Abstract
The aim of this study was to determine the neural correlates of different stages of episodic memory function and their modulation by Alzheimer's Disease (AD). Several decades of work have supported the role of the medial temporal lobes (MTL) in episodic memory function. However, more recent work, derived in part from functional neuroimaging studies, has suggested that other brain structures make up a large-scale network that appear to support successful encoding and retrieval of episodic memories. Furthermore, controversy exists as to whether dissociable MTL regions support qualitatively different aspects of memory (hippocampus: contextual memory or ‘recollection’; perirhinal/lateral entorhinal cortex: item memory or ‘familiarity’). There is limited neuropsychological support for these models and most work in AD only has examined free recall memory measures. We studied the relationship between performance on different stages of the Rey Auditory Verbal Learning Test (AVLT), a 15-item word list learning task, and structural MRI measures in mild AD patients. Structural measures included hippocampal volume and cortical thickness of several ROIs known to undergo atrophy in AD. Correlation and multiple regression analyses, controlling for age, education, and gender, were performed in 146 mild AD patients (MMSE 23.3 ± 2.0). To evaluate the robustness of these relationships, similar analyses were performed with additional standardized verbal memory measures. Early immediate recall trials (e.g. Trial 1 of the AVLT) were not associated with the size of MTL regions, but correlated most strongly with inferior parietal, middle frontal gyrus, and temporal pole ROIs. After repeated exposure (e.g. Trial 5 of the AVLT), immediate recall was correlated with both MTL and a similar distribution of isocortical structures, but most strongly the temporal pole. For delayed recall, only the hippocampus correlated with performance. In contrast, for delayed recognition discrimination, the perirhinal/entorhinal cortex correlated more strongly than hippocampus; no other isocortical regions were strongly associated with performance. Convergent results were found for immediate and delayed trials of the other memory tests. The current results suggest that a richer understanding of the memory deficits in AD can be gained by examining multiple measures, which tap different aspects of memory function. Furthermore, the present findings are consistent with models hypothesizing that different stages of verbal list learning map onto dissociable brain regions. These data have implications for understanding the anatomic basis of processes underlying episodic memory, particularly related to a division of labor within the medial temporal lobes and within the large-scale MTL-cortical memory network.
Keywords: memory performance, recollection, familiarity, Alzheimer's Disease, medial temporal lobe
Introduction
Decades of research support the central role of the medial temporal lobes (MTL), particularly the hippocampus, in episodic memory function (Squire et al., 2004). Although the most profound forms of amnesia are associated with lesions to these structures (Scoville and Milner, 1957), a variety of other cortical regions also play important roles. For example, the prefrontal cortex contributes to controlled-strategic processing at encoding and retrieval, supporting accurate memory performance (Alexander et al., 2009, Blumenfeld and Ranganath, 2007). Working memory, which is in part subserved by frontal and parietal regions (Champod and Petrides, 2007), also generally modulates episodic memory performance by promoting efficient encoding of information to later be remembered. Additionally, the semantic memory system is involved in modulating the depth and organization of encoding, impacting the strength of a memory trace (Craik and Lockhart, 1972, Goldblum et al., 1998). While the left ventrolateral prefrontal cortex has been implicated as critical to semantic access at memory encoding (Logan et al., 2002, Otten et al., 2001), such as the high level conceptual representations thought supported by the temporal pole (Lambon Ralph et al., 2009, Lambon Ralph et al., Patterson et al., 2007), are also likely essential to robust encoding.
Alzheimer's disease (AD) is the most common form of acquired amnesia. Given the early and profound involvement of neuropathology, particularly neurofibrillary tangles, in the MTL of AD patients (Arnold et al., 1991, Braak and Braak, 1991, Delacourte et al., 1999, Price et al., 1991), much of the work examining the anatomic basis of memory impairment in this condition has focused on the hippocampus and other MTL structures. However, AD pathology, as reflected in the clinical phenotype, involves multiple neural networks supporting other cognitive domains such as executive functioning, language, semantic memory, and visuospatial processing (Arnold et al., 1991). The influence of these additional domains of impairment is often neglected when considering episodic memory performance measures.
A variety of standardized episodic memory measures, frequently involving verbal list learning tasks, have been utilized for clinical diagnosis and monitoring of disease progression in AD. These tasks typically produce several memory measures, including immediate recall (commonly with repeated exposure), delayed free recall, and often recognition memory. However, much of the work in AD has focused on delayed free recall or treated these different measures as instances of a monolithic process. In fact, a number of studies have combined many of these measures into summary statistics to capture “episodic memory.” While such an approach is not unreasonable in that it may reduce the variability inherent in individual tests and may be useful in screening for any form of memory impairment, it reflects a process purity that is only approximate at best.
For example, a major debate in the memory literature is whether recollection, associated with detailed contextual retrieval of a prior event, is differentially represented in the MTL relative to familiarity, or an acontextual sense of prior encounter (Aggleton and Brown, 2006, Eichenbaum et al., 2007, Squire et al., 2007, Yonelinas, 2002). A variety of lines of research have supported an anatomic mapping of recollection to the hippocampus and familiarity to extrahippocampal MTL structures, particularly perirhinal and lateral entorhinal cortices [(Bowles et al., 2007, Davachi et al., 2003, Fortin et al., 2004, Montaldi et al., 2006, Wolk et al., in press); for review, see (Aggleton and Brown, 2006)]. As free recall and recognition memory are likely differentially dependent on these memory processes, these measures may reflect differential localization of pathology within the MTL. Moreover, immediate recall after a single trial is likely related more to working memory than to so-called long-term memory processes involved in recall with repeated learning or after a delay. Thus, performance on an initial learning trial(s) would likely be predicted to relate to frontoparietal or other nodes of the working memory system.
Since one major goal of psychometric testing is to reveal specific changes in underlying brain systems, we sought here to determine the neural correlates of the different measures associated with three common verbal memory measures that are typically part of clinical research batteries in AD. Specifically, we focused on correlates of immediate recall (early versus later trials), delayed recall, and recognition memory. These measures were derived from three standardized psychometric memory instruments. Drawing on the concept in both pathology (Arnold et al., 1991, Braak and Braak, 1991, Brun and Gustafson, 1976) and imaging studies (Baron et al., 2001,Fox et al., 2001, Frisoni et al., 2002, Thompson et al., 2001) that early in its course AD is not a diffuse process but is in fact associated with abnormalities in a relatively stereotyped set of brain regions, we utilized a relatively novel region of interest (ROI) approach in which a set of regions that have been previously demonstrated to be sensitive to mild AD – the “Cortical Signature of AD” (Bakkour et al., 2009, Dickerson et al., 2009) – were defined on the cortical surface. These regions, along with the hippocampus, represent structures most likely to be associated with the pathology of AD and serve as a type of lesion model for understanding the brain-behavior relationships of the memory measures.
