Abstract
Purpose
To investigate the biologic and immunologic effects of preoperative trastuzumab in patients with ductal carcinoma in situ (DCIS) of the breast.
Experimental Design
Patients with DCIS were enrolled on this open-label phase II trial and tested for HER2. Trastuzumab was by intravenous infusion (8 mg/kg) and patients had surgery 14 to 28 days following treatment. Tissue and peripheral blood samples were obtained before therapy and at the time of surgery to examine residual disease and immunologic response.
Results
Median age of the 69 enrolled patients was 53 years, mean mammographic size of the DCIS lesions was 5.2+/-1.2 cm, and 24 patients (35%) were found to have HER2 overexpression/amplification (12 received trastuzumab and 12 untreated patients provided tissue for blinded controlled biomarker analyses). No overt histologic evidence of response was noted. No significant change in mean pre-therapy staining for Ki-67 (44.3+/-3.4%) and cleaved caspase-3 (2.6+/- 0.8%) was noted when surgical specimens from patient samples treated were compared with those not treated with drug. Trastuzumab significantly augmented antibody-dependent cell mediated cytotoxicity (ADCC) in 100% of patients; this was demonstrated to be mediated through CD56+ degranulating natural killer cells (P<0.01). One patient developed a significant anti-HER2 humoral CD4 T-cell response.
Conclusions
Single-dose monotherapy with trastuzumab for patients with HER2-positive DCIS does not result in significant clinically overt histologic, antiproliferative, or apoptotic changes but results in the ability to mount ADCC mediated through natural killer cells and may also induce T-cell dependent humoral immunity. Further studies of trastuzumab for DCIS appear warranted.
Keywords: DCIS, Trastuzumab, Apoptosis, Neoadjuvant Systemic Therapy, Immune Response
Introduction
Ductal carcinoma in situ (DCIS) is the fourth leading cause of cancer among women in the United States with approximately 64,000 cases diagnosed annually(1). There is a critical need for the development and investigation of agents that can either eradicate DCIS before the need for surgery and radiation or can prevent frankly invasive disease in most patients.
Several lines of evidence suggest that it is logical to study trastuzumab, a monoclonal antibody targeting HER2/neu (HER2), for the treatment of DCIS. Neoadjuvant trastuzumab may be able to prevent the development of invasive breast cancer in patients with DCIS in much the same way that tamoxifen markedly decreases the risk of breast cancer development in women with high-risk proliferative lesions such as atypical ductal hyperplasia (2). A further rationale for studying neoadjuvant trastuzumab for DCIS relates to the scarcity of effective medical treatments for estrogen receptor (ER)-negative DCIS. Additional support for studying the efficacy of trastuzumab against DCIS comes from the promising results of trials of neoadjuvant trastuzumab for invasive breast cancer. Gennari et al showed that administration of a short preoperative course of single-agent trastuzumab to 11 patients with early-stage invasive breast cancer resulted in a complete pathologic response in one patient and partial responses in four (3). The in vivo mechanism of action of trastuzumab is not completely understood. In animal models, the activity of trastuzumab has been demonstrated to be dependent on the engagement of Fc-receptor expressing lymphocytes suggesting that ADCC is a major mechanism of action (4). Other investigators have demonstrated development of a T cell dependent humoral response (5). We hypothesized that trastuzumab will have substantial activity against DCIS, perhaps even more than it has against invasive breast cancer because the volume of disease in DCIS patients is normally much lower. This study is the first prospective trial of neoadjuvant trastuzumab in patients diagnosed with DCIS that overexpresses HER2 to test this hypothesis and to study individual patients’ immune responses with therapy to elucidate potential mechanisms of trastuzumab activity. This trial utilized a blinded controlled analysis of selected biomarkers in treated cases before and after therapy and in untreated patients with HER2-positive DCIS.
Materials and Methods
This study was a prospective, open-label, phase II trial approved by and conducted at The University of Texas M. D. Anderson Cancer Center between March 2005 and February 2009.
Patient selection and eligibility
Patients with histologically confirmed DCIS that had measurable residual microcalcifications on mammography following initial diagnostic biopsy were counseled regarding the study. To be eligible, patients were required to have adequate bone marrow, renal, cardiac, and liver function, and women of child bearing potential were required to have a negative urine or serum pregnancy test. Eligible patients were required give informed consent prior to testing their DCIS for HER2 status. Once consent was obtained, HER2 status was determined using immunohistochemical (IHC) analysis (AB8 Neomarkers, Labvision, Fremont, California) or fluorescence in situ hybridization (FISH; PathVysion assay kit; Vysis Inc, Downers Grove, IL). DCIS was considered to be positive if determined to be HER2 3+ by IHC or positive for HER2 gene amplification (HER2/CEP17 ratio > 2.0) by FISH.
