Abstract
Background and Aims
Gastric stem cells are located in the isthmus of the gastric glands, and give rise to epithelial progenitors that undergo bipolar migration and differentiation into pit and oxyntic lineages. While gastric mucus neck cells, located below the isthmus, express trefoil factor family 2 (TFF2) protein, TFF2 mRNA transcripts are concentrated in cells above the neck region in normal corpus mucosa, suggesting that TFF2 transcription is a marker of gastric progenitor cells.
Methods
Using a BAC strategy, we generated a transgenic mouse with a tamoxifen-inducible Cre under the control of the TFF2 promoter (TFF2-BAC-CreERT2) and analyzed the lineage derivation from TFF2 mRNA transcript-expressing (TTE) cells.
Results
TTE cells were localized to the isthmus, above and distinct from TFF2 protein-expressing mucus neck cells. Lineage tracing revealed that these cells migrated towards the bottom of the gland within 20 days, giving rise to parietal, mucous neck and chief cells, but not to ECL cells. Surface mucus cells were not derived from TTE cells, and the progeny of the TTE lineage did not survive beyond 200 days. TTE cells were localized in the isthmus adjacent to Dclk1+ putative progenitor cells. Induction of spasmolytic polypeptide-expressing metaplasia (SPEM) with DMP-777-induced acute parietal cell loss revealed that this metaplastic phenotype might arise in part through transdiferentiation of chief cells as opposed to expansion of mucus neck or progenitor cells.
Conclusion
TFF2-transcript-expressing cells are progenitors for mucus neck, parietal and zymogenic, but not for pit or ECL cell lineages in the oxyntic gastric mucosa.
Keywords: TFF2, Trefoil Factor Family, progenitor cell, stem cell, oxyntic lineage, parietal cell, chief cell, mucus neck cell
Introduction
Tissue stem cells are difficult to identify morphologically and not easily distinguished from other epithelial cells. They are characterized by multipotentiality, and ability to self renew. Tissue stem or progenitor cells reside within a ‘niche’, an area providing an optimal microenvironment for normal differentiation.1 Stem cells are present in small numbers and are thought to remain largely quiescent or undergo division at a very slow rate, such that they are negative for proliferation markers. Instead, proliferation markers such as Ki67, proliferating cell nuclear antigen (PCNA) or 5-bromo-2-deoxyuridine (BrdU) label transit-amplifying progenitor cells, immediate descendents of stem cells located adjacent to the stem cells. Transit–amplifying-progenitor cells divide quickly and are responsible for the bulk of cell division, but seem to have a limited lifespan and are replaced periodically by descendents of the true stem cell. However, recent data suggest that some classes of intestinal stem cells may be actively dividing with a higher cellular turnover rate than previously supposed.2
In the gastric oxyntic glands, the proliferative zone encompassing the putative gastric stem cell is localized to the isthmus of the glands. From the isthmus, cells migrate bidirectionally, upwards to differentiate into gastric surface mucus cells that coat the gastric pits, and downwards to give rise to gastric parietal and zymogenic cells.3 Analysis of genetic mosaic mice4,5 and of somatic mitochondrial mutations in humans6 suggest that most gastric units are monoclonal, mostly, with all epithelial cells derived from a single stem cell.7 Although the gastric stem cell has been the subject of investigation for several decades, it has not yet been identified except perhaps through studies using tritiated-thymidine-labeling, electron microscopic autoradiography, and chemical mutagenesis.8–12 These studies have helped to define pre-pit, pre-neck and pre-parietal cells, which derive from a more undifferentiated granule-free cell, potential stem cell of the gastric epithelium. As defined originally by EM, these rare cells are located in the center of the isthmus and are generally stationary, and divide rarely to give rise to progenitor cells.8–11 Thus, in these early studies, zymogenic or chief cells were derived from neck cells, while parietal cells developed from a separate progenitor, although this model was more recently modified to suggest some overlap in chief and parietal cell origins in humans.3
A well-validated stem cell marker, LGR5, shows lineage labeling in some antral gastric glands and might mark the antral stem or progenitor cells.13,14 In the oxyntic mucosa, similar lineage tracing has not been achieved, although a potential candidate, Dckl1, was identified from sequencing of laser capture microdissected mouse gastric and small intestinal epithelial progenitors.15 However, to date, direct evidence for the presence of long-lived progenitors has been limited, although one study involving chemical mutagenesis of random epithelial cells showed the presence of clones containing only a single mature cell type indicating the presence of committed progenitors in the gastric epithelium.12
Trefoil factor family 2 (TFF2), also known as spasmolytic polypeptide, is a small peptide that is co-expressed with Mucin 6 (Muc6) in discrete cell populations. There are three known members of the TFF family (TFF1,-2, and -3), which all contain three-looped structural motifs resembling a three-leaf trefoil. Under normal conditions, TFF2 is expressed and secreted by gastric mucous neck cells and deep antral gland mucous cells, and data from knockout mice indicate that it plays an important role in gastric cytoprotection and repair.16 Trefoils are strongly induced after epithelial damage and facilitate both short-term (restitution) and long term (glandular re-epithelialization) repair processes by stimulating cell migration,17 inhibiting apoptosis18 and reducing antigen access to the healing epithelium.19 In addition, they are regulated by pro-inflammatory and anti-inflammatory cytokine expression,20,21 and have been postulated to participate in the mucosal immune response by modulating immunocyte migration, possibly through the CXCR4 receptor.22 While a number of potential biological roles for TFF2 have been reported, a precise understanding of the function of TFF2 has remained elusive. There appears to be a relationship between TFF2 gene expression and expansion of the proliferative zone of the mucosa, raising the possibility that TFF2 may be involved in regulating epithelial proliferation or differentiation in response to injury. In addition, loss of parietal cells induces the development of a TFF2-expressing metaplastic lineage (SPEM) in both mice and humans.23
While TFF2 protein expression is confined to mucous neck cells, in situ hybridization studies have demonstrated that TFF2 mRNA expression is above the neck region in the gastric isthmus,24 raising the possibility that TFF2 mRNA transcript expression is a marker of gastric stem or progenitor cells. Using a tamoxifen-inducible Cre recombinase under the control of the TFF2 promoter we have identified a population of TFF2 transcript-expressing (TTE) cells as specific progenitors for parietal, mucous neck and zymogenic cell lineages, but not for pit or ECL cell lineages in the oxyntic gastric mucosa. Thus, while previous studies suggested that corpus mucosa stem cells gave rise to three second order transit amplifying cell populations (pre-pit, pre-neck and pre-parietal), these results are consistent with a model that the TTE cell represents a gland lineage progenitor cell population that then gives rise to both mucous neck and parietal cells.
