Abstract
Removal of the anterior temporal lobe (ATL) is an effective surgical treatment for intractable temporal lobe epilepsy but carries a risk of language and verbal memory deficits. Preoperative localization of functional zones in the ATL might help reduce these risks, yet fMRI protocols in current widespread use produce very little activation in this region. Based on recent evidence suggesting a role for the ATL in semantic integration, we designed an fMRI protocol comparing comprehension of brief narratives (Story task) with a semantically shallow control task involving serial arithmetic (Math task). The Story > Math contrast elicited strong activation throughout the ATL, lateral temporal lobe, and medial temporal lobe bilaterally in an initial cohort of 18 healthy participants. The task protocol was then implemented at 6 other imaging centers using identical methods. Data from a second cohort of participants scanned at these centers closely replicated the results from the initial cohort. The Story-Math protocol provides a reliable method for activation of surgical regions of interest in the ATL. The bilateral activation supports previous claims that conceptual processing involves both temporal lobes. Used in combination with language lateralization measures, reliable ATL activation maps may be useful for predicting cognitive outcome in ATL surgery, though the validity of this approach needs to be established in a prospective surgical series.
Keywords: Language, semantics, anterior temporal lobe, fMRI, epilepsy
Introduction
Partial removal of the anterior temporal lobe (ATL) remains the most common surgical procedure performed for treatment of intractable epilepsy and is effective at stopping seizures in a majority of patients (Tellez-Zenteno et al., 2005; Wiebe et al., 2001). Postoperative decline in naming or verbal memory ability is observed in roughly 30–50% of patients after left ATL resection (Baxendale et al., 2006; Bell et al., 2000; Binder et al., 2008a; Chelune et al., 1993; Gleissner et al., 2004; Helmstaedter and Elger, 1996; Hermann et al., 1994; Langfitt and Rausch, 1996; Lee et al., 2002; Lineweaver et al., 2006; Sabsevitz et al., 2003; Stroup et al., 2003). The magnitude of these declines is related to the degree of language lateralization to the left hemisphere and can be predicted using preoperative functional magnetic resonance imaging (fMRI) (Binder et al., 2008a; Sabsevitz et al., 2003).
FMRI might also be useful for “tailoring” resections to avoid critical language zones in the ATL. This application of fMRI, however, rests on two critical assumptions: that activated areas are functionally necessary and should not be resected, and that inactive areas are functionally unimportant and therefore safe to resect. The former assumption is problematic because activation may reflect a variety of processes and does not necessarily indicate that the activated region is critical for the cognitive outcome of interest. The latter assumption is particularly problematic because different fMRI language contrasts vary dramatically in their ability to identify functionally active tissue in the ATL (Binder et al., 2008b; Visser et al., 2010). Though there is an extensive literature on activation of the medial ATL (anterior hippocampus and parahippoccampus) using episodic memory encoding andretrieval tasks (see, e.g., Hwang and Golby, 2006; Paller and Wagner, 2002; Rugg et al., 2002; Schacter and Addis, 2007 for reviews), methods for activating ventral, lateral, and polar regions of the ATL are less well defined. Most fMRI language paradigms in widespread clinical use produce little or no activation of these regions (Benson et al., 1999; Binder et al., 2008b; Jansen et al., 2006; Lehéricy et al., 2000). The validity of tailoring resections using fMRI maps thus depends on the activation protocol used to generate these maps. Another factor that can impair fMRI sensitivity in the ATL is signal loss due to macroscopic field gradients, which commonly affects specific regions of the ATL (Devlin et al., 2000; Ojemann et al., 1997).
Considerable functional imaging and neuropsychological evidence suggests a role for the ATL in semantic processing, that is, storage and retrieval of conceptual knowledge that underlies word meaning (Binder et al., 2009; Mummery et al., 2000; Patterson et al., 2007; Rosen et al., 2002; Visser et al., 2010). Damage to this semantic system has been proposed as a major cause of the naming deficits observed in patients with ATL damage (Bell et al., 2001; Lambon Ralph et al., 2001). Recent studies suggest several factors that influence the detection of these ATL areas with fMRI. First, the ATL is more strongly activated by sentences than by single words or strings of unrelated words (Friederici et al., 2000; Humphries et al., 2006; Humphries et al., 2005; Mazoyer et al., 1993; Vandenberghe et al., 2002; Visser et al., 2010; Xu et al., 2005). This observation suggests that at least some parts of the ATL are involved in multi-word integration processes unique to sentence comprehension tasks (Jung-Beeman, 2005). Comprehension of sentences, and particularly of longer forms such as narratives and discourse, requires not only rapid retrieval of conceptual representations but also integration of individual concepts to form complex scenes with actors, intentions, and events. Thus there is also evidence for involvement of the ATL in comprehension of social interactions (Olson et al., 2007; Ross and Olson, 2010; Zahn et al., 2007), which likely depends on a similar rapid integration of conceptual information. Activation in these studies typically involves polar and superior aspects of the ATL bilaterally. Second, ATL activation is more likely to be observed when an active control task is used as a baseline rather than a resting state (Binder et al., 2008b; Spitsyna et al., 2006; Stark and Squire, 2001; Visser et al., 2010). For example, Stark and Squire (2001) observed activation in medial ATL regions during a picture encoding task when an active decision task was used as a baseline but not when a “rest” baseline was used. Similarly, Spitsyna et al. (2006) observed activation in the anterior fusiform gyrus and ITG during a story comprehension task when an active decision task was used as a baseline but not when a “passive” baseline was used. This observation suggests that semantic and episodic memory processes carried out by the ATL occur even during resting or passive states, comprising a component of normal consciousness that supports planning, problem solving, daydreaming, and other high-level integrative processes that depend on semantic knowledge (Binder et al., 2009; Binder et al., 1999). Active, attentionally-demanding control tasks disrupt these ongoing “default” processes, which would otherwise mask ATL activation.
Given the potential clinical benefits of using fMRI to tailor ATL resections, there is a critical need to define a reliable and sensitive fMRI task paradigm for this purpose. Our aim in the current study was to develop such a method and test its effectiveness at identifying ATL language zones in individual subjects. Based on prior studies of the ATL reviewed above, a story comprehension task was selected to engage rapid integration of conceptual information, including social concepts. This task was contrasted with an active, attentionally demanding arithmetic task. Prior evidence indicates that calculation tasks, particularly addition and subtraction operations, do not engage the temporal lobe (Baldo and Dronkers, 2007; Cappelletti et al., 2001; Crutch and Warrington, 2002; Diesfeldt, 1993), thus this task was expected to interrupt ongoing “default mode” processing in the ATL and to cause minimal ATL activation. An additional feature of the arithmetic task is that it used verbal, sentence-like stimuli that could be matched to the stories on low-level features like auditory and phonological input. A second aim of the study was to test whether comparable results could be achieved at different centers with a variety of imaging hardware and software platforms, as this is a prerequisite for any test under consideration for clinical application. It was not our aim to test different pulse sequences for optimal ATL coverage, to investigate alternative data analysis methods, or to examine effects of thresholding at different levels, though these are all important topics for future research.
