Abstract
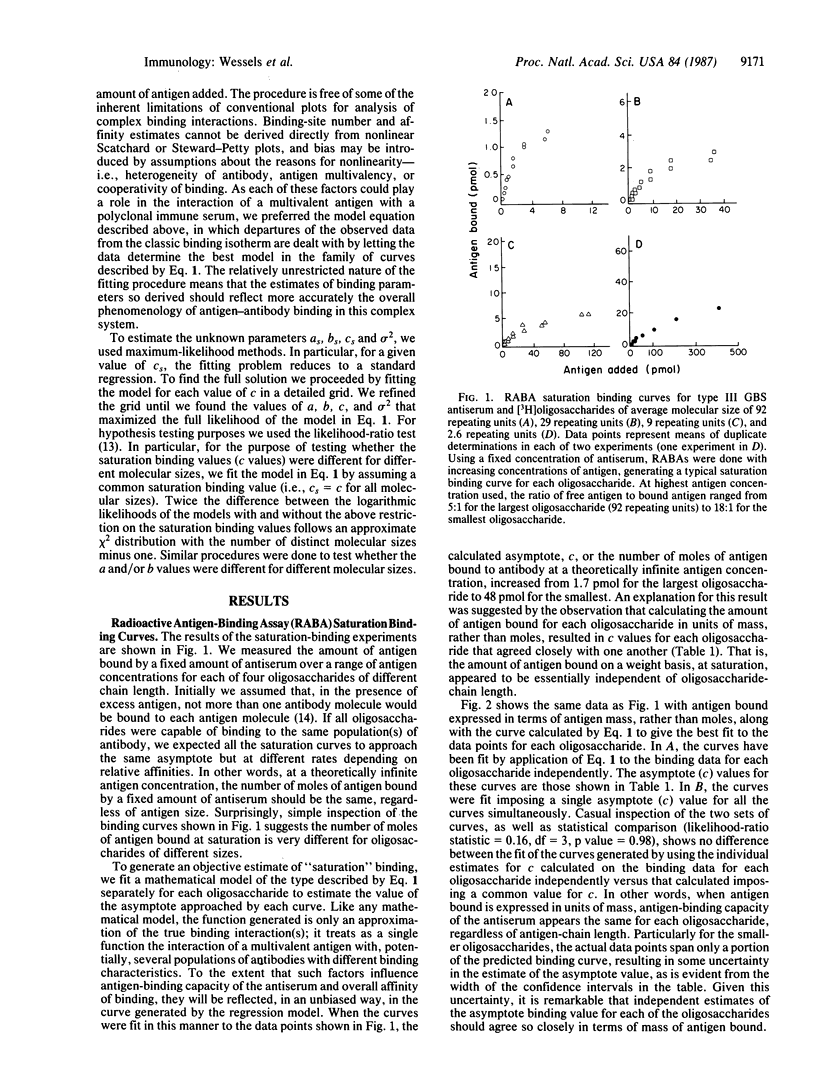
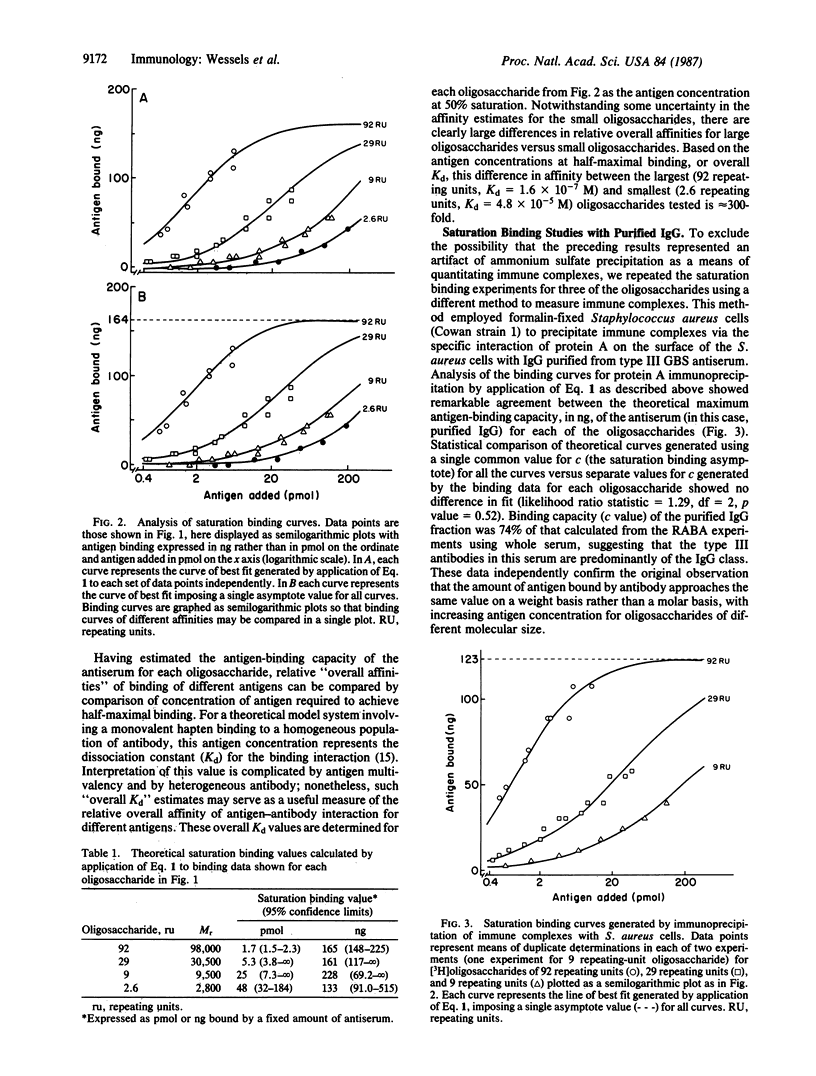
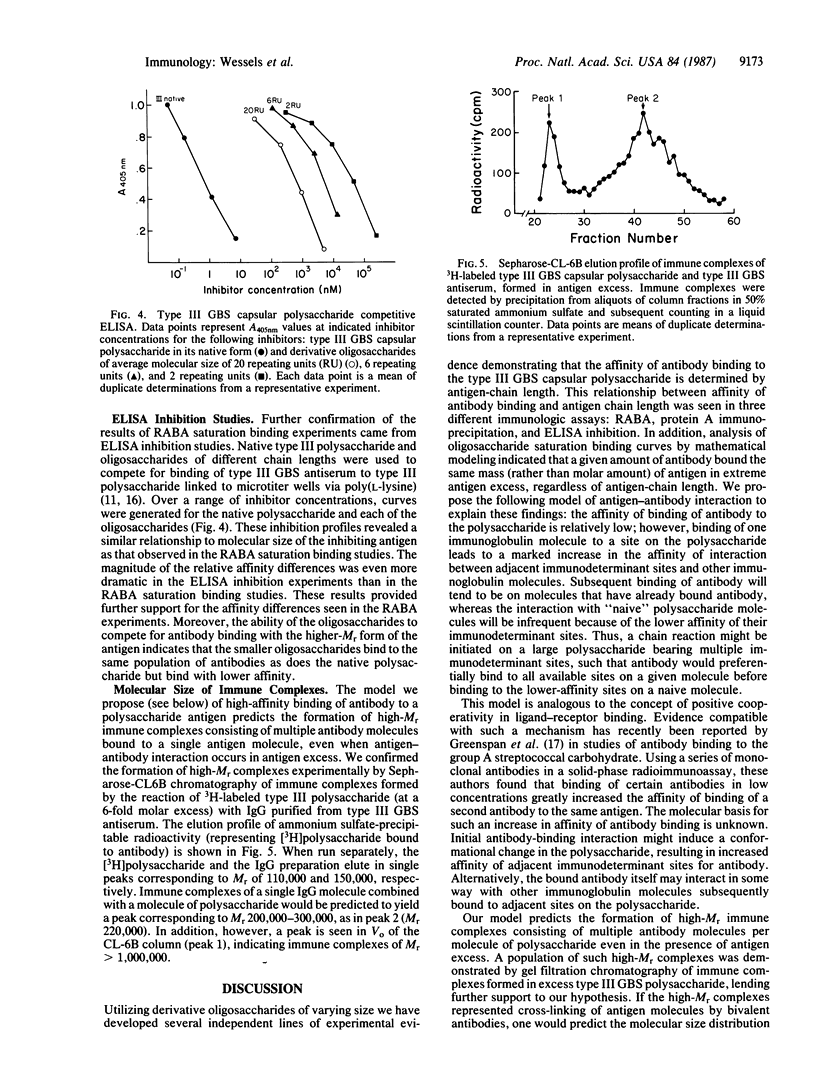
We recently reported that the single repeating-unit pentasaccharide of type III group B Streptococcus (GBS) capsular polysaccharide is only weakly reactive with type III GBS antiserum. To further elucidate the relationship between antigen-chain length and antigenicity, tritiated oligosaccharides derived from type III capsular polysaccharide were used to generate detailed saturation binding curves with a fixed concentration of rabbit antiserum in a radioactive antigen-binding assay. A graded increase in affinity of antigen-antibody binding was seen as oligosaccharide size increased from 2.6 repeating units to 92 repeating units. These differences in affinity of antibody binding to oligosaccharides of different molecular size were confirmed by immunoprecipitation and competitive ELISA, two independent assays of antigen-antibody binding. Analysis of the saturation binding experiment indicated a difference of 300-fold in antibody-binding affinity for the largest versus the smallest tested oligosaccharides. Unexpectedly, the saturation binding values approached by the individual curves were inversely related to oligosaccharide chain length on a molar basis but equivalent on a weight basis. This observation is compatible with a model in which binding of an immunoglobulin