Abstract
Early postoperative MRI after spinal surgery is difficult to interpret because of confounding postoperative mass effects and frequent occurrence of epidural hematomas. Purpose of this prospective study is to evaluate prevalence, extent and significance of hematoma in the first postoperative week in asymptomatic patients after decompression for lumbar stenosis and to determine the degree of clinically significant dura compression by comparing with the patients with postoperative symptoms. MRI was performed in 30 asymptomatic patients (47 levels) in the first week after lumbar spine decompression for degenerative stenosis. Eleven patients requiring surgical revision (16 levels) for symptomatic early postoperative hematoma were used for comparison. In both groups the cross-sectional area of the maximum dural compression (bony stenosis and dural sac expansion) was measured preoperatively and postoperatively by an experienced radiologist. Epidural hematoma was seen in 42.5% in asymptomatic patients (20/47 levels). The median area of postoperative hematoma at the operated level was 176 mm2 in asymptomatic patients and 365 mm2 in symptomatic patients. The median cross-sectional area of the dural sac at the operated level was 128.5 and 0 mm2 in asymptomatic and symptomatic patients, respectively, at the site of maximal compression. In the symptomatic group 75% of the patients had a maximal postoperative dural sac area of 58.5 mm2 or less, whereas in the asymptomatic group 75% of patients with epidural hematoma had an area of 75 mm2 or more. The size of hematoma and the degree of dural sac compression were significantly larger in patients with symptoms needing surgical revision. Dural sac area of less than 75 mm2 in early postoperative MRI was found to be the threshold for clinical significance.
Keywords: Epidural hematoma, Early postoperative MRI, Spinal stenosis, Neural compression
Introduction
Postoperative epidural hematoma as an early complication after decompression in lumbar stenosis occurs with a prevalence of 0.1–0.2% [1–4]. Magnetic resonance imaging (MRI) is recommended in patients with postoperative increasing pain and new neurological symptoms to rule out hematoma. However, early postoperative MRI is difficult to interpret due to postoperative changes [5, 6]. It is therefore questionable if MRI in the early postoperative period after decompression in lumbar spinal stenosis is useful. Reports in the literature describe mass effects and compression of dural sac in the early postoperative period in asymptomatic patients with normal postoperative course [5, 7]. These reports emphasize that early postoperative MRI must be interpreted with caution, since correlation between clinical and radiological picture is weak.
The sparse literature on characteristics of MRI in the early postoperative period after decompression in lumbar spinal stenosis reported various rates of hematoma [8, 9]. Only one study reported dimensions of hematoma and cross-sectional area (CSA) of dural sac pre- and postoperatively in patients with different surgical approaches [8, 9]. There is also lack of information on early postoperative MRI after lumbar decompression in patients with uneventful postoperative course. If hematoma is a common finding, how much compression of the neural structures can be tolerated? Is there a threshold for dural sac expansion which correlates with development of symptoms?
The purpose of this study is to detect the prevalence of early postoperative hematoma and the extent of dural compression in MRI in patients with uneventful postoperative course after decompression for degenerative lumbar spinal stenosis and to compare these findings to patients who needed revision surgery because of increasing pain or new neurologic deficit caused by hematoma compression.
Patients and methods
30 patients undergoing lumbar spine decompression without instrumentation for symptomatic degenerative stenosis were prospectively studied. Patients operated for lumbar disc herniation and patients requiring additional instrumentation were excluded. Local ethical commission approval for this study and written patients consent were obtained. In all patients the preoperative diagnosis of lumbar spinal stenosis was done by MRI. Patient characteristics are reported in Table 1. The methods of decompression included complete or subtotal laminectomy, laminotomy with midline resection, bilateral fenestration and unilateral fenestration with contralateral undercutting. The operated area was drained postoperatively with a subfascial suction drain during 24 h. All the patients were free of ischial pain or neurological symptoms (asymptomatic group) during early postoperative period and underwent standard MRI protocol between 1st and 4th postoperative day (sagittal T1 and T2, axial T2-sequence, GE 1.5T).