Methods
Participants and Centers
Participants in the study were 34 healthy, right-handed adults (17 women, 17 men), age 18–50 (mean 29). All spoke English fluently and had no history of neurological illness. In the initial development phase, 18 of these participants were scanned at the Medical College of Wisconsin (MCW). Subsequently, 2–3 additional participants were scanned at each of the following six centers to create a Multicenter cohort: Cleveland Clinic Foundation, Cleveland, OH (CCF); Georgia Institute of Technology, Atlanta, GA (GT); Medical University of South Carolina, Charleston, SC (MUSC); University of Cincinnati, Cincinnati, OH (UCin); University of Rochester, Rochester, NY (UR); and University of Washington, Seattle, WA (UW). The MCW and Multicenter cohorts were matched on age, sex, and education. All participants gave informed consent according to local institutional guidelines.
Tasks and Stimuli
Identical stimuli and E-Prime (Psychology Software Tools, www.pstnet.com) scripts were used at each center. All stimuli were presented aurally using MRI-compatible headphones adjusted to a comfortable listening level. Participants kept their eyes closed during all functional scans.
Story task
The language task required attentive comprehension of brief stories adapted from a collection of Aesop’s fables (www.aesopfables.com). Spoken versions of the stories were generated using a text-to-speech synthesizer (MacinTalk male “Alex” voice in Mac OS X 10.5), which enabled control over word rate, speaking style, and prosodic features. Stories were selected to be relatively brief (around 5–9 sentences) and engaging. All involved animal or human characters interacting in easily understandable social situations. Salient characteristics of the stories are listed in Table 1. After each story, the participant was queried about the general topic of the story in the form of a 2-alternative forced-choice question. For example, after a story about an eagle that saves a man who had done him a favor, participants were asked, “That was about revengeor reciprocity?” Participants pressed a button under the right index finger to select the first choice or a button under the right middle finger to select the second choice.
Table 1.
Story characteristics. Numbers represent the mean (sd) value across the 26 stories.
| Duration (sec) | 29.8 (14.5) |
| Number of words | 88.1 (41.4) |
| Number of sentences | 7.0 (3.0) |
| Mean sentence length (words) | 12.7 (2.7) |
| Percent content words | 47.8 (4.8) |
| Mean log content word frequency | 2.07 (0.20) |
| Mean noun imageability (1–7) | 5.67 (0.34) |
| Number of actors | 2.8 (1.5) |
| Number of events | 9.4 (3.4) |
Math task
The control task was designed to engage participants’ attention continuously in mental arithmetic. The materials were problems requiring serial addition and subtraction and were generated using the same text-to-speech method used for the Story task stimuli, with matched word and phoneme rate, speaking style, and prosodic features. Each series of arithmetic operations ended with the word “equals” followed by two alternative choices, e.g., “Four plus twelve minus two plus nineequals twenty-two or twenty-three?” The participant pressed a button under the right index finger to select the first choice or a button under the right middle finger to select the second choice.
Participants were expected to vary widely in ability, experience, and confidence with math problems. Thus, the presentation program was designed to automatically adjust the difficulty level of the problems to maintain accuracy at around 78% correct. The stimuli were divided into 20 levels of difficulty (1 = easiest, 20 = hardest) defined by the number of operations, size of the integers, and proportion of subtraction (relative to addition) operations. A training session outside the scanner was used to find an approximate level of difficulty for each participant. Further adjustment occurred automatically during scanning using a staircase method. The level increased in difficulty after 6 consecutive correct responses and decreased in difficulty after any incorrect response. The level at the start of each run was set to the level attained at the end of the preceding run.
Task training
A scripted training procedure was used to familiarize the participants with each task prior to scanning, with step-by-step instructions presented on a computer screen along with example stimuli and practice items. Instructions were visible to the participant and read aloud by the researcher throughout the training session. All training was performed by a neuropsychologist or behavioral neurologist with expertise in cognitive test administration.
Timing of task blocks
A total of 26 Story blocks and 26 Math blocks were presented over the course of 5 imaging runs, with the order of the blocks pseudorandomized. Each block was introduced with an auditory cue word, “Story” or “Math”, presented 3 sec prior to the beginning of the block. A response period of 2 sec was provided after each story or math question. The duration of the Math blocks varied across participants depending on the current difficulty level, which affected the number of operations presented in each math problem. To compensate for this variability and to ensure that an approximately equal number of images were acquired in the Story and Math conditions, each run ended with a Math block that continued until the end of the run. Total scanning time for all 5 runs was approximately 30 minutes.
Imaging Methods
Imaging was performed at 3 Tesla using GE Excite (MCW), Siemens Tim Trio (CCF, GT, MUSC, UR), or Philips Achieva (UCin, UW) scanners and head array receive coils with 8 or 12 channels. Two participants at UR were scanned with a 32-channel coil. FMRI used a gradient-echo echoplanar imaging (EPI) sequence. A short echo time (25 ms) and relatively small voxel size (2.5 mm cubic) were used to minimize signal dropout from intravoxel dephasing (Haacke et al., 1989; Merboldt et al., 2000; Morawetz et al., 2008). Other parameters included TR 3 sec, matrix 96 × 96, bandwidth ~250 kHz, partial Fourier factor of 0.82–0.875, and flip angle 84°. Gapless axial 2.5-mm slices were used in all cases. Number of slices ranged from 41 to 52. Complete coverage of the temporal lobes was required, and coverage included nearly the entire cerebrum, excluding only the most superior frontoparietal region in some participants. A high-resolution T1-weighted image (SPGR or MPRAGE) was acquired in each participant using approximately 1-mm cubic voxels.
Image Analysis
All data were de-identified and transferred to MCW for analysis with identical procedures, implemented inAFNI. Preprocessing steps included slice timing correction and rigid -body image registration of each EPI time series. Translation and rotation parameters estimated during registration were saved for use as noise covariates. EPI volumes were then registered to the T1 anatomical image using a modality-specific cost function based on weighted local Pearson coefficients (Saad et al., 2009). Image time points contaminated by residual artifactual transients were identified using automated routines in AFNI (‘3dToutcount’) and excluded from the analysis. This method resulted in an average exclusion of 2.7% of the image volumes.
BOLD signal changes were analyzed using a multiple linear regression model. The time series regressors coded: 1) story presentations, 2) math problem presentations (i.e., addition and subtraction steps up to the word “equals”), 3) story question presentations, 4) math question presentations, 5) response intervals following story questions, 6) response intervals following math questions, and 7) task cue words preceding story and math blocks. These indicator variables were convolved with a canonical hemodynamic response function. Six orthogonal motion vectors computed during image registration were included as covariates of no interest. Baseline trends were modeled using a third-order polynomial. A contrast was performed in each participant comparing the BOLD signal during story presentation (regressor 1) to the BOLD signal during math problems (regressor 2).
Activation in single-subject BOLD maps increases with image smoothness (Friedman et al., 2006a; Lowe and Sorenson, 1997; Parrish et al., 2000), and smoothness is known to vary across scanners (Friedman et al., 2006a). The smoothness of each dataset was determined from the time series of residual error values output from the multiple regression analysis, using the AFNI program 3dFWHMx. Average geometric mean smoothness across all datasets was 2.83 mm FWHM (range 2.4 to 3.5), with no difference across scanner vendors (GE = 2.76, Philips = 2.83, Siemens = 2.96). The raw data from each individual were then smoothed to a common level of 4 mm FWHM using AFNI’s 3dBlurtoFWHM, and these smoothness-normalized images were reanalyzed with the multiple regression model. Residual error time series images from this second analysis were used to confirm the target smoothness value of 4 mm. Individual beta coefficient contrast maps (difference between regressors 1 and 2) were then smoothed with a 7 mm FWHM kernel for construction of group activation maps. These individual maps were normalized with linear resampling to the standard stereotaxic space of Talairach and Tournoux (Talairach and Tournoux, 1988)and analyzed at the group level using a single sample t -test. Two group maps were generated, one from the 18 participants scanned at MCW and another from the 16 participants scanned at the other six sites. A direct, whole-brain, voxel-wise comparison between the MCW and Multicenter cohorts was also performed. For display purposes, group activation maps were thresholded at voxel-wise p <.005 and minimum cluster size of 2200 μl, which was determined by Monte-Carlo simulation to result in a whole-brain corrected p <.05 significance threshold.
