Abstract
A hallmark of autoimmune lymphoproliferative syndrome (ALPS), caused by mutation of the Fas death receptor, is massive lymphadenopathy from aberrant expansion of CD4−CD8− (double-negative, DN) T cells. Eomesodermin (Eomes) is a member of the T-box family of transcription factors and plays critical roles in effector cell function and memory cell fitness of CD8+ T lymphocytes. We now provide evidence that DN T cells exhibit dysregulated expression of Eomes in humans and mice with ALPS. We also find that T cell-specific deletion of Eomes prevents lymphoid hypertrophy and accumulation of DN T cells in Fas-mutant mice. Although Eomes has critical physiological roles in the function and homeostasis of CD8+ T cells, over-expression of Eomes appears to enable pathological induction or expansion of unusual CD8-related T cell subsets. Thus, antagonism of Eomes emerges as novel therapeutic target for DN T cell ablation in ALPS.
Introduction
Mice and humans with mutations in the death domain-containing receptor, Fas, suffer from dysregulated homeostasis of lymphocyte populations, often leading to massive lymphadenopathy and splenomegaly primarily composed of αβ T cell receptor-bearing CD4−CD8− (double-negative, DN) T cells. Autoimmune manifestations, including severe cytopenias, and heightened risk of lymphoma are also features of autoimmune lymphoproliferative syndrome (ALPS) (1, 2). Accumulation of DN T cells was recently described as a highly predictive “biomarker” of ALPS (2). This hallmark expansion of the DN subset and the subsequent enlargement of secondary lymphoid organs in ALPS lead to significant morbidity for affected patients.
The signaling and transcriptional events that induce the abnormal DN T cell fate, as well as the precise ontogeny of the DN T cells, are unknown. Previous reports suggest that DN T cell expansion requires deficiency of Fas only within the T cell compartment, as mice with B or dendritic cell-specific deletions of Fas do not amass the anomalous T cells (3, 4). DN T cells in ALPS are thought to arise from CD8+ T cells (3, 5–7), though there has been some debate as to whether the formerly CD8+ lymphocytes were self-reactive (3) or bore hypo-reactive T cell receptors (6, 7). Both models postulate that aberrantly-selected CD8+ T cells are cleared via a peripheral, Fas-dependent quality control mechanism (6–8). In the absence of Fas, it is presumed that the improperly selected CD8+ T cell has an opportunity to become a DN T cell and proliferate uncontrollably by an as-yet-unknown mechanism (3–7).
Eomesodermin (Eomes), a paralog of T-bet, is expressed in effector/memory CD8+ T cells and natural killer cells and plays redundant roles with T-bet in the induction of cytokine secretion and cytotoxic capacity of CD8+ T lymphocytes (9–11). Eomes expression is also a hallmark of non-canonical, interleukin-15 (IL-15)-responsive, “innate-like” CD8+ T cells that acquire functions, such as interferon-gamma (IFN-γ) production and cytolytic potential, during their development in the thymus (12–16). Previous studies suggested that ALPS DN T cells exhibit heightened sensitivity to the cytokine interleukin-15 (IL-15) (7). Additionally, Fas-mutant T cells were found to produce IFN-γ independently of the T-box transcription factor T-bet (17). Responsiveness to IL-15 and T-bet-independent induction of IFN-γ are both characteristics controlled by the transcription factor Eomes (11, 18). Here we report that Eomes dysregulation defines the DN T cells in lpr/lpr animals and in humans with ALPS. We sought to investigate the effects of T cell-specific deletion of Eomes on the abnormal T cells of Fas-deficiency, and our results suggest that Eomes is essential for the development or maintenance of this population.
Materials and Methods
Mice
All animals were housed at the University of Pennsylvania in specific pathogen-free conditions, and all experiments were performed in accordance with approved protocols by the University of Pennsylvania Institutional Animal Care and Use Committee. Mice harboring floxed alleles of Eomes (Eomes F/F) mated to mice expressing Cre-recombinase, driven by the Cd4 promoter (Cd4:Cre+) have been previously described (10). To study Fas-deficient, Eomes-deficient T cells, lpr/lpr mice were mated to Eomes F/F, Cd4:Cre+ mice. To study Fas-deficient, T-bet-deficient T cells, lpr/lpr mice were mated to Tbx21−/−, mice. To study Fas-deficient, IL-15-deficient mice, lpr/lpr animals were bred to Il15−/− animals.
Human samples
Human cells were obtained with informed consent and in accordance with the Institutional Review Boards of the Children's Hospital of Philadelphia and the National Institutes of Health.