Treatment schedule
Patients with HER2-positive DCIS were eligible to receive the study drug. Trastuzumab was provided free of charge to all patients by Genentech Inc (South San Francisco, CA). Trastuzumab was given at a dosage of 8 mg/kg over a 90-minute intravenous infusion and patients were scheduled for surgery 14 to 28 days following treatment. Surgical procedures were dictated by the extent of disease and patient preference. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria (version 3.0).
Correlative studies
Tissue processing and immunohistochemistry
Processing was performed according to routine clinical pathology tissue processing methods. IHC analyses for Ki-67, a nuclear marker for cell proliferation (Clone MIB-1, Dako, Glostrup, Denmark) and cleaved caspase-3 (Asp175), one of the key executioners of apoptosis (Cell Signaling Technology, Danvers, MA) were performed in duplicate using residual formalin-fixed paraffin-embedded (FFPE) biopsy tissue sections that contained DCIS before treatment and from the corresponding FFPE tissues that contained DCIS from the surgical resection.
Untreated control samples
Tissue samples collected from enrolled patients with HER2 overexpression/ amplification who gave informed consent to participate in the study but did not receive study drug, either because of surgical scheduling or patient preference, served as internal negative controls. All analyses were performed by investigators who were blinded to clinical information including receipt or non-receipt of study drug.
Isolation of sera and peripheral blood mononuclear cells (PBMC) from patients
Peripheral blood collected with anticoagulant was used to isolate peripheral blood mononuclear cells (PBMC) by standard Ficoll gradient centrifugation (Accu-Prep Lymphocites, Oslo, Norway). After centrifugation, PBMCs were washed with medium containing 10% fetal bovine serum (FBS), and resuspended in 90% FBS + 10% DMSO at a concentration of 10 × 106/ml.
Evaluation of antibody-dependent cellular cytotoxicity (ADCC)
Patient’s PBMCs were tested for their killing capacity against a HER2-overexpressing target cell line (MDA-MB-361) in the presence of simultaneously harvested autologous serum. The MDA-MB-361 cell line was validated by STR DNA fingerprinting using the AmpFℓSTR Identifier kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). ADCC by trastuzumab was measured in a 51Cr release assay using radiolabeled target cells from the HER2-overexpressing human breast cancer cell line MDA-MB-361. To this end, 1-3 million MDA-MB-361 target cells were labeled with 100 μCi (3.7 MBq) of Na51Cr (Perkin Elmer, Walthem, MA) for 1 h at 37°C, washed extensively, and used as targets. Five thousand 51Cr-labeled target cells per well were seeded into 96-well U-bottomed plates. Experiments were conducted in triplicate at PBMC (effector) to MDA-MB-361 (target) ratios of 12.5:1; 25:1, and 50:1, in 200 μl of RPMI 1640 containing 20% autologous human serum. The antibody source for evaluation of ADCC was made up of trastuzumab, autologous serum of treated patients, or both. Thus, all ADCC experiments were carried out in the presence of 20% autologous serum, with or without 10 μg/ml of trastuzumab added exogenously. After 4 h at 37°C in humidified air with 5% CO2, the release of 51Cr was determined by taking 100 μl of supernatant and counting using a Wizard 1470 Automate gamma counter (Perkin Elmer, Walthem, MA). Maximal and spontaneous 51Cr release values were obtained by adding either 1 % Tween detergent (Fisher Scientific, Pittsburgh, PA) or complete medium, respectively, to microtiter wells containing 5 × 103 labeled target cells. The percentage of lysis was calculated according to the standard formula [(cpm experimental) − (cpm spontaneous release)] / [(cpm maximum release) - (cpm spontaneous release)] × 100.
Flow cytometry analysis of PBMC effectors for ADCC
An eight-parameter flow cytometric analysis of thawed PBMC was performed using a three-laser FACSCanto II instrument equipped with DIVA software (BD Biosciences, San Jose, CA). To identify phenotype modifications of effector lymphocytes involved in trastuzumab-mediated cytotoxicity, as well as the frequency of effector natural killer (NK) and T cell populations among PBMCs 4×105 of the PBMCs prepared for use in the ADCC assay were left in parallel cultures with unlabeled MDA-MB-361 target cells at an effector-to-target ratio of 25:1 in the presence of 20% autologous serum or trastuzumab (10 μg/ml) or in their absence (negative control), in otherwise identical culture conditions of ADCC assay. To test CD107a mobilization, anti-CD107a-Alexa Fluor 488-conjugated (mouse IgG1, H4A3, eBioscience, San Diego, CA) was added at 1 μg/106 cells at the beginning of culture. One hour into the culture, GolgiStop was added (BD Pharmingen, San Diego, CA) following manufacturer’s instructions. At the end of culture (6 hours total), cells were harvested, washed, and directly stained with 1 μg/106 cells of each of the following mAbs: anti-CD3-PE (UCHT1, mouse IgG1), anti-CD56-APC (B159, mouse IgG1), anti-CD16-PECy7 (3G8, mouse IgG1), anti-CD69-APCCy7 (FN50, mouse IgG1), CD8-Pacific Blue (RPA-T8, mouse IgG1), CD4-PerCPCy5.5 (SK3, mouse IgG1) (All from BD Pharmingen, San Diego, CA) and live/dead Aqua cell stain (Invitrogen, Carlsbad, CA) or with isotype-matched mAbs of irrelevant specificity or with isotype-matched mAbs. Cells were then fixed and analyzed within 24 h.
