Abstract
The circuit changes that mediate parkinsonian tremor, while likely differing from those underlying akinesia and rigidity, are not precisely known. In this study, to identify a specific metabolic brain network associated with this disease manifestation, we used FDG PET to scan nine tremor dominant Parkinson’s disease (PD) patients at baseline and during ventral intermediate (Vim) thalamic nucleus during deep brain stimulation (DBS). Ordinal trends canonical variates analysis (OrT/CVA) was performed on the within-subject scan data to detect a significant spatial covariance pattern with consistent changes in subject expression during stimulation-mediated tremor suppression. The metabolic pattern was characterized by covarying increases in the activity of the cerebellum/dentate nucleus and primary motor cortex, and, to a less degree, the caudate/putamen. Vim stimulation resulted in consistent reductions in pattern expression (p<0.005, permutation test). In the absence of stimulation, pattern expression values (subject scores) correlated significantly (r=0.85, p<0.02 with concurrent accelerometric measurements of tremor amplitude.
To validate this spatial covariance pattern as an objective network biomarker of PD tremor, we prospectively quantified its expression on an individual subject basis in independent PD populations. The resulting subject scores for this PD tremor-related pattern (PDTP) were found to exhibit: (1) excellent test-retest reproducibility (p<0.0001); (2) significant correlation with independent clinical ratings of tremor (r=0.54, p<0.001) but not akinesia-rigidity; and (3) significant elevations (p<0.02) in tremor dominant relative to atremulous PD patients.
Following validation, we assessed the natural history of PDTP expression in early stage patients scanned longitudinally with FDG PET over a four year interval. Significant increases in PDTP expression (p<0.01) were evident in this cohort over time; rate of progression, however, was slower than for the PD-related akinesia/rigidity pattern (PDRP). We also determined whether PDTP expression is modulated by interventions specifically directed at parkinsonian tremor. While Vim DBS was associated with changes in PDTP (p<0.001) but not PDRP expression, subthalamic nucleus (STN) DBS reduced the activity of both networks (p<0.05). PDTP expression was suppressed more by Vim than by STN stimulation (p<0.05).
These findings suggest that parkinsonian tremor is mediated by a distinct metabolic network involving primarily cerebello-thalamo-cortical pathways. Indeed, effective treatment of this symptom is associated with significant reduction in PDTP expression. Quantification of treatment-mediated changes in both PDTP and PDRP scores can provide an objective means of evaluating the differential effects of novel antiparkinsonian interventions on the different motor features of the disorder.
Keywords: Parkinson’s disease, positron emission tomography, tremor, Vim DBS, STN DBS
Introduction
Resting tremor is one of the cardinal features of Parkinson's disease (PD) and is present in 75 to 100% of patients during the course of the illness (Rajput et al., 1991; Hughes et al., 1993). The pathophysiology of parkinsonian tremor is thought to be distinct from that of akinesia and rigidity, the other major clinical symptoms of the disease (e.g., Fishman, 2008; Zaidel et al., 2009). For instance, in PD, loss of nigral dopaminergic projections to the putamen correlates consistently with clinical ratings of akinesia and rigidity but not tremor (Eidelberg et al., 1995a; Benamer et al., 2003). Moreover, unlike akinetic-rigid manifestations of the disease, parkinsonian tremor is not uniformly responsive to dopaminergic therapy. Indeed, nigrostriatal dopaminergic loss appears to be a necessary but insufficient condition for the development of PD tremor (Fishman, 2008; Zaidel et al., 2009).
The ventral intermediate (Vim) nucleus of the thalamus has traditionally been regarded as the optimal target for the surgical relief of tremor (e.g., Machado et al., 2006). Neurons in this region receive projections from the deep cerebellar nuclei and discharge in synchrony with parkinsonian tremor (Lenz et al., 1994). Given that PD tremor can also be alleviated by lesions of other brain regions, including the pons and cerebellum (Boecker and Brooks, 1998), the Vim thalamic nucleus can be viewed as one of several interconnected nodes of a spatially distributed tremor circuit. Nevertheless, the precise anatomical/functional topography of this large-scale network is not known, particularly with respect to the relative contributions of the basal ganglia and cerebellum to this pathway (e.g., Volkmann et al., 1996; Deuschl et al., 2001; Timmermann et al., 2003; 2007; Zaidel et al., 2009).
The functional imaging hallmarks of parkinsonian tremor are also not fully defined, particularly from the circuit standpoint. Resting state imaging of glucose metabolism with 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) has provided a useful means of assessing disease-related changes in brain function at the network level (Eidelberg, 2009). Patient expression of a previously validated PD-related metabolic covariance pattern (PDRP) (Ma et al., 2007; Eidelberg, 2009) has been found to correlate with clinical ratings for akinesia and rigidity but not tremor (Eidelberg et al., 1994; 1995b; Feigin et al., 2001; Lozza et al., 2004). Moreover, PDRP expression has been found to be elevated to similar levels in PD patients with comparable degrees of bradykinesia, whether or not tremor is also present (Isaias et al., 2010; cf. Antonini et al., 1998). That said, the characterization of a specific metabolic network associated with PD tremor has been particularly challenging because of the much smaller signal associated with this disease manifestation. In an earlier study (Antonini et al., 1998), we sought to identify a significant PD tremor network that was independent of the dominant PDRP metabolic abnormalities. The analytical strategy that was used was cross-sectional, in that the tremor-related pattern was sought in FDG PET data from a combined group of patients with tremor and akinetic-rigid dominant symptoms. However, consistent with the relatively small effect of tremor on composite ratings of motor disability in PD (Martinez-Martin et al., 1994; Stochl et al., 2008), the signal associated with the corresponding metabolic network proved insufficient for prospective application.
In the current study, we approached this problem using a novel within-subject strategy in which tremor dominant PD patients underwent FDG PET scanning at baseline and again during deep brain stimulation (DBS) of the ventral intermediate (Vim) thalamic nucleus. Using a new voxel-based network approach (Habeck et al., 2005; Habeck and Stern, 2007; Carbon et al., 2010), we identified a distinct PD tremor-related metabolic pattern (PDTP) that was sufficiently stable to be applied on a prospective single case basis. The validity of PDTP expression as a quantitative network-based descriptor of this disease manifestation was demonstrated by the excellent reproducibility of this objective network measure, its consistent correlation with independent clinical tremor ratings, and its significant progression over time. We also assessed the potential of the PDTP for modulation by interventions directed specifically at this symptom.
Materials and methods
Pattern identification
Subjects
We studied nine PD patients (8 men and 1 woman, age 65.9±9.6 years [mean±SD], off-state Unified Parkinson’s Disease Rating Scale (UPDRS) motor ratings 36.6±14.2) who underwent clinically effective Vim DBS for tremor dominant symptoms (Table 1). Motor manifestations of PD were considered to be tremor dominant if the summed limb UPDRS tremor scores were ≥ 4 (items 20 and 21), with at least one limb scoring ≥ 2 (Antonini et al., 1998; Isaias et al., 2010). In this group, the stimulation parameters were: voltage 3.0±0.6 (V); pulse width 100±42.4 (µs); stimulation frequency 160±24.2 (Hz). Seven of the nine patients exhibited predominant tremor on the right side and had a stimulator placed unilaterally in the left Vim thalamic nucleus; the remaining two patients exhibited tremor dominant symptom on both the right and left body sides and underwent bilateral electrode implantation. Cerebral blood flow (H215O PET) data from these subjects have appeared previously (Fukuda et al., 2004).
Table 1.
Clinical features of DBS patients
| Vim DBS PD | STN DBS PD | Healthy Controls |
||
|---|---|---|---|---|
| n | 9 | 9 | 20 | |
| Age (years) | 65.9 (9.6)a | 59.5 (12.9) | 60.6 (13.0) | |
| M:F | 8:1 | 7:2 | 11:9 | |
| Disease duration | 8.6 (4.5) | 9.7 (4.2) | ||
| UPDRSb | ||||
| Total motor | OFF | 36.6 (14.2) | 32.3 (13.3) | |
| ON | 22.9 (12.4) | 20.9 (9.3) | ||
| Δ | −13.7 (8.6)** | −11.4 (9.2)** | ||
| Tremor | OFF† | 8.9 (3.7) | 5.1 (3.0) | |
| ON | 1.6 (2.9) | 1.9 (1.3) | ||
| Δ† | −7.3 (4.1)** | −3.2 (3.4)* | ||
| Akinesia-Rigidity | OFF | 13.2 (8.1) | 16.5 (6.9) | |
| ON | 10.6 (5.4) | 10.6 (6.5) | ||
| Δ | −2.6 (3.7) | −5.9 (7.6)* |
Mean ± SD
Composite UPDRS motor ratings in the baseline off-stimulation (OFF) state and in the stimulated state (ON). Both treatment states were evaluated 12 hrs after the cessation of antiparkinsonian medications.
Δ:ON-OFF
Significant group differences (Student’s t-test): †p<0.05
Significant ON-OFF differences (paired t-test): *p<0.05, **p<0.01
Metabolic imaging
The patients were scanned on two consecutive days in random order. On the first day, the stimulators were switched off (OFF) approximately 3 hours prior to PET; the stimulators were switched on after scanning. On the next day, scanning was conducted with the stimulator on (ON), with settings determined by the maximal tremor suppression that was achieved without pain or adventitious movements. Before each PET session, the patients fasted overnight; antiparkinsonian medications were withheld for at least 12 hours before imaging. In each PET session, the subjects were rated according to the UPDRS (Fahn S and Elton R, 1987) approximately 1 hour before imaging. In addition to a composite motor rating (the sum of items 18–31), we obtained separate subscale ratings for tremor (the sum of items 20 and 21) and akinesia/rigidity (the sum of items 18, 19, 22, and 27–31). Moreover, in seven of the patients, triaxial accelerometry (TRIAX) was used to measure tremor amplitude and frequency in the upper limbs contralateral to Vim stimulation. The details of the TRIAX recording procedures and data analysis are provided elsewhere (Fukuda et al., 2004). In each PET session (i.e., on and off stimulation), TRIAX recordings were acquired for at least 10 minutes to assure physiological stability (<5% variability) of the measured parameters during imaging.