Quantitative RT-PCR, cell sorting, and flow cytometry
Sorting indicated populations for qRT-PCR on murine cells was carried out on a BD FACSAria. qRT-PCR was carried out as previously described (10). Target gene probes were purchased from Applied Biosystems. The following antibodies (BD Pharmingen, unless otherwise indicated) were used for FACS staining: TCRβ APC or PE-Cy5, CD4 FITC or PE-Cy7, CD8α PerCP-Cy-5.5 or Alexa Fluor 700 (Biolegend), B220 PE or PE-Texas Red (Caltag Laboratories), CD19 APC-Cy7, and Eomes PE (eEioscience). Data were collected on a BD FACSCalibur, BD FACSAria, or BD LSRII (BD Biosciences). Data were analyzed with FlowJo software (Tree Star Inc.).
Autoantibody detection
Anti-nuclear antibodies were detected with an anti-nuclear antibody test kit (Antibodies Incorporated). Briefly, slides pre-coated with fixed, mitotic HEp-2 cells were exposed to sera from the indicated mice, followed by detection of anti-nuclear antibodies with FITC-labeled goat anti-mouse IgG. Slides were later mounted and fluorescent microscopy was performed.
Results and Discussion
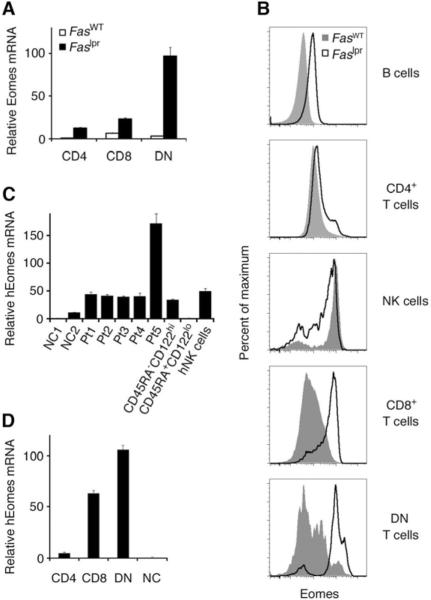
We examined the expression of Eomes in the T cell subsets of Fas-mutant lpr/lpr mice (from the non-autoimmune-prone C57BL/6 background) and found that Eomes mRNA (Fig. 1A) and protein (Fig. 1B) levels were substantially higher in Fas-mutant T cells compared to cells of wild-type mice. Expression of Eomes was most dysregulated in the DN T cell subset. Comparable results were obtained from the peripheral blood mononuclear cells of ALPS-FAS patients, who harbor confirmed mutations in the Fas gene (Fig. 1C, D and Table 1). Each patient exhibited elevated levels of Eomes in DN T cells (Fig. 1C). The patient with highest levels of Eomes in DN T cells (Pt5) had notably early and aggressive onset of disease (Fig. 1C and Table 1). As in lpr/lpr mice (Fig. 1A, B), expression of Eomes in ALPS patients was most dysregulated in the DN T cell subset (Fig. 1D). Levels of Eomes protein and mRNA in murine and human Fas-mutant DN T cells appeared comparable to or greater than levels found in NK cells and effector/memory CD8+ T cells (Fig. 1B, C).
FIGURE 1.
Dysregulated expression of Eomesodermin is a hallmark of double-negative (DN) T cells in both human and murine ALPS.
A, CD4+, CD8+, and CD4−CD8− (DN) T cells were sorted from wild-type and lpr/lpr mice (FasWT and Faslpr, respectively) and murine Eomes mRNA levels were analyzed by quantitative real-time RT-PCR (qRT-PCR). Values represent the mean ± S.E.M. of triplicate determinations normalized to hypoxanthine-guanine phosphoribosyltransferase. Results are representative of at least three independent experiments.
B, Intranuclear Eomes protein expression assessed by monoclonal antibody and flow cytometry of indicated subpopulations within the freshly isolated splenocytes of wild-type and lpr/lpr mice. Results are representative of three independent experiments.
C, qRT-PCR analysis of human Eomes (hEomes) mRNA in the sorted DN T cells of five ALPS patients (Pt1–Pt5) that have been diagnosed clinically and confirmed with genetic testing for Fas mutation (see Table 1 for additional clinical information). Comparison is made to bulk peripheral blood mononuclear cells (PBMCs) from two normal controls (NC1, NC2), sorted memory- (CD45RA−CD122hi) and naïve- (CD45RA+CD122lo) phenotype CD8+ T cells from normal controls, and sorted human natural killer (hNK) cells.
