Abstract
Behavioral research has yielded conflicting results regarding the architecture of short-term memory (STM). Whereas a consensus has emerged that within STM a single chunk within the focus of attention (FA) has a privileged status, it is unclear whether further distinctions exist. One proposal is that outside of FA, memory is all of one sort with a continuous progression from STM to long-term memory (LTM). On the other hand, sharp performance drop-offs when STM is loaded with more than 4±1 items suggest distinctions between STM and LTM. We use functional magnetic resonance imaging (fMRI) to adjudicate between these theories. A neural triple dissociation provided evidence for a 3-state model of memory. Critically, prefrontal cortex was selectively enhanced to retrieval from activated portions of LTM whereas the hippocampus was associated with retrieval of items within putative 4±1 capacity limits. We hypothesize that the associative properties of the hippocampus serve to inter-relate information actively maintained in STM which not only promotes strong STM, but also lays the foundations for subsequent LTM. By contrast, information not actively maintained in mind requires top-down retrieval processes mediated by the prefrontal cortex. These data provide key insights into the architecture of STM and its relationship to LTM.
Keywords: short-term memory (STM), working memory (WM), prefrontal cortex (PFC), medial temporal lobe (MTL), fMRI
Introduction
The ability to hold information online in an active state is central to much of cognition including problem solving, reading comprehension, and the formation of memories. Indeed, the capacity of online storage is predictive of a variety of higher order cognitive functions (Carpenter et al., 1990; Daneman and Carpenter, 1980; Daneman and Merikle, 1996). Despite the clear importance of the short-term storage system, fundamental questions regarding its architecture remain.
Since Baddeley (1986), the system that affords online maintenance of information and the processes that act upon that information have collectively been considered working memory. The storage aspect of working memory, or short-term memory (STM), has been thought to consist of separate buffers for different modalities of information. The information within such buffers has been thought of as one sort; that is, although individual items within a buffer may vary in activation strength, the qualitative state in which they are held is thought to be fundamentally similar.
More recent work, however, has demonstrated that short-term storage is not all of one sort. In particular, research has shown that a single chunk of information in STM has privileged access (Garavan, 1998; McElree, 2006; McElree and Dosher, 1989; Oberauer, 2002). This privileged chunk has been termed the focus of attention1 (FA) in STM. A series of behavioral studies has shown that the FA is accessed unusually quickly and that decisions regarding the information in the FA can be made immediately compared to other information in STM, which requires retrieval processes that take time. Recently, we have demonstrated a neural signature for the access of information in the FA consisting of functional connections between posterior parietal cortex and inferior temporal cortex (Nee and Jonides, 2008).
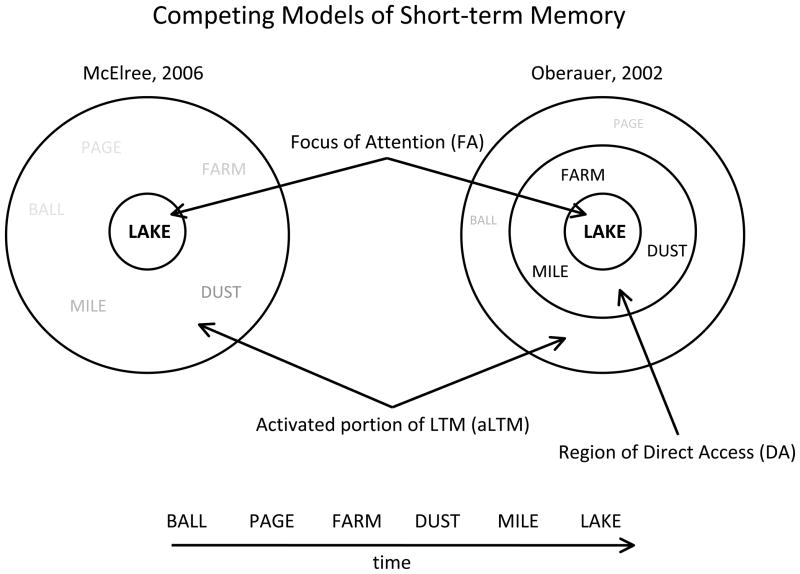
Outside of the FA, whether information within STM is homogeneous or not is subject to debate (Fig. 1). On the one hand, results from studies involving speeded decisions suggest that access to information outside of the FA follows a continuous pattern suggesting a homogeneous status of information with varying activation strengths (McElree, 2006; McElree and Dosher, 1989). On the other hand, several investigations have demonstrated sharp performance discontinuities when more than 4±1 items are loaded into STM (Cowan, 2001). Hence, by one account, STM is of 2-states consisting of the FA and other information that is assumed to be activated long-term memories (aLTM). By another, STM is of 3-states consisting of the FA, 3 or so other items in a region of direct access (DA), with other information in aLTM (Jonides et al., 2008; Oberauer, 2002). Thus far, no consensus has emerged based upon behavioral data. Imaging data may provide key insights into this issue, but to our knowledge, no experiment has successfully distinguished 2- and 3-state models of STM2.
Fig. 1.
Competing models of short-term memory. Left: The 2-state model of short-term memory consisting of a single chunk in the focus of attention with other information in short-term memory consisting of activated long-term memories with varying activation strengths. Right: The 3-state model of short-term memory where a region of direct access is thought to exist as an intermediary state between the focus of attention and activated long-term memory. In this account, the focus of attention + the region of direct access is hypothesized to have a capacity of 4±1 items.
It is important to note the distinction between “state” and “store”. It is becoming increasingly evident that shared neural substrates are involved in perception, short-term storage, and long-term storage (Jonides et al., 2005; Nee et al., 2008; Postle, 2006). Hence, different states of memory may nevertheless involve the same regions of memory storage. Storage, on the other hand, may vary fundamentally by stimulus-type (e.g. objects versus words). Of interest for present purposes are memory states.