We predicted that early immediate memory trials would be most dependent on regions supporting working memory and perhaps other aspects of controlled strategic processing and semantic elaboration, but that later trials would be more dependent on MTL structures. Delayed recall and recognition, which require the storage of information over time, were expected to be more dependent on MTL structures than the immediate trials. Finally, following the dual process model, we predicted that recognition memory performance, dependent on a combination of recollection and familiarity, would be more strongly associated with extrahippocampal MTL cortical atrophy while free recall, dependent solely on recollection, would be associated with hippocampal volume.
Materials and Methods
Participants
Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, as a $60-million, 5-year public-private partnership. The primary goal of ADNI has been to test whether imaging measures, biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
For the current analysis, we selected patients with a diagnosis of AD at baseline who underwent a 1.5 T MRI scan of adequate quality to obtain the below described morphometric measures (n=146). ADNI diagnosis of AD was made based on the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association criteria (McKhann et al., 1984). All AD patients in the cohort had a Clinical Dementia Rating (CDR) scale score of 0.5 to 1.0 for study inclusion (Morris, 1993). Other inclusion and exclusion criteria are described at: http://www.adni-info.org/index.php.
Psychometric Testing
We examined baseline and screening memory testing from the ADNI database. While our central analysis was focused on the Rey Auditory Verbal Learning Test [AVLT; (Rey, 1964)], we also examined brain-behavior relationships with the two other verbal memory instruments acquired as part of ADNI: the Logical Memory Test [LMT; (Wechsler, 1987)] and the 10-item word list memory task from the Alzheimer's Disease Assessment Scale-Cognitive [ADAS-Cog; (Rosen et al., 1984)]. Briefly, the AVLT consists of five learning trials in which a list of 15 words is read and the subject is asked to immediately recall as many items as possible. After an interference list of 15 novel words is read and recalled, subjects are then asked to recall words from the initial list (5-minute delayed recall). A 30-minute delayed recall trial and recognition test follow. For the recognition test, subjects are presented with a list of the 15 studied words and 15 nonstudied foils and are asked to circle all words previously studied. To account for false alarms to nonstudied items, we derived a measure of discriminability, d-prime (d′), which was calculated in a standard fashion based on classic signal detection theory (Snodgrass and Corwin, 1988). Additionally, because d′ is undefined when either proportion is 0 or 1, we used standard formulas to convert these values: Hits=(#Hits + 0.5)/(#studied items + 1) and FA=(#FA + 0.5)/(#unstudied items + 1).
The LMT is a modification of the episodic memory measure from the Wechsler Memory Scale-Revised (WMS-R) and involves the immediate and delayed (30 minutes) free recall of a short story that is read aloud to the patient and contains 25 elements of information (perfect score is 25). The 10-item word list memory task from the ADAS-cog involves 3 immediate free recall trials for words read by the subject prior to each trial and a 5-minute delayed recall trial.
MRI Imaging and Analysis
MRI scans for ADNI were collected on a 1.5 T scanner using a standardized protocol across sites. For the present analysis, the MPRAGE sequence was used with the following characteristics: sagittal plane, TR/TE/TI, 2400/3/1000 ms, flip angle 8°, 24 cm FOV, 192 × 192 in-plane matrix, 1.2 mm slice thickness (Jack et al., 2008).
T1 image volumes were examined quantitatively by a cortical surface-based reconstruction and analysis of cortical thickness. All baseline scans from patients with a diagnosis of AD that could be processed in a fully automated fashion with Freesurfer were used in this study. The general procedures for this processing method have been described in detail and applied and validated in a number of publications and presentations, and the technical details can be found in these manuscripts (Dale et al., 1999, Fischl and Dale, 2000, Fischl et al., 2002, Fischl et al., 1999, Fischl et al., 1999, Fischl et al., 2004). Cortical thickness measures were analyzed by both a hypothesis-driven and an exploratory approach. For the first approach, we utilized regions of interest (ROIs) that were previously determined to be reliably associated with AD, constituting the “cortical signature” of AD (Bakkour et al., 2009, Dickerson et al., 2009). Unlike most ROI analyses, these regions were defined in a data driven manner based on analysis of several datasets, as opposed to being determined strictly by anatomic boundaries. These ROIs encompass the following regions: rostral medial temporal cortex (encapsulating both the entorhinal and perirhinal cortices), rostral inferior temporal gyrus, temporal pole, angular gyrus, supramarginal gyrus, superior parietal lobule, precuneus, superior frontal gyrus, and inferior frontal sulcus/caudal middle frontal gyrus (see Figure 1). These nine ROIs were generated from an independent sample of 29 mild AD patients compared with 115 controls as described in detail in two previously published papers (Bakkour et al., 2009, Dickerson et al., 2009), and are available from one of the authors (BCD) on request [For additional detail on their definition and localization, see Figures 2 and 3 of the first paper (Dickerson et al., 2009)]. Given its significant early involvement of AD pathology, hippocampal volume was also determined and normalized to intracranial volume as previously described (Buckner et al., 2004).
Figure 1.
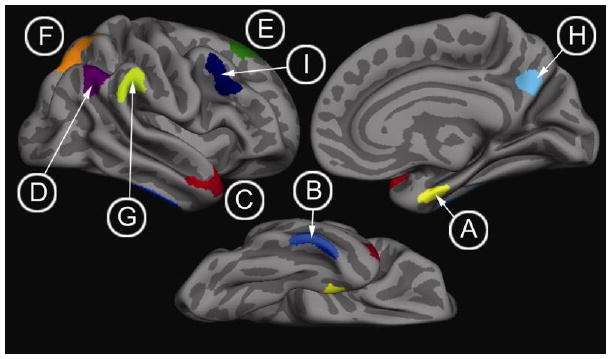
The ‘Cortical Signature of AD.’ ROIs derived from previous exploratory analyses that identified foci of thinning in mild AD (Bakkour et al., 2009, Dickerson et al., 2009). (A) rostral medial temporal cortex, (B) inferior temporal gyrus, (C) temporal pole, (D) angular gyrus, (E) superior frontal gyrus, (F) superior parietal lobule, (G) supramarginal gyrus, (H) precuneus, (I) inferior frontal sulcus/caudal middle frontal gyrus.
Figure 2.