Methods
For Bac recombineering a CreERT2FrtNeoFrt cassette was ligated into the p451 plasmid upstream of the ATG and 40bp downstream with a 198bp replacement of exon 1. BAC DNA was microinjected in the pronucleus of fertilized CBA × C57BL/6J oocytes. After back-crossing (F6) to C57BL/6, two of three founders were mated with B6.129S4-Gt(ROSA)26Sortm1Sor/J, referred to as Rosa26R-lacZ, or B6;129-Gt(ROSA)26Sortm2Sho/J, referred to as Rosa26R-GFP, (Jackson Laboratories) and histological analysis was performed. For detailed methods see supplementary information.
Results
TFF2 promoter-dependent Cre expression in the stomach, lung, kidney and duodenum
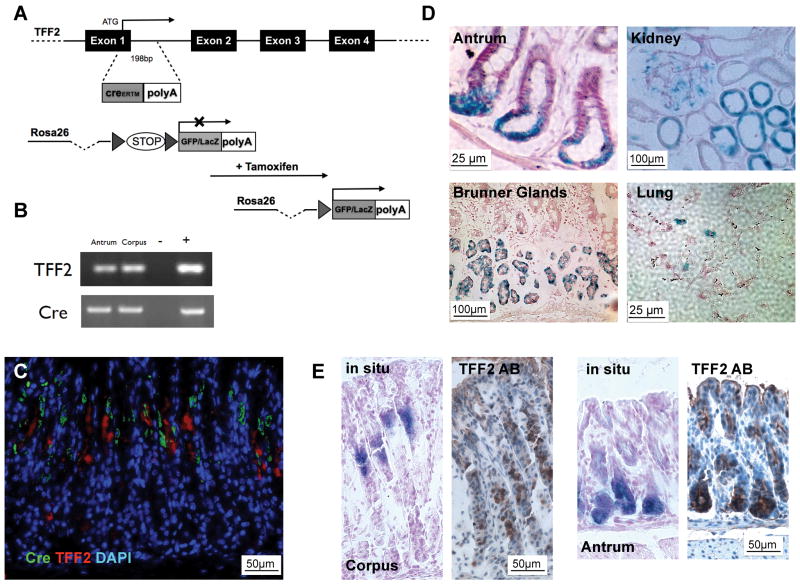
Lineage tracing, using inducible Cre recombinase, has become a powerful strategy to analyze the progeny of stem cells. Using a recombineering approach, we generated three positive founder lines of transgenic mice with a tamoxifen-inducible CreERT2 driven by a BAC-TFF2 promoter (Figure 1A). The resulting transgenic TFF2-CreERT2 mice exhibited TFF2 and Cre expression in the gastric corpus as analyzed by RT-PCR in the corpus and antrum (Figure 1B) and IHC (Figure 1C), generally located above the known localization of TFF2 protein expression, as also demonstrated by in situ hybridization and protein staining for TFF2 (Figure 1E). The transgenic mice showed no Cre expression in liver, spleen, muscle, and pancreas (data not shown). Taken together, TFF2-CreERT2 mice showed a pattern of Cre expression that was consistent with endogenous TFF2 mRNA expression, located above the TFF2 protein expression.
Figure 1.
(A) A CreERT2FrtNeoFrt cassette was inserted in a BAC containing the TFF2 gene. (B) RT-PCR of corpus and antrum of TFF2-CreERT2 mice with liver as negative(−) and TFF2-plasmid as positive(+) control. (C) Cre (green) and TFF2 (red) IHC in un-induced TFF2-CreERT2 mice (DAPI blue). (D) After tamoxifen dosing over three consecutive days LacZ+ cells were observed at the position 1 to 4 in the antral glands, in the Brunner glands of the duodenum, in the lungs, in the proximal tubules and glomeruli in the kidney. (E) Characteristic expression of TFF2 protein by IHC (right) and TFF2 RNA by in situ hybridization (left) in the corpus and antrum of C57BL/6 mice.
After backcrossing to a C57BL/6J background, we crossed the TFF2-CreERT2 transgenic mice to Rosa26R-lacZ or Rosa26R-GFP reporter mice. Injection of tamoxifen activates the CreERT2 enzyme in TTE cells, and Cre-mediated excision of the floxed STOP cassette in the Rosa26 reporter then irreversibly marks TTE cells and any subsequent progeny of these cells, facilitating lineage tracing (Figure 1A).
