Abstract
Tuberoinfundibular peptide of 39 residues (TIP39) is a neuropeptide localized to neural circuits subserving emotional processing. Recent work showed that mice with null mutation for the gene coding TIP39 (TIP39-KO mice) display increased susceptibility to environmental provocation. Based on this stressor-dependent phenotype, the neuroanatomical distribution of TIP39, and knowledge that novelty-induced arousal modulates memory functions via noradrenergic activation, we hypothesized that exposure to a novel environment differently affects memory performance of mice with or without TIP39 signaling, potentially by differences in sensitivity of the noradrenergic system. We tested TIP39-KO mice and mice with null mutation of its receptor, the parathyroid hormone 2 receptor (PTH2-R), in tasks of short-term declarative and social memory (object recognition and social recognition tests, respectively), and of working memory (Y-maze test) under conditions of novelty-induced arousal or acclimation to the test conditions. Mice lacking TIP39 signaling demonstrated memory impairment selectively under conditions of novelty-induced arousal. Acute administration of a PTH2-R antagonist in wild-type mice had a similar effect. The restoration of memory functions in TIP39-KO mice after injection of a β-adrenoreceptor-blocker, propranolol, suggested involvement of the noradrenergic system. Collectively, these results suggest that the TIP39/PTH2-R system modulates the effects of novelty exposure on memory performance, potentially by acting on noradrenergic signaling.
Keywords: TIP39, short-term memory, working memory, novelty-induced arousal, noradrenergic system
Introduction
Cognitive functions are affected by emotionally arousing events (McGaugh & Roozendaal, 2002b). In many psychiatric disorders, greater or inappropriate responses to arousing conditions may contribute to the memory impairments often observed (Holmes & Wellman, 2009; Wolf, 2008). Thus, because of its importance in mental health, it is critical to better understand the links between emotional state and cognition. Tremendous advances have been made in this area (see Pessoa, 2008 for a review), but the roles of specific neuromodulators are still not well understood. Because neuropeptides are mainly released during “burst activity”, they are good candidates for modulation of arousal effects on memory (Hokfelt et al., 2003; Ogren et al., 2010). Tuberoinfundibular peptide of 39 residues (TIP39; Usdin et al., 1999) could be such a modulator. TIP39 neurons project to brain regions involved in emotional and stress responses, such as the hypothalamus and medial prefrontal cortex, and areas that regulate noradrenergic signaling such as the amygdala and regions surrounding the locus coeruleus. These areas are enriched in its receptor, the parathyroid hormone 2 receptor (PTH2-R; Dobolyi et al., 2003; Dobolyi et al., 2010; Faber et al., 2007). Previous studies using direct peptide administration in rat brain (Labuda et al., 2004) or mice with TIP39 deletion (TIP39-KO – Fegley et al., 2008) provided evidence for TIP39 involvement in emotion-related functions. These studies inferred that TIP39 normally acts to negatively modulate stress behaviors during environmental challenge. These behavioral and neuroanatomical data suggest that TIP39 signaling could be involved in arousal-related memory modulation. Several years ago, McGaugh et al. (1975) suggested that hormones released during a learning experience might modulate memory functions. On one side, emotional arousal and high levels of norepinephrine have been shown to improve long-term memory function, and this norepinephrine/long-term memory relationship follows an inverted-U shape (i.e. memory consolidation – Cahill & McGaugh, 1998; McGaugh & Roozendaal, 2002a). On the other side, recent studies in humans and other animals provide support for the view that short-term and working memory are impaired in situation of stress or arousal, again, following an inverted-U shape relationship (Arnsten, 2009; Arnsten & Li, 2005; Okuda et al., 2004).
Based on the stressor-dependent phenotype of TIP39-KO mice, the brain distribution of PTH2-R and TIP39 fibers, and knowledge that arousing conditions alter working and short-term memory functions via noradrenergic activation, we hypothesized that arousal from novel environment exposure differentially affects these memory functions of mice with or without TIP39 signaling, potentially by differences in noradrenergic system sensitivity. To test this hypothesis, we employed both mutant mice and pharmacological manipulations of the TIP39/PTH2-R system: we used mice with deletion of TIP39’s coding sequence (TIP39-KO mice) or a PTH2-R null mutation (PTH2-R-KO), and wild-type (WT) mice with central infusion of a PTH2-R antagonist. We tested them in 3 separate behavioral assays of short-term and working memory with or without prior exposure to the experimental apparatus to vary the level of novelty-induced arousal. We then tested whether the noradrenergic system was involved in the memory performance change of TIP39-KO mice by the use of β-adrenoreceptor blockers. We show that mice lacking TIP39 or its receptor have an increased susceptibility to novelty arousal induced performance impairment in short term and working memory tasks, and suggest that TIP39 effects on noradrenergic activation may underlie this.
Materials and methods
Animals
Animals were housed under a reverse light-dark cycle (lights off at 0800h) in polycarbonate cages (35.5 × 14 × 12.5cm). For the object recognition test and the spontaneous alternation test animals were single-housed at least two weeks prior to testing to allow acclimation. For the social recognition test and the assessment of general health and olfactory abilities, animals were grouped housed (2–3 littermates per cage). Bedding, nesting material, food and water were provided ad libitum. Unless otherwise specified, tests were performed under red light during the dark phase, between 0900h and 1300h. Male WT and KO littermates generated from HET × HET matings were tested when 9–12 weeks old. Naïve animals were used for each test. All procedures were approved by the NIMH ACUC and strictly followed NIH/ILAR guidelines.