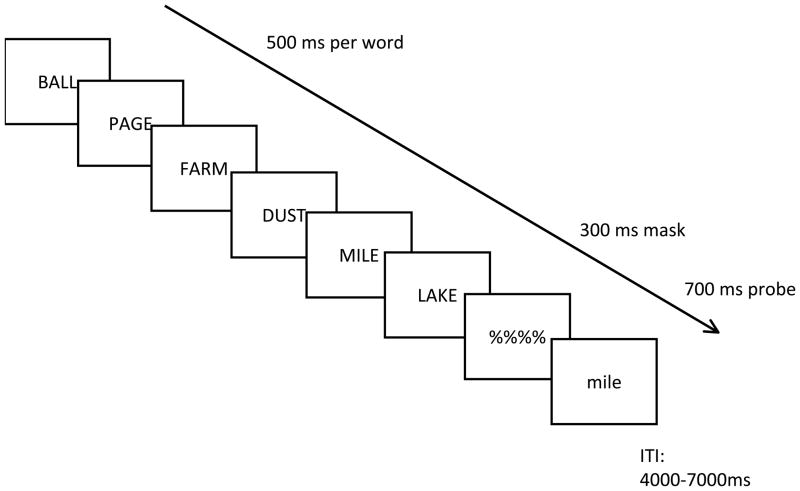
The present study used functional magnetic resonance imaging (fMRI) to explore the architecture of STM. We employed a simple item-recognition paradigm with rapid presentation similar to behavioral paradigms that have been used to explore the rate of access of information in STM (Fig. 2; McElree, 2006; McElree and Dosher, 1989). On each trial, six words were presented rapidly followed closely by a recognition probe. According to both 2- and 3-state models, the most recently presented item should be held in the FA. By the 2-state model of memory, the rest of the items should consist of aLTM, whereas the 3-state model would predict a discontinuity behaviorally and neurally when subjects are probed with early list items outside of the putative 4±1 capacity limit. Hence, our paradigm afforded the ability to examine whether a selective neural signature can be associated with retrieval of information in the FA, the putative DA, and the aLTM. As in our previous research, we expected to find a selective neural signature of FA-access in posterior parietal and inferior temporal cortex. However, the critical test was whether the DA and aLTM would dissociate. If a 2-state model is correct, then linear patterns of retrieval would be expected when comparing the FA, putative DA, and aLTM. By contrast, a 3-state model would predict a double dissociation of neural regions responding to DA- and aLTM-retrieval. In previous research we and others have demonstrated that retrieval of information outside of FA recruits left lateral prefrontal cortex (LPFC) and regions in the medial temporal lobe (Nee and Jonides, 2008; Oztekin et al., 2009b). Hence, how these regions respond to retrieval from DA and aLTM may be particularly informative.
Fig. 2.
Experimental protocol. On each trial subjects committed six rapidly presented words to memory and responded to a recognition probe after a very brief delay. The most recently presented item (serial position (sp) -1 was assumed to reside in the focus of attention. By the 3-state model of memory, sp -2 and -3 items would presumably reside in the region of direct access, which would be distinct from early list items.
Materials and Methods
Participants
We report data from 25 right-handed adults (14 female; ages 18–28; mean = 20.9 years old). They received $20/hr as well as a bonus for fast and accurate performance. An additional four subjects were recruited, but technical errors made their data unusable.
Materials and Procedure
Subjects performed an item-recognition task in which they were sequentially presented six items followed by a test probe (Fig. 2). The task was modeled after paradigms that have been used to examine different memory states of STM (McElree, 2006; McElree and Dosher, 1989; Nee and Jonides, 2008; Oztekin et al., 2009b). Subjects responded via keypress whether the test probe was a member of the memory set or not. Each trial began with a red fixation cross presented for 0.5 s to alert the subject to the start of the trial. Thereafter, 6 words were sequentially presented for 0.5 s each, followed by a mask presented for 0.3 s. A test probe was presented for 0.7 s that matched one of the memory items 50% of the time. All serial positions were probed equally. The inter-trial interval (ITI) was jittered between 3.5 and 6.5 s. Memory items were presented in uppercase and the test probe was presented in lowercase to minimize visual matching strategies. Words were drawn randomly without replacement from a set of 171 four-letter nouns, and the list was re-randomized after it was exhausted. Subjects performed 6 runs of 48 trials each. The day before scanning, subjects were given 2 runs of 48 trials to learn the task, and an additional run was given the day of scanning prior to entering the scanner.
Critically, rapid presentation of the memory set and the brief delay minimized the use of rehearsal and chunking strategies in accordance with previous work (Nee and Jonides, 2008; Oztekin et al., 2010; Oztekin et al., 2009b). As in previous research, the most recently presented item was assumed to reside in FA (McElree, 2006; Nee and Jonides, 2008; Oztekin et al., 2010; Oztekin et al., 2009b). Items outside of the FA were assumed to show decreasing memory strength with increasing distance from the end of the list (decreasing recency). The 6-item memory set afforded the ability to contrast items putatively in DA against items putatively in aLTM, with the assumption that DA would reflect serial position (sp) -2 and sp -3 items. As indicated by the behavioral data and consistent with previous work, aLTM appeared to consist of sp -4 and sp -5 items. The sp -6 item (i.e. the first item presented) was excluded in neural analyses due to ambiguity surrounding the primacy effect, as described in more detail below.
Prior to scanning, subjects performed the operation span (OSPAN) task as a measure of working memory capacity. In the OSPAN task, subjects solve math problems while maintaining a set of letters in mind (Unsworth et al., 2005). Capacity was measured by the number of letters subjects could successfully hold in mind while completing the math problems.
Image acquisition and preprocessing
Images were acquired on a GE Signa 3-T scanner equipped with a standard quadrature head coil. Head movement was minimized using foam padding and a cloth restraint strapped across participants’ foreheads.
Functional T2*-weighted images were acquired using a spiral sequence with 40 contiguous slices with 3.44 × 3.44 × 3 mm voxels (repetition time, or TR = 2,000 ms; echo time, or TE = 30 ms; flip angle = 90°; field of view, or FOV = 22 mm2). A T1-weighted gradient-echo anatomical overlay was acquired using the same FOV and slices (TR = 250 ms, TE = 5.7 ms, flip angle = 90°). Additionally, a 124-slice high-resolution T1-weighted anatomical image was collected using spoiled-gradient-recalled acquisition (SPGR) in steady-state imaging (TR = 9 ms, TE = 1.8 ms, flip angle = 15°, FOV = 25–26 mm2, slice thickness = 1.2 mm).
