Abstract
Background & Aims
The transition of gastric epithelial mucous neck cells (NCs) to digestive-enzyme–secreting zymogenic cells (ZCs) involves an increase in rough endoplasmic reticulum (rER) and formation of many large secretory vesicles. The transcription factor MIST1 is required for granulogenesis of ZCs. The transcription factor XBP1 binds the Mist1 promoter and induces its expression in vitro and expands the ER in other cell types. We investigated whether XBP1 activates Mist1 to regulate ZC differentiation.
Methods
Xbp1 was inducibly deleted in mice using a tamoxifen/Cre-loxP system; effects on ZC size and structure (ER and granule formation) and gastric differentiation were studied and quantified for up to 13 months after deletion using morphologic, immunofluorescence, quantitative reverse-transcriptase PCR, and immunoblot analyses. Interactions between XBP1 and the Mist1 promoter were studied by chromatin immunoprecipitation from mouse stomach and in XBP1-transfected gastric cell lines.
Results
Tamoxifen-induced deletion of Xbp1 (Xbp1Δ ) did not affect survival of ZCs but prevented formation of their structure. Xbp1Δ ZCs shrank 4-fold, compared to those of wild-type mice, with granulogenesis and cell shape abnormalities and disrupted rER. XBP1 was required and sufficient for transcriptional activation of MIST1. Despite severe structural defects, ZCs that developed in the absence of XBP1 expressed ZC markers (intrinsic factor, pepsinogen C) but did not lose expression of progenitor NC markers.
Conclusions
XBP1 controls the transcriptional regulation of ZC structural development; it expands the lamellar rough ER and induces MIST1 expression to regulate formation of large granules. XBP1 is also required for loss of mucous NC markers as ZCs form.
Keywords: maturation, stem cell, cell structures, gastric acid secretion
Introduction
The corpus of the mouse stomach is an excellent tissue for studying developmentally regulated transcription factors (TFs) in generation of secretory cell architecture, because the epithelium turns over continuously throughout adult life. In addition, the gastric epithelial stem cell gives rise to several diverse secretory lineages. Zymogenic cells (ZCs), for example, reside at the base of gastric epithelial glands and develop following a prolonged (~2 week) phase as progenitor cells, known as mucous neck cells (NCs), which in turn differentiate from the gastric epithelial stem cell1, 2. For the ZC lineage, thus, distance from the progenitor zone equates to differentiation stage.
The TF MIST 1 is involved in ZC differentiation. In Mist1−/− mice, ZCs delay turning off progenitor markers as they arise from NCs, though fully differentiated ZCs eventually form in normal numbers. However, all Mist1−/− ZCs are structurally defective with deficient apical cytoplasms and small secretory vesicles, though they show normal deposition of elaborate, lamellar rER3, 4. MIST1’s function as a secretory cell-specific structure-inducing TF is highly conserved: even in flies, the MIST1 ortholog DIMM mediates granule structure of peptide-secreting cells without affecting survival5, 6.
Despite the complex and interesting developmental patterning in the gastric epithelium, little is known about the underlying transcriptional and molecular mechanisms. Some progress has been made in understanding morphogens in gastric patterning. For example: EGFr ligands TGFα /EGF/Amphiregulin drive increased surface cell growth7, 8; the Hedgehog pathway seems required for inhibiting surface cell growth and promoting NC transition into ZCs9, 10; various cytokines, like IL1β and IL11, and in general the NFκ B signaling pathway, seem to be key in regulating growth and multiple differentiation pathways9, 11
Other than MIST1, only a handful of other TFs play a known role in differentiation of adult corpus epithelial lineages: FOXQ1, which regulates granule maturation in mucus-secreting surface (aka pit/foveolar) cells12; NGN3 and MASH113, 14, which regulate development of hormone-secreting endocrine cells; and KLF4, which apparently regulates differentiation of multiple secretory lineages15.
X-box binding protein 1 (XBP1) is a TF traditionally viewed as a key regulator of the unfolded protein response (UPR) during endoplasmic reticulum (ER) stress. XBP1 mRNA is spliced and thereby activated by IRE1, which governs part of the UPR16. XBP1 has also been described as a developmentally regulated transcription factor (TF) that induces ER expansion and may be required for differentiation of dedicated secretory cells, like antibody-secreting plasma cells and intestinal Paneth cells17–19. It is unclear, however, whether XBP1 is required for cell survival and fate determination like other developmentally regulated TFs or whether it plays a special role in establishing differentiated cell function rather than cell identity20, 21. Interestingly, an in vitro screen recently identified MIST1 as a transcriptional target of XBP1 in plasma cells22. It is not clear whether XBP1 is required for MIST1 induction, nor whether XBP1 targets MIST1 in vivo.
Here, we examine the role of XBP1 in gastric epithelial differentiation. Using inducible deletion with tamoxifen-Cre-loxP, we show XBP1 is required for nearly the entire structural development of ZCs, including elaboration of rER and formation of large secretory granules. XBP1 induces MIST1 in gastric epithelial cell lines and, in mice, is required for induction of Mist1 expression in ZCs. Interestingly, ZCs arising in the absence of XBP1 still induce normal ZC differentiation markers like gastric intrinsic factor, but they cannot extinguish expression of progenitor NC markers; in other words, they never terminally differentiate. Thus, XBP1 is absolutely required for structural differentiation and maturation of ZCs but is dispensable for survival and initial induction of the ZC fate. The results show for the first time that XBP1 is the principal governor of ZC structural maturation, plays a role in shutting off progenitor features, and is required for induction of Mist1 in vivo.
Results
Our earlier work had shown that ZCs expressed the highest levels of Xbp1 in the gastric epithelium4. To determine its role in ZC development, we deleted Xbp1 in adult stomachs by tamoxifen injections in Xbp1flox/flox mice23 expressing CAGGCreERTM (Chicken β-actin promoter)24. Based on our long term experience with expression patterns of this promoter in stomach and other tissues25, we titrated tamoxifen concentration and frequency to the minimum needed to induce lacZ expression in all ZCs and their mucous neck cell progenitors in CAGGCreERTM/R26R mice (SI, Fig. S1). The protocol resulted in loss of 79.1±5.7% of Xbp1 expression across the whole stomach by qRT-PCR (n=7 mice, 4 experiments; SI, Fig. S1).