TIP39 line
Mice generated from F1H4 ES cells (129S6/SvEvTac and C57BL/6Tac derived) and genotyped as previously described were backcrossed for 5 or 6 generations into the C57BL/6J background. No general defect or gross abnormalities could be found (Fegley et al., 2008) except for a germ cell maturation defect in TIP39-KO mice. However, serum testosterone is not significantly different than WT littermates (Usdin et al., 2008). Since olfaction and olfactory learning were not previously evaluated, we tested both genotypes in an olfactory habituation/dishabituation task (see Supplementary Materials and Methods) (Stack et al., 2008)
PTH2-R line
Mice with a null mutation of the PTH2-R were generated by first introducing loxP sites into intronic sequence flanking exon 5 of the receptor (Accession # NM_139270) in 129S6 X C57BL6/N F1 ES cells using standard mouse transgenic technology in the NIMH Intramural Program Transgenic Core Facility. The “floxed exon 5” mice were bred with a “Cre deleter” line (that express Cre recombinase in germ cells; Tg(Prm-cre)58Og (backcrossed to C57BL/6J more than 5 generations — O’gorman et al., 1997). Mice with permanent deletion of exon 5 were identified by PCR of genomic DNA (Dimitrov & Usdin, In press). These mice were then bred with C57Bl/6J mice, producing heterozygous exon 5 deleted (denoted PTH2-R KO)/WT mice. Animals used in this study were backcrossed to C57Bl/6J for 4 generations. Exon 5 encodes part of a transmembrane domain of this seven transmembrane domain G-protein coupled receptor, so its deletion is expected to ablate receptor function. We confirmed that exon 5 is absent from genomic DNA by PCR with primers in the flanking introns, and from brain mRNA by RT-PCR using primers in exons upstream and downstream of exon 5, and sequencing the PCR products. All DNA sequencing was performed by the NINDS Intramural Sequencing Core Facility. We confirmed that the mRNA produced encodes a nonfunctional receptor by producing PTH2-R cDNA expression constructs that differed only by the sequence absent in the PTH2-R mutant mice, transfecting tissue culture cells with these constructs, and measuring the ability of TIP39 to stimulate cAMP accumulation in the transfected cells. Cells transfected with the WT PTH2-R sequence produced a robust increase in cAMP accumulation and no stimulation was detected from the exon 5 deleted construct (data not shown). Mouse genotyping was performed by PCR analysis of mouse tail DNA with primers Pf1 5′-GGTGTAAGTTATCTGAAGTCACGGG-3, Pf2 5′-TTCTCTTCCTCCTCCTCCTCCTAC-3′ and Pr1 5′-CCCTGTCTGCTCTTTGCTTACG-3′, which produce bands of 635 and 507 basepairs from the WT and KO alleles, respectively.
The olfactory habituation/dishabituation task and an observational battery measuring general health and neurological function were performed (modified from Boyce-Rustay & Holmes, 2006 – see Supplementary Materials and Methods).
Behavioral assays of working and short-term memory: general procedures
Spontaneous alternation test
Spontaneous alternation, as an indicator of working memory, was assessed using a modified version of the free-choice trial procedure (Lalonde, 2002). The apparatus consists in a grey plastic maze formed of 3 arms (A, B and C) placed so as to form a Y shape (arm length: 40cm; width: 12.5cm; height: 20cm). Each animal was placed in the apparatus for 8 minutes during which it was allowed to explore the entire maze. This test is based on a strong tendency in rodents to alternate arm choices, explained by their natural propensity to explore a novel environment over a recently explored one. The series of arm entries (e.g. ACBCABCBCA) was scored from a video record by an observer unaware of the animal’s genotype. Alternation was defined as successive entry into the three arms, on overlapping triplet sets (e.g. in the sequence ACBCABCBCA, 5 alternations were recorded). Since traveling pattern and locomotor behavior might affect the results, we tested whether differences in locomotion could be noted between WT animals and PTH2-R-KO and TIP39-KO mice. No significant difference could be noted (data not shown). The percent alternation was calculated as the ratio of actual alternations to possible alternations (defined as the total number of arm entries minus two), multiplied by 100. The total number of arm entries was used as an indicator of locomotor activity.
Social recognition test
The social recognition test was based on a recently developed procedure (Macbeth et al., 2009). This procedure has been validated with the use of oxytocin- and oxytocin receptor-knockout mice known to present social recognition impairments. It consists of an acclimation period followed by two 5-minutes trials with a 30-minute inter-trial interval. At the beginning of the test, each mouse was placed individually in a new cage (35.5 × 14 × 12.5cm) with just enough bedding to cover the floor for 30 min to allow acclimation. Two empty wire corrals (10cm high; 4cm diameter – Kitchen Plus, Rockville MD) were then placed at the two opposite sides of the cage for another 30 minutes. After this period, an unfamiliar juvenile (J1) C57Bl/6J male (stimulus mouse) was placed in one of the corrals while the other corral was removed. The experimental animal was allowed to explore for 5 minutes (training trial), after which the stimulus mouse and the corral were removed for 30 minutes (delay time). Afterwards, the now-familiar juvenile (J1) was placed back under the same corral while a new unfamiliar juvenile (J2) was placed under the previously empty corral. The experimental animal was again allowed to explore for 5 minutes (testing trial). During each trial, the time the test mouse spent investigating a juvenile mouse was recorded with a stopwatch by an experimenter blind to its genotype. Placing the nose through the wire of the corral or directly touching any part of the juvenile with the nose and/or the paws were considered investigation. Mice with a total exploration time of less than 20 s in one of the two trials were excluded from the analysis. Recognition of the familiar juvenile during the second trial was assessed as a discrimination ratio (DR = time exploring (J2−J1)/time exploring (J2+J1)).
Object recognition test
The object recognition test was based on that first described by Ennaceur & Delacour (1988). The test was performed in a grey plastic arena (50 × 50 × 50 cm) and consisted of two trials of 10 minutes, with an inter-trial interval of 110 minutes. On the first trial – training (T1), each animal was placed in the center of the arena between two identical objects (A1 and A2) placed in direct contact with two opposite walls of the arena. On the second trial – testing (T2), the objects were replaced by a novel one (N) and a copy of the familiar objects of T1 (A3) to avoid odor cues. Objects varied in color, shape, size and material. Both objects and object location were counterbalanced in order to remove object and location preference effects. The basic measure in the object recognition task was the time the subjects spent exploring objects during the two trials. Exploration of an object was defined as directing the nose towards an object at a distance of less than 1cm and/or touching the object with nose and/or paws. Sitting on the object was not considered as exploratory behavior. Each trial was recorded from above by a video camera. Video records were analyzed off-line by an observer unaware of the genotype. Mice with a total exploration time of less than 20s in either T1 or T2 were excluded from the analysis. Recognition of the familiar object during the retrieval trial (T2) was assessed as a discrimination ratio (DR = time exploring (N−A)/time exploring (N+A)).
Memory performance under conditions of varied novelty-induced arousal
We varied the degree of novelty-induced arousal at the time of testing by repeatedly exposing the animals, or not exposing them at all, to the experimental context prior to testing (Cerbone & Sadile, 1994; De Boer et al., 1988; Maroun & Akirav, 2008; Okuda et al., 2004).
The social recognition and object recognition tests were performed in both lines of mice (TIP39 and PTH2-R lines). The spontaneous alternation test was performed only in the TIP39 line. All tests were performed under two conditions: repetitive pre-exposure to the experimental conditions (to reduce the level of novelty arousal) and absence of pre-exposure to the experimental condition (to induce novelty arousal).