FDG PET was performed in three dimensional (3D) mode using the GE Advance tomograph (General Electric Medical Systems, Milwaukee, Wisconsin) at North Shore University Hospital; the details of these procedures have been provided elsewhere (Ma et al., 2007). The studies were performed with the subjects’ eyes open in a dimly lit room and with minimal auditory stimulation. Ethical permission for the PET studies was obtained from the Institutional Review Board of North Shore University Hospital. Written consent was obtained from each subject after detailed explanation of the procedures.
Scan preprocessing was performed as described elsewhere (Huang et al., 2007b). In the two bilateral Vim DBS patients, images from the right hemisphere were flipped so that the operated side appeared on the left, along with the other stimulated hemispheres. Individual images were nonlinearly warped into Talairach space using a standard PET template, and smoothed with an isotropic Gaussian kernel (10 mm) in all directions to improve the signal-to-noise ratio.
Pattern derivation
To identify a specific metabolic brain network associated with PD tremor, we analyzed the on and off stimulation FDG PET scans from the nine Vim DBS patients using Ordinal Trends Canonical Variates Analysis (OrT/CVA) (Habeck et al., 2005; Moeller and Habeck, 2006) (software available at http://groups.google.com/group/gcva). OrT/CVA is a form of supervised principal component analysis (PCA) (Bair E, 2006) designed to identify linearly independent spatial covariance patterns for which subject expression increases (or decreases) in as many individuals as possible across scan conditions. OrT/CVA differs from voxel-wise univariate contrasts in that it requires that pattern expression exhibit an “ordinal trend”, the property of consistent change across conditions on a subject-by-subject (rather than on a group mean) basis. In addition to the identification of relevant spatial covariance patterns in the data, OrT/CVA quantifies the expression of the pattern(s) in each subject and condition. The significance of candidate patterns is assessed by permutation tests of the pattern expression measures (i.e., the principal component (PC) scalars or subject scores) to exclude the possibility that the observed changes across subjects/conditions had occurred by chance. Likewise, the reliability of the regional contributions to the candidate pattern (i.e., the voxel weights) is assessed using bootstrap estimation procedures (Habeck and Stern, 2007).
In the current study, a significant PD tremor-related metabolic pattern (PDTP) was sought among the linearly independent spatial covariance patterns (i.e., the orthogonal PCs) resulting from OrT/CVA of the scans acquired on and off Vim stimulation. The following model selection criteria were applied to the individual patterns: (1) the analysis was limited to the first 6 PCs, which typically account for at least 75% of the subject × region variance (Habeck and Stern, 2007); (2) subject scores for these PCs were entered singly and in all possible combinations into a series of logistic regression models, with stimulation condition (OFF, ON) as the dependent variable and the subject scores for each set of PCs as the independent variables for each model. The best model was considered to be that with the smallest Akaike information criterion (AIC) value. The selected PC(s) in this model were then used in linear combination to yield the spatial covariance pattern that was most closely related to the difference across stimulation conditions. The resulting pattern was considered to exhibit a significant ordinal trend if the associated subject scores differed from chance at p<0.05 (permutation test). To establish that the candidate pattern was indeed tremor-related we correlated subject scores measured in the baseline off-stimulation condition (i.e., without tremor suppression) with the simultaneously recorded TRIAX measurements. These correlations were assessed using regression analysis, with and without including DBS voltage as a covariate.
OrT/CVA covariance map(s) were displayed at a voxel weight threshold of Z=2.70, p<0.01 with a cluster cutoff of 50 voxels. Regions contributing to the pattern were considered significant for p<0.05 on bootstrap estimation. Because the tremor-related pattern was identified in the analysis of hemispheric PET data from predominantly unilateral Vim stimulation cases, the associated voxel weights were flipped to produce a symmetrical brain network for the quantification of pattern expression in whole-brain scan data from prospective subjects. We also determined the degree of similarity/difference between the PDTP and PDRP metabolic topographies by computing the variance shared (r2) between all the corresponding non-zero voxel weights on the two pattern images. Likewise, we compared the PDTP topography to that of a recently described normal movement-related covariance pattern (NMRP), identified using OrT/CVA of motor activation responses from healthy subjects (Carbon et al., 2010). In these analyses, the two pattern images (i.e., PDTP and PDRP; PDTP and NMRP) were spatially normalized and only voxels that differed from zero in both images were considered. Voxels from each pattern image were formatted into a single vector by appending successive rows in each plane of the image. The two vectors were then entered input into the MATLAB statistical routine ‘corr’ to calculate the correlation coefficient (r).
Pattern validation
We next performed a series of single case computations to quantify PDTP and PDRP expression in prospective imaging datasets. The resulting network values (subject scores) were correlated with UPDRS subscale ratings for tremor and akinesia/rigidity. All PDTP and PDRP scores were Z-transformed with respect to values from 20 age-matched healthy control subjects (11 men and 9 women, age 60.6±13.0 years) so the control group for each network had a mean value of zero and a standard deviation of one. These forward analyses were performed using an automated voxel-wise procedure (available at http://www.fil.ion.ucl.ac.uk/spm/ext/#SSM) as described in detail elsewhere (Ma et al., 2007; Spetsieris et al., 2009).
We determined the test-retest reliability of prospectively computed PDTP subject scores. PDTP expression was quantified in 14 PD patients (7 men and 7 women; age 64.1±8.9 years; motor UPDRS 22.0±14.5; Supplementary Table 1) who underwent repeat FDG PET imaging (Asanuma et al., 2006). Within-subject reproducibility of PDTP values in this group was assessed by computing the intraclass correlation coefficient (ICC) (Ma et al., 2007).
To determine the specificity of PDTP scores for parkinsonian tremor, we quantified the expression of this pattern in 41 subsequent PD patients (31 men and 10 women, age 59.8±9.1 years, motor UPDRS 27.7±16.2; Supplementary Table 1) who underwent FDG PET in the off-medication state. Computed PDTP scores for these subjects were correlated with UPDRS subscale ratings for tremor and akinetic-rigidity using multiple linear regression; disease duration and subject age and gender were used as covariates in this analysis. By including both subscale ratings and the PDTP scores in a single multiple regression model (West et al., 1996), we were able to directly contrast the magnitude of PDTP correlations with tremor vs. akinesia/rigidity.
We determined whether parkinsonian tremor was associated with elevated PDTP values measured using functional imaging modalities other than FDG PET. We therefore quantified PDTP expression in 18 other PD patients (14 men and 4 women, age 63.1±7.0 years, motor UPDRS 34.0±13.1; Supplementary Table 1) who underwent technetium-99m-ethylene cysteine dimmer single photon emission computed tomography ([99m]Tc-ECD SPECT) perfusion imaging in the off-medication state (Isaias et al., 2010). Nine of these subjects were classified as tremor predominant; the others were classified as akinetic-rigid predominant with little or no tremor. Prospectively computed PDTP scores for these patients were compared to corresponding values from nine healthy control subjects (5 men and 4 women, age 73.2±5.6 years) who also underwent ECD SPECT. This analysis was conducted using one-way analysis of variance (ANOVA) with post-hoc Bonferroni tests. Because the healthy control subjects were older (p<0.05, Student's t-test) than the patients one-way analysis of covariance (ANCOVA) was employed to adjust for the age difference.
Time course of pattern expression
To determine whether longitudinal changes in PDTP expression are sensitive to symptom progression, we computed PDTP and PDRP scores in FDG PET scans from 15 early stage PD patients (11 males and 4 females; age: 58.0±10.2 years; baseline motor UPDRS 8.2±4.5; Supplementary Table 1) who participated in our previously reported longitudinal imaging study (Huang et al., 2007b; Tang et al., 2010). In all subjects, PDTP and PDRP scores were separately quantified at each time point (0, 24, 48 months). Longitudinal changes in tremor and akinesia/rigidity subscale ratings and concurrent changes in PDTP/PDRP expression were evaluated using one-way repeated measure analysis of variance (RMANOVA). Annualized rates of progression over the three time points were estimated for each measure using an individual growth model (Singer and Willett, 2003)
Effects of treatment on pattern expression
To determine whether therapeutic interventions directed at parkinsonian tremor are associated with PDTP network modulation, we assessed changes in PDTP/PDRP expression during STN DBS and compared the results to the corresponding network changes observed during Vim stimulation. The STN DBS cohort was comprised of nine different tremor dominant PD patients (7 men and 2 women; age 59.5±12.9 years; motor UPDRS ratings 32.3±13.3) with bilaterally implanted electrodes (Table 1). In this group, the stimulation parameters were: voltage 3.1±0.6 (V), pulse width 78±19.0 (µs), stimulation frequency 165±29.6 (Hz). As in the Vim DBS group, these patients underwent FDG PET in the ON and OFF conditions in separate consecutive day imaging sessions. In both the Vim and STN DBS groups, PDTP and PDRP scores were computed on an individual hemisphere basis in each stimulation condition (Trošt et al., 2006; Tang et al., 2010). These calculations were performed using an automated voxel-wise algorithm (see above), blind to subject, DBS target (Vim, STN), and stimulation condition (OFF, ON).