Table 1.
Patient characteristics
| Asymptomatic | Reoperated | |
|---|---|---|
| No. of patients | 30 | 11 |
| Mean age (years) | 72.3 | 77.8 |
| Gender: female/male | 13/17 | 3/8 |
For comparison 11 patients who needed revision surgery in the early postoperative period because of symptoms due to postoperative epidural hematoma after decompression for lumbar spinal stenosis in the same institution were reviewed (reoperated group). These patients also had standard MRI protocol preoperatively and in the early postoperative period. The indications for revision and hematoma evacuation were increasing ischial pain in 5 patients and development of new neurological deficits in 6 patients.
In preoperative MRI of both groups the area of the stenosis (stenosis caused by bony margins, ligaments and disc), which we named “hard stenosis”, and the area of the dural sac at the most constricted level in transverse plane in T2-sequence were measured using digitized scans (Fig. 1). The measurements were performed in each operated level by an experienced radiologist who was blinded for the groups. The same measurements at the corresponding levels were repeated in both groups in the postoperative MRI (Fig. 2). The value of 0 mm2 was assigned when the dural sac could not be differentiated from hematoma and a complete compression was assumed. The ratios of postoperative to preoperative dural sac area and hard stenosis area in both groups were calculated. The size of postoperative hematoma in the transverse plane of the largest hematoma was chosen and the area calculated (Figs. 3, 4).
Fig. 1.
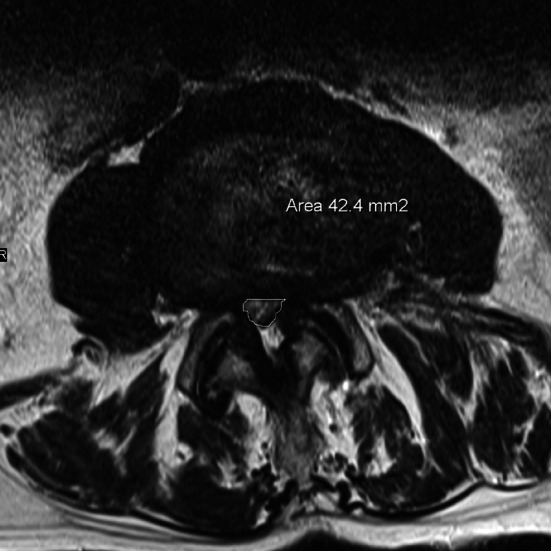
MRI of preoperative stenosis. Measurement of dural sac area
Fig. 2.
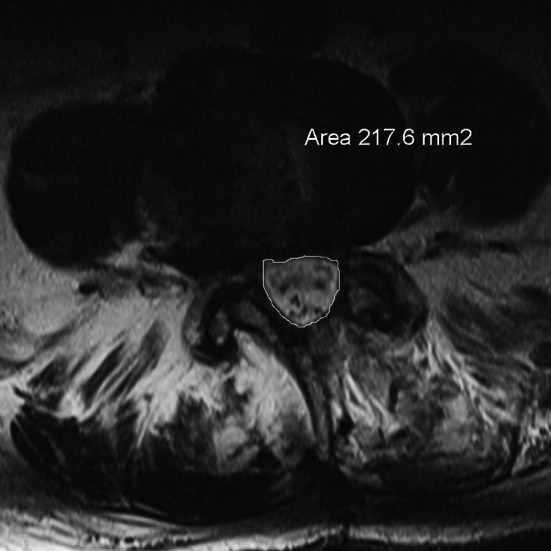
Postoperative MRI without epidural hematoma. Measurement of dural sac area
Fig. 3.
