Abstract
The cognitive impairment in individuals with schizophrenia includes deficits of working memory in dorsolateral prefrontal cortex and deficits of performance monitoring in medial prefrontal cortex (MPFC). Recent work suggests a more general role for MPFC in predicting the outcome of actions and then evaluating those predictions. Here we investigate, in individuals with schizophrenia, two specific effects associated with this role: the error likelihood effect (occurring on trials with correct performance, but features that predict a high probability of errors), and the error unexpectedness effect (occurring on trials with an error, but features that predict errors are of low probability). In a rapid event-related fMRI design with a modified version of the change-signal task, a cue incidentally predicting error likelihood was encoded into working memory by participants in order to perform a secondary delayed match-to-sample task. There were four key findings: 1) individuals with schizophrenia exhibited poorer working memory performance and reduced error signals in MPFC; 2) even in control and schizophrenia subgroups matched on working memory performance, the schizophrenia subgroup showed a deficit in error-likelihood prediction in MPFC at the time of the predictive cue; 3) the schizophrenia subgroup also showed a deficit in evaluative error-unexpectedness activity when errors were committed; and 4) a mediation analysis indicated that error-likelihood predictions successfully explained error-unexpectedness evaluations in both controls and patients. Collectively, these findings suggest that individuals with schizophrenia have a disturbance in the evaluation of outcomes that is the result of a primary deficit in the prediction of error likelihood in MPFC.
Keywords: fMRI, schizophrenia, medial prefrontal cortex, anterior cingulate cortex, error-likelihood, performance monitoring
1 Introduction
Individuals with schizophrenia have deficits in a variety of executive control processes (Cohen et al., 1999; Kerns, 2009; Kerns et al., 2008; Laurens et al., 2003; Lee and Park, 2005). The medial prefrontal cortex (MPFC), including the anterior cingulate cortex (ACC), is an important locus of many of these processes (Beckmann et al., 2009) and their dysfunction in schizophrenia (Sanders et al., 2002; Tamminga et al., 2000). Typically, these deficits in MPFC have been interpreted using theories of error detection (Polli et al., 2008; Silver and Goodman, 2007) or conflict monitoring (Kerns, 2009) that focus on the evaluation of performance. However, new work supports a unifying view of medial prefrontal function in terms of predictions of response-outcome associations followed by evaluations of those predictions after the outcomes have occurred (Alexander and Brown, in press; Brown and Braver, 2005; Jessup et al., 2010). This leads us to hypothesize that cognitive deficits in schizophrenia may result in part from failures of predicting and evaluating the outcomes of one s actions. Specifically, we test whether individuals with schizophrenia exhibit MPFC dysfunctions in predicting the likelihood of errors given cues in the environment (the error likelihood effect) and in subsequent evaluation of committed errors as a function of their expected likelihood of occurrence (the error unexpectedness effect).
Various theories of MPFC function have been proposed, including attention to action (Posner et al., 1988), error detection (Dehaene et al., 1994; Gehring et al., 1993), and conflict monitoring (Botvinick et al., 2001). More recently, MPFC has been recast as learning to predict the value of actions (Botvinick et al., 2004; Brown and Braver, 2008; Kennerley et al., 2009; Kennerley et al., 2006; Rudebeck et al., 2008; Rudebeck et al., 2006; Rushworth et al., 2007). These ideas have been formalized and extended in the “prediction of response-outcome” (PRO) model (Alexander and Brown, in press). According to this model, when a response is planned in a given context, the MPFC first signals an outcome prediction, and then after the response is performed, the prediction is evaluated by comparing it to the actual outcome to produce a discrepancy, or prediction error, signal (Figure 1A). This model can account for a variety of MPFC findings, both those central to past theories of MPFC function, including error effects (Gehring et al., 1993) and conflict effects (Botvinick et al., 1999), as well as more recent findings (e.g. Behrens et al., 2007; Brown, 2009; Jessup et al., 2010).
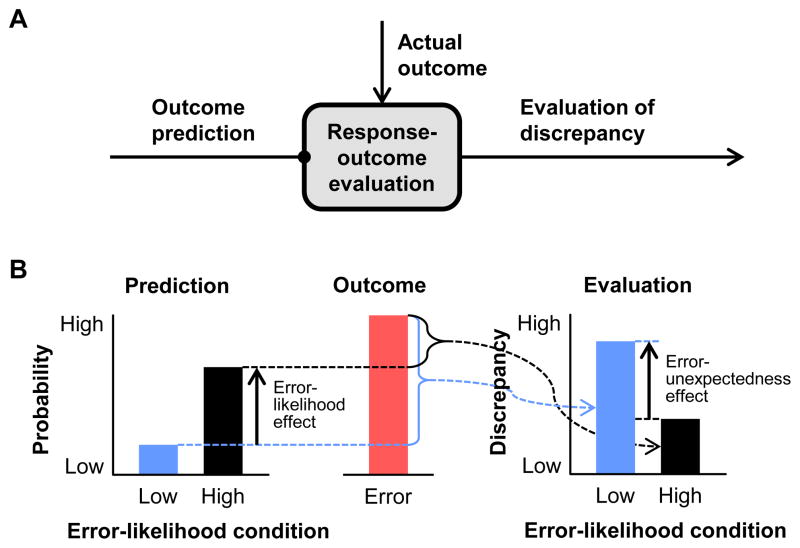
Figure 1.
Conceptual model of outcome prediction and evaluation and resulting neural effects. A) A response-outcome evaluation module computes the discrepancy between the predicted outcome and the actual outcome in the form of a prediction error, or unexpectedness, signal. B) The error-likelihood effect is the result of a greater predicted probability of error occurrence in the high error-likelihood condition than the low error-likelihood condition. When an error does occur, the error-unexpectedness effect is the result of the greater evaluated discrepancy between the actual and predicted outcomes in the low error-likelihood condition than the high error-likelihood condition.
To test for effects of error likelihood and error unexpectedness, we used a task design with two conditions indicated by separate cues, in which one cue signals that subsequent responses will be associated with a high frequency of errors (high error-likelihood condition), while the other indicates a low error-likelihood condition. According to the PRO model, once the participant has learned the task contingencies, the MPFC prediction signal at the time of the cue will be greater in the high error-likelihood condition, where errors (as well as correct outcomes) are likely, compared to the low error-likelihood condition, where only correct outcomes are likely (Figure 1B). This difference, the error-likelihood effect, has been found in MPFC in a change-signal task (Brown and Braver, 2005; Brown and Braver, 2007) (but see Aarts et al., 2008; Nieuwenhuis et al., 2007). Conversely, when an error does in fact occur, the PRO model predicts that the discrepancy, or prediction error, signal will be greater in the low error-likelihood condition (Figure 1B). This is because in the low error likelihood condition the occurrence of an error is not expected, while in the high error-likelihood condition, error occurrence is expected. This difference, the error-unexpectedness effect, has been found in MPFC in a change-signal task (Brown and Braver, 2005), as well as with the error-related negativity in a time-estimation task (Holroyd and Krigolson, 2007). According to the PRO model, error-likelihood predictions also mediate error-unexpectedness evaluations, because a greater disparity between predictions for two task conditions will subsequently lead to a greater disparity in prediction errors between those conditions when errors occur (Figure 2A, B, & E).
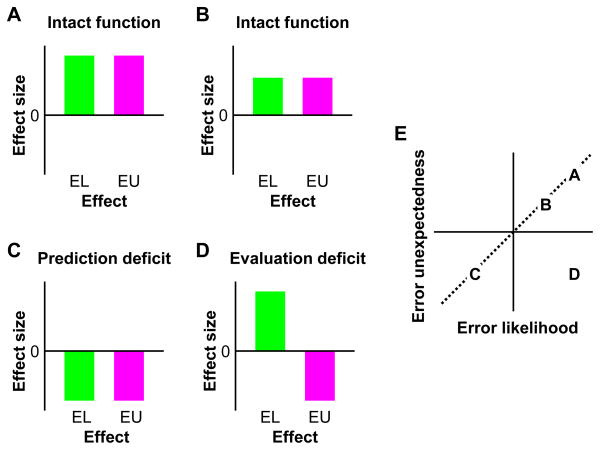
Figure 2.