HER2–specific CD4 IFN-γ enzyme-linked immunospots
Fourteen HER2-derived peptides, each known to bind to multiple HLA-DR molecules (5,6) were used to detect T-cell responses by the enzyme linked immunospot (ELISPOT) method (7). The peptides, which are designated by the position of the first amino acid (Pool I: p98, p369, p927; Pool II: p776, p62, p77; Pool III: p83, p88, p350; Pool IV: p976, p42, p688; and Pool V: p971, p1166) were all class II peptides that have been previously described (5,6,8,9). Both phorbol 12-myristate 13-acetate/ionomycin and tetanus toxoid were used as positive controls. In brief, cryopreserved PBMCs were cultured at 2 × 105 cells per well in 96-well plates for 7 days in medium containing pools of HER2 peptides (pools of 2 or 3 peptides, each at 10 μg/ml) or with HIV peptide control (negative control). In these cases, all of the time points were assessed with the same panels of peptides. Interleukin-2 (10 units/ml) was added at day 5. On day 7, peptide and irradiated autologous PBMCs (2 × 105 per well) were added as antigen-presenting cells. On day 8, the cells were gently transferred to the ELISPOT plate for detection of spots with a pair of IFNγ-specific antibodies (Mabtech, Mariemont, OH). ELISPOTs were developed, dried, and read with a C.T.L. Immunospot ELISPOT reader, using the programs Image Acquisition 4.4 and Immunospot 3 (Cellular Technology Ltd, Shaker Heights, OH). Peptide-specific immune reactivity was determined by subtracting the background spots in the HIV wells. A positive response was defined as peptide-specific spots that were statistically higher (triplicates) than control wells using a two-tailed t test (P < 0.05). A zero response was assigned if the peptide-specific wells were not different than control wells. The counts for each peptide were summed and presented as the total HER2 specific T cells per million PBMC assessed at each time point (6).
Isolation and detection of trastuzumab within the breast ductal fluids of a treated patient
Nipple aspirate fluid was obtained from patient number 7 at the time of surgery as previously described (10). The fluid droplets were collected and centrifuged at 1500 RPM for 10 minutes, and the supernatant was stored at −80°C until measurement. Detection and quantification of trastuzumab in the ductal fluid were kindly performed by the laboratory of Dr. David Jamieson at Northern Institute for Cancer Research, University of Newcastle upon Tyne utilizing a cell-based ELISA assay that measures the interaction between trastuzumab and formalin-fixed HER2+ SKBR3 cells (11).
Statistical considerations
Twelve evaluable patients were required to characterize the change in proliferation rate after treatment with a single dose of trastuzumab. In order to accrue 12 evaluable patients with DCIS, trastuzumab administration, surgery, and post surgery biomarkers testing, it was estimated that up to 71 patients needed to be registered in the study. A 6% absolute reduction in the proliferation rate, as measured by Ki-67, was designated as the study endpoint, providing sufficient preliminary data to pursue larger clinical studies with trastuzumab for DCIS. To compare ADCC and CD4+ T-cell response in each patient observed at pre- and post-treatment times, paired analysis was performed using Student’s t-test. Nonparametric Wilcoxon rank sum test was used to compare data between groups. Statistical tests were performed with Prism 4 software (San Diego, CA), and P < 0.05 was considered significant.
Results
Clinical and pathologic characteristics of enrolled subjects
Sixty-nine patients were enrolled in the trial (median age, 53 years). Overall, 24 (35%) patients had lesions with overexpression or amplification of HER2. HER2-overexpression/amplification correlated with histologic grade and ER expression. Overall, 6% of patients had grade I, 38% had grade II, and 56% had grade III lesions. Patients with high-grade lesions were more likely to exhibit HER2-overexpression/amplification (0% grade I, 27% grade II, and 44% in grade III, respectively; P = 0.10). Overall, 81% of the DCIS cases were ER positive. HER2- overexpression/amplification was significantly more likely in ER negative versus ER positive DCIS (62% vs. 28%, respectively; P=0.047).
Following receipt of HER2 testing results, 12 patients received study drug and 12 patients did not receive study drug either because of surgical scheduling constraints or a final decision not to take the study drug after receiving the HER2 results.