Functional data were spike-corrected to reduce the impact of artifacts using AFNI’s 3dDespike (http://afni.nimh.nih.gov/afni). Subsequent processing and analyses were done using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Functional images were corrected for differences in slice timing using sinc-interpolation (Oppenheim et al., 1999) and head movement using a least-squares approach and a 6 parameter rigid body spatial transformation. Structural data were co-registered to the functional data and segmented into gray and white-matter probability maps (Ashburner and Friston, 1997). These segmented images were used to calculate spatial normalization parameters to the MNI template, which were subsequently applied to the functional data. Eight-mm full-width/half-maximum isotropic Gaussian smoothing was applied to all functional images prior to analysis using SPM5. All analyses included a temporal high-pass filter (128 s), and each image was scaled to have a global mean intensity of 100.
Image analysis
Analyses were conducted using the general linear model implemented in SPM5. Predictors were locked to the onset of the recognition probe and convolved with a canonical hemodynamic response function (HRF) provided by SPM5. Accuracy of the HRF was confirmed in follow up analyses using finite impulse response (FIR) models within regions of interest (ROIs). Separate regressors were calculated for probes matching the FA (sp -1), DA (sp’s -2 and -3), and aLTM (-sp’s 4 and -5). This partition of memory states was based upon patterns demonstrated in the group-averaged behavioral data. Separate analyses using groupings tailored to putative capacity limits defined on a subject-by-subject basis provided confirmatory results. Error trials, negative probes, and probes matching the -6 back items were modeled separately. For subjects demonstrating greater than 3 mm of motion across a session or greater than 0.5 mm of motion between TRs, 24 motion regressors were included to capture linear, quadratic, differential, and squared differential residual motion variance (Lund et al., 2005).
Parameter estimates for retrieval from FA, DA, and aLTM for each subject were submitted to a second-level one-way ANOVA. This analysis was thresholded at p < 0.05 corrected for multiple comparisons using False-Discovery rate (FDR) with a 10-contiguous voxel-extent criterion. Significant clusters revealed by the ANOVA were subsequently tested using paired t-tests to determine the nature of condition differences.
Follow-up analyses were performed on left LPFC and the hippocampus in that these regions were critical ROIs for this study. Anatomical ROIs were created using the WFU Pick Atlas implemented in SPM5 (Maldjian et al., 2003). For left LPFC, the ROI included pars triangularis and pars opercularis of the left inferior frontal gyrus since this region was a major focus of activation in previous research (Nee and Jonides, 2008). The hippocampal ROI included the hippocampi bilaterally. Within each anatomical ROI, voxels demonstrating task-sensitivity were examined. Task-sensitive voxels were identified as those voxels showing greater activation for the average of all conditions of interest (FA, DA, and aLTM) compared to baseline at p < 0.05 uncorrected. Parameter estimates for each condition were then extracted from these ROIs and submitted to a region (left LPFC, hippocampus) x state (FA, DA, aLTM) ANOVA and paired t-tests.
Brain-behavior correlations were computed within the ROIs described above. Correlations were thresholded at p < 0.05 corrected for multiple comparisons using cluster extent according to simulations obtained via AFNI’s AlphaSim (http://afni.nimh.nih.gov/afni). Although all correlations are valid statistically, measures of effect size are not reported to avoid interpretation of potentially inflated effect sizes that occur as a result of thresholding (Yarkoni and Braver, in press). Scatter plots are included for the purpose of visual confirmation that correlations were not unduly driven by outliers. All correlations were re-tested using robust regression to reduce the impact of outliers to provide further assurance that the correlations were not driven by outliers.
Brain-brain correlations were computed across all voxels in the ROIs described above. Contrasts were created to isolate memory states of interest while avoiding common terms as much as possible. aLTM-related left LPFC activation was assessed using the contrast of aLTM-DA. DA-related hippocampal activation was assessed using the contrast of DA-FA. Notably, since DA activation is common to both contrasts a correlation could be the result of a left LPFC-hippocampal correlation on DA trials alone.
However, such a correlation did not exist (r = 0.04) and hence could not drive the results. Correlations were checked using robust regression to ensure that they were not driven by outlying values.
Results
Behavioral Results
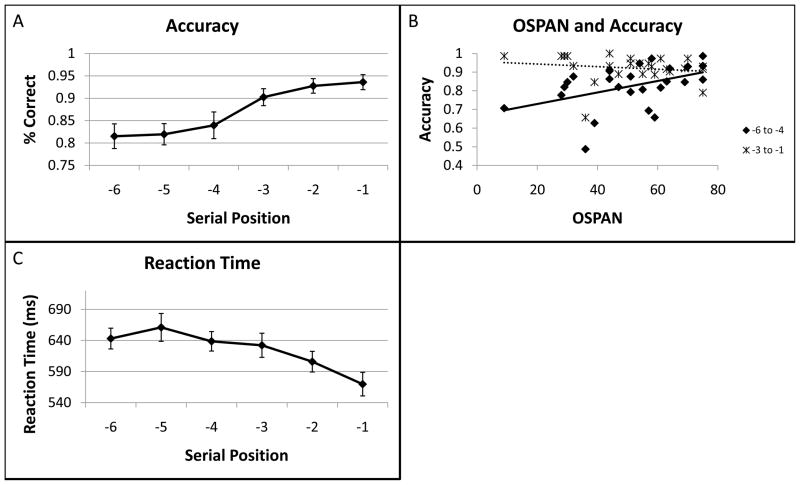
We began by examining behavioral results as a function of serial position. Accuracy was high overall (mean 89.9% correct) and varied as a function of serial position (F(5,120) = 10.68, p < 0.001; Fig. 3a). Sequential pair-wise comparisons revealed a sharp drop-off between serial position (sp) -3 and sp -4 (mean 6.3%, t(24) = 2.25, p < 0.05), but no other sequential pair-wise comparison was significant (all t(24) < 1.6, p > 0.1). Hence, whereas accuracy demonstrated a gradual decline with serial position, this decline was especially precipitous between sp -3 and -4. Highlighting this performance discontinuity, a sigmoidal model of the data provided a much stronger fit than a simple linear model (adjusted R2 = 0.998 for sigmoidal, adjusted R2 = 0.909 for linear). Such a performance discontinuity is consistent with previous reports demonstrating sharp declines when memory load reaches 4± 1 items (Cowan, 2001). To confirm this consistency, we correlated accuracy with working memory capacity as measured by the OSPAN task. Accuracy to early list items (sp’s -6 to -4) correlated positively with OSPAN (r = 0.45, p < 0.05), but not late list items (sp’s’ -3 to -1, r = −0.16, p > 0.4; Fig. 3b). This result confirms that drop-offs in performance are due to capacity limitations and that subjects with greater capacities show reduced drop-offs.