To visualize the location of TTE cells in the gastric corpus glands, we analyzed 2–3-month-old mice 48h after tamoxifen induction. Single cells with GFP or LacZ signals only rarely appeared in the isthmus region in gastric glands in the corpus (Figure 2A and B). Mostly, several cells (5–10) in the isthmus were labeled. Expression of LacZ or GFP was not observed in non-induced mice (Figure 2B), excluding any leakiness of the Cre expression. A single dose of tamoxifen activated Cre expression in up to 10% of the gastric glands and dosing over three consecutive days increased this to approximately 30% of glands (data not shown). Tamoxifen treatment itself did not induce any cellular changes in the stomach. The absence of uniform expression in all glands, which has been observed previously in lineage tracking experiments in the gut, has been attributed to issues regarding administration of tamoxifen or an incomplete or mosaic expression of the CreERT2 transgene. In the corpus, all of the Cre-GFP- or Cre-LacZ-labeled cells occurred at positions in the isthmus or upper neck region, similar to that observed in the past for TFF2 mRNA, whereas labeled cells were observed at the position 1 to 4 in the antral glands (Figure 1D). Thus, GFP and LacZ expression is equivalent to TFF2 mRNA, but not necessarily protein expression, at these early time points.
Figure 2.
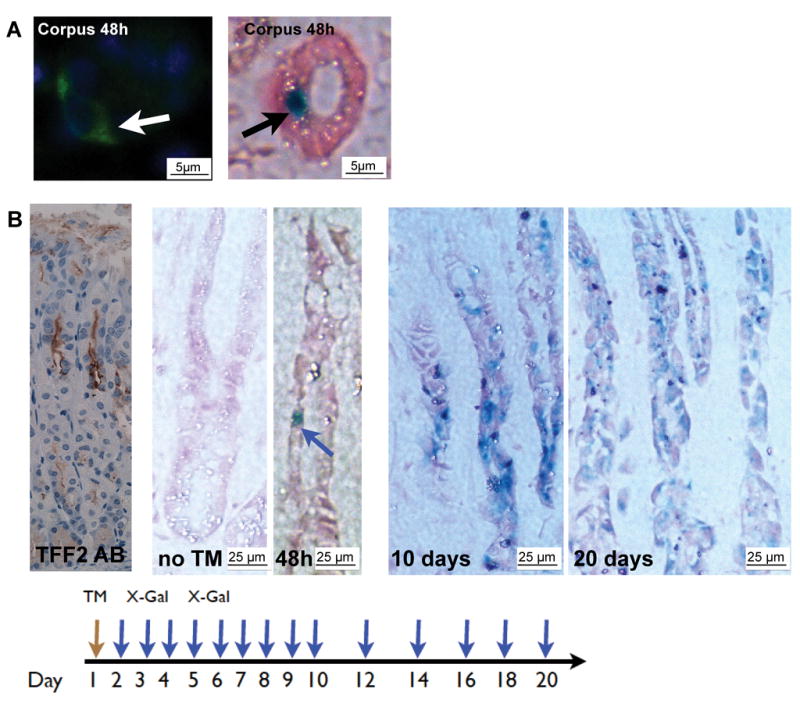
(A) 2–3-month-old mice were treated with tamoxifen and sacrificed 48h later. Single GFP+ and LacZ+ cells appeared in the isthmus of gastric glands in the corpus (arrows). (B) (Left) Characteristic TFF2 antibody staining in the corpus; TFF2-CreERT2 corpus without tamoxifen induction; LacZ staining of TFF2-CreERT2 mice after tamoxifen pulse (scheme on the bottom) showed increasing numbers of LacZ+ cells populating the gastric corpus glands.
We also observed LacZ expression in the Brunner glands of the duodenum (Figure 1D), where protein expression has been described previously. We detected rare LacZ positive cells in the lungs, where TFF2 is expressed in a subset of airway cells that display a mucus cell phenotype and where TFF2 expression is known to be upregulated during allergic airway inflammation (Figure 1D).25 We observed LacZ expression in the proximal tubules and glomeruli in the kidney, where TFF2 mRNA expression has been described despite lacking protein expression (Figure 1D).26 Surprisingly, we did not observe LacZ expression in the spleen, although we and others have previously described TFF2 gene expression in lymphocytes.22 Nevertheless, we observed GFP positive lymphocytic cells in the gastric tissue (data not shown).
TFF2 lineage tracing in the stomach
Next, thirty (30) 2–3 month old TFF2-CreER2 transgenic mice were induced with tamoxifen and sacrificed daily on days 1 through 10, and every other day up to 20 days (Figure 2, and data not shown). One day after induction, occasional single LacZ positive cells were detected in the corpus isthmus region, but were absent lower down in the glands. At 5 to 10 days post-induction, we observed multiple LacZ positive cells in the mucus neck cell region and in the middle of the gastric glands, and occasionally we found LacZ positive parietal cells in the upper gastric glands (Figures 3 & 4). Within 20 days most of the gastric corpus glands demonstrated multiple LacZ positive cells extending to the bottom of the glands. For each of the time points, 50 glands were counted in the corpus to determine the percentage containing LacZ+ cells. After 1, 5, 10, and 20 days, the percentage of LacZ+ in the gastric glands was 5% (+/−0,7%), 12% (+/−2,1%), 24% (+/−4.3%), and 32% (+/−3,8%), respectively. Thus, TTE cells appeared capable of giving rise to increasing numbers of cells and multiple gland lineages over time in the gastric oxyntic mucosa. In contrast, no lineage tracing was observed in the gastric antrum or duodenum, where a similar number of LacZ positive cells at the same position were observed at 1, 5, 10 and 20 days. Interestingly, we never observed lineage tracing into surface cells of the gastric corpus glands, indicating that TFF2-transcript expression marks a progenitor cell for the gland cells, but not the pit cells.