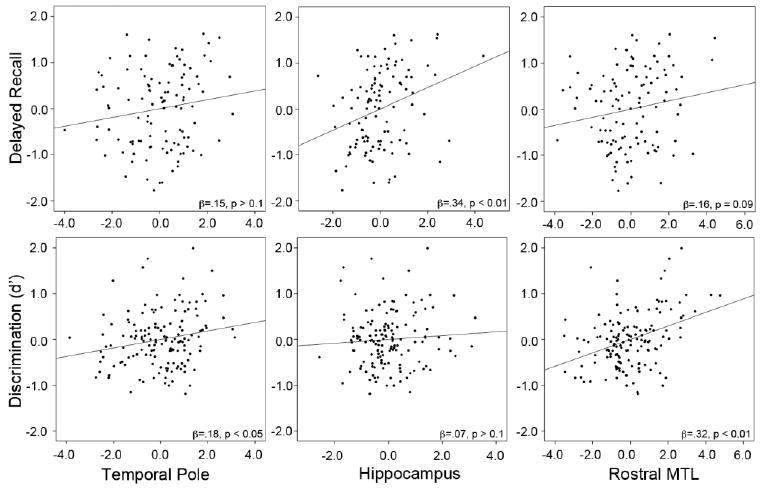
Partial regression plots of linear relationships of select ROIs with recall and recognition memory measures. For the top row, delayed recall is the mean proportion recalled across the three memory tasks, natural log-transformed to best approximate normality. Plots were generated from separate regression models for each of the individual ROIs (temporal pole, hippocampus, and rostral medial temporal lobe) in which age, education, and gender were first entered into the model. In the second row, d′ is the AVLT measure of recognition memory discrimination. In these models, AVLT 30-minute delayed recall was included as a covariate with age, education and gender. Beta coefficients and p-values are presented.
Figure 3.
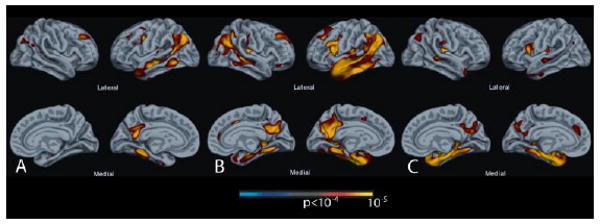
Exploratory analysis across the entire cerebral cortex of cortical thickness associated with (A) Trial 1 of the AVLT, (B) Trial 5 of the AVLT, and (C) delayed recognition memory. These results are convergent with hypothesis-driven analyses in demonstrating the specificity of regional atrophy in left-lateralized lateral temporoparietal, posterior cingulate/precuneus (PCC), and frontal regions in initial encoding (A), similar regions along with the medial temporal lobe in encoding after repetition (B), and only medial temporal, PCC, and inferior frontal regions in delayed retrieval (C). For these exploratory analyses, a statistical threshold of p < 10-4 was used.
For the exploratory analyses, general linear models were implemented with each behavioral variable of interest as the independent variable, cortical thickness as the dependent variable, and age and education as covariates. The results of these analyses were inspected via maps of the statistical significance at each surface point overlaid on the average cortical surface template. For these exploratory analyses, a statistical threshold of p < 10-4 was used.
Statistical Analysis
Pearson correlations between ROIs and individual AVLT measures were calculated, controlling for the effects of age, education, and gender. Statistical significance was Bonferroni-corrected for 10 comparisons (10 ROIs). To follow up on these correlations, hierarchical regression models were developed in which the covariates of age, education, and gender were entered first and then morphometric measures of all the ROIs were regressed in a step-wise fashion; the default value of p<0.05 to enter an independent variable into the model was employed. Memory measures from each of the psychometric tests served as dependent variables, allowing for determination of the brain regions that uniquely contributed to prediction on each measure. Statistical analyses were performed using standard methods in SPSS 16.0 (Chicago, IL).
Results
Demographic Data
A total of 146 patients (74 Female; 72 Male) with AD [mean 75.4 ± 7.4 (SD) years; mean education 14.9 ± 3.2 (SD) years] were included in the analyses. The racial and ethnic distribution of this group was consistent with the overall ADNI cohort [93.2% Caucasian; 4.1% African American; 1.4% Asian American; 2.7% Hispanic; (Petersen et al., 2010)]. Consistent with the mild status of these patients (72 with CDR=0.5; 74 with CDR=1), the mean Mini Mental State Examination (MMSE) and CDR-sum of boxes (CDR-SOB) were 23.3 ± 2.0 (SD) and 4.32 ± 1.6 (SD), respectively. Ninety-five (65.1%) had at least one copy of the ε4 allele of the apolipoprotein E (ApoE) gene.
AVLT Measures
To examine the relationships of the different AVLT trials with brain regions sensitive to AD pathology, we performed correlations with each of the “AD signature” regions and hippocampus, controlling for age, education, and gender. As can be observed in Table 1, the early immediate recall trials (Trials 1 and 2) correlated most strongly with cortical thickness of the temporal pole and supramarginal gyrus, regions that may be involved in semantic retrieval and maintenance of auditory-verbal working memory. There was no evidence of a significant relationship between memory performance on these early trials and MTL atrophy. By immediate recall Trial 3 and throughout the rest of the immediate trials, MTL structures—both hippocampus and rostral MTL cortex—displayed a trend towards correlation with performance, but did not always reach significance with Bonferroni correction. However, temporal pole continued to be the region that showed the strongest correlations. Not surprisingly, total learning, a commonly used clinical measure summing all of the learning trials, correlated with both MTL and several isocortical structures, including the middle frontal gyrus and temporal pole.
Table 1. Correlations of AVLT measures with AD signature ROIs, controlled for age, gender, and education.
| ROI | AVLT Trial 1 | AVLT Trial2 | AVLT Trial 3 | AVLT Trial 4 | AVLT Trial 5 | Total Learning | Delayed Recall (5 min) | Delayed Recall (30 min) | Recognition Memory (d′) |
|---|---|---|---|---|---|---|---|---|---|
| Hippo | .14 | .11 | .29 | *.21 | *.22 | .24 | .28 | .37 | .27 |
| MTL | .14 | .13 | .28 | .26 | *.19 | .25 | .12 | .16 | .40 |
| SFG | .14 | .07 | .10 | .11 | .10 | .12 | -.02 | -.07 | .11 |
| ITG | *.21 | *.17 | *.19 | .11 | .13 | *.19 | .04 | .00 | .09 |
| SPL | *.19 | .01 | .07 | .08 | .09 | .10 | -.01 | -.02 | .06 |
| PRC | .14 | -.01 | .09 | .08 | .07 | .09 | .03 | -.04 | .07 |
| MFG | *.23 | .15 | *.19 | .24 | *.19 | .24 | .05 | -.04 | .14 |
| TP | .31 | .26 | .37 | .30 | .28 | .36 | .10 | .09 | *.22 |
| AG | *.22 | .06 | .04 | .10 | .09 | .12 | -.05 | -.06 | .02 |
| SMG | .26 | .16 | .15 | .15 | *.17 | *.21 | .01 | .01 | .13 |
Note: Hippo: hippocampus; MTL: rostral medial temporal cortex; SFG: superior frontal gyrus; ITG: rostral inferior temporal gyrus; SPL: superior parietal lobule; PRC: precuneus; MFG: caudal middle frontal gyrus; TP: temporal pole; AG: angular gyrus; SMG: supramarginal gyrus. Bold: p < 0.05, Bonferroni-corrected for 10 comparisons;
p < 0.05 uncorrected.
