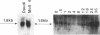Abstract
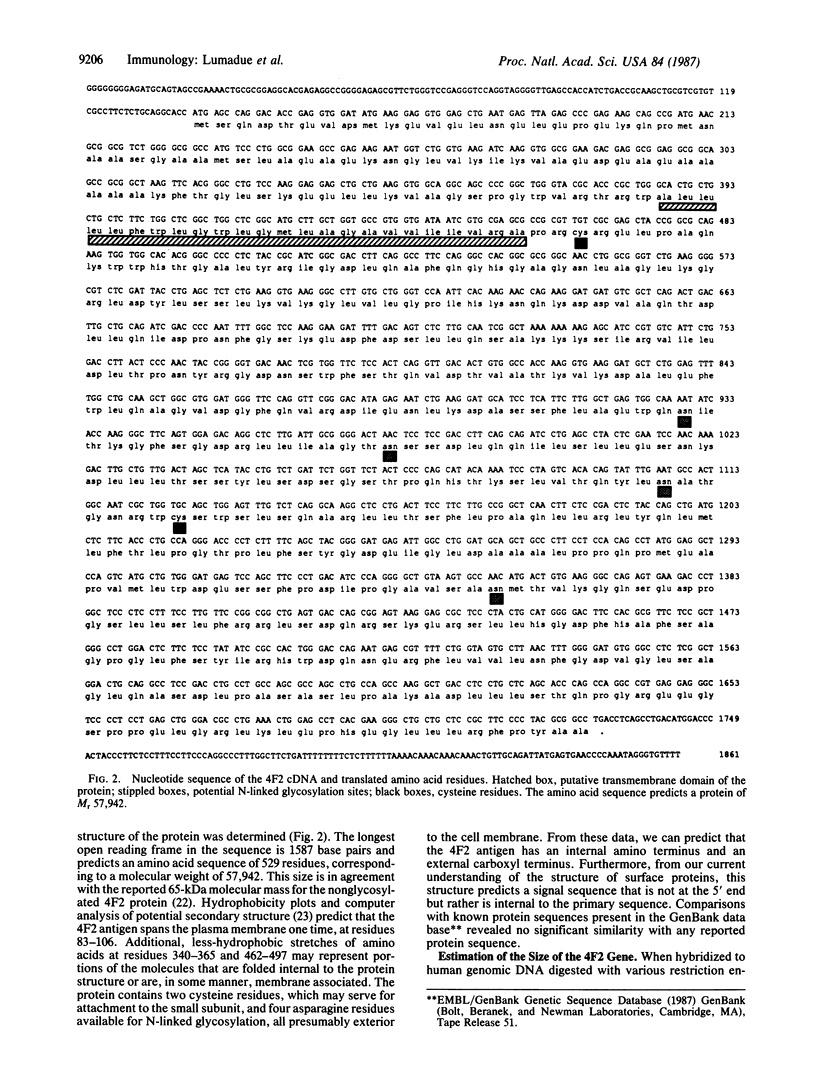
Among the earliest expressed antigens on the surface of activated human lymphocytes is the surface antigen 4F2. We have used DNA-mediated gene transfer and fluorescence-activated cell sorting to obtain cell lines that contain the gene encoding the large subunit of the human 4F2 antigen in a mouse L-cell background. Human DNAs cloned from these cell lines were subsequently used as hybridization probes to isolate a full-length cDNA clone expressing 4F2. Sequence analysis of the coding region has revealed an amino acid sequence of 529 residues. Hydrophobicity plotting has predicted a probable structure for the protein that includes an external carboxyl terminus, an internal leader sequence, a single hydrophobic transmembrane domain, and two possible membrane-associated domains. The 4F2 cDNA detects a single 1.8-kilobase mRNA in T-cell and B-cell lines. RNA gel blot analysis of RNA derived from quiescent and serum-stimulated Swiss 3T3 fibroblasts reveals a cell-cycle modulation of 4F2 gene expression: the mRNA is present in quiescent fibroblasts but increases 8-fold 24-36 hr after stimulation, at the time of maximal DNA synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzarone B., Malpièce Y., Zaech P., Moretta L., Fauci A., Suarez H. Analysis of the expression of the 4F2 surface antigen in normal and neoplastic fibroblastic human cells of embryonic and adult origin. Exp Cell Res. 1985 Aug;159(2):451–462. doi: 10.1016/s0014-4827(85)80018-5. [DOI] [PubMed] [Google Scholar]
- Azzarone B., Suarez H., Mingari M. C., Moretta L., Fauci A. S. 4F2 monoclonal antibody recognizes a surface antigen on spread human fibroblasts of embryonic but not of adult origin. J Cell Biol. 1984 Mar;98(3):1133–1137. doi: 10.1083/jcb.98.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya-Grosjean L., Azzarone B., Maunoury R., Zaech P., Elia G., Zaniratti S., Benedetto A. SV40 immortalization of adult human mesenchymal cells from neuroretina. Biological, functional and molecular characterization. Int J Cancer. 1984 Mar 15;33(3):319–329. doi: 10.1002/ijc.2910330308. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Haynes B. F., Schroer J. A., Fauci A. S. Production of monoclonal antibodies reacting with peripheral blood mononuclear cell surface differentiation antigens. J Immunol. 1980 Mar;124(3):1237–1244. [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gerrard T. L., Jurgensen C. H., Fauci A. S. Modulation of human B cell responses by a monoclonal antibody to an activation antigen 4F2. Clin Exp Immunol. 1984 Jul;57(1):155–162. [PMC free article] [PubMed] [Google Scholar]