Fig. 3.
Behavioral results. A) Accuracy data as a function of serial position. A clear drop-off in performance was observed between serial position (sp) -3 and -4 consistent with 3-state models that posit a capacity of 4±1 items. B) Capacity as measured by the operation span (OSPAN) task correlated with accuracy to early list items (sp -6 to -4), but not late list items (sp -3 to -1). This is consistent with the proposal that drop-offs in performance for early list items were due to capacity limitations. C) Reaction time data as a function of serial position. A pronounced recency effect was observed for sp -1 consistent with the idea that this item resides in the focus of attention and has privileged access.
Reaction time (RT) data were analyzed on correct trials only. RT varied as a function of serial position (F(5,120) = 23.21, p < 0.001; Fig. 3c). In accordance with previous reports (Nee and Jonides, 2008), there were both recency (sp -1 < sp -2, mean difference 40.6 ms, t(24) = 4.0, p < 0.001) and primacy effects (sp -6 < sp -5, mean difference 19.2 ms, t(24) = 2.20, p < 0.05) in RT. The pronounced recency effect is consistent with the idea that the most recently presented item resides in the FA and has privileged access (Garavan, 1998; McElree, 2006; McElree and Dosher, 1989; Oberauer, 2002). OSPAN was unrelated to RT (correlation between OSPAN and each sp, all r < 0.25).
Based on the observed behavioral patterns and the extant literature, we grouped the serial positions into 3 separate hypothesized states: the FA was assigned to sp -1, the DA was assigned to sp -2 and -3, and the aLTM was assigned to sp -4 and -5. The accuracy and RT data produced conflicting patterns regarding sp -6 with a notable primacy effect in RT. It is possible that due to heightened distinctiveness the first item was encoded into DA, although by the accuracy data it is clear that this was not always the case. Due to this ambiguity sp -6 was left out of memory state groupings. We note in passing that including sp -6 with aLTM does not qualitatively change behavioral and neural results. Also of note, analyses that tailored memory states to individual capacities produced similar results to those reported here (see Supplemental Information).
Neural Results
A one-way ANOVA revealed several regions showing differences as a function of memory state retrieval (Fig. 4; Table 1). Regions varying as a function of memory state included medial and lateral prefrontal cortex, medial and lateral posterior parietal cortex, and medial and lateral temporal cortex. Differences were most prominent in the left hemisphere, consistent with the verbal nature of the task.
Fig. 4.
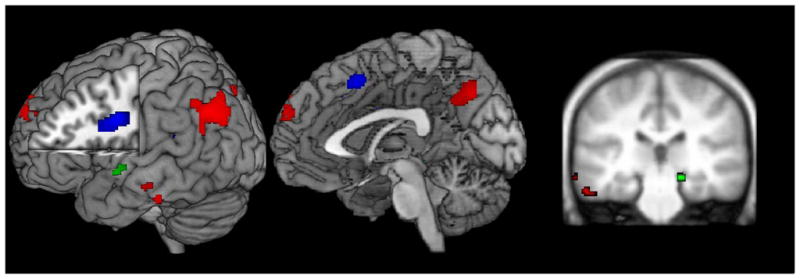
Regions demonstrating an effect of memory state. Red: regions showing greater activation for retrieval of the focus of attention (FA) compared to the region of direct access (DA) and the activation portion of long-term memory (aLTM). Activations included posterior parietal cortex and inferior temporal cortex replicating previous research (Nee and Jonides, 2008). Green: regions showing greater activation for retrieval of DA compared to FA and aLTM. These activations included the right hippocampus. Blue: regions showing greater activation for retrieval of aLTM compared to FA and DA. These activations included left lateral prefrontal cortex. This triple dissociation is consistent with 3-state models of memory.
Table 1.
| x | y | z | extent | F | Z | region |
|---|---|---|---|---|---|---|
| Focus of Attention Selective (FA > DA = aLTM) | ||||||
| −56 | −64 | 38 | 513 | 15.33 | 4.54 | left inferior parietal lobule |
| −50 | −60 | 24 | 19 | 10.07 | 3.64 | left post superior temporal gyrus |
| −60 | −30 | −22 | 24 | 9.86 | 3.59 | left inferior temporal gyrus |
| Region of Direct Access Selective (DA > FA = aLTM) | ||||||
| 18 | −28 | −12 | 18 | 11.4 | 3.89 | right hippocampus |
| Activated Long-Term Memory Selective (aLTM > FA = DA) | ||||||
| −44 | 16 | 14 | 20 | 9.99 | 3.62 | left inferior frontal gyrus |
| “Default” (FA > DA > aLTM) | ||||||
| −4 | 62 | 28 | 193 | 14.34 | 4.39 | anterior superior frontal gyrus |
| −2 | −64 | 44 | 185 | 14.31 | 4.38 | precuneus/posterior cingulate |
| −14 | −78 | 44 | 41 | 12.27 | 4.05 | precuneus/superior parietal lobule |
| −22 | −4 | −16 | 64 | 11.97 | 3.99 | left amygdala |
| −68 | −22 | −12 | 10 | 11.36 | 3.88 | left middle temporal gyrus |
| “Task-Positive/Cognitive Control” (aLTM > DA > FA) | ||||||
| −38 | 0 | 30 | 263 | 21.94 | 5.39 | left inferior frontal sulcus |
| −2 | 14 | 50 | 224 | 18.23 | 4.94 | dorsal anteror cingulate/pre-SMA |
| −10 | 32 | 24 | 44 | 11.49 | 3.91 | left anterior cingulate |
| 10 | 24 | 40 | 15 | 10.43 | 3.71 | right dorsal anterior cingulate |
| −42 | −42 | 20 | 14 | 9.68 | 3.55 | left posterior insula |
| Other (aLTM = DA > FA) | ||||||
| 10 | −2 | 26 | 42 | 13.19 | 4.2 | right caudate |
| Other (FA = DA > aLTM) | ||||||
| 22 | 0 | −14 | 10 | 10.22 | 3.66 | right amygdala |
| −56 | −2 | −2 | 19 | 9.7 | 3.56 | left ant superior temporal gyrus |
Regions demonstrating a main effect of memory state at p < 0.05 corrected by False-Discovery Rate with 10 contiguous voxels or more. Follow up paired t-tests on each region revealed by the ANOVA revealed the nature of the state differences. x, y, and z denote peaks in MNI space. FA, focus of attention; DA, region of direct access; aLTM, activated portion of long-term memory.
