Abstract
Mammographic breast density is one of the strongest risk factors for breast cancer. Unfortunately, the biologic basis underlying this association is unknown. This study compared aromatase expression or immunoreactivity (IR) in core biopsies from mammographically dense versus non-dense regions of the breast to examine whether estrogen synthesis in the breast is associated with mammographic breast density (MBD) and one possible mechanism through which it may influence breast cancer. Eligible participants were 40+ yrs, had a screening mammogram with visible MBD and no prior cancer or current endocrine therapy. Mammograms were used to identify dense and non-dense regions and ultrasound-guided core biopsies were performed to obtain tissue from these regions. Immunostaining for aromatase employed the streptavidin-biotin amplification method and #677 mouse monoclonal antibody. Aromatase IR was scored in terms of extent and intensity of staining for each cell type (stroma, epithelium, adipocytes) on the histologic section. A modified histological (H)-score provided quantitation of aromatase IR in each cell type and overall. Repeated measures analyses evaluated average differences (βH) in H-score in dense versus non-dense tissue within and across cell types. Forty nine women mean age 50 yrs (range: 40 to 82), participated. Aromatase IR was increased in dense (vs. non-dense) tissue in both the stroma (βH =0.58) and epithelium (βH =0.12) (p<0.01). Adipocytes from non-dense tissue, however, had a greater IR compared to those from dense tissue (βH =-0.24, p<0.01). An overall H-score which integrated results from all cell types demonstrated that aromatase IR was twice as great for dense (mean H-score=0.90, SD=0.53) vs. non-dense (mean H-score=0.45, SD=0.39) breast tissue (βH =0.45; p<0.001). Overall, aromatase IR was greater for mammographically dense vs. non-dense tissue and may partly explain how MBD influences breast cancer.
Keywords: Aromatase, Breast density, Dense area
Introduction
Mammographic breast density (MBD) is one of the strongest known breast cancer risk factors, with 3-5 fold increased risk associated with the highest vs. lowest density categories [1]. While the mechanism through which mammographically dense tissue influences breast cancer risk is not known, MBD has been hypothesized to reflect the influence of estrogens on the breast [2]. This hypothesis is supported by the consistent association of MBD with reproductive factors such as menopause, parity, age at first birth, and exogenous hormones, including postmenopausal hormone therapy (PMH) and tamoxifen [1]. Against this hypothesis are the studies correlating plasma levels of estrogens and MBD with the majority demonstrating little to no correlation [3-5]. In addition, estradiol measured in peripheral blood has been shown to influence the risk of developing breast cancer independently of mammographic density [6].
We have postulated that local production of estrogen in the breast itself may be responsible for breast density and that uptake of estrogens from plasma plays only a minor role. A variety of data support the possibility that local estrogen production is the key mediator of estrogen levels in postmenopausal breast tissue. Levels of estrogen in postmenopausal breast tissue are equivalent to those in pre-menopausal women even though plasma levels are 10-50 fold higher in pre-menopausal women [7]. Miller et al. using elegant radiometric assays have demonstrated that local estrogen synthesis in the breast accounts for a substantial fraction of the estrogen present. Recently, it was shown that 60% of breast tissue estrogen originates from local production whereas 40% results from uptake from plasma [8].
The estrogen synthesized in breast tissue largely results from activity of the aromatase enzyme which converts the major androgens, androstenedione and testosterone to the estrogens, estrone and estradiol. Overexpression of aromatase in mouse models has led to the formation of benign breast tumors [9]. In aromatase transfected MCF-7 breast tumor xenografts, local estrogen production in breast stimulates tumor growth to a greater extent than estrogen taken up from plasma [10]. Aromatase inhibitors (AIs) block local synthesis of estrogen in extracts of human breast tumors [11]. These agents serve as the most efficacious endocrine therapy for estrogen receptor-positive postmenopausal breast cancer [12, 13]. While the precise relationships between tissue estrogen uptake and local synthesis remain unknown, it is clear that local estrogen synthesis occurs and that aberrant local estrogen biosynthesis could affect breast cancer development.
We hypothesized that MBD is strongly influenced by aromatase expression and local estrogen synthesis in cancer-free breast tissue. If true, increased aromatase activity may be one mechanism through which MBD influences breast cancer risk and/or recurrence. We evaluated this hypothesis using core biopsies obtained from dense and non-dense areas of the breasts of healthy women.
Materials and Methods
Study population
Asymptomatic healthy volunteers were recruited through advertisements from 2006-2008 at the Mayo Clinic, Rochester. This study was approved by the Mayo Clinic Rochester Institutional Review Board. Eligible women were aged 40 years or older with no personal history of breast cancer, a normal screening mammogram within six months of biopsy, and mammographically dense and non-dense areas that could be biopsied for the study. Patients were ineligible if they were currently using postmenopausal hormones, oral contraceptives, other endocrine therapy or anticoagulants. Other exclusions included history of bleeding complications or allergy to local anesthetic agents. A total of 206 women inquired about study participation; of these, 126 (61%) were ineligible, 21 (10.2%) expressed interest but did not participate, 3 (1.5%) were scheduled for the biopsy but did not show for their appointment and 56 (27.2%) completed the study. Since our comparisons in the current study required tissue from both dense and non-dense regions in individual participants, 7 participants were excluded who only had cores available from a non-dense (n=6) or dense (n=1) region of their breasts. All volunteers completed a self-administered questionnaire on reproductive and lifestyle factors.