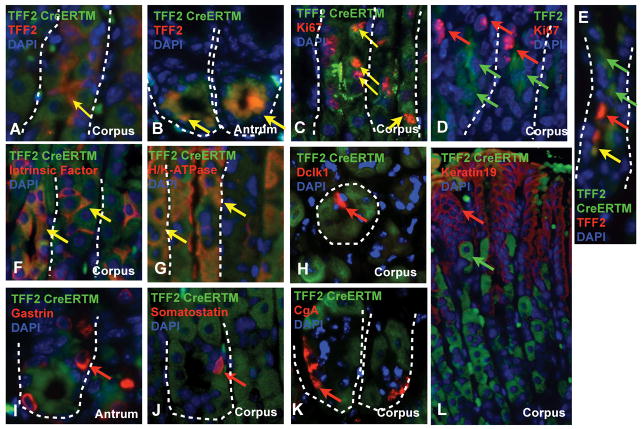
Figure 3.
Double staining for different characteristic cell markers (TFF2-CreERT2 clones: green; AB: red; DAPI blue; TFF2-Ab: green). Representative pictures of (A) TFF2 in the corpus and (B) in the antrum 20 days after TM). (C) Ki67 staining 5d after tamoxifen in the corpus. (D) TFF2 protein expressing mucus neck cells do not co-stain with Ki67. (E) GFP clones 3d after tamoxifen are located above TFF2 protein expressing cells in the corpus. GFP-reporter double staining with (F) Intrinsic Factor+ (IF) and (G) H/K-ATPase+ cells 40 days after TM. (H) Dclk1, (L) Cytokeratin-19, (K) Chromogranin-A (CgA), (J) Somatostatin, and (I) Gastrin did not co-localize with GFP. Arrows indicate single GFP+ clones (green), single AB positive clones (red), and double positive clones (yellow).
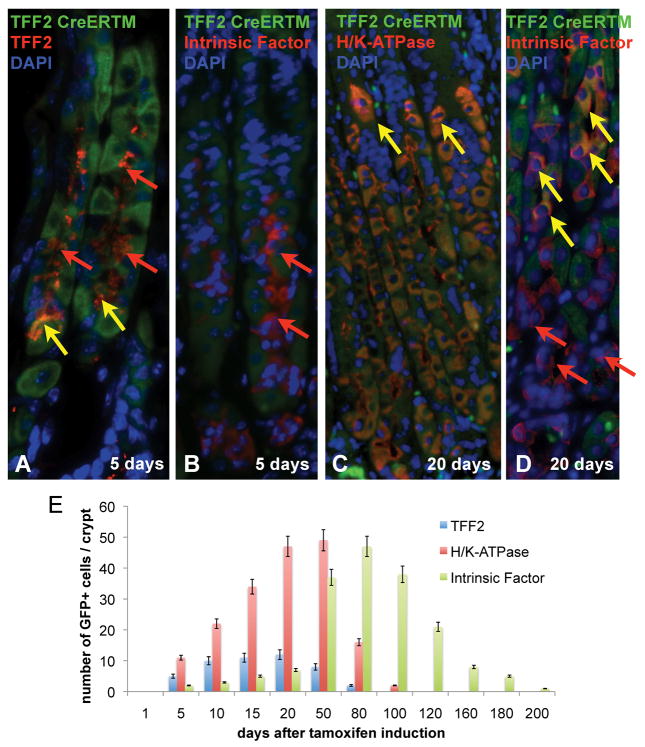
Figure 4.
Labeling of long-lived parietal and chief cells: (A) 5 days after tamoxifen induction TFF2+ but no Intrinsic factor+ (IF) staining GFP+ clones. 20 days after tamoxifen induction clonal cells in the labeled glands stain for H/KATPase and less for Intrinsic factor (IF). Arrows indicate single GFP+ clones (green), single AB positive clones (red), and double positive clones (yellow) (B) Quantitative evaluation of GFP-expressing mucus neck (TFF2), parietal (H/K-ATPase), and chief cells (IF) in 10 labeled glands (Mean with SEM).
TFF2 labels a progenitor cell of the oxyntic mucosa of the stomach
Having demonstrated lineage tracing for TTE cells that included the lower portions of the gastric corpus glands, we next performed double staining for known gastric cell types. In the isthmus and neck region 1–5 days after tamoxifen induction, GFP expressing clones co-stained for Ki67 (Figure 3C), indicating that these cells were actively proliferating. Proliferating cells could be observed up to 20 days post induction, with the most double-positive cells being present within the first 10 days. GFP or Cre expression before 10 days was rarely co-localized with TFF2 protein expression and was constantly observed above the TFF2 protein expressing mucus neck cells in the gastric corpus glands (Figure 3E). 5 days after tamoxifen induction, double-labeling of induced stomach mucosa revealed co-staining of TFF2-protein-expressing mucus neck cells with the GFP marker (Figure 3A). These data are consistent with a downward migration of TTE cells, and confirm a distinct localization for TFF2-RNA transcript and TFF2 protein expression, as reported previously by in situ hybridization (Figure 1E).27,28
Of note, TFF2 protein expressing cells did not show proliferation when co-stained with Ki67 (Figure 3D), suggesting a distinction between proliferating progenitors and mucous neck cells. In the antrum, TFF2 protein and GFP expression of the clones was co-localized at all the times (Figure 3B) but the TFF2 positive cells did not express proliferative markers (data not shown).
