Abstract
Transpedicular fixation can be challenging in the osteoporotic spine as reduced bone mineral density compromises the mechanical stability of the pedicle screw. Here, we sought to investigate the biomechanical and histological properties of stabilization of expandable pedicle screw (EPS) in the osteoporotic spine in sheep. EPSs and standard pedicle screws, SINO screws, were inserted on the vertebral bodies in four female ovariectomized sheep. Pull-out and cyclic bending resistance test were performed to compare the holding strength of these pedicle screws. High-resolution micro-computed tomography (CT) was performed for three-dimensional image reconstruction. We found that the EPSs provided a 59.6% increase in the pull-out strength over the SINO screws. Moreover, the EPSs withstood a greater number of cycles or load with less displacement before loosening. Micro-CT image reconstruction showed that the tissue mineral density, bone volume fraction, bone surface/bone volume ratio, trabecular thickness, and trabecular separation were significantly better in the expandable portion of the EPSs than those in the anterior portion of the SINO screws (P < 0.05). Furthermore, the trabecular architecture in the screw–bone interface was denser in the expandable portion of the EPS than that in the anterior portion of the SINO screw. Histologically, newly formed bone tissues grew into the center of EPS and were in close contact with the EPS. Our results show that the EPS demonstrates improved biomechanical and histological properties over the standard screw in the osteoporotic spine. The EPS may be of value in treating patients with osteoporosis and warrants further clinical studies.
Keywords: Expandable pedicle screw, Osteoporosis, Screw–bone interface, Micro-CT, 3D-parameters
Introduction
With rapid advances in the field of spinal surgery and with the aging of the population, an increasing proportion of elderly patients undergo surgical treatment for their spinal disorders [8]. Pedicle screw instrumentation is one of the most commonly used and rapidly growing forms of stabilization for spinal fusion. Transpedicular fixation, however, can be very challenging in the osteoporotic spine as mechanical stability of the pedicle screws is determined by bone mineral density (BMD)[9, 27]. In addition, poor rigidity of the bone–screw contact can lead to loosening of the implant in osteoporotic patients.
There have been numerous studies on the pull-out strength of pedicle screws in the lumbar spine for the optimization of the screw size, the depth of insertion or direction of the screw, and the screw design [4, 11, 21, 29]. Moreover, the pedicle screw has been augmented with polymethylmethacrylate, hydroxyapatite grout, calcium phosphate cement, or butyl-2-cyanoacrylate in an attempt to improve the strength of the screw–bone interface in the osteoporotic spine [1, 7, 18, 20, 25, 28, 30]. The effectiveness of these screw modification and augmentation strategies, however, has been limited by such complications and problems as increased risks of pedicle fracture with resultant neural injury for larger screws, anterior body penetration with ensuing vascular or visceral injury for longer screws [17], potential problems associated with the leakage of cement in the spinal canal [34], and uncertain long-term rigidity on the course of substitution for absorbable cements.
To address these issues, several investigators [5, 6, 13] have developed expandable pedicle screws (EPS), which have been shown to result in a 30% increase in bone pull-out strength compared with conventional pedicle screws, including an approximately 50% increase in pull-out strength in the osteoporotic bone. Bone fixation strength testing indicated that the EPS may be particularly useful in situations of expected compromised fixation strength such as osteoporosis [5, 13]. To our knowledge, there has been no previous report on the maintenance of stability in vivo by the EPS in osteoporosis. In this study, we sought to investigate the biomechanical and histological properties of stabilization of the EPS in the osteoporotic spine in sheep.
Materials and methods
Screws
The titanium alloy EPS is barrel-shaped and has an outer diameter of 4.5 mm and a 1.0 mm internal bore (Fig. 1). The anterior portion of the screw is split lengthwise by a groove to form two anterior fins. A smaller gauge can be inserted into the interior of the EPS and opens the fins concentrically as it is advanced. This system increases the diameter of the expanding screw tip while the diameter of the posterior portion of the screw remains constant during its expansion. Standard 4.5 mm pedicle screws, the Spinal Implant New Option (SINO) screws (Medtronic-weigao Orthopedic Device, Shandong, China) were selected as controls.
Fig. 1.