Ascertainment of dense and non-dense cores
Using mammogram films taken within six months, the study radiologist identified areas of high and low density in the right breast, the upper outer quadrant in most cases. If the patient had a previous benign surgical biopsy in the right breast, the left breast was selected for biopsy. The areas identified mammographically as dense and non dense were then localized by ultrasound in a similar fashion to routine clinical practice where mammographic findings are further evaluated with US. Sonographically, the dense tissue selected for biopsy was either homogenously hyperechoic (relative to subcutaneous breast fat) or a heterogenous mixture of hyperechoic tissue and hypoechoic ducts. Sonographically, the non dense tissue selected for biopsy was isoechoic to subcutaneous breast fat. Using a 14-gauge needle, an ultrasound-guided core-needle biopsy was performed in the identified dense and non-dense regions. A biopsy was not done if a mass was identified or if dense tissue was isoechoic to fat preventing the ultrasound localization of the dense area, which did not occur in any of our 49 participants. Four cores were taken from each region; three cores were formalin fixed and embedded in one paraffin block and used for the immunohistochemistry portion of the study; the remaining core was frozen and sectioned for the assessment of aromatase message by PCR. Thirty serial sections were cut from each paraffin-embedded block. Slides (sections 1, 15 and 30) cut from both the dense and non-dense paraffin-embedded blocks were stained with hematoxylin and eosin (H&E) and histologically examined to ensure that sections contained benign tissue.
Determination of cell type
For purposes of determination of proportion of normal stroma, ductal epithelium and adipocytes, two, five micron sections from each of the dense and non-dense paraffin blocks were stained with H&E and evaluated by an expert pathologist. Using a method described previously [14], one pathologist (H.S.) visually estimated the proportion of stroma, normal ductal epithelium and adipocytes on each H&E section using the percentage of area covered in the 10x field of the microscope. Stromal cells were defined histologically as fibroblasts on hematoxylin-eosin stained glass slides. Few foci of inflammatory cells such as lymphocytes and macrophages were identified in these areas. The proportions of stroma and epithelium on each slide were categorized as: <1%, 1-24%, 25-50%, 51%+ cells; an additional category, 76%+ was used for classifying adipocytes. Classification of these proportions was performed by the pathologist independent of aromatase staining in that tissue.
Immunohistochemistry and interpretation
Immunostaining for aromatase immunoreactivity (IR) employed the streptavidin-biotin amplification method using mouse monoclonal antibody #677 generated against native or non-denatured peptides. Briefly, after deparaffinization, sections were washed with PBS and treated with 0.3% hydrogen peroxide in methanol for 20 minutes at room temperature. Normal rabbit serum (1%) was applied to the sections for 20 minutes at room temperature, and then primary antibody was reacted to the tissue sections for 18 hours at 4 °C. Sections were subsequently incubated with biotinylated anti-mouse immunoglobulin for 20 minutes, followed by exposure to peroxidase-conjugated streptavidin for 20 minutes at room temperature. Immunoreactivity was detected by immersing the tissue sections in 3,3′-diaminobenzidine(DAB) solution (1 mM DAB, 50 mM tris-HCl buffer (pH 7.6), 10 mM sodium azide, and 0.006% hydrogen peroxidase). Hematoxylin was used as nuclear counterstain. Negative controls for immunostaining were performed by adding 0.01M PBS instead of primary antibody. No specific immunoreactivity was detected in the control sections.
Subjective scoring of aromatase immunoreactivity was performed with assessment of the percentage or extent of cells that stained positive and the average intensity of staining in the cells of each specific type. The average degree of intensity of staining was categorized as none=0, weak=1, moderate=2, strong=3 and the extent used the categories <1%, 1-24%, 25-50% and 51%+ cells for stromal and epithelial cells and an additional category, 76%+ for adipocytes.
Modified H-score
A modified H-score (histological scoring system) previously described by Santen and colleagues [14] was used to semi-quantitate the amount of aromatase staining from dense and non-dense regions of the breast. Briefly, the H-score represents the product of the degree of intensity of staining, the extent of cells positive for aromatase and the percent of specific cell type in the section. The median of categories for percent cell type and extent of staining was used to calculate this score (i.e. 0.5% for <1%; 12.5% for category 1-24%; 37.5% for category 25-50%, etc.). The global H-score for sections from dense and non-dense regions of the breast represents the sum of individual H-scores for the three tissue types for all 49 women. H-scores were then calculated separately for epithelium, stroma and adipocytes. Analyses within each cell type were only performed for women with sections from both dense and non-dense breasts that had at least 1% cells present for the cell type of interest.
Statistical analysis
The distributions of the proportions of each cell type (stroma, epithelium and fat) on dense and non-dense sections were compared within woman. Signed rank tests were used to determine if there were differences in percent of each cell type by dense and non-dense regions. Primary analyses examined differences in the summary aromatase IR, assessed as the (global) H-score, summed across all cell types. Secondary analyses examined differences in aromatase IR as the H-score, for each of the three cell types, where at least 1% of cells were present on the dense and non-dense paired sections. All analyses were conducted among all women and separately within menopausal subgroups. Linear mixed models were used to evaluate the differences in aromatase IR, including extent and intensity of stain and H-score, in dense vs. non-dense regions. These models included a random per-person intercept term that accounted for the correlations between the regions within a person. Parameter estimates indicated the average difference between dense and non-dense aromatase IR.