molecule to an antigenic site on the polysaccharide facilitates subsequent binding of antibody to that antigen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Edwards M. S., Kasper D. L. Immunogenicity of polysaccharides from type III, group B Streptococcus. J Clin Invest. 1978 Apr;61(4):1107–1110. doi: 10.1172/JCI109011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- Berman E. Determination of the structure of three oligosaccharides from normal human urine by using 60-MHz, carbon-13 nuclear magnetic resonance spectroscopy. Carbohydr Res. 1983 Jul 16;118:9–20. doi: 10.1016/0008-6215(83)88030-6. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Nitecki D. E., Stoltenberg I. M. Immunochemical studies on the poly-gamma-D-glutamyl capsule of Bacillus anthracis. 3. The activity with rabbit antisera of peptides derived from the homologous polypeptide. Biochemistry. 1968 Feb;7(2):706–710. [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Greenspan N. S., Monafo W. J., Davie J. M. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol. 1987 Jan 1;138(1):285–292. [PubMed] [Google Scholar]
- Jennings H. J., Rosell K. G., Kasper D. L. Structural determination and serology of the native polysaccharide antigen of type-III group B Streptococcus. Can J Biochem. 1980 Feb;58(2):112–120. doi: 10.1139/o80-016. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Roy R., Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. J Immunol. 