Abstract
Poets and philosophers have long acknowledged moral sentiments as key motivators of human social behavior. Prosocial sentiments, which include guilt, pity and embarrassment, enable us to care about others and to be concerned about our mistakes. Functional imaging studies have implicated frontopolar, ventromedial frontal and basal forebrain regions in the experience of prosocial sentiments. Patients with lesions of the frontopolar and ventromedial frontal areas were observed to behave inappropriately and less prosocially, which could be attributed to a generalized emotional blunting. Direct experimental evidence for brain regions distinctively associated with moral sentiment impairments is lacking, however. We investigated this issue in patients with the behavioral variant of frontotemporal dementia, a disorder in which early and selective impairments of social conduct are consistently observed. Using a novel moral sentiment task, we show that the degree of impairment of prosocial sentiments is associated with the degree of damage to frontopolar cortex and septal area, as assessed with 18-Fluoro-Deoxy-Glucose-Positron Emission Tomography, an established measure of neurodegenerative damage. This effect was dissociable from impairment of other-critical feelings (anger and disgust), which was in turn associated with dorsomedial prefrontal and amygdala dysfunction. Our findings suggest a critical role of the frontopolar cortex and septal region in enabling prosocial sentiments, a fundamental component of moral conscience.
Keywords: frontopolar cortex, prefrontal cortex, subgenual, septal area, amygdala, orbitofrontal cortex, moral sentiment, emotion
1. Introduction
Moral sentiments are vital ingredients of moral conscience (Smith, 1759/1966; Frank, 1988). Guilt, pity, and embarrassment, in pa rticular, are known as prosocial sentiments because they induce people to seek forgiveness following misbehavior, to comfort those who suffer and to abide to social standards (Eisenberg, 2000) (see Supplementary Information). The dominant role of prosocial sentiments is vividly illustrated by Hamlet, who ponders whether it would be preferable to die (“not to be”) than to live (“to be”) with his guilty conscience after committing a murder (“to bear the whips and scorns of time”; Shakespeare’s Hamlet, Act III, Sc. I).
Clinical observations suggest that damage to several brain regions, including ventral medial and polar sectors of the prefrontal cortex (PFC) (Eslinger and Damasio, 1985; Koenigs et al., 2007), the anterior temporal lobes (Edwards-Lee et al., 1997; Mendez, 2006; Rankin et al., 2006) and basal forebrain, including the septal area (Moll et al., 2005b), may lead to impairments of moral judgment and conduct. However, direct evidence for the neuroanatomical bases of prosocial sentiments in patients wit h brain lesions is scarce, and very few studies have employed stimulus -response tests in this context (e.g., Krajbich et al., 2009). Inferences about impaired prosocial sentiments have relied on behavioral questionnaires, caregiver ratings, and qualitative observations, which are heavily influenced by the expectations of observers and by the frequency of socially inappropriate behavior during daily activities. Most importantly, studies using caregiver ratings of prosocial sentiments have not reported whether these were impaired relatively to other feelings. Therefore, the purported prosocial impairment in such cases has been interpreted as due to a general (i.e., nonspecific) emotional blunting (Bechara et al., 2000). Naturally, impaired prosocial feelings, as compared to impaired other-critical feelings (e.g., anger, disgust), should be expected to cause severe antisocial behaviors.
Functional magnetic resonance imaging (fMRI) studies in healthy participants have implicated ventral and dorsal sectors of the PFC and the temporal lobes, as well as the amygdala, anterior insula and other subcortical regions, in interpersonal and emotional contexts associated with autonomic arousal, mental state inference, empathic responses and self-other distinction (Berthoz, 2006; Carr et al., 2003; Critchley et al., 2004; Decety et al., 2004; Ochsner et al., 2004; Singer et al., 2004; Lamm et al., 2007). Studies specifically addressing prosocial feelings and behaviors have implicated the medial frontopolar cortex (FPC) and subgenual cortex, as well as the superior temporal sulcus (STS) region and the ventral striatum, septal/basal forebrain area and hypothalamus. These investigations employed different study designs, including social scenarios evocative of guilt, pity and embarrassment (Berthoz et al., 2002; Moll et al., 2002; Moll et al., 2007; Takahashi et al., 2004; Zahn et al., 2009c), charitable donations ( Harbaugh et al., 2007; Hsu et al., 2008; Moll et al., 2006), and economic decisions involving regret, trust and cooperation (Coricelli et al., 2005; Krueger et al., 2007a; Rilling et al., 2002). In contrast, stimuli linked to social and object aversion, such as injustice and gore, typically evoke other-critical feelings (contempt, anger, disgust) (Haidt, 2003; Rozin et al., 1999). Other-critical feelings tend to promote interpersonal aggression and social disengagement (Moll and Schulkin, 2009), and their anatomical bases are more well -established; regions consistently associated with other -critical feelings include the dorsomedial (dmPFC) and lateral sectors of the PFC [especially the lateral orbitofrontal cortex (OFC) and the adjoining agranular insula) and the amygdala (Buckholz et al., 2008; Decety et al., 2004; Mitchell et al., 2006; Moll et al., 2005a; 2007). However, because functional imaging studies in normal individuals are unsuitable to identify which brain regions are necessary for a given cognitive ability (Price et al., 1999), studies in patients with brain lesions are indispensable for establishing which brain regions are necessary for representing prosocial and other-critical sentiments.