Hypothetical results for intact and disrupted function of MPFC response-outcome model. A & B) Following Figure 1B, the magnitude of error-likelihood and error-unexpectedness effects are correlated when outcome prediction and evaluation are intact. C) If prediction is impaired then the error-likelihood effect may be abnormal. This leads to an abnormal error-unexpectedness effect, yet the relationship between likelihood and unexpectedness remains. D) If evaluation is impaired, then error-unexpectedness alone may be abnormal, and the intact relationship between likelihood and unexpectedness will be violated. E) The relationship between error-likelihood and error-unexpectedness effects for the cases in panels A-D. Dashed line indicates idealized relationship in an intact system. EL = error-likelihood; EU = error-unexpectedness.
With this model in mind, we can now reconsider the MPFC dysfunctions found in individuals with schizophrenia. Error-detection deficits have been found in schizophrenia patients in the form of a decreased error-related negativity (Mathalon et al., 2002; Morris et al., 2006) and decreased ACC activity (Carter et al., 2001; Laurens et al., 2003; Polli et al., 2008). In addition, conflict monitoring deficits in MPFC have been found in schizophrenia using both event-related potentials (McNeely et al., 2003) and fMRI (Kerns et al., 2005). These findings have been interpreted as indicating an underlying impairment in the monitoring and evaluation of performance (Kerns et al., 2008). However, an alternative, equally consistent hypothesis, suggested by the PRO model, is that these deficits reflect an underlying impairment in predicting the outcome of actions.
These two possibilities, a deficit in prediction or a deficit in evaluation, can be distinguished using the error-likelihood and error-unexpectedness effects in MPFC (Figure 2). If the deficit is primarily a failure of prediction, then the error-likelihood effect will be abnormal since this effect directly reflects the accuracy of outcome predictions. Additionally, these prediction failures will lead to discrepancies between the predicted and actual outcomes, causing an abnormal error-unexpectedness effect. However, the mediation of error unexpectedness by error likelihood will remain, since the process of evaluation is intact, and thus the prediction errors, while abnormal, will nonetheless still be consistent with the predictions. For example, a failure of prediction could cause the prediction signal to be greater in the low error-likelihood condition than in the high error-likelihood condition, leading to an inverted error-likelihood effect. As a result, when errors do occur, the discrepancy signal will be greater in the high error-likelihood condition than in the low error-likelihood condition, and so the error-unexpectedness effect will also be inverted (Figure 2C & E).
On the other hand, if the deficit is primarily a failure of evaluation, then while the predictions and thus the error-likelihood effect will be unchanged, the error-unexpectedness effect will be abnormal, since the prediction errors are utilized in an inappropriate way by the system. Furthermore, the mediation of error-unexpectedness by error-likelihood will be lost given that the discrepancy signals will not accurately reflect the difference between the predicted and actual outcomes due to deficits in monitoring the actual outcomes. For example, a failure of evaluation could cause the discrepancy signal to incorrectly be greater in the high error-likelihood condition than in the low error-likelihood condition. This would lead to the error-unexpectedness effect being inverted, but the prediction signals and the error-likelihood effect would be unaffected (Figure 2D & E).
To evaluate these hypotheses, we used a rapid event-related fMRI design with a modified version of the change-signal task; a task constructed to separate out error-likelihood effects from effects of conflict and error (Brown and Braver, 2005). We added a delay between the cue incidentally predicting error likelihood and the go signal, and we also added a secondary delayed match-to-sample (DMTS) task with the cue acting as the sample (Figure 3). The delay, along with the inclusion of partial trials, enabled us to separately estimate the brain activity at the time of the cue and the response (Ollinger et al., 2001a; Ollinger et al., 2001b). This allowed us to evaluate whether error-likelihood signals in MPFC occurred at the time of the predictive cue or at the time of the actual response, a distinction not allowed by the original task design (Brown and Braver, 2005; Nieuwenhuis et al., 2007).
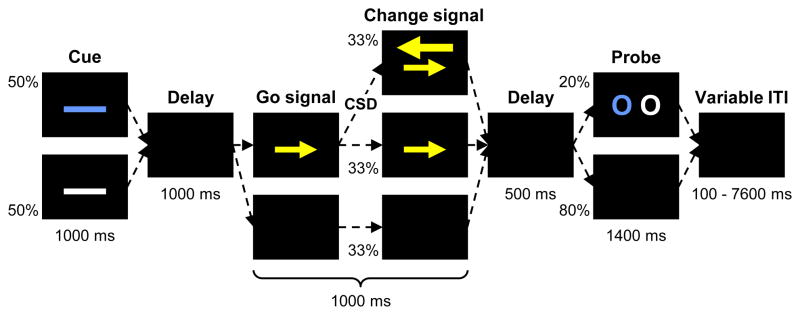
Figure 3.
Schematic of a trial with a change-signal task (CST) embedded within a delayed-match-to-sample task (DMTS). Each trial began with a high (blue) or low (white) error-likelihood cue, the sample for the DMTS task. After a delay, there was either a go signal, a go signal followed by a change signal, or a continued delay. Participants were to respond to the go signal as quickly as possible with a compatible response, but to respond to the change signal instead if it subsequently appeared. The duration of the change signal delay (CSD) was determined by the error-likelihood cue and the participant s past performance. After a further delay, either the DMTS probe was presented or the delay continued. Finally, there was a variable inter-trial interval (ITI) before the next trial began. Percentages indicate proportion of trials for which each event occurred. Times in milliseconds indicate duration of each event.
The DMTS task was included to encourage participants to encode the cue into working memory. Since the cue was not available at the time of the go and change signals, it was necessary to have it in working memory in order to learn the association between the cue and the error-likelihood condition. Previous work has suggested that information in working memory can modulate predictive MPFC activity (Aarts et al., 2008; Sohn et al., 2007). However, it is also well established that working memory, particularly working memory for task context, is impaired in schizophrenia (Barch et al., 2001; Braver et al., 1999; Cohen et al., 1999; Lee and Park, 2005; MacDonald and Carter, 2003; Meyer-Lindenberg et al., 2001). Thus it was important to exclude the possibility that deficits in error prediction and evaluation were simply due to an underlying working memory deficit. To this end, we used subgroups of patients and controls with matched DMTS accuracy to remove memory for the cue as a confound.
2 Methods
The Institutional Review Board of Washington University in St. Louis approved the experimental procedure reported here.
2.1 Participants
Thirteen non-psychiatric controls and fifteen individuals with DSM-IV schizophrenia were recruited from the local population around Washington University. Both groups were evaluated with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al., November 2002). Individuals from both participant groups were excluded if they met criteria for substance dependence in the past six months or abuse within the past month. Controls were additionally excluded if they met criteria for any psychotic disorder, including bipolar disorder. Individuals with schizophrenia were medicated outpatients taking typical or atypical antipsychotics. One control was excluded from data analysis for failing to conform to the task instructions in the change-signal task, leaving data from twelve controls. One patient was excluded from further analysis for failure to perform the full complement of runs, leaving data from fourteen patients. Controls and patients were matched on handedness (all right handed), gender, χ2(1, N = 25) = .02, p = .90, race, χ2(2, N = 25) = 1.65, p = .44, age, t(23) = .15, p = .88, and highest parental education level, t(17) = −0.39, p = .70 (Table 1). Some analyses were performed on subgroups matched on accuracy in the delayed match-to-sample task. These subgroups were also matched on handedness (all right handed), gender, χ2(1, N = 17) = .01, p = .91, race, χ2(2, N = 17) = 1.61, p = .45, age, t(15) = 1.68, p = .11, and highest parental education level, t(13) = −0.58, p = .57 (Table 1).
Table 1.
Demographic Characteristics
| Full groups |
Matched subgroups |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Controls | Patients | Controls | Patients | ||||
| N | (%) | N | (%) | N | (%) | N | (%) | |
| Participants | 12 | 14 | 9 | 9 | ||||
| Male gendera | 9 | (75) | 9 | (69) | 7 | (78) | 7 | (88) |
| White racea,b | 6 | (50) | 4 | (29) | 4 | (44) | 2 | (25) |
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| Age (years)a | 41.4 | (6.1) | 40.8 | (11.8) | 40.8 | (6.0) | 34.1 | (10.0) |
| HPEL (years)c | 13.1 | (1.0) | 13.5 | (2.2) | 13.1 | (1.0) | 13.7 | (2.7) |
Note. The three most accurate controls and the five least accurate patients on the delayed match-to-sample task were dropped to create the matched subgroups. HPEL = Highest parental education level.
