Abstract
Background and Aims
Fibrosis progression might be accelerated in patients that are co-infected with HIV and hepatitis C virus (HIV/HCV). However, no studies have directly compared fibrosis progression by paired liver biopsy between patients infected with HIV and HCV vs. those infected with only HCV.
Methods
Liver biopsy samples were collected from patients with HIV/HCV (n=306) and those with HCV; biopsies from 59 without a sustained virologic response (SVR) or cirrhosis were matched with those from patients with only HCV (controls) for initial fibrosis stage, demographics, and HCV treatment. For HIV/HCV patients, categorical variables at baseline and the area under the curve of continuous variables per unit time were analyzed for associations with fibrosis progression.
Results
Liver biopsies from HIV/HCV patients had more piecemeal necrosis than controls (P=.001) and increased lobular inflammation (P=.002); HIV/HCV patients also had shorter intervals between liver biopsies (4.7 vs. 5.9 yrs, P<.0001). Between the 1st and 2nd biopsies, fibrosis remained unchanged or progressed 1 or 2 units in 55%, 18%, and 18% of HIV/HCV patients, respectively, compared with 45%, 30%, and 9% of controls. The fibrosis progression rate was similar between HIV/HCV and control patients (0.12±0.40 vs. 0.091±0.29 units/yr; P=.72). In paired biopsies from 66 patients, including those with SVR, there were no associations between fibrosis progression and demographics; numbers of CD4+ T cells; levels of aspartate aminotransferase or alanine aminotransferase; use of highly-active anti-retroviral therapy; response to HCV therapy (no treatment, SVR, or non-response); baseline levels of FIB-4; or histological features including inflammation, fibrosis, or steatosis.
Conclusions
Based on analysis of liver biopsy samples, fibrosis progression was similar between HIV/HCV- and HCV-infected patients; no clinical or laboratory parameters predicted disease progression.
Keywords: Hepatitis C disease progression, co-infection, human immunodeficiency virus
Introduction
With the advent of highly active antiretroviral therapy (HAART), which combines various nucleoside/nucleotide analogue reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs), the morbidity and mortality related to human immunodeficiency virus (HIV) have significantly decreased [1]. As a result, patients are now living longer with HIV infection and other co-morbidities and hepatic events have emerged as a key issue in the management of HIV-infected patients [2]. Due to shared routes of transmission, coinfection with chronic hepatitis C virus (HCV) in those infected with HIV is common [3].
Several studies have examined the natural history of HCV in monoinfected subjects. These have found that disease progression was associated with age at infection, male gender, overweight, excess alcohol consumption, hepatic inflammation, steatosis, and presence of fibrosis [4–8]. Conversely, treatment induced sustained virologic response (SVR) can be associated with improved histology [9]. While some studies assessed fibrosis progression based on estimated disease duration in cross sectional analysis of single biopsies [4], others used a paired biopsy approach [5–9] comparing change in fibrosis over a known time period.
Although early reports suggested that the natural history of HCV in those coinfected with HIV was more progressive [10,11], more recent studies suggest that fibrosis progression in those with controlled HIV is similar to those with HCV alone [12,13], affected by excess alcohol use, age at infection, baseline fibrosis, inflammation and steatosis as well as SVR to anti-HCV therapy [10–12, 14–19] and unique factors associated with HIV therapy [12,14,19–22]. Although early studies suggested that low CD4 levels were associated with advanced fibrosis [10,14,15], more recent data in patients without HIV suggest that advanced fibrosis may result in low CD4 rather than vice versa [23]. Studies on disease progression by paired biopsy analysis in those with coinfection suggest that 16–50% have fibrosis progression [15,21,22,24]. However, few studies directly compare fibrosis progression by paired biopsy between those with coinfection and those with HCV alone [25,26]. To address this gap in knowledge, we performed a longitudinal cohort study to compare fibrosis progression by paired biopsy in patients with HIV-HCV and HCV alone and assessed factors associated with fibrosis progression in those with HIV-HCV coinfection.
Patients and Methods
Study Population
This single center study derived its population from HIV-HCV coinfected and HCV monoinfected patients seen between 1998 and 2009. All patients were age > 18 and positive for HCV RNA by commercial assay. All those with HIV were positive for anti-HIV antibodies. Patients were excluded from analysis if they had a prothrombin time prolonged > 2 seconds from control, presence of ascites, thrombocytopenia (platelet < 70,000), active or recent (within 3 months) opportunistic infection related to HIV, renal failure defined as a creatinine > 2.5, were HBV surface antigen positive, had any other form of chronic liver disease, or had inability to give informed consent. Those with HIV and advanced disease with less than 1 year expected survival were also excluded. Alcohol abuse was defined as more than 50 g/day. Those without cirrhosis on initial biopsy were offered a follow-up biopsy to assess disease progression.