A different pattern emerged for the delayed memory trials: only MTL structures were related to performance measures. For both the 5-minute and 30-minute delayed recall trials, the hippocampus was the only region that correlated with performance. There was little evidence of any isocortical region being associated with delayed recall trials. In contrast, delayed recognition discrimination correlated most strongly with the rostral MTL with a weaker correlation with the hippocampus (see Supplementary Table 1 for correlation analysis with the other memory measures).
Immediate Recall
To follow up on the above dissociations, a series of hierarchical regression models were developed in which covariates of age, education, and gender were entered first and then all ROIs were regressed in a step-wise fashion. In each case, the memory measure of interest served as the dependent variable.
Given the apparent dissociation in the above analysis related to the earlier and later immediate memory trials, we collapsed Trials 1 and 2 (‘Early Trials’) and Trials 4 and 5 (‘Late Trials’). Consistent with the above correlation analysis, the only ROI to survive the step-wise analysis of the Early Trials was the temporal pole (β = .33, p < .001). With inclusion of the temporal pole in the model, no other region provided additional explanatory value (β's < .12). Similarly, the best model for the Late Trials included only the temporal pole (β = .32, p < .001). However, in this case, there was a trend for a relationship with hippocampus (β = .16, p = .09) when the temporal pole was included in the model. Total learning produced an analogous result with only temporal pole (β = .38, p < .001) remaining in the model.
To further investigate the robustness of these finding we also examined immediate recall trials of the word-list memory task of the ADAS-Cog and the LMT. With the first immediate recall trial of the ADAS-cog word-list as the dependent variable, the best model included both the temporal pole (β = .36, p < .001) and the middle frontal gyrus (β = .17, p < .05) ROIs. Notably, MTL structures did not offer evidence of further modulating performance in this model (rostral MTL: β = .00; hippocampus: β = -.04). On the second trial, only the temporal pole was included in the most explanatory model (β = .38, p < 0.001). In distinction from the first trial, hippocampal volume now approached significance (β = .16, p = 0.09). With the third immediate recall trial as the dependent variable, again, only the temporal pole was included in the model (β = .40, p < 0.001), but the middle frontal gyrus (β = .16, p = 0.05) and rostral MTL cortex (β = .18, p = 0.07) approached significance. Remarkably similar to the foregoing analyses, the best model for immediate recall on the LMT also included only the temporal pole (β = .33, p < 0.001). No other ROI approached significance in this model (see Supplementary Table 2 for a summary of these regression analyses).
Delayed Recall
The same regression models as above were applied to the delayed recall measures of the AVLT. To mitigate against floor effects, only patients who recalled at least one item at the 5-minute (n=93) and 30-minute delays (n=41) were included in the analyses; however, the results were essentially identical when the entire cohort was included. For the 5-minute delayed recall, the most predictive model included only the hippocampus (β = .27, p < 0.05). No other ROI was close to significant when also included in this model (β's = -.09 to. 06). Likewise, with 30-minute delayed recall as the dependent variable, the hippocampus (β = .47, p < 0.05) was the only ROI included in the best model.
The analogous regressions were also performed for the delayed recall measure on the ADAS-cog and LMT, again restricting to those who recalled at least one item (n=90 and 65, respectively). As with the AVLT, the most explanatory model for both delayed recall measures included only the hippocampal ROI (β = .44, p < 0.001 and β = .38, p = 0.01, respectively). Finally, to further broaden the range of performance, the mean proportion correctly freely recalled across the three memory tests was calculated and natural log transformed to obtain a more normal distribution. Again, only patients who recalled at least one item on any of the three tests (n=110) were included. Consistent with all of the foregoing analyses, the best predictive model included the hippocampal ROI (β = .38, p < 0.001). Interestingly, an inverse relationship with superior frontal gyrus was also included in this model (β = -.21, p < 0.05). The partial regression plot of this relationship with hippocampus can be observed in Figure 2. The above model was essentially unchanged with removal of the one possible outlier (see Supplementary Table 3 for a summary of these regression analyses).
Recognition Memory
We also examined recognition memory performance on the AVLT with the same regression model. In distinction from the delayed recall measures, only the rostral MTL (β = .40, p < 0.001) was maintained in the best explanatory model; no other ROI was near to significance when included in the model (β's = -.16 to .06). We also hypothesized that performance on the recognition task relative to free recall at the 30-minute delay would reflect the degree to which familiarity or item-based memory contributed to performance of the task for that individual. In other words, for a given level of free recall, a putative measure of recollection, recognition discrimination would reflect the additional contribution of familiarity. To test the relationship with this potentially more “pure” item memory measure, we entered the delayed 30-minute recall score as a covariate. As would be expected, the delayed recall score strongly predicted discrimination performance (β = .51, p < 0.001). Nonetheless, the rostral MTL (β = .32, p < 0.001) remained the only ROI included in the best explanatory model (see Figure 2). Thus, the additional variance in discrimination performance after controlling for free recall (i.e. recollection-based memory) was related to the rostral MTL cortex encompassing entorhinal and perirhinal cortices.
Exploratory Analyses
To corroborate and complement the above ROI analyses, we also performed a global analysis of cortical thickness in relation to several of the key memory measures. As an exemplar of early learning, Trial 1 of the AVLT served as the dependent variable for the first map presented in Figure 3A. Consistent with the ROI analysis, left-hemisphere predominant atrophy of the middle frontal gyrus, inferior parietal lobule, and several temporal lobe regions, including temporal pole, were associated with immediate recall performance, but there was no relationship with extrahippocampal MTL structures. A similar isocortical distribution was seen for an exemplar of later learning (Trial 5 of the AVLT; see Figure 3B), but in this case MTL thinning was also related to performance. Finally, an analysis of delayed recognition memory discrimination revealed a more focused MTL distribution of thinning related to performance (see Figure 3C). In particular, non-MTL isocortical regions appeared to have much less influence than on the learning trials. Strikingly, if the 30-minute delayed recall score was included as a covariate for the recognition discrimination analysis to produce a more “pure” item, or familiarity, memory measure as described above, a very focal region encompassing the perirhinal/entorhinal region was the only area to be related to performance (see Figure 4). Note that a recall measure was not analyzed given the stronger relationship with hippocampus in the ROI analysis, a region not represented by cortical thickness.
Figure 4.