We investigated the neural basis of impaired prosocial and other-critical sentiments in 21 patients with the behavioral variant of frontotemporal dementia (bvFTD) and 30 normal controls with a novel experimental test (the Moral Sentiment Task) and resting -state 18-Fluoro-Deoxy-Glucose Positron Emission Tomography (FDG -PET). BvFTD is characterized by progressive frontotemporal neurodegeneration and early social impairments (Neary et al., 1998). Inappropriate social behavior and feelings in patients with bvFTD have been associated with ventral, dorsomedial frontal and anterior temporal lobe atrophy (Liu et al., 2004; Rosen et al., 2002; Snowden et al., 2001; Williams et al, 2005). BvFTD offers a unique opportunity to investigate which regions are specific for enabling prosocial sentiments due to differences in regional distribution of neuropathology. We hypothesized that the degree of impairment of prosocial feelings would be associated with the degree of damage to frontopolar, ventral frontal and basal forebrain regions, and that these effects would be at least partially dissociable from the brain networks enabling general emotional arousal or the other-critical emotions (anger/disgust). Although both prosocial and other-critical sentiments were investigated, the main focus of the present analyses was on prosocial sentiments because (1) their neuroanatomical bases have been much less explored by both functional imaging and lesion studies, and (2) reduced prosocial feelings most likely play a crucial role in explaining callous and non-empathic behaviors in brain-damaged patients.
2. Material and methods
2.1. Patients and controls
Patients were referred by specialists to participate in a larger observational study at the clinical center of the National Institutes of Health (NIH) intramural program in Bethesda, Maryland, USA. Thirty-one consecutive patients with a clinical diagnosis of bvFTD were enrolled in the study. The diagnosis of bvFTD was made on the basis of clinical and neuropsychological data, as well as on visual inspection of MRI and FDG-PET, following the consensus criteria of the Lund-Manchester Group (Neary et al., 1998) and the Work Group on Frontotemporal Dementia and Pick’s Disease (McKhann et al., 2001). Dementia severity was assessed with the Mattis Dementia Rating Scale -2, a reliable and valid measure of global cognitive dysfunction (Kertesz et al., 2003). Normal controls were also evaluated with the Mattis scale in or der to rule out cognitive impairment. Patients stayed at NIH for one week and were accompanied by their main caregiver (for further details on the patient cohort see Zahn et al., 2009b). They were clinically assessed by a senior neurologist and a senior n europsychologist (J.G.) and underwent extensive neuropsychological testing as well as MRI and resting FDG -PET. General inclusion criteria were strong right -handedness and English as first language. The leading symptoms of bvFTD were progressive behavioral abnormalities with an early loss of insight as noted by caregivers. All patients showed abnormalities within frontotemporal areas on visual inspection of FDG -PET, MRI, or both. Substance abuse was ruled out by a negative history antedating the onset of the behavioral changes and by close monitoring over the one week period of the study. Ten patients were excluded prior to statistical analysis due to technical problems with imaging (n = 8) or MST (n = 2) data. The final bvFTD sample therefore included 15 men and 6 women (age = 60.6 ± 8.0 years; education = 15.4 ± 3.2 years). Thirty age- and education-matched volunteers (15 men) served as controls for standardization of the MST (age: 61.5 ± 8.5 years; education: 16.9 ± 2.6 years). FDG-PET scans were obtained from 12 controls matched in gender, age and education. All controls had a normal neurological examination and no history of psychiatric or neurological disorders. Controls were compensated for participation according to NIH standards. Written informed consent was obtained from all participants according to the NINDS Institutional Review Board procedures.
2.2. Moral sentiment task
The MST (obtainable from the first author [ jorge.moll@idor.org] upon request) was designed as a self-paced computerized test programmed on Experimental Run Time System (Berisoft Cooperation, Germany, http://www.erts.de). Ninety-eight written scenarios (“stimuli”) were presented, one at a time, at the top of a screen for participants to decide which of four words at the bottom (choice options) best described the feeling they would experience in that given situation. Each stimulus was associated with a “target”, based on normative data from controls. Targets included the following classes of sentiments: guilt, pity and embarrassment (prosocial sentiments), anger and disgust (other-critical sentiments), fear, and neutral (Figure 1). A previous study on moral sentiments showed these stimuli to be powerful elicitors of experienced moral sentiments (Moll et al., 2007). Non-target feelings belonged to the same list of targets. All items were presented in fixed randomized order (14 stimuli for each sentiment plus 14 neutral stimuli). Colors and positions were counterbalanced across the different sentiments. A NEUTRAL tag was present in each trial as one of the four options. Instructions for the MST are provided in the Supplementary Methods.
Figure 1.