ROI analysis
Given our interest in mapping ATL regions typically resected for treatment of epilepsy, a region of interest was created to measure extent of activation in this region. This ROI was created using structural MRI data from 23 left ATL resection patients treated at MCW. Preoperative and postoperative structural scans were aligned and digitally subtracted to identify resected voxels in each patient. These images were then transformed into standard space and overlapped across patients. The resulting overlap map was thresholded to identify voxels resected in at least 20% (5 of 23) of the patients. This ROI was applied to the stereotaxically transformed activation map of each individual in the present study. Voxels within the ROI that survived a voxel-wise threshold of p <.01 and cluster size threshold of 400 μl (whole-brain corrected p <.05) were counted in each participant.
To further characterize the spatial location and reliability of activation across individual participants in the ATL, the ATL ROI was further subdivided into 8 subregions using landmarks on the N27 brain, as shown in Figure 1. These subregions included the superior temporal pole, consisting of the superior temporal gyrus anterior to a coronal plane at the level of the limen insula (coronal plane 1); the inferior temporal pole, consisting of the middle temporal gyrus anterior to coronal plane 1; anterior lateral cortex, consisting of STG and MTG between the coronal plane 1 and a coronal plane through the posterior edge of the interpeduncular fossa (coronal plane 2); posterior lateral cortex, consisting of STG and MTG posterior to coronal plane 2; anterior ventral cortex, consisting of ITG and fusiform gyrus between coronal planes 1 and 2; posterior ventral cortex, consisting of ITG and fusiform gyrus posterior to coronal plane 2; anterior medial cortex, consisting of amygdala, hippocampus, and parahippocampus between coronal planes 1 and 2; and posterior medial cortex (pMed), consisting of hippocampus and parahippocampus posterior to coronal plane 2. The two coronal planes were oriented perpendicular to the long axis of the temporal lobe. Voxels surviving the whole-brain corrected threshold were counted in each of these 8 subregions in each participant.
Figure 1.
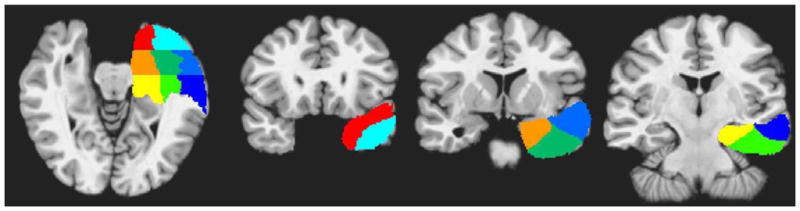
Anterior temporal subregions used to quantify activation extent and reliability of activation across individual participants. Red = superior temporal pole, cyan = inferior temporal pole, light blue = anterior lateral, dark blue = posterior lateral, dark green = anterior ventral, light green = posterior ventral, orange = anterior medial, yellow = posterior medial. See Methods for anatomical definitions of each subregion.
Finally, susceptibility-related signal loss in the ATL was quantified in each subject by calculating individual signal-to-fluctuation-noise-ratio (SFNR) maps following previously described methods (Friedman and Glover, 2006; Friedman et al., 2006b). Fluctuation noise was determined in each voxel using the standard deviation of the residual error time series images. Mean signal in each voxel was divided by these noise values to produce the SNFR maps, which thus represent signal magnitude relative to unexplained temporal signal fluctuation. These maps were thresholded to include only voxels in the ATL ROI with SFNR values below 20. Though this cutoff is necessarily arbitrary, previous observations (confirmed in the present study) suggest that SNFR values averaged over the brain at 3T are typically around 100. A value of 20 thus represents about 20% of a typical average SNFR value and at 3T may be a minimal level for detection of activation effects (Friedman et al., 2006b).
Results
Task Performance
The Math task was adjusted dynamically by increasing the difficulty level after six consecutive correct responses and decreasing the difficulty level after any incorrect response. Across all participants, this resulted in an average of 77.0% (SD 11.2) correct and an average difficulty level of 10.5 (SD 3.7) on a scale from 1 to 20. There were no differences between MCW and Multicenter participants on either of these measures (both p > 0.1). In the initial version of the task, the two response options always differed by one integer. One participant who achieved a very high difficulty level reported using a strategy of monitoring whether the result of each addition or subtraction step was odd or even. The program was changed after this so that response options could differ by 1, 2, or 10, the last to defeat a possible alternative strategy of monitoring only the modulo 10 remainder of each operation.
The Story stimuli used common vocabulary words and were easy to understand, but the probe questions were designed to be difficult so that participants would be forced to attend closely to the stories. Mean accuracy on this task was 75.7% (SD 12.1), with no difference between MCW and Multicenter participants (p >.1).
FMRI Results: MCW Cohort
Areas engaged selectively by the Story task relative to the Math task are shown in Figure 2. Stereotaxic coordinates of activation peaks are listed in Table 2. The largest activation cluster involves the left temporal lobe, including the entire temporal pole and anterior aspects of the lateral, ventral, and medial temporal cortex, including anterior superior temporal (STG), middle temporal (MTG), inferior temporal (ITG), and fusiform gyri. Lateral temporal activation extends posteriorly along most of the superior temporal sulcus (STS). Strong medial temporal activation involves the uncus, amygdala, and anterior hippocampus, extending posteriorly into the parahippocampus and posterior fusiform gyrus. Similar activation is observed in homologous regions of the right temporal lobe.
Figure 2.
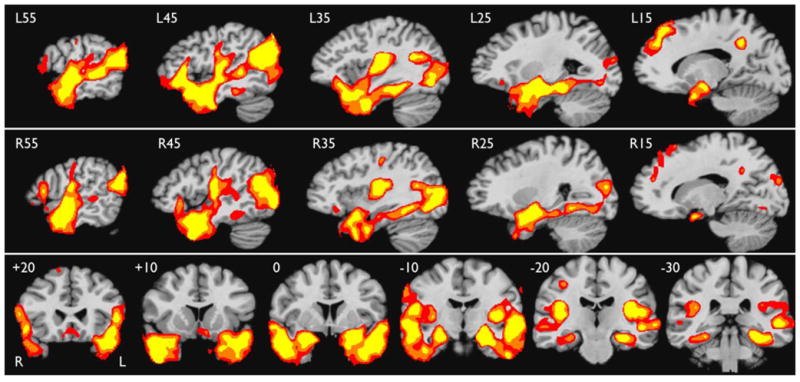
Areas of greater activation for the Story condition relative to the Math condition in the MCW cohort (thresholded at whole-brain corrected p < .05), shown in serial sagittal sections through the left (top row) and right (middle row) hemisphere, and in serial coronal sections covering the temporal lobes (bottom row). Colors indicate voxel-wise thresholds at p < .005 (red), p< .0005 (orange), and p < .00005 (yellow). Stereotaxic locations are given in the upper left corner of each slice.
Table 2.