Spontaneous alternation test: for the “repetitive pre-exposure” condition, each mouse (WT n=9; TIP39-KO n=6) was handled by an experimenter and placed in an unfamiliar arena in the testing room for 7 days before the testing day. Once per day, each animal was handled for 1 minute and then brought to the testing room where it was placed for 5 minutes in an unfamiliar square arena (50 × 50 × 50cm) made of the same material and of the same color as the Y-maze. For the novelty-induced arousal condition, mice (WT n=8; TIP39-KO n=7) were placed in an empty unfamiliar cage for 20 minutes on the day of testing before being brought to the unfamiliar testing room and placed in the Y-maze.
Social recognition test: for the “repetitive pre-exposure” condition, each mouse (TIP39 line: WT n=8; KO n=8; PTH2-R line: WT n=8; KO n=10) was placed in a new cage with the two empty corrals for 30 minutes for 5 days before the testing day. For the novelty-induced arousal condition, mice (TIP39 line: WT n=8; KO n=8; PTH2-R line: WT n=6; KO n=8) were not pre-exposed to the testing conditions before the testing day.
Object recognition test: for the “repetitive pre-exposure” condition, mice (TIP39 line: WT n=8; KO n=9; PTH2-R line: WT n=7; KO n=8) were handled and placed in the testing arena for 7 days before testing. Twice per day, on each of these 7 days, each animal was removed from its cage, handled for 1 minute, brought to the testing room and placed in the empty testing arena for 3 minutes. The animal was then brought back to its home cage. For the novelty-induced arousal condition, mice (TIP39 line: WT n=8; KO n=8; PTH2-R line: WT n=7; KO n=8) were not pre-exposed to the testing conditions.
Memory performance under novelty-induced arousal conditions after PTH2 receptor antagonist infusion
Surgery
WT mice were anesthetized with isoflurane and placed in a stereotaxic apparatus. A vertical incision was made in the skin to expose the skull. A steel guide cannula (26 gauge; 2mm projection below pedestal; Plastics One, Roanoke, VA) was implanted into the left lateral ventricle and fixed to the skull with dental cement. Coordinates from bregma were mediolateral, +1mm; antero-posterior, −0.4mm. To prevent occlusion, a dummy cannula was inserted into the guide cannula. After surgery, mice were given analgesic (ketoprofen, sc; 2 mg/kg) and allowed to recover in their home cage for at least 6 days. During this period, mice were gently handled daily to minimize the stress associated with the infusion procedure during the experiment and to make sure that the effects observed would be linked with the arousal induced by the experimental procedure per se and not by the infusion procedure. At the end of the experiment, animals were euthanized and brains removed and postfixed in paraformaldehyde (PFA). Brains were sectioned at 35 μm. Sections were visually examined to verify that the cannula tip was located in the lateral ventricle.
Infusion and testing
The spontaneous alternation test and the object recognition test were performed on non pre-exposed animals as described in the previous section. However, because of experimental considerations, the tests were performed during the light phase of the light/dark cycle, at low light intensity (9 lux). Kuo & Usdin (2007) developed a PTH2 receptor antagonist: HYWH-TIP39. In cultured cells HYWH-TIP39 completely blocks activation of the PTH2 receptor by TIP39. Animals were infused i.c.v. with 1μl of HYWH-TIP39 (0.5 nmole; a dose that had no effect in the open field or elevated plus maze (unpublished data) and which would completely block PTH2R’s if it were homogenously distributed over a volume equivalent to the brain) or vehicle (30% DMSO, 2mM TRIS-HCl, pH 7.5) over 1 minute. The cannula was left in place for 2 minutes to prevent backflow, after which the dummy cannula was replaced. For the spontaneous alternation test, after the infusion, animals (n=8 per group) were returned to their home cage for 18 minutes before being tested in the Y-maze for 8 minutes as described above. The delay time between the infusion and the testing was based on preliminary experiments showing that the PTH2-R antagonist requires several minutes to have a significant effect (data not shown). For the object recognition test, the infusion took place directly after the training trial (T1), after which animals (n=8 per group) were placed back in their home cage. After 110 minutes, animals were tested (T2) as described above.
Object recognition test under conditions of novelty-induced arousal and β-adrenoreceptor block
The object recognition test under conditions of novelty-induced arousal (no prior-exposure to the experimental condition) was performed as described above using two groups of WT and TIP39-KO mice, to test the effect of propranolol and nadolol. Both drugs have a similar potency as β-receptor antagonists. However, propranolol readily passes the blood-brain barrier and thus acts on both peripheral and central β-adrenergic receptor. Nadolol has limited ability to cross blood-brain barrier and thus acts primarily at peripheral β-adrenergic receptor (McDevitt, 1987). Nadolol is reported to have higher or equal potency than propranolol as a β-adrenergic receptor antagonist (Escoubet et al., 1986). To ensure that any lack of effect of nadolol was not simply due to lesser occupancy of peripheral β-adrenergic receptor by nadolol, we administered nadolol at higher dose than propranolol. At the end of the training trial (T1), the first group of WT and TIP39-KO mice (n=7 per genotype and per treatment) was injected i.p. with saline or propranolol (Sigma, St. Louis MO, USA; 2mg/kg), while the second group (n=6 or 7 per genotype and per treatment) received vehicle or nadolol (Sigma; dissolved in PBS/0.2% ethanol, adjusted to pH 7.0 with HCl; 10mg/kg). After injection, animals were returned their home cage for 110 minutes until tested (T2) for 10 minutes.
Statistical analysis
Data were analyzed using Prism 4 software (GraphPad Software Inc., SanDiego, CA, USA). All data were tested for normality with the Kolmogorov-Smirnof test and then analyzed using t-tests or various analyses of variance (ANOVA) followed by Bonferonni posthoc comparisons when necessary (see Results section). Statistical significance was set as p≤0.05 and trend as p≤0.1. All data are presented as mean ± standard error of the mean.
Results
General health and olfactory habituation/dishabituation task in the TIP39 and PTH2-R lines
No general defect or gross abnormalities could be found in PTH2-R-KO mice when compared to WT littermates (Table S1). Similarly, in the olfactory habituation/dishabituation task, TIP39-KO and PTH2-R-KO mice were not different than WT (Figure S1).