Hemispheric changes in pattern expression with stimulation (ON – OFF) were compared with analogous changes (RETEST – TEST) measured in the 14 PD patients described above who underwent repeat FDG PET without intervention. In this control group, changes in pattern expression in each hemisphere were averaged and compared to the corresponding hemispheric changes measured in the two DBS treatment groups. Differences in network modulation (i.e., between-session changes in pattern expression) across the three groups (Vim DBS, STN DBS, control) were compared using one-way ANOVA followed by post-hoc Bonferroni tests. The network analyses were followed up with mass-univariate procedures to identify regions in which the two DBS interventions gave rise to similar metabolic changes (i.e., areas in which both Vim DBS and STN DBS led to either increases or decreases in regional glucose utilization). This was achieved using conjunction analysis in SPM5 (Friston et al., 2005); the results were considered significant at p<0.05 (family wise error [FWE]-corrected). All statistical analyses were performed using SPSS software (SPSS, Chicago, IL) and SAS 9.1 (SAS Institute Inc.), and were considered significant for p<0.05 (two-tailed).
Results
Parkinson's disease-related tremor pattern
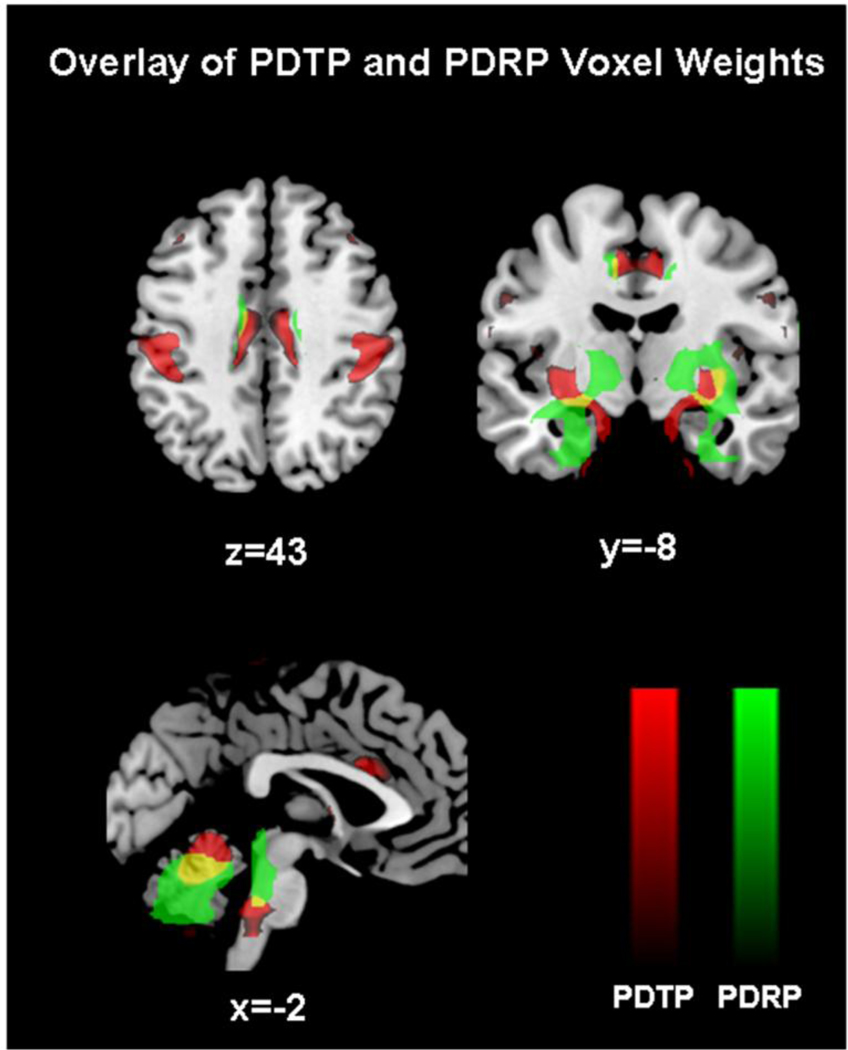
Network analysis of the FDG PET scans acquired on and off Vim stimulation revealed a significant spatial covariance pattern (Figure 1A) characterized by increased metabolic activity in the anterior cerebellum (lobule IV–V) and dentate nucleus, primary motor cortex, and, to a lesser degree, in the caudate and putamen (Table 2). Voxel weights on the pattern were stable by bootstrap estimation (p<0.05). Voxel-wise correlation of the regional loadings on this pattern disclosed an 18% correspondence with the PDRP topography (Figure 2) and no correspondence (0.01%) with the normal movement-related activation pattern (NMRP) topography.
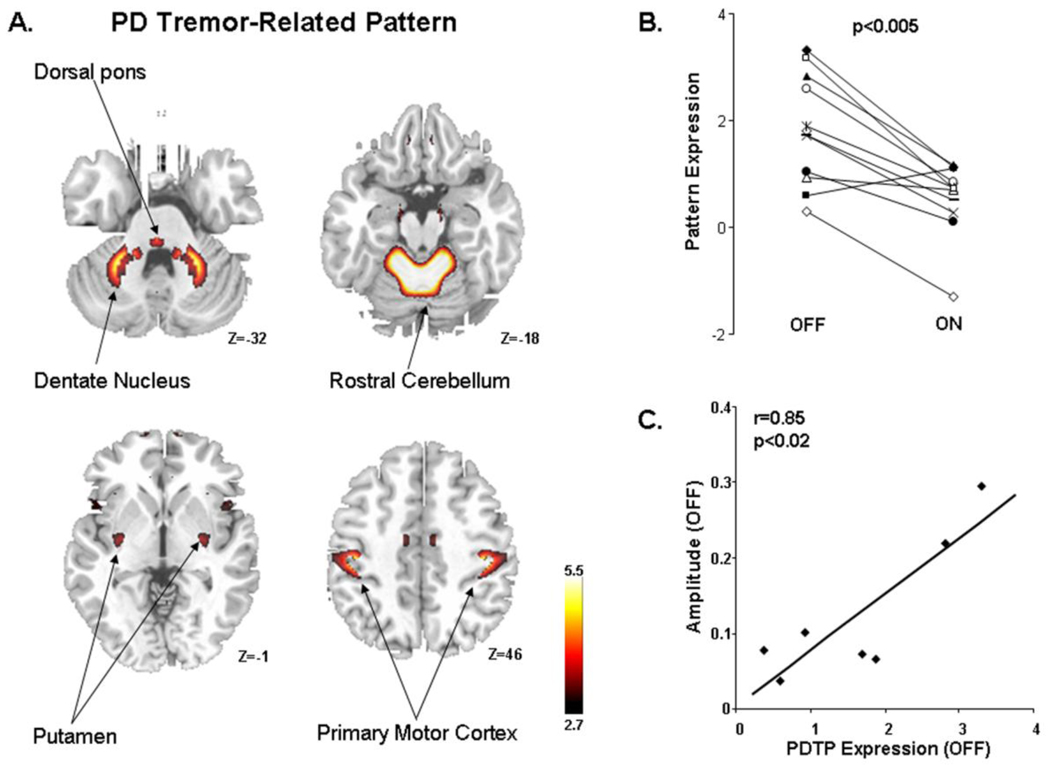
Figure 1. Parkinson’s disease tremor-related pattern (PDTP).
A. Spatial covariance pattern identified by ordinal trends canonical variate analysis (OrT/CVA) of FDG PET data from nine tremor dominant PD patients scanned on and off Vim stimulation (see text). The pattern was characterized by increased metabolic activity in the primary motor cortex, anterior cerebellum/dorsal pons, and the caudate/putamen. [The covariance map was overlaid on T1-weighted MR-template images. Voxel weights were thresholded at Z=2.70, p<0.01. The display represents regions that were demonstrated to be reliable (p<0.05) by bootstrap resampling].
B. The expression of this PD tremor-related metabolic pattern (PDTP) was reduced by Vim stimulation in 10 of the 11 treated hemispheres (p<0.005, permutation test).
C. Baseline PDTP expression (i.e., off-stimulation pattern scores) correlated (r=0.85, p<0.02) with concurrent accelerometric measurements of tremor amplitude (see text).
Table 2.
Regions contributing to Parkinson's disease tremor-related metabolic pattern (PDTP)
| Regions | Coordinatesa |
Zmax | ||
|---|---|---|---|---|
| x | y | z | ||
| Cerebellum (lobule IV/V)b | 10 | −46 | −14 | 5.08*** |
| Dentate Nucleus | 14 | −40 | −32 | 3.25** |
| Putamen | −32 | −8 | 4 | 2.74* |
| Cingulate cortex (BA 24/32) | 0 | 24 | 24 | 3.71** |
| Sensorimotor cortex (BA 4/1, 2, 3) | −28 | −24 | 48 | 3.73** |
Montreal Neurological Institute (MNI) standard space.
According to the atlas of Schmahmann (Schmahmann et al., 2000).
p<0.01,
p<0.001,
p<0.0001 (see text).
Figure 2. Comparison of PDTP and PDRP spatial topographies.
Display of brain areas contributing to the PDTP (red) and PDRP (green) metabolic networks. Areas of overlap between the two patterns (yellow) were evident in the cerebellum, pons, and putamen. For each pattern, the voxel displays were thresholded at Z=2.70, p<0.01 and superimposed on a standard magnetic resonance imaging template.