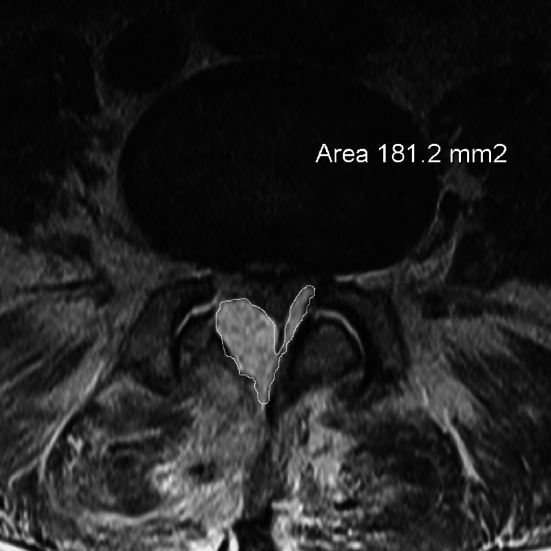
Postoperative MRI in an asymptomatic patient with hematoma. Measurement of epidural hematoma
Fig. 4.
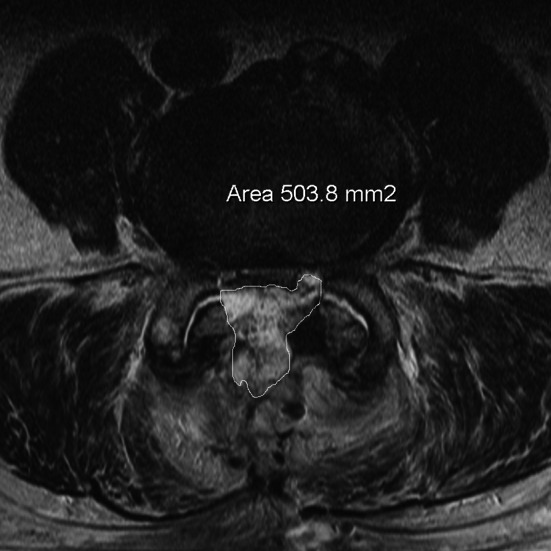
Postoperative MRI in a patient with compressive symptoms. Measurement of epidural hematoma
Statistics
For continuous variables, summary statistics are presented in the form of median (Q1–Q3), and p values are given from the Mann–Whitney U test. Comparisons of binary variables were performed using Fisher’s exact test. In this exploratory study, no correction for multiple testing has been made. Exact p values for the Mann–Whitney U test were computed using the coin package in R.
P values were calculated from Mann–Whitney U test (for continuous variable) or Fisher’s exact test (for binary variables). As several variables (for example, preoperative area dural sac) were not normally distributed, median and interquartile range (IQR, Q1, Q3) are given, rather than mean and standard error.
Results
In preoperative MRI of 3 patients in the asymptomatic group and of 2 patients in the revision group the measurements were not applicable because examinations were done in other institutions so that digitalized measurements were not feasible. For this reason preoperative data on 6 levels in the asymptomatic groups and 4 levels in the revised group were missing. In postoperative MRI one patient in the asymptomatic group did not complete the examination, for which reason postoperative data for 2 levels were not available (Table 2).
Table 2.
Levels of distribution and levels evaluated
| Asymptomatic group | Revision group | |
|---|---|---|
| No. of levels | ||
| L1/2 | 2 | 0 |
| L2/3 | 8 | 2 |
| L3/4 | 17 | 6 |
| L4/5 | 20 | 9 |
| L5/S1 | 2 | 2 |
| Drop out patient | ||
| Preop | 3 | 2 |
| Postop | 1 | 0 |
| Drop out levels | ||
| Preop | 6 | 4 |
| Postop | 2 | 0 |
| Evaluated levels | ||
| Preop | 43 | 15 |
| Postop | 47 | 19 |
On average 1.56 levels per patient in asymptomatic group and 1.72 levels per patient in revision group were decompressed.