Novelty-induced arousal selectively impairs working and short-term memory in TIP39-KO and PTH2-R-KO mice
TIP39 line
Spontaneous alternation test
Data were analyzed in a two-way ANOVA with the genotype and the prior exposure to testing condition as independent variables. The total number of arm entries (WT: pre-exposed: 68.67 ± 4.33; not pre-exposed: 65.13 ± 2.92; KO: pre-exposed: 68.17 ± 3.94; not pre-exposed: 71.29 ± 4.17) was not affected by the genotype (F1,26=0.69, p=0.41) or by the exposure condition (F1,26=0.51, p=0.48). In contrast, the percent alternation was significantly affected by genotype (F1,26=6.73, p=0.02), and the exposure condition (F1,26=10.7, p=0.003) and there was a significant interaction between these factors (F1,26=10.7, p=0.003). Post-hoc analysis revealed that the percent alternation did not differ between WT and TIP39-KO mice that were pre-exposed to the experimental condition (WT: 64.14 ± 1.74 % vs. KO: 65.89 ± 2.38 %; t14=0.478, p>0.05). However, when animals were not pre-exposed to the testing condition, TIP39-KO mice showed a significantly lower level of alternation than WT (WT: 64.14 ± 2.42 % vs. KO: 49.13 ± 3.55 %; t14=4.191, p<0.001).
Social recognition test
Two WT animals were removed from the analysis for exploring the stimulus animal less than 20 seconds during the training trial. Two-way ANOVA with genotype and the prior exposure to testing conditions showed that the total time spent exploring the juvenile during training was significantly affected by the exposure condition (F1,28=6.442, p=0.018). Animals explored less when previously exposed to the testing condition (Table 1). There was no overall genotype effect for time of exploration during the training or the testing trial (F1,28=0.749, p=0.395; F1,28=0.177, p=0.677, respectively).
Table 1.
Total time of exploration of the juveniles and the objects during the training and the testing trial of the social recognition (SRT) and object recognition (ORT) tests, respectively, by TIP39-line mice when previously or not previously exposed to the experiment conditions. Data are presented as Mean ± SEM.
| Training | Testing | |||
|---|---|---|---|---|
| SRT | WT | Prior exposure | 60.72 ± 6.40a | 43.60 ± 6.57 |
| No- Prior exposure | 66.64 ± 3.67b | 49.22 ± 5.64 | ||
| TIP39- | Prior exposure | 46.01 ± 5.84a | 41.52 ± 5.89 | |
| KO | No- Prior exposure | 70.86 ± 7.20b | 56.36 ± 5.85 | |
| ORT | WT | Prior exposure | 84.05 ± 9.07c | 72.23 ± 5.94e |
| No- Prior exposure | 70.54 ± 3.69d | 68.08 ± 2.41f | ||
| TIP39- | Prior exposure | 89.70 ± 5.34c | 89.46 ± 6.72e | |
| KO | No- Prior exposure | 66.79 ± 4.78d | 62.52 ± 1.75f |
overall effect of the “prior exposure” condition during the training phase of the social recognition test p=0.018;
overall effect of the “prior exposure” condition during the training phase of the object recognition test p=0.005;
overall effect of the “prior exposure” condition during the training phase of the object recognition test p=0.004
We analyzed the discrimination ratio (DR) with a 2-way ANOVA with the genotype and the pre-exposure condition as independent variables. No overall genotype effect was observed (F1,28=1.49, p=0.233) but an overall effect of the exposure condition was found (F1,28=5.201, p=0.031). We also observed a strong trend for an interaction between these factors (F1,28=3.672, p=0.063). We used a one-sample t-test to determine whether the DR was different from chance level (zero). The DR of WT mice was significantly or tended to be above chance level in both conditions (pre-exposed t5=2.249; p=0.074; non pre-exposed t7=2.439; p=0.045). The same was true for pre-exposed TIP39-KO mice (t7=3.895; p=0.006). However, the DR of non pre-exposed TIP39-KO mice was not above chance level (t7=0.560; p=0.593 – Figure 1a).
Figure 1.
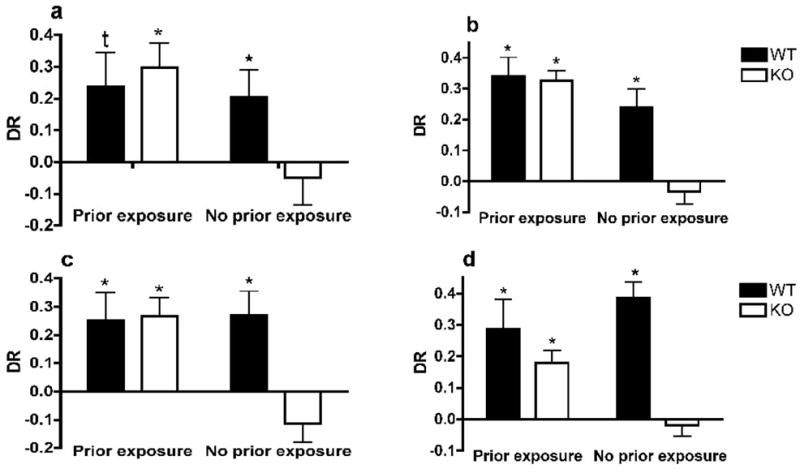
Object recognition test
The two-way ANOVA showed that the exploration time in both the training and the testing trials was significantly affected by the prior exposure/no exposure factor (F1,32=9.088, p=0.005; F1,32=10.081, p=0.004, respectively); animals spent more time exploring the objects when they were pre-exposed to the experimental conditions (Table 1). There was no overall genotype effect (training: F1,32=0.025, p=0.876; testing: F1,32=1.420, p=0.243) but a significant interaction was present in the time spent exploring the objects during the testing trial (F1,32=5.417, p=0.027): TIP39-KO mice explored the objects less when not pre-exposed to the experimental conditions relative to the pre-exposed animals (p=0.002).
Analysis of the DR with a 2-way ANOVA showed significant effects of the genotype, exposure condition and interaction between the two factors (F1,32=8.651, p=0.006; F1,32=21.888, p<0.001; F1,32=6.883, p=0.014, respectively). Posthoc analysis showed that the DR of WT mice was significantly higher that the one of TIP39-KO mice specifically under conditions of no pre-exposure to the testing conditions (t15=3.88, p<0.01). Analysis of the DR with the one-sample t-test showed that both pre-exposed and non pre-exposed WT mice had a preference for the novel object (t7=5.660; p=0.001; t7=4.091; p=0.005, respectively). Pre-exposed TIP39-KO mice showed a preference for the novel object (t8=9.746; p<0.001) but non pre-exposed TIP39-KO mice did not show this preference (t7=0.776; p=0.463 – see Figure 1b).