The expression of this pattern in the individual subjects (Figure 1B) exhibited a significant ordinal trend (p<0.005, permutation test), in that network activity values declined with stimulation in 10/11 treated hemispheres. Moreover, in the baseline (OFF) condition, hemispheric pattern expression (Figure 1C) correlated with concurrent TRIAX measurements of tremor amplitude in the contralateral upper limb (r=0.85, p<0.02). Nonetheless, tremor amplitude did not correlate with PDRP values measured in the same hemispheres (p=0.26). There was no correlation (p>0.26) between changes in pattern expression across conditions and individual differences in the stimulation parameters (DBS voltage and stimulation frequency) that were employed. Based upon the association of this spatial covariance pattern with parkinsonian tremor the bilateralized form of this metabolic network was termed the PD tremor-related pattern (PDTP).
Pattern validation
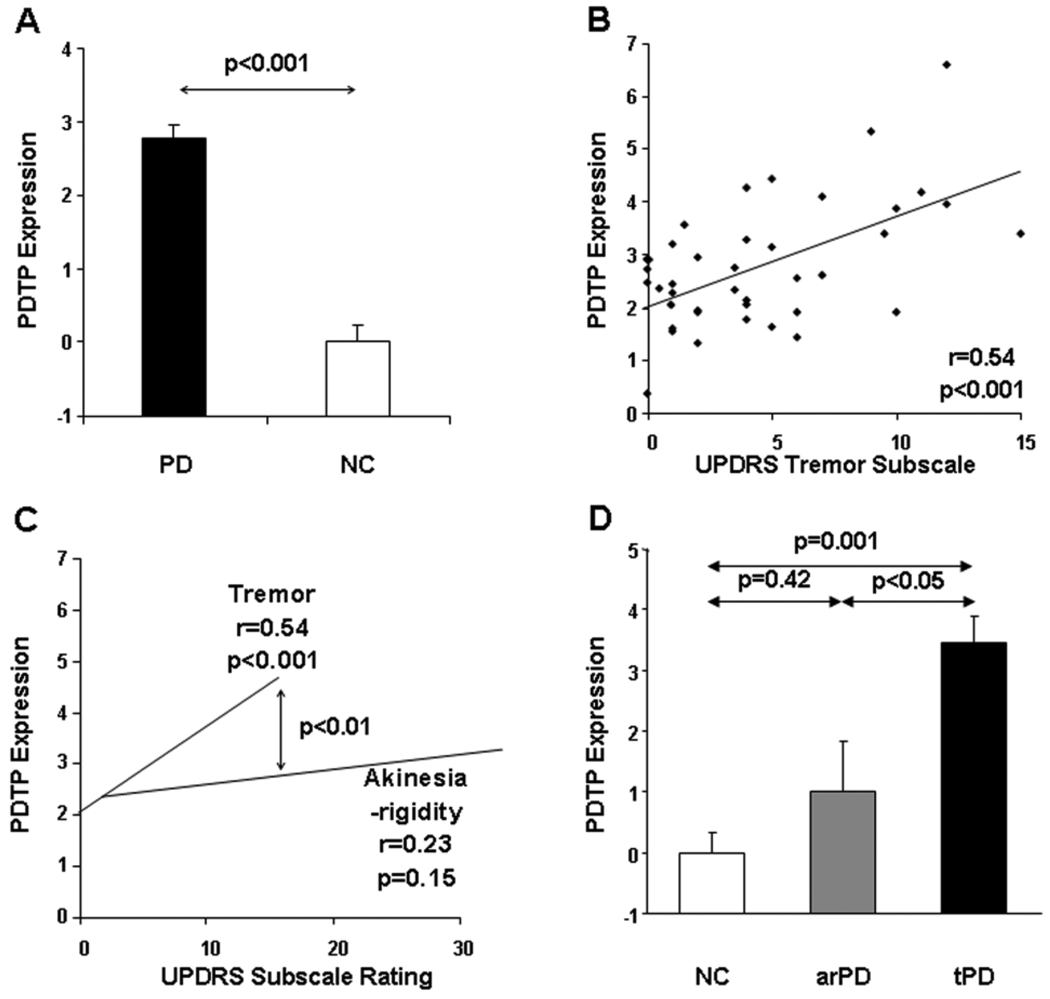
In an independent PD patient population (n=14), PDTP scores exhibited excellent test-retest reproducibility (ICC=0.86, p<0.0001) over an 8-week interval. PDTP expression was then quantified in another independent PD patient cohort (n=41) scanned with FDG PET, and the resulting network values were compared to those from the healthy volunteer subjects (n=20). We found that the resulting PDTP scores (Figure 3A) were abnormally elevated in this patient group (p<0.001, Student’s t-tests). These values were found to correlate with UPDRS tremor subscale ratings (r=0.54, p<0.001; Figure 3B). This correlation remained significant after adjusting for individual differences in disease duration, subject age and gender (r=0.56, p<0.001), as well as following the exclusion of the five subjects without clinically discernible tremor (r=0.56, p<0.001). Nonetheless, the correlation between PDTP expression and akinesia-rigidity subscale ratings was not significant (r=0.23, p=0.15) and was of smaller magnitude (p<0.01; multiple regression) than that observed with tremor ratings (Figure 3C).
Figure 3. Validation of PDTP expression as a network correlate of parkinsonian tremor.
A. Bar graph showing mean PDTP (±SE) in a prospective group of 41 PD patients (black bars) and 20 age-matched healthy control subjects (white bars). The expression of this disease-related pattern was elevated in this testing group (p<0.001, relative to controls).
B. PDTP expression correlated (r=0.54, p<0.001) with UPDRS subscale ratings for tremor in the PD group.
C. The correlation of PDTP scores with tremor was significantly greater in magnitude (p<0.01; multiple regression analysis) than with subscale ratings for akinesia-rigidity (see text).
D. Bar graph showing mean PDTP subject scores (±SE) in tremor dominant and akinesia-rigidity dominant PD patients (arPD and tPD, respectively), and in normal control (NC) subjects undergoing perfusion imaging with ECD SPECT (see text). PDTP expression was significantly higher in the tPD patients than in the arPD (p<0.05) and NC groups (p=0.001).
PDTP scores were also quantified in tremor and akinesia-rigidity dominant PD cohorts and in healthy volunteers scanned with ECD SPECT (n=9 in each group). We found a significant difference in pattern expression across the three groups (F(2,26) = 11.36, p<0.001; one-way ANOVA). Indeed, the tremor dominant patients exhibited increased PDTP expression (Figure 3D) relative to their akinetic-rigid counterparts (p<0.02) as well as the healthy controls (p<0.001), while the PDTP expression did not differ (p=0.38) between the akinetic-rigid patients and healthy controls. The results remained significant following adjustment for group differences in age (whole model: p=0.001; tremor vs. akinetic-rigid: p<0.02; tremor vs. control: p=0.01; akinetic-rigid vs. control: p=0.99).
Effects of disease progression
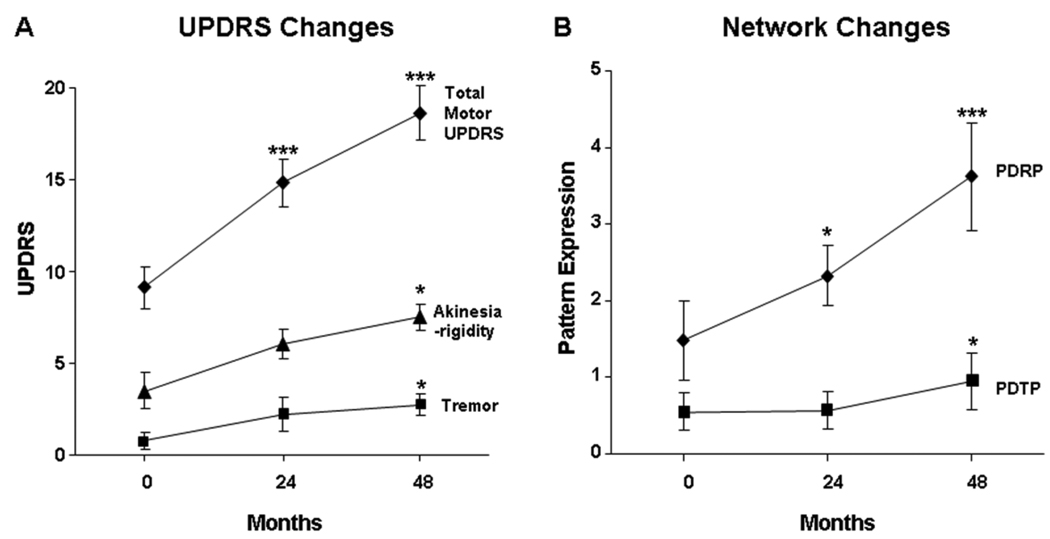
We assessed longitudinal changes in UPDRS tremor and akinesia-rigidity subscale ratings (Figure 4A), and the corresponding changes in PDTP and PDRP expression (Figure 4B), in the disease progression cohort described above (see Methods). Over time, there was significant worsening in akinesia-rigidity (F(2, 12)=5.6, p=0.02; one-way RMANOVA) and tremor (F(2, 15)=6.4, p=0.01), corresponding to a progression rate of 0.95 points/year for the former (p<0.01, individual growth model) and 0.41 points/year for the latter (p<0.005). For both subscores, significant increases were present only at 48 months relative to baseline (p<0.05; post-hoc Bonferroni test). These changes paralleled with concurrent progression in the activity of both PD-related metabolic networks (PDTP: F(2, 23)=4.67, p=0.01; PDRP: F(2,23)=29.9, p<0.0001; one-way RMANOVA). The longitudinal time course was however different for the two patterns (interaction effect: F(2, 23)=6.0, p<0.01; 2×3 RMANOVA), with PDTP expression progressing at a considerably slower rate (0.10 point/year, p<0.05; individual growth model) than the PDRP (0.51 point per year, p<0.0001). Relative to baseline, there were no changes in PDTP expression at 24 months (p=0.99; post-hoc Bonferroni test) and a significant increase at 48 months (p<0.05). By contrast, there were significant increases in PDRP expression at both the second (p<0.05) and third time points (p<0.0001) relative to baseline.