On average, surgery was performed at 18.08 +/- 5.21 days following administration of trastuzumab. Patients receiving trastuzumab had large DCIS lesions with the mean mammographic extent of disease measuring 5.15 +/- 1.15 cm. Mastectomy was performed in the majority of patients (n=8/12) with immediate breast reconstruction in all but one patient. The mean final pathologic size of the DCIS for these 12 patients was 5.15 +/- 3.73 cm. In addition to DCIS, 42% (n=5) of the patients had evidence of invasive breast cancer on their pathologic specimen. The mean size of invasive cancer in these patients was 0.28 cm and none of these patients was found to have sentinel lymph node metastases.
Toxicity and adverse events
Three patients (25%) had an adverse event reported during the study period. Two patients experienced a grade I or II infusion reaction consisting of chills or chills and fever, which resolved with administration of diphenhydramine and acetaminophen or hydrocortisone and meperidine; respectively. One 49 year-old participant with a distant history of adult onset seizure developed grade I right upper lip numbness 3-days after infusion of study drug which resolved without further intervention. Complete neurologic work-up was within normal limits.
Pathologic response
Each of the 12 treated patients had residual DCIS at the time of surgery without overt histopathologic evidence of response to treatment.
Proliferative and apoptotic studies
Pre and post-treatment tissue with DCIS was studied for proliferation and apoptosis in patients treated with trastuzumab. Tissue from the additional 12 patients who did not receive study drug was evaluated as an internal control. The mean percent Ki-67 staining was 44.29 +/- 3.42% and the mean percent cleaved caspase 3 staining was 2.59 +/- 0.78% for DCIS tissue from all initial biopsy specimens. Following administration of trastuzumab, Ki-67 staining increased on average by 16.11% and cleaved caspase-3 staining increased on average by 2.3% as measured in tissue before and after treatment (Figure 1). For patients not receiving study drug, Ki-67 staining increased on average by 17.23% and cleaved caspase-3 staining increased on average by 4.15% (Figure 1). No significant differences were identified with respect to percent change in Ki-67 staining (P= 0.75) or cleaved caspase-3 staining (P=0.65) between patients who did and did not receive trastuzumab.
Figure 1.
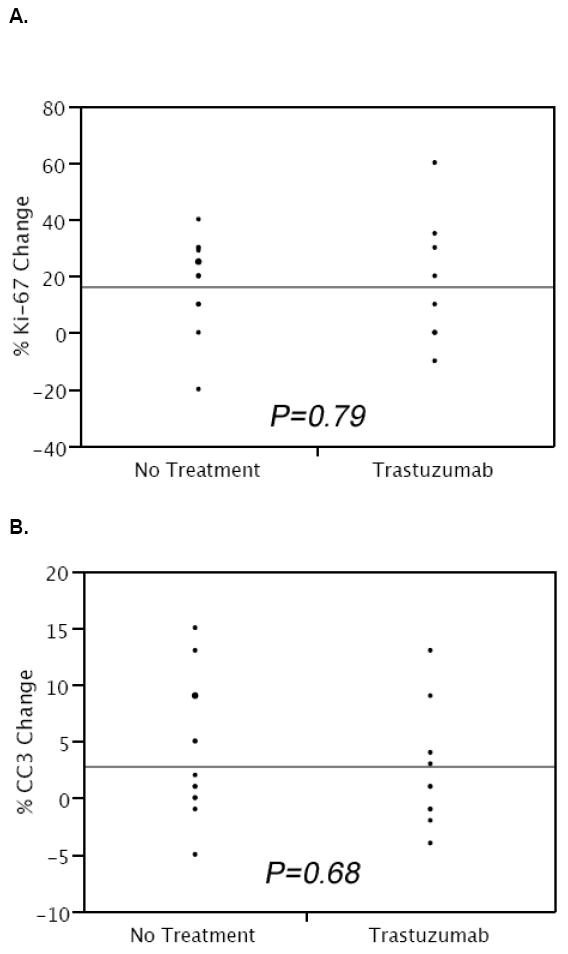
Change in proliferation (A) and apoptosis (B) from initial core biopsy compared with the patient’s surgical specimen in enrolled patients untreated and treated with preoperative trastuzumab (CC3=cleaved caspase-3).
Immune Response Correlative Studies
To investigate the role of ADCC in patients with DCIS enrolled in this study, cell-mediated cytotoxic activity was evaluated ex-vivo in 51Cr-release cytotoxicity assays using patient PBMCs in the presence of autologous serum versus HER2-overexpressing breast cancer target cells. As demonstrated in Figure 2, ADCC exerted by PBMC in the presence of autologous sera increased significantly for 9 of 9 (100%) patients tested where sufficient cells were available following the single dose of trastuzumab (P<0.0035). Taking into account all evaluable patients, the increase was significant, but there was considerable heterogeneity in the response. These data confirm that trastuzumab was present in the serum of patients receiving the drug and suggest that ADCC may occur in vivo and contribute to trastuzumab’s mechanism of action.