PTH2-R line
Social recognition test
The two-way ANOVA showed that non pre-exposed animals had higher exploration behavior during training compared to pre-exposed animals (training trial: F1,31=30.032, p<0.001; testing trial: F1,31=12.443, p=0.001). We also found an overall genotype effect on the total exploration time during the training trial: PTH2-R-KO mice explored the juvenile J1 more than WT (F1,31=7.611, p=0.01). This genotype difference disappeared during the testing trial (F1,31=0.163, p=0.69 – Table 2).
Table 2.
Total time of exploration of the juveniles and the objects during the training and the testing trial of the social recognition (SRT) and object recognition (ORT) tests, respectively, by PTH2-R-line mice when previously or not previously exposed to the experimental conditions. Data are presented as Mean ± SEM.
| Training | Testing | |||
|---|---|---|---|---|
| SRT | WT | Prior exposure | 42.03 ± 5.75a | 31.47 ± 4.35c |
| No- Prior exposure | 63.52 ± 3.02b | 51.65 ± 4.63d | ||
| PTH2-R-KO | Prior exposure | 50.15 ± 4.15a | 33.68 ± 5.05c | |
| No- Prior exposure | 82.53 ± 5.28b | 54.08 ± 7.52d | ||
| ORT | WT | Prior exposure | 54.03 ± 7.26e | 61.07 ± 6.30 |
| No- Prior exposure | 68.12 ± 5.66 | 66.44 ± 5.71 | ||
| PTH2-R-KO | Prior exposure | 79.65 ± 5.87f | 67.36 ± 4.44 | |
| No- Prior exposure | 67.44 ± 5.62 | 62.14 ± 3.68 |
overall effect of the “prior exposure” condition during the training phase of the social recognition test p<0.001;
overall effect of the “prior exposure” condition during the testing phase of the social recognition test p=0.001;
PTH2-R-KO mice have a higher level of exploration compared with WT mice when previously exposed to the experimental conditions p=0.015.
A 2-way ANOVA on the DR showed significant effects of the genotype, exposure condition and interaction between the two factors (F1,31=5.192, p=0.031; F1,31=5.120, p=0.032; F1,31=6.096, p=0.02). Posthoc analysis showed that the DR of WT mice was significantly higher that the one of PTH2-R-KO mice specifically under conditions of no pre-exposure to the testing conditions (t13=3.16, p<0.01). Using a one-sample t-test, we observed that in both conditions (pre-exposure and no pre-exposure) WT animals recognized the familiar juvenile (J1) over the unfamiliar one, their DR being significantly above chance level (t7=2.560; p=0.038; t5=3.058; p=0.028, respectively). Pre-exposed PTH2-R-KO mice demonstrated memory of the juvenile J1 (t9=4.156; p=0.002) while the non pre-exposed ones failed, as shown by a DR not different from chance level (t7=−1.679; p=0.137 – Figure 1c).
Object recognition test
Two-way ANOVA showed that the exposure condition did not affect the total time spent exploring the objects during the training or the testing trials (F1,30=0.023, p=0.88; F1,30=0.00, p=0.99, respectively). There was no overall genotype effect during the testing trial (F1,30=0.028, p=0.87) and a trend during the training trial was observed (F1,30=4.159, p=0.052). The significant genotype by exposure condition interaction on total exploration time (F1,30=4.628, p=0.041) is explained by the significantly higher level of exploration of PTH2-R-KO mice compared with WT mice when previously exposed to the experiment conditions (p=0.015 – see Table 2).
A 2-way ANOVA showed that the exposure condition did not significantly affect the DR (F1,30=0.689, p=0.414). However, a significant genotype effect (F1,30=19.316, p<0.001) and genotype × exposure condition effect (F1,30=6.423, p=0.018) were found. Posthoc analysis showed that the DR of WT mice was significantly higher that the one of PTH2-R-KO mice specifically under conditions of no pre-exposure to the testing conditions (t14=4.90, p<0.001). A one-sample t-test showed that both pre-exposed and non pre-exposed WT mice preferred the new object over the familiar one (t6=2.984, p=0.025; t6=7.646, p=0.001, respectively). Pre-exposed PTH2-R-KO mice recognized the familiar object (t7=4.257, p=0.004) but non pre-exposed ones failed to discriminate between the new and the familiar object (t7=−0.525, p=0.616 – see Figure 1d).
Infusion of the PTH2 receptor antagonist HYWH-TIP39 impairs memory under conditions of novelty-induced arousal
Data were analyzed using a t-test to compare the performance of mice infused with the PTH2 receptor antagonist to that of mice infused with vehicle.
Spontaneous alternation test
The total number of arm entries was not affected by antagonist infusion (vehicle: 59.88 ± 3.87, antagonist: 62.88 ± 4.89; t14=0.48, p=0.638). The percent alternation was significantly different between the two conditions: vehicle infused animals alternated more than antagonist infused animals (t14=4.59, p<0.001 – Fig 2a).
Figure 2.
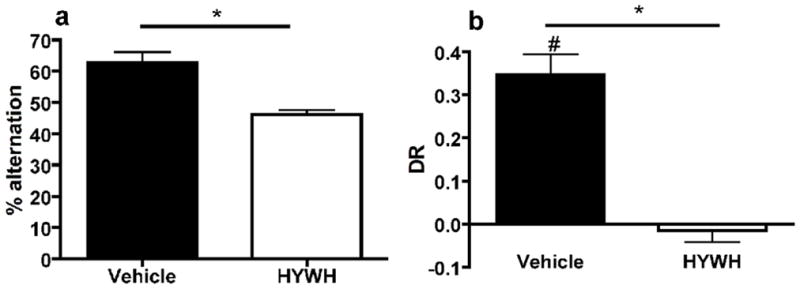
Object recognition test
Antagonist infusion did not affect the time spent exploring the objects during the testing trial T2 (vehicle: 62.10 ± 6.55sec, antagonist: 60.41 ± 3.94sec; t14= 0.339, p=0.74). The antagonist significantly affected the DR, vehicle infused animals had a higher DR than antagonist infused animals (t14= 6.562, p<0.001 – see Figure 2b). One-sample t-tests showed that vehicle infused animals had a DR significantly above chance level (t7=7.236; p<0.001) while this was not the case for the antagonist infused ones (t7=−0.543; p=0.604).