Figure 4. Changes in PDTP expression with disease progression.
A. Mean (±SE) off-state total motor UPDRS ratings (diamonds), and akinesia-rigidity (triangles) and tremor subscale ratings (squares), from a longitudinal cohort of early stage PD patients (n=15) followed at baseline, 24, and 48 months (Huang et al., 2007b). These ratings worsened over time (total motor UPDRS, p<0.0001; akinesia-rigidity: p<0.05; tremor: p=0.01; one-way RMANOVA), but at different progression rates (see text). Relative to baseline, significant increaes in the akinesia-rigidity and tremor ratings (p<0.05; post-hoc Bonferroni test) were evident only at the 48 month time point. *p<0.05, ***p<0.0001, post-hoc Bonferroni test relative to baseline.
B. Mean (±SE) PDTP (squares) and PDRP (diamonds) scores at baseline, 24 and 48 months. The expression of both patterns increases significantly over time (PDTP: p=0.01; PDRP: p<0.0001; one-way RMANOVA). The time course of network activity differed for the two patterns (p<0.01), with a slower rate of progression for PDTP (0.10 point/year, p<0.05) relative to PDRP (0.51 point/year, p<0.0001). Relative to baseline, there was no change in PDTP expression at 24 months (p=0.99; post-hoc Bonferroni test), although a significant increase was evident at 48 months (p<0.05). However, significant increases in PDRP expression were evident at both the second (p<0.05) and third time points (p<0.0001). *p<0.05, ***p<0.001, post-hoc Bonferroni tests with respect to baseline.
Effects of treatment on pattern expression
The effects of stimulation on the total motor UPDRS and the tremor and akinesia-rigidity subscale ratings are summarized in Table 1. At baseline, total motor UPDRS ratings did not differ across the two DBS groups (p=0.56, Student’s t-test). However, at baseline, tremor ratings were relatively greater for the Vim DBS group (p<0.05). Total motor UPDRS ratings and tremor subscale ratings declined with stimulation in both stimulation groups (p<0.01; paired Student’s t-tests). By contrast, significant reduction in the akinesia-rigidity subscale ratings was evident only for the STN DBS group (p<0.05). Although reductions in the total motor UPDRS did not differ between interventions (p=0.62), the decline in tremor ratings was found to be greater for the Vim relative to the STN DBS groups (p<0.05).
Network changes
At baseline, there was evidence of a significant group difference in the expression of both PD-related metabolic patterns (PDTP: F(2,48) = 12.8, p<0.001; PDRP: F(2,48) = 45.4, p<0.001; one-way ANOVA). Baseline PDTP expression (Figure 5A) was elevated relative to controls in both the Vim (p<0.002, post-hoc Bonferroni test) and the STN DBS cohorts (p<0.001). Baseline PDRP expression (Figure 5B) was also abnormally elevated (p<0.001) in both patient groups, although these network values were relatively higher (p<0.008) in the STN DBS group.
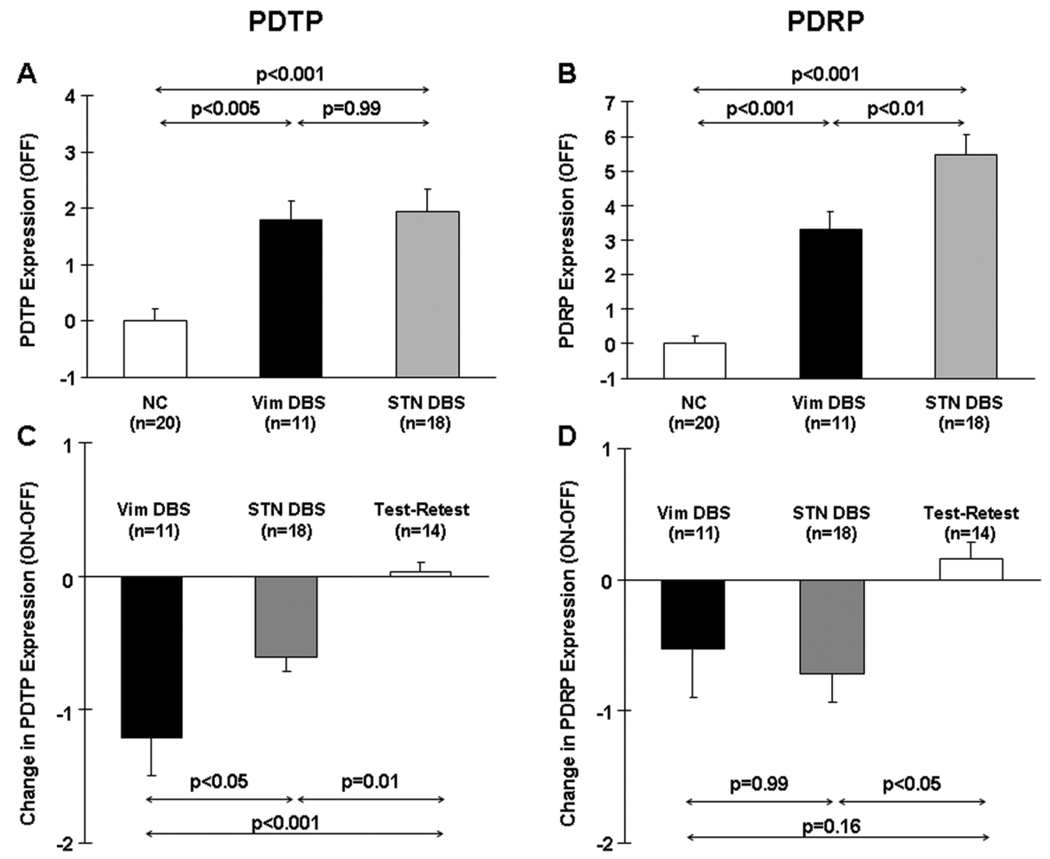
Figure 5. Changes in metabolic network activity with deep brain stimulation for PD tremor.
A. Bar graphs showing mean baseline PDTP expression (±SE) in the Vim DBS patients (black), the STN DBS patients (gray), and the healthy control subjects (white). There was a significant difference in PDTP expression across the three groups (p<0.001; one-way ANOVA), with comparable elevations in baseline pattern expression in both the Vim DBS (p<0.005) and STN DBS groups (p<0.001) relative to controls.
B. Baseline PDRP expression also differed across the three groups (p<0.001), with higher expression in both treatment groups relative to controls (p<0.001). Nonetheless, PDRP expression was higher in the STN than in the Vim DBS group (p<0.01).
C. Treatment-mediated changes (ON–OFF) in mean PDTP expression (±SE) in the Vim DBS patients (black), the STN DBS patients (gray), and the test-retest PD control subjects (white). Changes in PDTP expression were different across the three groups (p<0.001; one-way ANOVA), with stimulation-mediated declines in network activity in both DBS groups (Vim: p<0.001; STN: p=0.01, relative to the test-retest control group). PDTP modulation was greater with Vim than STN stimulation (p<0.05).
D. There was also a significant group difference in treatment-mediated PDRP modulation (p=0.02). Treatment-mediated reductions in PDRP expression reached significance (p<0.05) with STN stimulation, but not with Vim stimulation (p=0.16).
Significant differences in stimulation-mediated PDTP modulation (Figure 5C) were observed across the three groups (F(2,42)=13.6, p<0.001, one-way ANOVA), with greater changes in the stimulation groups relative to the test-retest controls (Vim DBS: p<0.001; STN DBS: p=0.01, post-hoc Bonferroni tests). The PDTP changes were greater in magnitude in the Vim DBS group relative to the STN DBS group (p=0.04). Significant group differences in stimulation-mediated PDRP modulation (Figure 5D) were also noted (F(2,42) = 4.3, p=0.02). During STN stimulation, significant treatment-mediated changes in PDRP expression were evident with respect to test-retest controls (p=0.02, post-hoc Bonferroni test). Changes in PDRP expression were, however, not significant during Vim stimulation (p=0.16).
Regional changes
Given that significant improvement in tremor ratings and PDTP suppression was observed with both Vim and STN stimulation, we sought to identify brain regions in which treatment-mediated changes in metabolic activity occurred with both interventions. Voxel-wise analysis of treatment-mediated metabolic changes in the two stimulation groups revealed a single, highly significant cluster in the sensorimotor cortex (SMC: x=40, y=−32, z=64; Zmax=6.01, p<0.05, FWE-corrected), corresponding to shared reductions (ON<OFF) in this region with both interventions (Supplementary Figure 1A). Post-hoc analysis revealed significant reductions in metabolic activity in this region during stimulation (Supplementary Figure 1B; Vim DBS: p<0.01; STN DBS: p<0.05, paired t-test). Metabolic activity in this cluster differed across the three groups (Vim DBS, STN DBS, healthy controls) in both the OFF and ON conditions (OFF: F(2, 48)=31.1, p<0.001; ON: F(2, 48)=15.0, p<0.001, one-way ANOVA). Post-hoc analysis revealed that relative to the control group, regional metabolic activity at baseline was similarly elevated in both stimulation groups (p<0.001). During stimulation, metabolic activity in this region remained abnormally elevated in the STN DBS group. By contrast, during stimulation, the mean value for the Vim DBS group fell to within 1 SD of normal. No regions were identified in which treatment-mediated increases in metabolic activity were present with the two interventions.