Effects of loss of Xbp1 are specific to the zymogenic lineage
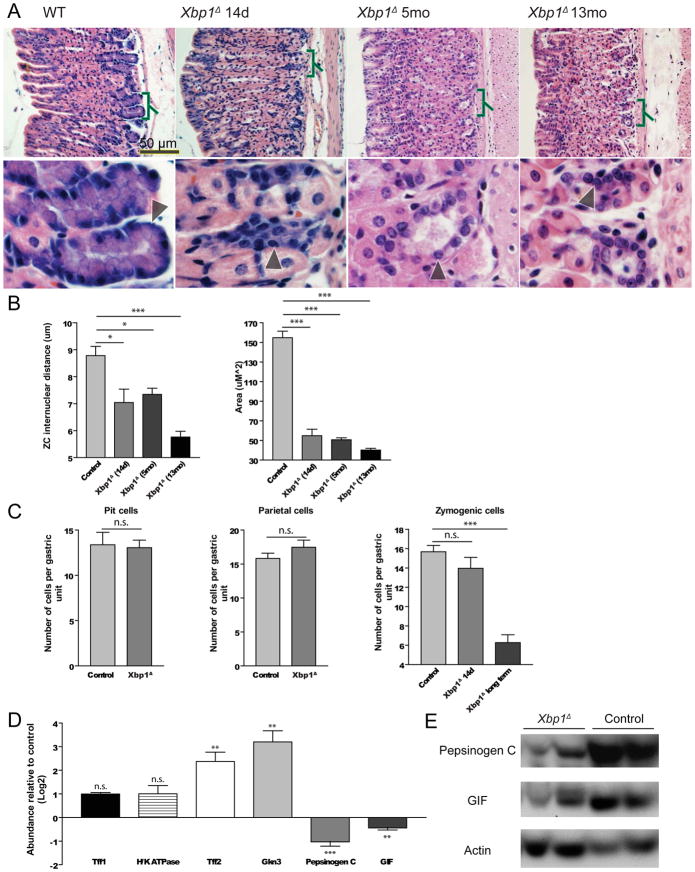
Induced Xbp1 deletion (hereafter designated Xbp1Δ ) caused dramatic reorganization of the basal, ZC-containing zone of gastric units (Fig. 1A). We examined every possible control: ±Cre allele, ±floxed Xbp1 alleles, and ±tamoxifen. The ZC phenotype was observed only in tamoxifen treated CAGGCreERTM; Xbpflox/flox stomachs. Deletion of Xbp1 caused a substantial reduction in size and number of histologically identifiable ZCs. By 14 days post deletion, the average cross-sectional area of Xbp1Δ ZCs was decreased 2.8-fold and 3.9-fold by 13 months (the longest timepoint studied). The census of NCs, the progenitors of ZCs, showed considerable variability across gastric region and from mouse to mouse even in wildtype mice, precluding statistical analysis. However, there was a statistically significant increase of the NC markers, TFF2 and GKN326, by qRT-PCR in the gastric corpus (Fig. 1D). Other gastric lineages (e.g., parietal, pit, endocrine cells) were not affected by Xbp1 deletion histologically (Fig. 1A,C, S2) or by qRT-PCR for specific markers (Fig. 1D). At no time were inflammatory cells observed. Consistent with the reduction in ZC size, there was a modest but statistically significant decrease in two ZC markers, GIF and PGC, in the corpus by qRT-PCR and by western blot (Fig. 1D–E).
Figure 1. Xbp1 is required for ZC lineage cellular structure and function.
A, H&E staining of adult gastric units (oriented with gastric lumen to left), with: control, CAGGCreERTM; Xbpflox/flox stomachs, 14 days, 5 months, and 13 months after tamoxifen injection. Lower panels magnify bracketed regions. Arrowheads indicate individual ZCs. B, Internuclear distance (μm) and cross-sectional area (μm2) measured from H&E sections (n=3 independent experiments; means ±SD; “Control” here and elsewhere denotes data from all mice in the given experiment that were not homozygous for floxed Xbp1 or did not have a Cre allele or were treated with vehicle rather than tamoxifen). C, Cell census scored from H&E (n=3 independent experiments). D, Transcripts in whole stomach corpora by qRT-PCR (n=7 mice; means ±SD)); For all figures: “n.s.”-Not significant; “*”-p<0.05; “**”-p<0.01;”***”, p<0.001. E, Western blot of ZC markers from two Xbp1Δ and two control mice.
XBP1 is necessary for rER and secretory vesicles in Zymogenic Cells
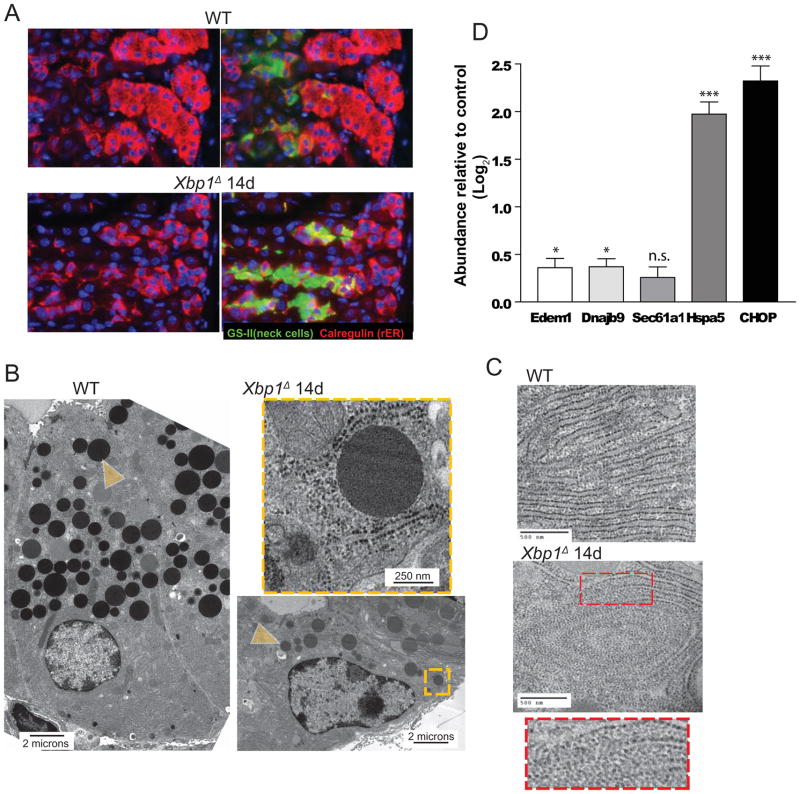
XBP1 regulates rER organization and deposition: as wildtype ZCs arise, they show a dramatic increase in rER relative to their NC progenitors (Fig. 2A upper panel). In contrast, Xbp1Δ ZCs showed nearly no increase in rER compared to progenitors (Fig. 2A). tEM confirmed sparse, disorganized rER in Xbp1Δ and abundant, lamellar rER in wildtype ZCs (Fig. 2B–C). Interestingly, we noted frequent free ribosomes, organized in a whorled pattern but no longer associated with ER, in Xbp1Δ ZCs (Fig. 2C). ER stress markers were either slightly (EDEM1 and DNAJB9; p<0.05) or markedly (CHOP and HSPA5; p<0.001) increased in corpora of Xbp1Δ mice (Fig. 2D).