- Goto M., Zvaifler N. J. Characterization of the natural killer-like lymphocytes in rheumatoid synovial fluid. J Immunol. 1985 Mar;134(3):1483–1486. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J Immunol. 1982 Aug;129(2):623–628. [PubMed] [Google Scholar]
- Kamarck M. E., Barbosa J. A., Ruddle F. H. Somatic cell genetic analysis of HLA-A, B, C and human beta 2-microglobulin expression. Somatic Cell Genet. 1982 May;8(3):385–402. doi: 10.1007/BF01538895. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Kühn L. C., Barbosa J. A., Kamarck M. E., Ruddle F. H. An approach to the cloning of cell surface protein genes. Selection by cell sorting of mouse L-cells that express HLA or 4F2 antigens after transformation with total human DNA. Mol Biol Med. 1983 Oct;1(3):335–352. [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- London L., Perussia B., Trinchieri G. Induction of proliferation in vitro of resting human natural killer cells: expression of surface activation antigens. J Immunol. 1985 Feb;134(2):718–727. [PubMed] [Google Scholar]
- Michalak M., Quackenbush E. J., Letarte M. Inhibition of Na+/Ca2+ exchanger activity in cardiac and skeletal muscle sarcolemmal vesicles by monoclonal antibody 44D7. J Biol Chem. 1986 Jan 5;261(1):92–95. [PubMed] [Google Scholar]
- Miskimins W. K., McClelland A., Roberts M. P., Ruddle F. H. Cell proliferation and expression of the transferrin receptor gene: promoter sequence homologies and protein interactions. J Cell Biol. 1986 Nov;103(5):1781–1788. doi: 10.1083/jcb.103.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. A., Eisinger M., Haynes B. F., Berger C. L., Edelson R. L. Monoclonal antibody 4F2 reactive with basal layer keratinocytes: studies in the normal and a hyperproliferative state. J Invest Dermatol. 1984 Sep;83(3):210–213. doi: 10.1111/1523-1747.ep12263581. [DOI] [PubMed] [Google Scholar]
- Peters P. G., Kamarck M. E., Hemler M. E., Strominger J. L., Ruddle F. H. Genetic and biochemical characterization of a human surface determinant on somatic cell hybrids: the 4F2 antigen. Somatic Cell Genet. 1982 Nov;8(6):825–834. doi: 10.1007/BF01543022. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Lane L. K., Lingrel J. B. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986 May 22;321(6068):429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]