Information missing for one patient;
One patient was Hispanic, all other non-white participants were black;
Information missing for 4 controls and 3 patients.
2.2 Procedure
After receiving spoken instructions and completing surveys for several individual difference measures, participants performed a small number of practice trials to insure that they understood the task instructions and the response mappings.
During fMRI data collection, participants performed five blocks of 66 trials of a modified change-signal task (CST) embedded within a delayed match-to-sample task (DMTS) (see Figure 3). Visual stimuli were presented using PsyScope software running on an Apple PowerMac G4. Stimuli were projected to participants with an AmPro LCD projector (Model 150) onto a screen positioned at the head end of the bore. Participants viewed the screen through a mirror attached to the head coil. A fiber-optic, light-sensitive key press that interfaced with the PsyScope button box was used to record participants behavioral performance.
Each trial began with the presentation of a blue or white colored bar for 1000 ms. Participants were instructed to remember the color of the bar for the duration of the trial. Initially unbeknownst to the participant, the color also specified whether the subsequent change-signal task would be of high or low error likelihood, in trials for which a change signal was later presented. Thus this stimulus acted as an incidental error-likelihood cue for the change-signal task. Each color was presented randomly on 50% of trials. The association between color and error likelihood was counter-balanced across participants.
The error-likelihood cue was followed by a 1000 ms empty delay. What followed the delay depended on whether it was a CST go trial, a CST change trial, or a trial in which the CST was omitted, with each occurring on 33% of trials. When the CST was omitted, the empty delay continued for another 1000 ms. On go and change trials, a go signal consisting of a yellow arrow was presented. The arrow pointed to the right or left, with each occurring on 50% of trials. Participants were to respond as quickly as possible with a compatible right or left index finger click of a response key. However, on change trials, after a change-signal delay (CSD), a change signal, a second larger yellow arrow, appeared above the go signal pointing in the opposite direction. In this case, participants were to withhold their initial response, and instead respond to the change signal with the opposite finger.
The likelihood of errors in the change trials was manipulated by altering the CSD. The CSD was determined separately for the high and low error-likelihood conditions for each participant, using a trial-to-trial asymmetric adaptive staircase procedure with a maximum step of 50 ms (Brown and Braver, 2005). The target error rate was 50% for the high error-likelihood condition and 10% for the low error-likelihood condition. On both go and change trials, the arrow(s) were removed from the screen 1000 ms after the onset of the go signal. If the participant failed to respond in those 1000 ms, then the message FASTER appeared on the screen during the middle 300 ms of the subsequent 500 ms delay.
The change-signal task was followed by a 500 ms empty delay. After this delay, the DMTS task occurred on 20% of trials, while it was omitted and the blank screen continued for 1400 ms on 80% of trials. On DMTS trials, a probe consisting of adjacent blue and white circles was presented for 1400 ms. The location, right or left, of the colors was counter-balanced across trials. Participants were to respond to this two-alternative forced choice query with a compatible right or left index finger click of a response key to indicate the color of the error-likelihood cue presented at the beginning of the trial. The trial ended, and an exponentially-distributed inter-trial interval (ITI) of 100, 2600, 5100, or 7600 ms followed before the next trial began.
The error-likelihood cue (high or low), CST condition (go, change, or omit), go signal direction, DMTS condition (probe or omit), and DMTS probe positions were pseudo-randomly ordered across trials. The pseudo-random trial ordering, the use of variable-length ITIs, and the use of partial trials for both the CST and the DMTS task were designed to facilitate separate estimation of the event-related brain activations associated with the cue, the CST signals and response, and the DMTS probe and response (Dale, 1999; Ollinger et al., 2001a; Ollinger et al., 2001b).
2.3 fMRI analysis
2.3.1 Image acquisition and preprocessing
Imaging data were collected on a 1.5 Tesla Siemens Magnetom Vision. For each participant, functional blood oxygenation-level dependent (BOLD) data were collected using gradient-echo echo-planar imaging for 5 blocks of 188 whole brain volumes (echo time [TE] = 37 ms, repetition time [TR] = 2568.8 ms, flip angle = 90°) with 19 axial slices (64 by 64 grid, 3.75 by 3.75 by 7 mm voxels, interleaved order, no spacing between slices). A structural scan was collected using three dimensional MP-RAGE imaging (TE = 4 ms, TR = 9.7 ms, flip angle = 10°) with 128 sagittal slices (224 by 256 grid, 1 by 1 by 1.25 mm voxels, no spacing between slices).
Preprocessing of the data was done using SPM5 (Wellcome Trust Centre for Neuroimaging, 2005) with default parameters except where otherwise specified. The structural scans were skull-stripped using BET2 (Péchaud et al., 2006) with default parameters. The functional images were slice timing corrected using Fourier phase shift interpolation with the first slice as reference, and then motion corrected and resliced using least squares 6-parameter rigid-body transformation. The functional images were then coregistered with the skull-stripped structural scan using affine transformation, and then registered to MNI space and resliced using segmentation and normalization by first segmenting the structural scans. Finally, the normalized images were smoothed using an 8 mm3 FWHM Gaussian kernel.
2.3.2 Intrasubject analysis
First-level analysis of the preprocessed fMRI data was performed using SPM5. A general linear model (GLM) was run for each participant using SPM5 s canonical hemodynamic response function with no derivatives, a micro-time resolution of 16 time-bins per scan, a high-pass filter cutoff at 128 seconds using a residual forming matrix, autoregressive AR(1) to account for serial correlations, and restricted maximum likelihood (ReML) for model estimation. The model included a constant term, 24 motion regressors generated from the six parameters of the motion correction performed during preprocessing, and 11 event-related regressors to model task-relevant activations. The motion regressors, consisting of the linear movement parameters, the squares of those parameters, the sequential differences of those parameters, and the squares of the sequential differences, were included to account for participant head movement during scanning (Friston, 1996), which can be a particular concern with patient populations (Mayer et al., 2007).
The 11 event-related regressors modeled the task as follows. Two cue-related regressors, time-locked to the onset of the cue, modeled high and low error-likelihood (HighCue and LowCue). Seven CST-related regressors, time-locked to the onset of the go signal, modeled high and low error-likelihood, go and change trials, and correct and error responses (HighGoCorrect, LowGoCorrect, HighChangeCorrect, LowChangeCorrect, HighChangeError, LowChangeError, and GoError/NoResponse). All errors on go trials and all failures to respond on go and change trials were modeled as a single category due to their infrequency. Finally, two DMTS-related regressors, time-locked to the onset of the probe, modeled correct and error responses (DMTSCorrect and DMTSError).
After the GLMs were run, contrasts of interest were defined for changes in brain activity at the time of the error-likelihood cue and the change-signal task. The error-likelihood effect at the time of the cue is: HighCue – LowCue. The error-likelihood effect at the time of the go signal and response is: HighGoCorrect – LowGoCorrect. The error effect, collapsing across error likelihood condition, is: (LowChangeError + HighChangeError) – (LowChangeCorrect + HighChangeCorrect). And the error-unexpectedness effect is: (LowChangeError – LowChangeCorrect) – (HighChangeError – HighChangeCorrect).
2.3.3 Group analysis
The per-participant contrasts were the basis for second-level random effects analyses performed with ReML estimation in SPM5 assuming independence and unequal variance between groups. The voxels included in the statistical tests were constrained using small-volume correction to an anterior dorsomedial region of prefrontal cortex (Supplementary Figure S1). The volume of interest, containing 10,748 2 mm3 voxels was defined using the WFU PickAtlas Tool (Maldjian et al., 2003), the Talairach Daemon (Lancaster et al., 2000), and MarsBaR (Brett et al., 2002). The volume consisted of the union of the anterior cingulate, cingulate gyrus, and medial frontal gyrus using 1 mm three-dimensional dilation, further constrained to those voxels with x-coordinates between –15 and 15 inclusive, y-coordinates greater than or equal to zero, and z-coordinates greater than or equal to zero, all specified in MNI space.