Figure 2. Treatment with trastuzumab augments cell-mediated cytotoxicity of Ductal Carcinoma in situ (DCIS) patients against HER2-overexpressing target cells in an ADCC assay.
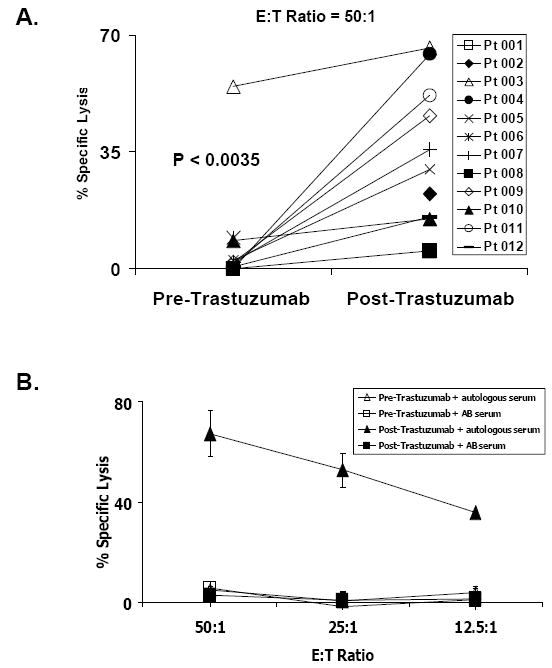
A, Basal and post therapy cytotoxic activity for each patient as percentage of Cr51-labeled MDA-MB361 target cells lysis at 50:1 effector to target ratio is shown. B, a representative experiment with increasing E:T ratios is shown (patient 004) using PBMC and autologous sera collected before or after therapy with trastuzumab.
To identify the effector cells involved in trastuzumab-mediated cytotoxicity, flow cytometry was used to analyze the phenotype of lymphocytes cultured for the ADCC assay. Classical ADCC is mediated by NK cells therefore we focused the analysis on this population, defined as CD56+CD16+/-. As demonstrated in Figure 3a, which depicts one representative patient, following administration of trastuzumab, there was increased expression of cell surface secreted CD107a, a molecule linked to the killing activity of cytotoxic lymphocytes. The percentage of CD56+CD107a+ degranulating NK cells was calculated by subtracting the percentage of CD56+CD107a+ using normal donor AB serum from the percentage of CD56+CD107a+ using autologous serum (Figure 3b). For the patient depicted in Figure 3a, this increased from 0.41% to 5.28% following administration of trastuzumab. The data for all patients is depicted in Figure 3b which demonstrates a significant increase in the percentage of CD56+CD107a+ degranulating NK cells for all 12 patients (100%) tested following the single dose of trastuzumab (P<0.00005). These data suggest that ADCC induced by trastuzumab treatment in DCIS patients is mediated by degranulating CD56+ NK cells.
Figure 3. Cell-mediated cytotoxicity induced by Trastuzumab treatment in DCIS patients is mediated by degranulating CD56+ NK cells.
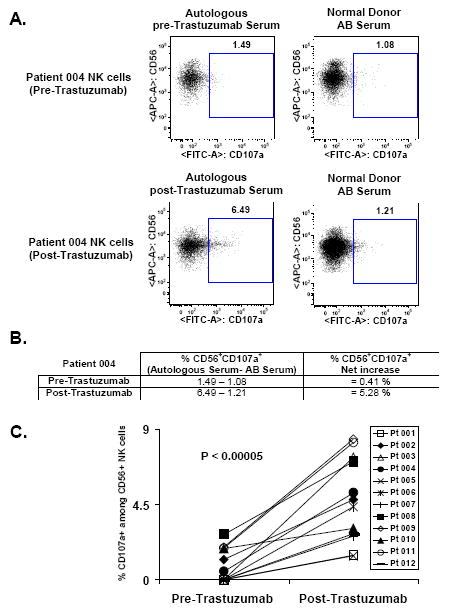
Mononuclear cells and HER2 overexpressing target cells MDA-MB361 were cultured similarly to ADCC assay at 25:1 effector to target ratio, in the presence of Pre-Trastuzumab or Post-Trastuzumab autologous serum. At the end of the culture, cells were stained with CD56 and CD107a and analyzed by multiparametric flow cytometry with gating on CD56+CD16+/- NK cells. A, a representative experiment is shown (patient 004). B, the net increase of CD56+CD107a+ degranulating NK cells for each time point of all patients is calculated by subtracting the percentage of CD56+CD107a+ using normal donor AB serum from the percentage of CD56+CD107a+ using autologous serum, here depicted with patient 004. C, Basal and posttherapy percentage of CD56+CD107a+ for each patient is shown.