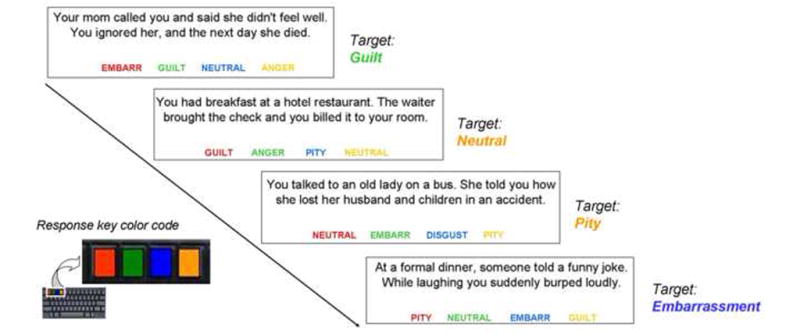
Moral sentiment task design. Participants viewed 98 social scenarios on the screen, together with response options: the target (correct choice, based on normative data) and three non-targets (“distracters”). They were told to report what they would feel in each given situation. A fixed color coding of keyboard keys and response options helped to minimize task-related visuomotor load. The position of the target word option was randomized across trials. The neutral option (with equal frequency as the other options) was present in all trials. Any given word could be the target on each trial with equal probability. The scenario examples do not include samples from all experimental conditions.
The accuracy of each stimulus category was assessed with an index score which penalized scores for a given category in case of omission or commission “errors” (these errors are statistical deviations from the healthy control group norm, being otherwise compatible with a patient’s subjective experience). To that aim, we first computed the number of correct choices for a given category (e.g., number of times “GUILT” was assigned to a scenario that is known to be consistently associated with guilt in controls) and then weighted this result by the inverse of the number of times that same option was assigned to a wrong category (e.g., number of times GUILT was erroneously associated with a scenario belonging to a different class) plus one (to avoid division by zero). For example, if a participant chose GUILT appropriately in 12 out of the 14 guilt scenarios, and mistakenly chose GUILT instead of the correct target in two other scenario categories, her score would be 12/(2 + 1) = 4. These raw scores were z-normalized for the whole sample (21 bvFTD patients and 30 controls). The normalized scores for guilt, pity and embarrassment were averaged to generate a composite prosocial sentiment score for each subject. Normalized scores for anger and disgust were averaged to generate a composite other-critical score for each subject. This was done to increase the power of the measures, based on psychological and functional imaging evidence that prosocial and other-critical sentiments rely on partially separate neural networks, which are nonetheless consistent among feelings belonging to each of these categories (Moll et al., 2007). Fear was left out of the analyses because (1) fearful stimuli in our task did not invoke social fear, (2) current theoretical models of other-critical emotions include anger and disgust (Haidt et al., 2003; Rozin et al., 1999), and (3) we had previous evidence from social script studies linking the neural representations of anger and disgust, but not fear (Moll et al., 2005, 2007). Mean number of words and characters per statement were equivalent for the prosocial and other-critical categories (respectively: mean word count = 20.0 vs. 20.1, t = – 0.14, p = 0.89; mean character count = 83.9 vs. 84.8, t = – 1.01, p = 0.31). Mean correct category assignment of social scenarios by controls (0.89 ± 0.12) was similar to our previous study on normal controls (Moll et al., 2007). Controls scored the prosocial and other-critical categories with comparable accuracy, indicating that these categories were matched in difficulty (t = 0.36, p = 0.74).
2.3. Behavioral data analysis
Neuropsychological data were analyzed with SPSS v. 16 (www.spss.com) and R v. 2.8.0 (www.r-project.org). The z-normalized scores on prosocial and other-critical sentiments from patients and controls were assessed by Welch t-tests, with the alpha level set to p = 0.05. All variables were assessed for multicollinearity (see Supplementary Methods). Two-tailed significances were adopted for all analyses. Clinical and behavioral symptoms were assessed with standard instruments (for further details, see Zahn et al., 2009b).
2.4. Image acquisition and analysis
2.4.1. Image acquisition
Structural MR imaging was performed on a General Electric 1.5 Tesla scanner (3D-T1-SPGR sequence, 120 contiguous slices, slice thickness = 1.5 mm, in-plane resolution = 0.9375 mm × 0.9375 mm, flip angle = 20°). All FDG -PET scans were acquired with a GE Advance PET Scanner. Subjects were fasting since midnight before the scan and had no caffeine, alcohol, or nicotine for 24 hours before the scan. An arterial line was inserted for arterial blood sampling. The subjects were given an intravenous injection of 5 millicuries of FDG while they wore eye patches and ear plugs. Starting with the time of injection and continuing through the cerebral uptake period and subsequent scan, 25 arterial blood specimens were taken at fixed intervals for assay of plasma radioactivity and glucose content. A transmission scan was used to correct the emission data. At the end of the 45 minute uptake period, the emission PET scan was performed (15 minutes for one set of 35 brain slices).
2.4.2. Imaging analysis
Imaging data were analyzed using Statistical Parametric Mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm/software/spm5 ). The following preprocessing steps were applied: normalization with 2x2x2 mm (8 mm 3) voxel size and smoothing (FWHM = 12 mm). Normalization was performed by first normalizing the 3D MRI using the SPM5 T1- template and then applying the same transformation to the FDG-PET images. Global normalization was performed using cerebellar metabolism as a scaling factor. Three statistical models were used to assess inter-group and intra-group PET metabolism differences. In the first model, bvFTD and controls were entered as categorical variables and t-tests were used to compare the regional metabolism between groups (voxel-level significance threshold: p < 0.001, uncorrected). This statistical map was used as an inclusive mask for the subsequent parametric analysis, to minimize the number of comparisons over the whole brain. The second model (parametric) employed multiple regression (SPM5 design matrix) to probe the association between regional cerebral metabolism in bvFTD with (i) prosocial scores (adjusting for other -critical and dementia severity scores) and (ii) other-critical scores (adjusting for prosocial and dementia severity scores). The relationship between metabolism and the combined (additive) effects of prosocial and other-critical scores was also investigated (see Supplementary Results). An additional analysis explored the correlations between FPC and septal metabolism with guilt, pity and embarrassment separately, controlling for dementia severity.