The prevalence of epidural hematomas per operated level was significantly different, 42.5% (20/47) in the asymptomatic group versus 84.4% (16/19) in the revision group (p = 0.0025; Table 3).
Table 3.
Postoperative hematoma at the operated levels
| Asymptomatic group | Revision group | p value | |
|---|---|---|---|
| Epidural hematoma | |||
| No | 27 | 3 | 0.0025 |
| Yes | 20 | 16 | |
The differences of preoperative hard stenosis and preoperative dural sac compression in both the groups were statistically not significant (p = 0.86; Table 4).
Table 4.
Statistical comparison between asymptomatic group and revision group (all levels)
| Groups | Q1 area (mm2) | Median (mean) area (mm2) | Q3 area (mm2) | p value | |
|---|---|---|---|---|---|
| Preop stenosis hard area (mm2) | Asymptomatic | 78.00 | 102.00 (111.80) | 134.00 | 0.6251 |
| Revision | 68.00 | 90.00 (128.7) | 149.00 | ||
| Postop stenosis hard area (mm2) | Asymptomatic | 242.50 | 300.00 (314.90) | 366.00 | 0.0095 |
| Revision | 306.00 | 411.00 (398.50) | 490.00 | ||
| Quotient hard | Asymptomatic | 2.09 | 2.82 | 4.16 | 0.4720 |
| Revision | 1.73 | 3.44 | 5.94 | ||
| Preop dural sac area (mm2) | Asymptomatic | 56.50 | 73.00 (80.60) | 96.00 | 0.1428 |
| Revision | 61.50 | 90.00 (119.00) | 149.00 | ||
| Postop dural sac area (mm2) | Asymptomatic | 96.50 | 128.50 (134.00) | 179.75 | <0.0001 |
| Revision | 0.00 | 0.00 (51.00) | 58.50 | ||
| Quotient dural sac | Asymptomatic | 1.21 | 1.78 | 2.19 | <0.0001 |
| Revision | 0.00 | 0.25 | 0.62 |
The difference between the postoperative dural sac area in asymptomatic patients and reoperated patients was statistically significant (p < 0.0001; Table 4). The median of dural sac area was 128.5 mm2 (mean 134 mm2) in asymptomatic patients and 0 mm2 (mean 51 mm2) in reoperated patients. The difference in the ratio of postoperative dural sac area and preoperative dural sac area was statistically significant between the two groups, being 1.78 for asymptomatic patients and 0.25 for patients who needed revision surgery (p < 0.0001).
In the revision group 75% of the patients had a maximal postoperative dural sac area of 58.5 mm2 or less (ratio 0.62), whereas in the asymptomatic group 75% of patients had an area of 96.5 mm2 or more (ratio 1.21; Table 4).
Our data suggested that the reoperated group had larger area of surgical bony decompression (hard stenosis) in comparison to the asymptomatic group. The difference was statistically significant (p = 0.0095; Table 4).
If we exclude the levels without hematoma and consider only the levels where postoperative hematoma was present, postoperative dural sac area and ratio of post-operative to preoperative dural sac area were statistically different between the two groups (Mann–Whitney U test, p < 0.0001; Table 5). Further, the area of surgical bony decompression was significantly larger in the revision group compared to the asymptomatic group (p = 0.055).
Table 5.