Of 306 coinfected subjects biopsied, 66 without cirrhosis at baseline underwent a second biopsy as part of a prospective study and composed the paired biopsy group. Because HCV therapy can modify disease progression [9,16–18], we included only the 59 who were HCV treatment naïve or prior non-responders (NR) to at least a 12 week course of therapy. Because NR have a similar progression to those who are treatment naïve [22], these groups were combined. During the same time period, 233 patients with HCV alone who were also treatment naïve or prior NR underwent paired biopsy at our center as part of routine care. From this group, we retrospectively identified a control group at random using a computer generated algorithm matched to the coinfected group for age (± 4 years) and baseline Knodell fibrosis. In addition, we identified a more strictly defined control group matched on age, gender, race, and baseline Knodell fibrosis.
Liver Histology
A percutaneous liver biopsy was performed in the standard fashion. Formalin-fixed, paraffin-embedded liver tissue was stained by hematoxylin-eosin and Masson’s trichrome. In both coinfected and monoinfected subjects, histologic activity index (HAI) for total inflammation (piecemeal necrosis, lobular inflammation, and portal inflammation) and fibrosis was assessed by Knodell score [27] and deemed adequate for size by one of two dedicated liver pathologists (MAC and ASM). In those coinfected with HIV-HCV, we also used the Ishak histologic activity index (HAI) for inflammation and fibrosis [28] and the modified Brunt scoring system for steatosis [29] to assess predictors of fibrosis disease progression.
Variables Examined
At the time of initial biopsy, the following information was collected: age, gender, race, and for those with coinfection pathologic alcohol use, the presence of diabetes, hypertension, lipodystrophy, dyslipidemia, and both past and current antiretroviral use. Prior to biopsy, biochemical tests for complete blood count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, albumin, complete blood count, HCV RNA and HCV genotype were performed by commercial assays. For those with HIV coinfection, CD4 and CD8 lymphocyte counts and HIV RNA were also obtained. Coinfected patients were seen every 3–6 months. AST to platelet ratio (APRI) and FIB-4 were calculated as previously described [30,31]. This study was approved by the Office of Research Subjects Protection at the Virginia Commonwealth University Health System.
Statistical Analysis
Demographic, clinical, laboratory, and histologic data are presented as mean and standard deviation for approximately normally distributed data, median and interquartile range [IQR] for skewed quantitative data, and proportions for categorical data as indicated. The primary outcome was defined as a worsening in Knodell fibrosis between biopsies. For those with no (stage 0) fibrosis, we assessed for a ≥ 1 stage increase. In those with portal fibrosis (stage 1), we assessed for either a one stage decrease or a ≥1 stage increase. In those bridging fibrosis (stage 3), we assessed for a ≥1 stage decrease or a one stage increase to cirrhosis (stage 4). To account for varying time between biopsies, a fibrosis progression rate (FPR) was calculated by subtracting the first biopsy score from the second and dividing by the years between biopsies. In coinfected patients, area under the curve (AUC) per unit time was computed for quantitative variables that were measured longitudinally. Subsequently, comparisons of individual quantitative variables between two groups were assessed by the Mann-Whitney test and comparisons of categorical variables by the Fisher exact test. In addition, Knodell fibrosis stage was cross-tabulated between first and second biopsy separately for the matched coinfected and monoinfected participants; and Ishak fibrosis stage was cross-tabulated between first and second biopsy for coinfected patients.
Results
Baseline Characteristics
Of 306 coinfected patients undergoing liver biopsy, 66 had more than one biopsy (Table 1). The majority of coinfected patients were on HAART (82%) with a mean CD4 count of 515 (cells/μl) and 62% had HIV less than 400 copies/ml. Of those on HAART, 98% were on a NRTI, 39% on NNRTI, and 53% on a PI. Those with more than one biopsy differed in age (43 vs. 47 years (p=.02), serum ALT (80 vs. 61; p=.007) and as expected absence of cirrhosis.
Table 1.