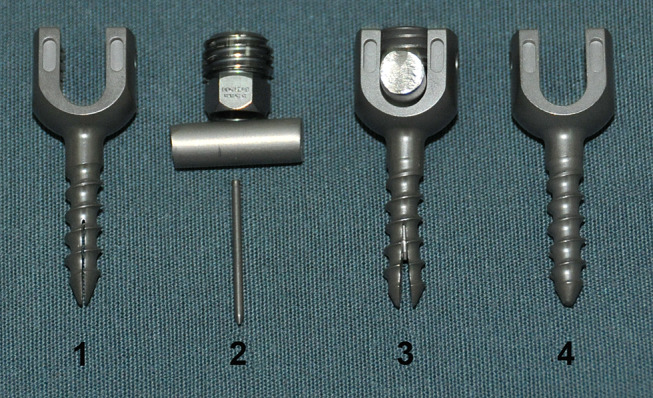
Comparison of the structure of the expandable pedicle screw (EPS) to the standard pedicle screw (SINO screw). The non-expanded EPS is shown in 1; the smaller gauge screw, rod and the accessory screw of the EPS are shown in 2; the expanded EPS is shown in 3; the standard SINO pedicle screw is shown in 4
Animals
Four adult sheep aged 3.8 ± 1.5 years with a mean weight of 45.5 ± 2.5 kg were used in the study. Osteoporosis was induced by ovariectomy followed by a 12-month low calcium diet containing 50% food pellet with 0.2% calcium and 50% wheaten chaff with 0.3% calcium as previously described [14, 16, 19, 32, 35] and was confirmed by dual energy X-ray absorptiometry (Lunar, Madison, WI) prior to and 12-month after ovariectomy. After they were anesthetized, the paravertebral muscles were exposed posteriorly and dissected. The lumbar spine from L1 to L5 was exposed and each vertebra body (L1 to L5) was drilled and tapped using the bilateral intrapedicular approach. Either an EPS or a SINO screw was placed into each pedicle at each vertebra from L1 to L5 and the EPSs were then expanded. A total of 40 screws were used with 10 screws in each animal, including 5 EPSs on the right side and 5 SINO screws on the left side (Fig. 2). Followed by a six-month low calcium diet, all animals were killed and the lumbar spines including the screws were harvested (Fig. 3).
Fig. 2.
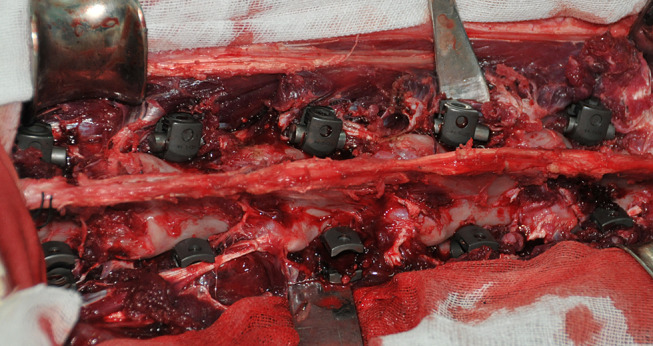
Insertion of EPSs and SINO screws in the osteoporotic spine in sheep
Fig. 3.
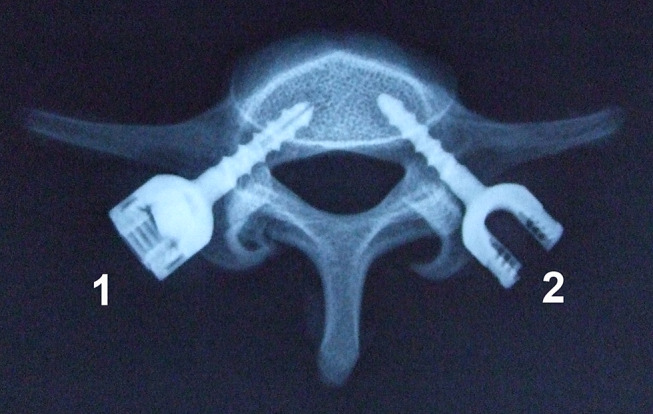
An X-ray film of the fifth lumbar vertebrae with an inserted EPS (1) and SINO screw (2)
The study protocol was approved by the local Institutional Review Board at the authors’ institution and was in accordance with the Guidelines for Animal Care and Studies by the Fourth Military Medical University, Xi’an, China.
