Abstract
A prospective, randomized study was performed in an ovine model to compare the efficacy of an anorganic bovine-derived hydroxyapatite matrix combined with a synthetic 15 amino acid residue (ABM/P-15) in facilitating lumbar interbody fusion when compared with autogenous bone harvested from the iliac crest. P-15 is a biomimetic to the cell-binding site of Type-I collagen for bone-forming cells. When combined with ABM, it creates the necessary scaffold to initiate cell invasion, binding, and subsequent osteogenesis. In this study, six adult ewes underwent anterior-lateral interbody fusion at L3/L4 and L4/L5 using PEEK interbody rings filled with autogenous bone at one level and ABM/P-15 at the other level and no additional instrumentation. Clinical CT scans were obtained at 3 and 6 months; micro-CT scans and histomorphometry analyses were performed after euthanization at 6 months. Clinical CT scan analysis showed that all autograft and ABM/P-15 treated levels had radiographically fused outside of the rings at the 3-month study time point. Although the clinical CT scans of the autograft treatment group showed significantly better fusion within the PEEK rings than ABM/P-15 at 3 months, micro-CT scans, clinical CT scans, and histomorphometric analyses showed there were no statistical differences between the two treatment groups at 6 months. Thus, ABM/P-15 was as successful as autogenous bone graft in producing lumbar spinal fusion in an ovine model, and it should be further evaluated in clinical studies.
Keywords: Lumbar interbody fusion, Sheep, Autogenous bone graft, P-15, Biomimetic, Bone graft substitute
Introduction
Orthopaedic spinal procedures often utilize bone grafts to facilitate fusion, an essential aspect of successful surgical outcomes. Autograft bone, most commonly harvested from the iliac crest, has remained the historic gold standard graft material [1]. Its use, however, is associated with additional morbidities, such as increased blood loss, wound complications, local sensory deficits, and persistent donor site pain [2]. This has led to the use of various bone graft substitutes. These products include osteoinductive materials, such as recombinant human bone morphogenetic proteins (rhBMPs), demineralized bone matrix (DBM), and allograft bone, as well as osteoconductive materials, such as hydroxyapatite and other synthetic calcium-phosphate substitutes. Potentially osteogenic products, including autologous bone marrow and other blood concentrates, have also been employed but not thoroughly studied or proven.
Recently, a composite material formulation was investigated as a potential alternative graft material. This product combines P-15, a synthetic 15-amino acid residue with a sequence identical to the cell-binding domain found on the α1(I) chain of Type-I collagen [3], onto an anorganic bovine-derived hydroxyapatite matrix (ABM) through a chemisorptive-coupling procedure [4]. The result is a complex that remains stable under physiologic conditions [5]. A delivery agent is also frequently used. One such material, carboxymethylcellulose, is a hydrogel that when combined with ABM/P-15 yields a putty-like material that improves handling characteristics without impeding its osteogenic effects [6, 7].
The combination of ABM and P-15 results in a composite bone graft substitute with both osteoinductive and osteoconductive properties. The mineral component (ABM) provides the necessary calcium phosphate and three-dimensional matrix needed for cellular invasion, while P-15 modulates cell binding by acting as an attachment factor for the α2-β1 integrin on the surface of bone-forming cells. Attachment of these cells initiates a cascade of intra-cellular signaling that leads to extracellular matrix and factor production, cell differentiation, and subsequent osteogenesis [5, 8–13]. In an in vitro study by Yang et al. [13] they showed that ABM/P-15 significantly stimulated human osteoblast synthesis of bioactive BMP-2 and alkaline phosphatase-specific activity of human bone marrow cells under both basal and osteogenic conditions. They also observed insignificant cell growth on the ABM alone. Overall, the properties of ABM/P-15 provide a novel approach to facilitating bone growth while avoiding the additional morbidities associated with the harvest of autograft bone or the potential side effects and high cost of osteoinductive agents.
P-15 has been shown to be effective in several preclinical models [14–16]. A study by Thorwarth et al. [15] studied the effectiveness of P-15 in porcine skull defects while Scarano et al. [14] studied ABM/P-15 in 8-mm tibial defects in rabbits. Both studies concluded that P-15 is an appropriate bone graft substitute to create new bone. Furthermore, ABM/P-15 has been used in humans for the regeneration of bone in periodontal defects for almost 10 years [17–26].
Based on the preclinical and human dental findings of ABM/P-15, we hypothesized that ABM/P-15 would also be successful in clinical orthopaedic use. As P-15 has not been previously tested in a lumbar spine model, the purpose of this study was to analyze the efficacy of ABM/P-15 in facilitating lumbar interbody fusion in an ovine model as compared to autogenous bone, the historic gold standard.
