Abstract
Activity based anorexia (ABA) is a model that mimics the self-starvation and hyperactivity features of anorexia nervosa (AN). This study investigated whether a history of ABA will enhance food avoidance learning and retard its extinction in female rats. We compared the acquisition and extinction of a conditioned taste aversion (CTA) in naive (ad lib with no access to RW), ABA, and pair-fed to the food intake of ABA (with access to a locked RW) female Sprague-Dawley rats. The CTA conditioning was conducted after the ABA and pair-fed rats had recovered to their pre-food restriction body weights. For CTA learning, 0.3M sucrose consumption was followed by low doses LiCl (0.009M or 0.018M at 1.33ml/100g of body weight, IP) injection. The results revealed that the ABA rats acquired an aversion to sucrose significantly sooner than the naive controls. Furthermore, they completely avoided sucrose while the naive and pair-fed controls still sampled it by the end of 10 conditioning trials. When extinction was assessed by 1-bottle and 2-bottle tests, the ABA rats extinguished more slowly than the controls. However, the differences in sucrose aversion extinction between the ABA and control rats were only significant in 1-bottle test. These data suggest that experience with AN-like behaviors results in an acquired aversion to a preferred food sooner and a longer retention of the negative food associations. These findings have implication for understanding the persistence of aberrant eating behaviors in eating disorders.
Keywords: anorexia nervosa, exercise, running wheel, conditioned taste aversion, food avoidance
1. Introduction
Anorexia nervosa (AN) is a severe illness of unknown etiology and has one of the highest mortality rates of all psychiatric disorders [1–3]. Onset of AN normally occurs in adolescence and young adulthood, and the disorders is found much more frequently in women than in men [4]. Subjects with AN are frequently ambivalent or refuse treatment [5]. Although AN affects a relatively small number of individuals, estimated 0.3–2% among women [6–8], treatment is extremely difficult and financially burdensome [5]. Moreover, relapse is common and 20–30% of AN patients become chronically ill [9, 10]. Thus, the identification of improved research-based treatment options is critical.
The defining characteristics of AN are self-imposed starvation and fear of fatness [11–13]. Patients are motivated to restrict their eating, particularly the consumption of highly palatable, high energy density foods [12–14]. They continue to avoid consuming food even when already severely underweight. Some researchers have suggested that starvation reduces negative mood or even becomes conditioned and reinforcing for AN subjects [12, 13]. In addition to eating restraint, hyperactivity is featured in up to 75% of AN. Indeed, excessive exercise has been reported to precede, follow, or coincide with the onset of strict dieting/food restriction [15–17]. In this sense, hyperactivity not only promotes the progression, but likely also impedes the successful treatment and recovery of AN [18, 19].
Neuroendocrinological studies in acute AN have found normal homeostatic physiological responses to starvation. These responses include elevated levels of orexigenic neuropeptide Y(NPY) in cerebrospinal fluid (CSF) and reduced levels of anorexigenic leptin in both CSF and plasma [20]. In contrast, dysregulation of reward and mood related systems have been identified in AN or subjects recovered from AN [12]. Compared to healthy controls, AN subjects and those recovered from AN have reduced CSF dopamine (DA) metabolites [21]. Along these line, decreased activity of the ventral striatum during reward processing has been found in subjects who have recovered from AN [22]. Similarly, underweight and malnourished anorexic individuals have abnormal serotonergic activity [23]. These data suggest altered reward and motivational functions in AN. Combined with the food avoidance and hyperactivity, it is likely that a motivational shift away from food but toward exercise occurs in some AN or hyperactive subjects with AN history. There are, however, few experimental studies examining this assertion.
One animal model that mimics both the behavioral and physiological aspects of AN is activity based anorexia (ABA). When given access to running wheels (RWs), rodents run voluntarily and, when food is available ad libitum, animals are able to maintain energy homeostasis. In the ABA model, rats have free access to RWs coupled with restricted access e.g. 1or 2 h/day, to food. Under these conditions, rats increase wheel running (WR) activity but decrease food intake [24–28]. Similar to AN, rats experience starvation and hyperactivity simultaneously and fail to maintain energy homeostasis. They lose weight dramatically and even die of starvation if the ABA protocol is not terminated [28]. From a behavioral standpoint this suggests that like AN, there is also a shift in motivation from food to exercise in the ABA model.
Although the basis of neither ABA nor AN are fully understood and the initiating mechanisms may be different, the ABA model can be used to study the consequences of combining increased activity and decreased food intake that may contribute to the maintenance or relapse of AN. Given that AN or weight restored AN subjects maintain an altered perception toward palatable food [12, 29], we hypothesized that such a motivational shift away from food as occurs with ABA may facilitate the development of learned aversions towards foods. This hypothesis was tested by examining conditioned taste aversion (CTA) acquisition and extinction in rats that had experienced ABA but were now weight restored.
2. Materials and methods
2.1. Subject and housing
A total of forty-three, adolescent (40–45 days old) female Sprague Dawley (Harlan) rats weighing 111–146 g were used in this study. Rats were individually housed and maintained on a 12:12 h light/dark cycle with the dark onset at 1:00 PM throughout the entire experiment. Initially, all rats were housed in a room equipped with RW activity recording system for establishing activity based anorexia. A 25% weight loss of body weight before the WR + restricted food access schedule is a commonly used criterion for ABA [26], and this criterion was used in our study. Once this criterion for ABA was reached, animals were transferred to another room for the weight restoration and CTA procedures. Harlan standard rodent chow diet 2018 (Harlan Teklad; 3.3 Kcal/g, fixed formula diet of 18% protein, 5% fat) and water were available ad libitum unless otherwise noted. Other feeding schedules are specified below. Animal usage and all procedures were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and are in compliance with NIH guidelines.