Pull-out and cyclic bending resistance tests
Vertebral bodies were collected immediately after killing and were snap frozen. Axial pull-out tests were conducted in eight EPSs and eight SINO screws. After the removal of the posterior elements, the vertebra and pedicle screw unit was placed in the holding device. Through a clamp holding the end of the screw, a straight axial pull-out force was applied using a material testing machine (MTS 858, MTS Systems, Minneapolis, MN, USA). Each screw was extracted from the pedicle at a constant rate of 5 mm min−1 until purchase failure, which is defined as the maximum pull-out strength reached before the load decreased abruptly.
In addition, six EPSs and six SINO screws with the vertebral bodies were employed in cyclic bending resistance test. The vertebra was embedded in polymethylmethacrylate bone cement and fixed on a specially made fixation plate (Fig. 4). While the load was applied downward by a rod which was perpendicular to the screw, caudocephalad cyclic loading was applied to the head of the screw perpendicularly with an initial load of 10 N. The load was first increased from 10 to 25 N, and then decreased to 10 N (10 → 25 → 10). Subsequently, the load was increased by 25 N in each cyclic loading until it reached 200 N. Each test was conducted at a rate of 5 Hz for 100 cycles. Displacement of the pedicle screw was continuously recorded with a data acquisition system. If the screw became loosened (displacement 2 mm) when the loading cycles were less than 800 times, we recorded the peak load and the loading cycles. If the screw did not loosen (displacement < 2 mm) after the loading cycles reached 800 times, we recorded the displacement at the end of the test.
Fig. 4.
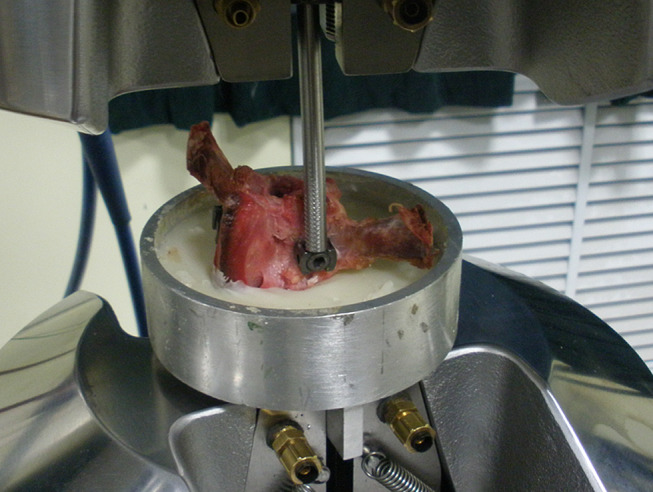
Cyclic bending resistance test
Three-dimensional imaging and reconstruction
For three-dimensional imaging and reconstruction, six vertebral bodies were incised into cylinders 20 mm in diameter centered by the EPS or SINO. The specimens of the EPS group (n = 6) and the SINO group (n = 6) were mounted on a stage and scanned using a micro-CT scanner (Explore Locus SP, GE Healthcare). The images used a voxel size of 21 μm in all three axes. Three-dimensional image of the bone and screws in the specimens were acquired and reconstructed. The same size of regions of interest (ROI) surrounding the EPS and SINO screws was reconstructed and analyzed in the same threshold. The ROI was defined from the region in the first three threads of the anterior portions of the screw. A software was used to generate three-dimensional parameters of each ROI automatically. Each of these parameters was compared between the EPS group and the SINO group.
Histological evaluation
After micro-CT scanning, all specimens of the EPS group (n = 6) were prepared for histological examination. They were fixed in 10% phosphate-buffered formalin (pH 7.25) for 7 days and dehydrated by alcohol solution with a gradient concentration of 70, 80, 90, 99, 100, and 100% (v/v) for 18 h in each, which were then embedded in polyester resin for 3 weeks. Thick sections (40–100 μm) were cut with a band saw (Leica-LA 2500, Germany) strictly perpendicular to the axis of the implant, which were then stained with Ponceau. A thorough microscopic analysis was performed on sections using transmitted light microscopy (Leica-LA) combined with a digital camera (Pixera Pro600cl, USA).