Materials and methods
All animal procedures were approved by the Institutional Animal Care and Use Committee at Colorado State University. Six skeletally mature ewes each underwent two-level lumbar spine fusion (L3/L4, L4/L5) using polyetheretherketone (PEEK-Optima, Invibio, West Conshohocken, PA) interbody spacers filled with either autogenous bone from the iliac crest or ABM/P-15 (P-15™, Cerapedics, Inc., Westminster, CO) formulated in a carboxymethylcellulose hydrogel matrix. Treatments were randomized with regard to the segment. A retroperitoneal lateral approach enabled discectomy, distraction of the vertebral bodies, preparation of the endplates, and placement of the interbody device. In order to optimize visualization and provide a more rigorous model for lumbar fusion, PEEK rings were utilized rather than titanium, and instrumentation was not used [27]. The PEEK rings were “D” shaped devices, 8 mm thick with a major circumference of 18 mm, 4 mm thick walls, and an internal volume of 0.4 cm3. The PEEK rings were filled with 0.4 mL of the autograft bone or ABM/P-15.
The animals were anesthetized at 3 months post-surgery and clinical CT scans were taken of the lumbar spine region. At 6 months post-surgery, the animals were euthanized, the lumbar spines were isolated, and repeat clinical CT scans were obtained. The experimental spine segments were separated, trimmed, and placed in fixative. The individual fusion segments were then micro-CT scanned (Scanco Micro-CT scanner, Zurich, Switzerland) and processed for undemineralized histology and histomorphometry analysis. The percentage of bone present within the PEEK rings was measured during histomorphometry. The histomorphometric data was statistically analyzed using a between subjects t test to compare the area of new bone formation. The p values of <0.05 were considered significant.
The clinical CT scans obtained at 3 and 6 months were analyzed in a blinded fashion by an orthopaedic surgeon (VP) using a 0–4 grading scale for fusion mass (Table 1). An experienced physician used this method due to its relevance in a clinical setting. The grading scale was based on those used in two previous ovine lumbar fusion studies [28, 29]. Each of the six levels per treatment was graded at three different positions within the PEEK ring. These positions were 2, 4, and 6-mm caudal from the cranial end of the PEEK ring. Thus, each treatment resulted in a total of 18 grades within the PEEK rings. Each treatment level also received one grade for the fusion mass outside of the PEEK ring, considering all three positions analyzed. Care was taken to distinguish residual ABM/P-15 from the assessment of new bone formation; given that the residual particles were more radio-opaque, they were easily excluded from the grading. The grading of the clinical CT scans was statistically analyzed using a Pearson’s chi-squared test. p values of <0.05 were considered significant.
Table 1.
Clinical CT Scan Grading
| Grade | Within the PEEK ring | Outside of the PEEK ring |
|---|---|---|
| 0 | No new bone formation | Same |
| 1 | ≤25% new bone formation | Same |
| 2 | 26–50% new bone formation | Same |
| 3 | 51–75% new bone formation; minimal radiographic fusion | Same and some bone bridging the vertebral bodies |
| 4 | 76–100% new bone formation; solid radiographic fusion | Same and large quantity of bone bridging the vertebral bodies that extends beyond the original vertebral border |
The 6-month micro-CT scans were graded with the same 0–4 scale (Table 1) by an orthopaedic surgeon (VP). However, only one grade was given for inside the PEEK ring, and one grade for outside the PEEK ring. These values were assigned based on the analysis of one 2-dimensional image from a sagittal plane view and one 2-dimensional image from a transverse plane view. The grading of the micro-CT scans was statistically analyzed using a Pearson’s Chi-squared test. p values of <0.05 were considered significant.