Statistical comparison between asymptomatic group and revision group (only the levels with hematoma in both groups)
| Groups | Q1 area (mm2) | Median (mean) area (mm2) | Q3 area (mm2) | p value | |
|---|---|---|---|---|---|
| Preop stenosis hard area (mm2) | Asymptomatic | 75.00 | 92.00 (96.50) | 131.00 | 0.2858 |
| Revision | 61.75 | 80.00 (85.80) | 92.75 | ||
| Postop stenosis hard area (mm2) | Asymptomatic | 247.00 | 326.50 (345.30) | 412.75 | 0.0546 |
| Revision | 301.25 | 448.00 (411.00) | 512.00 | ||
| Quotient hard | Asymptomatic | 2.21 | 3.47 | 4.72 | 0.2032 |
| Revision | 3.06 | 4.35 | 6.38 | ||
| Preop dural sac area (mm2) | Asymptomatic | 50.00 | 69.00 (68.70) | 84.00 | 0.2096 |
| Revision | 60.00 | 78.00 (84.00) | 92.75 | ||
| Postop dural sac area (mm2) | Asymptomatic | 75.00 | 88.00 (84.1) | 115. 00 | 0.0000 |
| Revision | 0.00 | 0.00 (17.50) | 26.25 | ||
| Quotient dural sac | Asymptomatic | 0.88 | 1.38 | 1.88 | 0.0000 |
| Revision | 0.00 | 0.00 | 0.38 |
In 75% of operated levels with postoperative hematoma in asymptomatic patients (interquartile at 25%, Q1) the dural sac area was greater than 75 mm2. The median of dural sac area of asymptomatic patients with hematoma was 88 mm2 (mean 84.1 mm2) and in reoperated patients 0 mm2 (mean 17.5 mm2; Table 5).
The median size of hematomas differed significantly between the two groups, median being 175.5 mm2 in asymptomatic and 364.5 mm2 in revision group (Mann–Whitney U test, p = 0.0001; Table 6).
Table 6.
Size of postoperative hematoma
| Q1 area (mm2) |
Median area (mm2) |
Q3 area (mm2) |
p value | |
|---|---|---|---|---|
| Area (mm2) | ||||
| Asymptomatic | 107.00 | 175.50 | 216.50 | 0.0001 |
| Revision | 259.25 | 364.50 | 473.25 | |
In the asymptomatic group the hematoma area was greater than 216.5 mm2 in 25% of the evaluated levels and in the revision group the hematoma area was greater than 259.2 mm2 in 75% of the evaluated levels.
Discussion
Findings in this prospective study of patients undergoing decompression for lumbar canal stenosis support the usefulness of early postoperative MR imaging.
Epidural hematomas are frequent in early postoperative MRI after decompression surgery in lumbar spine in asymptomatic patients with varying prevalence [6–8, 10, 11]. In our series postoperative epidural hematoma with compression of dural sac was seen also in patients with asymptomatic postoperative course. In our study 42.5% of operated patients with uneventful course had an epidural hematoma. However, the hematoma and dural sac compression were significantly larger in patients with postoperative symptoms requiring revision. We found a statistically significant difference between the postoperative dural sac areas in asymptomatic patients compared to patients who needed revision surgery.
In asymptomatic patients with postoperative hematoma, the preoperative and postoperative cross-sectional areas of the dural sac were similar. This could indicate that resected parts were replaced by epidural hematoma and no major dural tube expansion occurred in the early postoperative period in these patients.
Until now it is not known, what are the differences in MRI between the patients requiring revision surgery for symptomatic epidural compression by hematoma in the early postoperative period and those with uneventful course, since mass effect in early postoperative MRI is a common finding and can be regarded as “normal” [6, 7]. In the literature the correlation between clinical symptoms and radiological findings of early postoperative MRI is found to be low [5, 7, 8].
Most studies concerning early postoperative MRI included a variety of pathologies, e.g. disc herniations and patients with instrumentation which may have additional signal changes [8–12]. Since discectomy and instrumentation represent additional factors which obscure the early postoperative signals we analyzed only patients undergoing decompression for lumbar canal stenosis without discectomy or instrumentation. In the patients with spinal instrumentation postoperative measurements could be difficult because of obscuring signals.