β-adrenoreceptor blocker restored short-term memory in TIP39-KO mice under conditions of novelty-induced arousal
Data were analyzed using a two-way ANOVA with genotype and drug treatment conditions. There was no effect of the propranolol injection or the genotype on the total exploration time in the testing trial (Table 3). Significant overall effects of the genotype and the drug on the DR were found (F1,26=7.515, p=0.012 and F1,26=8.454, p=0.008, respectively). Interaction between the two factors was almost significant (F1,26=3.998, p=0.059). The DR of WT mice injected with saline or propranolol was above chance level (one-sample t-test; t6=4.47; p=0.004 and t6=3.43; p=0.014; respectively). TIP39-KO mice injected with saline did not demonstrate a preference for the novel object (t6=0.33; p=0.754). However, a preference was observed in TIP39-KO mice injected with propranolol (t6=5.22; p=0.002 – Figure 3a).
Table 3.
Total time of exploration of the objects during the training and the testing trial of the object recognition test by TIP39-line mice. Injection of vehicle, propranolol or nadolol was performed after the training phase. Data are presented as Mean ± SEM.
| Training | Testing | ||
|---|---|---|---|
| WT | Vehicle | 73.65 ± 4.23 | 67.28 ± 7.04 |
| Propranolol | 57.07 ± 6.47 | 55.34 ± 2.93 | |
| TIP39-KO | Vehicle | 64.11 ± 5.43 | 53.41 ± 5.90 |
| Propranolol | 68.67 ± 4.98 | 58.23 ± 7.57 | |
| WT | Vehicle | 89.90 ± 4.14 | 74.71 ± 4.26 |
| Nadolol | 90.24 ± 2.48 | 75.78 ± 4.13 | |
| TIP39-KO | Vehicle | 80.51 ± 3.23 | 77.28 ± 9.91 |
| Nadolol | 86.24 ± 1.92 | 71.76 ± 6.05 |
Figure 3.
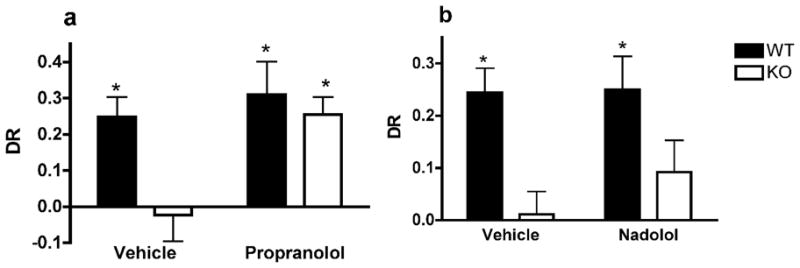
Neither the genotype or the nadolol injection had a main effect on the total exploration time in the testing trial (F1,23=0.022, p=0.913; F1,23=0.116, p=0.737, respectively – see Table 3). An overall genotype effect on the DR (F1,23=12.843, p=0.002) showed that WT animals have a higher DR than KO ones. No drug effect was found (F1,23=0.645, p=0.431 – Figure 3b).
Discussion
To test the involvement of the TIP39/PTH2-R system in modulation of arousal effects on memory performance, we used behavioral tasks that evaluate short-term and working memory under conditions that varied in novelty-induced arousal. Mice lacking TIP39 signaling failed in these memory tasks under conditions of novelty-induced arousal. This memory impairment was rectified by β-adrenergic receptor block, suggesting that (1) TIP39 signaling modulates effects of arousal on memory and (2) this modulatory effect involves noradrenergic signaling.
We first observed that WT and mice lacking TIP39 signaling (TIP39-KO and PTH2-R-KO) were able to spontaneously alternate and to remember a conspecific and an object presented up to 110 minutes earlier if they were repeatedly pre-exposed to the experimental conditions. This shows that TIP39 signaling does not significantly affect intrinsic memory mechanisms. To produce novelty-induced arousal we used a previously developed paradigm in which, rats not pre-exposed to the experimental conditions had higher corticosterone (Okuda et al., 2004) and were more anxious in an open-field (Maroun & Akirav, 2008) than rats pre-exposed to the experimental conditions. In the present work, we did not directly measure the effect of pre-exposure/no pre-exposure to the experimental procedures on the arousal state of our mice. However, based on the rat data (De Boer et al., 1988; Maroun & Akirav, 2008; Okuda et al., 2004) we inferred that the repeated pre-exposure to the testing environment reduced the novelty-induced arousal level of mice at the time of testing. We observed that non pre-exposed mice that lack TIP39 signaling had decreased spontaneous alternation and failed to investigate a novel object or mouse more than a familiar one, indicative of short-term and working memory impairment. This strongly suggests that the novelty-induced arousal differentially affects memory functions of mice with or without TIP39 signaling. While we cannot completely exclude the possibility that the difference in behavior between mice with and without TIP39 signaling that appears in mice not pre-exposed to the training apparatus is due to a process other than arousal, such as the cognitive structure formed by the prior experience, this seems unlikely to us.
These working and short-term memory impairments in mice lacking TIP39 signaling are likely to be explained by changes in memory processes rather than by other performance parameters such as exploration or motivation. During the object recognition test, non pre-exposed mice explored objects less than pre-exposed ones. This is consistent with previous rat data showing increased locomotion with novel context exposure (Borella et al., 2003; Okuda et al., 2004) resulting in lower object investigation. In the social recognition test, an opposite effect was found: non-exposed animals explored stimulus juveniles more than mice previously exposed to the testing conditions. The opposite effect of pre-exposure on exploration observed in the object recognition test and the social recognition test might be attributed to the relative size of the testing apparatuses: the object recognition testing arena was relatively big and the objects were small, leaving space for the animal to demonstrate high locomotion. The social recognition testing cage was small and the corrals used half of the space, which might reduce locomotion. However, we did not evaluate locomotor behavior to test this idea. Differences in exploration level were present in mice with or without TIP39 signaling and did not influence WT memory, indicating that exploration level variations did not account for the memory impairment observed in KO mice. Similarly, quantitative differences in motivation to perform the tasks could not explain our findings since all genotypes explored the objects or juveniles for the same amount of time, and made the same number of Y-maze arm entries. Impairment in short-term and working memory when learning occurs under arousing conditions is well described (Heinrichs, 2003; Okuda et al., 2004; Scullion et al., 2009). Considering the inverted-U shape relationship between arousal and memory performance (Arnsten, 2009), we suggest that in WT mice, the exposure to the novel environment did not result in a sufficient increase in arousal to significantly affect memory. In contrast, in mice lacking TIP39 signaling, the observed memory impairment was likely due to a greater impact of the novelty-induced arousal, compared to WT mice. However, the absence of pre-exposure to the experimental conditions could have resulted not only in increased arousal but also in increased anxiety. We showed previously that TIP39-KO mice have a higher anxiety level under stressful conditions (Fegley et al., 2008). Thus, being exposed to a novel environment during testing might have increased the anxiety level of mice without TIP39 signaling, leading to working and short-term memory impairments. This idea could be tested with the administration of anxiolytic drugs prior to testing.
