Abstract
Worsening renal function (RF) and improved RF during the treatment of decompensated heart failure have traditionally been thought of as hemodynamically distinct events. We hypothesized that if pulmonary artery catheter derived measures are relevant in the evaluation of cardiorenal interactions comparison of patients with improved vs. worsening RF should highlight any important hemodynamic differences. All subjects in the ESCAPE trial limited data set with admission and discharge creatinine values available were included (401 patients). There were no differences in baseline, final, or change in pulmonary artery catheter derived hemodynamic variables, inotrope and intravenous vasodilator use, or survival between patients with improved and worsening RF (p=NS for all). Both groups were equally likely to be in the bottom quartile of cardiac index (CI) (p=0.32), have a 25% improvement in CI (p=0.97), or have any worsening in CI (p=0.90). When patients with any significant change in renal function (positive or negative) were compared to patients with stable renal function, strong associations between variables such as reduced CI (OR=2.2, p=0.02), increased intravenous inotrope and vasodilator use (OR=2.9, p<0.001), and worsened all cause mortality (HR=1.8, p=0.01) became apparent. Contrary to traditionally held views, patients with improved RF and worsening RF have similar hemodynamic parameters and outcomes. Combining these groups identifies a hemodynamically compromised population with significantly worse survival than patients with stable renal function. In Conclusion, changes in renal function, regardless of direction, likely identify a population with an advanced disease state and poor prognosis.
Keywords: Cardio-renal syndrome, worsening renal function, improved renal function, acute heart failure, kidney
Worsening renal function (RF) complicates approximately one third of acute decompensated heart failure admissions and has been associated with increased length of stay, readmission rate, and decreased short and long term survival (1-4). Traditional teaching has held that the hemodynamic profile associated with worsening RF is that of decreased cardiac output and intravascular volume depletion, concepts which have not born out in recent publications (5-7). Likely arising from similar logic, improved renal function RF has been suggested to result from a treatment induced increase in “forward flow” to the kidney. Since the current literature regarding the profile of patients developing worsening RF has been largely inconsistent with previously proposed mechanisms, we hypothesized that this may also be true of improved RF. Our first aim was to directly compare patients with improved RF to those with worsening RF. Since the hemodynamic changes offered to explain worsening RF and improved RF are largely discordant we felt this would optimize the probability of documenting any important hemodynamic differences. Additionally, a requisite for improvement in renal function during the treatment of decompensated heart failure is reversible renal dysfunction at baseline. Unless this renal dysfunction was present at birth, by definition this indicates that worsening RF had occurred prior to admission. Resultantly, we hypothesized that the outcomes of patients with improved RF and worsening RF may be similar and our second aim was to investigate if any change in renal function, either improved or worsened, may be the prognostically more relevant variable to investigate.
Methods
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) Trial was a National Heart, Lung and Blood Institute (NHLBI) sponsored, randomized, multicenter trial of therapy guided by pulmonary artery catheter (PAC) vs. clinical assessment in hospitalized patients with acute decompensated heart failure. Methods and results have been previously published (8,9). Briefly, 433 patients were enrolled at 26 sites from January 2000 to November 2003. Inclusion criteria included an ejection fraction of 30% or less, systolic blood pressure of 125 mmHg or less, treatment in the preceding month with >160 mg of furosemide (or equivalent), and at least 1 sign and 1 symptom of congestion(6). Exclusion criteria included an admission creatinine level ≥ 3.5 mg/dL, use of dopamine or dobutamine ≥3 ug/kg/min, or any use of milrinone before randomization. Patients were randomized to therapy guided by clinical assessment alone vs. PAC and clinical assessment. Treatment goals were resolution of the signs and symptoms of congestion. In patients randomized to the PAC arm, additional goals of treatment were a pulmonary capillary wedge pressure ≤15 mmHg and a right atrial pressure ≤8 mmHg. Routine use of inotropes was “explicitly” discouraged but diuretics and vasodilating agents were recommended. The ESCAPE trial was conducted and supported by the NHLBI in collaboration with the ESCAPE study investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the ESCAPE investigators or the NHLBI.
A relative change in GFR was used rather than an absolute change in serum creatinine to account for the non-linear relationship between creatinine and renal function (10). A significant change in renal function, regardless of direction, was defined as a ≥20% change in GFR and patients meeting this criteria were categorized as having Dynamic RF. Patients not meeting this criteria were classified as Stable RF. GFR was estimated using the four variable Modification of Diet in Renal Disease study equation (11). The in-hospital loop diuretic dose represents the maximum total IV loop diuretic dose received in any one day of the study period.