2.2. Activity-based anorexia during adolescence
Due to limited number of running wheel chambers, animals were tested in 2 squads at separate times. All three groups were included in both squads and the groups were initially divided as follows; naive (n=16), ABA (n=14), and pair-fed (n=13). The naive rats were single housed in plastic tubs with corncob bedding (Harlan), and with food and water available ad libitum. The ABA and pair-fed rats were housed in a nest box (15 × 25 × 15 cm) with a RW (diameter 36 cm and width 11 cm, Wahmann, Timonium, MD) attached. The nest box has a wire mesh bottom. Below the wire mesh was a tray to collect food spillage. Body weight and food intake subtract spillage were measured for the nearest 0.1 gram daily at 7:30AM. During habituation, the wheels were locked for both groups.
Following a 7-day habituation period, wheels were unlocked for the ABA rats, whereas the wheels remained locked for the pair-fed animals. Wheel running activity was recorded in real time, and analyzed later through a computer with MedPC IV software (Med Associates Inc, Georgia, VT). The ABA rats were habituated to running wheel access for 7 (squad 1) or 10 (squad 2) days. The longer baseline running in squad 2 was to adjust to the daylight saving time change that occurred during the experiment. After such baseline running, the ABA rats were switched to a food restriction schedule until a 25% reduction of baseline body weight (average of the last 3 days before food restriction) was achieved or for as long as 10 days. Food restriction was imposed to both ABA and pair-fed groups. Initially (squad 1), access to food was allowed during the first 2 hrs of the dark cycle. To initiate restricted food access, food was removed at 3:00PM the day before the restriction schedule began. On the restriction day, rats in the ABA group received access to food between 1:00 to 3:00 PM. Later, it was determined that 1h food access (first hour of the dark cycle) was necessary to achieve 25 % weight loss in the ABA paradigm. Thus, after 5 days the access time was reduced to 1 h (1:00 to 2:00 PM). Rats in squad 2 had 1 h access to food during the food restriction schedule. Thus, food was removed at 2:00PM the day before the restriction schedule began. On the restriction day, rats in the ABA group received access to food between 1:00 to 2:00 PM. With this protocol, 2 and 6 rats reached the 25% body weight reduction criterion for ABA in squad 1 and 2, respectively. The pair-fed rats received the amount of food consumed by the ABA rats, and thus their food restriction schedule began one day after the ABA group. The pair-fed rats consumed all the food that was given. There was never evidence of spillage when they were being weighed.
Once an individual rat reached the 25% body weight loss (or at the end of 10 days restricted food access), it was removed from the RW box, transferred to another room, and single housed in a stainless steel wired cage. The naive and pair-fed rats were also transferred to the same room and housing condition in a timely manner that coordinated with the ABA group. Rats were 65–70 days old by this time. Once in the new room, all groups of rats had chow and water ad libitum until the ABA rats increased their body weight to the pre-food restriction baseline (took 3–9 days, mean= 6.3±0.5).
2.3. Conditioned taste aversion
Conditioned taste aversion acquisition and extinction procedures began as the ABA rats recovered to their pre-food restriction body weight for at least 2 consecutive days. In squad 1, all rats began the procedures on the same day. More rats reached the activity based anorexia criteria in squad 2, and there was more variability in body weight recovery. Thus, one subset of rats began CTA training 2 days earlier than the other subset. The experiment was conducted while rats were water deprived for 20 hrs. Distilled water was provided at two time points, for 5 min beginning at 7:30AM and for 1 hr beginning at 11:00AM. The CTA conditioning and extinction were conducted at the 5 min time point.
Acquisition
The rats underwent 10 conditioning days. Each conditioning day was separated by a water day. Prior to beginning the acquisition of the CTA, baseline 5 min water (distilled water; dH2O) intake was measured for 4 consecutive days. On the conditioning days, the 5 min access to dH2O was replaced by 0.3M sucrose solution. Five minutes after the end of sucrose presentation, rats were given an intraperitoneal (IP) injection of lithium chloride (LiCl; 1.33ml/100g body weight). As a control, 5 rats from the naive group were given IP saline at the same time. To determine whether there was difference in the strength of the association between groups, we used low doses of LiCl, 0.009M and 0.018M. The 0.009M LiCl has been demonstrated to be a threshold dose for forming conditioned aversions [30, 31]. Rats did not decrease their intake to more than 50% of baseline sucrose with this dose of LiCl. Furthermore, in order to test the speed of CTA extinction, a higher dose of LiCl must be used to form a complete avoidance to the sucrose solution. Thus, after a few trials, the LiCl concentration was increased to 0.018M. In squad 1, the first 3 trials were with 0.009M LiCl and the next 7 trials were with 0.018M LiCl. In squad 2, rats received 0.009M LiCl for the first 4 trials, and so they received only 6 trials of 0.018M LiCl. The total acquisition period for the 10 trials was 20 days.
Extinction
The CTA was extinguished by given 0.3M sucrose access without subsequent injection. There were both 1-bottle test and 2-bottle test methods for extinction. During the 1-bottle test, 0.3M sucrose was provided for 5 min. For the 2-bottle test, water and 0.3M sucrose were presented simultaneously for 5 min. The side of the cage where water and sucrose was placed was counterbalanced. The last conditioning day was followed by a water day, and the extinction schedule began on the next day. There were 3 extinction cycles. The first cycle included one 1-bottle test followed by one 2-bottle test. The next 2 cycles included two 1-bottle test days followed by one 2-bottle test day. As during acquisition, the schedule that sucrose and water were presented on alternate days was maintained. Thus, the extinction schedule lasted 16 days. This protocol of CTA extinction has been demonstrated previously [32].
2.4. Estrous cycle
In squad 1, vaginal cytology samples were collected daily at 7:00 AM. Phases of estrous cycle were identified according to standard criteria [33]. The vaginal cytology confirmed that access to unlocked wheel for the ABA group began when the rats had begun estrous cycling. Estrous cycle stopped when rats lost substantial weight during excess WR and food restriction. The estrous cycle returned once the rats recovered to baseline body weight. Daily vaginal cytology observations did not suggest that CTA learning was affected by different stages of the estrous cycle.