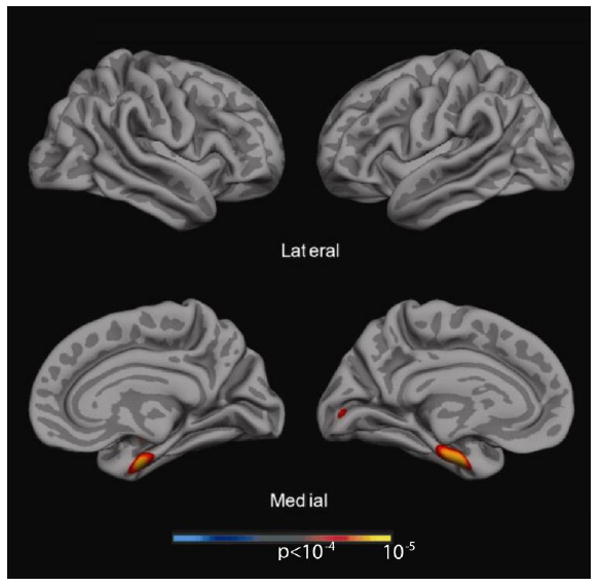
Exploratory analysis across the entire cerebral cortex of cortical thickness associated with recognition memory covaried for 30-minute delayed recall. This map demonstrates that only a very specific rostral MTL region was associated with recognition performance without the other cortical regions in Figure 3C. A statistical threshold of p < 10-4 was used for this analysis.
Discussion
Successful episodic memory function involves the coordination of a number of different cognitive processes that likely are subserved by the interaction of several large-scale neural networks. The present results support this contention by revealing dissociable anatomic substrates that differentially support memory performance depending on the stage of processing: encoding upon initial exposure, learning with repeated study, and delayed recall and recognition. Given the substantially disparate computations required at these stages of memory processing, from the initial exposure of a to-be-remembered event to its eventual retrieval, it is not surprising that the underlying neuroanatomy would vary significantly. While a considerable functional imaging literature has investigated the neural correlates of these processes, neuropsychological studies, such as the current work, have been less frequent. The ADNI cohort offers a large and relatively homogenous cohort to study these issues. In addition to providing insight into the substrates supporting episodic memory, the current findings have practical implications for the relative sensitivity of different memory measures to pathology in a number of key regions associated with AD. An understanding of these relationships may inform on the value of these commonly used measures in the early detection of AD and differentiation from other neurodegenerative conditions.
Process specificity within the MTL-cortical memory system
We found that performance on the initial encoding trials of the AVLT were associated with a broad set of isocortical regions that have been implicated in working memory, language, and semantic processing. Auditory-verbal working memory is likely critical to the initial trials of a list-learning memory task and crucial for the transfer of information into a long-term store. Regions found to correlate with the initial trial of the AVLT in the parietal lobe, including the supramarginal and angular gyrus, have been variably associated with the storage function of the phonological loop supporting auditory-verbal working memory (Baddeley, 1986, Buchsbaum and D'Esposito, 2009, Markowitsch et al., 1999, Peters et al., 2009).
Further, the exploratory global analysis of the first immediate memory trial delineates a number of regions that have also been proposed to be supportive of verbal working memory. In addition to the above noted parietal regions, thickness of the left superior temporal gyrus/sulcus and dorsolateral prefrontal cortex (e.g. caudal middle frontal gyrus) were associated with Trial 1 performance. A number of functional imaging studies attempting to localize the phonologic store in verbal working memory tasks have reported activations in posterior superior temporal regions, in addition to inferior parietal regions [for review, see (Buchsbaum and D'Esposito, 2008)]. Dorsolateral prefrontal cortex has been generally implicated as serving the central executive function in Baddeley's classic models of working memory (Baddeley, 1986, Baddeley, 2003). Finally, consistent with the localization of the phonologic loop, these anatomic correlations predominantly involved the left hemisphere despite the relative symmetry of atrophy in AD. Although by Trial 5 medial temporal lobe regions also appear to modulate performance, there remains a strong association with the above regions underscoring the salient role of working memory processes in list learning and, more generally, episodic memory function.
However, the region that most consistently predicted immediate recall performance (early and late trials) on all three memory tests was the temporal pole. In addition to the role of working memory in the successful encoding of information, a rich literature supports the importance of deep, semantic processing of information as a critical factor in the strength of a memory trace for later retrieval (Craik and Lockhart, 1972, Goldblum et al., 1998). The temporal pole has been implicated in high level, modality-invariant conceptual processing (Lambon Ralph et al., 2009, Lambon Ralph et al. , Patterson et al., 2007), and this function may contribute to a deeper level of processing and semantic organization that supports robust memory encoding. Interestingly, the AVLT and ADAS-cog list-learning tasks consist of semantically unrelated items while the LMT involves memory for a story. Despite potential differences in the need to impose a semantic organization on a list of unrelated words versus a story, the temporal pole was a strong predictor of immediate recall in each case. Lending further support to these ideas, prior work has suggested that the semantic impairments of AD may contribute to deficits in both verbal working memory and episodic memory (Goldblum et al., 1998, Peters et al., 2009).
One of the most striking findings related to the immediate memory measures is the lack of influence of medial temporal structures during the early trials and their increased explanatory value in later trials. This pattern appears quite consistent with the notion that performance on initial trials depends on working memory maintenance and aspects of strategic processing while transfer of information to long-term memory, supported by medial temporal structures, contributes to performance on later trials to a greater extent and serves as a better indicator of actual learning (Alexander et al., 2003). Both working and episodic memory deficits have long been accepted as relatively early features of AD (Baddeley et al., 1991, Becker et al., 1988) and the present data suggest that the relative contribution of these processes to list-learning is dependent on the stage of processing.
In contrast to the immediate memory trials, delayed memory performance was explained almost exclusively by MTL structures. Indeed, on the delayed free recall trials, the hippocampus was the only structure that correlated with performance on the AVLT and that remained in the best regression models for all three tests. Moreover, when we combined recall across the three memory tests to mitigate against floor effects and create a greater range of performance, the regression analysis recapitulated the findings of the individual tests and, again, hippocampus was the only region to predict delayed recall. The dissociation of involvement of non-MTL isocortical structures is also illustrated by the exploratory cortical thickness maps when comparing immediate memory trials versus delayed recognition (see Figure 3). Recognition discrimination is not associated with the lateral temporal and parietal regions observed on the immediate memory trials, but rather much more prominent MTL cortical effects.