Location of activation peaks in stereotaxic coordinates.
| Location | X | Y | Z | Z-score |
|---|---|---|---|---|
| Left Anterior Temporal | ||||
| L temporal pole | −43 | 13 | −19 | 5.78 |
| L temporal pole | −23 | 12 | −35 | 3.86 |
| L ant. MTG | −49 | −3 | −21 | 6.51 |
| L ant. MTG | −63 | −12 | −23 | 3.96 |
| L ant. STS | −54 | −6 | −8 | 6.48 |
| L ant. STS | −58 | −14 | −3 | 5.86 |
| L ant. STG | −49 | 7 | −12 | 5.05 |
| L anteromedial STG | −18 | 5 | −24 | 5.30 |
| L anteromedial STG | −31 | 0 | −10 | 5.07 |
| L ant. OTS | −38 | −12 | −24 | 3.89 |
| L ant. FG | −36 | −4 | −31 | 5.11 |
| L ant. FG | −26 | 2 | −42 | 3.70 |
| L amygdala | −21 | −5 | −6 | 5.80 |
| L ant. hippocampus | −23 | −11 | −18 | 5.73 |
| Right Anterior Temporal | ||||
| R temporal pole | 60 | 7 | −14 | 4.09 |
| R temporal pole | 26 | 13 | −38 | 3.87 |
| R ant. MTG | 49 | 5 | −21 | 6.56 |
| R ant. MTG | 46 | 22 | −22 | 4.07 |
| R ant. STS | 51 | −6 | −11 | 5.92 |
| R ant. STG | 55 | −4 | 0 | 5.62 |
| R ant. ITG | 49 | 6 | −35 | 5.63 |
| R ant. ITG | 40 | 8 | −40 | 5.41 |
| R ant. ITG | 41 | −10 | −28 | 4.45 |
| R ant. ITG | 57 | −11 | −31 | 3.72 |
| R ant. FG | 31 | −6 | −30 | 4.88 |
| R uncus | 27 | 5 | −22 | 4.88 |
| R uncus | −16 | 38 | 50 | 4.59 |
| R amygdala | 24 | −5 | −13 | 5.56 |
| Other Temporal | ||||
| L parahippocampus | −30 | −31 | −15 | 5.64 |
| L MTG | −50 | −38 | 2 | 5.29 |
| L MTG | −68 | −34 | 1 | 3.81 |
| R Heschl’s gyrus | 42 | −16 | 8 | 5.70 |
| R parahippocampus | 27 | −28 | −13 | 4.19 |
| R post. ITG | 40 | −60 | −3 | 4.62 |
| R FG | 32 | −49 | −8 | 4.50 |
| R OTS | 42 | −37 | −14 | 4.30 |
| R MTG | 64 | −37 | 4 | 3.54 |
| Posterior Parietal and Occipital | ||||
| L AG | −45 | −62 | 21 | 6.34 |
| L AG | −59 | −60 | 13 | 4.87 |
| L MOG | −37 | −73 | 1 | 4.51 |
| L MOG | −27 | −87 | 15 | 3.82 |
| L post. FG | −32 | −71 | −8 | 5.74 |
| R AG | 50 | −66 | 27 | 6.36 |
| R MOG | 39 | −79 | −1 | 5.44 |
| R MOG | 40 | −72 | 11 | 5.28 |
| R MOG | 32 | −85 | 10 | 5.26 |
| R MOG | 42 | −84 | 11 | 3.92 |
| R post. FG | 25 | −69 | −10 | 4.01 |
| Central | ||||
| L parietal operculum | −34 | −18 | 19 | 5.55 |
| L post. insula | −39 | −12 | 8 | 5.42 |
| L inf. PoCG | −43 | −13 | 24 | 4.46 |
| L central sulcus | −48 | −16 | 33 | 3.55 |
| R parietal operculum | 53 | −10 | 17 | 5.06 |
| R parietal operculum | 36 | −28 | 17 | 4.35 |
| R central sulcus | 35 | −21 | 43 | 3.87 |
| R central sulcus | 43 | −15 | 32 | 3.75 |
| Frontal | ||||
| L SFG | −12 | 41 | 38 | 5.63 |
| L IFG pars orbitalis | −44 | 28 | −7 | 5.41 |
| L IFG pars triangularis | −45 | 23 | 5 | 5.12 |
| L rostral cingulate | −1 | 45 | −2 | 3.78 |
| L gyrus rectus | −6 | 17 | −12 | 3.75 |
| R SFG | 13 | 45 | 24 | 4.18 |
| R SFG | 12 | 28 | 55 | 3.93 |
| R IFG pars orbitalis | 48 | 20 | −6 | 4.43 |
| R IFG pars orbitalis | 37 | 27 | −10 | 3.48 |
| R IFG pars triangularis | 56 | 19 | 10 | 4.27 |
| R rostral cingulate | 10 | 43 | −5 | 3.47 |
| Posterior Medial | ||||
| L post. cingulate | −11 | −48 | 36 | 5.78 |
| L post. cingulate | −3 | −52 | 29 | 5.31 |
| R post. cingulate | 10 | −52 | 32 | 5.23 |
Abbreviations: AG = angular gyrus, ant. = anterior, FG = fusiform gyrus, IFG = inferior frontal gyrus, ITG = inferior temporal gyrus, MOG = middle occipital gyrus, MTG = middle temporal gyrus, OTS = occipitotemporal sulcus, PoCG = postcentral gyrus, post. = posterior, SFG = superior frontal gyrus, STG = superior temporal gyrus, STS = superior temporal sulcus
A second prominent region of activation involves the lateral occipital lobe and angular gyrus bilaterally, with slightly more angular gyrus activation on the left. Left prefrontal activation involves the anterior and ventral inferior frontal gyrus (IFG), medial and dorsal aspects of the superior frontal gyrus (SFG), and ventromedial prefrontal cortex. Similar, though less extensive activations are observed in homologous right frontal regions. Other activated areas include the anterior parietal operculum bilaterally and the posterior cingulate gyrus bilaterally.
Figure 3 shows the degree of overlap in stereotaxic space between the activation produced by the Story–Math contrast and the ATL ROI representing voxels typically resected in left ATL surgery (see Methods). For comparison, the overlap between this typical resection volume and activation elicited by two other language comprehension protocols is also shown (Binder et al., 2008b). Overlapwith the resection volume is much more extensive for the Story – Math protocol than for the others, particularly in the temporal pole, which is only minimally activated by the other protocols. Interestingly, however, the Semantic Decision–Tone Decision protocol, which has been the subject of numerous prior studies in healthy and epilepsy populations (Binder et al., 1997; Binder et al., 2008a; Binder et al., 1996; Binder et al., 2008b; Sabsevitz et al., 2003; Springer et al., 1999; Szaflarski et al., 2008), produces stronger activation in more posterior-ventral aspects of the resection zone. Together, the two protocols activate largely complementary regions of the ATL that cover nearly all cortex in the typical resection volume.
Figure 3.