2.5. Statistical analysis
Data in this study included daily body weight, food intake, WR activity, and 5 min water or sucrose intake. Changes in each parameter were analyzed using repeated measures ANOVA. Post hoc comparisons were made with Fisher LSD test following significant main or interactive ANOVA effects. During the feeding restricted access phase of activity based anorexia establishment, individual rats reached the 25% weight loss criterion at different times (4–10 days). For statistical analyses, data from each rat were averaged across days of the food restriction period. Relative body weight losses from baseline (average of last 3 ad libitum days) during food restriction were compared using independent t-tests. Food intake and WR activity during feeding restriction were compared with baseline values using dependent t-tests.
Conditioned taste aversion data from 1 pair-fed rat was excluded because it had urinary symptoms as well as reduced water and food intake and lost a significant amount of weight during the CTA procedure. For CTA acquisition and extinction, data for squad 1 & 2 are combined because comparisons between the squads showed no difference (repeated measure ANOVA: naive, p=0.85; ABA, p=0.23; pair-fed, p=0.49). At first, sucrose intakes from 4 groups of rats (naive-sal, n=5; naive-LiCl, n=11; ABA, n=14; pair-fed, n=12) were analyzed by group × trial (4 ×10) repeated measure ANOVA. No significant differences in CTA acquisition between the ABA and the control groups were revealed. When only rats that lost 25% of pre-restriction body weight in the ABA group (n=8) and rats paired to them in the pair-fed group (n=7) were included in the final analysis, significant differences in CTA acquisition were revealed. Correlation coefficients and t-test of slope =0 were used to determine the relationship between ABA duration and sucrose suppression or preference ratio. Similar correlations were performed to determine the relationship between body weight/duration of weight recovery and sucrose suppression/preference ratio. All statistical analyses were performed with Statistica 7.0 software (StatSoft Inc.) and significance was set at α = 0.05. Data are expressed as mean values ± SE.
3. Results
3.1. Establishment of activity based anorexia
The 3 groups of rats were initially divided so that the mean body weights for each group were equal. The results of activity, food intake, and body weight are shown in Table 1. After baseline running, rats in the ABA group weighed significantly less than both naive and pair-fed controls. Body weight between naïve and pair-fed rats did not differ. One way ANOVA comparing average food intake of the three days of pre-restriction baseline period revealed a significant group effect, F(2, 40)=4.22, p<0.05. Naive rats consumed less food than pair-fed (p<0.01) and ABA (p=0.058) rats.
Table 1.
Development of activity based anorexia.
| ABA (n=14) | pair-fed (n=13) | naive (n=16) | ||||
|---|---|---|---|---|---|---|
| baseline | restriction | baseline | restriction | baseline | ad libitum | |
| daily activity (revolution) | 5455 ± 806 | 10549 ± 922* | – | – | – | – |
| daily food intake (g) suppression % | 16.1 ± 0.7 | 7.3 ± 0.6** | 16.8 ± 0.6 | 7.4 ± 0.6 | 14.7 ± 0.2 | 15.3 ± 0.2 |
| 54.2±4.2 | 55.5 ± 4 | |||||
| body weight (g) weight loss/gain % | 162.9 ± 2.6† | 126.4 ± 2.8 | 176.3 ± 4.1 | 164.9 ± 3.5 | 176.1 ± 2.9 | 199.8 ± 3.2 |
| −22.2 ±1.7‡ | −6.4 ±1.2 | +13.5 ± 0.8 | ||||
Values are means ± SE. The baseline refers to the average of the last 3 days values before the restricted food access began for the ABA and the pair-fed groups.
Restricted food access increased mean daily activity compared to mean baseline values, t(13)=5.37, p<0.001.
ABA rats consumed significantly less food during restricted access than their baseline 24 h food intake, t(13)=9.97, p<0.0001.
At baseline running, the ABA rats weighed significantly less than both the pair-fed and naïve rats, F(2, 40)=5.67, p<0.01.
Although consumed the same amount of food during restricted access, the ABA rats lost significantly more percentages of baseline weight than the pair-fed controls at the end of the restriction period, t(25)=7.36, p<0.0001.
Rats in the ABA group demonstrated significant increases of WR activity, decreases of food intake, and decreases of body weight during times of restricted food access (see Table 1). Consistent with previous studies, the ABA rats significantly increased WR activity during restricted food access. However, food intake was 54% less than their baseline 24 h food intake. As a result, the mean body weight of the ABA rats was significantly reduced by the end of the period of restricted food access. Eight of the ABA rats (2 in squad 1 and 6 in squad 2) reached the 25% reduction of baseline body weight criterion for activity based anorexia. The 8 ABA rats consumed significantly less food (5.9 ± 0.7 vs. 9 ± 0.4, t-test, p<0.004) and lost more body weight (27.3 ± 0.7% vs. 15.5 ± 1.2%, t-test, p<0.0001) than the 6 rats (designated as semi-ABA) in the same WR + restricted food access protocol. However, WR activity before (ABA, 6315 ± 1115 vs. semi-ABA, 4309 ± 1076; t-test, p=0.23) and during (ABA, 10279 ± 865 vs. semi-ABA, 10909 ± 1927; t-test, p=0.75) the restriction period did not differ between the two subsets of rats.
The pair-fed rats consumed the same amount of food as those in the ABA protocol (and similar percentage of food suppression, 55%), but their body weight reduction was significantly less than that of the ABA rats (p<0.0001). None of the pair-fed rats lost 25% of baseline body weight. In fact, the pair-fed rats also lost less body weight than the semi-ABA rats (pair-fed, 6.4 ± 1.2% vs. semi-ABA, 15.52 ± 1.2%, t<0.0003).