Figure 2. Xbp1 is required for rER expansion and secretory vesicle formation.
A, Immunofluorescent staining for GSII (labels mucous neck cells, ZC progenitors, green), and Calregulin (ER marker, red), (nuclei, blue with bisbenzimide). Control (WT) ZCs induce abundant ER after emerging from neck progenitor cells. Xbp1Δ ZCs lack rER expansion. B, Xbp1Δ ZCs have decreased secretory vesicles (e.g., yellow arrowhead) by tEM. Orange box magnified in inset. C, High magnification view of rER in control vs. Xbp1Δ ZC with inset showing magnified view of box in middle panel. Note loose whorls of free ribosomes with loss of associated ER in Xbp1Δ ZC vs. lamellar rER in control. D, Expression levels of ER stress markers (n=7 mice; means ±SD).
XBP1 directly binds the MIST1 promoter and is sufficient to induce MIST1
The deficiency in rER in ZCs lacking Xbp1 is consistent with the known role of XBP1 in direct transcriptional regulation of cellular effectors that establish ER17, 27. However, we also noted defects in granulogenesis which typifies loss of function of the only other TF known to regulate ZC development, MIST13, 4. Mist1 was recently identified as a potential direct transcriptional target of XBP1 in vitro22. Hence, we reasoned that XBP1 might act through parallel pathways: one wherein it directly induces cellular effectors of rER biogenesis and one wherein it regulates vesicular structure indirectly by inducing Mist1.
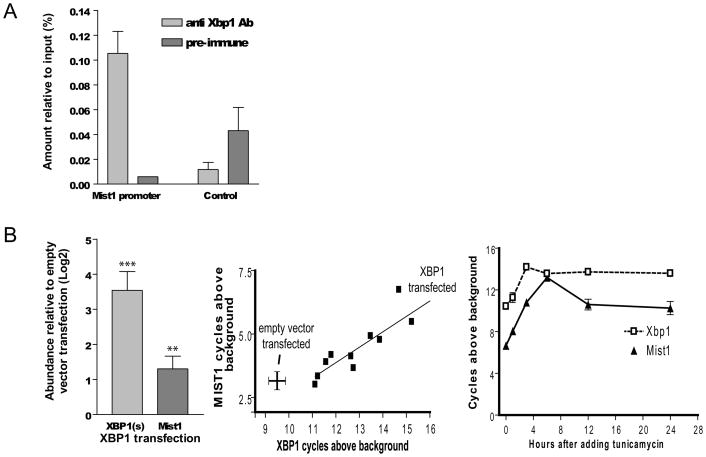
To test that hypothesis, we first asked whether XBP1 binds the Mist1 promoter in the stomach in vivo. Chromatin immunoprecipitation (ChIP) on adult mouse stomach indicated XBP1 bound a consensus cis-regulatory sequence in the Mist1 promoter but not a reference control site (Fig. 3A). Next, we transfected a cDNA plasmid encoding the active, spliced form of XBP1 into AGS cells. MIST1 expression was significantly increased by XBP1 transfection (Fig. 3B left panel). XBP1 is expressed at high levels in cultured cells; hence, to circumvent confounding by endogenous XBP1, we repeated the transfections to varying final XBP1 levels to confirm that MIST1 levels always correlated positively with XBP1 levels, independent of transfection efficiency [note the linear relationship (r2=0.81; p<0.001) between levels of XBP1 and MIST1 (Fig. 3B middle panel)]. XBP1 is also induced by ER stress. Accordingly, untransfected cells treated with tunicamycin for 12 hours increased XBP1 and MIST1 expression (Fig. 3B right panel).
Figure 3. XBP1 directly binds Mist1 promoter in the mouse stomach and XBP1 is sufficient to induce MIST1 expression in gastric cells.
A, Chromatin immunoprecipitation (ChIP) from mouse stomach. Nuclear proteins from mouse stomach were immunoprecipitated with either anti-XBP1 or control pre-immune antibody and fragmented genomic DNA amplified in qRT-PCR reactions (means ±SD). Note signal in the sample immunoprecipitated with anti-XBP1 antibody and amplified by primers flanking the XBP1 binding site in the Mist1 promoter is enriched relative to control antibody immunoprecipitate; there is no such enriched amplification using primers flanking the control genomic site that lacks consensus XBP1 binding sites. B, Left panel, qRT-PCR for XBP1(s) (active, spliced form) and MIST1 transcripts in XBP1 transfected AGS normalized to empty vector transfected AGS cells (n=11 independent experiments; means ±SD). Middle panel, qRT-PCR for overall levels of MIST1 and XBP1 transcripts in the multiple independent XBP1 transfection experiments summarized in left panel. Note that despite high levels of endogenous XBP1 (9.6 PCR cycles above background in vector transfected controls), increasing XBP1 levels by transfection results in higher MIST1 expression across a wide range of XBP1 levels (r2=0.81, p<0.0002; crosshairs = SEM). Data represented as PCR cycles above control (water) wells after normalization to 18s rRNA ( ref. 3; hence, each axis represents ~log2 relative message abundance. Right panel, XBP1 and MIST1 by qRT-PCR (means ±SD) following treatment with 1 μg/ml tunicamycin treatment (n=3 independent experiments).