Values reported are mean ± standard deviation for continuous variables and percentile for categorical variables, unless otherwise noted. Independent Student's t-test or the Mann-Whitney U test was used to compare means of independent continuous variables. Pearson's Chi Square was used to evaluate categorical variables. Trend analysis of ordinal variables was done using linear-by-linear association. Wilcoxon Signed Rank tests were used for comparison of GFR over time within worsening RF or improved RF groups. In order to highlight the similarity or dissimilarity between patients with improved RF and worsening RF, these groups were compared directly for the primary analysis. Contingent upon satisfying our hypothesis that the groups would be similar hemodynamically, the improved RF and worsening RF groups were combined and compared to patients with no change in renal function. Cox proportional hazard modeling was used to evaluate the univariate hazard ratio of predictors of mortality and rehospitalization. Patients alive or not rehospitalized at 180 days, respectively, were censored. Cox proportional modeling was used to test the independence of renal variables in their association with mortality. Candidate variables for multivariate Cox proportional modeling were obtained via entry of all univariate baseline predictors of mortality with a p<0.2 and ≤5% missing values. Using backwards elimination, starting with the variable with the largest p-value, variables altering the hazard ratio (HR) by more than 10% were retained in the final model. Statistical analysis was performed with SPSS version 17.0 (SPSS Inc, Chicago, Illinois) and significance defined as 2-tailed p<0.05.
Results
Of the 433 patients enrolled in the ESCAPE trial, 401 had admission and discharge serum creatinine values available and these subjects were included in subsequent analysis. Baseline characteristics of the trial population have been previously reported (9). Additionally, Nohria et al. have described the lack of association between PAC derived hemodynamic variables and the development of worsening RF in this population (6). Overall, worsening RF occurred in 21.2% (n=85) and improved RF occurred in 16.2% (n=65) of the population.
Overall, baseline demographics, comorbidities, symptom severity, physical exam, and medication use were similar between patients with improved RF and worsening RF. Notable exceptions were a greater admission systolic blood pressure, an increased prevalence of hypertension, and a higher incidence of suspected ascites in patients with worsening RF (Table 1).
Table1. Baseline characteristics.
| Characteristics | Worsening RF (n=85) |
Improved RF (n=65) |
p | Stable RF (n=251) |
Dynamic RF (n=150) |
p |
|---|---|---|---|---|---|---|
| Age (years) | 55 ± 13 | 58 ± 15 | 0.109 | 56 ± 14 | 56 ± 14 | 0.946 |
| Males | 73% | 85% | 0.087 | 72% | 78% | 0.192 |
| White race | 51% | 60% | 0.251 | 63% | 55% | 0.12 |
| Ischemic etiology of heart failure | 55% | 47% | 0.342 | 47% | 51% | 0.447 |
| Hypertension | 50% | 31% | 0.022* | 50% | 42% | 0.108 |
| Diabetes mellitus | 39% | 25% | 0.067 | 32% | 33% | 0.799 |
| Myocardial infarction | 48% | 42% | 0.511 | 43% | 45% | 0.663 |
| Coronary bypass grafting | 33% | 25% | 0.272 | 30% | 30% | 0.908 |
| Gout | 17% | 22% | 0.423 | 18% | 19% | 0.883 |
| Dyspnea at rest | 53% | 59% | 0.5 | 60% | 55% | 0.36 |
| Fatigue at rest | 67% | 72% | 0.49 | 64% | 69% | 0.254 |
| Orthopnea | 87% | 82% | 0.352 | 86% | 85% | 0.714 |
| NYHA class (mean) | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.855 | 3.8 ± 0.4 | 3.9 ± 0.3 | 0.18 |
| Six minute walk (feet) | 417 ± 418 | 428 ± 381 | 0.569 | 416 ± 429 | 422 ± 401 | 0.572 |