We conducted additional analyses to evaluate the potential influence of blinding on our results, or whether our results differed by the proportions of cells of each type on the section. Although efforts were taken, the pathologist cannot be completely blinded to dense or non-dense status of the sections due to the general composition of dense tissue being more likely to be stromal and epithelial cells and non-dense tissue to be adipocytes. If there was evidence of a bias, we would expect the associations observed to be attenuated in samples that had comparable proportions of cells on each section, where it is less likely that the pathologist could easily determine dense and non-dense status and bias the estimate of aromatase IR. Thus, we performed analyses within each cell type for samples with at least 25% cells of each type on both the dense and non-dense sections. Differences in extent, intensity of stain and overall H-score among dense vs. non-dense cells were then estimated.
Results
Participant characteristics are described in Table 1 for the forty nine volunteers with core biopsies from mammographically dense and non-dense tissue. All were Caucasian and both premenopausal (n=20; 59%) and postmenopausal (n=29; 41%) women were represented. Mean age was 50 years (range: 40-82) and BMI was 26.6 (range: 18.8-48.2). The majority of women had never used postmenopausal hormone therapy (76%) but had used oral contraceptives (77%) in the past. A small proportion of participants had breasts that were almost all fat (4% had BI-RADS=1) or extremely dense (4% had BI-RADS=4), since areas of high and low densities were required for the biopsies.
Table 1.
Characteristics of 49 women with dense and non-dense cores
| Characteristic | Category | N (%) or Mean |
|---|---|---|
| Age at biopsy (years) | 40-49 50-59 60-69 70-79 80-89 |
31 (63.3) 10 (20.4) 4 (8.2) 3 (6.1) 1 (2.0) |
| BMI (kg/m2) | 18-24 25-30 31-36 37+ |
22 (44.9) 19 (38.8) 7 (14.3) 1 (2.0) |
| Menopausal Status | Premenopausal Postmenopausal |
20 (41) 29 (59) |
| Parity | Nulliparous 1 2 3+ Missing |
9 (18.4) 4 (8.2) 20 (40.8) 14 (28.6) 2 (4.1) |
| Oral contraceptives | Former Never Missing** |
38 (77) 10 (20) 1 (2) |
| Age at first birth* in years | Mean (SD) | 26.3 (5.9) |
| Postmenopausal Hormones | Former Never Missing** |
11 (22) 37 (76) 1 (2) |
| BI-RADS | 1 2 3 4 |
2( 4) 21 (43) 24 (49) 2 (4) |
Parous women only
Not currently using therapy but unknown whether former or never user.
The proportion of each cell type in the paired dense and non-dense specimens is provided in Table 2. As expected, there was a greater proportion of stroma and epithelium on dense sections compared to non-dense sections and a lesser proportion of adipocytes (Table 2). Considering the stroma, 40/49 (82%) patients had a greater proportion of stroma (at least one category higher) on the dense section than the corresponding non-dense section. Considering the epithelium, 34/49 (69%) patients had greater percent epithelium (at least one category higher) on the dense section relative to the non-dense section. However, 43/49 (88%) patients had greater percent adipocytes on the non-dense section compared to the corresponding dense section. Also, 42 women had at least 1% stromal cells on both paired sections, 20 had at least 1% epithelial cells on both sections and 46 women had at least 1% adipocytes, allowing for analyses of aromatase IR within cell type.
Table 2.
Distribution of the proportion of stroma, epithelium and adipocytes in the dense and non-dense matched core biopsies of the breasts of 49 women
| Cell type | Proportion of cells in Dense Core |
Proportion of Cells in the Non-dense Core |
P* | ||||
|---|---|---|---|---|---|---|---|
| <1% | 1-25% | 26-50% | 51%+ | 76%+ | |||
| Stroma | <1% | 0 | 0 | 0 | 0 | <0.001 | |
| 1-25% | 2 | 1 | 0 | 1 | |||
| 26-50% | 1 | 3 | 2 | 1 | |||
| 51%+ | 4 | 26 | 4 | 4 | |||
| Epithelium | <1% | 2 | 0 | 0 | 0 | <0.001 | |
| 1-25% | 18 | 12 | 0 | 0 | |||
| 26-50% | 9 | 7 | 1 | 0 | |||
| 51%+ | 0 | 0 | 0 | 0 | |||
| Adipocytes | <1% | 0 | 0 | 0 | 1 | 2 | <0.001 |
| 1-25% | 0 | 1 | 4 | 1 | 19 | ||
| 26-50% | 0 | 0 | 1 | 3 | 10 | ||
| 51%-75% | 0 | 0 | 1 | 1 | 3 | ||
| 76%+ | 0 | 0 | 0 | 0 | 2 | ||
NOTE: Italicized numbers for stroma and epithelium reflect the women who have greater stroma and epithelium on dense core compared to non-dense core. Italicized numbers for adipocytes reflect greater proportion of cells on the non-dense vs. dense cores.
P-value from signed rank test examining the proportions of stroma, epithelium and adipocytes in the dense vs. non-dense cores.