We report only areas surviving a family-wise error (FWE) corrected threshold of p = 0.05 either across the whole brain or across the following regions of interest defined a priori on the basis of coordinates from previous studies and used as the center of spheres (see Supplementary Methods for details on the regions of interest). For the purpose of visual displays only, a threshold of p = 0.05, uncorrected, and a cluster threshold = 20 voxels was employed.
2.4.3. Anatomical localization
Localization was performed using anatomical landmarks and by looking at activations in the Montreal Neurological Imaging (MNI) space projected onto a standard MNI template. Talairach transformed coordinates using Brett’s formula (http://www.mrc-cbu.cam.ac.uk/Imaging/common/mnispace.shtml ) were used to identify corresponding Brodmann areas (BA) using the Talairach Daemon software version 2 (http://ric.uthscsa.edu/projects/talairachdaemon.html ). All coordinates are in Montreal Neurological Institute Standard Space. MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/) was used to display statistical masks overlaid on a high-resolution T1-weighted MRI template (Rorden and Brett, 2000).
3. Results
3.1. Behavioral results
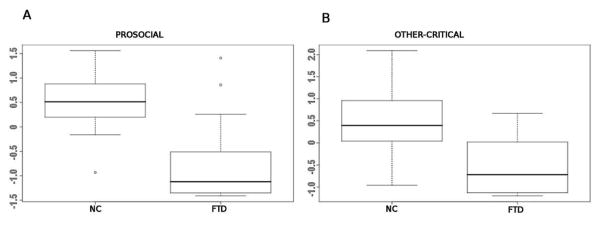
As expected, bvFTD patients showed pronouncedly reduced prosocial sentiment scores as compared with controls (mean ± standard deviation (SD): −0.76 ± 0.82 vs. 0.52 ± 0.49, t = 6.4, p < 0.000001; Figure 2a). Patients also showed reduced other-critical scores (combined anger and disgust scores) compared with controls (−0.54 ± 0.61 vs. 0.47 ± 0.71, t = 5.4, p < 0.00001; Figure 2b). These results indicate that, as a group, patients were markedly impaired both on prosocial and other -critical sentiments.
Figure 2.
(a) Box plots showing normalized scores for prosocial sentiments, compared between bvFTD patients and controls. Patients with bvFTD were significantly impaired in prosocial sentiments. (b) Box plots showing normalized scores for other -critical sentiments, compared between bvFTD and controls. Patients with bvFTD were also significantly impaired in other-critical sentiments.
3.2. Neuroimaging results
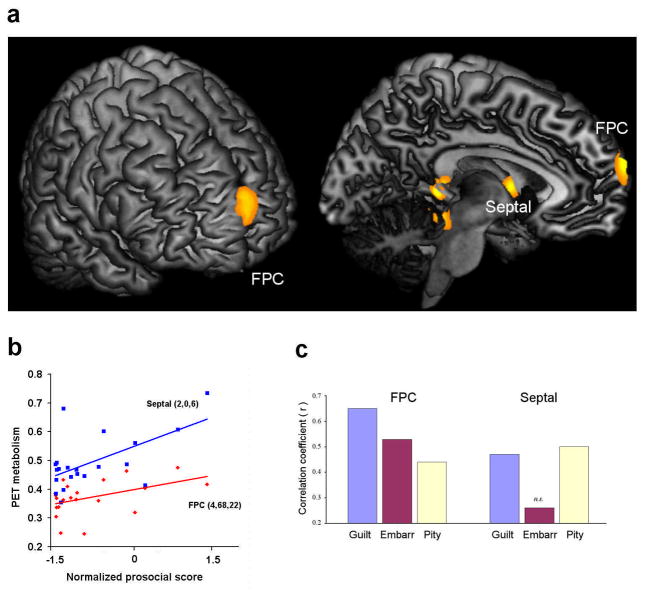
Patients showed the expected frontotemporal and subcortical hypometabolism in comparison to controls (see supplementary information). However, our main analyses aimed at exploring whether regional brain dysfunction (FDG -PET regional hypometabolism) would be parametrically associated with impaired performance on the MST within the bvFTD group. For this purpose, a multiple regression design was first employed within the patient group to explore the effects of regional hypometabolism on prosocial sentiment impairment, controlling for other -critical scores and dementia severity. Lower scores on prosocial sentiments were associated with hypometabolism in the medial FPC [Brodmann’s area (BA) 10], mainly in the right hemisphere, and in the septal area (Figure 3a,b and Table 1), supporting the prediction that these regions are necessary and selective for prosocial sentiments.
Figure 3.