Results
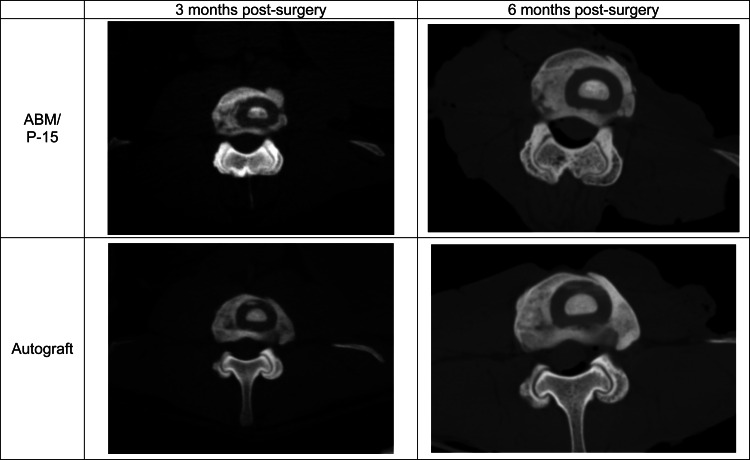
All animals survived to the 6-month study endpoint. At 3 months post-surgery, clinical CT scan analysis within the PEEK rings revealed that the autograft treatment group had significantly more new bone formation compared to ABM/P-15 as determined by the grading scale used (p = 0.0012; Table 2). However, at 3 months post-surgery clinical CT scan analysis outside of the PEEK rings revealed that 4 of 6 levels in both treatment groups had grade 4 fusions and the remaining 2 levels received grade 3 fusions. Thus, there was no statistical difference between autograft and ABM/P-15 treatments outside of the PEEK rings at 3 months post-surgery, and all treated levels had obtained radiographic fusion. At 6 months post-surgery, clinical CT scan analysis within the PEEK rings showed no statistical difference between autograft and ABM/P-15 treatments (p = 0.2065). Of the 18 total grades per treatment (six levels were evaluated at three different positions), 16 of the autograft and 13 of the ABM/P-15, respectively, were a grade 4 fusion. The remaining levels for both groups were scored a grade 3. Six-month post-surgery clinical CT scan analysis outside of the PEEK rings showed solid, grade 4 fusions at all levels, and thus no significant difference. Figure 1 shows clinical CT scans at both time points.
Table 2.
Clinical CT grades within PEEK rings at 3 months post-surgery
| Grade | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Autograft | 0 | 6 | 0 | 12 |
| ABM/P-15 | 2 | 13 | 2 | 1 |
Each column presents the number of levels for each treatment group scored as 1–4 with the grading scale shown in Table 1. The autograft groups received significantly higher grades than the ABM/P-15 group (p = 0.0012)
Fig. 1.
CT scans of the same level at both time points. Large amount of new bone formation outside of the PEEK rings in both treatments can be seen starting at 3 months post-surgery. All treatments appear to have solid bone formation inside the PEEK rings at 6 months
Micro-CT scan analysis at 6 months post-surgery also showed no statistically significant difference between autograft and ABM/P-15 inside of the PEEK rings, as 5 of 6 levels in the autograft group and 6 of 6 levels in the ABM/P-15 group received a grade 4 fusion score (p = 0.2963). There was also no significant difference between autograft and ABM/P-15 outside of the PEEK rings. Both treatment groups had 5 of 6 levels scored as grade 4 fusions; one autograft level showed grade 3 fusion and one ABM/P-15 received a grade 2 fusion (p = 0.3679). Representative micro-CT scans of both treatment groups are shown in Fig. 2.
Fig. 2.
The micro-CT images show dense bone matrix within and outside of the PEEK rings of both treatments. The sagittal plane views show bone formation bridging the intervertebral space. The residual ABM/P-15 can be seen in the top images within the PEEK rings as a hyper-opacity
In summary, the clinical CT analyses showed that the autograft treatment had higher fusion within the PEEK rings at 3 months post-surgery; however, according to both clinical CT and micro-CT analyses, both treatment groups showed equivalent, solid fusion within and outside the PEEK rings at 6 months.
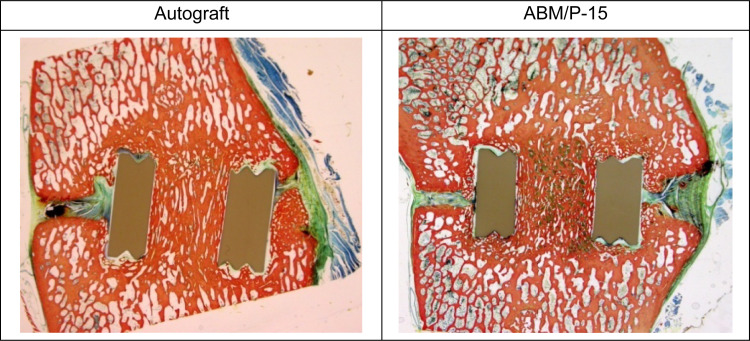
The histomorphometric data of the fused bone segments at 6 months post-surgery demonstrated no statistically significant group differences between the two graft materials used inside of the PEEK devices (p = 0.1231; Table 3); that is, both autogenous bone graft and ABM/P-15 were equivalent in supporting spine fusions. The histology shows that the bone trabeculation within the rings of both treatments is denser than the adjacent cancellous bone, as shown by objective, visual assessment of amount of bone per area, proving solid bone formation within the rings (Fig. 3). Most of the remaining area within the PEEK cages was found to be bone marrow or fibrous tissue.