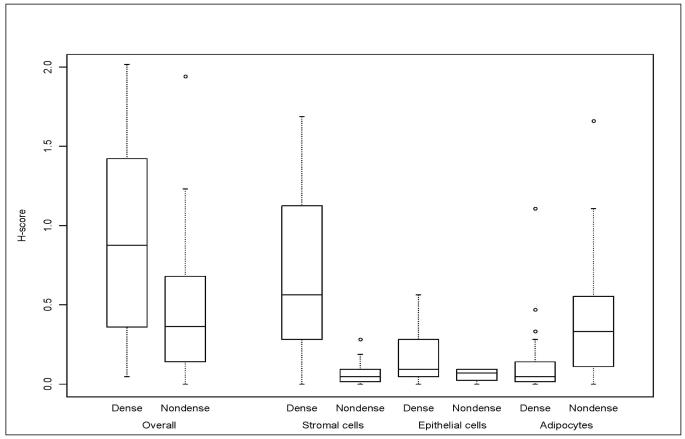
Overall aromatase IR across all cell types was on average two-fold greater in dense (mean global H-score=0.90 (SD=0.53)) vs. non-dense (mean global H-score=0.45 (SD=0.39)) tissue ((βH =0.45, p=0.0003) (Figure 1 and Table 3)). The H-scores corresponding to dense and non-dense tissue for the three cell types are displayed in Figure 1. Aromatase IR was greater among stromal cells on dense vs. non-dense sections, as reflected in the greater extent, intensity of stain and H-score corresponding to dense sections (βH=0.58 (SE=0.07)) (Table 3). Aromatase IR was also greater for epithelial cells from dense vs. non-dense sections (βH=0.12 (SE=0.04); (Table 3; Figure 1)). However, contrary to the findings among stromal and epithelial cells, aromatase IR was lower in adipocytes from dense tissue (βH= −0.24; SE=0.06) (Table 3; Figure 1). Results were similar for premenopausal and postmenopausal subgroups (Supplementary Tables 1a and 1b), although there no longer was statistical significance for differences in aromatase IR among epithelium from dense and non dense tissue for the premenopausal subgroup, likely due to reduced power in this small subset of women.
Fig. 1.
Aromatase immunoreactivity in dense and non-dense sections overall (global H score) and within cell type (H-scores for stroma, epithelium and adipocytes)
Table 3.
Differences in aromatase immunoreactivity in dense vs. non-dense cores across all cell types and within stroma, epithelium and adipoctyes
| N (pairs) |
Dense Mean (SD) |
Non-dense Mean (SD) |
Unadjusted mean difference estimate (SE) |
P | Adjusteda mean difference estimate (SE) |
Adjusted P | |
|---|---|---|---|---|---|---|---|
|
All cell types combined Modified H-scoreb |
49 | 0.90 (0.53) | 0.45 (0.39) | 0.45 (0.08) | <0.01 | ------ | ------ |
| Stroma | 42 | ||||||
| Extent (%)c | 0.46 (0.23) | 0.21 (0.14) | 0.25 (0.04) | <0.01 | 0.19 (0.05) | <0.01 | |
| Intensityd | 1.95 (0.70) | 1.26 (0.66) | 0.69 (0.12) | <0.01 | 0.66 (0.18) | <0.01 | |
| Modified H- scoree |
0.65 (0.45) | 0.08 (0.09) | 0.58 (0.07) | <0.01 | ------ | ------ | |
| Epithelium | 20 | ||||||
| Extent (%)c | 0.38 (0.25) | 0.29 (0.21) | 0.09 (0.05) | 0.08 | 0.10 (0.06) | 0.11 | |
| Intensityd | 1.70 (0.98) | 1.35 (0.75) | 0.35 (0.20) | 0.09 | 0.33 (0.23) | 0.17 | |
| Modified H-scoree | 0.18 (0.18) | 0.06 (0.04) | 0.12 (0.04) | <0.01 | ------ | ------ | |
| Adipocytes | 46 | ||||||
| Extent (%)c | 0.27 (0.21) | 0.29 (0.22) | −0.02 (0.04) | 0.54 | 0.05 (0.06) | 0.40 | |
| Intensityd | 1.11 (0.57) | 1.22 (0.73) | −0.11 (0.12) | 0.36 | 0.19 (0.19) | 0.31 | |
| Modified H-scoree | 0.12 (0.20) | 0.37 (0.38) | −0.24 (0.06) | <0.01 | ------ | ------ |
Adjusted for proportion of cells of each type.
Overall modified H-score is sum of H-scores across all cell types in all 49 women.
Analyses of extent of staining are based on comparisons of medians of categories <1%, 1-24%, 25-50%, 51%+ for stroma and epithelium and <1%, 1-24%, 25-50%, 51%-75%, 76%+ for adipocytes.
Analyses of intensity of staining are based on categories of no to strong staining (0-3).
Modified H-score is product of percent of specific cell type, extent and intensity of aromatase stain.
As both stromal and epithelial aromatase IR were increased in dense versus non-dense tissue, we sought to determine which contributed the greater amount to overall aromatase IR. We found that stromal cells from dense tissue had a three-fold higher overall IR (mean H-score=0.65) compared to epithelial cells also from dense tissue (mean H-score=0.18). The IR corresponding to stromal and epithelial cells from non-dense tissue, however, was almost identical (mean H-score, 0.08 and 0.06, respectively).