ELISPOT assays were used to determine CD4 T-cell responses against multiple HER2-derived class II peptides. The peptides were evaluated as pools and the composition of each of the 5 pools. There were 9 evaluable patients who had sufficient PBMC available pre- and post-treatment. The frequency of HER2 peptide-pool-specific T cells for each patient is shown in Table 1. Table1 depicts the pre- and post-treatment HER2-specific T cell levels in the 9 patients with evaluable PBMC at both time points. Each point is the total CD4 T-cell frequency calculated by summing the counts for each peptide and subtracting out counts against the control peptide. Only one patient had a significant CD4 T cell response (Pool II and V) suggesting that a single dose of trastuzumab did not promote a generalized T-cell dependent response in these patients with DCIS.
Table 1. Frequency of HER2 peptide pool-specific T cells.
Frequencies of T cells to specific HER2-derived peptide pools pretreatment (pre) and after a single dose of trastuzumab (post). Values are expressed as the number of CD4+ T cells per million PBMC, calculated from triplicate determinations. All values are statistically higher than the no-antigen containing control wells.
| Peptide | Patient 3 | Patient 4 | Patient 5 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Pool I | 0 | 875 | 0 | 815 | 0 | 0 | 0 | 0 | 0 | 10 | 370 | 355 | 25 | 0 | 225 | 475 |
| Pool II | 350 | 465 | 2150 | 1635 | 0 | 415 | 710 | 310 | 0 | 30 | 1160 | 1225 | 465 | 65 | 630 | 340 |
| Pool III | 725 | 460 | 220 | 365 | 0 | 0 | 0 | 0 | 0 | 25 | 515 | 105 | 130 | 0 | 160 | 10 |
| Pool IV | 0 | 85 | 305 | 465 | 0 | 0 | 0 | 0 | 0 | 20 | 770 | 405 | 290 | 0 | 45 | 25 |
| Pool V | 0 | 185 | 460 | 965 | 0 | 0 | 5 | 0 | 0 | 25 | 135 | 0 | 80 | 65 | 0 | 5 |
| Peptide | Patient 12* | |||||||||||||||
| Pre | Post | |||||||||||||||
| Pool I | 425 | 465 | ||||||||||||||
| Pool II | 95 | 1650 | ||||||||||||||
| p776 | 135 | 905 | ||||||||||||||
| p62 | 215 | 540 | ||||||||||||||
| p77 | 465 | 945 | ||||||||||||||
| Pool III | 170 | 185 | ||||||||||||||
| Pool IV | 140 | 130 | ||||||||||||||
| Pool V | 160 | 975 | ||||||||||||||
| p971 | 465 | 640 | ||||||||||||||
| p1166 | 230 | 1550 | ||||||||||||||
Only patient 12 had statistically significant increased reactivity in pool II and V. The positive pools in patient 12 were retested using the individual peptides from each pool. Peptides p776 and p62 both demonstrated specific significantly increased activity (P<0.05) peptides p971 from pool V was not significant and p1166 from pool V showed a trend toward significance on repeat single peptide specific testing (P=0.07)
Detection of Trastuzumab in Ductal Fluids
Prior to this publication, it was unknown whether trastuzumab could pass through the basement membrane or be secreted into the breast ducts in humans. In one treated patient, ductal fluids were obtained at the time of surgery on day 18 following administration of trastuzumab. Trastuzumab was demonstrated to be present within the ducts at a level of 161 μg/ml.
DISCUSSION
This study is the first to report on the use of neoadjuvant trastuzumab for patients with HER2 positive DCIS. We have found that single dose monotherapy with trastuzumab for patients with HER2-positive DCIS does not result in significant clinically overt histologic, proliferative, or apoptotic changes but results in the specific ability to mount ADCC mediated through NK cells and may also induce humoral immunity in a T-cell dependent manner.
Prior to this study, it was not known what alterations in the histologic appearance might be seen utilizing trastuzumab for DCIS. In the neoadjuvant setting for invasive cancer, histologic changes seen after administration of trastuzumab with chemotherapy can range from minimal effect to marked decreases in size and cellularity to complete eradication of any evidence of disease (complete pathologic response) (12). Following one cycle of trastuzumab given in the present study for DCIS, no particular morphologic changes were noted. Changes in proliferation (Ki-67) and apoptosis (cleaved caspase-3) were therefore investigated to better evaluate the tissue effects of a single dose of trastuzumab on DCIS. Compared with patients not receiving trastuzumab, there was no significant change in proliferation or apoptosis. Two previous studies enrolling patients with HER2-overexpressing invasive breast cancer have evaluated proliferation following short-course trastuzumab therapy. Mohsin et al evaluated a three week short-course of trastuzumab in patients with locally advanced invasive breast cancer and, consistent with our findings, noted no change in proliferation as measured by Ki-67 (13). A second study by Gennari et al evaluated 11 patients that received a loading dose of trastuzumab followed by three weekly doses (3). They also noted no change in proliferation. These data are in contrast to in vitro data investigating potential mechanisms of action of trastuzumab in the laboratory. In vitro, blockade of the HER2 receptor results in upregulation of the cell cycle inhibitor p27, which can inhibit cyclin E/cdk2 complexes resulting in a G1 cell cycle arrest, with a concomitant reduction in proliferation (14,15).