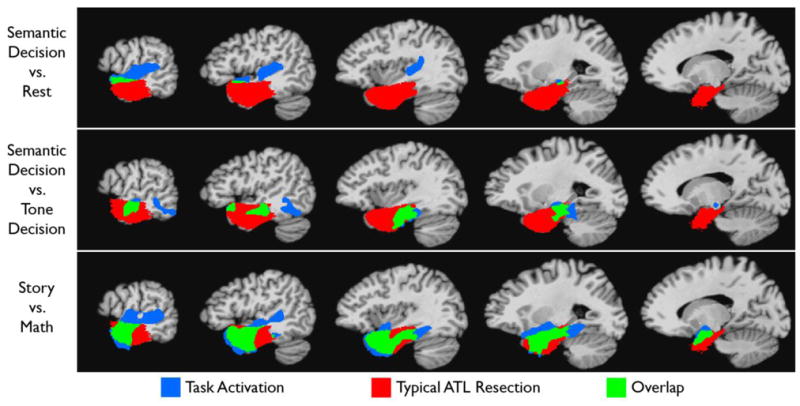
Overlap between activated areas for three fMRI language paradigms and regions typically resected in left anterior temporal lobe surgery (see Methods). Blue indicates temporal lobe regions activated with each paradigm (all thresholded at whole-brain corrected p < .05) that do not fall within the typical resection zone (see (Binder et al., 2008b) for a description of the Semantic Decision vs. Rest and the Semantic Decision vs. Tone Decision studies). Green indicates activated regions that overlap with the typical resection zone.
FMRI Results: Multicenter Cohort
Figure 4 shows the group activation map for 16 subjects from the six other imaging centers. The results are very similar to those obtained at MCW, including bilateral activation of the ATL, medial temporal lobe, STS, angular gyrus and lateral occipital lobe, SFG, IFG, parietal operculum, and posterior cingulate gyrus, with a somewhat larger extent of activation on the left in most areas. A voxel-wise, whole brain comparison of the MCW and Multicenter cohorts was also performed. This showed no differences in activation between the groups in any temporal lobe areas even at a relaxed voxel-wise threshold of p <.01 and small-volume cluster correction (563 μl) based on the surgical ATL ROI. The Multicenter cohort showed stronger activation in the ventromedial prefrontal region, likely reflecting greater susceptibility-induced signal dropout in this region in the MCW cohort.
Figure 4.
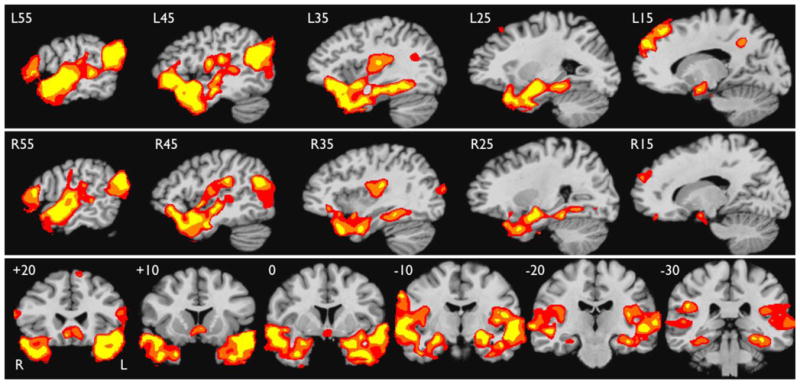
Areas of greater activation for the Story condition relative to the Math condition in the Multicenter cohort, shown in serial sagittal sections through the left (top row) and right (middle row) hemisphere, and coronal sections through the temporal lobes. Formatting as in Figure 2.
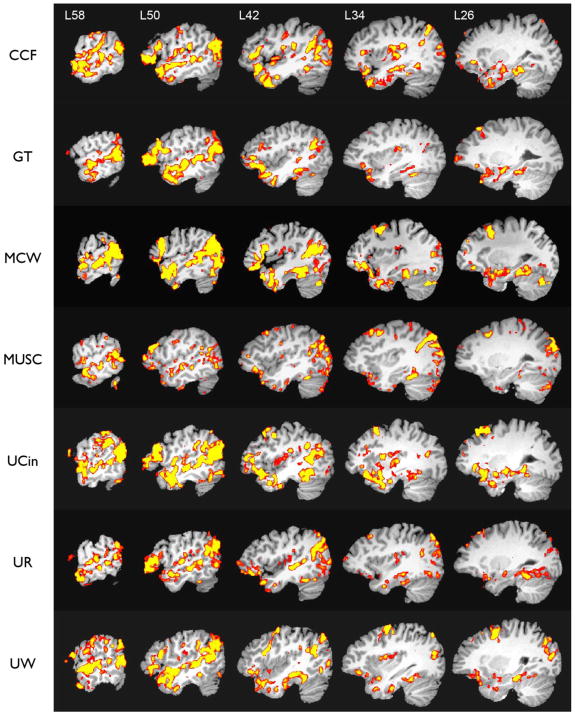
Representative single-subject activation maps (corrected p <.05 threshold) from each of the centers are shown in Figure 5. Robust ATL activation is observed in each case.
Figure 5.
Representative individual subject activation maps (one subject from each center), shown in serial sagittal sections through the left hemisphere after stereotaxic normalization. Colors indicate areas of greater activation for the Story condition relative to the Math condition, thresholded at whole-brain corrected p< .05.
ATL ROI Analysis of Individual Data
The left ATL resection volume shown in Figure 3 was used as an ROI applied to each individual’s activation map. Extent of ATL activation in each subject was determined by counting the number of voxels within this ROI that survived a whole-brain corrected p <.05 threshold. The mean extent of activation was 10,877 μl (SD 4,674), which represents an average of 38.1% of the grey matter voxels in the ROI. There was no difference in extent of activation between the MCW and Multicenter cohorts (unpaired t = 1.57, p >.1).
Table 3 summarizes the individual activation data for 8 anatomically defined subregions within the surgical ROI (see Figure 1). A subregion was counted as showing activation in an individual if the volume of activation was at least 156 μl, roughly equivalent to 10 voxels in the original images. Superior and inferior temporal pole regions were activated in 33 of 34 participants, and anterolateral cortex (STG and MTG) in 100%. Ventral regions (ITG and fusiform gyrus) were activated in over 80% of participants, and anteromedial regions (amygdala, anterior hippocampus, anterior parahippocampus) in 79%.
Table 3.
Individual participant activation in 8 left ATL subregions.
| Region | Activation volume in μl, mean (sd) | Participants with activation |
|---|---|---|
| Superior temporal pole | 2167 (1234) | 97% |
| Inferior temporal pole | 2173 (1264) | 97% |
| Lateral-anterior | 2705 (1055) | 100% |
| Lateral-posterior | 716 (575) | 91% |
| Ventral-anterior | 752 (577) | 85% |
| Ventral-posterior | 891 (668) | 82% |
| Medial-anterior | 806 (664) | 79% |
| Medial-posterior | 435 (397) | 68% |
Finally, Figure 6 shows ATL regions adversely affected by susceptibility -related signal loss. Individual maps of signal-to-fluctuation-noise-ratio (SFNR) were calculated following previously described methods (Friedman and Glover, 2006; Friedman et al., 2006b) and thresholded to include only voxels in the ATL ROI with SFNR values below 20. The overlap maps in Figure 6 show that signal loss consistently affected portions of the ATL bilaterally, most notably the medial temporal pole and adjacent uncus, and a lateral region overlying the petrous temporal bone that includes portions of the inferior temporal and fusiform gyri. This signal dropout affected an average of 12.4% of voxels in the left ATL ROI (SD 6.2). There was no difference in extent of ATL signal dropout between the MCW and Multicenter cohorts (unpaired t = 0.748, p >.1).
Figure 6.