XBP1 is required for induction of but not maintenance of MIST1
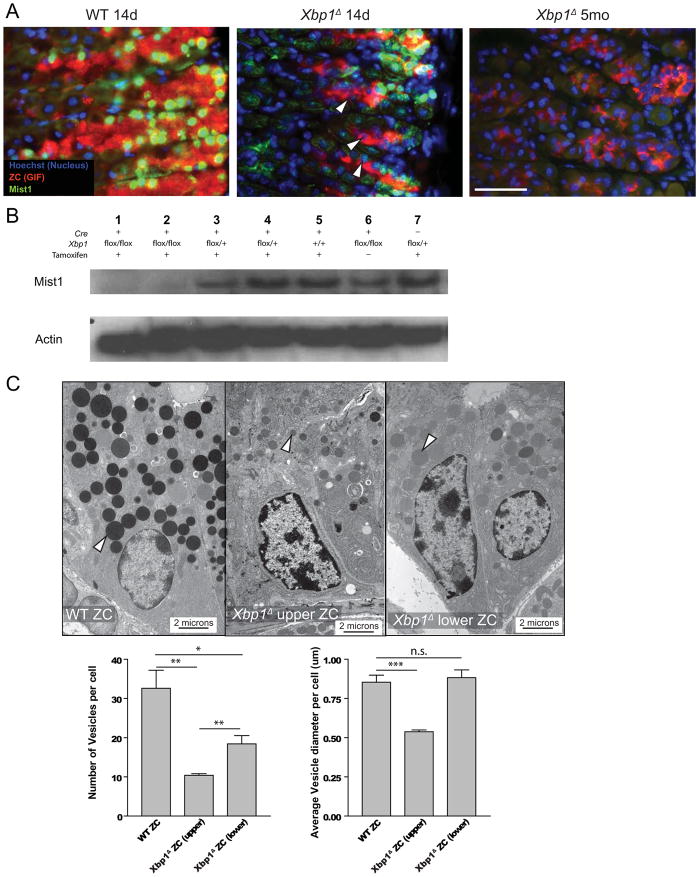
To determine whether XBP1 is necessary in vivo for induction of MIST1, we followed MIST1 expression by immunofluorescence in Xbp1Δ ZCs. As expected, MIST1 levels were dramatically reduced by western blot and immunofluorescence in Xbp1Δ ZCs (Fig. 4A–B). By qRT-PCR, Mist1 expression was 25.2% of control levels by 14d and absent by five months (not shown). However, we noted that some ZCs at the base at 14d still expressed detectable MIST1 (Fig. 4A). By tEM, those older, basally located ZCs were small and dramatically rER-deficient but did not have as dramatic vesicular defects as the newly emerged ZCs closer to the progenitor zone. Vesicles in the first 5 ZCs closest to the progenitor zone were 60% smaller than wildtype, whereas basal ZC vesicles were essentially unchanged. In addition, although basal ZCs had 44% fewer vesicles/cell relative to wildtype (32.6±9.0 vs.18.4±4.2), emerging ZCs had even fewer vesicles: 10.4±0.8/cell, a 68% decrease (Fig. 4C).
Figure 4. Xbp1 is necessary for activation of Mist1 in ZCs and Xbp1 regulates number and size of secretory vesicles partially through Mist1.
A, All ZCs (red, GIF) express MIST1 (green) in control gastric units (nuclei: blue). In Xbp1Δ units, early, immature ZCs (i.e., those closest to the progenitor zone to the left) do not express MIST1 (arrowheads, middle panel), whereas mature ZCs (to the right) that were MIST1 positive at the time of tamoxifen treatment remain MIST1 positive. By 5 months, no ZCs express MIST1 (right panel). Green channel exposure was equivalent in all three panels. B, Western blot using anti-MIST1 antibody with two individual 14d Xbp1Δ mice with various controls (Actin=loading control). C. tEM of ZCs illustrating secretory vesicle (white arrowheads) phenotypes. Note, relative to WT ZCs, the upper (i.e., newly formed) ZCs from an Xbp1Δ stomach have scant, small vesicles; the lower (older) ZC formed prior to Xbp1 deletion has fewer but similar sized vesicles. Upper right panel, lower ZC from Xbp1Δ stomach. Graphs at bottom quantify mean vesicle number (±SD) and diameter per cell (±SEM), scored from tEM micrographs.
One explanation for the more pronounced vesicular phenotype in newly emerged ZCs is that XBP1 is required for the induction of Mist1 transcription but not for its maintenance. Normally, the transition from NCs to ZCs is characterized by induction of abundant MIST1, but ZCs forming from NCs in Xbp1Δ mice would be Xbp1 null, and, if XBP1 was absolutely required for direct activation of Mist1, the newly formed ZCs in the transition zone would always lack MIST1. On the other hand, ZC lifespan is several months1, so cells nearer the base would already be ZCs and already have MIST1 expression at the time of Xbp1 deletion. If XBP1 was not required for maintenance of MIST1, one would expect that it would take several months for complete MIST1 loss in basal ZCs. Supporting that interpretation, Xbp1Δ ZCs at 5 and 13 months showed complete loss of MIST1, even at the base (Fig. 3A).