Characteristics of coinfected patients with a single biopsy and with more than one biopsy at the time of the first liver biopsy.
| Characteristic | Single biopsy (n=240) | Paired biopsy (n=66) | P |
|---|---|---|---|
| Age (years)1 | 47 (8.5) | 43 (8.7) | 0.022 |
| Male (%)2 | 78 (72–83) | 73 (60–83) | 0.42 |
| Caucasian (%)2 | 20 (15–26) | 20 (11–31) | 1 |
| Weight (kg)2 | 79 (16) | 77 (16) | 0.62 |
| Body mass index1 | 26 (4.7) | 26 (5.2) | 0.72 |
| Alcohol abuse (%)2 | 28 (22–34) | 21 (12–33) | 0.34 |
| HCV genotype 1 (%)2 | 80 (75–85) | 83 (72–91) | 0.72 |
| HAART use (%)2 | 83 (78–87) | 79 (67–88) | 0.47 |
| NRTI (%)2 | 81 (75–86) | 79 (67–88) | 0.73 |
| NNRTI (%)2 | 33 (27–39) | 30 (20–43) | 0.77 |
| PI (%)2 | 45 (39–52) | 38 (26–51) | 0.33 |
| CD4 (cells/microliter)3 | 440 (270–670) | 500 (290–700) | 0.32 |
| HIV < 400 (%)2 | 65 (58–71) | 51 (36–66) | 0.1 |
| AST (U/L)3 | 58 (43–95) | 72 (48–100) | 0.094 |
| ALT (U/L)3 | 61 (42–90) | 80 (50–110) | 0.0075 |
| ALP (U/L)3 | 110 (82–140) | 110 (91–150) | 0.24 |
| Platelet (cells/microliter)1 | 210 (84) | 210 (69) | 0.63 |
| APRI3 | 0.58 (0.35–1.1) | 0.69 (0.46–1.3) | 0.25 |
| FIB-43 | 1.9 (1.2–3.2) | 1.8 (1.1–2.9) | 0.55 |
| Total HAI inflammation1 | 6.4 (2.9) | 6.3 (3) | 0.9 |
| Ishak fibrosis stage (%)1 | |||
| 0 | 0: 17 (12–22) | 0: 15 (8–26) | |
| 1 | 1: 36 (30–43) | 1: 36 (25–49) | |
| 2 | 2: 19 (14–25) | 2: 24 (15–36) | |
| 3 | 3: 9 (6–14) | 3: 15 (8–26) | |
| 4 | 4: 8 (5–12) | 4: 9 (3–19) | |
| 5 | 5: 6 (3–10) | 5: 0 | |
| 6 | 6: 5 (3–9) | 6: 0 | 0.083 |
| Steatosis > 5% (%)2 | 18 (13–24) | 20 (11–32) | 0.72 |
| Biopsy length (mm)1 | 20 (7.8) | 22 (9.2) | 0.35 |
Mean (SD).
Percent (95% confidence interval).
Median (IQR).
The 59 without SVR and with >1 biopsy were matched to HCV monoinfected patients by age and baseline fibrosis (Table 2). Coinfected patients had higher piecemeal necrosis (p=.014) and lobular inflammation (p<.001). On initial biopsy, the Knodell fibrosis distribution was 24% no fibrosis (score 0), 48% portal fibrosis (score 1), and 27% bridging fibrosis (score 3). The HAI averaged 6.3 ± 3.0 and >5% steatosis was observed in 20%.
Table 2.
Comparison of HIV-HCV coinfected patients without sustained virologic response to matched HCV controls.
| Characteristic | HIV - HCV (n=59)1 | HCV (n=59) | P |
|---|---|---|---|
| Age (years)1 | 44 (8) | 44 (7.7) | 0.99 |
| Male (%)2 | 71 (58–82) | 68 (54–79) | 0.84 |
| Caucasian (%)2 | 15 (7–27) | 56 (42–69) | <0.001 |
| AST (U/L)3 | 74 (50–100) | 60 (42–94) | 0.37 |
| ALT (U/L)3 | 80 (51–110) | 87 (61–150) | 0.081 |
| Treatment naive (%)3 | 54 (41–67) | 19 (10–31) | <0.001 |
| Total HAI inflammation1 | 6.4 (3) | 5.4 (1.8) | 0.034 |
| Piecemeal necrosis1 | 2 (1.4) | 1.3 (0.9) | 0.014 |
| Lobular inflammation1 | 2 (1.2) | 1.3 (0.91) | <0.001 |
| Portal inflammation1 | 2.4 (0.99) | 2.7 (0.81) | 0.13 |
| Knodell fibrosis score (%)2 | |||
| 0 | 0: 24 (14–37) | 0: 24 (14–37) | |
| 1 | 1: 49 (36–63) | 1: 49 (36–63) | |
| 3 | 3: 27 (16–40) | 3: 27 (16–40) | 1 |
| Interval between biopsies (years)1 | 4.7 (2.3) | 5.8 (1.7) | <0.001 |
| Knodell fibrosis change between biopsies1 | 0.36 (1.2) | 0.51 (1.2) | 0.48 |
| Change per year in Knodell fibrosis score1 | 0.12 (0.4) | 0.091 (0.29) | 0.7 |
Mean (SD);
Percent (95% confidence interval);
Median (IQR).