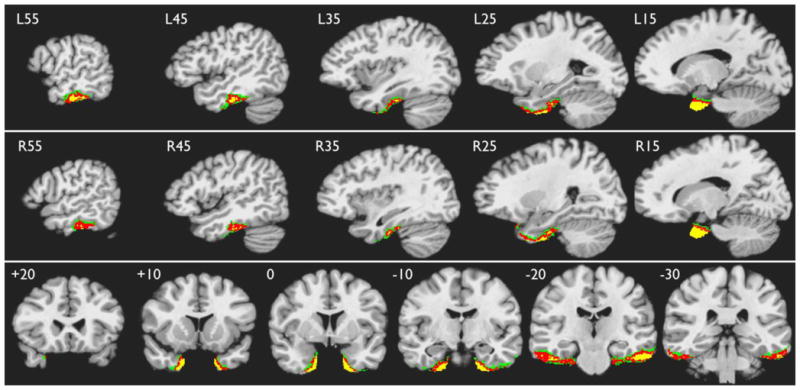
ATL areas showing consistent signal loss across participants. Green indicates overlap in at least 50%, red in 75%, and yellow in 90% of participants.
Discussion
We demonstrate here an fMRI task contrast that produces strong activation of the ATL. The ATL plays a central role in integration of semantic and syntactic information and is particularly responsive to meaningful sentences (Binder et al., 2009; Friederici et al., 2000; Humphries et al., 2006; Humphries et al., 2005; Mazoyer et al., 1993; Mummery et al., 2000; Patterson et al., 2007; Rosen et al., 2002; Vandenberghe et al., 2002; Visser et al., 2010; Xu et al., 2005). Some evidence suggests that the ATL, particularly the temporal pole and superior aspects of the ATL, play a special role in processing social concepts (Olson et al., 2007; Ross and Olson, 2010; Zahn et al., 2007). Parts of the ATL and medial temporal lobe appear to be active during resting and other passive cognitive states (Binder et al., 2008b; Spitsyna et al., 2006; Stark and Squire, 2001; Visser et al., 2010). Based on these prior observations, the protocol was designed to elicit maximal ATL activation by contrasting a story comprehension task, which should maximally engage systems required for complex semantic and syntactic integration, with a continuous, attentionally demanding, non-semantic task, which should maximally prevent such processes from occurring during intervals between the stories. The resulting temporal lobe activation is relatively symmetric, supporting the longstanding view that conceptual processing involves both hemispheres (Beeman and Chiarello, 1998; Bottini et al., 1994; Hodges et al., 1992; Jung-Beeman, 2005; Paivio, 1986; Tranel et al., 1997).
We also demonstrate successful replication of this fMRI protocol across six other imaging centers. Uniformity of results was achieved by implementing uniform task training procedures administered by clinicians with expertise in cognitive testing, identical fMRI tasks, and identical analysis procedures for all fMRI data, including compensation for variation in image smoothness across scanners. Replication across centers is a prerequisite for conducting multicenter imaging studies. The establishment of multicenter imaging consortia is becoming increasingly widespread (Evans and Group, 2006; Friedman and Glover, 2006; Mueller et al., 2005). Such consortia offer a powerful and efficient method for carrying out imaging studies that require large subject samples, and are particularly useful for patient studies. Our consortium plans to use the present ATL activation technique to examine the utility of presurgical fMRI mapping in patients undergoing left ATL surgery. Given an annual recruitment rate of roughly 5–10 patients at each of seven centers, it becomes feasible to enroll several hundred such patients in a matter of just a few years.
Retrieval of conceptual knowledge is central to many language tasks, including naming (Levelt, 1989). Anomic deficits from left ATL damage are generally considered to reflect damage to either the semantic system or its connections with the phonological system (Bell et al., 2001; Hadar et al., 1987; Hodges et al., 1995; Lambon Ralph et al., 2001). Despite evidence that the right temporal lobe plays a role in semantic retrieval, however, naming deficits are generally not observed after right ATL surgery or right temporal lobe stroke. Similarly, naming deficits in patients with semantic dementia, a degenerative condition affecting the temporal lobes, are relatively more severe and rapidly progressive when atrophy is lateralized to the left. Lambon-Ralph and colleagues (2001) proposed that this dependence of naming on the left temporal lobe reflects hemispheric asymmetry in the connection between semantic and phonological systems. According to this model, the phonological system is represented primarily in the language-dominant (usually left) hemisphere and receives more input from the dominant-hemisphere semantic system than from the nondominant hemisphere.
Expanding on this theory, we propose that the Story–Math protocol demonstrated here might be used in combination with an fMRI index of language lateralization for presurgical temporal lobe language mapping. The Story–Math contrast would be used to identify functionally relevant semantic networks in the ATL, while the language lateralization index would assess how strongly connected these regions are likely to be with phonological retrieval and production components of the language network, and therefore how critical they are for naming. The Semantic Decision–Tone Decision contrast mentioned above produces a strongly left-lateralized activation pattern, and has been shown to correlate with Wada language testing, naming outcome, and verbal memory outcome in left ATL surgery patients (Binder et al., 1997; Binder et al., 2008a; Binder et al., 1996; Binder et al., 2008b; Sabsevitz et al., 2003; Springer et al., 1999; Szaflarski et al., 2008). On the other hand, language lateralization accounted for only about 40% of the variance in naming outcome in the study by Sabsevitz et al. (2003). We hypothesize that naming outcome in left ATL surgery will be more precisely predicted by an interaction between the degree of language lateralization to the left hemisphere and the extent of damage to the ATL semantic network. If language is represented in the right hemisphere or bilaterally, damage to left ATL networks will not produce naming deficits, whereas if language is lateralized to the left, then naming decline will be proportional to the degree of damage to the left ATL semantic system. Interestingly, the Semantic Decision–Tone Decision contrast activates more posterior left temporal lobe regions that are largely complementary to those activated by the Story–Math contrast, perhaps because the former emphasizes retrieval of concrete lexical concepts that are stored more posteriorly in the ventral temporal lobe (Kan et al., 2003; Sabsevitz et al., 2005; Wise et al., 2000). The combination of these mapping protocols in the presurgical setting would therefore provide a more complete mapping of the typical ATL resection zone as well as highly complementary information about the location of semantic and phonological language networks.
As mentioned in the introduction, an important caveat in all presurgical applications of fMRI is that activation may reflect a variety of processes and does not necessarily indicate that the activated region is critical for the cognitive outcome of interest. We have proposed that the ATL activation elicited in the present study mainly reflects semantic processes, consistent with a large body of neuropsychological and imaging research on functions of the ATL (Binder et al., 2009; Mummery et al., 2000; Nestor et al., 2006; Noppeney et al., 2007; Patterson et al., 2007; Pobric et al., 2007; Rosen et al., 2002; Ross and Olson, 2010; Visser et al., 2010; Zahn et al., 2007). Comprehension of spoken narratives also requires auditory and phonetic perception, syntactic analysis, and working memory, but we believe the math task controls for most of these non-semantic processes. The math stimuli were generated by the same text-to-speech synthesis method used for the stories, enabling close matching of auditory and phonetic features, and the two conditions were matched for word presentation rate. Furthermore, the math stimuli have a simple grammatical structure similar toa subject -verb-object sentence (Baldo and Dronkers, 2007; Hauser et al., 2002)and make strong demands o n verbal working memory. In addition to controlling for auditory, phonological, simple syntactic, and working memory processes, the math task requires only simple arithmetic and numerical concepts that do not appear to engage the ATL (Baldo and Dronkers, 2007; Cappelletti et al., 2001; Crutch and Warrington, 2002; Diesfeldt, 1993), thereby enabling a strong semantic contrast with the story task. On the other hand, the stories employ more complex syntactic structures, thus some of the observed activation may represent syntactic processes. The superior aspect of the ATL is sensitive to the presence of syntactic structure in word strings (Humphries et al., 2006; Humphries et al., 2005; Mazoyer et al., 1993; Stowe et al., 1999), but whether this effect is syntactic or semantic in nature is under debate (Humphries et al., 2006; Rogalsky and Hickok, 2009; Vandenberghe et al., 2002). Syntactic complexity effects are frequently observed in other brain regions, such as the inferior frontal gyrus and temporoparietal cortex (Ben-Shachar et al., 2004; Caplan, 2001; Caplan et al., 2008; Friederici and Kotz, 2003;Keller et al., 2001; Wartenburger et al., 2004), but seldom in the ATL.