Ross et al. [6] in a prospective study of 15 patients found extensive soft-tissue changes in MRI obtained immediately postoperatively. They concluded that these changes severely limit the usefulness of MRI in the evaluation of early postoperative symptoms. They also stated that identification of hemorrhage is possible due to the distinctive signal on T1-weighted images. After decompression the regions of the missing laminas, ligamentum flavum and spinous process were replaced by a variable amount of posterior soft-tissue edema exhibiting heterogenous intermediate signals at T1-weighted pulse sequences and isointense to increased signals at T2-weighted sequences.
Awwad et al. [5] reviewed the findings of immediate postoperative MRI of 10 patients who had decompression surgery without postoperative adverse symptoms. In 9 out of 10 patients severe thecal sac compression was present, greater than that on preoperative MRI. The authors concluded that severe spinal canal compression can be a normal finding in postlaminectomy spine and that the MRI appearance in such instances is not significant in the absence of compressive clinical symptoms. They stated that these findings on immediately postoperative MRI may lead to incorrect conclusion that there is a surgical complication needing evacuation.
Kotilainen et al. [7] performed MRI on the first postoperative day after percutaneous nucleotomy or microdiscectomy in 44 patients. In 86% of the patients an extradural hematoma was found. All patients who underwent decompression for lumbar disc herniation and with large hematoma in early postoperative MR imaging had a complete resolution of sciatica. In patients with medium hematoma and small hematoma sciatica resolved in a lower rate, 88 and 74%, respectively. Further, patients with no hematoma in early postoperative MR imaging showed a reduction in sciatica only in 67%. The author concluded that the presence of hematoma was not associated with poor short-term prognosis.
Sokolovsky et al. [8] in a prospective study determined in 57 patients the incidence, volume and extent of postoperative epidural hematoma resulting in dural sac compression with MRI 2 to 5 days after surgery. This group included various pathologies with and without fusion. As much as 58% of patients developed an epidural hematoma. The postoperative dural sac area ranged from 44 to 194% of preoperative area. On average the postoperative dural sac area was 32% smaller than preoperative at the maximum site of compression due to the mass effect of subfascial hematoma. In our series, we found a statistically significant increase of the dural sac area postoperatively with a median of 178% in all asymptomatic patients.
In a second study Sokolovsky et al. [9] compared the asymptomatic patient group with two retrospective groups with severe peri-incisional pain (12 patients) and postoperative cauda equine syndrome (5 patients). In this study absolute measurements of dural sac area did not differ significantly between groups. They found that the critical ratio was the only measurement to differ significantly among the 3 groups. Mean critical ratio was 0.8 for asymptomatic patients, 0.5 for patients with pain and 0.2 for patients with cauda equine symptoms.
Schönstrom et al. [13, 14] in an in vitro study calculated the CSA at which a further constriction caused a pressure increase among the nerve roots and called this the critical size. The average corresponding critical size was 76.9 mm2 at the level L2, 71.5 mm2 at level L3 and 64.8 mm2 at L4. Measuring the CSA with CT or MR has been shown to be a reliable method to diagnose lumbar stenosis [13–16].
Until now there are no reports determining an absolute critical value of the cross-sectional area of dural sac in early postoperative MRI which could indicate clinical significance. We found in 75% of the patients in revision group a maximal postoperative dural sac area of 58.5 mm2 or less (ratio 0.62), whereas in the asymptomatic group only 25% of patients had an area of 96.5 mm2 or less (ratio 1.21). This indicates that in the investigated patients a critical value in between 58.5 and 96.5 mm2 exists which may produce symptoms. Probably this value is similar to that proposed in preoperative images by different authors to differentiate moderate from severe stenosis, approximately 75 mm2 [14]. In fact, considering only levels with hematoma in our asymptomatic group 75% patients had a dural sac area at least of 75 mm2 (Q1, Table 5).
Bolender et al. [15] found central lumbar stenosis if the cross-sectional area of the dural sac was 100 mm2 or less, early stenosis or likely stenosis if the area was 100–130 mm2. Normal canal dimensions were given with a mean of 180 ± 50mm2.