XBP1 is partially required for ZC fate specification
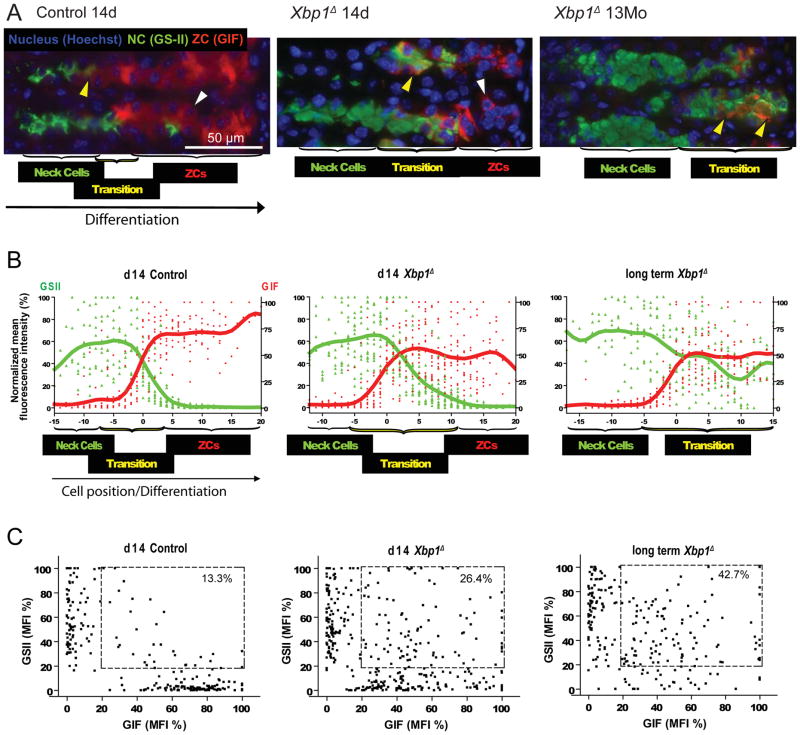
By histology, qRT-PCR, and western blot, we observed a modest decrease in ZC markers in Xbp1Δ mice, whereas markers for their progenitor NCs were increased. This phenotype could have resulted either from the inhibition of differentiation from NCs to ZCs or from increased proliferation of NCs with increased apoptosis of ZCs. Thus, we immunostained and quantified markers of proliferation, cell death and NC/ZC differentiation. In control gastric units, differentiation from NCs to ZCs is abrupt. Thus, the vast majority of cells are either NCs or ZCs with only 13.3% of total cells in the lineage unit co-expressing progenitor and differentiated markers (Fig. 5A–C; refs.3, 4). Xbp1Δ units at 14d, on the other hand, showed a large increase in such transitional cells co-expressing NC and ZC markers (26.4%), although there were still discrete NC and mature ZC populations that expressed predominately one marker and not the other (Fig. 5A–C). At later timepoints, when all ZCs would have arisen in the absence of XBP1, there were essentially no definitive ZCs formed. There were large NC and transitional populations (42.7%) but only rare mature ZCs expressing the ZC marker and not the progenitor marker (Fig. 5A–C). Thus, XBP1 is required for extinguishing progenitor cell features but not induction of ZC markers; without XBP1, NCs form only transitional cells. At no time point do Xbp1Δ ZCs show significantly increased cell death either by morphology or TUNEL stain, and loss of XBP1 does not affect proliferation in the gastric unit, as assessed by Brdu immunolabeling (Fig. S3).
Figure 5. Xbp1 is required for complete ZC maturation, specifically to turn off NC genes.
A, Immunofluorescent staining for GSII (progenitor cell marker, green), GIF (ZC marker, red), and Hoechst 33258 (nucleus, blue). Transitional cells (e.g., yellow arrowheads) co-expressing progenitor and differentiated markers are increased in Xbp1Δ stomachs by 14 days (middle panel) and completely fill the basal zone by 13 months (right panel) after tamoxifen injection. ZCs only expressing GIF (white arrowheads) are decreased by 14 days (middle panel) and rare by 13 months (right panel). B, Compiled results from multiple gastric units as in (A). Each point represents the normalized green or red channel mean fluorescent intensity (MFI) for a given cell in a unit, expressed as a function of the maximal MFI in that unit. Units are aligned by setting the first cell with background- subtracted GIF MFI ≥ 25 as cell #0. Cells to the right (i.e., more differentiated) are given positive cell positions with highest number being the cell farthest toward the base of the unit (i.e., the oldest). C, the same data are expressed on a scatter plot, ignoring cell position. Cells with both progenitor and differentiated cell marker expression (i.e., transitional cell phenotype; boxed) are increased in Xbp1Δ units; note also that longterm Xbp1Δ deletion leads to failure in formation of a cell population expressing exclusively ZC markers without NC markers
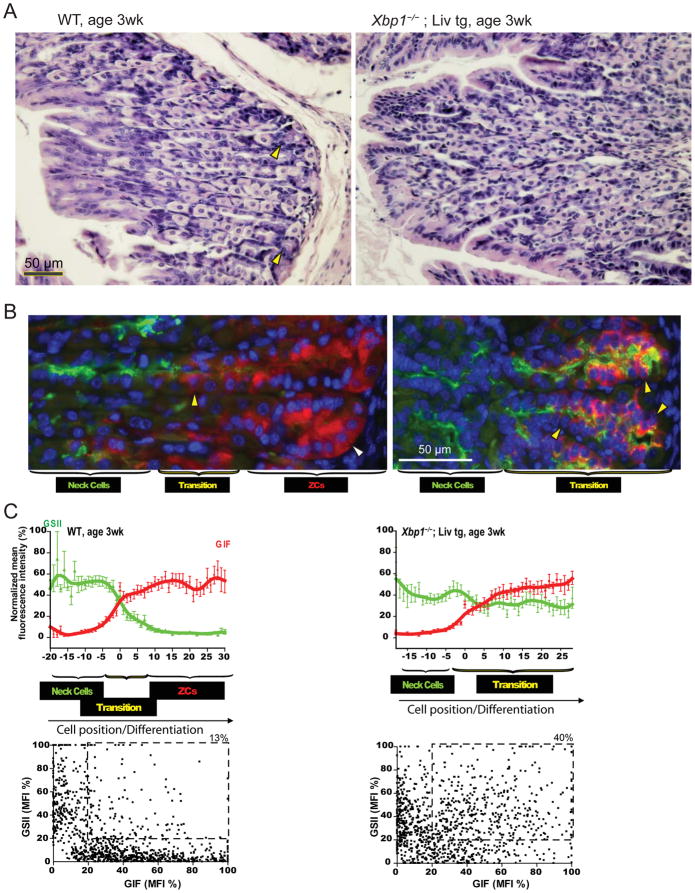
To further examine XBP1 in determining ZC cell identity, we examined ZC development in germline Xbp1 null mice. Xbp1−/− mice die in utero; however, Xbp1−/− mice expressing liver-specific transgenic Xbp1 (Xbp1−/−;LivXBP1) survive to birth, although most die within a few days (Fig. S4 17). We examined a rare, 3 week-old Xbp1−/−;LivXBP1. By 3 weeks, wildtype gastric units already showed substantial separation into progenitor, transition, and mature ZC zones (Fig. 6A–B left panels, quantified in 6C). On the other hand, Xbp1−/−;LivXBP1 units lacked a distinct ZC zone and had abundant transitional cells (Fig. 6A–B right panels, quantified in 6C), again consistent with a role of XBP1 in regulating terminal differentiation of ZCs.