Discussion
In this study, we used an innovative covariance mapping approach to identify and validate a distinct tremor-related metabolic network in PD patients scanned on and off Vim thalamic stimulation. The PDTP was characterized by network-related increases in the metabolic activity of the cerebellum/dorsal pons and primary motor cortex, and to a lesser degree in the caudate/putamen. The expression of this pattern in individual patients correlated with independent clinical ratings for tremor, but not akinesia-rigidity. This contrasted with PDRP expression, which has been found to correlate with ratings for akinesia/rigidity, but not tremor (Eidelberg et al., 1994; 1995a; Antonini et al., 1998). Indeed, PDTP expression was selectively elevated in tremor dominant patients relative to their akinetic-rigid atremulous counterparts. Furthermore, the expression of this pattern increased with advancing disease, but at a slower rate than for the akinesia-related PDRP. Imaging studies of DBS interventions directed at parkinsonian tremor revealed significant reductions in PDTP expression during either Vim or STN stimulation. By contrast, significant PDRP modulation and concomitant improvement in akinesia/rigidity occurred only with STN stimulation. In aggregate, the findings suggest that the PDTP represents a distinct functional topography of PD, which may serve as a quantitative descriptor of the effects of antiparkinsonian interventions directed at tremor pathways. Moreover, the quantification of changes in PDTP and PDRP expression during treatment may help objectively parcellate the effects of novel antiparkinsonian therapies on the major motor manifestations of the illness.
The pathophysiology of parkinsonian tremor remains unclear. Convergent lines of evidence suggest that resting tremor in PD is not a direct reflection of dopamine deficiency (Fishman, 2008). Tremor has been found to be independent of other motor manifestations of the disease (see e.g., Eidelberg et al., 1994) and has a relatively small impact on the variability of clinical ratings data (Martinez-Martin et al., 1994; Stochl et al., 2008). This is consistent with the results of dopaminergic imaging studies. In contrast to akinesia and rigidity, tremor ratings in PD patients do not correlate with dopaminergic imaging measures of presynaptic nigrostriatal dysfunction (Ishikawa et al., 1996; Kazumata et al., 1997; Benamer et al., 2003). These findings accord with experimental animal studies (Poirier et al., 1966; Pechadre et al., 1976; Ohye et al., 1988) that have associated parkinsonian-like tremor with combined lesions of nigrostriatal dopaminergic projections and cerebello-rubral outflow pathways. Thus, nigrostriatal dopamine loss appears to be necessary but not sufficient for the development of PD tremor.
Characterization of the PD tremor network
In the present study, the PDTP topography was characterized by significant metabolic contributions from the cerebellum and from the primary motor cortex and striatum. The stability of this regional pattern was verified using non-parametric resampling methods (Suckling and Bullmore, 2004). Moreover, network activity values proved to have excellent within-subject reproducibility in a prospective test-retest validation sample (cf. Ma et al., 2007; Huang et al., 2007a). Perhaps most pertinent was the observation in the Vim DBS derivation cohort that baseline PDTP expression correlated with individual differences in tremor amplitude measured concurrently in the absence of stimulation. This suggests that PDTP expression is directly linked to tremor and is not indicative of stimulation per se.
To substantiate these findings, we prospectively quantified PDTP expression an independent PD patient sample and assessed the relationship between this network measure and UPDRS subscale ratings for akinesia-rigidity and tremor. Indeed, the resulting PDTP scores proved to correlate strongly with the latter but not with the former. By contrast, PDRP ratings in this cohort did not correlate with tremor ratings. Further evidence of the specificity of PDTP expression for tremor was provided by the ECD SPECT data which verified the presence of significant pattern elevation in tremor predominant patients. As with the PDRP and PDCP topographies (Ma and Eidelberg, 2007; Hirano et al., 2008), PDTP scores measured in the off-state cerebral blood flow scans are coupled to the corresponding network values measured in scans of glucose metabolism acquired in the same subjects (data not shown). It is therefore not surprising that PDTP expression could be successfully quantified in ECD SPECT perfusion scans (cf. Eckert et al., 2007). Presumably, as shown previously (Ma et al., 2010), similar network measurements will also be accessible using arterial spin labeling (ASL) perfusion MRI techniques.
Changes in network activity with disease progression and treatment
We also found that longitudinal changes in PDTP expression were sensitive to symptom progression. Indeed, in a previously reported early stage PD cohort who underwent longitudinal FDG PET imaging (Huang et al., 2007b; Tang et al., 2010), PDTP expression increased over time, but at a significantly slower rate than for the concurrent PDRP measurements. The progression of PDTP activity paralleled the slow rate of change in tremor ratings over the four years of observation. By contrast, the faster longitudinal increase in PDRP activity comports with the more rapid deterioration in akinesia-rigidity reported in this disease (Louis et al., 1999). The distinct time courses of PDTP and PDRP progression lend further credence to the notion that discrete pathophysiological mechanisms underlie PD tremor and the other motor manifestations of the disorder.
To determine whether and to what degree the PDTP network can be modulated by treatment, we contrasted two DBS procedures known to alleviate parkinsonian tremor (Machado et al., 2006; Blahak et al., 2007). We found that improvement in tremor ratings (Table 1) was greater following Vim as compared to STN stimulation (p<0.05), which accords with concurrent treatment-mediated changes in PDTP activity measured in the same subjects. That said, consistent with the reported efficacy of Vim stimulation for parkinsonian tremor (Lyons et al., 2001; Rehncrona et al., 2003), the difference in clinical response across interventions may in part be attributed to baseline effects. Thus, the current findings do not permit a definitive statement to be made regarding the relative utility of one or the other DBS targets for the relief of PD tremor. Nonetheless, the observation that Vim stimulation gives rise to marginal improvement in akinesia-rigidity and only a modest degree of PDRP modulation underscores the specificity of this intervention for tremor pathways.
By contrast, the mechanisms underlying the effects of STN stimulation on tremor are less clear and have been related to activation of the surrounding white matter, i.e., the fields of Forel, the prelemniscal radiation, and the zona incerta (see e.g., Herzog et al., 2007). It is important to consider the possibility that PDTP modulation with STN stimulation is mediated by antidromic effects on the primary motor cortex through the hyperdirect pathway (Nambu, 2004). Indeed, voxel-wise conjunction analysis disclosed shared metabolic reductions in this region during stimulation, suggesting that it may be a common final pathway for the anti-tremor effects observed with both Vim and STN DBS. It is conceivable that this “back door” approach to the PDTP circuit is associated with weaker network effects than the direct depolarization of thalamic cell bodies by Vim DBS. Importantly, STN DBS also affects the activity of subthalamic projections to the internal globus pallidus (GPi), thereby reducing inhibitory pallido-thalamic output and concomitantly the activity of the PDRP network (Lin et al., 2008; cf. Asanuma et al., 2006; Pourfar et al., 2009). On this basis, it is not surprising that by modulating the activity of both PDRP and PDTP, STN stimulation can improve both akinesia-rigidity and tremor in PD patients. Moreover, the cerebellum has recently been found to receive substantial disynaptic projections from the STN (Bostan et al., 2010). This pathway may represent an additional means by which STN interventions can influence these two PD-related metabolic networks.
Anatomical and functional basis for the PD tremor network
The akinetic-rigid manifestations of PD have been associated with discrete functional abnormalities of cortico-striatopallido-thalamocortical (CSPTC) motor circuits (DeLong and Wichmann, 2007). These changes, however, do not readily account for other disease manifestations such as tremor (Zaidel et al., 2009). Indeed, tremor generation has been linked to abnormal activity in cerebello-thalamo-cortical (CbTC) pathways (Volkmann et al., 1996; Timmermann et al., 2003), and the role of the basal ganglia in mediating this symptom has remained the subject of debate (see Deuschl et al., 2000; Timmermann et al., 2007 for review). Indeed, prior imaging studies have shown that both lesioning and high frequency stimulation of the Vim thalamic nucleus results in localized reductions in neural activity in the primary motor cortex and the anterior cerebellum (Baron et al., 1992; Deiber et al., 1993; Boecker et al., 1997; Wielepp et al., 2001; Fukuda et al., 2004). In keeping with these findings, magnetoencephalography (MEG) studies with EMG back-averaging disclosed a tremor-coherent oscillatory network involving the primary motor cortex, thalamus, and cerebellum, which also contribute significantly to the PDTP metabolic topography. Interestingly, the PDTP metabolic topography also included significant contributions from the striatum, albeit of lower magnitude than the other nodes of this network. In the primate, the striatum receives cerebellar output via the ventrolateral and intralaminar thalamic nuclear groups (Hoshi et al., 2005), and metabolic activity in the putamen was found to correlate with tremor ratings in another FDG PET study (Lozza et al., 2004). In aggregate, findings from both MEG and PET suggest that the regional nodes of the PD tremor network are defined by abnormal synchronization of firing, leading to localized increases in synaptic activity and concomitant elevations in glucose metabolism. While the observed tremor-related changes are most prominent in the primary motor cortex and cerebellum, these PDTP regions interconnect through the Vim thalamus and putamen, thus describing a distinct large-scale metabolic network associated with this disease manifestation.