Other authors also defined the size of spinal canal of more than 130 mm2 as normal, between 130 and 100 mm2 as borderline or early stenosis. Values below 100 mm2 are generally accepted as stenosis and values below 75 mm2 [14, 16–19] or 70 mm2 [20, 21] as absolute stenosis.
According to our results a dural sac area of 75 mm2 in the early postoperative period probably represents a threshold which could help to differentiate patients at risk for development of new symptoms from those with uneventful outcome in the early postoperative period.
In our study the median size of hematomas differed significantly between the two groups. The median of hematoma in the revision group was approximately twice as large as in the asymptomatic group.
In the asymptomatic group 25% of patients had a hematoma area greater than 216.5 mm2 and 75% of patients in the revision group had a hematoma area which was larger than 259.2 mm2. This means that in the investigated patients there could be a critical hematoma area between 216.5 and 259.2 mm2 which can lead to clinically significant dural sac compression.
Further, in our study there was evidence of a statistically significant difference in postoperative bony areas between the groups. The revision group had greater surgical bony decompression compared to the asymptomatic group. This probably indicates that more extensive bony resection may lead to more exposure of epidural veins and soft tissue which can provoke larger hematomas.
We conclude that MRI is useful for adequate evaluation of postoperative epidural hematoma and dural sac compression in early postoperative period after lumbar spinal decompression for degenerative stenosis. Early postoperative epidural hematoma was seen in 42.5% in patients without any symptoms. The size of hematoma correlates with the development of symptoms. The size of hematoma and the degree of dural sac compression were significantly larger in patients with symptoms. The median area of postoperative hematoma at the operated level was 176 mm2 in asymptomatic patients and 365 mm2 in symptomatic patients. The median cross-sectional area of the dural sac at the operated level was 128.5 and 0 mm2 in asymptomatic and symptomatic patients. Dural sac area of less than 75 mm2 in early postoperative MRI indicated clinical significance.
Acknowledgments
The authors gratefully acknowledge the assistance of Sarah Haile, PhD in statistical analyses.
References
- 1.Awad KN, Kebaish KM, Donigan J, Cohen DB, Kostuik JP. Analysis of risk factors for the development of post-operative spinal epidural haematoma. J Bone Jt Surg Br. 2005;87-B:1248–1252. doi: 10.1302/0301-620X.87B9.16518. [DOI] [PubMed] [Google Scholar]
- 2.Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus. 2004;16(6):e1. doi: 10.3171/foc.2004.16.6.1. [DOI] [PubMed] [Google Scholar]
- 3.Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine. 2002;27(15):1670–1673. doi: 10.1097/00007632-200208010-00016. [DOI] [PubMed] [Google Scholar]
- 4.Lawton MT, Porter RW, Heiserman JE, et al. Surgical management of spinal epidural hematoma: relationship between surgical timing an neurological outcome. J Neurosurg. 1995;83:1–7. doi: 10.3171/jns.1995.83.1.0001. [DOI] [PubMed] [Google Scholar]
- 5.Awwad EE, Smith KR. MRI of marked dural sac compression by surgicel in the immediately postoperative period after uncomplicated lumbar laminectomy. J Comput Assist Tomogr. 1999;23(6):969–975. doi: 10.1097/00004728-199911000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Ross JS, Masaryk TJ, Modic MT, Bohlman H, Delamater R, Wilber G. Lumbar spine: postoperative assessment with surface-coil MR imaging. Radiology. 1987;164:851–860. doi: 10.1148/radiology.164.3.3615887. [DOI] [PubMed] [Google Scholar]