The statistical threshold for significance was set at the cluster-level to p < .05, with family-wise error (FWE) correction using random field theory. Clusters were defined using a voxel-wise height threshold of p < .001, uncorrected. Smoothness, which varied slightly between analyses, was approximately 10 x 10 x 10 mm with 60 RESELs in each volume. The skull-stripped single-subject MNI CH2BET template was used as the background brain image in all figures. Anatomical labeling was done using the Talairach Daemon (Lancaster et al., 2000) and xjView (Cui et al., 2009).
2.3.4 ROI analysis
Follow-up ROI analyses were performed using SPM5 and MarsBaR 0.41 (Brett et al., 2002) within significant clusters identified in the 2nd-level analyses described above. The ROI analyses provide further descriptive details about the patterns of activations within these clusters. It should be noted that when the follow-up contrasts are not orthogonal to the contrasts used to identify the cluster, the results are not independent (Lieberman et al., 2009). Each ROI was defined as the set of significant clusters within the search volume for a particular 2nd-level test of a particular contrast. Mean parameters for controls and patients were estimated within each ROI and reported as percent magnetic resonance (MR) signal change calculated as the mean magnitude of the event regressor relative to the mean magnitude of the constant term regressor.
2.3.5 Mediation analysis
Mediation analysis provides an approach to testing hypotheses about pathways relating independent variables to dependent variables by way of intervening mediator variables (Baron and Kenny, 1986). Here, the error-likelihood condition (high or low) is the independent variable, the medial prefrontal error-likelihood prediction signal is the mediator variable, and the medial prefrontal error-unexpectedness evaluation signal is the dependent variable. Since error-likelihood was manipulated within-subject, the tests determining mediation are: 1) the prediction signal varies as a function of error-likelihood, i.e. there is an error-likelihood effect; 2) the evaluation signal varies as a function of error-likelihood, i.e. there is an error-unexpectedness effect; and most critically, 3) the difference in error-unexpectedness between conditions is predicted by the difference in error-likelihood, i.e. the error-likelihood effect predicts the error-unexpectedness effect (Judd et al., 2001).
Using the Mediation Effect Parametric Mapping approach, an ROI is identified using one of the contrasts of interest, and then the contrast values for individual participants from this ROI are used as the parameters in a linear regression on the other contrast of interest to construct a statistical parametric map identifying voxels that complete the mediation relationship (Wager et al., 2008). An error-likelihood ROI was used to search for mediated error-unexpectedness regions, and an error-unexpectedness ROI was used to search for mediating error-likelihood regions. All mediation analyses were done in the MPFC search volume described above using p < .005, uncorrected, and 3 contiguous voxels, to balance the need for sensitivity with avoidance of false positives (Wager et al., 2008).
3 Results
3.1 Behavioral performance
Behavioral measures for the delayed match-to-sample task and change-signal task are shown in Table 2 with statistics reported in the text.
Table 2.
Behavioral Performance
| Task | Full groups |
Matched subgroups |
||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Controls |
Patients |
Controls |
Patients |
||||
| Condition | M | (SD) | M | (SD) | M | (SD) | M | (SD) |
| DMTS | ||||||||
| Accuracy (% correct) | 90.0 | (10.8) | 64.8 | (27.3) | 86.9 | (10.9) | 82.2 | (8.8) |
| Reaction time (ms) | 644 | (101) | 765 | (207) | 664 | (97) | 646 | (107) |
| CST | ||||||||
| CSD (ms) | ||||||||
| High | 534 | (100) | 414 | (135) | 517 | (112) | 472 | (118) |
| Low | 318 | (100) | 239 | (99) | 300 | (110) | 268 | (93) |
| Accuracy (% correct) | ||||||||
| High/Go | 89.9 | (6.9) | 76.6 | (26.3) | 91.1 | (6.3) | 88.4 | (12.9) |
| High/Change | 39.0 | (7.0) | 33.5 | (14.9) | 40.6 | (7.1) | 40.4 | (7.4) |
| Low/Go | 91.1 | (6.5) | 76.3 | (29.2) | 91.5 | (6.4) | 88.6 | (13.1) |
| Low/Change | 94.2 | (6.1) | 74.8 | (29.9) | 93.0 | (6.5) | 88.9 | (11.3) |
| Reaction time (ms) | ||||||||
| High/Go | 775 | (111) | 730 | (113) | 750 | (118) | 720 | (97) |
| High/Change | 929 | (75) | 885 | (89) | 918 | (84) | 896 | (71) |
| Low/Go | 788 | (108) | 732 | (100) | 768 | (118) | 729 | (98) |
| Low/Change | 769 | (78) | 778 | (107) | 765 | (90) | 747 | (68) |
Note. The three most accurate controls and the five least accurate patients on the DMTS task were dropped to create the matched subgroups.
DMTS = delayed match-to-sample task; CSD = change-signal delay; CST = change-signal task; High = high difficulty; Low = low difficulty; Go = CST go trials; Change = CST change trials.
3.1.1 Delayed match-to-sample task
As predicted based on past work, controls were more accurate than patients in the DMTS task, t(24) = 2.99, p = .006. To test whether the patients differed from the controls in error-likelihood prediction apart from their clear differences in working memory performance, we established subgroups of controls and patients who were matched on DMTS accuracy, t(16) = 1.00, p = .33. These subgroups were formed by removing the three most accurate controls and the five least accurate patients on the DMTS task, leaving nine controls and nine patients. As reported in the methods section and shown in Table 1, the demographics for these subgroups remained equated. Neither the full groups, t(24) = −1.85, p = .08, nor subgroups, t(16) = .39, p = .70, differed significantly in DMTS reaction time.
3.1.2 Change-signal task
The change-signal delay (CSD) was adjusted for each participant for each error-likelihood condition so as to maintain the target error rates on change trials. As a result, the delays were longer for the high error-likelihood condition with both the full groups, F(1, 24) = 311.12, p < .001, and the subgroups, F(1, 16) = 242.95, p < .001. CSDs were longer for the controls than patients for the full groups, F(1, 24) = 5.63, p = .03, but not for the subgroups, F(1, 16) = .60, p = .45. There were no interactions of group and condition for either full groups, F(1, 24) = 3.25, p = .08, or subgroups, F(1, 16) = .26, p = .62.
Since accuracy on change trials was manipulated by adjusting the CSD to achieve target error rates, it is unsurprising that, for both the full groups and subgroups, accuracy was lower for the high error-likelihood change trials than for the other conditions, leading to main effects of task condition, full groups: F(1, 24) = 148.33, p < .001, subgroups: F(1, 16) = 95.80, p < .001, and error likelihood, full groups: F(1, 24) = 176.33, p < .001, subgroups: F(1, 16) = 360.92, p < .001, and an interaction of the two, full groups: F(1, 24) = 286.75, p < .001, subgroups: F(1, 16) = 423.46, p < .001. With the full groups, controls were marginally more accurate than patients across conditions, F(1, 24) = 3.51, p = .07, but less so in the high error-likelihood change trials, leading to an interaction of group with error likelihood, F(1, 24) = 4.52, p = .04, and a three-way interaction of group, task condition, and error likelihood, F(1, 24) = 4.93, p = .04, but no interaction of group with task condition, F(1, 24) = .17, p = .69. However, with the matched subgroups, controls and patients did not differ significantly in accuracy, F(1, 16) = .59, p = .45, and group did not interact with task condition, F(1, 24) = .021, p = .89, nor error likelihood, F(1, 16) = .62, p = .44, and there was no three-way interaction, F(1, 16) =.54, p = .47.
Reaction time was measured from the onset of the go signal, yet accurate responses on change trials depended on responding to the change signal which occurred after the CSD. As a result, for both the full groups and subgroups, reaction times were longer for the high error-likelihood change trials than for the other conditions, leading to main effects of task condition, full groups: F(1, 24) = 37.81, p < .001, subgroups: F(1, 16) = 21.68, p < .001, and error likelihood, full groups: F(1, 24) = 109.97, p < .001, subgroups: F(1, 16) = 123.08, p < .001, and an interaction of the two, full groups: F(1, 24) = 79.95, p < .001, subgroups: F(1, 16) = 162.86, p < .001. With the full groups, controls were slightly faster than patients in every condition except the low error-likelihood change trials, leading to a three-way interaction of group, task condition, and error likelihood, F(1, 24) = 4.48, p = .04, but no main effect of group, F(1, 24) = .93, p = .34, nor interactions of group with task condition, F(1, 24) = 1.41, p = .25, or error likelihood, F(1, 24) = 3.01, p = .10. But with the matched subgroups, controls and patients did not differ significantly in reaction time, F(1, 16) = .48, p = .50, and group did not interact with task condition, F(1, 16) = .14, p = .72, or error likelihood, F(1, 16) = .03, p = .87, and there was no three-way interaction, F(1, 16) = .26, p = .61.