1985 Apr;134(4):2651–2657. [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferle R. E., Jennings H. J., Wessels M. R., Katzenellenbogen E., Roy R., Kasper D. L. Immunochemical analysis of the types Ia and Ib group B streptococcal polysaccharides. J Immunol. 1985 Dec;135(6):4164–4170. [PubMed] [Google Scholar]
- Sharon J., Kabat E. A., Morrison S. L. Immunochemical characterization of binding sites of hybridoma antibodies specific for alpha (1 leads to 6) linked dextran. Mol Immunol. 1982 Mar;19(3):375–388. doi: 10.1016/0161-5890(82)90203-6. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The antigen-binding characteristics of antibody pools of different relative affinity. Immunology. 1972 Dec;23(6):881–887. [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Kasper D. L. Molecular size affects antigenicity of type III, group B Streptococcus capsular polysaccharide. Trans Assoc Am Physicians. 1985;98:384–391. [PubMed] [Google Scholar]
- Wessels M. R., Pozsgay V., Kasper D. L., Jennings H. J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987 Jun 15;262(17):8262–8267. [PubMed] [Google Scholar]
- Yoshima H., Matsumoto A., Mizuochi T., Kawasaki T., Kobata A. Comparative study of the carbohydrate moieties of rat and human plasma alpha 1-acid glycoproteins. J Biol Chem. 1981 Aug 25;256(16):8476–8484. [PubMed] [Google Scholar]