Change in hepatic fibrosis between first and second biopsy
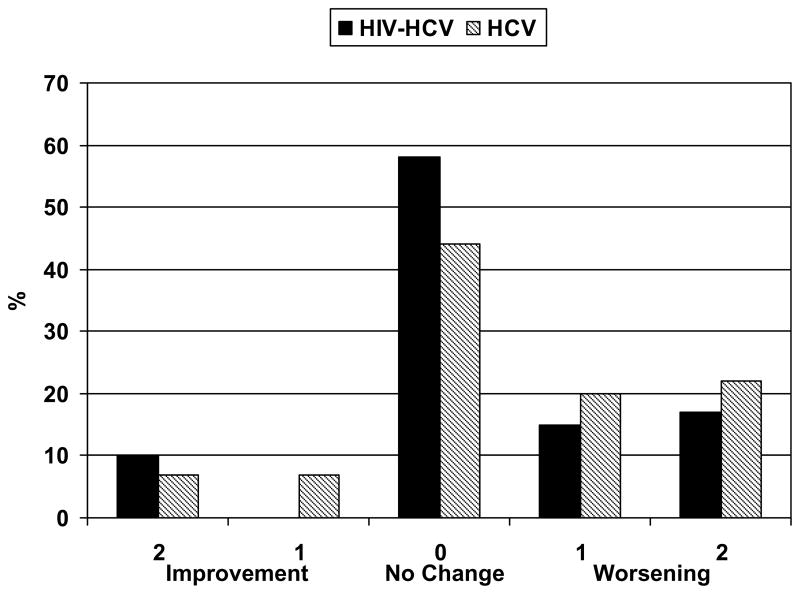
The interval between biopsies was less in those with coinfection 4.7 ± 2.3 years compared to those with HCV alone 5.8 ± 1.7 (p<.001). Tables 3a and 3b show the change in fibrosis for both those with HIV-HCV (3a) and HCV alone (3b). Figure 1 shows the proportion of patients in each group that improved fibrosis, remained unchanged, or worsened fibrosis. Between biopsies, fibrosis remained unchanged, progressed 1 and 2 units in 55%, 18% and 18% respectively in coinfected patients compared to 45%, 30% and 9% in HCV controls (NS). Between the first and second biopsy, the fibrosis progression rate was similar between HIV/HCV and HCV alone (0.12±0.40 vs. 0.091±0.29 units/yr; p=.72). When coinfected patients were matched on all baseline factors (fibrosis stage, gender, race, and prior exposure to HCV therapy), we were able to compare 38 matched pairs with similar results (fibrosis progression 0.15±0.46 vs. 0.18±0.31 fibrosis units/yr; p=0.7).
Table 3a.
Knodell fibrosis stages at initial and final biopsy in coinfected patients without sustained virologic response*
| Second Biopsy | |||||
|---|---|---|---|---|---|
| First Biopsy | 0 | 1 | 3 | 4 | Total |
| 0 | 4 | 6 | 4 | 0 | 14 |
| 1 | 0 | 23 | 6 | 0 | 29 |
| 3 | 0 | 6 | 7 | 3 | 16 |
| Total | 3 | 35 | 17 | 3 | 59 |
shaded cells represent a one or more increase in fibrosis.
Table 3b.
Knodell fibrosis stages at initial and final biopsy in HCV control patients without sustained virologic response*.
| Second Biopsy | |||||
|---|---|---|---|---|---|
| First Biopsy | 0 | 1 | 3 | 4 | Total |
| 0 | 4 | 8 | 2 | 0 | 14 |
| 1 | 4 | 14 | 9 | 2 | 29 |
| 3 | 0 | 4 | 8 | 4 | 16 |
| Total | 8 | 26 | 19 | 6 | 59 |
shaded cells represent a one or more increase in fibrosis
Figure 1.
Frequency of changes in fibrosis between HIV-HCV coinfected and HCV monoinfected patients without sustained virologic response.
Correlates of fibrosis progression in those with HIV-HCV coinfection
Table 4 shows the change in Ishak fibrosis in all 66 coinfected patients who underwent more than 1 biopsy including those with SVR. A change in Ishak fibrosis of two or more units, the primary endpoint, was observed in 11% while 21% had a one point worsening, 39% remained unchanged, 21% had a one point improvement, and 8% had a two or more point improvement.
Table 4.