| Maximal oxygen consumption (mL/kg/min) | 9.7 ± 1.9 | 10.9 ± 5.0 | 0.831 | 9.9 ± 3.2 | 10.3 ± 3.7 | 0.655 |
| Ejection fraction (%) | 18.3 ± 6.0 | 18.2 ± 6.3 | 0.94 | 20.1 ± 6.6 | 18.3 ± 6.1 | 0.008* |
| Systolic blood pressure (mm Hg) | 110 ± 18 | 101 ± 16 | 0.001* | 106 ± 15 | 106 ± 18 | 0.775 |
| Heart rate (bpm) | 84 ± 16 | 80 ± 16 | 0.111 | 81 ± 14 | 82 ± 16 | 0.569 |
| Respiration rate (breaths/min) | 21 ± 4 | 20± 4 | 0.862 | 21 ± 4 | 20 ± 4 | 0.722 |
| Jugular venous pressure > 12 cm | 25% | 21% | 0.509 | 19% | 23% | 0.294 |
| Rales > 1/3 lung fields | 18% | 11% | 0.241 | 14% | 15% | 0.726 |
| Ascites ≥ moderate | 29% | 14% | 0.036* | 18% | 22% | 0.241 |
| Severe edema | 17% | 11% | 0.322 | 10% | 14% | 0.258 |
| Cool extremities | 14% | 22% | 0.229 | 16% | 18% | 0.684 |
| Medications (baseline) | ||||||
| ACE inhibitor / ARB | 89% | 89% | 0.972 | 90% | 89% | 0.721 |
| β-Blocker | 54% | 67% | 0.107 | 64% | 60% | 0.423 |
| Loop diuretic (mg)† | 200 (120, 400) |
160 (120, 320) |
0.16 | 200 (100, 320) |
190 (120, 320) |
0.334 |
| Spironolactone | 38% | 32% | 0.664 | 33% | 34% | 0.726 |
| Thiazide diuretic | 16% | 12% | 0.438 | 11% | 14% | 0.33 |
| Medications (in-hospital) | ||||||
| ACE inhibitor / ARB | 93% | 88% | 0.27 | 93% | 91% | 0.345 |
| Loop diuretic (mg) † | 280 (160, 450) |
190 (120, 400) |
0.056 | 200 (100, 340) |
240 (140, 425) |
0.039* |
| Thiazide diuretic | 46% | 27% | 0.017* | 24% | 38% | 0.003* |
| Inotropes | 45% | 55% | 0.221 | 38% | 49% | 0.028* |
| Vasodilators | 37% | 43% | 0.486 | 22% | 39% | >0.001* |
| Inotropes or vasodilators | 71% | 78% | 0.3 | 50% | 74% | >0.001* |
| Laboratory Findings | ||||||
| Hemoglobin (g/dL) | 12.5 ± 1.8 | 12.6 ± 1.9 | 0.565 | 12.6 ± 1.8 | 12.5 ± 1.8 | 0.748 |
| Serum sodium (mEq/L) | 137 ± 5 | 135 ± 6 | 0.002* | 137 ± 4 | 136 ± 5 | 0.189 |
| Serum creatinine (mg/dL) | 1.27 ± 0.51 | 2.08 ± 0.83 | >0.001* | 1.44 ± 0.49 | 1.62 ± 0.78 | 0.126 |
| Glomerular filtration rate (mL/min) | 69.5 ± 29.5 | 39.9 ± 17.2 | >0.001* | 57.2 ± 23.0 | 56.7 ± 28.9 | 0.837 |
| B-Type Natriuretic peptide (pg/mL) | 1076 ± 1301 | 1309 ± 1953 | 0.48 | 906 ± 1144 | 1174 ± 1605 | 0.209 |
| Norepinephrine (pg/mL) | 547 ± 366 | 977 ± 579 | >0.001* | 660 ± 535 | 721 ± 508 | 0.175 |
ACE: Angiotensin converting enzyme, ARB: Angiotensin receptor blocker, Dynamic RF: ≥ 20% increase or decrease in glomerular filtration rate from admission to discharge, RF: Renal function, NYHA: New York Heart Association, Stable RF: ≤ 20% change in renal function from admission to discharge.
Represents a significant p-value.
Loop diuretic dose reported as median with interquartile range. Baseline loop dose represents daily oral dose prior to hospitalization. In-hospital loop dose represents the maximum IV loop diuretic dose received on any one day of the study. All other values represent mean +/- standard deviation or %.
There were no PAC derived variables that demonstrated any significant discriminative ability between improved RF and worsening RF (Table 2). Contrary to the postulated association between cardiac index and changes in renal function; baseline, final, and admission to final change in cardiac index was no different in patients with improved RF or worsening RF (Table 2). Both groups were equally likely to be in the bottom quartile of cardiac index (p=0.32), have a 25% improvement in cardiac index (p=0.97), or have any worsening in cardiac index during hospitalization (p=0.90). Similarly, baseline, final, and change in right atrial pressure and pulmonary capillary wedge pressure did not differentiate patients with improved RF or worsening RF (Table 2).
Table 2. Pulmonary artery catheter derived parameters at admission, catheter removal, and the associated change.