Statistical analysis
Statistical analysis was performed using the SPSS11.5 software. The data were expressed as mean ± SD. After Levene’s test, the pull-out data were compared using a paired Student’s t test. The displacement of screws in the cyclic bending resistance test was compared using Mann–Whitney U test. For three-dimensional image analysis, the parameters of the two groups were compared using Mann–Whitney U test. P < 0.05 was regarded as statistically significant.
Results
We successfully established a sheep osteoporosis model by ovariectomy and low calcium diet. Dual energy X-ray absorptiometry showed that the BMD value of the lumbar vertebrae 12 months after ovariectomy and low calcium diet was 0.755 ± 0.031 g cm−2, which was significantly less than that of the pre-ovariectomy BMD of 0.951 ± 0.047 g cm−2, and the decrease of BMD for each animal ranged from 24.7 to 27.8% (mean: 25.98%) (Table 1). We carried out pull-out tests of EPS and SINO screws and found that the mean pull-out strengths for the EPS and SINO screws were 951.83 ± 49.64 and 596.38 ± 51.21 N, respectively. Furthermore, EPSs provided a 59.6% increase in pull-out strength over the SINO screws and this increase was statistically significant (P < 0.05). The results of our cyclic bending resistance test of the EPSs and SINO screws further revealed that approximately 83.3% of the SINO screws became loosened with a displacement equal to 2.0 mm before the loading cycles reached 800 times while only 16.7% of the EPSs became loosened. Before the loading cycles reached 800 times, the displacement of the pedicles was 1.978 ± 0.053 mm for the SINO screws and 1.274 ± 0.368 mm for the EPSs (Table 2). The difference in displacement between the two types of screws was statistically significant (P < 0.05).
Table 1.
BMD (g cm−2) for lumbar spines before ovariectomy (OVX) and 12 months postoperatively
| No. | 1 | 2 | 3 | 4 | Mean ± SD |
|---|---|---|---|---|---|
| Pre-OVX | 0.907 | 0.934 | 1.017 | 0.945 | 0.951 ± 0.047 |
| Post-OVX | 0.723 | 0.741 | 0.796 | 0.758 | 0.755 ± 0.031 |
| Decrease (%) | 25.4 | 26.0 | 27.8 | 24.7 | 25.98 ± 1.33 |
Table 2.
Cyclic bending resistance of SINO screws and EPSs
| No. | SINO screws | EPSs | ||||
|---|---|---|---|---|---|---|
| Cycle | Displacement (mm) | Load (N) | Cycle | Displacement (mm) | Load (N) | |
| 1 | 800 | 1.868 | 200 | 800 | 1.289 | 200 |
| 2 | 565 | 2 | 150 | 800 | 1.114 | 200 |
| 3 | 570 | 2 | 150 | 800 | 1.012 | 200 |
| 4 | 486 | 2 | 125 | 549 | 2 | 150 |
| 5 | 625 | 2 | 175 | 800 | 1.159 | 200 |
| 6 | 716 | 2 | 200 | 800 | 1.070 | 200 |
| Mean ± SD | 627 ± 113 | 1.978 ± 0.053 | 167 ± 30 | 758 ± 102 | 1.274 ± 0.368* | 191 ± 20 |
* P < 0.05 compared with the SINO group
A three-dimensional rendering of the micro-CT data of the screw–bone interface showed that the trabecular architecture of the expandable portions of the EPSs in the screw–bone interface was denser than that of the anterior portion of the SINO screws, especially within the spiral markings (Fig. 5). The same size of ROI adjacent to the expandable portions of the EPS and the anterior portion of the SINO screws were reconstructed and analyzed using the same thresholds. We found that tissue mineral density (TMD) (480 ± 70 mg cc−1), bone volume fraction (BVF) (56 ± 8%), and trabecular thickness (Tb.Th) (0.36 ± 0.12 mm) of ROIs in the expandable portions of the EPSs were significantly greater than those of the anterior portions of the SINO screws (TMD, 390 ± 30 mg cc−1; BVF, 36 ± 7%; Tb.Th, 0.23 ± 0.04 mm) (P < 0.05) (Table 3). On the other hand, the bone surface/bone volume ratio (BS/BV) (6.1 ± 1.8 mm−1) and trabecular separation (Tb.Sp) (0.25 ± 0.06 mm) were significantly lower in the expandable portions of the EPSs than those of the anterior portions of the SINO screws (BS/BV, 9.0 ± 1.5 mm−1 and Tb.Sp, 0.43 ± 0.11 mm) (P < 0.05). Furthermore, there was no significant difference in trabecular number (Tb.N) and structure model index (SMI) between the EPS group (Tb.N, 1.57 ± 0.24 mm−1 and SMI, 2.1 ± 0.7) and the SINO group (Tb.N, 1.7 ± 0.5 mm−1 and SMI, 2.5 ± 0.7) (P > 0.05).