Twenty days (20 d) after tamoxifen induction, we continued to detect TFF2 protein and GFP double positive cells in the mucus neck region of the glands (data not shown). However, below the neck region we found abundant GFP+ parietal cells that were identified by H/K-ATPase staining (Figure 3G). These double positive parietal cells were mostly found below the isthmus of the gastric corpus glands, although a small subset was also present above the isthmus. In contrast to human gastric corpus glands, parietal cells in the mouse have been described to migrate bidirectionally.29 In addition, we observed at this 20d time point rare Intrinsic Factor (IF) and GFP double positive gastric chief or zymogenic cells (Figure 3F). Of note, in the marked glands we never observed labeling of only one of the oxyntic lineages (i.e. parietal, neck and zymogenic) 20 days after induction, and glands if labeled showed Cre-labeling of all three. Importantly, gastric corpus pit cells, as labeled here with cytokeratin-19 staining (Figure 3L), did not co-localize with GFP expressing clones, indicating that these cells did not derive from TTE cells in the isthmus region. Interestingly, no neuroendocrine cells co-stained with the GFP-expressing clones 20 and 60 days after induction. Staining for chromogranin-A (CgA) did not show any GFP expressing clones among the ECL-cells or somatostatin-expressing D cells (Figure 3J+K).
In humans and mice, each gastric unit in the corpus region is considered to be monoclonal, with all cellular progeny derived from a single stem cell.30 While Lgr5 is not expressed in the isthmus of the gastric corpus14, a putative stem or progenitor cell marker, doublecortin CaM kinase-like-1 (Dclk1), was reported in 2007 to be expressed in this region.15 Staining for Dclk1 in our model of TTE lineage tracing at 5, 10, 20, and 60 days post induction did not demonstrate any co-localization with GFP (Figure 3H). Nevertheless, we observed a close proximity between Dclk1 expressing cells and GFP expressing clones at 24–48 hrs after tamoxifen induction.
In the antrum, TFF2 protein staining co-localized with the GFP-expressing TTE clones at 5, 10, 20 (Figure 3B) and up to 40 days at the bottom of the glands up to position +4, but there was no expansion or migration of GFP or LacZ positive clones. Staining for gastrin peptide expression in antral G-cells showed no co-localization with GFP expressing clones (Figure 3I) at any time point.
Taken together, these data suggest that TFF2 mRNA transcript expression labels a multilineage progenitor cell for the gastric oxyntic mucosa, but indicate that TTE cell is not a progenitor cell for pit cells or neuroendocrine cells in the corpus.
Longevity of chief cells and parietal cell lineages
We next analyzed the longevity of the progenitor cells and their progeny. As described above, 20 days after tamoxifen induction, most parietal cells in the labeled glands were derived from the TTE progenitor cell, but only a few IF-positive chief cells below the isthmus were labeled at this time point (Figure 4A). Fifty (50) days after tamoxifen induction, most of the IF-expressing chief cells were GFP(+), indicating that differentiation of TTE cells to chief cells is delayed relative to neck and parietal cell differentiation. Therefore, we induced thirty (30) TFF2-CreERT2/Rosa26R-GFP mice with 3 consecutive pulses of tamoxifen and analyzed GFP expression after 5, 50, 80, 100, 120, 160 and 180 days (Figure 5) to clarify the time dependent migration of the TTE cell progeny.
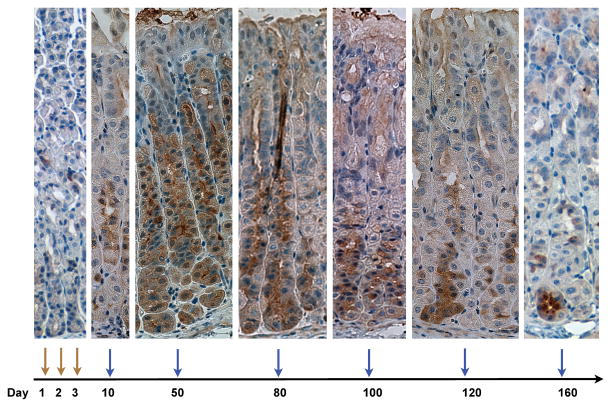
Figure 5.
Long term labeling of TTE progenitor cells: Following three consecutive doses of tamoxifen, GFP expression was analyzed in TFF2-CreERT2/Rosa26R-GFP at 5,10,50,80,100,120, and 160 days (scheme at the bottom), to follow migration of TFF2 expressing cell progeny.
As expected from earlier experiments, we observed GFP expression (Figure 5) in mucus neck cells after 5 days. Fifty (50) days after tamoxifen induction, nearly 100% of parietal cells and a majority of chief cells stained for GFP (Figure 5). In contrast, 80 days after tamoxifen induction, only rare GFP(+) parietal cells could be detected, while the majority of chief cells in the gastric gland were still GFP(+) (Figure 5, co-staining with IF not shown). At 100, 120, and 160 days after tamoxifen induction, we observed a gradual decrease in GFP expression over time in the gastric corpus glands that were labeled from top to bottom, reflecting the migration of chief cells to the bottom of the glands and their continuous replacement by non-labeled chief cells. Finally, after 180 days only 5% of cells were labeled, and after 200 days only 2% of the glands showed GFP expressing cells, indicating the complete loss of the labeled cells at these later time points (Figure 5).
Figure 4E shows a quantitative evaluation of GFP-expressing mucus neck cells (TFF2), parietal cells (H/K-ATPase) and chief cells (IF) at various time points. Five (5) days after tamoxifen induction, a mean of 10 parietal cells, 2–3 mucus neck cells cells and 0–1 chief cells were found in ten GFP-labeled and double stained glands. The number of mucus neck cells among the GFP clones increased up to 20 days after tamoxifen induction (12 cells/gland), and the number of parietal cells increased up to 50 days after induction (49 cells/gland). Later on, at day 80 following tamoxifen induction, chief cells were maximally derived from the GFP-labeled progenitors (47 cells/gland), and at subsequent time points, GFP-labeled chiefs cells decreased, as described above. These observations indicate that a single parietal cell on average survives for 30–50 days and a single chief cells for approximately 120 to 140 days. In addition, the data suggest that a GFP-labeled TFF2 mRNA transcript expressing progenitor cell might survive for approximately 20 days before being replaced by a new progenitor cell.