Before beginning CTA, rats in the ABA protocol and pair-fed condition restored their weight to pre-restriction baseline, but body weight between the three groups differed significantly [one way ANOVA, F(2, 40)=23.43, p<0.0001: naive, 201.3 ± 2.9g vs. ABA, 172.3 ± 2.3g vs. pair-fed, 188.5 ± 3.9g]. However, they weighed the same at the end of the CTA acquisition and extinction protocols [one way ANOVA, F(2, 40)=0.06, p=0.94: naive, 237.4 ± 4g vs. ABA, 237.3 ± 3.4g vs. pair-fed, 239.2 ± 5.4g].
3.2. CTA acquisition
Baseline 5 min water intakes were the average intakes of the last 2 water trials before the conditioning trials began. This baseline intake was not different among the groups [one way ANOVA, F(3, 27)=0.61, p=0.61].
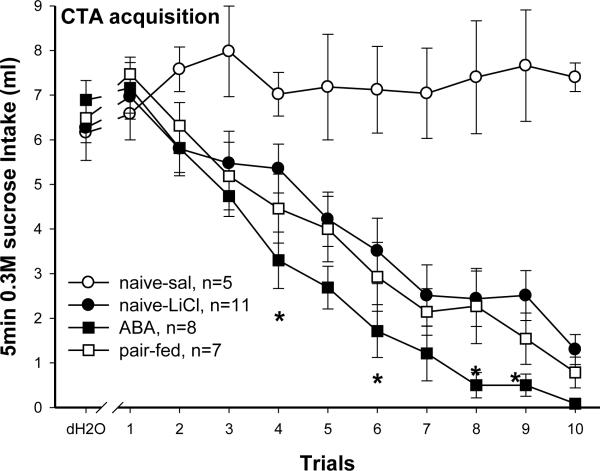
All rats that received LiCl injections (naive-LiCl, pair-fed, and ABA) after 0.3M sucrose consumption progressively decreased their sucrose intake (Fig. 1). Group by trial (4 × 10) repeated measure ANOVA revealed significant effects of group [F(3, 27)=14.23, p<0.0001], trial [F(9, 243)=38.601, p<0.0001], and group × trial interaction [F(27, 243)=4.34, p<0.0001]. By design, naive rats that received saline injections after sucrose (naive-sal) never learned an aversion so their sucrose intakes were maintained at levels higher than their baseline water intake. Post hoc tests on the group effect revealed that overall sucrose intakes were naive-sal>naive-LiCl ≥ pair-fed ≥ ABA (each group vs. naive-sal, p<0.0001). Sucrose intake on the first conditioning trial did not differ between the 4 groups. Compared with intakes of naive-sal rats, naive-LiCl rats consumed significantly less sucrose on trial 3 (p<0.05) and 5–10 (p<0.003), and pair-fed (p<0.01) and ABA (p<0.003) rats consumed significantly less on trial 3–10. Thus, all 3 groups that received sucrose-LiCl pairings acquired a CTA. Furthermore, post hoc tests on the group effect also revealed that the ABA rats learned sucrose aversion significantly sooner than the naive-LiCl rats (p<0.05). Suppression of sucrose intake in ABA rats was more than 50% by the fourth conditioning trial (the last trial of 0.009M LiCl) while the same level of suppression was not reached until the 6th and 7th trial for pair-fed and naive rats, respectively. Sucrose intakes on trial 4, 6, 8, and 9 (p<0.05 for each trial) in the ABA rats were significantly less than in the naive-LiCl rats. By the 10th trial, only the ABA rats exhibited complete sucrose avoidance.
Fig. 1.
Conditioned taste aversion to 0.3M sucrose. Values are means ± SE. Data include baseline 5 min water intake (dH2O) and 5 min intake of sucrose during 10 pairing trials. Naive rats paired with saline (naive-sal) after sucrose intake did not acquire an aversion. The other 3 groups received LiCl injections after sucrose, and all acquired a CTA (see section 3.2). The ABA rats decreased sucrose intake significantly more than the naive-LiCl rats on trials 4, 6, 8, and 9. They also completely avoided while the controls still sampled sucrose by the last conditioning trial. *p<0.05, naive-LiCl vs. ABA.
The 6 rats that went through the WR + restricted food access protocol (semi-ABA) but did not reach the 25% body weight loss criteria, acquired a CTA to sucrose in a similar manner to the naive-LiCl rats [repeated measure ANOVA (2 × 10) comparing 10 CTA trials in naive-LiCl and the semi-ABA rats, F(9, 144)= 0.46, p=0.9]. When compared with the 8 ABA rats, repeated measure ANOVA (2 × 10) revealed a significant effect of group × trial interaction [F(9, 108)=2.4, p<0.05]. Sucrose intakes on trials 4 and 5 (p<0.05) were significantly more in the semi-ABA than in the ABA rats. Further, similar to the naive-LiCl rats, the semi-ABA rats did not suppress more than 50% of their initial sucrose intake until trial 7. By the end of 10 conditioning trials, the semi-ABA rats still drank 1.5 ml of sucrose. Thus, although the semi-ABA rats went through the same WR + restricted food access protocol, they acquired a CTA more slowly than did the ABA rats.
3.3. CTA extinction
The results of 1-bottle and 2-bottle tests both revealed that the ABA rats increased sucrose intakes more slowly than the controls. However, a significant difference between the ABA and control rats was only shown in 1-bottle test.