To evaluate the potential influence of blinding on our results, we examined samples from the 11 women who had at least 25% stroma on each of her dense and non-dense sections and the 21 who had at least 25% fat on both sections. Only one woman had >25% epithelium on both sections, limiting examination. The differences in extent (βExtent=0.28 (SE=0.06)), intensity (βIntensity=0.68 (SE=0.25)) and H-score (βH=0.58 (SE=0.13)) for aromatase IR in the stroma were essentially the same, and even greater, in this subset relative to all women combined (Supplementary Table 2; Table 3). Thus, there does not appear to be evidence of bias resulting from the recognition of section type in regards to the stroma results. However, the differences in overall aromatase IR (H-score) among adipocytes from dense and non-dense sections were attenuated (βH = −0.12 (SE=0.08)) in the 21 paired sections with >25% fat, and the association was no longer significant (p=0.15) (Supplementary Table 2). This could suggest that knowledge of section type influenced the pathologist evaluation of IR in adipocytes. However, since overall aromatase IR was found to be higher in dense relative to non-dense sections, any potential bias due to adipocytes would serve to attenuate the magnitude of this difference. Thus, our findings on the entire sample are likely conservative and the actual difference in overall aromatase IR is likely greater.
Discussion
This study provides the first evidence that aromatase IR is increased in mammo-graphically dense breast tissue when compared to non-dense tissue in the same patient. Using a modified H-score, overall mean aromatase IR was two-fold greater in tissue derived from dense compared to non-dense regions of the breast. Key components of this increase were the stromal and epithelial cells. Stromal cells from dense regions were found to have higher levels of aromatase IR than epithelium. These data have important potential implications since lifetime exposure to estrogen is thought to be a major mediator of breast cancer development. Increased aromatase IR would result in enhanced estrogen synthesis and greater exposure of breast tissue to this sex steroid. Accordingly, these data provide a potential biological basis by which mammographic density could influence breast cancer risk and underscore the importance of the stroma to mammographic density
Numerous studies suggest that mammographic density represents the amount or proportion of stroma and epithelium in the breast [15-19]. We show an increased proportion of stroma and epithelium but decreased fat in biopsies specifically taken from dense compared to non-dense regions of the breast, which support these earlier studies. Mammographically dense tissue has been hypothesized to reflect increased cellular proliferation of stroma and epithelium, however, studies correlating cell proliferation markers (Ki-67 or DNA S-phase %) with mammographic density in healthy women have been inconsistent [18-22].
A recent study of noncancerous tissue from mastectomy specimens showed that the amount and percent of fibrous stroma and percent density were increased among women currently taking postmenopausal hormones, but not among women who were former or never PMH users [22]. Since neither estrogen receptor nor progesterone receptor activity was seen associated with these increases, the authors suggested increased stroma among women on PMH could be due to alternative methods of up-regulation of endocrine pathways, such as aromatase activity [22]. Our findings that the greatest difference in aromatase IR was among stroma from dense vs. non-dense tissue coupled with the fact that stromal cells from dense tissue showed the highest aromatase IR of all cell types would support this hypothesis. Also, stromal cells (myofibroblasts) from breast tumors and benign breast tissue have been shown to synthesize estrogens in vitro and demonstrate increased aromatase enzyme activity in response to stimulators of aromatase transcription. Interestingly, epithelial breast cells have not been responsive to these activators of transcription [23]. Furthermore, a recent cell culture-based study indicates that high cell density alone can significantly increase aromatase transcription in primary stromal cells isolated from cancer-free women [24]. Stroma, then, is capable of estrogen synthesis, and elevated estrogen production by stromal cells can promote the growth of estrogen-dependent tumor cells [25, 26]
Our findings of increased aromatase IR in stromal cells from dense tissue are also consistent with work by Bulun and colleagues [27-29], who quantified P450 aromatase transcript levels and histologic components among tissue samples from both women undergoing reduction mammoplasty and breast cancer cases. They found that the breast quadrant with the highest P450 transcript levels corresponded with the highest proportion of stromal cells relative to adipocytes [27, 29]. Our dense regions also showed higher levels of aromatase IR and proportion of stromal cells relative to adipocytes compared to non-dense regions. These studies also suggested the highest P450 transcript levels occurred in high risk regions; among cases, this corresponded with the quadrant where the tumor arose [27] and among controls, this was the outer (vs. inner) quadrant where the majority of tumors tend to occur [28, 29]. The increased aromatase IR in mammographically dense tissue in our study would suggest that dense tissue is at increased risk for breast cancer relative to non-dense tissue.
The finding of higher aromatase IR in adipose tissue in non-dense vs. dense tissue appears to be a paradoxical finding. Non-dense tissue contains a higher proportion of adipose tissue than stroma, as was noted in this study. From this, one might speculate that non-dense tissue would have higher aromatase activity, which was seen here. As noted in the results, though, differences in aromatase IR among adipose tissue from dense and non-dense sections were no longer present when analyses were performed in the subset of 21 individuals who had at least 25% adipocytes on each section. In contrast, the differences in the aromatase IR among stroma from dense and non-dense sections were similar when performed in the subset of 11 women with >25% stromal cells on each section. This would suggest that differences in aromatase IR seen in adipose tissue in the entire sample of 49 women were driven by the differential proportion of adipocytes on dense vs. non dense sections. Even if this is the case, the differences in stromal IR were robust to the proportion of stromal cells on each section. However, since estradiol from any cell type can diffuse locally to the other cells, we hypothesize that the most important parameter is the total amount of aromatase present, and not the particular cell type responsible. Thus, these findings emphasize the need to integrate the findings from all three cellular compartments and to determine overall aromatase activity.