(a) Areas of PET hypometabolism associated with reduced prosocial sentiments in bvFTD patients (adjusted for dementia severity and other -critical feelings). The FPC and the septal area were the only regions surviving FWE - correction over the whole-brain or a priori ROIs. Statistical maps are overlaid onto an anatomical template, with display threshold of uncorrected p < 0.05, k ≥ 20 voxels for visualization purposes. (b) Linear regression plots (not adjusted for dementia severity and other -critical sentiments) of regional PET metabolism and prosocial scores for the FPC ( r = 0.45, p = 0.041) and septal area (r = 0.60, p = 0.004). These scatter plots are for the purpose of display only, and were not the basis of any inferences in this study. (c) Correlations between FPC and septal metabolism with guilt, pity and embarrassment scores. FPC metabolism was significantly correlated with guilt (r = 0.65, p = 0.002), pity (r = 0.44, p = 0.052) and embarrassment (r = 0.53, p = .016). Septal metabolism was significantly correlated with guilt (r = 0.46, p = 0.039) and pity (r = 0.50, p = 0.025), but not with embarrassment (r = 0.26, p = 0.26).
Table. Effects of regional hypometabolism on prosocial sentiment impairment.
Relationship between regional PET hypometabolism and impaired prosocial or other-critical sentiments were assessed using multiple regression analyses. Prosocial effects were evaluated by entering prosocial scores as the variable of interest, and other -critical and dementia scores as covariates. Other-critical effects were evaluated by entering other -critical scores as the variable of interest, and prosocial and dementia scores as covariates. Results of FWE-correction over a priori regions of interest defined on the basis of previous studies are reported (see Methods).
| Cluster (k) | p (FWE-cor) | T | p (unc) | MNI local maxima (x, y, z) | |||
|---|---|---|---|---|---|---|---|
| Prosocial | |||||||
| FPC (BA 10) | 51 | 0.009 | 3.71 | 0.001 | 4 | 68 | 22 |
| Septal region | 23 | 0.047 | 2.27 | 0.018 | 2 | 0 | 6 |
| Other-critical | |||||||
| dmPFC (BA 8/9) | 38 | 0.016 | 3.38 | 0.002 | 4 | 48 | 40 |
| Amygdala | 34 | 0.022 | 2.82 | 0.006 | 20 | 6 | −18 |
One related issue is whether the above brain regions are equally important for guilt, pity and embarrassment, as emphasized by recent fMRI studies (Moll et al., 2007). As a further differentiation of the PFC, the FPC performs an important role in the prospective assessments and valuation of social choices (Christoff and Gabrieli, 2000; Moll et al., 2007), key abilities for the successful engagement of prosocial behaviors (Moll et al., 2006). The septal area, in turn, is believed to be involved in social attachment and empathic concern for others (Insel and Young, 2001; Moll et al., 2006), which are important for feelings of guilt and pity, but not for embarrassment, a prosocial sentiment that it is associated with concerns about one’s own self -image related to violations of social conventions (Einsenberg, 2000). This aspect was investigated by computing the correlations between FPC and septal metabolism with guilt, pity and embarrassment separately, controlling for dementia severity. Individual scores on each of these three sentiments were significantly correlated with the degree of FPC hypometabolism. Hypometabolism in the septal area was significantly correlated with impaired guilt and pity, but not with embarrassment (Figure 3c).
Next, the relationship between brain hypometabolism and other-critical sentiments were explored with multiple regression, entering prosocial scores and dementia severity as control variables. Lower other-critical scores were associated with reduced metabolism in two predicted frontal and limbic regions, the dmPFC (mainly in the right hemisphere; BA 8/9; p = 0.016, FWE-corrected) and the right amygdala (p = 0.022, FWE-corrected) (Figure 4, Table 1). Hypometabolism in the lateral OFC/anterior insula correlated both with other-critical and prosocial scores (Supplementary Figure 2). These effects support the notion that the dmPFC and amygdala play an important role in social aversion (Buckoltz et al., 2008; Decety et al., 2004; Moll et al., 2005a; Sanfey et al., 2003).
Figure 4.
(a) Areas of PET hypometabolism associated with reduced other -critical sentiments (adjusted for dementia severity and prosocial sentiments). The right amygdala and the dmPFC were the only regions surviving FWE -corrected p = 0.05 over the whole-brain or a priori ROIs. Statistical maps are overlaid onto an anatomical template, with display threshold of uncorrected p < 0.05, k ≥ 20 voxels for visualization purposes. (b) Linear regression plots (not adjusted for dementia severity and prosocial sentiments) of regional PET metabolism and other-critical scores for the dmPFC (r = 0.54, p = 0.011) and right amygdala (r = 0.50, p = 0.022). These scatter plots are for the purpose of display only, and were not the basis of any inferences in this study.
4. Discussion
The main finding of this investigation was that the resting PET hypometabolism, a sensitive marker of neurodegeneration (Kanda et al., 2008), in the medial FPC and in the septal area was associated with the level of prosocial sentiment impairment in patients with bvFTD. The specific role of the FPC and septa l area in this regard was further corroborated by the finding that decreased other-critical scores resulted from hypometabolism in distinct PFC and subcortical regions, namely the dmPFC and the right amygdala.