1-bottle tests (Fig. 2)
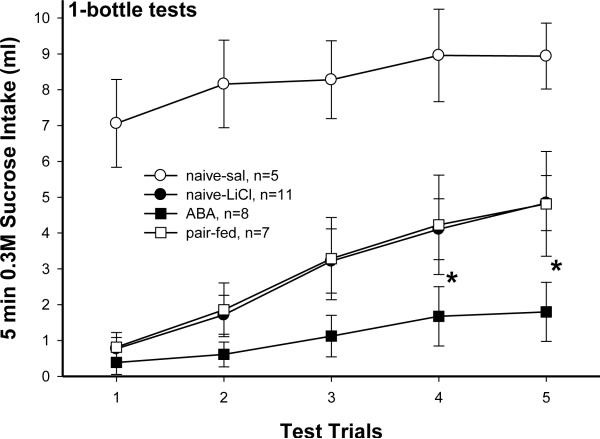
Fig. 2.
Sucrose CTA extinction during 5 trials of 1-bottle tests. Values are means ± SE. Naive rats paired with saline during CTA acquisition never formed an aversion to sucrose so their intakes maintained large. Aversion to sucrose in all 3 LiCl paired groups extinguished over trials. The naive-LiCl and pair-fed rats extinguished their sucrose aversion at similar rate. Rats with ABA experience extinguished sucrose aversion significantly more slowly than the other 2 LiCl paired groups. *p<0.05, naive-licl or pair-fed vs. ABA.
Rats in the naive-sal group never learned aversion to sucrose so their initial sucrose intake was significantly higher than that of the other 3 groups [4×5 repeated measure ANOVA: group effect F(F(3, 27)=11.81, p<0.0001]. All 4 groups increased intake of 0.3M sucrose over trials [trial effect, F(4, 108)=28.31, p<0.0001; group × trial interaction, F(12, 108)=1.94, p<0.05]. For the naive-sal rats, intakes on trial 4 and 5 were significantly more than on trial 1 (p<0.05). The naive-LiCl and pair-fed rats recovered their sucrose intake similarly. Compared with trial 1, they consumed significantly more sucrose from trial 3–5 (p<0.001). In contrast, the ABA rats did not significantly increase sucrose intake until trial 4 and 5 (p<0.05). Furthermore, intakes on trial 4 and 5 were significantly less in ABA rats than in naive-LiCl (p<0.05 and 0.01) and pair-fed rats (p<0.05). The results for the semi-ABA rats were similar to the naive-LiCl and pair-fed rats (data not presented).
2-bottle tests (Fig. 3)
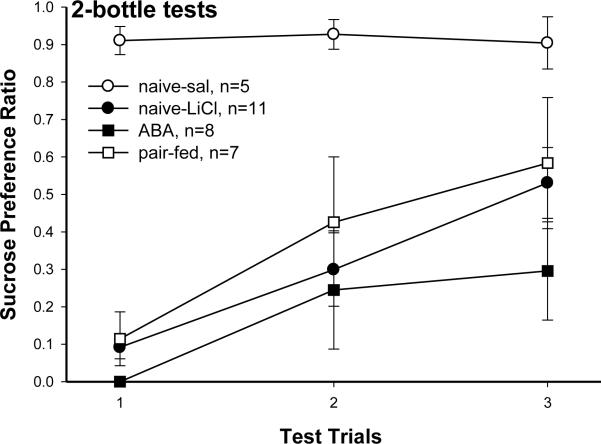
Fig. 3.
Sucrose CTA extinction during 3 trials of 2-bottle tests. Data are presented as sucrose preference ratio (=sucrose intake/ total 5 min intakes of dH2O and sucrose). Values are means ± SE. Naive rats paired with saline during CTA acquisition never formed an aversion to sucrose so their preference to sucrose maintained high. All 3 groups paired with LiCl during CTA acquisition showed gradual extinction as the sucrose preference ratio increased across trials. Although not significantly different, the ABA rats increased their sucrose preference more slowly than the other 2 groups.
Data from 2-bottle tests are expressed as a sucrose preference ratio. Preference ratio was calculated by dividing the 5 min sucrose intake by 5 min total intake (sucrose + water). Again, as the naive-sal rats never formed aversion, they highly preferred sucrose. This preference was significantly more than those of the 3 groups that had learned sucrose aversion in all 3 trials of 2-bottle test [4×3 repeated measure ANOVA: group effect, F(3, 27)=11.1, p<0.0001]. Preferences to sucrose in those 3 groups did not differ, and the preferences gradually increased over trials [trial effect, F(2, 54)=11.65, p<0.0001; group × trial, F(6, 54)=1.36, p=0.25 ]. Although no significant group effect was found, the sucrose preference ratio for the ABA rats (0.3 ± 0.13) was lower than both the naive-LiCl (0.53 ± 0.09) and pair-fed (0.58 ± 0.17) rats on the third 2-bottle test. Again, the results for the semi-ABA rats were similar to the naive-LiCl and pair-fed rats (data not presented).
4. Discussion
The results of this study demonstrated that food aversion learning is enhanced after experiencing restricted food access along with excessive exercise. The paradigm used to induce ABA in this study included free access to RW while access to food was restricted to 1–2 h. After the ABA rats restored baseline body weight, all rats (naive, pair-fed, and ABA) were trained to learn a CTA to sucrose. Enhanced CTA acquisition and retarded extinction was not shown in pair-fed rats or rats that experienced the WR + restricted food access schedule but failed to reach the ABA criterion (semi-ABA). The finding that the enhanced CTA was specific in WR rats that reached the 25% weight loss criterion suggest that a certain degree of starvation combined with excessive activity is required to produce such an effect. Such data further the notion that exercise is highly reinforcing under the condition of starvation [13]. The increased reinforcing value of exercise or severe weight loss per se could in turn support a hedonic or motivational shift away from food and, may even facilitate or promote food avoidance [13, 34]. Although others have investigated the formation of a CTA to a novel food when the novel food (i.e., dog biscuits) was used in ABA paradigm [35], this is the first study to investigate how a history with ABA with standard chow alters the acquisition and retention of a CTA using 0.3M sucrose as the conditioned stimulus.