| Worsening RF |
Improved RF |
Stable RF | Dynamic RF |
|||
|---|---|---|---|---|---|---|
| Characteristics | p | p | ||||
| Hemodynamics (baseline) | (n=41) | (n=42) | (n=99) | (n=83) | ||
| Right atrial pressure (mm Hg) | 13.2 ± 6.3 | 12.7 ± 7.4 | 0.612 | 13.4 ± 10.4 | 12.9 ± 6.8 | 0.664 |
| Pulmonary artery systolic pressure (mm Hg) | 57.4 ± 13.8 | 55.1 ± 12.7 | 0.447 | 54.4 ± 15.7 | 56.2 ± 13.2 | 0.407 |
| Pulmonary capillary wedge pressure (mm Hg) | 26.3 ± 7.1 | 25.4 ± 10.2 | 0.717 | 23.7 ± 9.8 | 25.9 ± 8.8 | 0.219 |
| Cardiac index (L/min/m2) | 1.82 ± 0.63 | 1.91 ± 0.55 | 0.249 | 2.08 ± 0.60 | 1.86 ± 0.59 | 0.019* |
| Systemic vascular resistance (dyn-s/cm5) | 1554 ± 1064 | 1490 ± 679 | 0.97 | 1464 ± 802 | 1522 ± 885 | 0.974 |
| Hemodynamics (PAC removal) | (n=37) | (n=37) | (n=89) | (n=72) | ||
| Right atrial pressure (mm Hg) | 10.5 ± 5.4 | 9.0 ± 5.3 | 0.216 | 8.6 ± 5.5 | 9.7 ± 5.4 | 0.137 |
| Pulmonary artery systolic pressure (mm Hg) | 49.0 ± 12.3 | 46.4 ± 11.8 | 0.381 | 44.6 ± 13.0 | 47.8 ± 12.1 | 0.113 |
| Pulmonary capillary wedge pressure (mm Hg) | 18.5 ± 6.5 | 16.4 ± 7.5 | 0.323 | 17.0 ± 8.2 | 17.4 ± 7.0 | 0.844 |
| Cardiac index (L/min/m2) | 2.26 ± 0.71 | 2.27 ± 0.53 | 0.915 | 2.41 ± 0.65 | 2.26 ± 0.62 | 0.07 |
| Systemic vascular resistance (dyn-s/cm5) | 1091 ± 466 | 1156 ± 488 | 0.746 | 1119 ± 491 | 1126 ± 475 | 0.82 |
| Hemodynamics (change) | (n=36) | (n=35) | (n=85) | (n=68) | ||
| Right atrial pressure (mm Hg) | -2.8 ± 7.4 | -3.9 ± 7.5 | 0.712 | -4.8 ± 10.5 | -3.4 ± 7.4 | 0.547 |
| Pulmonary artery systolic pressure (mm Hg) | -9.6 ± 13.5 | -9.0 ± 11.9 | 0.851 | -9.7 ± 12.4 | -9.3 ± 12.7 | 0.338 |
| Pulmonary capillary wedge pressure (mm Hg) | -8.3 ± 7.1 | -7.5 ± 10.0 | 0.729 | -6.9 ± 9.3 | -7.9 ± 8.6 | 0.526 |
| Cardiac index (L/min/m2) | 0.46 ± 0.69 | 0.47 ± 0.59 | 0.909 | 0.36 ± 0.70 | 0.46 ± 0.63 | 0.092 |
| Systemic vascular resistance (dyn-s/cm5) | -484 ± 1121 | -384 ± 549 | 0.641 | -336 ± 757 | -431 ± 858 | 0.372 |
Dynamic RF: ≥ 20% increase or decrease in glomerular filtration rate from admission to discharge, RF:Renal function, Stable RF: ≤ 20% change in renal function from admission to discharge. Values represent mean +/-standard deviation or %.
Represents a significant p-value.
Overall, treatment characteristics of patients with improved RF and worsening RF were similar. There were no differences in baseline or in hospital medications with the exception of a higher utilization of thiazide diuretics in the group with worsening RF (Table 1). Notably, loop diuretic dosage, intravenous vasodilator use, and inotrope use were similar between both groups (Table 1).
As might be expected by the nature of the dichotomy, renal variables differed substantially between groups. Mean admission glomerular filtration rate (GFR) was significantly lower in the group developing improved RF (39.9 ± 17.2 mL/min vs. 69.5 ± 29.5 mL/min, p<0.001). However, by the time of discharge, GFR was higher in the improved RF group compared to the worsening RF group (57.1 ± 22.2 mL/min vs. 45.7 ± 22.4 mL/min, p=0.002). In patients with 6 month creatinine values available, the group with improved RF had a significant decrease in GFR from discharge to 6 months (59.2 ± 24.6 mL/min vs. 47.5 ± 20.9 mL/min, p<0.001), but GFR remained significantly higher than the admission value (40.8 ± 18.8 mL/min vs. 47.5 ± 20.9 mL/min, p<0.001). From discharge to 6 months, GFR in the worsening RF group improved somewhat (47.7 ± 22.7 mL/min vs. 60.2 ± 29.6 mL/min, p<0.001), but did not recover fully to baseline levels (73.0 ± 29.6 mL/min vs. 60.2 ± 29.6 mL/min, p<0.001).
Patients with improved RF had significantly higher admission norepinephrine levels (Table 1) and were much more likely to have values in the highest quartile at admission compared to patients with worsening RF (OR=6.3, p<0.001). Serum sodium was also lower in the group with improved RF (Table 1). The differences in sodium (p=0.99) and norepinephrine (p=0.38) were no longer significantly different at discharge.