Figure 6. Xbp1 germline knockout mice confirm Xbp1 is required for ZC maturation.
A, H&E staining showing WT stomach has abundant parietal cells and cytoplasm-rich ZCs in the base (yellow arrowhead, left panel) at 3w post-natally, whereas Xbp1−/− ;LivXBP1 units show abundant parietal cells without obvious ZCs. B, Immunofluorescent staining shows that, whereas definitive ZCs are already developed in control stomach (white arrowheads) in three week old mice, Xbp1−/−;LivXBP1 mice show only cells co-expressing GIF/GSII (yellow arrowheads) at the base, similar to Xbp1Δ stomach 13 months following tamoxifen injection (cf. Figure 5A). C, Differentiation patterns across multiple units are quantified as for Figure 5B,C (upper panels: means±SD)
Discussion
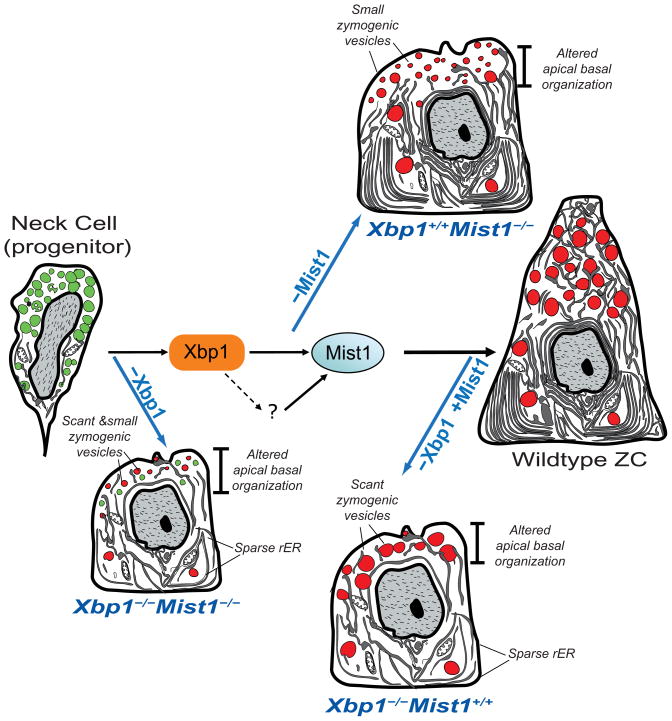
Here we show that XBP1 governs nearly the entire morphogenetic program in gastric ZCs (Fig. 7). It likely acts through direct cellular effectors that regulate rER formation and via MIST1, which in turn regulates secretory vesicle maturation via RAB26/3D28 (Fig. 7).
Figure 7. Xbp1 regulates the principal aspects of ZC structural development: secretory vesicle size/number and rER expansion.
Secretory vesicle size is regulated through Mist1, which is a direct transcriptional target of Xbp1. There is likely another factor(s) that maintains Mist1 expression once induced (“?” in the diagram), because loss of Xbp1 does not abrogate Mist1 once Mist1 is already expressed. If Xbp1 is deleted before Mist1 is expressed, ZCs have scant, small vesicles and sparse ER; if after, ZCs have scant, normal-sized vesicles and sparse ER. If Mist1 alone is deleted, cells have normal rER but small vesicles.
That Xbp1 is upstream of Mist1 in vivo and is required for Mist1 activation have not previously been demonstrated. However, XBP1 is dispensable for maintenance of MIST1, as loss of XBP1 in mature ZCs did not uniformly lead to loss of MIST1 until months following tamoxifen induced deletion. At such late timepoints, most, if not all ZCs, would have been generated in the absence of XBP1. Interestingly, despite large deficits in rER resulting from loss of XBP1 and despite the reduction in number of secretory vesicles, mature MIST1+/XBP1 cells at 14d following tamoxifen still had vesicles similar in size to wildtype. That suggests MIST1 regulates vesicle size independent of the secreted proteins available for packaging into those vesicles.
Although the terminal ZC fate is apparently initially specified in the absence of XBP1 (i.e., ZC specific markers are still upregulated), newly forming Xbp1Δ ZCs cannot extinguish expression of progenitor cell proteins; thus, they remain stuck in transition. XBP1 is thought to be a transcriptional activator, so there might be downstream targets of XBP1 that themselves turn off progenitor cell gene expression. It is also possible that loss of XBP1 leads to defects in cellular structures, like the ubiquitin-proteasomal or autophagic machinery, that are needed to degrade NC proteins. However, ZCs live for months, so it is difficult to imagine that continued expression of NC proteins would not also involve continued transcription of the genes encoding those proteins.
The increase in cells expressing NC markers at the base is reminiscent of pseudopyloric or spasmolytic polypeptide expressing metaplasia (SPEM)29, 30. In SPEM, loss of parietal cells correlates with changes in zymogenic lineage differentiation; however, SPEM further correlates with increased foveolar cells and expansion of proliferating cells towards the base of the unit29, 30. In SPEM, the entire zymogenic lineage (both progenitor NC and mature ZC) assumes a transitional morphology: there are neither NCs expressing only NC markers nor mature ZCs expressing only ZC markers3. Thus, compared with previous studies, the current results are unusual in that only ZC terminal maturation is profoundly affected without dramatic effects on any other cell lineage in the gastric units. In some ways, the Xbp1Δ phenotype is a more dramatic example of the Mist1−/− phenotype, as those mice show increased transitional cells, though all ZCs eventually turn off NC gene expression4.
The molecular underpinnings of SPEM are almost wholly unknown. Our recent analysis of hundreds of human gastric samples exemplifying the progression of changes from chronic gastritis to carcinoma showed that loss of MIST1 expression is one of the first molecular markers of altered NC/ZC differentiation in SPEM and that MIST1 expression is lost in over 99% of gastric cancers 31. Thus, it will be interesting to determine whether loss of XBP1 expression is a key early event in SPEM, which might explain both decreased MIST1 levels and the co-expression of NC and ZC markers that characterize this precancerous lesion.
