svetlana Senina, Guillaume Delmas, Enriko Dolgov, Jennifer L. Dankmeyer, Michael M. Wormald, Steven Tobery, Anthony Bassett, Lawrence W. Kummer, and Frank M. Szaba. This research was supported by PHS grants R01-AI061577, U54-AI057158, and the Joint Science and Technology Office/Defense Threat Reduction Agency project number TMTI10049_09_RT_T.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brubaker RR. Yersinia pestis and bubonic plague. The prokaryotes, an evolving electronic resource for the microbiological community. 2000 Available from: http://141.150.157.117:8080/prokPUB/chaprender/jsp/showchap.jsp?chapnum=265.
- 3.Pollitzer R. Plague. Geneva: 1954. [Google Scholar]
- 4.Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. Plague Manual: Epidemiology, Distribution, Surveillance and Control WHO/CDS/CSR/EDC/99.2. World Health Organization; 1999. [Google Scholar]
- 5.Prentice MB, Rahalison L. Plague. Lancet. 2007 Apr 7;369(9568):1196–1207. doi: 10.1016/S0140-6736(07)60566-2. [DOI] [PubMed] [Google Scholar]
- 6.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000 May 3;283(17):2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 7.Kool JL. Risk of person-to-person transmission of pneumonic plague. Clin Infect Dis. 2005 Apr 15;40(8):1166–1172. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 8.Dennis DT. Plague as a biological weapon. In: Fong IW, Alibek K, editors. Bioterrorism and infectious agents: a new dilemma for the 21st century: Springer Science. 2005. pp. 37–64. [Google Scholar]
- 9.Williamson ED, Flick-Smith HC, Lebutt C, Rowland CA, Jones SM, Waters EL, et al. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005 Jun;73(6):3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SR. Development of a recombinant vaccine against aerosolized plague. Vaccine. 2007 Apr 20;25(16):3115–3117. doi: 10.1016/j.vaccine.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Williamson ED. Plague. Vaccine. 2009 Nov 5;(27) Suppl 4:D56–D60. doi: 10.1016/j.vaccine.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Heath DG, Anderson GW, Jr, Mauro JM, Welkos SL, Andrews GP, Adamovicz J, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998;16(11–12):1131–1137. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones SM, Day F, Stagg AJ, Williamson ED. Protection conferred by a fully recombinant sub-unit vaccine against Yersinia pestis in male and female mice of four inbred strains. Vaccine. 2000;19(2–3):358–366. doi: 10.1016/s0264-410x(00)00108-0. [DOI] [PubMed] [Google Scholar]
- 14.Pitt ML. Animals Models and Correlates of Protection for Plague Vaccines Workshop. Gaithersburg, MD: 2004. Oct 13–14, Non-human primates as a model for pneumonic plague. http://www.fda.gov/cber/minutes/plague101304t.pdf, editor. [Google Scholar]
- 15.Bashaw J, Norris S, Weeks S, Trevino S, Adamovicz JJ, Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol. 2007 May;14(5):605–616. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdy M. The "Animal Rule". Washington, DC. 5th Annual Biodefense Vaccines & Therapeutics Policy, Funding, and Development Conference.2007. [Google Scholar]
- 17.New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible, final rule. Federal Register. 2002;67:37988–37998. [PubMed] [Google Scholar]
- 18.Williamson ED, Duchars MG, Kohberger R. Predictive models and correlates of protection for testing biodefence vaccines. Expert Rev Vaccines. 2010 May;9(5):527–537. doi: 10.1586/erv.10.22. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GW, Jr, Worsham PL, Bolt CR, Andrews GP, Welkos SL, Friedlander AM, et al. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am J Trop Med Hyg. 1997 Apr;56(4):471–473. doi: 10.4269/ajtmh.1997.56.471. [DOI] [PubMed] [Google Scholar]
- 20.Green M, Rogers D, Russell P, Stagg AJ, Bell DL, Eley SM, et al. The SCID/Beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol Med Microbiol. 1999;23(2):107–113. doi: 10.1111/j.1574-695X.1999.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 21.Hill J, Copse C, Leary S, Stagg AJ, Williamson ED, Titball RW. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect Immun. 2003 Apr;71(4):2234–2238. doi: 10.1128/IAI.71.4.2234-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J, Eyles JE, Elvin SJ, Healey GD, Lukaszewski RA, Titball RW. Administration of antibody to the lung protects mice against pneumonic plague. Infect Immun. 2006 May;74(5):3068–3070. doi: 10.1128/IAI.74.5.3068-3070.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008 Mar;7(2):209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson ED, Flick-Smith HC, Waters E, Miller J, Hodgson I, Le Butt CS, et al. Immunogenicity of the rF1+rV vaccine for plague with identification of potential immune correlates. Microb Pathog. 2007 Jan;42(1):11–21. doi: 10.1016/j.micpath.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Garmory HS, Griffin KF, Brown KA, Titball RW. Oral immunisation with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine. 2003;21(21–22):3051–3057. doi: 10.1016/s0264-410x(03)00112-9. [DOI] [PubMed] [Google Scholar]