The body weight and food intake results during baseline WR for the ABA rats were consistent with previous studies. It has been demonstrated that both adolescent and adult male rats significantly suppress food intake for as long as 14 days with WR activity [36, 37]. Reductions of food intake and body weight are most remarkable within the first 4 days of WR activity [36–38]. Food intake returns to the level of pre-wheel running or sedentary controls after 14 days of WR, but body weight is maintained at significantly lower levels than in controls without RW access [36, 38]. Compared to pre-wheel running baseline, average food intake of the first 3 days of wheel running was significantly reduced in the WR rats (16.24 ± 0.4g vs. 13.26 ± 0.4g). Food intake before the beginning of restricted access was at the level of pre-wheel running (16.24 ± 0.4g vs. pre-restricted food access, 16.1 ± 0.7g), but, as shown in Table 1, the rats still weighed significantly less than the pair-fed and naive controls by the end of baseline WR period. Thus, as with male rats [38], food intake and body weight are also remarkably reduced during the early stage of WR activity in adolescent female rats.
The results of this study also demonstrate significant individual differences in the response to the WR + restricted food access protocol. Among the 14 rats that were placed in such protocol, only 8 reached the 25% weight loss criterion for ABA. These rats suppressed food intake more than did the 6 rats that did not reach the criterion for ABA during the restricted food access period. The WR activity during restricted food access between the two subsets of rats was not different. These results reflect the individual differences that make some rats more susceptible to ABA and, this may underlie the physiological mechanisms that make individuals more likely to engage in aberrant eating behaviors. Furthermore, these results also agree with the finding that genetic factors contribute to the susceptibility for developing AN [39]. Since there were differences in the duration of food restriction and weight recovery among the ABA rats, one would expect that differences in the two factors might affect CTA acquisition and extinction outcomes. However, there was not a significant correlation between restriction duration and final sucrose suppression during acquisition (p=0.86) or sucrose preference ratio at the end of extinction (p=0.58) in the 8 ABA experienced rats. There was also no relationship between the duration of recovery and sucrose suppression (p=0.24) or preference ratio (p=0.32). Thus, it appears that as long as the rats experienced ABA, CTA acquisition and extinction were not affected by the duration of food restriction or of weight restoration
One limitation of this study was that although we had a pair-fed group to match the amount of food consumed by the ABA, we did not include a control group that was weight-matched to the weight loss in the ABA group. Having this group would allow us to determine whether the difference between groups was influenced by the ABA experience or the resulting weight loss. In order to understand how periods of weight reduction can influence the association properties of a CTA, including a food restricted, weight-matched group will be necessary in future studies.
The conditioned aversion paradigm used in this study was sensitive for detecting differences in CTA learning among the groups. The threshold dose, 0.009M, of LiCl has been shown to produce less than 50% suppression of baseline intake of the conditioned stimulus (CS, 0.3M sucrose in this case) after 8 trials of pairing in naive rats [30, 31]. In this study, neither the naive nor the pair-fed controls reached 50% level of suppression on sucrose intake during the 0.009M LiCl trials. On the other hand, the ABA rats suppressed more than 50% of baseline sucrose intake by the last 0.009M LiCl trial. Furthermore, prior work has demonstrated that rats do not normally acquire complete avoidance to a CS with up to 8 pairings with 0.018M LiCl [30]. This is consistent with the results for the pair-fed and naive rats in the current study. In contrast, the ABA rats completely avoided the sucrose CS within 6 pairings with 0.018M LiCl. Thus, these results revealed that the ABA rats learned a CTA sooner and to a greater degree than did the controls. In addition, the CTA paradigm with low doses of LiCl was sensitive to detect the differences in CTA learning between rats with and without a history of activity based anorexia.
The results of 1-bottle tests demonstrated that the ABA rats extinguished a CTA significantly more slowly than the controls (Fig. 2.). Although the sucrose preference ratio was lower in the ABA rats than in controls (Fig. 3.), the results of the 2-bottle test did not show significant differences between groups. In this study, the rats were trained for a CTA with a CS presented alone during acquisition. For extinction, the 1-bottle but not 2-bottle test retained this one bottle presentation context. This contextual difference in assessing the strength of a CTA may contribute to the inconsistent level of group differences between the results of 1-bottle and 2-bottle tests. This finding is consistent with the claim that 1-bottle test is more sensitive for detecting between-group differences in determining the strength of CTA learning than 2-bottle test [40–42].
At the time that the CTA acquisition and extinction procedures were conducted the weights of the ABA rats had returned to their pre-restriction levels but were still less than the weights of the controls at the time of testing. Whether this reduced level of body weight contributed to the CTA acquisition or extinction data is unclear. It has been suggested that rats are more resistant to CTA learning while they are food deprived or are in negative energy balance [43]. Based on this assertion, the ABA rats would be expected to acquire a CTA more slowly than the other rats because they would consume more calories to compensate for the energy deficit. This was not the case. The results showed that the ABA rats learned a CTA sooner than the controls, even though they weighed significantly less at the beginning of the CTA procedures. Moreover, there were no significant correlations between beginning body weight and final sucrose suppression during acquisition (p=0.48) or the sucrose preference ratio on the last 2-bottle test during extinction (p=0.91). This suggests that a difference in body weight or negative energy balance at the beginning of the CTA was unlikely to contribute to the sucrose aversion displayed by the ABA experienced rats.