Despite not seeing a decrease in proliferation, Mohsin et al reported clinical responses as evidenced by significant tumor regression (13). These authors performed additional studies to investigate the mechanism of this cytotoxic effect and demonstrated a significant increase in apoptosis. These data are notable because in in vitro models using cultured breast cancer cells, trastuzumab has not been demonstrated to be capable of inducing apoptosis (13). In the current study, we did not see evidence of increased apoptosis in DCIS patients treated with trastuzumab. Both our study and the one by Mohsin et al were investigating apoptosis in vivo and both employed the same assay suggesting the differences cannot be explained by methodology. It is likely that this difference is due to the fact that the current study enrolled patients with non-invasive cancer whereas the study by Mohsin et al, enrolled patients with very large advanced invasive tumors that were more susceptible to therapy. It is possible that longer treatment may be required to see changes in histology or biomarkers in patients with DCIS treated with trastuzumab.
These data also highlight the fact that the mechanism by which trastuzumab exerts its therapeutic action remains incompletely understood. Mechanisms of action of trastuzumab include inhibition of HER2 extracellular domain proteolysis, disruption of downstream signaling pathways, G1 cell-cycle arrest, inhibition of DNA repair, suppression of angiogenesis, and induction of ADCC (16,17). One mechanism of action that has been shown to be significant in vivo first in a mouse model and then in the short-course trastuzumab trial conducted by Gennari et al, is ADCC (3,18). Our data also demonstrated ADCC activity by PBMC obtained from patients treated with trastuzumab in the presence of autologous serum. This finding confirmed that there was trastuzumab in the serum of these patients which is consistent with the known long half-life of this agent (11). It also confirmed that ADCC is a potential mechanism of action by which trastuzumab may exert cytotoxic activity. It is important to note however that these data are from an ex vivo assay and do not confirm that ADCC was occurred in our patients. The fact that apoptosis, which is considered the final pathway for ADCC-mediated cytotoxicity, did not occur suggests that significant ADCC was not occurring in vivo in these patients (13).
To further assess the immune response, we looked for evidence that HER2-specific immunity was being induced. In a study evaluating patients with HER2-overexpressing metastatic breast cancer treated with trastuzumab and chemotherapy, Taylor et al demonstrated a significant increase in the anti-HER2 humoral response that was associated with an increase in HER2-specific CD4+ T cell responses (5). This suggests that the therapeutic antibody trastuzumab can not only provide passive immunotherapy through ADCC but also induce active immunity by promoting a cellular response. In the current study, only one patient had a significant CD4+ T cell response suggesting that a single dose of trastuzumab does not promote a generalized T-cell response in most patients with DCIS.
As DCIS by pathologic definition remains within the breast ducts, it was of interest to determine if trastuzumab could be detected within non-lactating human ductal fluids. Immunoglobulins are known to be secreted in the milk and trastuzumab has been detected in milk of lactating monkeys given intravenous trastuzumab (19). In the present study, trastuzumab was shown, using a new cell-based ELISA for the quantification of trastuzumab in human plasma (11), to cross the basement membrane in a non-lactating patient with DCIS and enter the breast ducts. The assay, developed and validated by Jamieson et al., specifically measures the interaction between trastuzumab and HER2 (11). The present study is the first published study to demonstrate the presence of trastuzumab in human ductal fluids and shows that trastuzumab can theoretically act on cancer cells located within the breast ducts.
Some clinicians have voiced concern that preoperative studies of DCIS run the risk of undertreating women who have a concurrent occult invasive breast cancer. In the current study, 45% of patients did in fact have an occult small invasive cancer detected in the surgical specimen. There was an inherent selection bias due to surgery scheduling issues in favor of enrolling patients with very large DCIS in the current study. Patients who were going to schedule a mastectomy with immediate reconstruction (87.5% of patients chose immediate reconstruction with their mastectomy) were more likely to participate in the study than patients with much smaller DCIS who could be immediately scheduled for a segmental resection. The average size of the DCIS was over 5 cm in this study, and it has been established that larger size is an independent risk factor for finding occult invasive cancer (20). Another potential selection bias for identification of occult invasive cancer in a high proportion of treated patients in this study may involve the overexpression of HER2. In a recent study published by Roses et al, invasive disease was found in association with HER2-overexpressing DCIS at a higher frequency than with DCIS that did not overexpress HER2 (21). Despite these legitimate concerns, the current study is important because we have demonstrated that a DCIS “window study” can be performed safely. It is likely that many similar preoperative trial designs will be utilized in future studies of DCIS. Missing an occult invasive malignancy is of concern, and it is anticipated that more stringent DCIS size eligibility criteria and the use of potentially better imaging will markedly decrease the risk of missing a concurrent occult invasive component.