Aromatase expression as detected by immunoreactivity reflects the amount of aromatase protein present. Various mechanisms such as rate of transcription, half life of message, rate of translation, and half life of protein stability could result in the increased aromatase protein demonstrated by immunohistochemistry. We utilized a nested PCR assay [30] to quantitate aromatase message in paired dense and non-dense tissue. As this is highly labor intensive, assays were performed in a pilot fashion on a small sample of participants (n=13). We found no evidence for correlation between aromatase IR and message assessed by PCR in these 13 samples (Pearson correlation=0.22, P-value=0.27). However, laser capture of the stroma, adipocytes and epithelial cells are necessary to verify these preliminary results. Finding no correlation would suggest mechanisms other than increased aromatase message were responsible for the increased aromatase protein but further investigations are required for clarification.
A potential weakness of our study is the lack of information regarding sulfatase activity. Controversy exists whether the major contributor to local estrogen biosynthesis in breast is aromatase or sulfatase. Theoretical calculations based on reported Km and Vmax levels suggest that sulfatase might contribute slightly more to estrogen production in breast than aromatase, but this has not been experimentally substantiated [31]. Also, sulfatase is primarily expressed in ductal cells but not in stromal cells of breast tissue [32, 33]. The other consideration is that aromatase activity is high in cells with close proximity to tumor cells, particularly the stromal component, suggesting a significant role of this enzyme in stimulating tumor growth [23]. Isolated stromal cells respond substantially to enhancers of promotion such as phorbol esters, glucocorticoids and cyclic AMP whereas epithelial cells do not. These data suggest that regulation of stromal aromatase activity might be an important factor in mediating breast density.
If aromatase expression is increased in dense tissue, then we might expect aromatase inhibitors (AIs) to change overall breast density. Four small studies to date have examined changes in percent MBD in response to letrozole, with mixed results. We examined changes in percent MBD in 106 breast cancer patients who were randomized to either letrozole (N=56) or placebo (N=48) after 5 years of tamoxifen [34]. We found no difference in change in MBD after 9-15 months of letrozole vs. placebo. However, all women had previously been on tamoxifen, which is known to significantly reduce mammographic density [35]; therefore baseline MBD may have been too low to detect additional decreases resulting from AI therapy [34]. A recent prospective trial of 2.5 mg daily letrozole (N=31) vs. placebo (N=19) among healthy postmenopausal women, required women to have at least 25% density at baseline, to allow detection of reductions in density. But, there was no evidence for change in percent MBD with AI therapy compared to placebo after 12 or 24 months of therapy [36]. Two additional studies examined the influence of letrozole on MBD among postmenopausal women taking PMH. Fabian and colleagues showed no change in percent MBD among 42 high risk women on either estrogen alone or combination PMH (estrogen and progestins) after taking 2.5 mg letrozole per day for six months [37]. However, in a retrospective study of 18 women on low dose, combination therapy, those who took 2.5 mg letrozole three times weekly for a median of 24 (range, 2-63) months, were found to significantly reduce their percent MBD compared to 22 women who did not take letrozole (6.8% vs. 1.4% reduction, respectively) [38]. The inconsistent findings of these two studies of women on PMH may be due to differential duration of therapy, as six months may be too early to see resulting changes in MBD. In fact, the reductions in density among healthy women randomized to tamoxifen as part of the IBIS-1 prevention study were seen at 12-18 months of therapy [35]. Conversely, if MBD causes higher aromatase expression (i.e. MBD sits upstream of the functional pathway) and not vice versa, then we may not expect changes in MBD with aromatase inhibition. Large studies are underway to comprehensively examine the influence of AI therapy on MBD among women who were never on prior endocrine therapy and on differential duration of therapy to help clarify the role of MBD as a potential biomarker for response to AI therapy.
We recognize that the study population was a select group of healthy and motivated volunteers, limiting the generalizability of results. Our findings will need to be validated in other populations and using other measures of aromatase expression. And, as noted above, sulfatase should also be examined to determine whether it is increased in dense tissue. Finally, our study design does not permit complete blinding of the pathologist to cell type and consequently, dense vs. non-dense tissue classification. Knowledge of the tissue type could influence our findings. However, our pathologist (HS) is experienced in assessment of aromatase IR. Also, our analyses performed in paired samples with greater than 25% of stroma showed no evidence for bias of this nature. Any potential bias due to differential reading in the adipocytes would serve to weaken our findings, suggesting our differences in overall aromatase IR by dense vs. non-dense type are likely conservative. Unfortunately we could not evaluate the influence that blinding had on epithelial cells. There are also several strengths to this study, including the innovative study design which controls for genes and other factors influencing the aromatase pathway using within-individual comparisons. Also, this is the first study to examine breast tissue derived from mammographically dense and non-dense regions of healthy women. Our findings are novel and provide evidence that aromatase in breast tissue, especially the stroma, may be responsible for some of the variation in MBD and suggest one mechanism by which MBD may increase breast cancer risk.
Supplementary Material
Fig. 2.