We note that the thalamus itself did not contribute to the PDTP regional topography. Interestingly, a post-hoc volume-of-interest (VOI) analysis did reveal a stimulation-related (ON>OFF) increase in Vim thalamic metabolic activity (p<0.02, paired Student’s t-test). This is consistent with prior reports of increased regional cerebral blood flow and metabolism at the Vim electrode insertion site (Rezai et al., 1999; Perlmutter et al., 2002; Haslinger et al., 2003; Fukuda et al., 2004). Nonetheless, we found that Vim thalamic metabolic activity in our DBS cohort did not differ from normal control values in either stimulation condition (OFF: p=0.74; ON: p=0.58), and failed to correlate with UPDRS tremor subscale ratings (n=41; r=0.21, p=0.19) in prospective scan data. Moreover, the stimulation-mediated changes observed in the Vim thalamus did not correlate (r=0.18, p=0.65) with concurrently measured PDTP changes. These findings suggest that the regional thalamic changes occurring with stimulation are not critical to the tremor-related spatial covariance pattern identified with OrT CVA. It is likely that the increases in thalamic blood flow and metabolic activity observed with Vim stimulation reflect direct effects on local cell membrane potentials at the electrode tip, rather than functional effects at downstream thalamic output pathways. By contrast, the ventrolateral thalamus, particularly the pallido-receptive Voa/Vop nuclei, contributes functionally to the PDRP topography (Lin et al., 2008; Eidelberg et al., 1997). Indeed, the observed topographic difference between PDTP and PDRP is compatible to the known segregation of cerebellar- and pallidal-receiving circuits at the thalamic level (Middleton and Strick, 2000).
In addition, we noted that network-related activation of the sensorimotor cortex and cerebellum is a known accompaniment of normal movement. Nevertheless, we found no spatial homology between the PDTP topography and the previously characterized normal movement-related activation pattern (NMRP) (Carbon et al., 2010). These results suggest that the PDTP is a truly abnormal metabolic network and cannot be construed simply as an overactive fragment of the normal motor circuit. Similarly, partial coherence analysis of MEG data from patients with PD tremor suggests that the tremor-related regional changes are not the consequence of increased somatosensory input from rhythmic muscle activity (Timmermann et al., 2003). Finally, the specificity of the PDTP for parkinsonian tremor is not known. Indeed, a moderate degree of pattern expression has recently been observed in the ECD SPECT scans of patients with essential tremor (S. Hirano and I. Isaias, personal communication). Whether different tremor disorders are associated with unique metabolic networks or with subtle variants of the PDTP topography is a topic for future investigation.
Conclusion
These findings suggest that parkinsonian tremor is mediated by a distinct metabolic network involving primarily cerebello-thalamo-cortical pathways. Indeed, effective treatment of this symptom is associated with significant reduction in PDTP activity. Quantification of discrete treatment-mediated changes in PDTP and PDRP activity can provide an objective means of evaluating the effects of novel antiparkinsonian interventions on the different motor features of the disorder.
Supplementary Material
A. Stimulation-mediated reductions in sensorimotor cortex (SMC) metabolic activity (BA 4/3) was a common feature of both Vim and STN stimulation (p<0.05, FWE-corrected; SPM conjunction analysis). [SPM{t} maps were superimposed on a single-subject MRI brain template. Stimulation-mediated metabolic reductions (ON<OFF) common to the two DBS interventions were thresholded at T=3.00, p<0.05 (FWE-corrected)].
B. Globally adjusted metabolic values (mean±SE) for the SMC cluster depicted in A. Regional metabolic activity for each stimulation condition (OFF: black; ON: gray) in the two DBS groups, and in healthy control subjects (white) are presented as bar graphs. SMC metabolism was abnormally elevated (p<0.001) at baseline in both treatment groups. Stimulation was associated with significant metabolic reductions (p<0.05) with either intervention, but approximated normal levels (i.e., fell below 1 SD of the normal mean) only in the Vim DBS group.
Acknowledgments
This work is dedicated to the memory of our colleague, Dr. Harald Fodstad (1940–2008) who performed the Vim DBS surgeries. The project was supported by R01 NS 35069, the General Clinical Research Center of The Feinstein Institute for Medical Research (M01 RR018535), and by a grant from the Emily and Jerry Spiegel Foundation. We thank Dr. Thomas Chaly for radiochemistry support, Mr. Claude Margouleff for technical support, and Ms. Toni Fitzpatrick for assisting in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/conflict of interest statement
The authors do not have any conflicts to disclose.
References
- Antonini A, Moeller JR, Nakamura T, Spetsieris P, Dhawan V, Eidelberg D. The metabolic anatomy of tremor in Parkinson's disease. Neurology. 1998;51:803–810. doi: 10.1212/wnl.51.3.803. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Tang C, Ma Y, Dhawan V, Mattis P, Edwards C, Kaplitt MG, Feigin A, Eidelberg D. Network modulation in the treatment of Parkinson's disease. Brain. 2006;129:2667–2678. doi: 10.1093/brain/awl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair E HT, Paul D, Tibshirani R. Prediction by supervised principal components. J Am Stat Assoc. 2006;101:119–137. [Google Scholar]
- Baron JC, Levasseur M, Mazoyer B, Legault-Demare F, Mauguiere F, Pappata S, Jedynak P, Derome P, Cambier J, Tran-Dinh S, et al. Thalamocortical diaschisis: positron emission tomography in humans. J Neurol Neurosurg Psychiatry. 1992;55:935–942. doi: 10.1136/jnnp.55.10.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benamer HT, Oertel WH, Patterson J, Hadley DM, Pogarell O, Hoffken H, Gerstner A, Grosset DG. Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3-month observations. Mov Disord. 2003;18:977–984. doi: 10.1002/mds.10482. [DOI] [PubMed] [Google Scholar]
- Blahak C, Wohrle JC, Capelle HH, Bazner H, Grips E, Weigel R, Hennerici MG, Krauss JK. Tremor reduction by subthalamic nucleus stimulation and medication in advanced Parkinson's disease. J Neurol. 2007;254:169–178. doi: 10.1007/s00415-006-0305-x. [DOI] [PubMed] [Google Scholar]
- Boecker H, Brooks DJ. Functional imaging of tremor. Movement Disorders. 1998;13 Suppl 3:64–72. doi: 10.1002/mds.870131311. [DOI] [PubMed] [Google Scholar]
- Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thomas DG, Marsden CD, Brooks DJ. Stereotactic thalamotomy in tremor-dominant Parkinson's disease: an H2(15)O PET motor activation study. Annals of Neurology. 1997;41:108–111. doi: 10.1002/ana.410410118. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Argyelan M, Habeck C, Ghilardi MF, Fitzpatrick T, Dhawan V, Pourfar M, Bressman SB, Eidelberg D. Increased sensorimotor network activity in DYT1 dystonia: a functional imaging stud. Brain. 2010;133:690–700. doi: 10.1093/brain/awq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Pollak P, Passingham R, Landais P, Gervason C, Cinotti L, Friston K, Frackowiak R, Mauguiere F, Benabid AL. Thalamic stimulation and suppression of parkinsonian tremor. Evidence of a cerebellar deactivation using positron emission tomography. Brain. 1993;116(Pt 1):267–279. doi: 10.1093/brain/116.1.267. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Baron R, Lindemann M, Wilms H, Krack P. The pathophysiology of parkinsonian tremor: a review. Journal of Neurology. 2000;247 Suppl 5:V33–V48. doi: 10.1007/pl00007781. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Lindemann M, Krack P. The pathophysiology of tremor. Muscle Nerve. 2001;24:716–735. doi: 10.1002/mus.1063. [DOI] [PubMed] [Google Scholar]
- Eckert T, Van Laere K, Tang C, Lewis DE, Edwards C, Santens P, Eidelberg D. Quantification of Parkinson's disease-related network expression with ECD SPECT. Eur J Nucl Med Mol Imaging. 2007;34:496–501. doi: 10.1007/s00259-006-0261-9. [DOI] [PubMed] [Google Scholar]
- Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S. The metabolic topography of parkinsonism. Journal of Cerebral Blood Flow & Metabolism. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, Belakhlef A, Mandel F, Przedborski S, Fahn S. Early differential diagnosis of Parkinson's disease with 18F-fluorodeoxyglucose and positron emission tomography. Neurology. 1995a;45:1995–2004. doi: 10.1212/wnl.45.11.1995. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T, Robeson W, Dahl JR, Margouleff D. Assessment of disease severity in parkinsonism with fluorine-18-fluorodeoxyglucose and PET. J Nucl Med. 1995b;36:378–383. [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Kazumata K, Antonini A, Sterio D, Dhawan V, Spetsieris P, Alterman R, Kelly PJ, Dogali M, Fazzini E, Beric A. Metabolic correlates of pallidal neuronal activity in Parkinson's disease. Brain. 1997;120:1315–1324. doi: 10.1093/brain/120.8.1315. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. M.o.t.U.D.C. In: Recent Developments in Parkinson's Disease. Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Vol 2. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, Moeller JR, Eidelberg D. Metabolic correlates of levodopa response in Parkinson's disease. Neurology. 2001;57:2083–2088. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- Fishman PS. Paradoxical aspects of parkinsonian tremor. Movement Disorders. 2008;23:168–173. doi: 10.1002/mds.21736. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Barnes A, Simon ES, Holmes A, Dhawan V, Giladi N, Fodstad H, Ma Y, Eidelberg D. Thalamic stimulation for parkinsonian tremor: correlation between regional cerebral blood flow and physiological tremor characteristics. Neuroimage. 2004;21:608–615. doi: 10.1016/j.neuroimage.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural Computation. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- Habeck C, Stern Y. Neural network approaches and their reproducibility in the study of verbal working memory and Alzheimer’s disease. Clin Neurosci Res. 2007;6:381–390. doi: 10.1016/j.cnr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Boecker H, Buchel C, Vesper J, Tronnier VM, Pfister R, Alesch F, Moringlane JR, Krauss JK, Conrad B, Schwaiger M, Ceballos-Baumann AO. Differential modulation of subcortical target and cortex during deep brain stimulation. Neuroimage. 2003;18:517–524. doi: 10.1016/s1053-8119(02)00043-5. [DOI] [PubMed] [Google Scholar]