Fig. 5.
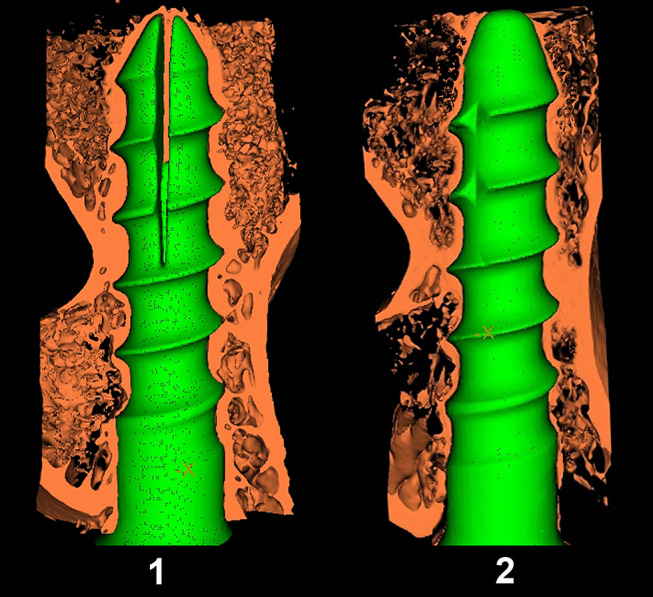
Three-dimensional reconstructed micro-CT image of the bony trabeculae (1/2) with screws in the EPS group (1) and SINO group (2) (Resolution, 21 μm)
Table 3.
ROI parameters of the expandable portion of EPSs and the anterior portion of SINO crews (mean ± SD)
| Parameters | EPS | SINO |
|---|---|---|
| TMD (mg cc−1) | 480 ± 70* | 390 ± 30 |
| BVF (%) | 56 ± 8* | 36 ± 7 |
| Tb.Th (mm) | 0.36 ± 0.12* | 0.23 ± 0.04 |
| BS/BV (mm−1) | 6.1 ± 1.8* | 9.0 ± 1.5 |
| Tb.Sp (mm) | 0.25 ± 0.06* | 0.43 ± 0.11 |
| Tb.N (mm−1) | 1.57 ± 0.24 | 1.7 ± 0.5 |
| SMI (%) | 2.1 ± 0.7 | 2.5 ± 0.7 |
TMD tissue mineral density, BVF bone volume fraction, Tb.Th trabecular thickness, BS/BV bone surface/bone volume ratio, Tb.Sp trabecular separation, Tb.N trabecular number, SMI structure model index
* P < 0.05 compared with the SINO group
Histologically, newly formed bone tissues extended along the expandable fissures and grew into the center of EPS (Fig. 6). Both the newly formed bone and the bone surrounding the interface directly contacted the EPS without any intervening connective tissue layer (Fig. 7). Though there existed significant osteoporosis around the EPS, the newly formed bone as well as the bone in the bone–screw interface tightly contacted the screw and constituted two compartments.
Fig. 6.

Newly formed bone extended into the groove of the anterior fins (Ponceau ×50)
Fig. 7.