Importantly, the subgroups of patients and controls selected by matching solely on DMTS accuracy did not differ on any of the other measures for either task. This suggests that the critical fMRI contrasts comparing the groups are not confounded by differences in time-on-task or error rate.
3.2 fMRI results
The imaging analysis was confined to the dorsomedial prefrontal volume of interest described in the methods and shown in Supplementary Figure S1.
3.2.1 Error effects
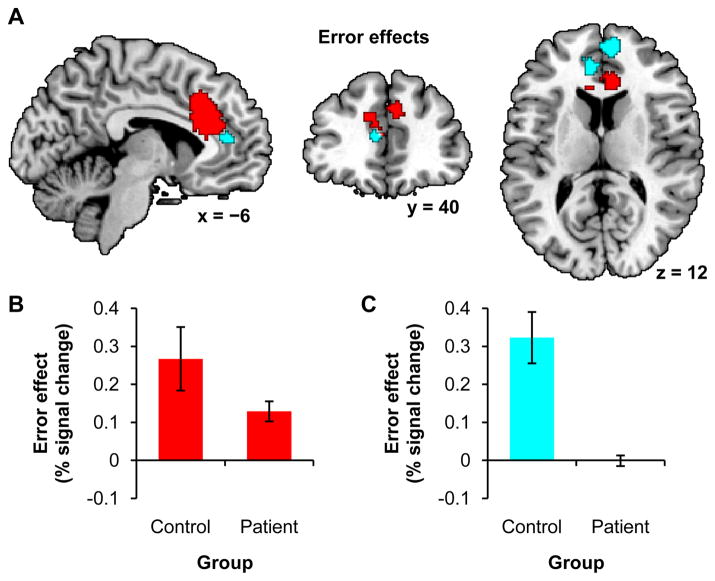
For comparison to past studies, we first looked at error effects using the full groups of participants. We found a large bilateral cluster in medial frontal gyrus and anterior cingulate showing a positive error effect across controls and patients, p < .05, FWE (Table 3 and Figure 4 in red). We then looked for areas showing an interaction between error effect and group. We identified clusters in right medial frontal gyrus and left anterior cingulate and medial frontal gyrus where controls had a greater error effect than patients, p < .05, FWE (Table 3 and Figure 4 in cyan). No clusters were identified where patients had a greater error effect than controls.
Table 3.
fMRI Results
| Groups | BA | Peak MNI coordinates |
Peak Z-score | Cluster p-value | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|---|
| Effect (Comparison) | |||||||
| Brain region(s) | x | y | z | ||||
| Full groups | |||||||
| Error (Main effect: All > 0) | |||||||
| B. MFG/ACC | 32/24/9 | −12 | 38 | 24 | 4.10 | < .001 | 1393 |
| Error (Interaction: Control > Patient) | |||||||
| R. MFG | 10 | 4 | 56 | 12 | 3.84 | .007 | 90 |
| L. ACC/MFG | 32/10 | −10 | 50 | 8 | 3.81 | .02 | 64 |
| Matched subgroups | |||||||
| Error-likelihood (Interaction: Control > Patient) | |||||||
| B. ACC/MFG | 32/10 | 14 | 40 | 6 | 4.08 | < .001 | 168 |
| Error-unexpectedness (Interaction: Control > Patient) | |||||||
| L. ACC | 32/24 | −10 | 22 | 24 | 3.60 | .006 | 68 |
| B. MFG | 9 | 8 | 44 | 32 | 3.72 | .05 | 33 |
Note. Clusters are significant within a predefined dorsomedial prefrontal search volume at p < .05, FWE, with a height threshold of p < .001.
BA = Brodmann area; MNI = Montreal Neurological Institute; B = bilateral; R = right; L = left; ACC = anterior cingulate cortex; MFG = medial frontal gyrus.
Figure 4.
Regions showing error effects with all participants. A) Red clusters show a main effect of error across both groups. Cyan clusters show an interaction, with a greater error effect for controls than patients. Clusters are significant within a predefined dorsomedial prefrontal search volume at p < .05, FWE, with a height threshold of p < .001. Coordinates are in MNI space. B and C) Post hoc ROI analyses showing the error effects for patients and controls in the red and cyan regions respectively. Error bars indicate SEM.
Subsequent analyses were performed with the DMTS accuracy-matched subgroups. A smaller cluster, consisting of a subset of the region identified with the full groups, again showed a positive error effect across controls and patients, p < .05, FWE (Supplementary Table S1 and Supplementary Figure S2 in red). And a cluster, highly overlapping with the right medial frontal gyrus cluster identified with the full groups, showed a greater error effect for controls than patients with the subgroups, p < .05, FWE (Supplementary Table S1 and Supplementary Figure S2 in cyan). And again, no clusters were identified where patients had a greater error effect than controls. These findings established our ability to detect activations in both controls and patients, and replicated past findings showing diminished medial prefrontal error effects in individuals with schizophrenia.
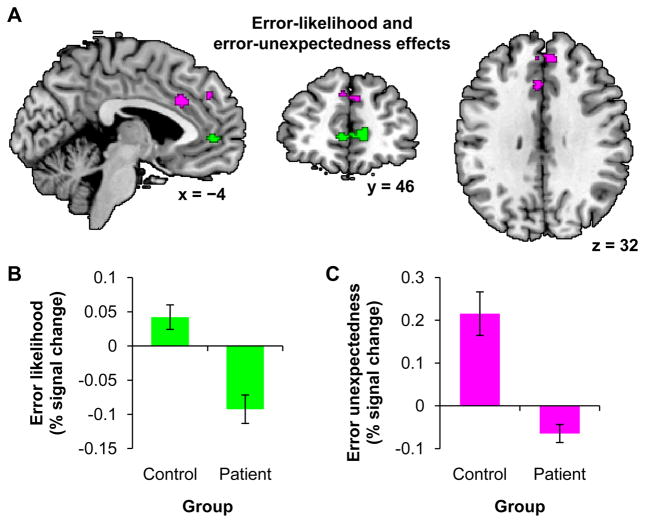
3.2.2 Error-likelihood and error-unexpectedness effects
We then focused on the error-likelihood and error-unexpectedness effects in the subgroups matched on DMTS accuracy. The experimental design allowed us to test for error-likelihood effects both at the presentation of the error-likelihood cue, and at the presentation of the go signal and subsequent response. At the time of the cue, we found no clusters with a positive or negative error-likelihood effect across both subgroups, and we found no clusters where patients had a great error-likelihood effect than controls. However, we did find a significant cluster in bilateral anterior cingulate and medial frontal gyrus where controls had a greater error-likelihood effect than patients, p < .05, FWE (Table 3 and Figure 5 in green). At the time of the go signal and response, no clusters were identified with a significant positive or negative error-likelihood effect across both subgroups, nor were any clusters identified where one group showed a greater error-likelihood effect than the other group.
Figure 5.
Regions showing error-likelihood and error-unexpectedness effects with DMTS accuracy-matched subgroups. A) Green clusters show a greater error-likelihood effect for controls than patients. Violet clusters show a greater error-unexpectedness effect for controls than patients. Clusters are significant within a predefined dorsomedial prefrontal search volume at p < .05, FWE, with a height threshold of p < .001. Coordinates are in MNI space. B and C) Post hoc ROI analyses showing the error-likelihood and error-unexpectedness effects for patients and controls in the green and violet regions respectively. Error bars indicate SEM.
We found no clusters with positive or negative error-unexpectedness effects across both subgroups, nor did we find clusters with a greater error-unexpectedness effect in patients than controls. However, we did find clusters in left anterior cingulate and bilateral medial frontal gyrus where controls had a greater error-unexpectedness effect than patients, p < .05, FWE (Table 3 and Figure 5 in violet).