Follow-up analyses revealed regions selective to retrieval from particular memory states, as well as regions that varied linearly with recency. Each region revealed by the whole-brain ANOVA was subjected to paired t-tests to understand the nature of the memory state differences. FA-selective regions (FA > DA = aLTM) included left posterior parietal cortex, left inferior temporal cortex, and left posterior superior temporal gyrus. The right hippocampus demonstrated a DA-selective pattern (DA > FA = aLTM). The left inferior frontal gyrus demonstrated an aLTM-selective pattern (aLTM > DA = FA). Taken together these regions demonstrated a triple dissociation, with different regions selective to retrieval from different memory states.
Regions demonstrating decreased activation with decreasing recency (FA > DA > aLTM) included the precuneus, posterior cingulate, and anterior medial frontal cortex. These patterns are consistent with the involvement of these regions in the “default network” (Gusnard and Raichle, 2001). The anterior cingulate, inferior frontal sulcus, and insula demonstrated increased activation with decreasing recency (aLTM > DA > FA) consistent with their prominence in task-positive or cognitive control situations (Fox et al., 2005).
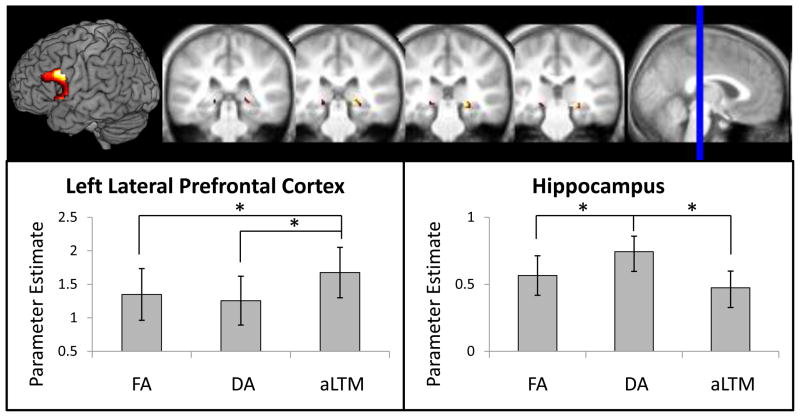
To further examine the dissociation between DA-related hippocampal activation and aLTM-related left LPFC activation, we performed ROI analyses on task-sensitive voxels within anatomically defined regions (see Methods; Fig. 5). These unbiased ROIs allowed us further anatomical precision and the ability to confirm the robustness of the whole-brain patterns. A two-way ANOVA revealed a significant region (left LPFC, hippocampus) x memory state (FA, DA, aLTM) interaction (F(2,48) = 10.22, p < 0.001). In accordance with the whole-brain analyses, left LPFC demonstrated greater activation for retrieval of aLTM compared to FA (t(24) = 2.97, p < 0.01) and DA (t(24) = 4.70, p < 0.001). The hippocampus demonstrated greater activation to DA than FA (t(24) = 2.29, p < 0.05) and aLTM (t(24) = 2.89, p < 0.01).
Fig. 5.
Parameter estimates from anatomical/functional regions of interest. Colored regions are task-sensitive voxels within anatomical regions of interest in the left lateral prefrontal cortex and hippocampus that were interrogated. The left lateral prefrontal cortex showed greater activation for retrieval of activated long-term memory (aLTM) compared to the focus of attention (FA) and the region of direct access (DA). By contrast, the hippocampus showed greater activation for DA compared to FA and aLTM. These data demonstrated a double dissociation between retrieval activations underlying DA and aLTM as predicted by 3-state models of memory. Error bars represent standard error of the mean.
Brain-Behavior Correlations
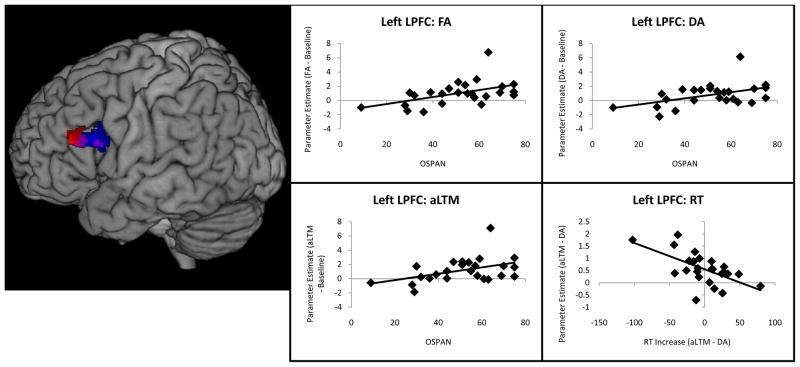
To examine the relationship between neural activation and behavior, we performed correlations within ROIs in left LPFC and the hippocampus. First, we examined whether capacity as measured by OSPAN was predictive of neural activation. A positive correlation between OSPAN and activation in left LPFC was found, indicating that increased left LPFC activation was related to increased working memory capacity. This correlation was not specific to retrieval from any one memory state, but was related generally to retrieval over-and-above baseline (Fig. 6). Next, we examined whether aLTM-specific activation (aLTM-DA) in left LPFC was associated with behavioral indices of aLTM retrieval. aLTM retrieval was measured behaviorally as RT to probes testing aLTM partialing out RT to DA probes (i.e. the residual after regressing aLTM RT on DA RT). High scores on this measure indicate increased slowing (i.e. worse performance) for aLTM-retrieval. Left LPFC activation was negatively related to this behavioral metric indicating that increased left LPFC activation was associated with better performance. Within our hippocampal ROI, we did not find correlations between hippocampal activation and behavior. This may have been due to the smaller extent of the hippocampal ROI. An expanded ROI including the entire right hippocampus anatomically revealed a significant positive correlation between DA-related activation (DA-FA) and OSPAN (p < 0.05 cluster corrected). However, this region did not show a univariate difference between memory states, so this result should be taken with caution. All reported correlations were significant using robust regression to reduce the impact of outliers (all p < 0.05).