This result is consistent with a number of studies of AD patients that have demonstrated a relationship between the hippocampus and delayed recall (Killiany et al., 2002, Kramer et al., 2004, Petersen et al., 2000, Vargha-Khadem et al., 1997). However, most of this prior work has focused only on the MTL, particularly hippocampus, and, thus, has not been able to assess dissociations with other regions associated with AD atrophy, as was done here. Nonetheless, the current result does bear resemblance to a prior study with a smaller cohort of AD patients in which only hippocampus correlated with delayed recall while total cortical grey matter volume, and not hippocampus, predicted immediate memory performance (Kramer et al., 2004). Similarly, Kohler and colleagues described stronger relationships between medial temporal volumes and delayed memory than for immediate memory trials (Kohler et al., 1998). Finally, another recent study examined a subset of the ADNI AD cohort (n=36) explored here and correlated total learning on the AVLT and delayed recognition memory to MRI and FDG PET landmark-defined ROIs. As found here, MTL regions only weakly correlated with total learning, but were the only regions to correlate with delayed memory (Walhovd et al., 2008). In that study, although total learning correlated with parietal and frontal regions, in contrast to the current results the posterior cingulate was the best predictor. However, their analysis did not include non-MTL temporal structures (e.g. temporal pole) or lateral frontal cortex.
Further, similar to their findings, precuneus and posterior cingulate associations were observed in our exploratory analysis of the immediate memory trials. These medial parietal regions have been associated with the ‘default mode’ (Raichle et al., 2001), display increased activity during successful memory retrieval (Wheeler and Buckner, 2004), and, based on measures of functional connectivity, represent nodes in a distributed hippocampal memory network (Vincent et al., 2006). While the significance is unclear, it is interesting to note that atrophy in these medial parietal regions appears to be particularly associated with immediate rather than delayed memory retrieval in this cohort. That precuneus and posterior cingulate pathology is associated with poorer immediate memory is consistent with functional imaging work suggesting aberrant encoding deactivations of these regions accompanies memory loss associated with aging and AD (Celone et al., 2006, Miller et al., 2008).
Division of labor within the MTL
As with delayed recall, recognition memory was also most strongly associated with MTL structures, but in this case the extrahippocampal, rostral MTL cortical region provided the highest explanatory power. Impressively, hippocampal volume offered no additional predictive information when included in the regression model with this ROI. The dissociation of the rostral MTL region, which encompasses the entorhinal and perirhinal cortices, and hippocampus in relationship to recognition and delayed recall is consistent with the ‘dual process model.’ Figure 5 provides an illustration of the data highlighting this behavioral-anatomic dissociation in a manner that is strikingly parallel to that reported in a recent paper on normal aging (Yonelinas et al., 2007). This account argues that memory may be subserved by the dissociable processes of recollection and familiarity (Brown and Aggleton, 2001, Eichenbaum et al., 1994, Jacoby, 1991, Mandler, 1980, Yonelinas, 2002). Recollection is conceived of as reflecting conscious, contextual retrieval of a prior episode or event, while familiarity is described as an acontextual sense of prior exposure. A number of studies have supported an anatomic dissociation such that recollection is dependent on the hippocampus while familiarity is subserved by extrahippocampal MTL cortex regions, particularly perirhinal cortex and perhaps the lateral entorhinal region (Aggleton and Brown, 2006, Eichenbaum et al., 2007, Yonelinas, 2002).
Figure 5.
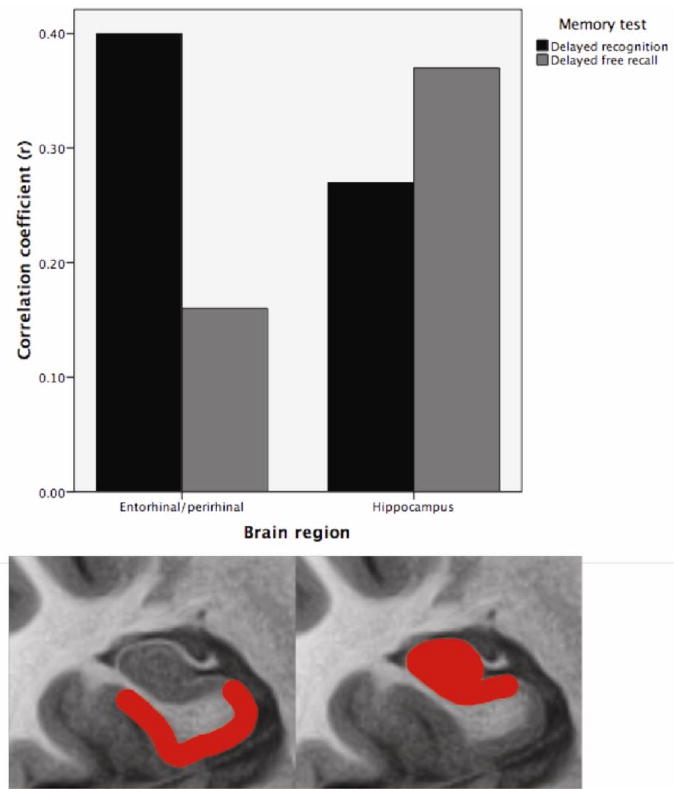
Pearson correlations for hippocampus and perirhinal/entorhinal cortex (rostral MTL) with AVLT 30-minute delayed recall and recognition discrimination, controlled for age, education, and gender. Hippocampal atrophy is more strongly related to impaired delayed recall while perirhinal/entorhinal cortex atrophy is more strongly associated with impaired recognition memory performance. The bottom section of the figure represents the ‘idealized’ anatomic localization of the two regions being compared; however, the actual ROI for perirhinal/entorhinal cortex is displayed in Figure 1A (rostral medial temporal cortex).
This view of memory is contentious [see (Squire et al., 2007)], and neuropsychological data demonstrating a double dissociation of the memory processes with their putative underlying anatomic substrates have been scant. The current data appear to provide such a dissociation in that free recall, often conceptualized as solely dependent on recollection (Yonelinas, 2002), correlated with hippocampus, but not rostral MTL cortex. In contrast, recognition memory discrimination, thought dependent on a mixture of recollection and familiarity, was more strongly associated the perirhinal/entorhinal atrophy than that of the hippocampus. Moreover, for any given level of recall performance, differences in discrimination are likely to reflect the degree to which familiarity supports additional memory accuracy during the recognition phase. In other words, better recognition memory in the setting of poor recall would suggest a greater preservation of familiarity-based memory. Consistent with this notion and the anatomic mappings of the dual process model, controlling for delayed recall resulted in only the rostral MTL cortex being associated with recognition memory accuracy (see Figures 2 and 4). In this context, the hippocampal ROI no longer demonstrated any predictive value on its own.