Interpretation of studies using mice with targeted mutations is potentially complicated by the effects of genes closely linked to or flanking the targeted locus, because the genetic background of ES lines used for targeting frequently differs from the lines into which they are introduced (Crusio, 2004; Wolfer et al., 2002). In the present work, we obtained equivalent results from mouse lines with null mutation of TIP39 or of its receptor, which are on different chromosomes, providing very strong support for the suggestion that the phenotypes observed derive from changes in TIP39/PTH2-R signaling. Data from acute receptor antagonist administration are consistent with this. Mice acutely infused with the PTH2-R antagonist prior to testing had a low discrimination ratio in the object recognition test and a reduced rate of Y-maze alternation, compared to vehicle-infused mice. This result supports the idea that the phenotype of TIP39-KO and PTH2-R-KO mice is the direct result of the TIP39 signaling absence during the experiment, and not of potential compensatory or developmental effects in these conventional knockouts with life-long gene function loss. Altogether, our results are consistent with the idea that TIP39 signaling modulates memory impairment selectively under conditions of novelty-induced arousal.
We hypothesized that the working and short-term memory impairments in mice lacking TIP39 signaling is due to increased sensitivity to novelty exposure. In absence of TIP39 signaling, the exposure to a novel environment during testing would lead to greater activation of the sympathetic nervous system, central noradrenergic pathways, and/or the pituitary-adrenal system of mice, resulting in increased noradrenaline and/or CRF secretion that ultimately affects memory. Many studies have implicated norepinephrine in memory modulation (King & Williams, 2009; Okuda et al., 2004; Roozendaal et al., 2007; Scullion et al., 2009). To further examine this idea, we administered two β-adrenoreceptor antagonists, propranolol and nadolol, immediately after the training phase of the object recognition test performed without any prior exposure to the experimental conditions. Propranolol fully restored memory function in TIP39-KO mice while nadolol failed. The antagonists have a similar β-adrenoreceptor specificity (Frishman, 1981), but in contrast to nadolol, propranolol enters the brain well and acts centrally. Our results support the idea of noradrenergic involvement in TIP39 effects on working and short-term memory impairment under novelty-induced arousal conditions. They also indicate that central β-adrenergic receptors play a greater role in mediating the differences between the effects of arousal on memory function in WT and mice lacking TIP39 signaling than peripheral ones. Norepinephrine released from the locus coeruleus under arousing conditions affects structures involved in cognitive processes, including the amygdala, hippocampus (Jones et al., 1977) and prefrontal cortex (Arnsten, 2009; Hains & Arnsten, 2008). It has been shown that stress-induced catecholamine elevations impair prefrontal cortex integrity, and thus working memory functions (Arnsten, 2009; Hains & Arnsten, 2008). Thus, a possible pathway by which TIP39 signaling affects memory under conditions of novelty-induced arousal could involve a noradrenergic activation of the prefrontal cortex or other forebrain areas.
However, with the present results, we cannot conclude that there is a direct effect of TIP39 signaling on the noradrenergic system. Other neuromodulators influence short-term and working memory (e.g. dopaminergic or serotonergic pathways - Chamberlain et al., 2006) and their effects are also subjected to noradrenergic modulation (Sara, 2009). Glucocorticoid activation has also been shown to affect memory performance on its own or in synergy with a noradrenergic activation (Okuda et al., 2004; Roozendaal et al., 2008). Considering this, additional approaches will be required to elucidate the molecular mechanisms underlying our behavioral data. Specifically, we demonstrated previously that TIP39 and the PTH2-R are present in several brain regions that contribute to HPA axis regulation and that injection of TIP39 within the hypothalamic paraventricular nucleus increases plasma corticosterone (Dimitrov & Usdin, In press). In future studies, we will investigate the possibility that TIP39 effects on the HPA axis contribute to its modulation of cognitive performance. Moreover, it will also be important to determine whether TIP39 signaling is involved in the modulation of memory processes other than working and short-term memories (e.g. long-term memory).
Supplementary Material
Olfactory habituation/dishabituation of adult WT and KO males of the TIP39 (n=10 per genotype) (a) and PTH2-R (n=8 per genotype) (b) lines. Animals were presented with 3 consecutive cotton-tipped applicators either dipped into water or swiped across the floor of cages of two unfamiliar single-housed adult males (cage 1 and cage 2). (a) No genotype effect was found (F1,18= 0.953, p=0.342). Both WT and TIP39-KO mice showed habituation to a neutral (Exposure effect: F2,18= 9.734, p<0.001) or social odor cue (Cage 1, exposure effect: F2,18= 11.818, p<0.001; Cage 2, exposure effect: F2,18= 4.479, p=0.018) and discriminated between odors (Exposure 3 – water vs. Exposure 1 – Cage 1: p<0.001; Exposure 3 – Cage 1 vs. Exposure 1 – Cage 2: p = 0.015), which demonstrates no olfaction or olfactory learning impairment in the TIP39-KO.
(b) No overall genotype effect was found (F1,14= 0.620, p=0.444). Both WT and PTH2-R-KO showed habituation to a neutral (Exposure effect: F2,14= 90.394, p<0.001) or to a social odor cue (Cage 1, exposure effect: F2,14= 94.068, p<0.001; Cage 2, exposure effect: F2,14= 105.855, p<0.001) and discriminated between odors (Exposure 3 – water vs. Exposure 1 – Cage 1: p<0.001; Exposure 3 – Cage 1 vs. Exposure 1 – Cage 2: p<0.001), which demonstrates no olfaction or olfactory learning impairment in the WT or the PTH2-R-KO mice.
Acknowledgments
We thank Jim Pickel for generation of knockout mice, Elka Scordalakes and Eugene Dimitrov for help and advice, and Andrew Holmes for advice and helpful comments on the manuscript. This research was supported by the Intramural Program of the NIH, National Institute of Mental Health.
Footnotes
The authors declare that there are no potential conflicts of interest.