Ishak Fibrosis stages at initial and final biopsy in all coinfected patients.
| Second biopsy | ||||||||
|---|---|---|---|---|---|---|---|---|
| First biopsy | 0 | 1 | 2 | 3 | 4 | 5 | 6 | Total |
| 0 | 5 | 3 | 1 | 0 | 1 | 0 | 0 | 10 |
| 1 | 4 | 11 | 6 | 2 | 0 | 1 | 0 | 24 |
| 2 | 1 | 6 | 6 | 3 | 0 | 0 | 0 | 16 |
| 3 | 0 | 3 | 2 | 3 | 0 | 0 | 2 | 10 |
| 4 | 1 | 0 | 0 | 2 | 1 | 2 | 0 | 6 |
| Total | 11 | 23 | 15 | 10 | 2 | 3 | 2 | 66 |
shaded cells represent a two or more increase in fibrosis.
For the same 66 patients assessed in Table 4, Table 5 compares those who had fibrosis progression of at least 2 Ishak points against those that did not. Other than a lower body mass index (BMI) (22 vs. 27; p=0.01), there were no other associations between fibrosis progression and demographics, baseline or changes (AUC) in CD4, AST, or ALT, HAART use, response to HCV therapy (no treatment, SVR, or NR), baseline FIB-4, APRI or histology including inflammation, fibrosis, or steatosis.
Table 5.
Comparison of coinfected patients with at least 2 Ishak fibrosis point progression and those that did not progress.
| Characteristic | Non-Progressors (n=59)1 | Progressors (n=7) | P | N |
|---|---|---|---|---|
| Age (years)1 | 44 (8) | 43 (14) | 0.88 | 59 & 7 |
| Male (%)2 | 73 (60–84) | 71 (29–96) | 1 | 59 & 7 |
| Caucasian (%)2 | 20 (11–33) | 14 (0–58) | 1 | 59 & 7 |
| Weight (kg)1 | 78 (16) | 67 (12) | 0.061 | 58 & 7 |
| Body mass index1 | 27 (5.2) | 22 (2.6) | 0.011 | 53 & 7 |
| Alcohol abuse (%)2 | 22 (12–35) | 14 (0–58) | 1 | 59 & 7 |
| HCV genotype 1 (%)2 | 94 (84–99) | 86 (42–100) | 0.41 | 52 & 7 |
| HAART use (%)2 | 78 (65–88) | 86 (42–100) | 1 | 59 & 7 |
| NRTI (%)2 | 78 (65–88) | 86 (42–100) | 1 | 59 & 7 |
| NNRTI (%)2 | 29 (18–42) | 43 (10–82) | 0.43 | 59 & 7 |
| PI (%)3 | 37 (25–51) | 43 (10–82) | 1 | 59 & 7 |
| HIV < 400 (%)2 | 50 (35–65) | 60 (15–95) | 1 | 44 & 5 |
| HIV titer longitudinal summary2 | ||||
| 0–400 | 0–400: 51 (37–64) | 0–400: 57 (18–90) | ||
| mixed4 | mixed: 25 (15–38) | mixed: 43 (10–82) | ||
| 400+ | 400+: 24 (14–37) | 400+: 0 | 0.34 | 59 & 7 |
| CD4 (cells/microliter)3 | 510 (290–700) | 380 (320–470) | 0.32 | 52 & 7 |
| CD4 (cells/microliter) | ||||
| AUC3 | 510 (350–740) | 350 (300–570) | 0.28 | 59 & 7 |
| APRI3 | 0.66 (0.44–1.3) | 0.84 (0.55–1.4) | 0.59 | 56 & 7 |
| APRI AUC3 | 0.65 (0.42–1.1) | 0.76 (0.64–0.92) | 0.39 | 59 & 7 |
| Platelet (cells/microliter)1 | 220 (68) | 190 (80) | 1 | 56 & 7 |
| Platelet (cells/microliter) | ||||
| AUC1 | 210 (56) | 180 (64) | 0.3 | 59 & 7 |
| ALT (U/L)3 | 82 (51–120) | 64 (38–100) | 0.27 | 56 & 7 |
| ALT AUC3 | 70 (52–92) | 56 (52–73) | 0.29 | 59 & 7 |
| AST (U/L)3 | 68 (48–110) | 77 (66–85) | 0.98 | 56 & 7 |
| AST AUC3 | 66 (50–93) | 62 (59–77) | 0.81 | 59 & 7 |
| ALP (U/L)3 | 110 (90–140) | 130 (110–160) | 0.27 | 56 & 7 |
| ALP AUC3 | 100 (85–140) | 140 (100–150) | 0.26 | 59 & 7 |
| FIB-43 | 1.8 (1.1–2.9) | 1.8 (1.4–5) | 0.62 | 56 & 7 |
| FIB-4 AUC3 | 1.8 (1.3–2.4) | 2.2 (1.5–3.3) | 0.4 | 58 & 7 |
| Total HAI inflammation1 | 6.5 (2.9) | 4.4 (3.1) | 0.09 | 59 & 7 |
| Piecemeal necrosis1 | 2.1 (1.3) | 1 (1.4) | 0.059 | 59 & 7 |
| Lobular inflammation1 | 2 (1.2) | 1.6 (0.98) | 0.33 | 59 & 7 |
| Portal inflammation1 | 2.4 (1) | 1.9 (1.1) | 0.16 | 59 & 7 |
| Ishak fibrosis stage (%)2 | ||||
| 0 | 0: 14 (6–25) | 0: 29 (4–71) | 0.28 | 59 & 7 |
| 1 | 1: 36 (24–49) | 1: 43 (10–82) | ||
| 2 | 2: 27 (16–40) | 2: 0 | ||
| 3 | 3: 14 (6–25) | 3: 29 (4–71) | ||
| 4 | 4: 10 (4–21) | 4: 0 | ||
| 5 | 5: 0 | 5: 0 | ||
| 6 | 6: 0 | 6: 0 | ||
| Steatosis > 5% (%)2 | 20 (11–33) | 17 (0–64) | 1 | 59 & 6 |
| Interval between biopsies (years)1 | 4.6 (2.3) | 5.1 (2.6) | 0.78 | 59 & 7 |
| Not treated for HCV (%)2 | 49 (36–63) | 57 (18–90) | 1 | 59 & 7 |
| Sustained viral response (%)2 | 10 (4–21) | 0 (0–41) | 1 | 59 & 7 |
Mean (SD).