Overall, short and long term outcomes of these groups were also similar with no differences in length of stay (p=0.87) or rehospitalization (p=0.47) between patients with improved RF and worsening RF. Additionally, mortality was similar in the group developing improved RF compared to those with worsening RF (HR=1.1, p=0.85). One notable difference was a significantly greater weight loss (5.4 ± 5.2 kg vs. 2.7 ± 5.0 kg, p=0.002) and higher rate of weight loss (0.75 ± 0.78 kg/day vs. 0.34 ± 0.68 kg/day, p=0.002) in the worsening RF compared to the improved RF group.
Patients with worsening RF had no significant differences in baseline, final, or change in hemodynamic parameters when compared to patients without worsening RF (p=NS for all, data not shown). The group with improved RF shared the same lack of associations when compared to patients without improved RF (p=NS for all, data not shown). As such, analysis of patients with a 20% change in GFR (Dynamic RF), regardless of improvement or deterioration, was undertaken to explore the hypothesis that susceptibility to changes in renal function rather than the actual direction of change may be a more relevant clinical variable.
Compared to patients with stable renal function (Stable RF), patients with Dynamic RF had a significantly lower baseline ejection fraction and cardiac index (Tables 1,2). Additionally, they were more likely to be in the bottom quartile of cardiac index (OR=2.2, p=0.021) or have a 25% improvement in cardiac index from admission to PAC removal (OR=3.3, p=0.001). Other hemodynamic variables were similar (Table 2). Notable differences in treatment characteristics were a significantly greater use of intravenous vasodilators and inotropes in patients with Dynamic RF (Table 1). Moreover, the dose of loop diuretics was greater and adjunctive thiazide diuretics were more commonly used (Table 1). Length of stay was significantly longer in the group with Dynamic RF (9.8 vs. 8.0 days, p<0.001).
The univariate associations between improved RF and mortality (HR=0.16, p=0.11) and between worsening RF and mortality (HR=1.5, p=0.14) were not statistically significant. However, comparison of patients with improved RF or worsening RF to the group with Stable RF demonstrated significant associations with mortality (improved RF HR=1.9, p=0.032; worsening RF HR=1.7, p=0.043). Similarly, the group with Dynamic RF had a substantially increased mortality rate compared to patients with Stable RF (HR=1.8, p=0.01) (Figure 1).
Figure 1.
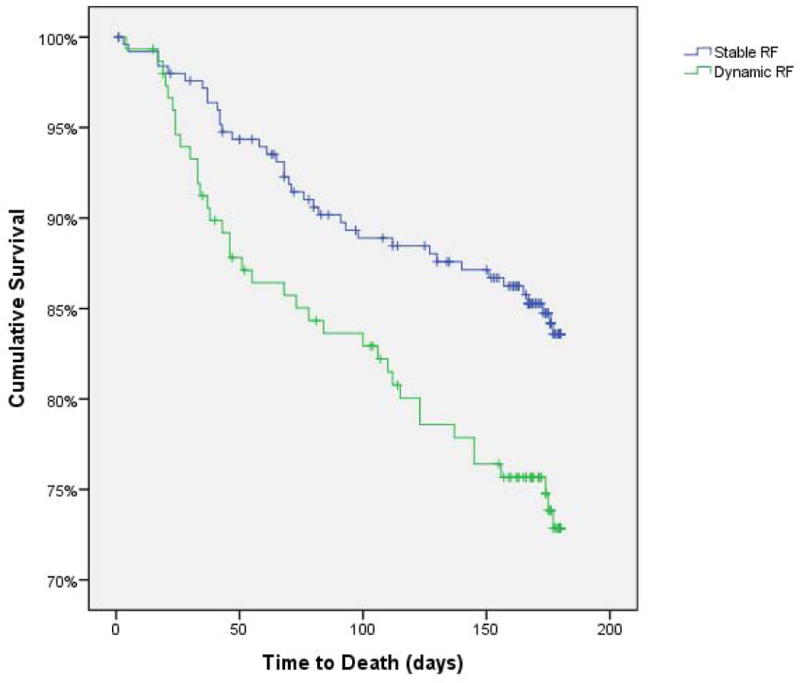
Kaplan-Meier survival curves grouped by Stable RF versus Dynamic RF. Dynamic RF: ≥20% increase or decrease in glomerular filtration rate from admission to discharge, Stable RF: ≤20% change in renal function from admission to discharge.