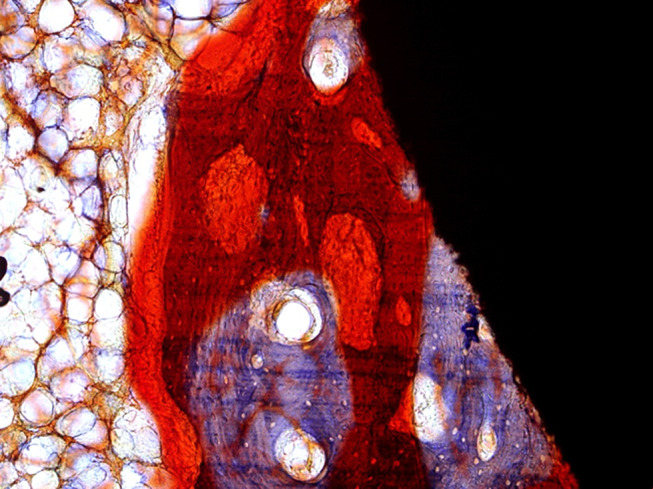
The bone surrounding the interface directly contacted the EPS without any connective tissue layer (Ponceau ×100)
Discussion
Instrumentation with pedicle screws has become a routine method in spinal surgery during the last decade. The stability of the instrumentation depends on the purchase of the pedicle screws in the pedicle and the vertebral body. Despite the fact that satisfactory clinical outcomes have been achieved with pedicle screw instrumentation, loosening of the screws, especially in the osteoporotic spine, still constitutes a significant problem [23, 26] because it can lead to loss of correction or nonunion. To improve the stability of pedicle screw instrumentation, we have designed EPS and demonstrated that the instant fixation strength of the EPS was significantly higher than non-EPS. However, instrumentation needs long-term stability, which depends on the screw–bone interface binding. To the best of our knowledge, there has been no previous study on the histological and micro-architectural appearances of the interface around the EPS, especially in the osteoporotic spine.
Clinically, EPS should be inserted into the vertebra transpedicularly. Sheep spines have been accepted as a reasonable substitute for human spines [15, 19, 22, 31, 32, 35, 37] because their geometric properties are comparable to those of humans. Besides, sheep may be the biggest available animal model for spine surgery. Even with an outer diameter of 6.5 mm, currently existing EPSs are much bigger than the width of the pedicles in sheep. In the current study, we have reduced the outer diameter of the newly designed EPS to 4.5 mm to match the width of the pedicle in sheep.
In our earlier study [13], we evaluated the EPSs using fresh pedicles from calf lumbar vertebrae and compared their biomechanical properties with those of conventional pedicle screws and showed that the turning back torque and pull-out force of the EPS were significantly greater than those of conventional pedicle screws. The use of a pedicle screw that expands radially at the screw tip diameter may improve fixation strength by allowing greater bone contact without a concurrent increase in the diameter or length of pedicle insertion. In this investigation, we found that the EPSs provided an approximately 60% increase in the pull-out strength compared with their paired SINO screws. In the cyclic bending resistance test, the EPSs were found to withstand a greater number of cycles or a greater load with less displacement before loosening (Table 2). These findings together suggest that EPS has better biomechanical properties than SINO screws and may offer a more effective option for surgical treatment of the osteoporotic spine.
Most of earlier studies used histomorphometry as the standard method to investigate the screw–bone interface [3, 33, 36]. Although histomorphometry can show interfacial properties, it has a fundamental limitation: the method is based on two-dimensional analysis and unavoidably loses much spatial information. In addition, histomorphometry cannot accomplish precise quantitative analysis of such parameters as TMD and BMD. Micro-CT makes it an ideal technique to investigate spatial properties at the bone–screw interface and enables three-dimensional quantitative analysis [24]. Studies indicated that micro-CT at a voxel size of 24 μm enabled the detection of bone changes earlier than histomorphometry in a model of osteoporosis in rats killed at different time points [2, 12]. Using micro-CT in the present study, we showed that the EPS had a very good screw–bone contact. Histologically, newly formed bone tissues grew into the groove of the fins. These bone tissues constructed two compartments and wrapped up the pins tightly. As shown by the unique three-dimensional structure, compared with conventional pedicle screws, the bone and the screw grew into each other, which could further improve the pull-out strength of the EPS and lead to greater fixation despite the continued presence of osteoporosis. Our results demonstrated that the parameters in the expandable portions of the EPS, including TMD, BVF, BS/BV, Tb.Th and Tb.Sp, were significantly better than those in the anterior portions of the SINO screws, indicating an improved biomechanical performance of the EPS compared with the SINO screws.