- Herzog J, Hamel W, Wenzelburger R, Potter M, Pinsker MO, Bartussek J, Morsnowski A, Steigerwald F, Deuschl G, Volkmann J. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130:1608–1625. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- Hirano S, Asanuma K, Ma Y, Tang C, Feigin A, Dhawan V, Carbon M, Eidelberg D. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson's disease. J Neurosci. 2008;28:4201–4209. doi: 10.1523/JNEUROSCI.0582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nature Neuroscience. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage. 2007a;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Tang C, Feigin A, Lesser M, Ma Y, Pourfar M, Dhawan V, Eidelberg D. Changes in network activity with the progression of Parkinson's disease. Brain. 2007b;130:1834–1846. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Archives of Neurology. 1993;50:140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- Isaias I, Marotta G, Hirano S. Imaging Essential Tremor. Movement Disorders. 2010;25:679–686. doi: 10.1002/mds.22870. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Dhawan V, Kazumata K, Chaly T, Mandel F, Neumeyer J, Margouleff C, Babchyck B, Zanzi I, Eidelberg D. Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med. 1996;37:1760–1765. [PubMed] [Google Scholar]
- Kazumata K, Antonini A, Dhawan V, Moeller JR, Alterman RL, Kelly P, Sterio D, Fazzini E, Beric A, Eidelberg D. Preoperative indicators of clinical outcome following stereotaxic pallidotomy. Neurology. 1997;49:1083–1090. doi: 10.1212/wnl.49.4.1083. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain. 1994;117(Pt 3):531–543. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- Lin TP, Carbon M, Tang C, Mogilner AY, Sterio D, Beric A, Dhawan V, Eidelberg D. Metabolic correlates of subthalamic nucleus activity in Parkinson's disease. Brain. 2008;131:1373–1380. doi: 10.1093/brain/awn031. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K. Progression of parkinsonian signs in Parkinson disease. Arch Neurol. 1999;56:334–337. doi: 10.1001/archneur.56.3.334. [DOI] [PubMed] [Google Scholar]
- Lozza C, Baron JC, Eidelberg D, Mentis MJ, Carbon M, Marie RM. Executive processes in Parkinson's disease: FDG-PET and network analysis. Hum Brain Mapp. 2004;22:236–245. doi: 10.1002/hbm.20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KE, Koller WC, Wilkinson SB, Pahwa R. Long term safety and efficacy of unilateral deep brain stimulation of the thalamus for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 2001;71:682–684. doi: 10.1136/jnnp.71.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Eidelberg D. Multivariate brain mapping in clinical neuroscience research. Clin Neurosci Res. 2007;6:357–358. [Google Scholar]
- Ma Y, Huang C, Dyke JP, Pan H, Alsop D, Feigin A, Eidelberg D. Parkinson's disease spatial covariance pattern: noninvasive quantification with perfusion MRI. J Cereb Blood Flow Metab. 2010;30:505–509. doi: 10.1038/jcbfm.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado A, Rezai AR, Kopell BH, Gross RE, Sharan AD, Benabid AL. Deep brain stimulation for Parkinson's disease: surgical technique and perioperative management. Mov Disord. 2006;21 Suppl 14:S247–S258. doi: 10.1002/mds.20959. [DOI] [PubMed] [Google Scholar]
- Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson's Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research - Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Moeller J, Habeck C. Reciprocal Benefits of Mass-Univariate and Multivariate Modeling in Brain Mapping: Applications to event-related functional MRI, H215O-,and FDG PET. International Journal of Biomedical Imaging. 2006;2006:1–13. doi: 10.1155/IJBI/2006/79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A. A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res. 2004;143:461–466. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- Ohye C, Shibazaki T, Hirai T, Wada H, Kawashima Y, Hirato M, Matsumura M. A special role of the parvocellular red nucleus in lesion-induced spontaneous tremor in monkeys. Behavioural Brain Research. 1988;28:241–243. doi: 10.1016/0166-4328(88)90102-7. [DOI] [PubMed] [Google Scholar]
- Pechadre JC, Larochelle L, Poirier LJ. Parkinsonian akinesia, rigidity and tremor in the monkey. Histopathological and neuropharmacological study. Journal of the Neurological Sciences. 1976;28:147–157. doi: 10.1016/0022-510x(76)90100-3. [DOI] [PubMed] [Google Scholar]
- Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58:1388–1394. doi: 10.1212/wnl.58.9.1388. [DOI] [PubMed] [Google Scholar]
- Poirier LJ, Sourkes TL, Bouvier G, Boucher R, Carabin S. Striatal amines, experimental tremor and the effect of harmaline in the monkey. Brain. 1966;89:37–52. doi: 10.1093/brain/89.1.37. [DOI] [PubMed] [Google Scholar]
- Pourfar M, Tang C, Lin T, Dhawan V, Kaplitt MG, Eidelberg D. Assessing the microlesion effect of subthalamic deep brain stimulation surgery with FDG PET. J Neurosurg. 2009;110:1278–1282. doi: 10.3171/2008.12.JNS08991. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Rozdilsky B, Ang L. Occurrence of resting tremor in Parkinson's disease. Neurology. 1991;41:1298–1299. doi: 10.1212/wnl.41.8.1298. [DOI] [PubMed] [Google Scholar]
- Rehncrona S, Johnels B, Widner H, Tornqvist AL, Hariz M, Sydow O. Long-term efficacy of thalamic deep brain stimulation for tremor: double-blind assessments. Mov Disord. 2003;18:163–170. doi: 10.1002/mds.10309. [DOI] [PubMed] [Google Scholar]
- Rezai AR, Lozano AM, Crawley AP, Joy ML, Davis KD, Kwan CL, Dostrovsky JO, Tasker RR, Mikulis DJ. Thalamic stimulation and functional magnetic resonance imaging: localization of cortical and subcortical activation with implanted electrodes. Technical note. J Neurosurg. 1999;90:583–590. doi: 10.3171/jns.1999.90.3.0583. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- Spetsieris PG, Ma Y, Dhawan V, Eidelberg D. Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. Neuroimage. 2009;45:1241–1252. doi: 10.1016/j.neuroimage.2008.12.063. [DOI] [PubMed] [Google Scholar]
- Stochl J, Boomsma A, Ruzicka E, Brozova H, Blahus P. On the structure of motor symptoms of Parkinson's disease. Mov Disord. 2008;23:1307–1312. doi: 10.1002/mds.22029. [DOI] [PubMed] [Google Scholar]
- Suckling J, Bullmore E. Permutation tests for factorially designed neuroimaging experiments. Hum Brain Mapp. 2004;22:193–205. doi: 10.1002/hbm.20027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CC, Poston KL, Dhawan V, Eidelberg D. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann L, Florin E, Reck C. Pathological cerebral oscillatory activity in Parkinson's disease: a critical review on methods, data and hypotheses. Expert Rev Med Devices. 2007;4:651–661. doi: 10.1586/17434440.4.5.651. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Gross J, Dirks M, Volkmann J, Freund HJ, Schnitzler A. The cerebral oscillatory network of parkinsonian resting tremor. Brain. 2003;126:199–212. doi: 10.1093/brain/awg022. [DOI] [PubMed] [Google Scholar]
- Trošt M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J, Joliot M, Mogilner A, Ioannides AA, Lado F, Fazzini E, Ribary U, Llinas R. Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology. 1996;46:1359–1370. doi: 10.1212/wnl.46.5.1359. [DOI] [PubMed] [Google Scholar]
- West SG, Aiken LS, Krull JL. Experimental personality designs: analyzing categorical by continuous variable interactions. J Pers. 1996;64:1–48. doi: 10.1111/j.1467-6494.1996.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Wielepp JP, Burgunder JM, Pohle T, Ritter EP, Kinser JA, Krauss JK. Deactivation of thalamocortical activity is responsible for suppression of parkinsonian tremor by thalamic stimulation: a 99mTc-ECD SPECT study. Clin Neurol Neurosurg. 2001;103:228–231. doi: 10.1016/s0303-8467(01)00165-2. [DOI] [PubMed] [Google Scholar]
- Zaidel A, Arkadir D, Israel Z, Bergman H. Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol. 2009;22:387–393. doi: 10.1097/WCO.0b013e32832d9d67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Stimulation-mediated reductions in sensorimotor cortex (SMC) metabolic activity (BA 4/3) was a common feature of both Vim and STN stimulation (p<0.05, FWE-corrected; SPM conjunction analysis). [SPM{t} maps were superimposed on a single-subject MRI brain template. Stimulation-mediated metabolic reductions (ON<OFF) common to the two DBS interventions were thresholded at T=3.00, p<0.05 (FWE-corrected)].
B. Globally adjusted metabolic values (mean±SE) for the SMC cluster depicted in A. Regional metabolic activity for each stimulation condition (OFF: black; ON: gray) in the two DBS groups, and in healthy control subjects (white) are presented as bar graphs. SMC metabolism was abnormally elevated (p<0.001) at baseline in both treatment groups. Stimulation was associated with significant metabolic reductions (p<0.05) with either intervention, but approximated normal levels (i.e., fell below 1 SD of the normal mean) only in the Vim DBS group.