- 7.Kotilainen E, Alanen A, Erkintalo M, Helenius H, Valtonen S. Postoperative hematomas after successful lumbar microdiscectomy or percutaneous nucleotomy: a magnetic resonance imaging study. Surg Neurol. 1994;41:98–105. doi: 10.1016/0090-3019(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 8.Sokolovsky MJ, Garvey TA, Perl John II, Sokolovsky MA, Cho W, Mehbod AA, Dykes DC, Transfeldt EE. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine. 2008;33(1):108–113. doi: 10.1097/BRS.0b013e31815e39af. [DOI] [PubMed] [Google Scholar]
- 9.Sokolovsky MJ, Garvey TA, Perl J, et al. Postoperative lumbar epidural hematoma: does size really matter? Spine. 2008;33(1):114–119. doi: 10.1097/BRS.0b013e31815e3a26. [DOI] [PubMed] [Google Scholar]
- 10.Dina TS, Boden SD, Davis DO. Lumbar spine after surgery for herniated disk: imaging findings in the early postoperative period. AJR. 1995;164(3):665–671. doi: 10.2214/ajr.164.3.7863890. [DOI] [PubMed] [Google Scholar]
- 11.Montaldi S, Frankhauser H, Schnyder P, de Tribolet N. Computed tomography of postoperative intervertebral disc and lumbar spinal canal: investigation of twenty-five patients after successful operation for lumbar disc herniation. Neurosurgery. 1988;22:1014–1021. doi: 10.1227/00006123-198806010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Ikuta K, Tono O, Tanaka T, Arima J, Nakano S, Sasaki K, Oga M. Evaluation of postoperative spinal epidural hematoma after microendoscopic posterior decompression for lumbar spinal stenosis: a clinical and magnetic resonance imaging study. J Neurosurg Spine. 2006;5:404–409. doi: 10.3171/spi.2006.5.5.404. [DOI] [PubMed] [Google Scholar]
- 13.Schönstrom NS, Bolender NF, Spengler DM. Pressure changes within the cauda equine following constriction of dura sac: an in vitro experimental study. Spine. 1984;9:604–607. doi: 10.1097/00007632-198409000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Schönstrom N, Hansson T. Pressure changes following constriction of the cauda equine: an experimental study in situ. Spine. 1988;13:385–388. doi: 10.1097/00007632-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Bolender NF, Schönstöm NSR, Spengler DM. Role of computed tomography and myelography in the diagnosis of the central spinal stenosis. J Bone Jt Surg Am. 1985;67:240–246. [PubMed] [Google Scholar]
- 16.Speciale AC, Pietrobon R, Urban C, et al. Observer variability in assessing lumbar spinal stenosis severity on magnetic resonance imaging and its relation to cross-sectional spinal canal area. Spine. 2002;27(10):1082–1086. doi: 10.1097/00007632-200205150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Hamanishi C, Matukura N, Fujita M, Tomihara M, Tanaka S. Cross-sectional area of the stenotic dural tube measured from transverse views of magnetic resonance imaging. J Spinal Disord. 1994;7(5):388–393. doi: 10.1097/00002517-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Herno A, Airaksinen O, Saari T. Computed tomography after laminectomy for lumbar spinal stenosis. Spine. 1994;19(17):1975–1978. doi: 10.1097/00007632-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Mariconda M, Zanforlino G, Celestino G, Brancaleone S, Fava R, Milano C. Factors influencing the outcome of degenerative lumbar spinal stenosis. J Spinal Disord. 2000;13(2):131–137. doi: 10.1097/00002517-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Willen J, Danielson B, Gaulitz A, Niklason T, Schönström N, Hansson T (1997) Dynamic effects on the lumbar spinal canal. Axially loaded CT-myelography and MRI in patients with sciatica and/or neurogenic claudication. Spine 22(24):2968–2976 [DOI] [PubMed]
- 21.Ogikubo O, Forsberg L, Hansson T. The relationship between the cross-sectional area of the cauda equina and the preoperative symptoms in central lumbar spinal stenosis. Spine. 2007;32(13):1423–1428. doi: 10.1097/BRS.0b013e318060a5f5. [DOI] [PubMed] [Google Scholar]