Fig. 6.
Brain-behavioral correlations. The left lateral prefrontal cortex (LPFC) correlated with working memory capacity as measured by the operation span (OSPAN) task. This correlation was found across all probe types and demonstrated that increased frontal activation was related to higher working memory capacity (red). A more specific correlation was found between aLTM-related activation in left LPFC (aLTM-DA) and the reaction time (RT) to probes involving aLTM (aLTM RT partially out DA RT; blue). That is, greater aLTM-related activation in left LPFC was related to faster decisions for information in aLTM. Overlapping voxels for these correlations are depicted in purple.
Brain-Brain Correlations
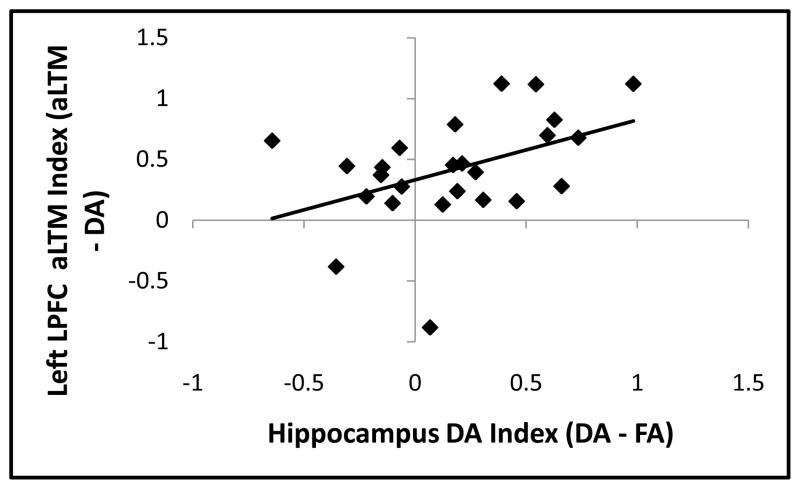
To explore the relationship between left LPFC and the hippocampus, we correlated aLTM-related left LPFC activation (aLTM-DA) with DA-related hippocampal activation (DA-FA; see Methods). On the one hand, the hippocampus and left LPFC may be inversely related. If the hippocampus and left LPFC support competing forms of retrieval, then subjects who rely to a greater degree on hippocampally-mediated retrieval may show less left LPFC-mediated retrieval and vice versa. On the other hand, previous research has demonstrated that with increasing retrieval demands, left LPFC and the hippocampus show enhanced functional connectivity suggesting complementary roles during retrieval (Nee and Jonides, 2008; Nee et al., 2007). Consistent with the latter proposal, aLTM-related left LPFC activation and DA-related hippocampal activation were positively correlated (r = 0.43, p < 0.05; also p < 0.05 after robust regression). Notably, this was not due to within-state correlations (i.e. the hippocampus and left LPFC were not correlated in response to DA probes, r = 0.04). Hence, subjects who showed strong DA-related hippocampal activation showed strong aLTM-related left LPFC activation.
Discussion
Our results demonstrate a neural triple dissociation contrasting retrieval processes acting upon different memory states in STM. Accessing information in FA recruits posterior parietal and inferior temporal cortex, replicating previous research (Nee and Jonides, 2008). Also replicating previous research, retrieval of information outside of FA recruits left LPFC and the medial temporal lobe (Nee and Jonides, 2008; Oztekin et al., 2009b). We find that activations in left LPFC and hippocampus are dissociable with left LPFC preferentially associated with retrieval acting upon aLTM, and the hippocampus preferentially associated with retrieval acting upon DA. This dissociation is difficult to reconcile with 2-state models of memory (McElree, 2006) and is consistent with 3-state models of memory (Jonides et al., 2008; Oberauer, 2002).
Retrieval of activated long-term memories
We found that retrieval of aLTM was associated with increased activation in left LPFC. This activation was centered in Brodmann Area 45 in pars triangularis. Much research has demonstrated that this same region is involved during retrieval when subjects resolve proactive interference (see Jonides and Nee, 2006 for a review). Left LPFC activation increases when subjects resolve proactive interference (Jonides et al., 1998), is absent in older adults who demonstrate increased proactive interference (Jonides et al., 2000), and lesions to this region cause severe and selective performance deficits when resolving proactive interference (Thompson-Schill et al., 2002). We and others have hypothesized that the role of left LPFC during retrieval is to associate information with its appropriate episodic context (Badre and Wagner, 2005; Jonides and Nee, 2006). Such a process is especially important when information is familiar yet its originating context is unclear. We suggest that information in aLTM is just the sort of information that requires this process. Information in aLTM is thought to be the source of proactive interference (Oberauer, 2001) and here we demonstrate that left LPFC is also recruited when proactive interference is minimal3. This suggests a common role of left LPFC during retrieval from aLTM either to reject intruding information as irrelevant or to confirm familiar information as relevant using controlled recollection processes (Badre and Wagner, 2005; Feredoes and Postle, 2010; Oztekin et al., 2009a) or post-retrieval feature selection (Badre et al., 2005).
An alternative possibility is that left LPFC activation reflects retrieval difficulty/effort. That is, left LPFC activation may subserve a general memory search function that scales with the difficulty/effort of the search. However, this account would predict greater activation for retrieval from DA compared to FA, which we did not find. Moreover, research using the recent probes task has demonstrated that repeated presentations of positive probes (recent positives) leads to improved performance relative to items that were not recently repeated (non-recent positives). Despite this improved performance, left LPFC shows greater activation for recent positives relative to non-recent positives, a finding that contradicts retrieval difficulty/effort accounts (Badre and Wagner, 2005; Nee et al., 2007). Instead, repeated presentations relate an item to a recent episode presumably held in aLTM (i.e. the previous trial) in addition to the current episode held in DA (i.e. the current trial). We theorize that such associations trigger a search of aLTM in addition to a search of DA thereby recruiting left LPFC for the former.