These data accord well with two other studies that have described similar dissociations. Yonelinas and colleagues also compared recall and recognition memory scores in older adults and found dissociable correlations with hippocampal and entorhinal volumes, respectively (Yonelinas et al., 2007). Another recent study using experimental measures of recollection and familiarity found a similar dissociation between hippocampal and extrahippocampal MTL volumes in a population of older adults and patients with AD and mild cognitive impairment (Wolk et al., in press). It is also worth noting that in the Walhovd and colleagues' study involving a subsample of the present dataset, the entorhinal cortex appeared to correlate more strongly with recognition memory than hippocampus, but none of these correlations reached significance perhaps related to the much smaller sample size (Walhovd et al., 2008). Finally, an additional caveat is that the present results cannot adjudicate between some alternative accounts to the dual process model. For example, Squire and colleagues have suggested that measures that dissociate between recollection and familiarity may be better classified along the orthogonal dimension of weak and strong memories, which map onto the perirhinal cortex and hippocampus, respectively (Squire et al., 2007). Further work will be needed to distinguish between these accounts, as well as to assess whether subregions within these structures correlate differentially with these processes.
Clinical implications
In addition to providing insight into the underlying substrates supporting different aspects of memory processing, the current findings have practical implications about the nature of these tests commonly used in the assessment of patients with AD and other conditions. For example, these data support the notion that measures of immediate memory are sensitive to pathology in a number of different brain regions, reflecting the role of several disparate cognitive processes involved in list learning. This finding may explain why immediate memory, usually quantified as total learning, has proven to be such a sensitive measure to the early stages of AD, as injury to a number of different neuroanatomical sites might modulate performance (Albert et al., 2001, Blacker et al., 2007, Petersen et al., 1994, Tierney et al., 2005). However, this property may also limit specificity, as a number of these structures might be selectively injured in other conditions. For example, a recent study found that list learning had the highest diagnostic accuracy in differentiating controls from MCI patients, but that a delayed recognition memory measure was superior in predicting conversion to AD (Rabin et al., 2009). Given the early and relatively selective medial temporal pathology of AD (Arnold et al., 1991, Braak and Braak, 1991), delayed memory tests may provide the most specific measures of early disease. It is particularly intriguing that recognition discrimination appears to be modulated by the rostral MTL cortex, which includes both perirhinal and entorhinal cortex. As noted above, this result echoes prior work suggesting that familiarity-based memory may be subserved by these structures (Aggleton and Brown, 2006, Eichenbaum et al., 2007, Wolk et al., in press, Yonelinas et al., 2007). These regions are often considered to be the earliest affected by neurofibrillary tangle pathology (Braak and Braak, 1991, Delacourte et al., 1999) and, thus, psychometric tests sensitive to this pathology may be particularly useful in early diagnosis. Yet clinical lore suggests that recognition memory tests are not particularly sensitive to early or prodromal AD. However, most recognition measures in standard psychometric batteries suffer from ceiling effects in more mild or ‘preclinical’ patients. We believe that tests designed specifically for these populations would be a worthy area of future investigation. For example, Guedj and colleagues tested MCI patients with impaired free recall on an experimental recognition memory test and found that those below a cut-off displayed an AD-specific PET pattern while those above had a pattern more consistent with age-associated memory loss (Guedj et al., 2006).
Caveats and Conclusions
The current analysis has a number of strengths as well as potential limitations. In addition to the large sample size of a well-characterized and relatively homogeneous cohort of patients with mild AD, some strengths include the novel approach of using a priori ROIs which have been previously determined to be particularly sensitive to AD. The ‘Cortical Signature of AD’ regions were defined and replicated in several samples of patients with mild AD and represent regions in which atrophy is consistently found in these populations (Bakkour et al., 2009, Dickerson et al., 2009). This approach, which focuses on regions known to undergo atrophy in the disease, is akin to lesion mapping approaches that consider the spatial distribution of lesioned brain regions in the sample under study (Schwartz et al., 2009). That is, in a lesion-symptom mapping study, it is important to delineate the areas of the brain with which it is possible to find behavioral relationships since most patient lesion studies do not include patients with lesions spanning every brain region, thus leaving some brain regions unexplored in any given sample. Following the same logic, since AD is associated with a fairly stereotypic pattern of cortical atrophy, we focused on the regions in which consistent disease effects are present. In addition, the use of disease-specific ROIs has the advantage over a traditional landmark-based anatomic approach in that the disease process may not respect these boundaries or only affect a portion of the region, limiting the power to find these relationships. Thus, associations reported here between impaired performance and regional cortical thinning are highly likely to be driven by the disease process itself and generalizable to other samples, whereas findings from exploratory analyses including regions not consistently affected by AD may be less generalizable. However, to the extent that additional regions also modulate these functions, this approach may produce Type II errors. Nonetheless, the ROI and exploratory approaches employed here yielded remarkably convergent results. Finally, it is worth pointing out that although a few investigations have demonstrated that MRI-derived hippocampal volume correlates with neurofibrillary tangle burden in AD patients (Csernansky et al., 2004, Gosche et al., 2002, Jack et al., 2002, Silbert et al., 2003), the histologic determinants of cortical thinning are unknown, and may include amyloid pathology, neurofibrillary pathology, or the loss of neuronal, glial, or other important cellular components such as neuropil volume, which reflects synaptic numbers and extent of dendritic branching, both of which are markedly reduced early in the course of AD (Coleman et al., 2004, Coleman and Flood, 1987, DeKosky and Scheff, 1990, DeKosky et al., 1996, Dickerson et al., 2009, Scheff et al., 1990).
In conclusion, we found dissociable relationships between regional brain atrophy and different aspects of memory function. These results provide insight into the sensitivity of these standard psychometric measures to the topographically diverse anatomic involvement of the AD pathologic process. A fuller understanding of these brain-behavior relationships is essential to the interpretation of deficits on these tests. Additionally, this work supports the notion that diverse cognitive processes support different stages of memory function. Working memory and semantic processing are likely to be particularly critical to the effective encoding of verbal information, while MTL structures support the long-term storage of this information. Further, a division of labor may exist within the MTL dependent on the nature of the memory trace. It is important to keep in mind that we do not mean to suggest that the relationships described here fully define the substrates of these memory processes, but that they represent critical nodes in the neural networks supporting them. For example, delayed recall is also clearly dependent on executive-control processes subserved by non-MTL systems. In other populations in which executive function is more disproportionately affected than AD, such as Frontotemporal Dementia, frontal ROIs might have greater predictive power for delayed recall. Thus, it will be important to study how selective these relationships are across disease populations to assist in understanding their value in differential diagnosis.