References
- Arnsten A. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten FT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Borella A, Sumangali R, Ko J, et al. Characterization of social behaviors and oxytocinergic neurons in the S-100 beta overexpressing mouse model of Down Syndrome. Behav Brain Res. 2003;141:229–236. doi: 10.1016/s0166-4328(02)00373-x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacol. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cerbone A, Sadile AG. Behavioral habituation to spatial novelty: interference and noninterference studies. Neurosci Biobehav Rev. 1994;18:497–518. doi: 10.1016/0149-7634(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Muller U, Robbins TW, et al. Neuropharmacological modulation of cognition. Curr Opin Neurol. 2006;16:607–612. doi: 10.1097/01.wco.0000247613.28859.77. [DOI] [PubMed] [Google Scholar]
- Crusio WE. Flanking gene and genetic background problems in genetically manipulated mice. Biol Psychiatry. 2004;56:381–385. doi: 10.1016/j.biopsych.2003.12.026. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Slangen JL, van der Gugten J. Adaptation of plasma catecholamine and corticosterone responses to short-term repeated noise stress in rats. Phys Behav. 1988;44:273–280. doi: 10.1016/0031-9384(88)90149-7. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Bodnar I, et al. Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience. 2003;122:1093–1105. doi: 10.1016/j.neuroscience.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol. 2010;90:29–59. doi: 10.1016/j.pneurobio.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Escoubet B, Leclercq JF, Maison-Blanche P, et al. Comparison of four beta-blockers as assessed by 24-h ECG recording. Clin Pharmacol Ther. 1986;39:361–368. doi: 10.1038/clpt.1986.55. [DOI] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, et al. Distribution of tuberinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol. 2007;502:563–583. doi: 10.1002/cne.21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley DB, Holmes A, Riordan T, et al. Increased fear- and stress-related anxiety-like behavior in mice lacking tuberinfundibular peptide of 39 residues. Genes Brain Behav. 2008;7:933–942. doi: 10.1111/j.1601-183X.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman WH. Beta-adrenoreceptor antagonists: new drugs and new indications. N Engl J Med. 1981;305:500–506. doi: 10.1056/NEJM198108273050907. [DOI] [PubMed] [Google Scholar]
- Hains AB, Arnsten AF. Molecular mechanisms of stress-induced prefrontal cortical impariment: implications for mental illness. Learn Mem. 2008;15:551–564. doi: 10.1101/lm.921708. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC. Modulation of social learning in rats by brain corticotropin-releasing factor. Brain Res. 2003;994:107–114. doi: 10.1016/j.brainres.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Bartfai T, Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2:463–472. doi: 10.1016/s1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobeha Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Halaris AE, McIlhany M, et al. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res. 1977;127:1–21. doi: 10.1016/0006-8993(77)90377-8. [DOI] [PubMed] [Google Scholar]
- King SO, II, Williams CL. Novelty-induced arousal enhances memory for cued classical fear conditioning: interactions between peripheral adrenergic and brainstem glutamatergic systems. Learn Mem. 2009;16:625–634. doi: 10.1101/lm.1513109. [DOI] [PubMed] [Google Scholar]
- Kuo J, Usdin TB. Development of a rat parathyroid hormone 2 receptor antagonist. Peptides. 2007;28:887–892. doi: 10.1016/j.peptides.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Dobolyi A, Usdin TB. Tuberoinfundibular peptide of 39 residues produces anxiolytic and antidepressant actions. Neuroreport. 2004;15:881–885. doi: 10.1097/00001756-200404090-00030. [DOI] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- MacBeth AH, Stepp Edds J, Young SW., III Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nat Protoc. 2009;4:1574–1581. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation memory. Neuropsychopharmacol. 2008;33:394–405. doi: 10.1038/sj.npp.1301401. [DOI] [PubMed] [Google Scholar]
- McDevitt DG. Comparison of pharmacokinetic properties of beta-adrenoreceptor blocking drugs. Eur Heart J. 1987;8 (Supplement M):9–14. doi: 10.1093/eurheartj/8.suppl_m.9. [DOI] [PubMed] [Google Scholar]
- McGaugh J, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002a;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh J, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002b;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, Gold PE, Van Buskirk R, et al. Modulating influences of hormones and catecholamines on memory storage processes. Prog Brain Res. 1975;42:151–162. doi: 10.1016/S0079-6123(08)63656-0. [DOI] [PubMed] [Google Scholar]
- O’Gorman S, Dagenais NA, Qian M, et al. Protamine-Cre recombinase transgene efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren S, Ove Kuteeva E, Elvander-Tottie E, et al. Neuropeptides in learning and memory processes with focus on galanin. Eur J Pharmacol. 2010;626:9–17. doi: 10.1016/j.ejphar.2009.09.070. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation and memory for emotionally arousing experiences. Prog Brain Res. 2007;167:79–95. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, et al. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Scullion GA, Kendall DA, Sunter D, et al. Central noradrenergic depletion by DSP-4 prevents stress-induced memory impairments in the object recognition task. Neuroscience. 2009;164:415–423. doi: 10.1016/j.neuroscience.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Stack cM, Lim MA, Cuasay K, et al. Deficit in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Hoare SRJ, Wang T, et al. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Paciga M, Riordan T, et al. Tuberoinfundibular peptide of 39 residues is required for germ cell development. Endocrinology. 2008;149:4292–4300. doi: 10.1210/en.2008-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst) 2008;127:513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking gene. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Olfactory habituation/dishabituation of adult WT and KO males of the TIP39 (n=10 per genotype) (a) and PTH2-R (n=8 per genotype) (b) lines. Animals were presented with 3 consecutive cotton-tipped applicators either dipped into water or swiped across the floor of cages of two unfamiliar single-housed adult males (cage 1 and cage 2). (a) No genotype effect was found (F1,18= 0.953, p=0.342). Both WT and TIP39-KO mice showed habituation to a neutral (Exposure effect: F2,18= 9.734, p<0.001) or social odor cue (Cage 1, exposure effect: F2,18= 11.818, p<0.001; Cage 2, exposure effect: F2,18= 4.479, p=0.018) and discriminated between odors (Exposure 3 – water vs. Exposure 1 – Cage 1: p<0.001; Exposure 3 – Cage 1 vs. Exposure 1 – Cage 2: p = 0.015), which demonstrates no olfaction or olfactory learning impairment in the TIP39-KO.
(b) No overall genotype effect was found (F1,14= 0.620, p=0.444). Both WT and PTH2-R-KO showed habituation to a neutral (Exposure effect: F2,14= 90.394, p<0.001) or to a social odor cue (Cage 1, exposure effect: F2,14= 94.068, p<0.001; Cage 2, exposure effect: F2,14= 105.855, p<0.001) and discriminated between odors (Exposure 3 – water vs. Exposure 1 – Cage 1: p<0.001; Exposure 3 – Cage 1 vs. Exposure 1 – Cage 2: p<0.001), which demonstrates no olfaction or olfactory learning impairment in the WT or the PTH2-R-KO mice.