We demonstrated that activation in left LPFC is associated with good working memory capacity generally. These positive correlations between left LPFC activation and OSPAN were found by contrasting retrieval with baseline. Unlike contrasts that compare retrieval from different memory states, contrasts against baseline are somewhat underspecified and likely contain contributions from encoding and maintenance. So, it is possible that encoding/maintenance processes were the driving force of these general correlations. Braver and colleagues have shown that subjects with high general fluid intelligence (gF) recruit left LPFC proactively during the encoding and maintenance of information (Braver et al., 2007). Moreover, gF is related to working memory capacity (Carpenter et al., 1990). Hence, the positive correlation between left LPFC activation and OSPAN across all probe types may reflect encoding and maintenance processes that do not differ between conditions, but that are enhanced in high capacity subjects. Over-and-above this general correlation with capacity, we also demonstrated a specific correlation between left LPFC activation and aLTM-retrieval. In both the general and specific case, greater left LPFC activation was associated with better performance. Taken together, our results suggest that left LPFC is an important determinant of working memory capacity and performance above of typical 4±1 capacity limits.
Although our data can only speak to retrieval operations acting upon aLTM, it is also important to understand the processes that underlie aLTM maintenance. Recent work has demonstrated that information outside of focal attention may not represented by neural activation (Lewis-Peacock et al., 2010). In this intriguing study, a pattern classifier was trained to identify neural representations of semantic, phonological, and visual information. Subjects were then given 2 sorts of information (e.g. phonological and visual) and told to rid their minds of one of the sorts of information while retaining the other (e.g. remember phonological and forget visual). Whereas patterns of the retained information continued to be present during maintenance, patterns of the discarded information dropped to baseline. When a cue told subjects to switch their focus of attention to the previously discarded information, the original pattern of activation was reinstantiated. Hence, the discarded information was still accessible, presumably in aLTM. Moreover, this suggests that aLTM is not represented by active neuronal firing and instead may be related to rapid synaptic changes that create representations that can be quickly reinstated, but are otherwise dormant (Jonides et al., 2008). Our data suggest that reinstatement of dormant patterns may involve top-down processes driven by left LPFC.
Retrieval of the region of direct access
Interestingly, retrieval of information in DA was associated with activation in the hippocampus. This result is potentially surprising in light of a large corpus of data associating the hippocampus with LTM (e.g. Eichenbaum et al., 2007). A priori, one might have expected the hippocampus to be associated preferentially with retrieval from aLTM. However, a growing body of research is demonstrating the importance of the hippocampus in STM, especially when information is novel or requires the maintenance of associations (Nee et al., 2008; Ranganath and Blumenfeld, 2005). Moreover, hippocampal activation while information is stored in STM predicts better subsequent LTM recognition (Ranganath et al., 2005). Finally, a recent study using a similar paradigm demonstrated hippocampal results consistent with those reported here (Oztekin et al., 2010). Hence, it is becoming increasingly clear that the hippocampus serves a role for both STM and LTM. We hypothesize, as others have, that this role is to create associations amongst items of information (Cohen et al., 1999; Diana et al., 2007; Konkel and Cohen, 2009).
Why would the associative nature of the hippocampus be selective to retrieval from DA? DA affords the online maintenance of a limited set of items in a highly active state. We hypothesize that this state makes the representations in DA amenable to associative processes since synchronous firing drives neural associations (Hebb, 1949). These associative processes not only strengthen STM, but also lay the traces for subsequent LTM. These associations may be unique to DA. aLTM is not actively maintained by neuronal firing (Lewis-Peacock et al., 2010) making it difficult to associate with active material. FA consists of a single chunk potentially isolating it from associative processes. This isolation could be realized neurally by different oscillatory patterns for FA and DA. That is, if FA and DA are distinguished by different frequencies of firing, asynchrony between these states may separate FA from DA and preclude associations between them. Although speculative, this account provides a potential avenue for future research investigating maintenance of FA and DA by different oscillatory states.
PFC-hippocampal relationship
We demonstrated that subjects who showed high aLTM-related PFC activation also showed high DA-related hippocampal activation. This is consistent with previous work that shows that both regions are co-active during retrieval (Nee and Jonides, 2008; Oztekin et al., 2009a; Oztekin et al., 2009b) and that these regions show enhanced connectivity as retrieval becomes more difficult (Nee and Jonides, 2008; Nee et al., 2007). Hence, both regions appear to work together to facilitate good STM performance underlying complementary, but dissociable roles.
Retrieval of the focus of attention
As in previous research, we found activations in posterior parietal and inferior temporal cortex associated with decisions regarding information in FA (Nee and Jonides, 2008). As before, activation in inferior temporal cortex was anterior to traditional representational cortices that would include the visual word form area (Cohen et al., 2000) and instead may relate to semantic/conceptual elaboration that is afforded by information in the focused state. Posterior parietal activation through its interconnections with inferior temporal cortices may provide top-down attentional processes that support this elaboration. Notably, these parietal activations were lateral to the intra-parietal sulcus region that has been shown to track capacity (Todd and Marois, 2004; Xu and Chun, 2006), in accordance with previous data (Nee and Jonides, 2008). The left posterior superior temporal gyrus also demonstrated selective FA-related enhancement, consistent with the role of this region in STM for particularly short retention intervals (Buchsbaum et al., 2010).
Alternative accounts posit no distinction between FA and DA, suggesting instead a 2-state model with a 4±1 capacity-limited state dissociable from LTM (Cowan, 2001). By this account, differences in STM may be a function of probabilistic encoding into STM with the likelihood of encoding an item into STM being a function of attention. For instance, the last item of a list may have a very high likelihood to be encoded into STM providing higher performance estimates when averaged across trials. This account suggests that performance measures (i.e. accuracy and RT) should provide an assay of encoding probabilities. Since our data demonstrated sharp discontinuities between DA and aLTM, FA-sensitive regions would be expected to show greater activation to DA than aLTM according to this model. However, inferior temporal cortex, posterior parietal cortex, and the posterior superior temporal gyrus all demonstrated activation patterns that did not differentiate DA and aLTM while showing heightened activation for retrieval from FA. A lack of a graded pattern in these regions contradicts models that do not posit a distinct FA.
Conclusion
Our results are consistent with a 3-state model of memory. Notably, we use the term “state” rather than “store”. This terminology highlights a distinction in how the information is represented rather than where. Although information within verbal STM may all be stored in the same regions of cortex, we have highlighted distinctions regarding the manner of its storage by showing that retrieval operations acting upon stored information can be triply dissociated. These dissociations highlight the sorts of processes best suited to act upon the stored information. We hypothesize that associative processes mediated by the hippocampus are best suited for information in DA. Attentional and elaborative processes underlie the FA and episodic contextual retrieval is associated with information in aLTM.