Percent (95% confidence interval).
Median (IQR).
“Mixed” means that over the course of the study a patient was sometimes over 400 and sometimes under.
Discussion
The decision to treat HCV in those coinfected with HIV depends on several factors including HCV genotype, control of HIV, patient compliance, and concerns of disease progression without therapy [32,33]. Because early studies suggested a more rapid progression of fibrosis in the coinfected [7,8], many experts suggested treating all coinfected patients [34], even in those without significant histologic injury. Because most studies of fibrosis progression in coinfected patients were either retrospective with only estimates of disease duration or after anti-HCV therapy [7–17], the natural history of HCV in those coinfected with HIV remains poorly defined. Although several studies included either paired biopsy analysis [15,21,22,24] or indirect comparisons to those with HCV alone [12,16,25,26], no studies of paired biopsies report direct comparisons of coinfected patients to matched patients with HCV alone.
The first important finding from our study is that although 20% of coinfected patients progressed over time (10% one stage and 10% two or more stages), this was not significantly different than that observed in HCV alone (17% one stage and 5% two stages) and similar to others with HCV alone [8]. Our observations in coinfected patients are lower than previously reported [6]. Schiavini et al reported that 18/36 (50%) of coinfected patients progressed at least one stage over 54 (IQR 50–86) months [15]; Bonnard that 9/32 (28%) coinfected subjects progressed at least two stages over a median 49 months [21]; and Sulkowski that in 174 coinfected patients, 37 (22%) and 41 (24%) had at least a two point fibrosis increase over a mean of 2.9 years (IQR 2.3 – 3.4) while 48% had no change [24]. In a more recent study, Macias observed that 28% progressed one fibrosis stage and 16% increased at least 2 stages over a period over three years [22]. To account for the differences in time between biopsies, we also calculated the FPR and again observed no significant differences in those with and without HIV supportive of other retrospective studies [12,26]. Interestingly, not only did we not observe a pattern of progression; 4 of our patients without SVR (7%) experienced improvement of 2 units in the Ishak fibrosis score. Fibrosis progression may not be linear over time and we had acknowledged this in our manuscript. In patients with only two fibrosis measurements, it would be impossible to differentiate between linear and nonlinear progression. In the twelve patients for whom we had more than two fibrosis measurements apiece, however, we did examine fibrosis progression and found no evidence of nonlinearity.
The second important finding was that other than a lower BMI, we were unable to identify a factor associated with fibrosis progression. Unlike studies in HCV alone [6,7], no baseline histologic feature (such as inflammation, steatosis, or fibrosis) was associated with fibrosis progression in our data. This is in contrast to Macias who observed that those with more necroinflammation at baseline were more likely to progress [22]. Unlike studies that found that coinfected patients with increased AST [24], decreased CD4 cell count [15] and HIV RNA [22] were at higher risk for disease progression, we were not able to identify any single demographic or laboratory test associated with fibrosis progression. Unlike Rodriguez-Torres [18] but similar to Sulkowski [24], we did not that response to anti-HCV therapy was associated with disease progression. However, because of the limited number of patients who achieved SVR in our cohort, our study was not powered to assess the impact of SVR on histology. Reasons for these differences may be related to the size and characteristics of the patient population, interval between biopsies, or liver biopsy sampling error. Similar to others [24], we did not find that CD4 count, HIV RNA level, steatosis, or any particular HAART regimen was associated with progression.