- 26.Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect Immun. 2005 Sep;73(9):6127–6137. doi: 10.1128/IAI.73.9.6127-6137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeks S, Hill J, Friedlander A, Welkos S. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb Pathog. 2002;32(5):227–237. doi: 10.1006/mpat.2002.0498. [DOI] [PubMed] [Google Scholar]
- 28.Zauberman A, Cohen S, Levy Y, Halperin G, Lazar S, Velan B, et al. Neutralization of Yersinia pestis-mediated macrophage cytotoxicity by anti-LcrV antibodies and its correlation with protective immunity in a mouse model of bubonic plague. Vaccine. 2008 Mar 20;26(13):1616–1625. doi: 10.1016/j.vaccine.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 29.Welkos S, Norris S, Adamovicz J. Modified caspase-3 assay indicates correlation of caspase-3 activity with immunity of nonhuman primates to Yersinia pestis infection. Clin Vaccine Immunol. 2008 Jul;15(7):1134–1137. doi: 10.1128/CVI.00091-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisele NA, Anderson DM. Dual-function antibodies to Yersinia pestis LcrV required for pulmonary clearance of plague. Clin Vaccine Immunol. 2009 Dec;16(12):1720–1727. doi: 10.1128/CVI.00333-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiley ST. Immune defense against pneumonic plague. Immunol Rev. 2008;225:256–271. doi: 10.1111/j.1600-065X.2008.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parent MA, Wilhelm LB, Kummer LW, Szaba FM, Mullarky IK, Smiley ST. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect Immun. 2006 Jun;74(6):3381–3386. doi: 10.1128/IAI.00185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kummer LW, Szaba FM, Parent MA, Adamovicz JJ, Hill J, Johnson LL, et al. Antibodies and cytokines independently protect against pneumonic plague. Vaccine. 2008;26:6901–6907. doi: 10.1016/j.vaccine.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chattopadhyay A, Park S, Delmas G, Suresh R, Senina S, Perlin DS, et al. Single-dose, virus-vectored vaccine protection against Yersinia pestis challenge: CD4+ cells are required at the time of challenge for optimal protection. Vaccine. 2008 Nov 25;26(50):6329–6337. doi: 10.1016/j.vaccine.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amemiya K, Meyers JL, Rogers TE, Fast RL, Bassett AD, Worsham PL, et al. CpG oligodeoxynucleotides augment the murine immune response to the Yersinia pestis F1-V vaccine in bubonic and pneumonic models of plague. Vaccine. 2009 Apr 6;27(16):2220–2229. doi: 10.1016/j.vaccine.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Hill J, Leary SE, Griffin KF, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997;65(11):4476–4482. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005 Nov;73(11):7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee-Lewis H, Anderson DM. Absence of inflammation and pneumonia during infection with nonpigmented Yersinia pestis reveals a new role for the pgm locus in pathogenesis. Infect Immun. 2010 Jan;78(1):220–230. doi: 10.1128/IAI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fetherston JD, Kirillina O, Bobrov AG, Paulley JT, Perry RD. The yersiniabactin transport system is critical for the pathogenesis of bubonic and pneumonic plague. Infect Immun. 2010 May;78(5):2045–2052. doi: 10.1128/IAI.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pujol C, Grabenstein JP, Perry RD, Bliska JB. Replication of Yersinia pestis in interferon gamma-activated macrophages requires ripA, a gene encoded in the pigmentation locus. Proc Natl Acad Sci USA. 2005 Sep 6;102(36):12909–12914. doi: 10.1073/pnas.0502849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci USA. 2005 Dec 6;102(49):17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007 Jan 26;315(5811):509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- 43.Bubeck SS, Cantwell AM, Dube PH. Delayed inflammatory response to primary pneumonic plague occurs in both outbred and inbred mice. Infect Immun. 2007 Feb;75(2):697–705. doi: 10.1128/IAI.00403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jawetz E, Meyer KF. Studies on plague immunity in experimental animals. II. Some factors of the immunity mechanism in bubonic plague. J Immunol. 1944;49:15–30. [Google Scholar]
- 45.Meyer KF. Immunity in plague: a critical consideration of some recent studies. J Immunol. 1950;64:139–163. [PubMed] [Google Scholar]
- 46.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61(1):23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakajima R, Motin VL, Brubaker RR. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63(8):3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, Oyston PC. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun. 2005 Nov;73(11):7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elvin SJ, Williamson ED. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb Pathog. 2004 Oct;37(4):177–184. doi: 10.1016/j.micpath.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003 Feb;3(2):133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]