As previously reported by Nohria et al, both admission and discharge GFR were strong univariate predictors of mortality (6). Despite the relatively strong correlation between admission and discharge GFR (r=0.80, p<0.001), they had discordant effects on the prognostic ability of worsening RF and improved RF. Using Stable RF as the comparison group, controlling for admission GFR caused improved RF to lose its significant association with mortality (HR=1.3, p=0.37) but the relationship between worsening RF and mortality was strengthened (HR=2.2, p=0.004). Similarly, after adjusting for discharge GFR, worsening RF was no longer significantly associated with mortality (HR=1.4, p=0.26), whereas the relationship between improved RF and mortality was unaffected (HR=1.9, p=0.033). Much of this effect is likely explained by the highly significant, but discordant, relationships between improved RF/worsening RF and GFR at admission versus discharge (Table 3). Addition of admission and/or discharge GFR to a model containing Dynamic RF did not eliminate the association between Dynamic RF and mortality (Table 4). After multivariate Cox regression using backwards elimination adjusting for baseline variables (ischemic etiology, history of coronary artery bypass grafting or myocardial infarction, jugular venous distention, ascites, edema, angiotensin converting enzyme inhibitor or receptor blocker use, beta blocker use, loop diuretic dose, thiazide diuretic use, systolic blood pressure, serum sodium, and admission GFR), Dynamic RF remained a significant predictor of mortality (p=0.01).
Table 3. Relationship between changes in renal function and renal function at baseline and discharge.
| Variable | Improved RF |
p | Worsening RF |
p |
|---|---|---|---|---|
| Admission glomerular filtration rate | r = -0.30 | <0.001* | r = 0.26 | <0.001* |
| Discharge glomerular filtration rate | r = 0.05 | 0.302 | r = -0.20 | <0.001* |
| Admission glomerular filtration rate <60 mL/min | OR = 4.4 | <0.001* | OR = 0.33 | <0.001* |
| Discharge glomerular filtration rate <60 mL/min | OR = 0.99 | 0. 961 | OR = 2.0 | 0.010* |
RF: Renal function
Represents a significant p-value.
Table 4. Cox regression results for dynamic renal function and mortality controlling for glomerular filtration rate at admission and discharge.
| Variable | Covariates | SE | HR | p | 95% CI |
|---|---|---|---|---|---|
| Dynamic RF | 0.229 | 1.79 | 0.011* | 1.14-2.81 | |
| Dynamic RF | 0.231 | 1.71 | 0.020* | 1.09-2.69 | |
| Admission GFR† | 0.054 | 0.83 | <0.001* | 0.75-0.92 | |
| Dynamic RF | 0.232 | 1.59 | 0.047* | 1.006-2.499 | |
| Discharge GFR† | 0.059 | 0.81 | <0.001* | 0.73-0.91 | |
| Dynamic RF | 0.232 | 1.67 | 0.027* | 1.06-2.63 | |
| Discharge GFR† | 0.086 | 0.9 | 0.207 | 0.76-1.06 | |
| Admission GFR† | 0.077 | 0.89 | 0.136 | 0.77-1.04 | |
CI: Confidence interval, Dynamic RF: ≥ 20% increase or decrease in estimated glomerular filtration rate, RF: Renal function, SE: Standard error.
Represents a significant p-value.
Hazard ratio (HR) calculated per 10 mL/min increase in glomerular filtration rate.
There appeared to be a dose response effect with regards to the association with mortality since escalating thresholds used to define Dynamic RF (p=0.013 for trend) or define improved RF (p=0.028 for trend) were associated with progressively greater odds for death at 180 days (Figure 2). This association did not meet statistical significance for worsening RF (p= 0.071).
Figure 2.
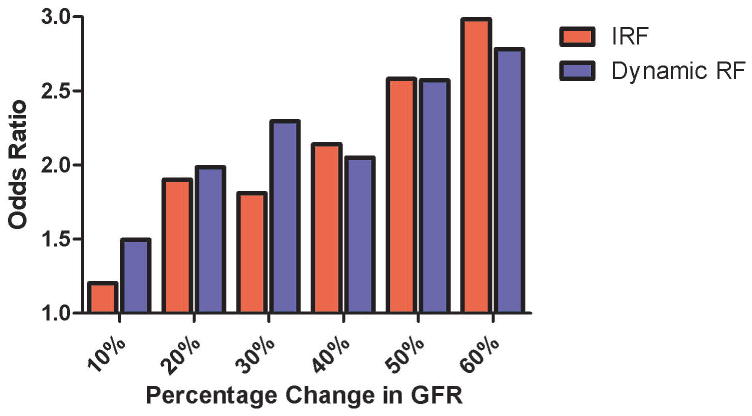
Odds ratio for 6 month mortality at increasing percentage change in GFR cut points. Dynamic RF: Increase or decrease in glomerular filtration rate from admission to discharge, GFR: Glomerular filtration rate, RF: Renal function. Dynamic RF p=0.013 for trend, improved RF p=0.028 for trend.