A semantic interpretation is also suggested by the bilateral pattern of ATL activation, which would be unlikely if the activation reflected phonological or syntactic processes. As mentioned above, there is extensive experimental and clinical evidence for bihemispheric involvement in conceptual processing (Awad et al., 2007; Beeman and Chiarello, 1998; Bottini et al., 1994; Chan et al., 2001; Chiarello, 1991; Coltheart, 1980; Coslett and Saffran, 1989; Hodges et al., 1992; Humphries et al., 2006; Jung-Beeman, 2005; Lambon Ralph et al., 2009; Mummery et al., 2000; Nestor et al., 2006; Noppeney et al., 2007; Paivio, 1986; Rodd et al., 2005; Rosen et al., 2002; Tranel et al., 1997; Van Overwalle, 2009; Xu et al., 2005). The right hemisphere may be particularly involved in processing figurative language, polysemy, and social concepts (Beeman and Chiarello, 1998; Bottini et al., 1994; Jung-Beeman, 2005; Olson et al., 2007; Thompson et al., 2003). Visser et al. (2010) found in a recent meta-analysis of neuroimaging studies that ATL activation is often bilateral when it is observed. This is often true even when linguistic materials such as sentences and narratives are used, as in the present study (Awad et al., 2007; Crinion et al., 2003; Fletcher et al., 1995; Giraud et al., 2004; Humphries et al., 2006; Rodd et al., 2005; Rogalsky and Hickok, 2009; Xu et al., 2005), though a few studies using sentences and narrative stimuli have reported left-lateralized ATL activation (Scott et al., 2000; Spitsyna et al., 2006). The reasons for this variable lateralization are unclear, but could be related to the semantic content of the materials. For example, the stimuli may portray social concepts (e.g., theory of mind, intention, emotion, morality) to varying degrees, or they may contain different amounts of metaphor, idiom, or implied meaning. Lateralization may also reflect the adequacy of controls for non-semantic processing. Studies showing left lateralized activation generally used non-phonological control stimuli (e.g., reversed or rotated speech) or speech stimuli that were not matched on word presentation rate. Thus, the left temporal lobe activation in these studies may partly reflect non-semantic phonological or syntactic processes.
Whether cognitive outcome after left ATL surgery is related to removal of activated regions in the ATL, and whether this relationship is modulated by language lateralization, can only be determined from a large prospective study of surgically treated patients. In adapting the Story-Math protocol for this purpose, we plan to implement an adjustment procedure for the Story condition similar to that used for the Math condition. Multiple levels of difficulty will be created, defined by the vocabulary level of the story materials and the relative similarity of meaning of the response choices, so that the difficulty of the task can be adjusted based on ongoing performance or on previously available neuropsychological data. With a sufficiently large sample of surgically treated patients, sensitive preoperative fMRI measures that are not used to influence the surgical resection, and cognitive outcome data, it will be possible to determine whether removal of “activated” voxels is correlated with outcome. These data will also be critical for determining the level of activation that is meaningful, and therefore how best to threshold fMRI language maps if these are to be used to guide resections, which at present is a vexing and largely unexamined problem.
Research Highlights.
An fMRI protocol emphasizing semantic integration activates the anterior temporal lobe.
ATL activation was consistently observed in individual participants.
The results were successfully replicated at 6 other imaging centers.
The methods provide a valuabletool for studying cognitive effects of ATL resection.
Acknowledgments
The authors thank Amanda Golsch; Edward T. Possing, MS; and Ron Pratt, PhD for help with data acquisition; and Sara Berentsen for additional data analysis. This research was supported by NINDS grants R01 NS035929 and R01 NS048281.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awad M, Warren JE, Scott SK, Turkheimer FE, Wise RJS. A common system for the comprehension and production of narrative speech. Journal of Neuroscience. 2007;27:11455–11464. doi: 10.1523/JNEUROSCI.5257-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Dronkers NF. Neural correlates of arithmetic and language comprehension: A common substrate? Neuropsychologia. 2007;45:229–235. doi: 10.1016/j.neuropsychologia.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Baxendale S, Thompson P, Harkness W, Duncan J. Predicting memory decline following epilepsy surgery: A multivariate approach. Epilepsia. 2006;47:1887–1894. doi: 10.1111/j.1528-1167.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- Beeman M, Chiarello C, editors. Right hemisphere language comprehension: Perspectives from cognitive neuroscience. Erlbaum; Mahwah, NJ: 1998. [Google Scholar]
- Bell BD, Davies KG, Hermann BP, Walters G. Confrontation naming after anterior temporal lobectomy is related to age of acquisition of the object names. Neuropsychologia. 2000;38:83–92. doi: 10.1016/s0028-3932(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Bell BD, Hermann BP, Woodard AR, Jones JE, Rutecki PA, Sheth R, Dow CC, Seidenberg M. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15:434–443. doi: 10.1037//0894-4105.15.4.434. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Palti D, Grodzinsky Y. The neural correlates of syntactic movement: Converging evidence from two fMRI experiments. Neuroimage. 2004;21:1320–1336. doi: 10.1016/j.neuroimage.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSeuer LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai R, Conant LL, Graves WW. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional MRI. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008a;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008b;49:1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RSJ. The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain. 1994;117:1241–1253. doi: 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Caplan D. Functional neuroimaging studies of syntactic processing. Journal of Psycholinguistic Research. 2001;30:297–320. doi: 10.1023/a:1010495018484. [DOI] [PubMed] [Google Scholar]
- Caplan D, Stanczak L, Waters G. Syntactic and thematic constraint effects on blood oxygenation level dependent signal correlates of comprehension of relative clauses. Journal of Cognitive Neuroscience. 2008;20:643–656. doi: 10.1162/jocn.2008.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Butterworth B, Kopelman M. Spared numerical abilities in a case of semantic dementia. Neuropsychologia. 2001;39:1224–1239. doi: 10.1016/s0028-3932(01)00035-5. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, Rossor AM, Stevens JM, Cipolotti L, Rossor MN. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Annals of Neurology. 2001;49:433–442. [PubMed] [Google Scholar]
- Chelune GJ, Naugle RI, Lüders H, Sedlak J, Awad IA. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology. 1993;7:41–52. [Google Scholar]
- Chiarello C. Interpretation of word meanings by the cerebral hemispheres: One is not enough. In: Schwanenflugel P, editor. The psychology of word meanings. Lawrence Erlbaum; Hillsdale, NJ: 1991. pp. 251–278. [Google Scholar]
- Coltheart M. Deep dyslexia: A right hemisphere hypothesis. In: Colheart M, Patterson K, Marshall JC, editors. Deep dyslexia. Routledge and Kegan Paul; London: 1980. [Google Scholar]
- Coslett HB, Saffran EM. Preserved object recognition and reading comprehension in optic aphasia. Brain. 1989;112:1091–1110. doi: 10.1093/brain/112.4.1091. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. Preserved calculation skills in a case of semantic dementia. Cortex. 2002;38:389–399. doi: 10.1016/s0010-9452(08)70667-1. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Diesfeldt HFA. Progressive decline of semantic memory with preservation of number processing and calculation. Behavioural Neurology. 1993;6:239–242. doi: 10.3233/BEN-1993-6411. [DOI] [PubMed] [Google Scholar]