TFF2 mRNA transcript expressing progenitor cells are not the cells of origin for SPEM
In the mouse stomach, oxyntic atrophy secondary to chronic H. felis infection is associated with loss of parietal cells and the emergence of spasmolytic polypeptide expressing metaplasia (SPEM).27 The expression of TFF2 in dysplastic cells has suggested that SPEM may be a precursor of dysplasia and neoplasia in H. felis–infected mice.23 While the connection between SPEM and dysplastic transformation remains to be further clarified, a number of studies have supported the notion that SPEM does not arise exclusively from expansion of mucus neck cells, but instead might result from dedifferentiation of chief cells.31
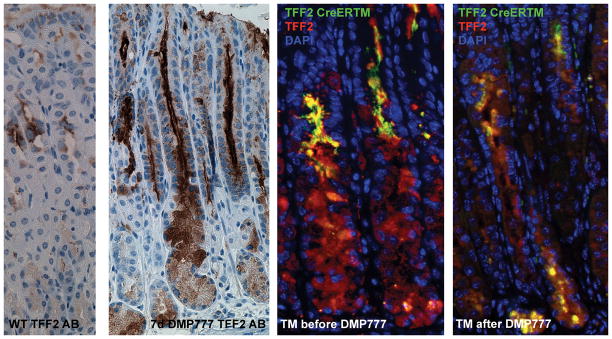
Previous studies by our group demonstrated that short-term treatment with DMP-777 caused hypergastrinemia, loss of parietal cells, and foveolar hyperplasia, as well as emergence of SPEM.27 Interestingly, one of the hallmarks of the SPEM lineage is the high level of expression of both TFF2 mRNA transcripts and TFF2 protein in the same cells.32,31 Thus, we sought to evaluate the effects of DMP-777 on the TFF2 lineage in the gastric oxyntic mucosa. DMP-777 was administered to two groups of three TFF2-CreERT2/Rosa26R-GFP mice for 7 days to evaluate changes that occurred during the onset of oxyntic atrophy, since TFF2-expressing SPEM was found to appear within 7 days of DMP-777 treatment. One group was induced with tamoxifen on the day before DMP-777 administration and the other group was induced after DMP-777 administration on day 7. Both groups were sacrificed on day 10. Compared to untreated mice, TFF2 expressing SPEM was observed in all DMP-777 treated mice (Figure 6). Staining with TFF2 antibodies revealed that TFF2 protein-expressing SPEM cells in the bottom of the glands did not express GFP when Cre was induced before DMP-777 treatment. However, we observed strong GFP expression in SPEM when Cre was induced by tamoxifen treatment after 7 days of DMP-777 treatment (Figure 6). These data indicate that SPEM at least in part arises initially from trans-differentiating chief cells in the bottom and not from progenitor cells in the isthmus of the gastric corpus glands.
Figure 6.
Effects of DMP-777 on the TFF2 lineage in the gastric oxyntic mucosa: TFF2 staining in the corpus of TFF2-CreERT2/Rosa26R-GFP without and with 7 days of DMP-777 treatment. Representative pictures (N=3) of TFF2 staining in TFF2-CreERT2/Rosa26R-GFP mice induced with tamoxifen one day before DMP-777 administration and after DMP-777 administration on day 7. Both groups were sacrificed on day 10.
Discussion
While active dividing stem cells (Lgr5+) for the intestine, colon, and antrum have been identified,13 specific short-lived oxyntic gland progenitors, distinct from stem cells, have not been characterized. Lineage relationships with the gastric oxyntic mucosa have been postulated but up until now not been validated. TFF2 peptide is clearly expressed in mucus neck cells that migrate out of the gastric isthmus. However, in this study we confirm early observations that TFF2 mRNA transcripts are present not in mucous neck cells that expressed the TFF2 peptide,28 but instead in progenitor cells in the gastric isthmus, within the stem cell region. TFF2 transcript-expressing (TTE) progenitors proliferative and migrate down toward the bottom of the oxyntic glands, and give rise to mucus neck, parietal and chief cells, but not to pit or enteroendocrine lineages. Thus, TFF2 mRNA expression defines a novel class of glandular progenitors.
While TTE cells are multipotent progenitors, they are clearly not stem cells, since they do not persist in the isthmus beyond 20 days and instead are frequently replaced by new progenitor cells, which most likely derive from an as yet undefined stem cell. In addition, they appear to be highly proliferative in contrast to the TFF2 protein expressing cells, since cell proliferation was most evident in TTE cells in the isthmus region above the neck of gastric corpus TFF2 glands. Of note, the identification of a marker for a distinct short-lived progenitor cell seems to be a novel observation, supporting the concept of active or quiescent stem cells giving rise to short-lived progenitor or transit amplifying cells which was suggested in previous studies.12 Nevertheless, we observed close proximity of TTE cells to cells that express Dclk-1, a relatively new putative stem cell marker.33 Dclk-1 was discovered in gut epithelial progenitor cells,15 and Karam at al.3 described a corpus mucosa stem cell in the same location that gives rise to progenitor cells as a granule-free cell in the isthmus, but this remains to be authenticated.