4.1. The role of the frontopolar cortex in prosocial sentiments
The role of FPC in social cognition has received growing attention (Christoff and Gabrieli, 2000; Heekeren et al., 2003; Greene et al., 2004; Wood and Grafman, 2006; Krueger et al., 2007b; Young et al., 2007). Remarkably, virtually all fMRI studies addressing neural responses to moral judgments (Moll et al., 2005b) and to social scenarios evocative of guilt, pity and embarrassment (Moll et al., 2007; Zahn et al., 2009c) found medial FPC activation. Furthermore, the FPC has been implicated in decisions involving costly punishment (de Quervain et al., 2004) and donation (Moll et al., 2006), as well as in inferring the emotional states of others (Amodio and Frith, 2006). Although the FPC is often affected in cases of vmPFC damage (Eslinger and Damasio, 1985; Koenigs and Tranel, 2007), few lesion studies investigated its role in the genesis of social and emotional impairments (Hornak et al., 2003; Moll and de Oliveira-Souza, 2007). A recent study on a similar patient group indicated reduced behavioral responses to embarrassment, but not to startle (Sturm et al., 2006). In addition, another recent study engaged six patients with vmPFC lesions in different economic decision tasks (dictator, ultimatum and trust games) and demonstrated that, relative to control subjects, they donated less, were less trustworthy and relatively insensitive to guilt (Krajbich et al., 2009). It should be emphasized that PFC lesions in Krajbich et al.’s study ranged from the vmPFC proper to the medial FPC in virtually all cases, as judged by visual inspection of the lesion overlap maps. Our study thus helps establish the FPC as an important neural component for enabling prosocial sentiments, including guilt, pity and embarrassment, and extends previous findings by improving anatomical localization of these effects within the PFC.
4.2. Social affiliation and the septal area
In addition to the FPC, we found a decrease in septal hypometabolism with increasing impairments on prosocial sentiments. Anatomically, the septal region contains the septum pellucidum, composed of thin layers of fibrous tissue and slender fascicles, and the septum verum, a collection of eleven small nuclei located anteriorly and inferiorly to the septum pellucidum (Andy and Stephan, 1968). Contrary to the notion that limbic structures are evolutionarily ancient or vestigial in humans (MacLean, 1973), the septal nuclei may actually have increased in size and complexity during hominin phylogenesis (Andy and Stephan, 1968; Joseph, 1996). The role of the septal area in social behavior is highlighted by the fact that septal damage can produce drastic behavioral abnormalities, leading to predatory aggression and changes in sexual behavior in humans and animals (Gorman and Cummings, 1992). Stimulation of this region, in contrast, induces pleasurable experiences and decreases intraspecies aggression (Bishop et al., 1963; Irvin et al., 1990). Some researchers have proposed that septal dysfunction may underlie the lack of empathy, guilt and remorse that is typically ob served in psychopathy (Gorenstein and Newman, 1980). The septal region and adjacent subgenual cingulate, preoptic and ventromedial hypothalamic areas are the main binding sites for oxytocin and vasopressin, which play central roles in social bonding (Insel and Young, 2001). Functional imaging studies looking at mother-offspring and romantic attachment, charitable donations and economic cooperation based on trust (Bartels and Zeki, 2004; Harbaugh et al., 2007; Hsu et al., 2008; Krueger et al., 2007; Moll et al., 2006; Mobbs et al., 2009) found activation in the septal region. The putative role of the septal area in enabling prosocial sentiments has so far remained under-recognized, however. Our finding that septal hypometabolism is associated with reduced guilt and pity supports a mutual role of the septal area and the FPC in prosocial behaviors, including empathic concern, altruism and related affiliative inclinations.
Previous fMRI studies implicate additional brain regions in prosocial sentiments, especially the subgenual cortex (Hsu et al., 2008; Moll et al., 2006; Zahn et al., 2009c). Our prediction that subgenual neurodegeneration would be associated with prosocial sentiment impairment was not confirmed, however. It is possible that the subgenual cortex plays a more elaborated role in prosocial sentiments, a notion supported by the role of the subgenual cortex in the pathophysiology of major depression (Drevets et al., 1998), decisions involving altruistic donations (Moll et al., 2006) and coding for individual differences in guilt-proneness and empathic concern (Zahn et al., 2009a).
4.3. Brain regions mediating other-critical feelings
In contrast to our findings regarding prosocial sentiments, hypometabolism in the right amygdala and dmPFC was associated with reduced other-critical scores. The amygdala, dmPFC (and neighboring paracingulate cortex) and lateral OFC/adjoining agranular anterior insula have been consistently implicated in imaging studies on the mechanisms of social punishment, social response reversal, racial bias, and competition (Decety et al., 2004; Kringelbach and Rolls, 2003; Lieberman et al., 2005; Moll et al., 2005a; Sanfey et al., 2003; Singer et al., 2006). A shared feature of these conditions is that they all involve some kind of external aversive signaling, inducing an agent to act upon the behavior of another agent or object (e.g., through punishment). Our data show that the right amygdala and dmPFC are necessary for appropriate other -critical sentiments.