Many biomechanical studies have demonstrated that pedicle screw fixation is highly correlated with BMD [27]. The screw–bone interface is a dynamic structure with differing mechanical characteristics in different portions of the implant surface. In the present study, the fins of the EPS were expanded. The bone tissues surrounding the expandable portions of the EPS were compressed to some extent because of micro-fractures and became denser than before, resulting in augmented fixation strength because of the increased interfacial BMD. According to Wolff’s law [10], bone tissue structure adapts to mechanical load to which it is exposed. It is also believed that changes of bone mass during bone remodeling can be achieved by changing bone density or geometry. The sustained pressure exerted by the expanded fins could act as regulators of bone remodeling, leading to increased interfacial BMD. As a result, the microstructure surrounding the expandable portions of the EPS was significantly better than that in the anterior portion of the SINO screw. Thus, the mechanical stability of EPS was maintained and the risks of loosening were effectively decreased.
The trabecular bone surrounding the expandable portions of the EPS was compressed to some extent and, consequently, became denser than it was before screw expansion. This mechanical interaction between the EPS and the trabecular bone is expected to markedly increase the fixation strength at the screw–bone interface [5, 6]. The expansion of the EPS should also improve fixation strength by allowing greater bone contact at the anterior without an increase in pedicle insertion diameter or screw length. Furthermore, newly formed bone tissues grew into the groove of the fins. These bone tissues constructed two compartments and increased the contact area of screw–bone interface. After insertion of screw, the screw–bone interface experienced a healing process of micro-fracture [3]. Osseointegration took place between the grooves of the fins in the screw–bone interface, effectively increasing the area of the osseointegration over that of SINO screw. We found that the EPSs provided a 59.6% increase in the pull-out strength over the SINO screws. In the cyclic bending resistance test, the EPSs were found to withstand a greater number of cycles with less displacement before loosening. In sum, the biomechanical and histological properties of stabilization of the EPS are significantly better than that of SINO screw.
The limitation of this study was the use of only four sheep. Though the number of specimens in biomechanical tests was less, they were sufficient for statistical comparison between the EPSs and SINO screws. In addition, according to Wolff’s law, it is believed that bone tissue structure adapted to mechanical load the pressure for which is provided by the expanded fins. This study does not find out how much the BMD increased acutely and chronically and how long the bone remodeling continued along the trajectory of EPS. However, further in vivo studies should be performed.
Conclusion
In summary, we show here that EPS could provide significant stabilization in the osteoporotic spine because of its superior biomechanical properties. Because of its increased fixation strength, EPSs may prevent screw loosening in patients with osteoporosis by providing early achievement and maintenance of stability between the pedicle screw and the bone. Therefore, EPS may be of value in treating patients with osteoporosis and warrants further clinical studies.
Contributor Information
Shiyong Wan, Email: wanshiyong2009@yahoo.com.cn.
Wei Lei, Phone: +86-29-84771011, FAX: +86-29-84771011, Email: wsyydwyt@yahoo.com.cn.
References
- 1.Bai B, Kummer FJ, Spivak J. Augmentation of anterior vertebral body screw fixation by an injectable, biodegradable calcium phosphate bone substitute. Spine (Phila Pa 1976) 2001;26:2679–2683. doi: 10.1097/00007632-200112150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Barbier A, Martel C, de Vernejoul MC, Tirode F, Nys M, Mocaer G, Morieux C, Murakami H, Lacheretz F. The visualization and evaluation of bone architecture in the rat using three-dimensional X-ray microcomputed tomography. J Bone Miner Metab. 1999;17:37–44. doi: 10.1007/s007740050061. [DOI] [PubMed] [Google Scholar]