While previous studies employed tritiated-thymidine and autoradiography to track progenitors,8–11 the current study utilized an inducible Cre expressing transgenic to define both the nature of the lineage relationships and the kinetics of cellular turnover in the gastric glands. Following tamoxifen induction, we observed the rapid appearance of TTE cell-derived LacZ or GFP positive progeny cells below the isthmus, confirming the active proliferative status of these cells. Within 5–20 days, LacZ or GFP positive progeny could be found distributed in the bottom part of the glands, but not in the pits of the corpus units, providing an estimate of the rate of self-renewal occurring in this region of the stomach. TTE cells give rise to (1) mucus neck cells, which survive for approximately 20 days, (2) parietal cells, which survive for approximately 40 days, and to (3) chief or zymogenic cells which survive for up to 140 days. The findings and time course are consistent with a model whereby mucus neck cells give rise to chief cells, as suggested previously.3 Nevertheless, lineage tracing of TTE cells cannot exactly discriminate the longevity of mucus neck cells or conclusively proof that neck cells give rise to chief cells, since we cannot be definitively exclude that TTE cells migrate downward and directly differentiate into chief cells (bypassing the mucus neck cell stage). The findings were different in the gastric antrum, where TFF2 protein and TFF2-Cre induced LacZ or GFP expression correlated quite well. TTE cells in the antrum did not appear to migrate, survived for up to 40 days, and therefore do not represent any type of stem or progenitor cell in the distal stomach.
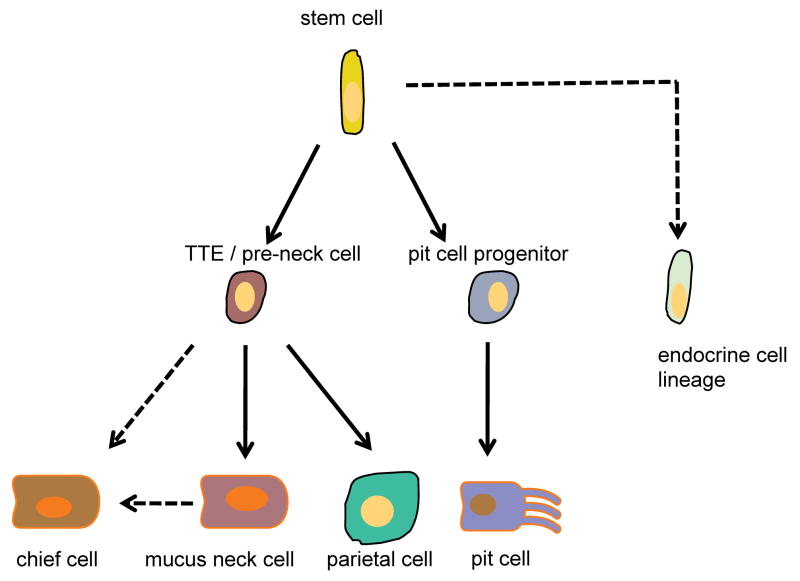
While the close relationship between progenitors of mucus neck and chief cells has been appreciated for a number of years, the finding that parietal cells also arise from the same progenitor has been postulated but not previously been shown. Thus, based on earlier observations,3 we propose a model (Figure 7) in which a common progenitor cell, the TTE cell, gives rise to all three major cell types of the oxyntic gland, namely parietal, mucus neck and chief cells. The TTE progenitor does not function as a progenitor cell for pit surface mucous cell lineage,34 nor for enteroendocrine cells, and thus the current model would suggest that these cell types arise from a stem cell either directly or through separate progenitors. Karam et al have shown that the pre-neck cell is proliferative, as we have shown for the TTE progenitor. Both cells give rise to the parietal and chief cell lineage, and therefore we conclude that the TFF2-Cre transgene labeles the pre-neck cell. This model raises some interesting possibilities with respect to the development of gastric atrophy, a histopathologic condition associated with a loss of both parietal and chief cells. Such a lesion could in theory occur due to an arrest in the differentiation of the TTE, especially since the lineages, which are spared in gastric atrophy are the foveolar and the endocrine lineages. Atrophy is also associated with an accumulation of metaplastic mucus neck cells that has been termed pseudopyloric metaplasia or SPEM. In the current study, we find that following DMP-777 treatment SPEM appears to arise in part from a second proliferative area at the bottom of the gastric glands.27 Our data suggest that the TFF2 expressing cells at the bottom of the glands might derive initially from trans-differentiation of chief cells rather than migration of progenitor cells from the isthmus, nevertheless additional work is needed to confirm these observations. 31 This finding also reflects the close relationship of mucus neck cells, which express TFF2 protein, and chief cells, which do not express TFF2, but can reactivate the TFF2 expression with a change of microenvironment conditions. Interestingly, an opposite experiment using Mist1-cre lineage tracing has recently demonstrated a similar finding of chief cell transdifferentiation into SPEM following treatment with DMP-777 (Nam and Goldenring, submitted).
Figure 7.
Proposed model of the oxyntic lineage: A common progenitor cell (TTE) gives rise to parietal, mucus neck and chief cells but not pit cell lineage. A stem cell, active or quiescent, will give rise to a pit lineage progenitor and the TTE oxyntic gland progenitor cell.
Finally, the observation that TFF2 transcript expression represents a marker for an oxyntic progenitor does not in itself elucidate any specific function for the TFF2 protein. Studies with TFF2 knockout mice suggested that mild changes in cellular differentiation and proliferation rates occurred with loss of gene function.11 Thus we would speculate that TFF2 mRNA and protein function might contribute in some way to cell fate decisions at the progenitor cell level. The finding of absent TFF2 protein expression in gastric progenitor cells also raises questions regarding the mechanism for the block in protein translation that may be specific to progenitor cells. Further studies will be needed to elucidate these mechanisms.
Supplementary Material
Acknowledgments
We thank Ashley Whelan and Bethany DiPrete for excellent technical assistance, Sachiyo Nomura for helping with the TFF2 in-situ, and Chen Yang for excellent help with IHC. Further, we thank all members of the Wang lab for helpful discussion.