D, CD4+, CD8+, and DN T cells were fractionated from the PBMCs of three ALPS patients (Pt1, Pt4, Pt5) and hEomes mRNA levels were compared to bulk PBMCs from healthy donors. Values represent the mean ± S.E.M. Results are representative of two independent experiments.
TABLE 1.
Patient characteristics.
| Subject | Age/Sex | Identified Fas Mutation | Diagnosis | % DN | LAD | Splenomeg. | IgG (mg/dL) | Autoimmune Manifestations | Therapy |
|---|---|---|---|---|---|---|---|---|---|
| NC1 | 40/F | none | helathy volunteer | <1% | - | - | - | - | none |
| NC2 | 47/M | none | helathy volunteer | <1% | - | - | - | - | none |
| Pt1 | 10/M | 942C->T, p.R234 stop (exon 9) | ALPS-FAS | 8% | ++ | + | 1300–1910 | Autoimmune cytopenia | MMF |
| Pt2 | 10/M | 952G->T, p.G237V (exon 9) | ALPS-FAS | 16% | +++ | asplenic | 2540–3600 | Autoimmune cytopenia | MMF |
| Pt3 | 11/F | 952G->T, p.G237V (exon 9) | ALPS-FAS | 7% | ++ | + | 507–1270 | No cytopenia | none |
| Pt4 | 12/M | 430del AAG, p.E63fs (exon 3) | ALPS-FAS | 10% | +++ | + | 300–1000 | Autoimmune cytopenia | MMF |
| Pt5 | 4/M | 383T->A, p.C47X (exon 2) | ALPS-FAS | 8% | ++++ | + | 2230–2520 | Autoimmune cytopenia, Guillain-Barré | none |
“% DN” denotes percent DN T cells among PBMCs. “LAD” denotes grades of lymphadenopathy:
= shotty nodes
= multiple nodes up to 2cm
= many nodes >2cm
= visible lymphadenopathy.
“% IgG” denotes serum IgG concentration. “Therapy” denotes chronic immunosuppressive regimen. “fs” denotes frameshift mutation. Pt1, Pt2, and Pt3 have previously been referred to in publication as NIH 080.8, NIH 128.1, and NIH 128.4, respectively.
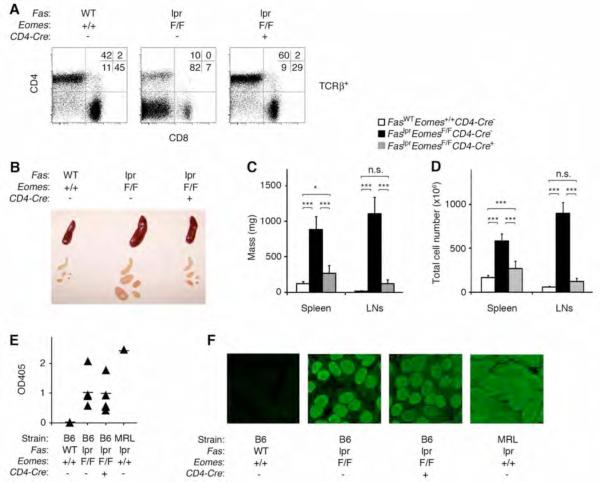
In view of the dysregulated expression of Eomes in DN T cells from patients and mice with ALPS, we took advantage of Cre-Lox technology to achieve a conditional knockout of the murine Eomes locus (Eomes F/F, Cd4:Cre+) to ask whether Eomes expression in T cells plays a causal role in the pathogenesis of ALPS (10). Deletion of Eomes in the T cell lineage of Fas-mutant mice resulted in substantial amelioration of the hallmark T cell dysregulation and lymphoproliferation of ALPS syndrome. In lpr/lpr, Eomes F/F, Cd4:Cre+ mice, accumulation of DN T cells was reduced to the percentage found in wild-type mice (Fig. 2A). The mass and cellularity of lymphoid tissue in lpr/lpr, Eomes F/F, Cd4:Cre+ mice was also substantially reduced compared to Eomes-proficient lpr/lpr mutants (Fig. 2B, C, D). Residual increase in mass and cellularity of lpr/lpr, Eomes F/F, Cd4:Cre+ spleens relative to wild-type mice (together with a similar trend in the lymph nodes) suggests an additional, Eomes-independent phenotype of cellular excess in lpr/lpr mice. This Fas-dependent, Eomes-independent abnormality is likely to be affecting apoptosis or proliferation of conventional leukocytes, in general, because the percentage of CD4+ T cells, CD8+ T cells, B cells, and non-B/non-T cells was not reproducibly different between wild-type and Eomes-deficient lpr/lpr mice (not shown).