While the role of the amygdala in other-critical sentiments is supported by several studies on social disapproval, prejudice and lack of trust (Calder et al., 2001; Lieberman et al., 2005; Sanfey et al., 2003), the theoretical basis for the association of dmPFC hypometabolism with impaired other-critical feelings is uncertain. fMRI studies found activity in this region in response to social feedback and when evaluating others, mainly when “others” are categorized as members of an out-group or dissimilar (Mitchell et al., 2006; Schiller et al., 2009; Takahashi et al., 2009). A recent fMRI study using scenarios similar to the ones herein employed showed a remarkable agreement with our findings: whereas the dmPFC showed increased responses to “other-anger”, the more anterior FPC responded more strongly to guilt and compassion or pity (Kédia et al., 2008). Nonetheless, lesion studies investigating the impact of dmPFC damage on moral cognition and emotion are lacking. One interesting possibility is whether other -critical sentiments could be reduced by modulating dmPFC activity, a finding that could have potential therapeutic implications for managing aggressive and antisocial behaviors. A notable exception for the brain regions predicted to be selectively associated with other-critical scores was the lateral OFC/anterior insula. Instead, we found that hypometabolism in this region was strongly but similarly associated with other -critical and with prosocial impairments. One possible explanation is that the lateral OFC/anterior insula are involved with more general aspects of behavioral reinforcement, response reversal and unpleasantness in social contexts, such as when observing other’s pain (Decety et al., 2004; Kringelbach and Rolls, 2003; Lamm et al., 2007; Singer et al., 2006), which might be important not only for other-critical feelings but also for certain aspects of prosocial sentiments (e.g., restraining from an action that could evoke feelings of guilt or embarrassment).
4.4. Emotional dysfunction and PFC-subcortical damage
Our results did not support the alternative hypothesis that the association between FPC-septal hypometabolism and reduced prosocial sentiments emerged from an overall emotional blunting in bvFTD because this association remained robust after controlling for other-critical ratings. This is in keeping with the hypothesized role of specific PFC and subcortical sub-regions in enabling prosocial sentiments (Moll and de Oliveira -Souza, 2007). Studies on patients with rostral ventromedial lesions, which often encompass the FPC, speak for a complex interplay of emotional impairments: while these patients have an increased preference for “utilitarian” choices in moral dilemmas (Koenigs et al., 2007), indicating reduced emotionality, they show increased costly punishment of non-cooperators in an economic game (Koenigs and Tranel, 2007), suggestive of heightened emotionality. On a more cautionary note, although the observed relationships between impairments of feelings and localized metabolic dysfunction go a step beyond fMRI evidence by implying possible causal effects, these associations are still correlational in nature and future prospective studies are needed to further support causal inferences. Taken together, these results support a functional subspecialization of PFC-subcortical regions in enabling distinct aspects of prosocial and other -critical subjective emotional states and behavioral inclinations.
5. Conclusions
The FPC and the septal area are necessary for prosocial sentiments. These effects are likely selective, because other-critical impairments seem to be associated with a distinct pattern of regional dysfunction affecting the dmPFC and the right amygdala. These findings are in line with the role of prosocial sentiments as the affective hub of moral conscience, given the prominence of their association with the FPC and the septal area — brain regions that have shown both allometric and non-allometric increases in humans (Stephan, 1983).
Supplementary Material
Acknowledgments
This study was supported by NINDS intramural funding to JG an d in part by the D’Or Institute for Research and Education, Rio de Janeiro, Brazil, to JM, RO -S, IEB and BT. RZ was supported by a Stepping Stone Fellowship at The University of Manchester, UK. We thank Eric Wassermann for the neurological exams, Kris Knutson and Griselda Garrido for imaging analysis advice, Michael Tierney and Karen DeTucci for testing patients, and Vijeth Iyengar for help with data pre -processing and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andy OJ, Stephan H. The septum in the human brain. J Comp Neurol. 1968;133:383 –410. doi: 10.1002/cne.901330308. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJR, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696 –1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Grezes J, Armony JL, Passingham RE, Dolan RJ. Affective response to one’s own moral violations. Neuroimage. 2006;31:945 –950. doi: 10.1016/j.neuroimage.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Bishop MP, Elder ST, Heath RG. Intracranial self -stimulation in man. Science. 1963;140:394–396. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Asplund CL, Dux PE, Zald DH, Gore JC, Jones OD, Marois R. The neural correlates of third-party punishment. Neuron. 2008;60:930–940. doi: 10.1016/j.neuron.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Lawrence AD, Young AW. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M-C, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8:1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189 –195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL, Sommerville JA, Chaminade T, Meltzoff AN. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage. 2004;23:744–745. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ-F, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–326. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, Mena I. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027 –1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annu Rev Psychol. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731 –1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Frank RH. Passions Within Reason: The Strategic Role of the Emotions. New York: WW Norton & Co; 1998. [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: a new perspective and a model for research. Psychol Rev. 1980;87:301 –315. [PubMed] [Google Scholar]
- Gorman DG, Cummings JL. Hypersexuality following septal injury. Arch Neurol. 1992;49:308–310. doi: 10.1001/archneur.1992.00530270128029. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Haidt J. In: The moral emotions. Handbook of Affective Sciences. Davidson RJ, Scherer KR, Goldsmith HH, editors. Oxford: Oxford University Press; 2003. pp. 852–870. [Google Scholar]
- Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622 –1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. Neuroreport. 2003;1:14(9):1215–1219. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092 –1095. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129 –136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693 –699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- Joseph R. Neuropsychiatry, Neuropsychology and Clinical Neuroscience. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- Kanda T, Ishii K, Uemura T, Miyamoto N, Yoshikawa T, Kono AK, Mori E. Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG -PET and voxel-based morphometric MR studies. Eur J Nucl Med Mol Imaging. 2008;35:2227 –2234. doi: 10.1007/s00259-008-0871-5. [DOI] [PubMed] [Google Scholar]
- Kédia G, Berthoz S, Wessa M, Hilton D, Martinot J-L. An agent harms a victim: a functional magnetic resonance imaging study on specific moral emotions. J Cogn Neurosci. 2008;20:1788–1798. doi: 10.1162/jocn.2008.20070. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, McCabe P, Munoz D. Behavioral quantitation is more sensitive than cognitive testing in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2003;17(4):223–229. doi: 10.1097/00002093-200310000-00005. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision -making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951 –956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio AR. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. J Neurosci. 2009;29(7):2188–2192. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20:1371 –1383. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, Heinecke A, Grafman J. Neural correlates of trust. Proc Natl Acad Sci USA 2007a. 104:20084–20089. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Moll J, Zahn R, Heinecke A, Grafman J. Event frequency modulates the processing of daily life activities in human medial prefrontal cortex. Cereb Cortex. 2007b;17:2346–2353. doi: 10.1093/cercor/bhl143. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Hariri A, Jarcho JM, Eisenberger NI, Bookheimer SY. An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nat Neurosci. 2003;8:720 –722. doi: 10.1038/nn1465. [DOI] [PubMed] [Google Scholar]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss -Coray C, Gearhart R, Phengrasamy L, Weiner M, Rosen HJ. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology. 2004;62:742 –748. doi: 10.1212/01.wnl.0000113729.77161.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean P. A Triune Concept of the Brain and Behavior. Oxford: University of Toronto Press; 1973. [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the work group on frontotemporal dementia and Pick’s disease. Arch Neurol. 2001;58:1803 –1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mendez MF. What frontotemporal dementia reveals about the neurobiological basis of morality. Med Hypotheses. 2006;67:411–418. doi: 10.1016/j.mehy.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655 –663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, Passamonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R. Moral judgments, emotions and the utilitarian brain. Trends Cogn Sci. 2007;11:319–321. doi: 10.1016/j.tics.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger P, Bramati I, Miranda J, Andreiuolo P. The neural correlates of moral sensitivity: A functional magnetic resonance imaging investigation of basic and moral emotions. J Neurosci. 2002;22:2370 –2736. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Garrido GJ, Bramati IE, Caparelli -Dáquer EMA, Paiva MLMF, Zahn R, Grafman J. The self as a moral agent: linking the neural bases of social agency and moral sensitivity. Soc Neurosci. 2007;2:336–352. doi: 10.1080/17470910701392024. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Tovar-Moll F, Ignácio FA, Bramati IE, Caparelli-Dáquer EMA, Eslinger PJ. The moral affiliations of disgust: A functional MRI study. Cog n Behav Neurol. 2005a;18:68–78. doi: 10.1097/01.wnn.0000152236.46475.a7. [DOI] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira -Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Schulkin J. Social attachment and aversion in human moral cognition. Neurosci Biobehav Rev. 2009;33:456–465. doi: 10.1016/j.neubiorev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nat Rev Neurosci. 2005b;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546 –1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746 –1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frakowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945 –2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman DA, Zeh TR, Pagnoni G, Berns GS, Kilts CD. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191 –200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno -Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612 –2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Lowery L, Imada S, Haidt J. The CAD triad hypothesis: a mapping between three moral emotions (contempt, anger, disgust) and three moral codes (community, autonomy, divinity) J Pers Soc Psychol. 1999;76:574–586. doi: 10.1037//0022-3514.76.4.574. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755 –1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schiller D, Freeman JB, Mitchell JP, Uleman JS, Phelps EA. A neural mechanism of first impressions. Nat Neurosci. 2009;12:508–514. doi: 10.1038/nn.2278. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466 –469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. The Theory of Moral Sentiments. New York: Kelly; 1759/1966. [Google Scholar]
- Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–332. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan H. Evolutionary trends in limbic structures. Neurosci Biobehav Rev. 1983;7:367 –374. doi: 10.1016/0149-7634(83)90041-6. [DOI] [PubMed] [Google Scholar]
- Sturm VE, Rosen HJ, Allison S, Miller BL, Levenson RW. Self -conscious emotion deficits in frontotemporal lobar degeneration. Brain. 2006;129:2508 –2516. doi: 10.1093/brain/awl145. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and Schadenfreude. Science. 2009;323:937–939. doi: 10.1126/science.1165604. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–974. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. Neuroimage. 2005;24:1042 –1051. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Wood JN, Grafman J. Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci. 2003;4:139–147. doi: 10.1038/nrn1033. [DOI] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proc Natl Acad Sci USA. 2007;104:8235 –8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, de Oliveira-Souza R, Bramati I, Garrido G, Moll J. Subgenual cingulate activity reflects individual differences in empathic concern. Neurosci Lett. 2009a;457:107–110. doi: 10.1016/j.neulet.2009.03.090. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009b;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey ED, Grafman J. The neural basis of human social values: evidence from functional MRI. Cereb Cortex. 2009c;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.