Table 3.
Percentage of fused bone at 6 months as determined by histomorphometric analysis
| Animal # | Autograft | ABM/P-15 |
|---|---|---|
| 1 | 57 | 30 |
| 2 | 60 | 63 |
| 3 | 78 | 50 |
| 4 | 69 | 58 |
| 5 | 72 | NAa |
| 6 | 52 | 62 |
| Mean score | 64.67* | 52.60* |
| SD | 9.91 | 13.63 |
aExcessive slip of the PEEK interbody cage prevented histomorphometric analysis
* p = 0.1231
Fig. 3.
Histology images showing elaborate, dense bone formation inside of the PEEK rings bridging the intervertebral space and proving successful interbody fusion. The ABM/P-15 treatment on the right also reveals some residual ABM/P-15 within the PEEK ring shown as the green stain
Lateral displacement of the PEEK rings, sometimes by several millimeters, occurred during the early post-surgery period, but was unrelated to the graft material used. In one ewe at the ABM/P-15 treated level, histomorphometry analysis was not possible due to a large device slip of 17.2 mm. However, during CT analysis solid fusion was visible in the part of the PEEK ring that was still within the vertebral border and in contact with original bone. Overall, the amount of device slip was not significantly different between the groups as determined by a between subjects t test for both time points (p = 0.2839 for 3 months, p = 0.3210 for 6 months). This study also showed that 9.0 ± 7.3% residual ABM/P-15 was present at 6 months post-surgery. This residual ABM/P-15 can be seen in the micro-CT scans (Fig. 2), and histology slides (Fig. 3).
Discussion
The use of bone graft can be an essential element in spine fusion procedures. Autogenous bone, most commonly from the iliac crest, remains the gold standard material. However, due to the increased morbidity associated with its harvest, including persistent pain at the donor site and wound complications, alternative products have been pursued. These materials, though, have not always been as successful in producing desired clinical outcomes and those, which have proven equivalent to autograft, such as BMPs, can be very costly.
Clinically, lumbar fusion rates have been found to be variable, with one literature review by Turner et al. [30] reporting that 68% of patients obtained satisfactory outcomes following fusion, with a range from 16 to 95%. A 20–40% failure rate was also reported that might have contributed to the lack of satisfaction. Furthermore, the most frequently reported complications were pseudarthrosis (14%) and chronic pain at the bone graft donor site (9%). Thus, patient satisfaction in lumbar fusions would likely rise if bone graft harvesting could be avoided by using a synthetic agent, like ABM/P-15, that is as effective as autologous bone.
P-15 has been shown to be effective in several preclinical models [14–16]. A study by Thorwarth et al. [15] studied the effectiveness of P-15 in porcine skull defects by creating four different treatment groups: autogenous bone, a porous algae-derived hydroxyapatite (adHA), P-15 and adHA, and P-15 and adHA combined with 25% autogenous bone. At 12 and 26 weeks post-surgery, micro-radiographic analysis showed no statistical differences between autograft alone, P-15 and adHA, and P-15 and adHA combined with 25% autogenous bone. This led to the conclusion that adHA/P-15 is an appropriate bone graft substitute for the correction of larger bony defects. In another study, Scarano et al. [14] tested the effectiveness of ABM/P-15 in 8-mm tibial defects in rabbits versus no added agent. Using histomorphometric analysis at 4 weeks post surgery, they found a statistical significance in favor of ABM/P-15 for its ability to generate new bone. Only one study, to our knowledge, has found unfavorable outcomes with ABM/P-15. Sarahrudi et al. [31] tested ABM/P-15 versus a control (no added agent) in the treatment of fixated 5-mm segmental femoral defects in rabbits. They showed that ABM/P-15 was equivalent to their control (no added agent) using radiographical analysis at 8 and 12 weeks, but that ABM/P-15 had a smaller amount of new bone formation as shown by histomorphometry at 4, 8, and 12 weeks. They hypothesized that the unfavorable results may have been due to the large defect size and possible biomechanical instability due to the fixation used.