Research Highlights
The volume of dissociable brain regions correlate with different stages of memory processing in patients with mild Alzheimer's Disease
The early immediate memory trials on a word list memory task correlate most strongly with brain regions associated with working and semantic memory; while later immediate memory trials (after repeated exposure) are additionally associated with medial temporal lobe structures
Measures of delayed memory correlate more exclusively with medial temporal lobe structures than immediate memory trials
There appears to be a division of labor within the medial temporal lobes for qualitatively different forms of delayed memory, as free recall measures correlate more strongly with the hippocampus and recognition discrimination with the entorhinal/perirhinal cortex, consistent with the ‘Dual Process Model’
Supplementary Material
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.This analysis was also supported by grants from the NIA R01-AG29411, R21-AG29840, P50-AG005134, K23-AG028018, P30AG010124 and the Alzheimer's Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cogn Sci. 2006 Oct;10(10):455–63. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001 Jul;7(5):631–9. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Stuss D, Gillingham S. Impaired list learning is not a general property of frontal lesions. J Cogn Neurosci. 2009 Jul;21(7):1422–34. doi: 10.1162/jocn.2009.21094. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991 Jan-Feb;1(1):103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford: Oxford University Press; 1986. [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003 Oct;4(10):829–39. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Della Sala S, Spinnler H. The two-component hypothesis of memory deficit in Alzheimer's disease. J Clin Exp Neuropsychol. 1991 Mar;13(2):372–80. doi: 10.1080/01688639108401051. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009 Mar 24;72(12):1048–55. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001 Aug;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Becker JT, Huff FJ, Nebes RD, Holland A, Boller F. Neuropsychological function in Alzheimer's disease. Pattern of impairment and rates of progression. Arch Neurol. 1988 Mar;45(3):263–8. doi: 10.1001/archneur.1988.00520270037018. [DOI] [PubMed] [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, et al. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007 Jun;64(6):862–71. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007 Jun;13(3):280–91. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, et al. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci U S A. 2007 Oct 9;104(41):16382–7. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brown CM, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer's disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 1976 Dec 31;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008 May;20(5):762–78. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D'Esposito M. Repetition suppression and reactivation in auditory-verbal short-term recognition memory. Cereb Cortex. 2009 Jun;19(6):1474–85. doi: 10.1093/cercor/bhn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004 Oct;23(2):724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006 Oct 4;26(40):10222–31. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14837–42. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004 Oct 12;63(7):1155–62. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987 Nov-Dec;8(6):521–45. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: a framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–84. [Google Scholar]
- Csernansky JG, Hamstra J, Wang L, McKeel D, Price JL, Gado M, et al. Correlations between antemortem hippocampal volume and postmortem neuropathology in AD subjects. Alzheimer Dis Assoc Disord. 2004 Oct-Dec;18(4):190–5. [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999 Feb;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):2157–62. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990 May;27(5):457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, Styren SD. Structural correlates of cognition in dementia: quantification and assessment of synapse change. Neurodegeneration. 1996 Dec;5(4):417–21. doi: 10.1006/neur.1996.0056. [DOI] [PubMed] [Google Scholar]
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999 Apr 12;52(6):1158–65. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009 Mar;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen N. Two functional components of the hippocampal memory system. Behavioral Brain Science. 1994;17:449–517. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–52. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000 Sep 26;97(20):11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002 Jan 31;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999 Feb;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004 Jan;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–91. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001 Jul 21;358(9277):201–5. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002 Dec;73(6):657–64. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum MC, Gomez CM, Dalla Barba G, Boller F, Deweer B, Hahn V, et al. The influence of semantic and perceptual encoding on recognition memory in Alzheimer's disease. Neuropsychologia. 1998 Aug;36(8):717–29. doi: 10.1016/s0028-3932(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002 May 28;58(10):1476–82. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- Guedj E, Barbeau EJ, Didic M, Felician O, de Laforte C, Ceccaldi M, et al. Identification of subgroups in amnestic mild cognitive impairment. Neurology. 2006 Jul 25;67(2):356–8. doi: 10.1212/01.wnl.0000225076.73312.d4. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008 Apr;27(4):685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002 Mar 12;58(5):750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. A process-dissociation framework: separating automatic from intentional uses of memory. Journal of Memory and Language. 1991;30:513–41. [Google Scholar]
- Killiany RJ, Hyman BT, Gomez-Isla T, Moss MB, Kikinis R, Jolesz F, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002 Apr 23;58(8):1188–96. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: an MR volumetry study in Alzheimer's disease. Neuropsychologia. 1998 Sep;36(9):901–14. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Schuff N, Reed BR, Mungas D, Du AT, Rosen HJ, et al. Hippocampal volume and retention in Alzheimer's disease. J Int Neuropsychol Soc. 2004 Jul;10(4):639–43. doi: 10.1017/S1355617704104050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex. 2009 Apr;19(4):832–8. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci U S A. Feb 9;107(6):2717–22. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002 Feb 28;33(5):827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgement of previous occurrence. Psychological Review. 1980;87:252–71. [Google Scholar]
- Markowitsch HJ, Kalbe E, Kessler J, von Stockhausen HM, Ghaemi M, Heiss WD. Short-term memory deficit after focal parietal damage. J Clin Exp Neuropsychol. 1999 Dec;21(6):784–97. doi: 10.1076/jcen.21.6.784.853. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:285–97. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2181–6. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16(5):504–20. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001 Feb;124(Pt 2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007 Dec;8(12):976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain. 2009 Jul;132(Pt 7):1833–46. doi: 10.1093/brain/awp075. [DOI] [PubMed] [Google Scholar]
- Peters F, Majerus S, De Baerdemaeker J, Salmon E, Collette F. Impaired semantic knowledge underlies the reduced verbal short-term storage capacity in Alzheimer's disease. Neuropsychologia. 2009 Dec;47(14):3067–73. doi: 10.1016/j.neuropsychologia.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010 Jan 19;74(3):201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Xu YC, Waring SC, O'Brien PC, Smith GE, et al. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54(3):581–7. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Kokmen E, Tangalos EG. Memory function in very early Alzheimer's disease. Neurology. 1994 May;44(5):867–72. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991 Jul-Aug;12(4):295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, et al. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer's disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2009 May;16(3):357–76. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie Paris. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry. 1984;141(11):1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer's disease. Neurobiol Aging. 1990 Jan-Feb;11(1):29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009 Dec;132(Pt 12):3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003 Aug 26;61(4):487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988 Mar;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007 Nov;8(11):872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Mega MS, Woods RP, Zoumalan CI, Lindshield CJ, Blanton RE, et al. Cortical change in Alzheimer's disease detected with a disease-specific population-based brain atlas. Cereb Cortex. 2001 Jan;11(1):1–16. doi: 10.1093/cercor/11.1.1. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005 Jun 14;64(11):1853–9. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–80. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006 Dec;96(6):3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, et al. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging. 2008 Oct 5; doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WMS-R Wechsler Memory Scale - Revised Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Dunfee KL, Dickerson BC, Aizenstein HJ, DeKosky ST. A medial temporal lobe division of Labor: Insights from memory in early Alzheimer's disease. doi: 10.1002/hipo.20779. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1134–40. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