The effects of deletion of sonic hedgehog (Shh) in parietal cells in the stomach10 are in some aspects similar to the Xbp1Δ phenotype. For example, transitional ZCs accumulate in the base in both cases, with preservation of normal NCs. Aspects of the ShhΔ mice not seen in Xbp1Δ stomachs are expansion of foveolar cells and increased proliferation. Nonetheless, it is possible that Hh signaling is aberrant in Xbp1Δ stomachs; indeed, we found increased levels of transcripts for the Hh signaling ligands and targets: Shh (2.1 fold, p<0.001), Ihh (1.7 fold, p<0.05), Ptc1 (2.9 fold, p<0.01) and Gli1 (2.7 fold, p<0.01) in Xbp1Δ mice. Hh signaling is complex in the gastric unit with multiple sources, age-dependent changes, and unclear cellular and molecular targets9, 32; however, an easy interpretation of the results is that the ZC structural and differentiation defects lead to compensatory increase in Hh signaling from other epithelial cells and thereby increase Hh target expression in the mesenchyme10. In other experiments, we have inducibly deleted the Hh ligands mediators Ptc1 and Smo, but we have not seen effects on ZC differentiation (unpublished results).
One caveat of our study is that the genetic tools are not currently available to target inducible Xbp1 deletion specifically to NCs; thus, even though the phenotype we see is entirely restricted to ZCs, it is possible that some component is due to deletion of Xbp1 in cells other than NCs and ZCs. We think such non-cell-autonomous contributions to the phenotype are minimal for several reasons. For one, Xbp1 expression is highest in general in large secretory cells, and our previous studies showed that its gastric expression is highest in the zymogenic lineage4. Two, we have extensive experience with the tamoxifen levels necessary to induce deletion in this mouse pedigree, and the levels we use target the NC/ZC lineage25 (SF 1). Three, at no time point following tamoxifen do we observe a change in mesenchymal cells, an influx of inflammatory cells, or a change in census or marker expression of other epithelial lineages in the stomach (Fig. 1C–D). Four, an entirely different Xbp1 deletion strategy, germline (as opposed to inducible) loss of Xbp1, also caused ZC specific effects.
The next step in dissecting zymogenic differentiation and understanding gastric metaplasia will be to determine what is upstream of XBP1. What upregulates Xbp1 expression and what leads to the sudden increase in expression of zymogenic lineage markers that occurs as cells migrate out of the neck cell zone? There are few clues from other tissues about upstream regulation of Xbp1. In plasma cells, it is known that the transcription factor Blimp1 is upstream and required for Xbp1 expression27, but Xbp1 is not a direct target. Clearly, there is a great deal more to learn about ZC differentiation, though with the Xbp1 → Mist1 sequence, we are beginning to parse that circuitry.
Materials and Methods
Mice
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Floxed Xbp1, CAGGCreERTM transgenic mice, and germline Xbp1−/− mice with liver Xbp1 transgene (Xbp1−/−;LivXBP1) were generated as described previously17, 23, 24, 33, 34. Xbp1flox/flox mice were crossed with CAGGCreERTM transgenics. Then CAGGCreERTM tg; Xbp1flox/+ mice were crossed with Xbp1flox/+ to generate CAGGCreERTM tg; Xbp1flox/flox as well as a variety of control mice. Tamoxifen (0.75 mg per 20 g body weight, Sigma, St. Louis, MO) was injected intraperitoneally for seven consecutive days to induce gene deletion. Mice were sacrificed on 7, 14, and 28 days, 5, 7, and 13 months after first tamoxifen injection.
Cell Imaging
tEM4, TUNEL, Brdu25 and other immunofluorescence studies were as described3, 4, 35; goat anti-calregulin (Santa Cruz Biotechnology, Santa Cruz, CA) was diluted 1:200. Immunofluorescent quantification to determine cytoplasmic fluorescence intensity was performed in ImageJ software, with methods described previously3; however, for the current study, the MFI for each cell was normalized to the maximum MFI for that channel in each unit to generate %maximal MFIs. In Xbp1Δ mice, 351 cells in 14 units from 2 mice were quantified; in controls: 225 cells, 9 units, 5 mice.
Stomach Chromatin Immunoprecipitation
Mouse stomach chromatin immunoprecipitation (ChIP) was performed after Wells and Farnham36. One stomach was dissected for one experiment, and the experiment was repeated with another mouse showing similar results. 10 μl of rabbit anti-XBP1 antibody (Santa Cruz Biotechnology) with protein A/G plus agarose (Santa Cruz Biotechnology, Santa Cruz, CA) was added to the tissue lysate for immunoprecipitation. A consensus XBP1 binding motif in the Mist1 promoter was described previously22, and, using ECR browser (http://ecrbrowser.dcode.org), we noted this site was conserved from human to mouse to opossum (not shown). Primers spanning the putative Xbp1 binding site were designed with Primer3 (http://frodo.wi.mit.edu/primer3/) (Supp. Table 1). QRT PCR with these primers was performed to assess quantity of genomic sequences immunoprecipitated by anti- XBP1 antibody, as well as a 1:10 dilution of the cell extract prior to immunoprecipitation, and a control genomic region lacking consensus XBP1 binding sites.
Cell Line and Transient Transfection
AGS cells (from ATCC, Manassas, VA; a human gastric carcinoma cell line) were grown and transfected by Nucleofection as described3 with 3 μg hXbp1(s) and 2 μg pmaxGFP (Amaxa-Lonza). qRT-PCR analysis was as described 28.
Western Blot and qRT-PCR for Stomach Tissue
For blots, corpus tissue was frozen in liquid nitrogen and ground with mortar and pestle with proteins separated on NuPAGE4–12% (Invitrogen) transferred to PVDF, and detected by Immobilon Chemiluminescence (Millipore). Primary antibodies were: rabbit anti-MIST1 (1:200), sheep anti-PGC (1:1,000), rabbit anti-GIF (1:20,000), and goat anti-Actin (1:1,000, Santa Cruz). Secondaries were: HRP conjugated donkey anti-rabbit (1:2,000, Jackson ImmunoResearch, West Grove, PA), donkey anti-sheep and anti-goat (both 1:2,000, Santa Cruz). For qRT-PCR, total RNAs from corpus were extracted and assayed as described25.