In summary, a medial prefrontal error-likelihood effect was found only at the time of the cue, and it was greater in controls than patients, and a medial prefrontal error-unexpectedness effect was also found to be greater in controls than patients. The pattern found for controls is consistent with the prediction of intact function shown in Figure 2A and 2B. The pattern found for patients is consistent with an outcome prediction deficit as shown in Figure 2C.
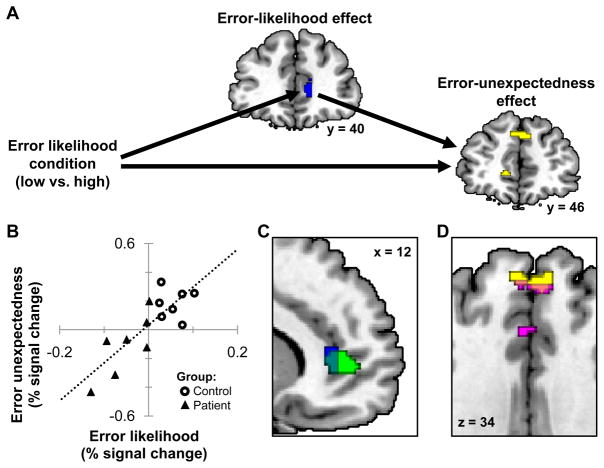
3.2.3 Mediation of error unexpectedness by error likelihood
To evaluate the relationship between brain regions sensitive to error likelihood and those sensitive to error unexpectedness, we performed a mediation analysis with error-likelihood condition as the independent variable. So as not to bias the results towards a finding of mediation, the potentially mediating brain region was identified by a positive error-likelihood effect solely for the control subgroup, p < .005, uncorrected, with at least 3 contiguous voxels (Table 4 and Figure 6A in blue). With this region as the potential mediator, we identified regions in bilateral medial prefrontal gyrus and left anterior cingulate and medial frontal gyrus showing evidence of a mediated error-unexpectedness effect across both subgroups, p < .005, uncorrected, with at least 3 contiguous voxels (Table 4 and Figure 6A in yellow). The mediation relationship between these regions across the control and patient subgroups is illustrated in Figure 6B. The fact that the y intercept is approximately zero indicates complete mediation (Judd et al., 2001). In other words, group differences in the MPFC region showing positive error-likelihood effects fully accounted for the group differences in MPFC regions showing group differences in error-unexpectedness effects. Note the consistency of these findings with the predictions shown in Figure 2E.
Table 4.
Mediation Analysis: Error-Unexpectedness Regions Mediated by Error-Likelihood ROI
| Effect (Comparison) | BA | Peak MNI coordinates |
Peak Z-score | Peak p-value | Cluster size (voxels) | ||
|---|---|---|---|---|---|---|---|
| Brain region(s) | x | y | z | ||||
| Error-likelihood (Contrast: Control > 0) | |||||||
| R. ACC | 32 | 12 | 40 | 8 | 3.53 | < .001 | 38 |
| Error-unexpectedness mediated by error-likelihood (Main effect: All > 0) | |||||||
| B. MFG | 9 | 6 | 44 | 34 | 3.41 | < .001 | 93 |
| L. ACC | 32 | −10 | 46 | 0 | 3.23 | .001 | 14 |
| L. MFG | 9 | −10 | 56 | 28 | 2.95 | .002 | 4 |
| L. MFG | 9 | −12 | 56 | 22 | 2.76 | .003 | 4 |
Note. Clusters are significant within a predefined medial prefrontal search volume at p < .005, uncorrected, with a cluster size threshold of 3.
BA = Brodmann area; MNI = Montreal Neurological Institute; B = bilateral; R = right; L = left; ACC = anterior cingulate cortex; MFG = medial frontal gyrus.
Figure 6.
Regions showing within-subject mediation of error-unexpectedess by error-likelihood with DMTS accuracy-matched subgroups. A) The blue ROI, identified as showing an error-likelihood effect in controls, mediates the relationship between the error-likelihood condition and error unexpectedness in the yellow clusters across controls and patients. Voxels are significant within a predefined dorsomedial prefrontal search volume at p < .005, uncorrected, with a cluster-size threshold of 3. B) Post hoc ROI analysis showing the relationship between the error-likelihood effect in the blue region and the error-unexpectedness effect in the yellow region for controls and patients. Each point represents one participant. C) Overlap of the blue region described above and the region previously identified by a greater error-likelihood effect in controls than patients (green). D) Overlap of the yellow region described above and the region previously identified by a greater error-unexpectedness effect in controls than patients (violet). All coordinates are in MNI space.
Since they were identified by different means, the regions identified here using mediation analysis could have been different regions than those previously identified as showing greater error-likelihood and error-unexpectedness effects in controls than patients. However, the error-likelihood region identified here (blue) overlaps with the region previously shown to have a greater error-likelihood effect for controls than patients (green) (Figure 6C). Further, the mediated error-unexpectedness region identified here (yellow) overlaps with the bilateral region previously shown to have a greater error-unexpectedness effect in controls than patients (violet) (Figure 6D).
An additional mediation analysis was performed by searching for mediating error-likelihood regions instead of mediated error-unexpectedness regions. In this case, again to avoid biasing the results towards a finding of mediation, the potentially mediated brain regions were identified by a positive error-unexpectedness effect solely for the control subgroup, p < .005, uncorrected, with at least 3 contiguous voxels (Supplementary Table S2 and Supplementary Figure S3A in yellow). With these regions as the potential recipients of mediation, we identified a region of right medial prefrontal gyrus showing evidence of a mediating error-likelihood effect across both subgroups (Supplementary Table S2 and Supplementary Figure S3A in blue). The mediation relationship between these regions across the control and patient subgroups is illustrated in Supplementary Figure S3B. For this analysis, the y intercept is greater than zero indicating incomplete mediation (Judd et al., 2001). As above, we compared the regions identified with this analysis to those identified as showing a difference between groups. The error-likelihood regions are nearby but not overlapping (Supplementary Figure S3C), but, as before, the error-unexpectedness regions overlap (Supplementary Figure S3D).
Overall, the mediation analyses provide strong evidence that for both controls and patients, the influence of the error-likelihood cue on the medial prefrontal region showing sensitivity to error unexpectedness is mediated by the medial prefrontal region showing sensitivity to error likelihood.
4 Discussion
We observed deficits in dorsomedial prefrontal error-detection activity in individuals with schizophrenia compared to controls, replicating a number of previous studies (e.g. Kerns et al., 2005; Laurens et al., 2003; Mathalon et al., 2002; Morris et al., 2006; Polli et al., 2008). Yet our results significantly extend and depart from this prior work, by demonstrating that the deficit might be best understood not as an impairment in the monitoring and evaluation of errors, but rather as an impairment in the prediction of response-outcome associations. Specifically, in addition to a basic reduction in error-related activity within MPFC, we also found evidence of reduced activation associated with error-likelihood and error-unexpectedness effects in individuals with schizophrenia, but an intact mediational relationship of error-likelihood on error-unexpectedness. These findings suggest that schizophrenia might be associated with seemingly deficient evaluation signals that arise despite an intact evaluation mechanism, as a consequence of the dependence of the evaluation signals on a faulty response-outcome prediction system. Below, we describe how our findings relate to prior research, and elaborate on the critical implications of the current results.
4.1 Relationship to prior studies of schizophrenia
4.1.1 Error-related studies
The basic error-related effects common to both groups were localized to dorsal regions of MPFC, whereas the MPFC region in which error-related activity was reduced in individuals with schizophrenia relative to controls was located more ventrally and anteriorly. This pattern is generally consistent with past findings, since prior studies have observed error-related MPFC activity in individuals with schizophrenia (Carter et al., 2001; Kerns et al., 2005; Laurens et al., 2003; Polli et al., 2008), although the activation is diminished relative to controls. The regions we identified with deficits in individuals with schizophrenia generally fell in the rostral ACC areas typically associated with affect and motivation as opposed to the caudal ACC areas associated with conflict monitoring and reinforcement learning (Polli et al., 2008; van Veen and Carter, 2002). However, the caudal regions, where we found no overall deficit in error-detection across error-likelihood conditions in schizophrenia, did show deficits in the error-unexpectedness effect, i.e. abnormally low error effects in the low error-likelihood condition compared to the high error-likelihood condition. Since we found different patterns of activity in the rostral and caudal areas, and we found abnormal activations in both areas in patients relative to controls, our findings are generally consistent with the existence of two error-related sub-systems, each with abnormal activity in schizophrenia, as found by Polli, et al. (2008).