The strength of our study is the direct comparison of coinfected patients to those with HCV alone, after matching by baseline fibrosis and prior response to HCV therapy. When also matched for age, gender, and race, we again observed no differences in fibrosis progression. However, our study has several important limitations that make direct comparison to other studies difficult. First, although we included all coinfected subjects who underwent more than one biopsy, referral bias and self-selection of subjects willing to undergo a second biopsy could have affected our results. Second, although all subjects were abstinent from alcohol for at least 6 months prior to initial biopsy, we were not able to take into account alcohol use in between biopsies and did not have accurate data on alcohol use in those with HCV alone. Third, although we did not identify any particular HAART regimen associated with fibrosis progression, the specific regimen for each patient was not controlled. Furthermore, we did not directly take into account compliance or change in HAART between biopsies; this could have affected our results. Nevertheless, changes in HIV RNA and CD4 count, which should reflect control of HIV, were not associated with fibrosis progression. Fourth, imprecisions in staging could have affected our results. Also, only Knodell fibrosis scores without specific steatosis grading were available on those with HCV alone. Therefore, changes in Ishak fibrosis scores and impact of steatosis, which could have affected results, were not used for comparison. However, more subjects had either no change or progression (95% in coinfection and 91% in HCV controls) than regression (5% in coinfection and 9% in controls). To minimize the effect of HCV treatment, we only included either those who were treatment naïve or prior NR in the comparative analysis. In support of this, prior studies in coinfected patients did not show an affect of HCV treatment on disease progression [22]. We recognize that our low SVR rate and limited number of treated subjects may have limited our ability to assess response to HCV therapy in coinfected patients. We were also not able to match for every possible variable that might have affected disease progression (such as BMI, steatosis, race and exact duration of prior HCV therapy in NR). It is also important to recognize that our coinfected population had mild disease on initial biopsy and well controlled HIV and that fibrosis progression may not be linear. Therefore, these results may not be generalizable to all coinfected patients. Finally, although the intervals between biopsies in the two groups were different, we adjusted for this by matching HCV controls to baseline fibrosis and determining the FPR. Although fibrosis progression may not be linear over time, it would be impossible to differentiate between linear and nonlinear progression in those with only two biopsies. In the twelve patients for whom we had more than two fibrosis measurements apiece, however, we did examine fibrosis progression and found no evidence of nonlinearity.
In summary, when matched for baseline fibrosis and demographic factors, while a proportion of coinfected patients progressed, fibrosis progression was no different than observed in HCV monoinfection. Because there were no clinical or laboratory parameters that predicted disease progression, we therefore recommend that all coinfected patients should be considered for repeat assessment of disease severity by liver biopsy at periodic intervals in order to identify those who progress [35]. Because a significant proportion did not progress, similar to those with HCV alone, decisions on whether or not to begin HCV therapy should depend on patient compliance and likelihood of SVR and not on concerns about rapid disease progression. Because SVR rates with current therapy are suboptimal [36,37], it is reasonable to defer HCV therapy in those with mild disease until better therapies for HCV are available.
Acknowledgments
This work was supported by a grant from the National Institute of Health (K23-DK064578) to Richard Sterling and by a grant to the General Clinical Research Center at Virginia Commonwealth University (M01RR00065). This work was presented in part at the European Association for the Study of Liver Diseases, Vienna, Austria, 2010 and Digestive Diseases Week, New Orleans, LA, 2010.
Footnotes
Clinical Trials # NCT00575315
Disclosures
This was an investigator initiated study supported by NIDDK to RKS.