ABM/P-15 has been used successfully in dentistry for the repair of periodontal defects. Yukna et al. [26] used ABM/P-15 as a bone replacement graft in human periodontal defects and found a significant improvement at 6 months and 3 years postoperative when compared to the time of surgery. These outcomes included increased mean clinical attachment level, a decrease in probing depth, and improved mean gingival recession. They concluded that ABM/P-15 might have a beneficial effect in the long-term clinical management of infrabony defects. In a separate study by Yukna et al. [25] they found that ABM/P-15 yielded better clinical results than ABM alone in infrabony periodontal defects, showing that P-15 is needed for optimal results in vivo. In fact, ABM/P-15 had ≥ 90% defect fill in 3.5 times more treatment areas than ABM alone, and ABM alone had twice as many failures, as defined by minimal response to treatment. In a more recent study by Kasaj et al. [18] they compared ABM/P-15 to open flap debridement for the regeneration of periodontal tissues in otherwise healthy adults. At 12 months, the ABM/P-15 group resulted in significant improvements in clinical outcomes versus open flap debridement alone as measured by increased clinical attachment level and probing depth reductions. All of these results suggest that ABM/P-15 improves clinical outcomes in the repair of human periodontal tissues.
The use of ABM/P-15 has only recently been applied to clinical orthopaedic models. A pilot study by Gomar et al. [9] used ABM/P-15 as a bone graft substitute to treat delayed or non-unions in various long bones in adult trauma. The study reported full consolidation in 90% of patients (20 of 22) at an average of 4.2 months. Full consolidation was determined by an independent surgeon radiographically as callus formation across the full bone thickness. Based on these results, the authors concluded that ABM/P-15 is a safe, economical, and clinically useful alternative to autograft in the repair of non-united fractures in humans.
To date, the sheep lumbar spine has been the experimental model most widely used in orthopaedic spine publications because it has been shown to be biomechanically comparable to the human lumbar spine [32–34]. However, this model can be challenging and studies have produced inconsistent success rates using autograft bone. Ovine single-level interbody fusions utilizing different radiolucent polymers with autograft augmentation, including 70/30 poly (L-lactide-co-D,L-lactide) and PEEK rings, have produced fusion rates of 50 and 71%, respectively [27, 28]. Other lumbar interbody fusion cages have also been investigated in sheep, including Bagby and Kuslich (BAK) cages (SpineTech, Minneapolis, MN) which yielded only a 20% rate of fusion at 6 months when supplemented with autograft [35]. This study used histological evaluation as the primary outcome measure. However, the study obtained a 100% fusion rate with rhBMP-2. Another ovine interbody lumbar fusion study by Toth et al. [29] used fenestrated titanium cages loaded with autograft bone as their control (without electrical stimulation), which yielded a fusion rate of 29% (2/7). This study also reported that electrical stimulation of 40 μA and 100 μA of the fenestrated titanium cages filled with autograft bone led to fusion rates of 71% (5/7) and 100% (8/8), respectively. These variable percentages of lumbar interbody fusion in ovine autograft models indicate that the radiographic fusion rate of 100% found in the present study in the ABM/P-15 and autograft augmented cages is noteworthy, especially as it was obtained in a traditionally difficult environment with uninstrumented, non-threaded cages.
The present study used multiple imaging modalities in an ovine model to analyze the efficacy of ABM/P-15 versus autogenous bone in lumbar interbody fusion. Due to the nature of the imaging used, clinical CT scans were the only parameters analyzed that were non-invasive and thus able to compare both time points. At 3 months post-surgery, the clinical CT scan analysis showed a significant difference between autograft and ABM/P-15 within the PEEK rings. However, no such difference was found outside of the PEEK rings. In fact, all levels had a grade 3 fusion (minimal radiographic fusion) or higher outside of the rings. Thus, at 3 months post-surgery all levels had at least 50–75% of the total intervertebral area occupied by new bone with some new bone bridging the intervertebral space outside of the vertebral border. At 6 months post-surgery, clinical CT scan analysis found no significant differences between ABM/P-15 and autogenous bone inside or outside of the PEEK rings. Again, all levels were found to be radiographically fused, with most levels (16/18 for autograft and 13/18 for ABM/P-15) achieving grade 4 solid fusions. In conclusion, clinical CT scan analysis showed no significant differences in the ability of ABM/P-15 and autograft to facilitate radiographic fusion at the 6-month study endpoint.
Micro-CT scan analysis at 6 months post-surgery also showed no significant differences between ABM/P-15 and autograft inside or outside of the PEEK ring. This high resolution imaging technique was able to show very dense new bone formation in the intervertebral space and confirm radiographic fusion at all levels. Histomorphometric analysis at 6 months post-surgery also showed no statistical difference between the two treatments based on percentage of new bone formation within the PEEK rings. Furthermore, the 9.0 ± 7.3% of residual ABM/P-15 revealed by histomorphometry may have continued to form new bone in vivo and added to the percentage of new bone formation, thus making the difference between the mean scores of the treatments even smaller with additional time (Table 3). The successful bone formation results achieved in this study with ABM/P-15 are consistent with results obtained in other preclinical models [14–16], dental models [17–26], and the pilot orthopaedic model [9].