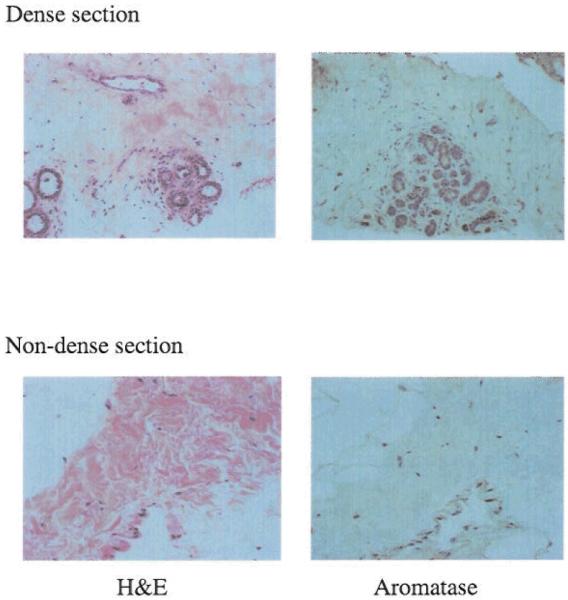
Magnified views of sections from dense and non-dense regions stained for H&E and aromatase
Acknowledgments
We acknowledge the participants in this study who provided samples for investigations of the determinants of breast density.
This work was financially supported by the Mayo Clinic Breast SPORE, NIH CA116201; NIH K12 RR24151 for the KL2 Clinical and Translational Science Award-Mentored Career Development Program; Friends for an Earlier Breast Cancer Test.
Contributor Information
Celine M. Vachon, Mayo Clinic, Rochester, Minnesota
Hironobu Sasano, Tohoku University School of Medicine, Tohoku, Japan.
Karthik Ghosh, Mayo Clinic, Rochester, Minnesota.
Kathleen R. Brandt, Mayo Clinic, Rochester, Minnesota
David A. Watson, Mayo Clinic, Rochester, Minnesota
Carol Reynolds, Mayo Clinic, Rochester, Minnesota.
Wilma L. Lingle, Mayo Clinic, Rochester, Minnesota
Paul E. Goss, Massachusetts General Hospital, Boston, Massachusetts
Rong Li, University of Texas Health Science Center at San Antonio, San Antonio, Texas.
Sarah E. Aiyar, University of Virginia Health System, Charlottesville, Virginia
Christopher G. Scott, Mayo Clinic, Rochester, Minnesota
V. Shane Pankratz, Mayo Clinic, Rochester, Minnesota.
Richard J. Santen, University of Virginia Health System, Charlottesville, Virginia
James N. Ingle, Mayo Clinic, Rochester, Minnesota
References
- 1.McCormack VA, Highnam R, Perry N, dos Santos Silva I. Comparison of a new and existing method of mammographic density measurement: intramethod reliability and associations with known risk factors. Cancer Epidemiol Biomarkers Prev. 2007;16:1148–1154. doi: 10.1158/1055-9965.EPI-07-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA. The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol. 2005;162:826–834. doi: 10.1093/aje/kwi286. [DOI] [PubMed] [Google Scholar]
- 4.Verheus M, Peeters PH, van Noord PA, van der Schouw YT, Grobbee DE, van Gils CH. No relationship between circulating levels of sex steroids and mammographic breast density: the Prospect-EPIC cohort. Breast Cancer Res. 2007;9:R53. doi: 10.1186/bcr1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack VA, Dowsett M, Folkerd E, Johnson N, Palles C, Coupland B, Holly JM, Vinnicombe SJ, Perry NM, dos Santos Silva I. Sex steroids, growth factors and mammographic density: a cross-sectional study of UK postmenopausal Caucasian and Afro-Caribbean women. Breast Cancer Res. 2009;11:R38. doi: 10.1186/bcr2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 7.Miller WR, O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunbier AK, Anderson H, Folkerd E, Ghazoui Z, Smith IE, Ellis MJ, Dowsett M, Neoadjuvant Letrozole Study Group Expression of estrogen responsive genes in breast cancers correlates with plasma estradiol levels in postmenopausal women. Cancer Res. 2009;69:63. [Google Scholar]
- 9.Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ. Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer. 2003;39:462–468. doi: 10.1016/s0959-8049(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 10.Yue W, Wang JP, Hamilton CJ, Demers LM, Santen RJ. In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res. 1998;58:927–932. [PubMed] [Google Scholar]
- 11.Lipton A, Harvey HA, Demers LM, Hanagan JR, Mulagha MT, Kochak GM, Fitzsimmons S, Sanders SI, Santen RJ. A phase I trial of CGS 16949A. A new aromatase inhibitor. Cancer. 1990;65:1279–1285. doi: 10.1002/1097-0142(19900315)65:6<1279::aid-cncr2820650604>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Brodie A, Lu Q, Long B. Aromatase and its inhibitors. J Steroid Biochem Mol Biol. 1999;69:205–210. doi: 10.1016/s0960-0760(99)00051-5. [DOI] [PubMed] [Google Scholar]
- 13.Morales L, Neven P, Paridaens R. Choosing between an aromatase inhibitor and tamoxifen in the adjuvant setting. Curr Opin Oncol. 2005;17:559–565. doi: 10.1097/01.cco.0000180434.31991.bf. [DOI] [PubMed] [Google Scholar]
- 14.Santen RJ, Martel J, Hoagland M, Naftolin F, Roa L, Harada N, Hafer L, Zaino R, Santner SJ. Stromal spindle cells contain aromatase in human breast tumors. J Clin Endocrinol Metab. 1994;79:627–632. doi: 10.1210/jcem.79.2.8045987. [DOI] [PubMed] [Google Scholar]
- 15.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006;12:186–193. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 16.Alowami S, Troup S, Al-Haddad S, Kirkpatrick I, Watson PH. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 18.Hawes D, Downey S, Pearce CL, Bartow S, Wan P, Pike MC, Wu AH. Dense breast stromal tissue shows greatly increased concentration of breast epithelium but no increase in its proliferative activity. Breast Cancer Res. 2006;8:R24. doi: 10.1186/bcr1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stomper PC, Penetrante RB, Edge SB, Arredondo MA, Blumenson LE, Stewart CC. Cellular proliferative activity of mammographic normal dense and fatty tissue determined by DNA S phase percentage. Breast Cancer Res Treat. 1996;37:229–236. doi: 10.1007/BF01806504. [DOI] [PubMed] [Google Scholar]