Author involvement: Study concept and design; RKS, AJS, MLS
Acquisition of data; RKS, PGS, RTS, VAL, MF, PP, MLS, AJS
Analysis and interpretation of data; RKS, JAW
Drafting of the manuscript; RKS
Critical revision of the manuscript for important intellectual content; MLS
Statistical analysis; JAW
Obtained funding; RKS
Study supervision: RKS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee LM, Karon JM, Selik R, et al. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285(10):1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Møller N, et al. Arch Intern Med. 2006 Aug 14–28;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;4:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 4.Natural history of liver fibrosis progression in patients with chronic hepatitis C. 9055. Vol. 349. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups; 1997. Mar 22, pp. 825–32. [DOI] [PubMed] [Google Scholar]
- 5.Sobesky R, Lebray P, Nalpas B, et al. World J Gastroenterol. 2008 Jun 28;14(24):3861–5. doi: 10.3748/wjg.14.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghany MG, Kleiner DE, Alter H, et al. Gastroenterology. 2003 Jan;124(1):97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 7.Ryder SD, Irving WL, Jones DA, et al. Trent Hepatitis C Study Group Gut. 2004 Mar;53(3):451–5. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Predicting progressive hepatic fibrosis stage on subsequent liver biopsy in chronic hepatitis C virus infection. 1. Vol. 12. 2005. Jan, pp. 74–80. [DOI] [PubMed] [Google Scholar]
- 9.George SL, Bacon BR, Brunt EM, et al. Hepatology. 2009 Mar;49(3):729–38. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benhamou Y, Bochet M, Di Martino V, et al. Hepatology. 1999 Oct;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 11.Mohsen AH, Easterbrook PJ, Taylor C, et al. Gut. 2003 Jul;52(7):1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bräu N, Salvatore M, Ríos-Bedoya CF, et al. J Hepatol. 2006 Jan;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Sterling RK, Contos MJ, Sanyal AJ, et al. The clinical spectrum of hepatitis C virus in HIV coinfection. J Acquir Immune Defic Syndr. 2003;32:30–37. doi: 10.1097/00126334-200301010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Benhamou Y, Di Martino V, Bochet M, et al. Hepatology. 2001 Aug;34(2):283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 15.Schiavini M, Angeli E, Mainini A, et al. HIV Med. 2006 Jul;7(5):331–7. doi: 10.1111/j.1468-1293.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 16.Impact of peginterferon alpha-2b and ribavirin treatment on liver tissue in patients with HCV or HCV-HIV co-infection. 6. Vol. 54. 2007. Jun, pp. 609–16. Epub 2006 Dec 27. [DOI] [PubMed] [Google Scholar]
- 17.Bani-Sadr F, Lapidus N, Bedossa P, et al. Clin Infect Dis. 2008 Mar 1;46(5):768–74. doi: 10.1086/527565. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Torres M, Rodríguez-Orengo JF, Ríos-Bedoya CF, et al. J Hepatol. 2007 Apr;46(4):613–9. doi: 10.1016/j.jhep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Pineda JA, Macías J. J Antimicrob Chemother. 2005 Apr;55(4):417–9. doi: 10.1093/jac/dkh555. [DOI] [PubMed] [Google Scholar]
- 20.Sterling RK, Wilson MS, Sanyal AJ, et al. Impact of highly active antiretroviral therapy on the spectrum of liver disease in HCV-HIV coinfection. Clin Gastroenterol Hepatol. 2004;2:432–439. doi: 10.1016/s1542-3565(04)00129-6. [DOI] [PubMed] [Google Scholar]
- 21.Bonnard P, Lescure FX, Amiel C, et al. J Viral Hepat. 2007 Nov;14(11):806–11. doi: 10.1111/j.1365-2893.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 22.Macías J, Berenguer J, Japón MA, et al. Hepatology. 2009 Oct;50(4):1056–63. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 23.McGovern BH, Golan Y, Lopez M, et al. Clin Infect Dis. 2007 Feb 1;44(3):431–7. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 24.Sulkowski MS, Mehta SH, Torbenson MS, et al. AIDS. 2007 Oct 18;21(16):2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 25.Thein HH, Yi Q, Dore GJ, Krahn MD. AIDS. 2008 Oct 1;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 26.Souza AR, Tovo CV, Mattos AA, Chaves S. Braz J Med Biol Res. 2008 Mar;41(3):223–8. doi: 10.1590/s0100-879x2006005000200. [DOI] [PubMed] [Google Scholar]
- 27.Knodell RG, Ishak KG, Black WC, et al. Hepatology. 1981 Sep–Oct;1(5):431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 28.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 29.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 31.Sterling RK, Lissen E, Clumeck N, et al. Hepatology. 2006 Jun;43(6):1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 32.Alberti A, Clumeck N, Collins S, et al. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005 May;42(5):615–24. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Tien PC. Veterans Affairs Hepatitis C Resource Center Program; National Hepatitis C Program Office Management and treatment of hepatitis C virus infection in HIV-infected adults: recommendations from the Veterans Affairs Hepatitis C Resource Center Program and National Hepatitis C Program Office. Am J Gastroenterol. 2005 Oct;100(10):2338–54. doi: 10.1111/j.1572-0241.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 34.Sulkowski MS. J Acquir Immune Defic Syndr. 2007 Jul 1;45(Suppl 2):S36–7. doi: 10.1097/QAI.0b013e318068d0f4. [DOI] [PubMed] [Google Scholar]
- 35.Sterling RK. Clin Infect Dis. 2005 Apr 15;40(Suppl 5):S270–5. doi: 10.1086/427439. [DOI] [PubMed] [Google Scholar]
- 36.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. N Engl J Med. 2004 Jul 29;351(5):438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 37.Chung RT, Andersen J, Volberding P, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]