4.1.2 Working memory studies
Before we address the critical issue of outcome prediction, we first address working memory maintenance. Patients were less accurate than controls on the delayed match-to-sample task, indicating that they were less likely to successfully hold the error-likelihood cue in memory over the period during which they performed the change-signal task. This is consistent with previous findings of schizophrenia-related deficits in working memory performance with visuospatial items, verbal items, and task context (Cohen et al., 1999; Lee and Park, 2005). The cue acted as an incidental contextual signal predictive of error likelihood on CST change trials, but participants were not told of this relationship. Thus, maintaining the cue in memory during the CST was a necessary prerequisite for learning the relationship between the cue and the error likelihood of the change trials. As such, to determine if there were deficits in the prediction and evaluation of the probability of error occurrence beyond the deficit found in maintenance of the cue, it was necessary to perform analyses using subgroups matched on accuracy in the DMTS task. The fact that we found a deficit of outcome prediction, even with groups matched on working memory performance, rules out working memory as the underlying cause of this deficit.
4.2 Implications of the current findings
To our knowledge this is the first demonstration of an impaired error-likelihood effect in individuals with schizophrenia. Importantly, the effect was observed when comparing control and schizophrenia subgroups that were otherwise well-matched in other aspects of task performance. Even more critically, the error-likelihood effect was observed to an advance cue stimulus that was temporally and statistically dissociated from the response. Thus, the effect could be conceptually distinguished from any response-related or evaluation deficits present in individuals with schizophrenia.
In contrast, the error-likelihood response to the cue in controls had been previously predicted by the PRO account, but heretofore had not been observed experimentally (Brown and Braver, 2005; Brown and Braver, 2007), and in fact, was relatively controversial on theoretical grounds (Nieuwenhuis et al., 2007). The successful observation of cue-related error-likelihood activity in the current study, but not previous ones, may have been due to a more robust methodology and experimental design that allowed for dissociating cue and response related effects – involving the use of partial trials and the necessity of maintaining cue information in working memory to satisfy incidental task demands. It may also have been due at least partly to abnormal function in schizophrenia, as the effect was found in the between-groups contrast but not in controls per se. As a result, while the small sample size and the use of a between-groups contrast suggest caution, the current findings provide additional support for the PRO account, as a comprehensive theory of MPFC function. Moreover, the results suggest a new interpretation regarding previous observations of anticipatory MPFC activation (e.g., (Sohn et al., 2007) as potentially reflecting error-likelihood predictions rather than preparatory conflict per se.
In addition to the error-likelihood effects, we also found that, compared to controls, individuals with schizophrenia exhibited an impaired error-unexpectedness effect in MPFC. The error-unexpectedness effect in controls is consistent with past findings of a greater error response to less expected errors (Brown and Braver, 2005; Holroyd and Krigolson, 2007). The lack of such an effect in individuals with schizophrenia could be treated as evidence of an evaluation (i.e., strict performance monitoring) deficit. However, the finding that the error-unexpectedness deficit was accompanied by an error-likelihood deficit suggested that the former might be a causal consequence of the latter. Our finding of a mediation pattern between the two effects statistically confirmed this inference. As such, the results are most consistent with the interpretation that the abnormal error-unexpectedness effect in individuals with schizophrenia is in fact a byproduct of their abnormal error-likelihood effect, and that the underlying dysfunction is occurring in prediction, not evaluation, of response-outcome associations. In principle, it could also be the other way around, i.e. that error effects provide a learning signal to entrain error likelihood effects in the first place, so that the lack of error likelihood effects may be due to a lack of error effects. This is not likely, however, because error effects are still found in individuals with schizophrenia (Figure 4B), even though they are somewhat weaker (Carter et al., 2001).
A critical implication of the current results is thus a shift of focus in the nature of performance monitoring in schizophrenia. Past work has focused primarily on deficits of performance evaluation in MPFC in schizophrenia, with the implication that it is the evaluation system itself that is malfunctioning (Kerns et al., 2005; Laurens et al., 2003; Polli et al., 2008). However, more recent work has suggested a role for MPFC in both prediction and evaluation of response-outcome associations (Alexander and Brown, in press; Rushworth et al., 2003). We have shown that incorrect predictions can lead to incorrect evaluations, even if the evaluation mechanism is intact. This suggests a need for a greater focus on the formation of expectations about outcomes and the reciprocal relationship between error predictions and error evaluations over the course of learning. While this finding was made in the context of schizophrenia, it potentially applies more generally in evaluating MPFC function and dysfunction.
Another implication of the current findings is that they add to the growing data suggesting that regional specialization is an important consideration in understanding the nature of MPFC function and dysfunction. The locations of the effects found in this study, as shown in Figure 7, can be compared to sub-regions associated with particular patterns of connectivity, as determined by diffusion tractography and meta-analysis of functional studies (Beckmann et al., 2009). The regions that showed basic error effects in both groups and also the error-unexpectedness effect in controls were located in the more dorsal and posterior sector of MPFC. These MPFC sectors coincide well with areas that have been shown to have strong connections to dorsolateral prefrontal cortex and premotor cortex, and often show conflict, error, reward, and emotional activation (Beckmann et al., 2009). In contrast, the greater error-likelihood effect for controls compared to patients was located in a more rostral and anterior sector of MPFC. This MPFC sector tends to have strong connections to orbital frontal cortex and hypothalamus, and often shows reward, reward expectation, and emotional activations (Beckmann et al., 2009). The MPFC regions that showed maximal between-groups differences in error-likelihood and error-unexpectedness effects were located somewhat differently from those that have been observed to show these effects in studies focusing exclusively on control participants (Brown and Braver, 2005; Brown and Braver, 2007). We currently do not have an explanation for this pattern. Thus, further work will be necessary to understand how consistently error-likelihood and error-unexpectedness effects occur in particular medial prefrontal sub-regions, and then to relate the connectivity of those subregions to the prediction and evaluation of response-outcome associations.
Figure 7.
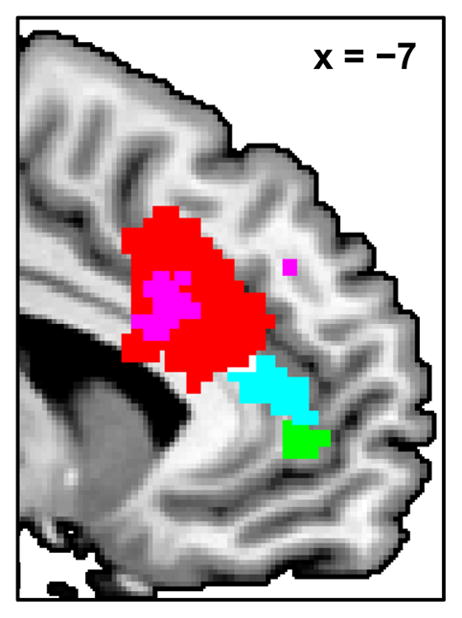
The results shown separately in Figures 4 and 5 for comparison of locations within medial frontal cortex: the main effect of error across both groups (red), and the greater effects of error (cyan), error likelihood (green), and error unexpectedness (violet) for controls compared to patients.
4.3 Study limitations and future directions
Due to the use of small sample sizes, the results of this study should be treated with caution. A further limitation of the current study, shared with most other cognitive neuroscience research on schizophrenia, is that the individuals with schizophrenia that were study participants were all outpatients taking typical or atypical anti-psychotic medications. Since the patients were taking different medications, we could not use dosage as a covariate. As a consequence, it is impossible to determine how much the pattern of current results is related to medication effects on cognitive and neural processing, rather than to the role of the disease per se. However, even in studies where unmedicated individuals have been examined, similar MPFC abnormalities have been observed (Nordahl et al., 2001; Snitz et al., 2005). These studies are becoming harder to do, because of ethical concerns. Nevertheless, it is still critical to examine the role of medication effects per se, if possible, through various approaches, such as using medication dosage as a covariate, studying genetic relatives of individuals with schizophrenia (i.e., unaffected siblings), etc.