Discussion
The primary finding of this study is the absence of substantial differences in standard PAC derived hemodynamic parameters between patients developing improved RF versus worsening RF. Despite the traditional assumptions that cardiac output driven increases or decreases in renal perfusion explain differing changes in renal function, PAC derived hemodynamic parameters, traditional baseline characteristics, treatment with inotropes or vasodilators, and outcomes were largely similar between these groups. In fact, patients with improved RF and worsening RF were hemodynamically more closely related to each other than to patients with Stable RF. After combining patients with worsening RF and improved RF into a single “cardio-renal” group, significant associations with variables such as reduced cardiac index, utilization of inotropes, and decreased survival became apparent.
The overall lack of substantial hemodynamic differences between the worsening RF and improved RF groups is not surprising given that recent publications have failed to validate the predicted relationship between PAC derived variables and worsening RF (5-7). It is however somewhat surprising that a poor prognostic indicator such as worsening RF has not co-segregated with hemodynamic markers of disease severity, such as a low cardiac index, even if the hemodynamic perturbations had no causal relationship. These observations can be interpreted to indicate the presence of a truly minimal, or paradoxical, association between worsening RF and parameters such as cardiac index. Alternatively, worsening RF may only be capturing a portion of the “cardio-renal” population and those patients left in the comparison group are obscuring any differences due to shared hemodynamic derangements with worsening RF patients. We believe the later hypothesis is supported by the above data.
It is however of interest that the worsening RF group had a significantly greater rate and quantity of weight loss compared to the improved RF group despite no difference in baseline, PAC removal, or baseline to PAC removal change in cardiac filling pressures. Given the well described disconnect between filling pressures and volume status, it is possible that relative intravascular volume depletion secondary to aggressive diuresis contributed to the deterioration in renal function in the worsening RF group (12-18). Additional non-PAC derived measures of intravascular volume merit further study for potential guidance of diuresis in these patients.
In this cohort, patients with any significant change in renal function (Dynamic RF) had a lower cardiac index, lower ejection fraction, were more apt to be treated with inotropes and intravenous vasodilators, received higher loop diuretic doses and more frequent adjunctive thiazide diuretics than patients with Stable RF. Any of the above cited associations could be employed in speculation on mechanisms related to improved RF or worsening RF and their associated mortality. It is equally likely, however, that the majority of the above factors are all simply describing a “sicker” patient population. Despite the mechanistic uncertainty underlying these changes in renal function, it seems clear that there is prognostic relevance in the changes. In this series, prognostic information was best captured by focusing on the dynamic, as opposed to the directional aspect, of changes in renal function. This was highlighted by the fact that both improved RF and worsening RF were not significantly associated with mortality when patients with changes in renal function of the opposite direction were left in the comparator group. However, comparison of patients with improved RF or worsening RF to patients with Stable RF revealed strong associations with mortality. From a prognostic standpoint, separation of patients into worsening versus improving renal function may be an artificial delineation.
Many published reports support the prognostic importance of chronic renal insufficiency; however, this analysis raises some question as to what actually constitutes chronic renal insufficiency in a heart failure population (19-25). Central to the theme of chronic kidney disease is the concept of loss of nephron mass since the majority of diseases associated with chronic kidney disease cause irreversible loss of functional nephrons (26). In this study, patients with improved RF had on average a 48% improvement in GFR from admission to discharge, but at 6 months GFR had once again decreased and was not significantly different from admission levels. Since a transient regeneration of nephrons is unlikely, a significant percentage of the renal impairment at admission, and quite possibly at 6 months, may be secondary to reversible decreases in renal function rather than irreversible parenchymal disease. Assuming the association between Dynamic RF and mortality stems from the advanced heart failure disease state severe enough to facilitate worsening in renal function at some time, these observations raise the question if a significant portion of the predictive ability of chronic renal insufficiency stems from its relationship to outpatient worsening RF, rather than intrinsic parenchymal disease. Given the interdependence between changes in renal function, admission, and discharge GFR, determination of the relative importance of parenchymal disease verses functional renal impairment is impossible from this data.
The most direct clinical implication of this study is the incremental prognostic information that Dynamic RF adds to established risk stratification tools. Additionally, this study further supports the concept that pulmonary artery catheters provide limited information related to renal function given that patients with improved RF and worsening RF serially had similar PAC derived variables. Reversible renal dysfunction appears to be common and further research is necessary to develop methods to prospectively identify it. If such tools become available they could help inform practical decisions such as cardiac transplantation listing status, cardiac versus combined cardiac renal transplantation, left ventricular assist device implantation, and hemodialysis initiation. Additionally, identification of outpatients with renal insufficiency that has the potential for improvement may represent a tremendous therapeutic target.