Grant Support: These studies were supported by grants to T. C. Wang from the National Institutes of Health grants RO1DK060758, 1U54CA126513, and R01CA120979 and to J.R.G. from a Department of Veterans Affairs Merit Review Award, RO1 DK071590, and the AGA Funderburg Award in Gastric Biology Related to Cancer. M. Quante is supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Germany.
Abbreviations used in this paper
- TFF2
Trefoil Factor Family 2
- TTE
TFF2 transcript expressing
- SPEM
Spasmolytic Polypeptide Expressing Metaplastic lineage
Footnotes
Disclosure: All authors have nothing to disclose.
Involvement of authors:
Michael Quante: study concept and design, acquisition of data, analysis and interpretation of data; drafting of manuscript
Frederic Marrache: acquisition of data
James R. Goldenring: analysis and interpretation of data; critical revision of manuscript for important intellectual content
Timothy C. Wang: study concept and design, analysis and interpretation of data; drafting of manuscript, study supervision, obtained funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K, Goldman J, Alison MR, Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–7. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–64. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–36. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 4.Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. doi: 10.1016/s0016-5085(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 5.Wong MH, Saam JR, Stappenbeck TS, Rexer CH, Gordon JI. Genetic mosaic analysis based on Cre recombinase and navigated laser capture microdissection. Proc Natl Acad Sci U S A. 2000;97:12601–6. doi: 10.1073/pnas.230237997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, Rodriguez-Justo M, Deheragoda M, Leedham SJ, Taylor RW, Lee CY, Preston SL, Lovell M, Hunt T, Elia G, Oukrif D, Harrison R, Novelli MR, Mitchell I, Stoker DL, Turnbull DM, Jankowski JA, Wright NA. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–10. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 7.Nomura S, Kaminishi M, Takagi N, Esumi H. Analysis of promoter region of X-linked pgk-1 gene polymorphisms: evidence for polyclonality of adult mouse gastric glands. Dig Dis Sci. 2004;49:218–23. doi: 10.1023/b:ddas.0000017441.06479.c8. [DOI] [PubMed] [Google Scholar]
- 8.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–40. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 9.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 10.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–96. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 11.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–79. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 12.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. AmJ Physiol Gastrointest Liver Physiol. 2002;283:G767–77. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 13.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 14.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Giannakis M, Stappenbeck TS, Mills JC, Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam M, Brent MR, Gordon JI. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 16.Farrell JJ, Taupin D, Koh TJ, Chen D, Zhao CM, Podolsky DK, Wang TC. TFF2/SP-deficient mice show decreased gastric proliferation, increased acid secretion, and increased susceptibility to NSAID injury. J Clin Invest. 2002;109:193–204. doi: 10.1172/JCI12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oertel M, Graness A, Thim L, Buhling F, Kalbacher H, Hoffmann W. Trefoil factor family-peptides promote migration of human bronchial epithelial cells: synergistic effect with epidermal growth factor. Am J Respir Cell Mol Biol. 2001;25:418–24. doi: 10.1165/ajrcmb.25.4.4429. [DOI] [PubMed] [Google Scholar]
- 18.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A. 2000;97:799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–73. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 20.Dossinger V, Kayademir T, Blin N, Gott P. Down-regulation of TFF expression in gastrointestinal cell lines by cytokines and nuclear factors. Cell Physiol Biochem. 2002;12:197–206. doi: 10.1159/000066279. [DOI] [PubMed] [Google Scholar]
- 21.Baus-Loncar M, Lubka M, Pusch CM, Otto WR, Poulsom R, Blin N. Cytokine regulation of the trefoil factor family binding protein GKN2 (GDDR/TFIZ1/blottin) in human gastrointestinal epithelial cells. Cell Physiol Biochem. 2007;20:193–204. doi: 10.1159/000104166. [DOI] [PubMed] [Google Scholar]
- 22.Dubeykovskaya Z, Dubeykovskiy A, Solal-Cohen J, Wang TC. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284:3650–62. doi: 10.1074/jbc.M804935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffrey GP, Oates PS, Wang TC, Babyatsky MW, Brand SJ. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994;106:336–45. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- 25.Nikolaidis NM, Wang TC, Hogan SP, Rothenberg ME. Allergen induced TFF2 is expressed by mucus-producing airway epithelial cells but is not a major regulator of inflammatory responses in the murine lung. Exp Lung Res. 2006;32:483–97. doi: 10.1080/01902140601059547. [DOI] [PubMed] [Google Scholar]
- 26.Hertel SC, Chwieralski CE, Hinz M, Rio MC, Tomasetto C, Hoffmann W. Profiling trefoil factor family (TFF) expression in the mouse: identification of an antisense TFF1-related transcript in the kidney and liver. Peptides. 2004;25:755–62. doi: 10.1016/j.peptides.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Nomura S, Yamaguchi H, Ogawa M, Wang TC, Lee JR, Goldenring JR. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–75. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 28.Goldenring JR, Poulsom R, Ray GS, Wright N, Meise KS, Coffey RJ., Jr Expression of trefoil peptides in the gastric mucosa of transgenic mice overexpressing transforming growth factor-alpha. Growth Factors. 1996;13:111–9. doi: 10.3109/08977199609034571. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann W. Regeneration of the gastric mucosa and its glands from stem cells. Curr Med Chem. 2008;15:3133–44. doi: 10.2174/092986708786848587. [DOI] [PubMed] [Google Scholar]
- 30.Nomura S, Esumi H, Job C, Tan SS. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–35. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 31.Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–22. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–94. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 33.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–7. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 34.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–23. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.