The pathophysiological mechanisms underlying AN and those for reduced food intake, increased exercise and the maintenance of low body weight in ABA are essentially different. Nevertheless, having experienced the consequences of such behaviors may serve to sustain the behaviors in both ABA and AN. Activity based anorexic rats have been shown to have significantly less adipose tissues, and lower plasma levels of leptin and insulin [44]. Centrally, hypothalamic gene expressions of orexigenic neuropeptides (NPY and agouti-related protein) are significantly elevated, while anorexigenic peptides (pro-opiomelanocortin and cocaine- and amphetamine-related transcript) are reduced in the ABA rats compared with ad lib or pair-fed controls [45]. On the other hand, mesolimbic reward systems have been implicated in the hyperactivity and starvation paradox of AN [12]. In particular, studies have demonstrated that DA receptor 1 and 2 antagonists can reduce food associated activity [34, 46], and DA receptor antagonists has been shown to attenuate body weight loss during ABA [47]. Further studies are required to determine whether the specific neural systems involved in reward are altered after animals have experienced ABA and whether such neural alterations supports enhanced conditioned food avoidance learning.
Activity based anorexia models the consequences of repetitive abnormal eating and exercise behaviors. This experience with ABA could have long term physiological and neuronal effects that affect learning and cognitive functions. For example, it has been demonstrated that rats with ABA experience during adolescence have increased anxiety like behaviors and alterations in the HPA axis in adulthood [48]. This is in agreement with the current study. As demonstrated with a CTA paradigm, the negative food association learning and retention is enhanced specifically in rats that experienced dramatic 25% loss of initial weight due to hyperactivity coupled with reduced food intake. In some AN patients, a negative food association could initially occur during the acute phase i.e. increased motivation to exercise but reduced reward value of food paired with visceral symptoms such as nausea and vomiting [49]. The neural alterations resulting from such pairing could in turn support the sustaining avoidance of palatable food or the susceptibility of forming conditioned food aversion in the future.
Overall, the findings from this study not only support the assertion that experience with severe starvation and exercise enhances negative food associations, but also suggest that the consequences of these behaviors are not transitory. Research of this nature is likely to provide insight into the circumstances that impede treatment and facilitate relapse in eating disorders.
Research Highlights.
Activity based anorexia rats show increased activity but reduced food intake.
Activity based anorexic rats dramatically reduced 25% of initial body weight.
These anorexic rats learned a conditioned taste aversion sooner than the controls.
The same anorexic rats extinguished the aversion more slowly than the controls.
Acknowledgement
This research was supported by NIH grant DK19302.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Birmingham CL, Su J, Hlynsky JA, Goldner EM, Gao M. The mortality rate from anorexia nervosa. Int J Eat Disord. 2005;38(2):143–6. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- [2].McKnight R, Boughton N. A patient's journey. Anorexia nervosa. Bmj. 2009;339:b3800. doi: 10.1136/bmj.b3800. [DOI] [PubMed] [Google Scholar]
- [3].Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002;159(8):1284–93. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- [4].Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H, et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav. 2003;79(1):25–37. doi: 10.1016/s0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- [5].Guarda AS. Treatment of anorexia nervosa: insights and obstacles. Physiol Behav. 2008;94(1):113–20. doi: 10.1016/j.physbeh.2007.11.020. [DOI] [PubMed] [Google Scholar]
- [6].Berkman ND, Lohr KN, Bulik CM. Outcomes of eating disorders: a systematic review of the literature. Int J Eat Disord. 2007;40(4):293–309. doi: 10.1002/eat.20369. [DOI] [PubMed] [Google Scholar]
- [7].Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry. 2006;19(4):389–94. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- [8].Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord. 2003;34(4):383–96. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- [9].Hsu LK, Crisp AH, Harding B. Outcome of anorexia nervosa. Lancet. 1979;1(8107):61–5. doi: 10.1016/s0140-6736(79)90060-6. [DOI] [PubMed] [Google Scholar]
- [10].Steinhausen HC. Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am. 2009;18(1):225–42. doi: 10.1016/j.chc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- [11].Becker AE, Thomas JJ, Pike KM. Should non-fat-phobic anorexia nervosa be included in DSM-V? Int J Eat Disord. 2009;42(7):620–35. doi: 10.1002/eat.20727. [DOI] [PubMed] [Google Scholar]
- [12].Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- [13].Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiol Behav. 2004;81(2):359–74. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [14].Schmidt U, Treasure J. Anorexia nervosa: valued and visible. A cognitive-interpersonal maintenance model and its implications for research and practice. Br J Clin Psychol. 2006;45(Pt 3):343–66. doi: 10.1348/014466505x53902. [DOI] [PubMed] [Google Scholar]
- [15].Davis C. Eating disorders and hyperactivity: a psychobiological perspective. Can J Psychiatry. 1997;42(2):168–75. doi: 10.1177/070674379704200207. [DOI] [PubMed] [Google Scholar]
- [16].Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach K, et al. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr Psychiatry. 1997;38(6):321–6. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- [17].Davis C, Kennedy SH, Ravelski E, Dionne M. The role of physical activity in the development and maintenance of eating disorders. Psychol Med. 1994;24(4):957–67. doi: 10.1017/s0033291700029044. [DOI] [PubMed] [Google Scholar]