The ESCAPE trial was not designed to evaluate improved RF or worsening RF and given that the treating physicians were not blinded to either renal or PAC data, it is likely that treatment strategies were significantly modified in response to those variables. Patients with advanced renal insufficiency were excluded limiting generalization to this group of patients. The presence of multicollinearity between improved RF, worsening RF, and admission and discharge GFR limits the ability to determine the relative contribution of each covariate. Consequently, these analyses should be interpreted as hypothesis generating and further research is required to determine the true importance of various causes of reduced renal function in heart failure populations. By nature of the trial design, PAC data is only available on slightly more than half the patients, limiting power. Additionally, given the small number of patients with improved RF and worsening RF, comparison of these groups may have missed smaller hemodynamic differences. While many of the associations in this study had very highly significant p-values and are unlikely to have arisen by chance, some of the more modestly significant associations may have arisen as a result of multiple comparisons and should be interpreted with caution.
Acknowledgments
Funding Sources: None
Footnotes
All authors report no conflicts of interest or relationship to industry relevant to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O'Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB, Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. doi: 10.1016/j.ahj.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, POSH Investigators Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH) Eur Heart J. 2006;27:1216–1222. doi: 10.1093/eurheartj/ehi859. [DOI] [PubMed] [Google Scholar]
- 3.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhuisen DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 7.Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–596. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah MR, O'Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 9.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, ESCAPE Investigators and ESCAPE Study Coordinators Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 10.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening Renal Function Defined as an Absolute Increase in Serum Creatinine is a Biased Metric for the Study of Cardio-Renal Interactions. Cardiology. 2010 doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Baek SM, Makabali GG, Bryan-Brown CW, Kusek JM, Shoemaker WC. Plasma expansion in surgical patients with high central venous pressure (CVP); the relationship of blood volume to hematocrit, CVP, pulmonary wedge pressure, and cardiorespiratory changes. Surgery. 1975;78:304–315. [PubMed] [Google Scholar]
- 13.Cohn JN. Central venous pressure as a guide to volume expansion. Ann Intern Med. 1967;66:1283–1287. doi: 10.7326/0003-4819-66-6-1283. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi H, Biuk-Aghai EN, Yu M, Ho HC, Chapital AD, Koss W, Takanishi DM., Jr Circulating blood volume measurements correlate poorly with pulmonary artery catheter measurements. Hawaii Med J. 2008;67:8–11. [PubMed] [Google Scholar]
- 15.Wiesenack C, Fiegl C, Keyser A, Prasser C, Keyl C. Assessment of fluid responsiveness in mechanically ventilated cardiac surgical patients. Eur J Anaesthesio. 2005;22:658–665. doi: 10.1017/s0265021505001092. [DOI] [PubMed] [Google Scholar]
- 16.Rex S, Brose S, Metzelder S, Huneke R, Schalte G, Autschbach R, Rossaint R, Buhre W. Prediction of fluid responsiveness in patients during cardiac surgery. Br J Anaesth. 2004;93:782–788. doi: 10.1093/bja/aeh280. [DOI] [PubMed] [Google Scholar]
- 17.Pinsky MR, Teboul JL. Assessment of indices of preload and volume responsiveness. Curr Opin Crit Care. 2005;11:235–239. doi: 10.1097/01.ccx.0000158848.56107.b1. [DOI] [PubMed] [Google Scholar]
- 18.Janssens U, Graf J. Volume status and central venous pressure. Anaesthesist. 2009;58:513–519. doi: 10.1007/s00101-009-1531-2. [DOI] [PubMed] [Google Scholar]
- 19.Akhter MW, Aronson D, Bitar F, Khan S, Singh H, Singh RP, Burger AJ, Elkayam U. Effect of elevated admission serum creatinine and its worsening on outcome in hospitalized patients with decompensated heart failure. Am J Cardiol. 2004;94:957–960. doi: 10.1016/j.amjcard.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 21.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, ADHERE Scientific Advisory Committee and Investigators High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Campbell RC, Sui X, Filippatos G, Love TE, Wahle C, Sanders PW, Ahmed A. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol Dial Transplant. 2009;24:186–193. doi: 10.1093/ndt/gfn445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochiai ME, Barretto AC, Oliveira MT, Jr, Munhoz RT, Morgado PC, Ramires JA. Uric acid renal excretion and renal insufficiency in decompensated severe heart failure. Eur J Heart Fail. 2005;7:468–474. doi: 10.1016/j.ejheart.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Naruse H, Ishii J, Kawai T, Hattori K, Ishikawa M, Okumura M, Kan S, Nakano T, Matsui S, Nomura M, Hishida H, Ozaki Y. Cystatin C in acute heart failure without advanced renal impairment. Am J Med. 2009;122:566–573. doi: 10.1016/j.amjmed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 26.Brenner BM, Rector FC. Brenner & Rector's the kidney. 8th. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]