Finally, the National Surgical Adjuvant Breast and Bowel (NSABP) is studying the potential efficacy and role of post-operative trastuzumab for DCIS in a phase III randomized trial for patients with DCIS treated with breast conservation surgery. Patients are being randomized to 6 weeks of whole-breast irradiation with or without concurrent trastuzumab given in two doses at week 1 and 3. The rationale for utilizing trastuzumab concurrently with radiation for HER2-overexpressing DCIS is that trastuzumab radiosensitizes only cells that overexpress HER2 and therefore will enhance the radiation sensitivity of carcinoma more than surrounding healthy tissues. The results of the present study provide no evidence that trastuzumab utilized with radiation will or will not be effective. Furthermore, the NSABP study is also designed to evaluate the long term potential effects of trastuzumab on the prevention of contralateral breast cancer.
In summary, this phase II, open-label clinical trial utilizing a single dose of intravenous trastuzumab for patients with HER2-positive DCIS did not result in significant clinically overt histologic, proliferative, or apoptotic changes but did result in the specific ability of treated patients to mount ADCC mediated through NK cells and induce humoral immunity in a T-cell-dependent manner in one patient. These findings warrant additional studies to determine if these short-term immune responses may prove useful in the prevention of recurrent or contralateral breast cancer.
Acknowledgments
Funding for the trial was provided by research grant funds received from Randalls Food Markets, Genentech, Inc., and NCI # CA16672. The investigators are grateful to Aurora Madrigal, RN, and Rebekah Hubbard for assisting with patient accrual and processing of specimens and to Dr. David Jamieson for performing the cell-based ELISA for the quantification of trastuzumab.
References
- 1.Kuerer HM, Albarracin CT, Yang WT, et al. Ductal carcinoma in situ: State of the science and roadmap to advance the field. J Clin Oncol. 2009;27:279–88. doi: 10.1200/JCO.2008.18.3103. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353(9169):1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 3.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Taylor C, Hershman D, Shah N, et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res. 2007;13:5133–43. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 6.Salazar LG, Fikes J, Southwood S, et al. Immunization of cancer patients with HER-2/neu-derived peptides demonstrating high-affinity binding to multiple class II alleles. Clin Cancer Res. 2003;9:5559–65. [PubMed] [Google Scholar]
- 7.Knutson KL, Schiffman K, Disis ML. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–84. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–32. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 9.Perez SA, Sotiropoulou PA, Sotiriadou NN, et al. HER-2/neu-derived peptide 884-899 is expressed by human breast, colorectal and pancreatic adenocarcinomas and is recognized by in-vitro-induced specific CD4(+) T cell clones. Cancer Immunol Immunother. 2002;50:615–24. doi: 10.1007/s002620100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuerer HM, Thompson PA, Krishnamurthy S, et al. High and differential expression of HER-2/neu extracellular domain in bilateral ductal fluids from women with unilateral invasive breast cancer. Clin Cancer Res. 2003;9:601–5. [PubMed] [Google Scholar]
- 11.Jamieson D, Cresti N, Verrill MW, Boddy AV. Development and validation of cell-based ELISA for the quantification of trastuzumab in human plasma. J Immunol Methods. 2009;345:106–11. doi: 10.1016/j.jim.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 13.Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol. 2005;23:2460–8. doi: 10.1200/JCO.2005.00.661. [DOI] [PubMed] [Google Scholar]
- 14.Le XF, Claret FX, Lammayot A, et al. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–50. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 15.Lane HA, Motoyama AB, Beuvink I, Hynes NE. Modulation of p27/Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signaling. Ann Oncol. 2001;12(Suppl 1):S21–2. doi: 10.1093/annonc/12.suppl_1.s21. [DOI] [PubMed] [Google Scholar]
- 16.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 17.Morrow PK, Zambrana F, Esteva FJ. Recent advances in systemic therapy: Advances in systemic therapy for HER2-positive metastatic breast cancer. Breast Cancer Res. 2009;11:207. doi: 10.1186/bcr2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416(6878):279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 19.Herceptin [Package Insert] Genentech, Inc.; South San Francisco, CA: Mar, 2009. [Google Scholar]
- 20.Yen TW, Hunt KK, Ross MI, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg. 2005;200:516–26. doi: 10.1016/j.jamcollsurg.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Roses RE, Paulson EC, Sharma A, et al. HER-2/neu overexpression as a predictor for the transition from in situ to invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1386–9. doi: 10.1158/1055-9965.EPI-08-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]