- Evans AC, Group BDC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: Functional imaging and lesion studies. Neuroimage. 2003;20:S8–S17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY. Auditory language comprehension: An event-related fMRI study on the processing of syntactic and lexical information. Brain and Language. 2000;74:289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Friedman L, GHG, Krenz D, Magnotta V, BIRN TF. Reducing inter-scanner variability of activation in a multicenter fMRI study: role of smoothness equalization. Neuroimage. 2006a;32:1656–1668. doi: 10.1016/j.neuroimage.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. Report on a multicenter fMRI quality assurance protocol. Journal of Magnetic Resonance Imaging. 2006;23:827–839. doi: 10.1002/jmri.20583. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH, Consortium TF. Reducing interscanner variability of activation in a multicenter fMRI study: controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006b;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Kell C, Thierfelder C, Sterzer P, Russ MD, Preibisch C, Kleinschmidt A. Contributions of sensory input, auditory search, and verbal comprehension to cortical activity during speech processing. Cerebral Cortex. 2004;14:247–255. doi: 10.1093/cercor/bhg124. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Helmstaedter C, Schramm J, Elger CE. Memory outcome after selective amygdalohippocampectomy in patients with temporal lobe epilepsy: One-year follow-up. Epilepsia. 2004;45:960–962. doi: 10.1111/j.0013-9580.2004.42203.x. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Tkach JA, Parrish TB. Reduction of T2* dephasing in gradient field-echo imaging. Radiology. 1989;170:457–462. doi: 10.1148/radiology.170.2.2911669. [DOI] [PubMed] [Google Scholar]
- Hadar U, Jones C, Mate-Kole C. The disconnection in anomic aphasia between semantic and phonological lexicons. Cortex. 1987;23:505–517. doi: 10.1016/s0010-9452(87)80011-4. [DOI] [PubMed] [Google Scholar]
- Hauser MD, Chomsky N, Fitch WT. The faculty of language: What is it, who has it, and how did it evolve? Science. 2002;298:1569–1579. doi: 10.1126/science.298.5598.1569. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Elger CE. Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia. 1996;37:171–180. doi: 10.1111/j.1528-1157.1996.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Clement L. Dysnomia after left anterior temporal lobectomy without functional mapping: frequency and correlates. Neurosurgery. 1994;35:52–57. doi: 10.1227/00006123-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: Implications for the organisation of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Swinney D, Love T, Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Human Brain Mapping. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Golby AJ. The brain basis for episodic memory: insights from functional MRI, intracranial EEG, and patients with epilepsy. Epilepsy and Behavior. 2006;8:115–126. doi: 10.1016/j.yebeh.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jansen A, Menke R, Sommer J, Forster AF, Bruchmann S, Hempleman J, Weber B, Knecht S. The assessment of hemispheric lateralization in functional MRI—Robustness and reproducibility. Neuroimage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;8:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, Thompson-Schill SL. Role of mental imagery in a property verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cognitive Neuropsychology. 2003;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Keller TA, Carpenter PA, Just MA. The neural bases of sentence comprehension: A fMRI examination of syntactic and lexical processing. Cerebral Cortex. 2001;11:223–237. doi: 10.1093/cercor/11.3.223. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland J, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cerebral Cortex. 2009;19:832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Archives of Neurology. 1996;53:72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- Lee TMC, Yip JTH, Jones-Gotman M. Memory deficits after resection of left or right anterior temporal lobe in humans: A meta-analytic review. Epilepsia. 2002;43:283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, Hertz-Pannier L, LeBihan D, Marsault C, Baulac M. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Speaking: From intention to articulation. MIT Press; Cambridge, MA: 1989. [Google Scholar]
- Lineweaver TT, Morris HH, Naugle RI, Najm IM, Diehl B, Bingaman W. Evaluating the contributions of state-of-the-art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia. 2006;47:1895–1903. doi: 10.1111/j.1528-1167.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Sorenson JA. Spatially filtering functional magnetic resonance imaging data. Magnetic Resonance in Medicine. 1997;37:723–729. doi: 10.1002/mrm.1910370514. [DOI] [PubMed] [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, Salamon G, Dehaene S, Cohen L, Mehler J. The cortical representation of speech. Journal of Cognitive Neuroscience. 1993;5:467–479. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- Merboldt KD, Finterbusch J, Frahm J. Reducing inhomogeneity artifacts in functional MRI of human brain activation: thin sections vs. gradient compensation. Journal of Magnetic Resonance. 2000;145:184–191. doi: 10.1006/jmre.2000.2105. [DOI] [PubMed] [Google Scholar]
- Morawetz C, Holz P, Lange C, Baudewig J, Weniger G, Irle E, Dechent P. Improved functional mapping of the human amygdala using a standard functional magnetic resonance imaging sequence with simple modifications. Magnetic Resonance Imaging. 2008;26:45–53. doi: 10.1016/j.mri.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clinics of North America. 2005;15:869–877. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36 –45. [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer’s disease and semantic dementia. Neuroimage. 2006;30:1010–1020. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss HE, Stamatakis EA, Bright P, Mummery CJ, Price CJ. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Paivio A. Mental representations: A dual-coding approach. Oxford University Press; New York: 1986. [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93 –102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Parrish TB, Gitelman DR, LaBar KS, Mesulam MM. Impact of signal-to-noise on functional MRI. Magnetic Resonance in Medicine. 2000;44:925–932. doi: 10.1002/1522-2594(200012)44:6<925::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric GG, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proceedings of the National Academy of the Sciences, USA. 2007;104:20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Hickok G. Selective attention to semantic and syntactic features modulates sentence processing networks in anterior temporal cortex. Cerebral Cortex. 2009;19:786–796. doi: 10.1093/cercor/bhn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49:3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RNA. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London, Series B. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, Mueller WM, Binder JR. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London: Series B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank C, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. Journal of Neuroscience. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe LA, Paans AMJ, Wijers AA, Zwarts F, Mulder G, Vaalburg W. Sentence comprehension and word repetition: a positron emission tomography investigation. Psychophysiology. 1999;36:786–801. [PubMed] [Google Scholar]
- Stroup E, Langfitt JT, Berg M, McDrmott M, Pilcher W, Como P. Predicting verbal memory declinefollowing anterior temporal lobectomy (ATL) Neurology. 2003;60:1266 –1273. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy and Behavior. 2008;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61:1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: A meta-analysis. Human Brain Mapping. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. Journal of Cognitive Neuroscience. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: A meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2010;22:1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Burchert F, Heinemann S, De Bleser R, Villringer A. Neural correlates of syntactic transformations. Human Brain Mapping. 2004;22:72–81. doi: 10.1002/hbm.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Blume W, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New England Journal of Medicine. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C, Scott SK. Noun imageability and the temporal lobes. Neuropsychologia. 2000;38:985–994. doi: 10.1016/s0028-3932(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]