Supplementary Material
Fig. 7.
Brain-brain correlations. Subjects that demonstrated strong DA-related activation in the hippocampus (DA-FA) also demonstrated strong aLTM-related activation in left LPFC (aLTM-DA). These correlations were not driven by within-state correlations (i.e. activation in the hippocampus to DA probes was unrelated to activation in the left LPFC to DA probes; r = 0.04). This pattern suggests that the left LPFC and hippocampus play complementary roles.
Acknowledgments
The authors are grateful to Nelson Cowan and two anonymous reviewers for insightful comments on a previous version of this manuscript. This research was supported in part by the National Science Foundation under Grant BCS 0822748 and in part by grant MH60655 from NIMH.
Footnotes
It should be noted that the term “focus of attention” has been used by different authors to describe potentially different states of short-term memory. Cowan (2001) also describes a 2-state model of memory with the focus of attention being a capacity-limited state that can hold 3 to 5 items. This is different from the 2-state model of McElree and colleagues (McElree & Dosher, 1989; McElree, 2006) that posits a single-item focus of attention (Figure 1). The model of Oberauer (2002) can be thought of as a blend of these two positions with the focus of attention corresponding to a single privileged item and a region of direct access containing an additional 2 to 4 items, both distinct from long-term memory. Hence, the focus of attention in the Cowan (2001) 2-state model corresponds to the focus of attention plus the region of direct access in the Oberauer (2002) 3-state model with no distinction between these two states. Here, we use the terminology of Oberauer (2002).
At the time of writing, we were made aware of a recent study that did not find evidence for a 3-state model of memory and concluded that memory is composed of 2-states (Oztekin et al., 2010). The present study used a larger sample and region of interest analyses to ensure that lack of evidence supporting a 3-state model, which requires a triple dissociation, would not be due to insufficient power.
Notably, although item-specific proactive interference is minimal there is most certainly a buildup of general proactive interference due to repeated trials of verbal information. Resolution of general proactive interference has also been shown to be mediated by left LPFC (Postle and Brush, 2004) and such general proactive interference may be partly responsible for the main effect of left LPFC activation across all memory states in addition to the selective increase for retrieval from aLTM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston K. Multimodal image coregistration and partitioning--a unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Frontal lobe mechanisms that resolve proactive interference. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. Oxford University Press; New York: 2007. pp. 76–106. [Google Scholar]
- Buchsbaum BR, Padmanabhan A, Berman KF. The Neural Substrates of Recognition Memory for Verbal Information: Spanning the Divide between Short- and Long-term Memory. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21496. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychol Rev. 1990;97:404–431. [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff M, Michel F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114–185. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: A meta-analysis. Psychonomic Bulletin and Review. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E, Postle BR. Prefrontal control of familiarity and recollection in working memory. J Cogn Neurosci. 2010;22:323–330. doi: 10.1162/jocn.2009.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Snyder A, Vincent J, Corbetta M, Van Essen D, Raichle M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan HP. Serial attention within working memory. Memory & Cognition. 1998;26:263–276. doi: 10.3758/bf03201138. [DOI] [PubMed] [Google Scholar]
- Gusnard D, Raichle M. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. Wiley; New York: 1949. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lacey S, Nee D. Processes of working memory in mind and brain. Current Directions in Psychological Science. 2005;14:2–5. [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Marshuetz C, Smith EE, Reuter-Lorenz PA, Koeppe RA, Hartley A. Age differences in behavior and PET activation reveal differences in interference resolution in verbal working memory. J Cogn Neurosci. 2000;12:188–196. doi: 10.1162/089892900561823. [DOI] [PubMed] [Google Scholar]
- Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Functionally distinct states of working memory retention are revealed by pattern classification of fMRI. Cognitive Neuroscience Society 2010 Annual Meeting; Montreal, Canada. 2010. [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McElree B. Accessing Recent Events. In: Ross B, editor. The Psychology of Learning and Motivation. Academic Press; San Diego, CA: 2006. pp. 155–200. [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: The time course of recognition. Journal of Experimental Psychology: General. 1989;118:346–373. [Google Scholar]
- Nee DE, Berman MG, Moore KS, Jonides J. Neuroscientific evidence about the distinction between short-and long-term memory. Current Directions in Psychological Science. 2008;17:102–106. doi: 10.1111/j.1467-8721.2008.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J. Neural correlates of access to short-term memory. Proc Natl Acad Sci U S A. 2008;105:14228–14233. doi: 10.1073/pnas.0802081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Jonides J, Berman MG. Neural mechanisms of proactive interference-resolution. Neuroimage. 2007;38:740–751. doi: 10.1016/j.neuroimage.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K. Removing irrelevant information from working memory: A cognitive aging study with the modified Sternberg task. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2001;27:948–957. [PubMed] [Google Scholar]
- Oberauer K. Access to information in working memory: exploring the focus of attention. J Exp Psychol Learn Mem Cogn. 2002;28:411–421. [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. 2. Prentice Hall; Upper Saddle River, N.J: 1999. [Google Scholar]
- Oztekin I, Curtis CE, McElree B. The medial temporal lobe and the left inferior prefrontal cortex jointly support interference resolution in verbal working memory. J Cogn Neurosci. 2009a;21:1967–1979. doi: 10.1162/jocn.2008.21146. [DOI] [PubMed] [Google Scholar]
- Oztekin I, Davachi L, McElree B. Are representations in working memory distinct from representations in long-term memory?: neural evidence in support of a single store. Psychol Sci. 2010;21:1123–1133. doi: 10.1177/0956797610376651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztekin I, McElree B, Staresina BP, Davachi L. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. J Cogn Neurosci. 2009b;21:581–593. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Brush LN. The neural bases of the effects of item-nonspecific proactive interference in working memory. Cogn Affect Behav Neurosci. 2004;4:379–392. doi: 10.3758/cabn.4.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–380. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. J Cogn Neurosci. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D'Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Heitz R, Schrock J, Engle R. An automated version of the operation span task. Behavior Research Methods. 2005;37:498. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Braver TS. Cognitive neuroscience approaches to individual differences in working memory and executive control: Conceptual and methodological issues. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of individual differences in cognition: Attention, memory and executive control. Springer; New York: in press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.