Supplementary Material
Acknowledgments
Grant support: JCM (ACS DDC-115769, NIH DK079798-1,2), AJB (T32 CA009547-21, 22), LHG (NIH AI32412), AHL (American Heart Association, AHA0835610P), SFK (NIH DK55489 and CA124586). WJH and JHG were supported in part by the Cancer Biology Pathway Program, Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes Jewish Hospital. There is no conflict of interest to disclose for all authors.
Abbreviations
- TF
Transcription Factor
- ZC
Zymogenic Cell
- NC
Mucous Neck Cell
- tEM
Transmission Electron Microscopy
- ChIP
Chromatin ImmunoPrecipitation
- Brdu
Bromodeoxyuridine
Footnotes
Contributions: WJH (study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; statistical analysis), EE (acquisition of data; analysis and interpretation of data), AJB (acquisition of data; analysis and interpretation of data), JHG (acquisition of data; analysis and interpretation of data), AHL (material support; revision of the manuscript for important intellectual content), GS (material support), SFK (material support; revision of the manuscript for important intellectual content), LHG (material support; revision of the manuscript for important intellectual content), JCM (study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 2.Mills JC, Andersson N, Stappenbeck TS, Chen CC, Gordon JI. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem. 2003;278:46138–46145. doi: 10.1074/jbc.M308385200. [DOI] [PubMed] [Google Scholar]
- 3.Bredemeyer AJ, Geahlen JH, Weis VG, Huh WJ, Zinselmeyer BH, Srivatsan S, Miller MJ, Shaw AS, Mills JC. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsey VG, Doherty JM, Chen CC, Stappenbeck TS, Konieczny SF, Mills JC. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 5.Hamanaka Y, Park D, Yin P, Annangudi SP, Edwards TN, Sweedler J, Meinertzhagen IA, Taghert PH. Transcriptional Orchestration of the Regulated Secretory Pathway in Neurons by the bHLH protein DIMM. Curr Biol. 2010;20:9–18. doi: 10.1016/j.cub.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park D, Shafer OT, Shepherd SP, Suh H, Trigg JS, Taghert PH. The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Mol Cell Biol. 2008;28:410–421. doi: 10.1128/MCB.01104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomura S, Settle SH, Leys CM, Means AL, Peek RM, Jr, Leach SD, Wright CV, Coffey RJ, Goldenring JR. Evidence for repatterning of the gastric fundic epithelium associated with Menetrier's disease and TGFalpha overexpression. Gastroenterology. 2005;128:1292–1305. doi: 10.1053/j.gastro.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Doljanin K, Skeljo MV, Yeomans ND, Giraud AS. Adaptation of the gastric epithelium to injury is maintained in vitro and is associated with increased TGF-alpha expression. J Gastroenterol Hepatol. 1996;11:259–263. doi: 10.1111/j.1440-1746.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 9.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 138:562–572. 572, e561–562. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–561. 561, e551–558. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibata W, Takaishi S, Muthupalani S, Pritchard DM, Whary MT, Rogers AB, Fox JG, Betz KS, Kaestner KH, Karin M, Wang TC. Conditional deletion of IkappaB-kinase-beta accelerates helicobacter-dependent gastric apoptosis, proliferation, and preneoplasia. Gastroenterology. 138:1022–1034. e1021–1010. doi: 10.1053/j.gastro.2009.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verzi MP, Khan AH, Ito S, Shivdasani RA. Transcription factor foxq1 controls mucin gene expression and granule content in mouse stomach surface mucous cells. Gastroenterology. 2008;135:591–600. doi: 10.1053/j.gastro.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokubu H, Ohtsuka T, Kageyama R. Mash1 is required for neuroendocrine cell development in the glandular stomach. Genes Cells. 2008;13:41–51. doi: 10.1111/j.1365-2443.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz JP, Perreault N, Goldstein BG, Actman L, McNally SR, Silberg DG, Furth EE, Kaestner KH. Loss of Klf4 in mice causes altered proliferation and differentiation and precancerous changes in the adult stomach. Gastroenterology. 2005;128:935–945. doi: 10.1053/j.gastro.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 17.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. Embo J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd DJ, McHeyzer-Williams LJ, Kowal C, Lee AH, Volpe BT, Diamond B, McHeyzer-Williams MG, Glimcher LH. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med. 2009;206:2151–2159. doi: 10.1084/jem.20090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. Embo J. 2009;28:1624–1636. doi: 10.1038/emboj.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 25.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menheniott TR, Peterson AJ, O'Connor L, Lee KS, Kalantzis A, Kondova I, Bontrop RE, Bell KM, Giraud AS. A novel gastrokine, Gkn3, marks gastric atrophy and shows evidence of adaptive gene loss in humans. Gastroenterology. 138:1823–1835. doi: 10.1053/j.gastro.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Blazewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol. doi: 10.1128/MCB.01328-09. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenring JR, Ray GS, Coffey RJ, Meunier PC, Haley PJ, Barnes TB, Car BD. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 30.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 31.Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK, Mills JC. The Transcription Factor MIST1 Is a Novel Human Gastric Chief Cell Marker Whose Expression Is Lost in Metaplasia, Dysplasia, and Carcinoma. Am J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiotani A, Uedo N, Iishi H, Tatsuta M, Ishiguro S, Nakae Y, Kamada T, Haruma K, Merchant JL. Re-expression of sonic hedgehog and reduction of CDX2 after Helicobacter pylori eradication prior to incomplete intestinal metaplasia. Int J Cancer. 2007;121:1182–1189. doi: 10.1002/ijc.22835. [DOI] [PubMed] [Google Scholar]
- 33.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–1378. doi: 10.1053/j.gastro.2008.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pin CL, Bonvissuto AC, Konieczny SF. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat Rec. 2000;259:157–167. doi: 10.1002/(SICI)1097-0185(20000601)259:2<157::AID-AR6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Wells J, Farnham PJ. Characterizing transcription factor binding sites using formaldehyde crosslinking and immunoprecipitation. Methods. 2002;26:48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.