FIGURE 2.
Eomes is required for the lymphoproliferative, but not autoimmune, component of murine ALPS.
A, Flow cytometry of freshly isolated, TCRβ+ lymph node cells from wild-type, lpr/lpr (with homozygous floxed alleles of Eomes but no Cre recombinase transgene), and lpr/lpr mice with T cell-specific deletion of Eomes (homozygous floxed alleles of Eomes and transgenic Cre recombinase driven by the Cd4 promoter). Results are representative of more than ten independent experiments. Of note, the percentage of CD4+ T cells, CD8+ T cells, B cells, and non-B/non-T populations was not reproducibly different between wild-type and Eomes-deficient lpr/lpr mice.
B, Photograph of spleens and lymph nodes from mice with the indicated genotypes. Results are representative of eight independent experiments.
C, Mass of spleens and pooled lymph nodes and D, total cell number contained therein from mice of the indicated genotypes. Bar graphs indicate mean values, and error bars represent standard error of the mean, n=8 mice per group. A one-way ANOVA with Tukey's post-comparison test was performed using Prism software. Labels of n.s. denote not significant (p>0.05); * denotes p<0.05; *** denotes p<0.001.
E, F Autoantibody production in 12- to 16-month-old female mice of the indicated genotypes. Results are representative of three independent experiments. E, Sera were isolated and analyzed for anti-double-stranded DNA antibodies by enzyme-linked immunosorbent assay (ELISA). Lupus-prone MRL-lpr/lpr mice serve as positive control for severe autoantibody titers. F, Anti-nuclear antibodies were detected by exposing HEp-2 cells to sera of indicated mice, followed by staining with a secondary, FITC-conjugated antibody reactive to mouse IgG.
Consistent with the finding that Fas deficiency might also alter homeostasis of conventional immune cell lineages independently of Eomes, we found that the autoimmune manifestations of ALPS were not affected by the T cell-specific deletion of Eomes (Fig. 2E, F). This result was not unexpected since independent lines of evidence have uncoupled DN T cell accumulation from the pathogenic autoantibody production of ALPS (3, 4, 9). While B cell or dendritic cell-specific deficiency of Fas is not sufficient to drive DN T cell expansion, either is sufficient to recapitulate the autoantibody production of ALPS (3, 4). CD4+ T cells, DCs and B cells are still present and Fas-deficient in lpr/lpr, Eomes F/F, Cd4:Cre+ mice, providing a sufficient cellular network for the elaboration of pathogenic autoantibodies. Taken together, these data suggest that the Eomes-dependent DN T cell population is responsible for the lymphoproliferative phenotype but does not appear to be required for the humoral autoimmunity characteristic of ALPS.
Previous evidence implicated T-bet in the pathogenic autoantibody production in Fas-mutant mice, owing to the B cell-autonomous role of T-bet in class switch recombination. However, germline deletion of Tbx21, the gene encoding T-bet, had little impact on the course of the T cell-mediated pathology of murine ALPS (17). Consistent with the prior result, there was only moderate dysregulation of T-bet mRNA expression in the cells of Fas-mutant mice (Supplemental Fig. 1A), and deletion of Tbx21 did not substantially affect the accumulation of DN T cells (Supplemental Fig. 1B). These data support a non-essential role for T-bet and an essential, non-redundant role for Eomes in driving DN T cell expansion and lymphoproliferation in ALPS.
In addition to their role in conferring functional competence to killer lymphocytes, Eomes and T-bet are responsible for enhancing expression of CD122, the receptor that confers responsiveness to IL-15 (11). IL-15 serves as a critical growth factor in the maturation and maintenance of memory CD8+ T cells, natural killer cells, and some atypical T cell subsets (19, 20). It was previously suggested that DN T cells from ALPS mice are more sensitive to IL-15 (7), an effect that might be mediated by Eomes. We, therefore, intercrossed Il15−/− mice with lpr/lpr mice. In contrast to the substantial protection associated with T cell-specific deletion of Eomes, deficiency of IL-15 afforded limited protection to lpr/lpr mice against accumulation of DN T cells and lymphadenopathy (Supplemental Fig. 2A, B). These data suggest that Eomes may direct other proliferative or survival mechanisms in DN T cells that transcend its effect on IL-15-responsiveness. Additionally, we found that IL-15 is not responsible for driving dysregulated Eomes expression in DN T cells of lpr/lpr mice (Supplemental Fig. 2C).