A separate direction for future investigation is to more directly examine the link between MPFC impairment in schizophrenia related to error prediction and both clinical symptoms and functional outcomes. In the current study, the sample sizes were too small to examine these relationships with sufficient statistical power, but should be addressed in further research. In prior studies, deficits of executive function have been linked to symptoms of schizophrenia, including formal thought disorder (Kerns and Berenbaum, 2003), and to functional outcomes of schizophrenia, including community functioning (Greenwood et al., 2005). More focused examinations of error-detection and conflict-monitoring have linked these to specific behavioral performance impairments in individuals with schizophrenia (Kerns et al., 2005), and with more general deficits of executive function (Silver and Goodman, 2007). The current results suggest that it will be profitable to extend these examinations to a more specific focus on response-outcome prediction, as a potential underlying mechanism that may help to understand the nature of cognitive dysfunction in schizophrenia.
Supplementary Material
Acknowledgments
We thank W. Alexander for helpful comments and N. Yodkovik, M. Hanewinkel, and C. McKenna for help with subject ascertainment and data collection. This research was supported in part by National Institutes of Health R03 DA023462-01 (JWB), Air Force Office of Scientific Research FA9550-07-1-0454 (JWB), a NARSAD Young Investigator Award and the Sydney R. Baer, Jr. Foundation (JWB), and National Institutes of Health T32 MH019879-15 (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts E, Roelofs A, van Turennout M. Anticipatory activity in anterior cingulate cortex can be independent of conflict and error likelihood. Journal of Neuroscience. 2008;28:4671–4678. doi: 10.1523/JNEUROSCI.4400-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WH, Brown JW. Computational models of performance monitoring and cognitive control. Topics in Cognitive Science. doi: 10.1111/j.1756-8765.2010.01085.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Iii, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Walton ME, Rushworth MFS. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Marseille Boîte À Région d'Intérêt (MarsBaR) (Version 0.41) [Computer software] 2002 http://marsbar.sourceforge.net/
- Brown JW. Conflict effects without conflict in anterior cingulate cortex: Multiple response effects and context specific representations. NeuroImage. 2009;47:334–341. doi: 10.1016/j.neuroimage.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Risk prediction and aversion by anterior cingulate cortex. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:266–277. doi: 10.3758/cabn.7.4.266. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Research. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: An event-related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Cui X, Li J, Song X. xjView (Version 8) [Computer software] 2009 http://www.alivelearn.net/xjview/
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: Nov, 2002. [Google Scholar]
- Friston KJ. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Greenwood KE, Landau S, Wykes T. Negative symptoms and specific cognitive impairments as combined targets for improved functional outcome within cognitive remediation therapy. Schizophrenia Bulletin. 2005;31:910–921. doi: 10.1093/schbul/sbi035. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Krigolson OE. Reward prediction error signals associated with a modified time estimation task. Psychophysiology. 2007;44:913–917. doi: 10.1111/j.1469-8986.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Jessup RK, Busemeyer JR, Brown JW. Error effects in anterior cingulate cortex reverse when error likelihood is high. Journal of Neuroscience. 2010;30:3467–3472. doi: 10.1523/JNEUROSCI.4130-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd CM, Kenny DA, McClelland GH. Estimating and testing mediation and moderation in within-subject designs. Psychological Methods. 2001;6:115–134. doi: 10.1037/1082-989x.6.2.115. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Dahmubed AF, Lara AH, Wallis JD. Neurons in the frontal lobe encode the value of multiple decision variables. Journal of Cognitive Neuroscience. 2009;21:1162–1178. doi: 10.1162/jocn.2009.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Walton ME, Behrens TEJ, Buckley MJ, Rushworth MFS. Optimal decision making and the anterior cingulate cortex. Nature Neuroscience. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- Kerns JG. Distinct conflict resolution deficits related to different facets of schizophrenia. Psychological Research. 2009;73:786–793. doi: 10.1007/s00426-008-0195-x. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Berenbaum H. The relationship between formal thought disorder and executive functioning component processes. Journal of Abnormal Psychology. 2003;112:339–352. doi: 10.1037/0021-843x.112.3.339. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. American Journal of Psychiatry. 2005;162:1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach Atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Ngan ETC, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren't voodoo: Commentary on Vul et al. (2009) Perspectives on Psychological Science. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, Carter CS. Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology. 2003;112:689–697. doi: 10.1037/0021-843X.112.4.689. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Fedor M, Faustman WO, Gray M, Askari N, Ford JM. Response-monitoring dysfunction in schizophrenia: An event-related brain potential study. Journal of Abnormal Psychology. 2002;111:22–41. [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Ling J, Cañive JM. Assessment and quantification of head motion in neuropsychiatric functional imaging research as applied to schizophrenia. Journal of the International Neuropsychological Society. 2007;13:839–845. doi: 10.1017/S1355617707071081. [DOI] [PubMed] [Google Scholar]
- McNeely HE, West R, Christensen BK, Alain C. Neurophysiological evidence for disturbances of conflict processing in patients with schizophrenia. Journal of Abnormal Psychology. 2003;112:679–688. doi: 10.1037/0021-843X.112.4.679. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Morris SE, Yee CM, Nuechterlein KH. Electrophysiological analysis of error monitoring in schizophrenia. Journal of Abnormal Psychology. 2006;115:239–250. doi: 10.1037/0021-843X.115.2.239. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Schweizer TS, Mars RB, Botvinick MM, Hajcak G. Error-likelihood prediction in the medial frontal cortex: A critical evaluation. Cerebral Cortex. 2007;17:1570–1581. doi: 10.1093/cercor/bhl068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl TE, Carter CS, Salo RE, Kraft L, Baldo J, Salamat S, Robertson L, Kusubov N. Anterior cingulate metabolism correlates with Stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25:139–148. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI - II. Analysis. NeuroImage. 2001a;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI - I. The method. NeuroImage. 2001b;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Péchaud M, Jenkinson M, Smith S. Brain Extraction Tool (BET) (Version 2) [Computer software] Oxford University Centre for Functional MRI of the Brain; Oxford, UK: 2006. [Google Scholar]
- Polli FE, Barton JJS, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, Raichle ME. Localization of cognitive operations in the human brain. Science. 1988;240:1627–1631. doi: 10.1126/science.3289116. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Behrens TE, Kennerley SW, Baxter MG, Buckley MJ, Walton ME, Rushworth MFS. Frontal cortex subregions play distinct roles in choices between actions and stimuli. Journal of Neuroscience. 2008;28:13775–13785. doi: 10.1523/JNEUROSCI.3541-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nature Neuroscience. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Hadland KA, Gaffan D, Passingham RE. The effect of cingulate cortex lesions on task switching and working memory. Journal of Cognitive Neuroscience. 2003;15:338–353. doi: 10.1162/089892903321593072. [DOI] [PubMed] [Google Scholar]
- Sanders GS, Gallup GG, Heinsen H, Hof PR, Schmitz C. Cognitive deficits, schizophrenia, and the anterior cingulate cortex. Trends in Cognitive Sciences. 2002;6:190–192. doi: 10.1016/s1364-6613(02)01892-2. [DOI] [PubMed] [Google Scholar]
- Silver H, Goodman C. Impairment in error monitoring predicts poor executive function in schizophrenia patients. Schizophrenia Research. 2007;94:156–163. doi: 10.1016/j.schres.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: Functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Albert MV, Jung K, Carter CS, Anderson JR. Anticipation of conflict monitoring in the anterior cingulate cortex and the prefrontal cortex. Proceedings of the National Academy of Sciences. 2007;104:10330–10334. doi: 10.1073/pnas.0703225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Vogel M, Gao XM, Lahti AC, Holcomb HH. The limbic cortex in schizophrenia: focus on the anterior cingulate. Brain Research Reviews. 2000;31:364–370. doi: 10.1016/s0165-0173(99)00053-3. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Centre for Neuroimaging. Statistical Parametric Mapping (SPM) (Version 5 r826) [Computer software] Wellcome Trust Centre for Neuroimaging; London: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.