While migration of the rings was noted in both groups, with lateral displacement by several millimeters, this occurred early in the post-surgical course and was likely due to the lack of additional instrumentation. Furthermore, the migration of the PEEK rings did not prevent successful fusion outcomes. Although the migration of the rings could have been avoided with instrumentation, we wanted a challenging model for the graft material in this pilot study. Given the ring displacements, it is possible that bone formation outside of the cage was due to the natural response upon trauma to the disc and a broader, larger callus formation similar to a hypertrophic non-union in long bone fracture repair. However, the homogenous and more densely trabeculated bone within the displaced rings implies that the graft material at least allowed osteoconduction and was likely the primary load bearing bone. Nonetheless, future studies should consider utilizing instrumentation and an earlier time point to better assess the progression of bone formation inside and outside the rings. Furthermore, with the added stability of instrumentation, the inclusion of non-destructive biomechanical testing prior to histology would be feasible. We also noted subsidence in nearly all of the fusions and occasional cage deformation (e.g., Fig. 2, bottom right panel) that likely would have been avoided by instrumented fusions. Even with the subsidence, we had successful fusion in all treatments and the actual contribution of subsidence to the fusion is unclear.
There are some limitations to this study. While an increase in sample size would be beneficial in furthering statistical significance, the study size and methods are consistent with previously investigated models [27–29, 35]. It could be argued that a 6-month follow-up is too long to find significant differences among the groups. Ideally, with unlimited funding, the addition of 6 weeks and 3 month histologic evaluations would have been ideal. However, we chose 3 and 6 months as our time points for this pilot study based on previous studies reporting variable rates of fusion ranging from 3 to 12 months [9, 15, 18, 26, 29, 35]. As for the high fusion rates, a control with empty PEEK rings would likely have resulted in a low fusion rate. However, a bone graft substitute is usually used with lumbar interbody fusions in clinical practice and our study was designed to be clinically relevant. Nonetheless, in retrospect a negative control would have strengthened our conclusions. We did expect a lower fusion rate when planning the study and we would consider using older animals in future studies. Similarly, perhaps larger numbers of animals or decreased volumes of autograft and ABM/P-15 would have led to some non-unions; however, the fact that these smooth, non-threaded cages moved prior to fusion shows that ABM/P-15 and autograft can result in fusion even in a potentially unstable environment. Next, our study design could be improved in future studies by leaving an untreated motion segment between fusion levels, as fusion has been shown to increase stress and degeneration at adjacent levels [36, 37]. Adjacent segment disease did not seem to be a problem in our study, though, given the high fusion rate at both levels. Lastly, a single orthopaedic surgeon in the present study performed the clinical CT and micro-CT analysis. However, the histomorphometry reported in this study used a different analysis by another individual and revealed similar results.
In summary, ABM/P-15 acts as a biomimetic for hard tissues and introduces a novel method of enhancing bone growth and fusion. It is a composite material possessing both osteoinductive and osteoconductive properties. The product relies on an attachment factor that, through mechanotransduction, enhances cellular attachment, intra-cellular stimulation, and transformation, ultimately resulting in enhanced osteogenesis. The synthetic material not only avoids the morbidities associated with the harvest of autogenous bone, but it can be manufactured inexpensively through solid-phase peptide synthesis. Furthermore, the sintering process involved in preparing the ABM serves to deproteinate the substance, eliminating the potential for disease transmission present with some materials, most notably allograft tissue [38].
ABM/P-15 provides an alternative composite bone graft substitute material that may be used to successfully enhance bone formation in spine fusion surgeries. Results in this ovine model indicate similar fusion rates for ABM/P-15 and autograft bone when used for lumbar fusion procedures. However, further investigation, including prospective, randomized, controlled human clinical trials must be performed in order to fully elucidate the benefits that ABM/P-15 may provide in orthopaedic practices.
Acknowledgments
The authors would like to acknowledge Hari Prasad for assistance with histological analysis. Cerapedics, Inc. of Westminster, CO provided the research funding for this study.