The events leading to expression of Eomes in DN T cells remain to be investigated. DN T cells are thought to arise from CD8+ T cells (5). A self-reactive T cell might undergo self-antigenic activation (3), leading to induction of Eomes as part of an incipient program of effector differentiation (11). Alternatively, a CD8+ T cell unfit to be engaged by self-peptide/MHC might degenerate into a state of autonomous survival (6), somewhat akin to Eomes-expressing central memory T cells, which survive independently of self-peptide/MHC (21). It is also possible that cytokine secretion during the pathogenesis of ALPS acts to induce Eomes (22), as interleukin-4 (IL-4) was recently found to drive expression of Eomes in a population of non-canonical, innate-like CD8+ T lymphocytes (14, 15). Though previous data argue against an absolute requirement for IL-4 in the accumulation of DN T cells in lpr/lpr mice (22), we cannot rule out a role for other cytokines or soluble factors in inducing and enhancing Eomes expression in Fas-mutant CD8+ and DN T cells.
It has recently been suggested that CD8+ T cells deficient in Eomes may fail to effectively compete for the memory T cell niche (23). The present results raise the possibility that gain-of-function of Eomes in Fas-deficient DN T cells promotes their preferential proliferation in lymphoid tissues. This hypothesis is consistent with both the prior evidence of increased proliferation and defective apoptosis of Fas-deficient DN T cells (24) as well as the enhanced proliferation and Bcl-2 expression of Eomes-proficient compared to Eomes-deficient memory CD8+ T cells (23). Future studies will be designed to identify the target genes of Eomes that are involved in ALPS DN T cell homeostasis, since it appears to be more complex a matter than Eomes simply regulating responsiveness to IL-15 (Supplemental Fig. 2A, B). Despite uncertainties surrounding the ontogeny of DN T cells, our finding that Eomes is essential and non-redundant for DN T cell development or maintenance offers a novel therapeutic target to reduce the DN T cell compartment and alleviate a major source of morbidity in children with ALPS.
Supplementary Material
Acknowledgements
We thank Janet Dale for assistance with obtaining human samples.
This work was supported by NIH grants AI042370, AI076458, and AI061699 and the Abramson Family (S.L.R.) and training grant T32 CA09140 (S.M.G.). Part of this research was also supported by the Intramural Research Program of the NIH, NIAID, Bethesda, MD 20892.
Abbreviations used in this paper
- 1) DN
Double-negative (CD4−CD8−)
- 2) ALPS
Autoimmune lymphoproliferative syndrome
- 3) Eomes
Eomesodermin
Footnotes
Competing financial interests The authors declare no competing financial interests.
References
- 1.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, Rieux-Laucat F, Siegel RM, Su HC, Teachey DT, Rao VK. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seif AE, Manno CS, Sheen C, Grupp SA, Teachey DT. Identifying autoimmune lymphoproliferative syndrome in children with Evans syndrome: a multi-institutional study. Blood. 2010;115:2142–2145. doi: 10.1182/blood-2009-08-239525. [DOI] [PubMed] [Google Scholar]
- 3.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, Liepa J, Wood GA, Casola S, Rajewsky K, Mak TW. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristeau-Leprince A, Mateo V, Lim A, Magerus-Chatinet A, Solary E, Fischer A, Rieux-Laucat F, Gougeon ML. Human TCR alpha/beta+ CD4−CD8− double-negative T cells in patients with autoimmune lymphoproliferative syndrome express restricted Vbeta TCR diversity and are clonally related to CD8+ T cells. J Immunol. 2008;181:440–448. doi: 10.4049/jimmunol.181.1.440. [DOI] [PubMed] [Google Scholar]
- 6.Pestano GA, Zhou Y, Trimble LA, Daley J, Weber GF, Cantor H. Inactivation of misselected CD8 T cells by CD8 gene methylation and cell death. Science. 1999;284:1187–1191. doi: 10.1126/science.284.5417.1187. [DOI] [PubMed] [Google Scholar]
- 7.Trimble LA, Prince KA, Pestano GA, Daley J, Cantor H. Fas-dependent elimination of nonselected CD8 cells and lpr disease. J Immunol. 2002;168:4960–4967. doi: 10.4049/jimmunol.168.10.4960. [DOI] [PubMed] [Google Scholar]
- 8.Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci U S A. 1996;93:2131–2136. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 10.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 12.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31:122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 19.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 22.Peng SL, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting Edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010 doi: 10.4049/jimmunol.1002042. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou T, Bluethmann H, Eldridge J, Berry K, Mountz JD. Origin of CD4−CD8−B220+ T cells in MRL-lpr/lpr mice. Clues from a T cell receptor beta transgenic mouse. J Immunol. 1993;150:3651–3667. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.