- 20.Khan QJ, Kimler BF, O’Dea AP, Zalles CM, Sharma P, Fabian CJ. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007;9:R35. doi: 10.1186/bcr1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheus M, Maskarinec G, Erber E, Steude JS, Killeen J, Hernandez BY, Cline JM. Mammographic density and epithelial histopathologic markers. BMC Cancer. 2009;9:182. doi: 10.1186/1471-2407-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey JA, Santen RJ, Petroni GR, Bovbjerg VE, Smolkin ME, Sheriff FS, Russo J. Histologic changes in the breast with menopausal hormone therapy use: correlation with breast density, estrogen receptor, progesterone receptor, and proliferation indices. Menopause. 2008;15:67–73. doi: 10.1097/gme.0b013e318054e29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santner SJ, Pauley RJ, Tait L, Kaseta J, Santen RJ. Aromatase activity and expression in breast cancer and benign breast tissue stromal cells. J Clin Endocrinol Metab. 1997;82:200–208. doi: 10.1210/jcem.82.1.3672. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh S, Choudary A, Ghosh S, Musi N, Hu Y, Li R. IKKbeta mediates cell shape-induced aromatase expression and estrogen biosynthesis in adipose stromal cells. Mol Endocrinol. 2009;23:662–670. doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Gurates B, Yang S, Sebastian S, Bulun SE. Malignant breast epithelial cells stimulate aromatase expression via promoter II in human adipose fibroblasts: an epithelial-stromal interaction in breast tumors mediated by CCAAT/enhancer binding protein beta. Cancer Res. 2001;61:2328–2334. [PubMed] [Google Scholar]
- 27.Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. J Clin Endocrinol Metab. 1993;77:1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 28.Bulun SE, Simpson ER. Regulation of aromatase expression in human tissues. Breast Cancer Res Treat. 1994;30:19–29. doi: 10.1007/BF00682738. [DOI] [PubMed] [Google Scholar]
- 29.Bulun SE, Sharda G, Rink J, Sharma S, Simpson ER. Distribution of aromatase P450 transcripts and adipose fibroblasts in the human breast. J Clin Endocrinol Metab. 1996;81:1273–1277. doi: 10.1210/jcem.81.3.8772611. [DOI] [PubMed] [Google Scholar]
- 30.Liu GJ, Wu YS, Brenin D, Yue W, Aiyar S, Gompel A, Wang JP, Tekmal RR, Santen RJ. Development of a high sensitivity, nested Q-PCR assay for mouse and human aromatase. Breast Cancer Res Treat. 2008;111:343–351. doi: 10.1007/s10549-007-9792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santner SJ, Feil PD, Santen RJ. In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab. 1984;59:29–33. doi: 10.1210/jcem-59-1-29. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res. 2003;63:2762–2770. [PubMed] [Google Scholar]
- 33.Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H. Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues. J Clin Endocrinol Metab. 2002;87:5760–5768. doi: 10.1210/jc.2002-020670. [DOI] [PubMed] [Google Scholar]
- 34.Vachon CM, Ingle JN, Suman VJ, Scott CG, Gottardt H, Olson JE, Goss PE. Pilot study of the impact of letrozole vs. placebo on breast density in women completing 5 years of tamoxifen. Breast. 2007;16:204–210. doi: 10.1016/j.breast.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96:621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 36.Cigler T, Tu D, Yaffe MJ, Findlay B, Verma S, Johnston D, Richardson H, Hu H, Qi S, Goss PE. A randomized, placebo-controlled trial (NCIC CTG MAP1) examining the effects of letrozole on mammographic breast density and other end organs in postmenopausal women. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0662-0. 2009 Dec 6 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Fabian CJ, Kimler BF, Zalles CM, Khan QJ, Mayo MS, Phillips TA, Simonsen M, Metheny T, Petroff BK. Reduction in proliferation with six months of letrozole in women on hormone replacement therapy. Breast Cancer Res Treat. 2007;106:75–84. doi: 10.1007/s10549-006-9476-5. [DOI] [PubMed] [Google Scholar]
- 38.Mousa NA, Crystal P, Wolfman WL, Bedaiwy MA, Casper RF. Aromatase inhibitors and mammographic breast density in postmenopausal women receiving hormone therapy. Menopause. 2008;15:875–884. doi: 10.1097/gme.0b013e31816956c3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.