References
- 1.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A:716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324–1331. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar RS, Qian JJ, Gough CA. The role in cell binding of a beta-bend within the triple helical region in collagen alpha 1 (I) chain: structural and biological evidence for conformational tautomerism on fiber surface. J Biomol Struct Dyn. 1997;14:547–560. doi: 10.1080/07391102.1997.10508155. [DOI] [PubMed] [Google Scholar]
- 4.Seghal D (1994) A method for the high efficiency of water-soluble carbodiimidemediated amidation. Anal Biochem 218:87–91 [DOI] [PubMed]
- 5.Bhatnagar RS, Qian JJ, Wedrychowska A, Sadeghi M, Wu YM, Smith N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999;5:53–65. doi: 10.1089/ten.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 6.Lewis KN, Thomas MV, Puleo DA. Mechanical and degradation behavior of polymer-calcium sulfate composites. J Mater Sci Mater Med. 2006;17:531–537. doi: 10.1007/s10856-006-8936-0. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H, Qian JJ, Bhatnagar RS, Li S (2003) Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem Biophys Res Commun 311:179–186. S0006291X03020308 [pii] [DOI] [PubMed]
- 8.Bhatnagar RS, Qian JJ, Wedrychowska A, Smith N. Construction of biomimetic envioments with a synthetic peptide anlogue of collagen. Mat Res Soc Symp Proc. 1998;530:43–54. [Google Scholar]
- 9.Gomar F, Orozco R, Villar JL, Arrizabalaga F. P-15 small peptide bone graft substitute in the treatment of non-unions and delayed union. A pilot clinical trial. Int Orthop. 2007;31:93–99. doi: 10.1007/s00264-006-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanks T, Atkinson BL (2004) Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials 25:4831–4836. doi:10.1016/j.biomaterials.2003.12.007 [DOI] [PubMed]
- 11.Kubler A, Neugebauer J, Oh JH, Scheer M, Zoller JE (2004) Growth and proliferation of human osteoblasts on different bone graft substitutes: an in vitro study. Implant Dent 13:171–179. 00008505-200413020-00015 [pii] [DOI] [PubMed]
- 12.Qian JJ, Bhatnagar RS (1996) Enhanced cell attachment to anorganic bone mineral in the presence of a synthetic peptide related to collagen. J Biomed Mater Res 31:545–554. doi:10.1002/(SICI)1097-4636(199608)31:4<545::AID-JBM15>3.0.CO;2-F [DOI] [PubMed]
- 13.Yang XB, Bhatnagar RS, Li S, Oreffo RO. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004;10:1148–1159. doi: 10.1089/ten.2004.10.1148. [DOI] [PubMed] [Google Scholar]
- 14.LG ScaranoA, Petrone G, Orsini G, Degidi M, Strocchi R, Piattelli A. Cortical bone regeneration with a synthetic cell-binding peptide: a histological and histomorphometric pilot study. Implant Dent. 2003;12:318–324. doi: 10.1097/01.ID.0000095467.48241.68. [DOI] [PubMed] [Google Scholar]
- 15.Thorwarth M, Schultze-Mosgau S, Wehrhan F, Srour S, Wiltfang J, Neukam FW, Schlegel KA (2005) Enhanced bone regeneration with a synthetic cell-binding peptide–in vivo results. Biochem Biophys Res Commun 329:789–795. doi:10.1016/j.bbrc.2005.01.157 [DOI] [PubMed]
- 16.Thorwarth M, Schultze-Mosgau S, Wehrhan F, Kessler P, Srour S, Wiltfang J, Andreas Schlegel K (2005) Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials 26:5648–5657. doi:10.1016/j.biomaterials.2005.02.023 [DOI] [PubMed]
- 17.Degidi M, Piattelli M, Scarano A, Iezzi G, Piattelli A. Maxillary sinus augmentation with a synthetic cell-binding peptide: histological and histomorphometrical results in humans. J Oral Implantol. 2004;30:376–383. doi: 10.1563/0720.1. [DOI] [PubMed] [Google Scholar]
- 18.Kasaj ARB, Reichert C, Willershausen B. Clinical evaluation of anorganic bovine-derived hydroxyapatite matrix/cell-binding peptide (P-15) in the treatment of human infrabony defects. Clin Oral Invest. 2008;12:241–247. doi: 10.1007/s00784-008-0191-y. [DOI] [PubMed] [Google Scholar]
- 19.Krauser JTRM, Wallace SS. Human histological and histomorphometric analysis comparing OsteoGraf/N with PepGen P-15 in the maxillary sinus elevation procedure: a case report. Implant Dent. 2000;9:298–302. doi: 10.1097/00008505-200009040-00004. [DOI] [PubMed] [Google Scholar]