- [18].Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychol Med. 2004;34(4):671–9. doi: 10.1017/S0033291703001168. [DOI] [PubMed] [Google Scholar]
- [19].Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10–15 years in a prospective study. Int J Eat Disord. 1997;22(4):339–60. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [20].Misra M, Klibanski A. Neuroendocrine Consequences of Anorexia Nervosa in Adolescents. Endocr Dev. 2010;17:197–214. doi: 10.1159/000262540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999;21(4):503–6. doi: 10.1016/S0893-133X(99)00053-6. [DOI] [PubMed] [Google Scholar]
- [22].Wagner A, Aizenstein H, Venkatraman VK, Fudge J, May JC, Mazurkewicz L, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164(12):1842–9. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- [23].Kaye WH, Frank GK, Bailer UF, Henry SE. Neurobiology of anorexia nervosa: clinical implications of alterations of the function of serotonin and other neuronal systems. Int J Eat Disord. 2005;37(Suppl):S15–9. doi: 10.1002/eat.20109. discussion S20–1. [DOI] [PubMed] [Google Scholar]
- [24].Beneke WM, Schulte SE, vander Tuig JG. An analysis of excessive running in the development of activity anorexia. Physiol Behav. 1995;58(3):451–7. doi: 10.1016/0031-9384(95)00083-u. [DOI] [PubMed] [Google Scholar]
- [25].Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci. 1999;113(5):1080–9. [PubMed] [Google Scholar]
- [26].Dixon DP, Ackert AM, Eckel LA. Development of, and recovery from, activity-based anorexia in female rats. Physiol Behav. 2003;80(2–3):273–9. doi: 10.1016/j.physbeh.2003.08.008. [DOI] [PubMed] [Google Scholar]
- [27].Kanarek RB, Collier GH. Self-starvation: a problem of overriding the satiety signal? Physiol Behav. 1983;30(2):307–11. doi: 10.1016/0031-9384(83)90024-0. [DOI] [PubMed] [Google Scholar]
- [28].Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64(3):414–21. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- [29].Wagner A, Aizenstein H, Mazurkewicz L, Fudge J, Frank GK, Putnam K, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- [30].Grigson PS. Conditioned taste aversions and drugs of abuse: a reinterpretation. Behav Neurosci. 1997;111(1):129–36. [PubMed] [Google Scholar]
- [31].Grigson PS, Lyuboslavsky PN, Tanase D, Wheeler RA. Water-deprivation prevents morphine-, but not LiCl-induced, suppression of sucrose intake. Physiol Behav. 1999;67(2):277–86. doi: 10.1016/s0031-9384(99)00080-3. [DOI] [PubMed] [Google Scholar]
- [32].Lundy RF, Jr., Caloiero V, Bradley C, Liang NC, Norgren R. Furosemide-induced food avoidance: evidence for a conditioned response. Physiol Behav. 2004;81(3):397–408. doi: 10.1016/j.physbeh.2004.01.016. [DOI] [PubMed] [Google Scholar]
- [33].Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70(3–4):397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- [34].Scheurink AJ, Boersma GJ, Nergardh R, Sodersten P. Neurobiology of hyperactivity and reward: agreeable restlessness in anorexia nervosa. Physiol Behav. 2010;100(5):490–5. doi: 10.1016/j.physbeh.2010.03.016. [DOI] [PubMed] [Google Scholar]
- [35].Sparkes S, Grant VL, Lett BT. Role of conditioned taste aversion in the development of activity anorexia. Appetite. 2003;41(2):161–5. doi: 10.1016/s0195-6663(03)00057-6. [DOI] [PubMed] [Google Scholar]
- [36].Looy H, Eikelboom R. Wheel running, food intake, and body weight in male rats. Physiol Behav. 1989;45(2):403–5. doi: 10.1016/0031-9384(89)90147-9. [DOI] [PubMed] [Google Scholar]
- [37].Levitsky DA. Feeding patterns of rats in response to fasts and changes in environmental conditions. Physiol Behav. 1970;5(3):291–300. doi: 10.1016/0031-9384(70)90101-0. [DOI] [PubMed] [Google Scholar]
- [38].Kawaguchi M, Scott KA, Moran TH, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1800–5. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- [39].Connan F, Campbell IC, Katzman M, Lightman SL, Treasure J. A neurodevelopmental model for anorexia nervosa. Physiol Behav. 2003;79(1):13–24. doi: 10.1016/s0031-9384(03)00101-x. [DOI] [PubMed] [Google Scholar]
- [40].Batsell WR, Best MR. One bottle too many? Method of testing determines the detection of overshadowing and retentionof taste aversions. Anim Learn Behav. 1993;2:154–158. [Google Scholar]
- [41].Grote F, Brown RT. Conditioned taste aversions: Two-stimulus tests are more sensitive than one-stimulus tests. Behav Res Methods Instrument. 1971;3:311–312. [Google Scholar]
- [42].Reilly S, Bornovalova MA. Conditioned taste aversion and amygdala lesions in the rat: a critical review. Neurosci Biobehav Rev. 2005;29(7):1067–88. doi: 10.1016/j.neubiorev.2005.03.025. [DOI] [PubMed] [Google Scholar]
- [43].Bell SM, Thiele TE, Seeley RJ, Bernstein IL, Woods SC. Effects of food deprivation on conditioned taste aversions in rats. Pharmacol Biochem Behav. 1998;60(2):459–66. doi: 10.1016/s0091-3057(98)00024-0. [DOI] [PubMed] [Google Scholar]
- [44].Verhagen LA, Luijendijk MC, Korte-Bouws GA, Korte SM, Adan RA. Dopamine and serotonin release in the nucleus accumbens during starvation-induced hyperactivity. Neuropsychopharmacol. 2009;19(5):309–16. doi: 10.1016/j.euroneuro.2008.12.008. [DOI] [PubMed] [Google Scholar]
- [45].de Rijke CE, Hillebrand JJ, Verhagen LA, Roeling TA, Adan RA. Hypothalamic neuropeptide expression following chronic food restriction in sedentary and wheel-running rats. J Mol Endocrinol. 2005;35(2):381–90. doi: 10.1677/jme.1.01808. [DOI] [PubMed] [Google Scholar]
- [46].Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31(7):1371–81. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- [47].Verhagen LA, Luijendijk MC, Hillebrand JJ, Adan RA. Dopamine antagonism inhibits anorectic behavior in an animal model for anorexia nervosa. Eur Neuropsychopharmacol. 2009;19(3):153–60. doi: 10.1016/j.euroneuro.2008.09.005. [DOI] [PubMed] [Google Scholar]
- [48].Kinzig KP, Hargrave SL. Adolescent activity-based anorexia increases anxiety-like behavior in adulthood. Physiol Behav. 2010;101(2):269–76. doi: 10.1016/j.physbeh.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rigaud D, Verges B, Colas-Linhart N, Petiet A, Moukkaddem M, Van Wymelbeke V, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92(5):1623–9. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]