- 20.Hahn J, Rohrer MD, Tofe AJ (2003) Clinical, radiographic, histologic, and histomorphometric comparison of PepGen P-15 particulate and PepGen P-15 flow in extraction sockets: a same-mouth case study. Implant Dent 12:170–174. 00008505-200312020-00016 [pii] [DOI] [PubMed]
- 21.Philippart P, Daubie V, Pochet R. Sinus grafting using recombinant human tissue factor, platelet-rich plasma gel, autologous bone, and anorganic bovine bone mineral xenograft: histologic analysis and case reports. Int J Oral Maxillofac Implants. 2005;20:274–281. [PubMed] [Google Scholar]
- 22.Gelbart M, Friedman R, Burlui V, Rohrer M, Atkinson B (2005) Maxillary sinus augmentation using a peptide-modified graft material in three mixtures: a prospective human case series of histologic and histomorphometric results. Implant Dent 14:185–193. 00008505-200506000-00013 [pii] [DOI] [PubMed]
- 23.Valentin AH, Weber J. Receptor technology–cell binding to P-15: a new method of regenerating bone quickly and safely-preliminary histomorphometrical and mechanical results in sinus floor augmentations. Keio J Med. 2004;53:166–171. doi: 10.2302/kjm.53.166. [DOI] [PubMed] [Google Scholar]
- 24.Yukna RA, Callan DP, Krauser JT, Evans GH, Aichelmann-Reidy ME, Moore K, Cruz R, Scott JB. Multi-center clinical evaluation of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) as a bone replacement graft material in human periodontal osseous defects. 6-month results. J Periodontol. 1998;69:655–663. doi: 10.1902/jop.1998.69.6.655. [DOI] [PubMed] [Google Scholar]
- 25.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects. 6-month results. J Periodontol. 2000;71:1671–1679. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 26.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Thirty-six month follow-up of 25 patients treated with combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell-binding peptide (P-15) bone replacement grafts in human infrabony defects. I. Clinical findings. J Periodontol. 2002;73:123–128. doi: 10.1902/jop.2002.73.1.123. [DOI] [PubMed] [Google Scholar]
- 27.Toth JM, Wang M, Estes BT, Scifert JL, Seim HB 3rd, Turner AS (2006) Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 27:324–334. doi:10.1016/j.biomaterials.2005.07.011 [DOI] [PubMed]
- 28.Toth JM, Estes BT, Wang M, Seim HB, 3rd, Scifert JL, Turner AS, Cornwall GB. Evaluation of 70/30 poly (l-lactide-co-D, l-lactide) for use as a resorbable interbody fusion cage. J Neurosurg. 2002;97:423–432. doi: 10.3171/jns.2002.97.2.0423. [DOI] [PubMed] [Google Scholar]
- 29.Toth JM, Seim HB, 3rd, Schwardt JD, Humphrey WB, Wallskog JA, Turner AS. Direct current electrical stimulation increases the fusion rate of spinal fusion cages. Spine. 2000;25:2580–2587. doi: 10.1097/00007632-200010150-00007. [DOI] [PubMed] [Google Scholar]
- 30.Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, Deyo R. Patient outcomes after lumbar spinal fusions. JAMA. 1992;268:907–911. doi: 10.1001/jama.268.7.907. [DOI] [PubMed] [Google Scholar]
- 31.Sarahrudi K, Mousavi M, Grossschmidt K, Sela N, Konig F, Vecsei V, Aharinejad S. Combination of anorganic bovine-derived hydroxyapatite with binding peptide does not enhance bone healing in critical-size defect in a rabbit model. J Orthop Res. 2008;26:759–763. doi: 10.1002/jor.20527. [DOI] [PubMed] [Google Scholar]
- 32.Sandhu HS, Kanim LEA, Girardi F, et al. Animal models of spinal instability and spinal fusion. In: An YH, Friedman RJ, et al., editors. Animal models in orthopaedic research. Boca Raton: CRC Press; 1999. [Google Scholar]
- 33.Smit TH. The use of a quadruped as an in vivo model for the study of the spine—biomechanical considerations. Eur Spine J. 2002;11:137–144. doi: 10.1007/s005860100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22:2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 35.Sandhu HS, Toth JM, Diwan AD, Seim HB, 3rd, Kanim LE, Kabo JM, Turner AS. Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine. 2002;27:567–575. doi: 10.1097/00007632-200203150-00003. [DOI] [PubMed] [Google Scholar]
- 36.GH ParkP, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 37.RM HillibrandAS. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4:190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Huffman EWD, Keil RL (2003) Determination of trace organic carbon and nitrogen in the presence of carbonates in anorganic bovine bone graft materials. Microchem J 249–256