- 3.Branemark R, Ohrnell LO, Nilsson P, Thomsen P. Biomechanical characterization of osseointegration during healing: an experimental in vivo study in the rat. Biomaterials. 1997;18:969–978. doi: 10.1016/S0142-9612(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 4.Becker S, Chavanne A, Spitaler R, Kropik K, Aigner N, Ogon M, Redl H. Assessment of different screw augmentation techniques and screw designs in osteoporotic spines. Eur Spine J. 2008;17:1462–1469. doi: 10.1007/s00586-008-0769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SD, Barbera J, Rubi M, Salkeld SL, Whitecloud TS., III Lumbosacral fixation using expandable pedicle screws: an alternative in reoperation and osteoporosis. Spine J. 2001;1:109–114. doi: 10.1016/S1529-9430(01)00020-1. [DOI] [PubMed] [Google Scholar]
- 6.Cook SD, Salkeld SL, Whitecloud TS, III, Barbera J. Biomechanical evaluation and preliminary clinical experience with an expansive pedicle screw design. J Spinal Disord. 2000;13:230–236. doi: 10.1097/00002517-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Fransen P. Increasing pedicle screw anchoring in the osteoporotic spine by cement injection through the implant. Technical note and report of three cases. J Neurosurg Spine. 2007;7:366–369. doi: 10.3171/SPI-07/09/366. [DOI] [PubMed] [Google Scholar]
- 8.Gaines RW., Jr The use of pedicle-screw internal fixation for the operative treatment of spinal disorders. J Bone Joint Surg Am. 2000;82A:1458–1476. doi: 10.2106/00004623-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Hirano T, Hasegawa K, Washio T, Hara T, Takahashi H. Fracture risk during pedicle screw insertion in osteoporotic spine. J Spinal Disord. 1998;11:493–497. doi: 10.1097/00002517-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Jovanović JD, Jovanović MLJ. Biomechanical model of vertebra based on bone remodeling. Med Biol. 2004;11:35–39. [Google Scholar]
- 11.Kwok AW, Finkelstein JA, Woodside T, Hearn TC, Hu RW. Insertional torque and pull-out strengths of conical and cylindrical pedicle screws in cadaveric bone. Spine. 1996;21:2429–2434. doi: 10.1097/00007632-199611010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Laib A, Barou O, Vico L, Lafage-Proust MH, Alexandre C, Rugsegger P. 3D micro-computed tomography of trabecular and cortical bone architecture with application to a rat model of immobilisation osteoporosis. Med Biol Eng Comput. 2000;38:326–332. doi: 10.1007/BF02347054. [DOI] [PubMed] [Google Scholar]
- 13.Lei W, Wu ZX. Biomechanical evaluation of an expansive pedicle screw in calf vertebrae. Eur Spine J. 2006;15:321–326. doi: 10.1007/s00586-004-0867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung KS, Siu WS, Cheung NM, Lui PY, Chow DH, James A, Qin L. Goats as an osteopenic animal model. J Bone Miner Res. 2001;16:2348–2355. doi: 10.1359/jbmr.2001.16.12.2348. [DOI] [PubMed] [Google Scholar]
- 15.Lill CA, Fluegel AK, Schneider E. Sheep model for fracture treatment in osteoporotic bone: a pilot study about different induction regimens. J Orthop Trauma. 2000;14:559–565. doi: 10.1097/00005131-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lill CA, Fluegel AK, Schneider E. Effect of ovariectomy, malnutrition and glucocorticoid application on bone properties in sheep: a pilot study. Osteoporos Int. 2002;13:480–486. doi: 10.1007/s001980200058. [DOI] [PubMed] [Google Scholar]
- 17.Lonstein JE, Denis F, Perra JH, Pinto MR, Smith MD, Winter RB. Complications associated with pedicle screws. J Bone Joint Surg Am. 1999;81:1519–1528. doi: 10.2106/00004623-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Lotz JC, Hu SS, Chiu DF, Yu M, Colliou O, Poser RD. Carbonated apatite cement augmentation of pedicle screw fixation in the lumbar spine. Spine. 1997;22:2716–2723. doi: 10.1097/00007632-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 19.MacLeay JM, Olson JD, Enns RM, Les CM, Toth CA, Wheeler DL, Turner AS. Dietary-induced metabolic acidosis decreases bone mineral density in mature ovariectomized ewes. Calcif Tissue Int. 2004;75:431–437. doi: 10.1007/s00223-004-0217-7. [DOI] [PubMed] [Google Scholar]
- 20.Milcan A, Ayan I, Zeren A, Sinmazcelik T, Yilmaz A, Zeren M, Kuyurtar F. Evaluation of cyanoacrylate augmentation of transpedicular screw pullout strength. J Spinal Disord Tech. 2005;18:511–514. doi: 10.1097/01.bsd.0000143311.70185.23. [DOI] [PubMed] [Google Scholar]
- 21.Mummaneni PV, Haddock SM, Liebschner MA, Keaveny TM, Rosenberg WS. Biomechanical evaluation of a double-threaded pedicle screw in elderly vertebrae. J Spinal Disord Tech. 2002;15:64–68. doi: 10.1097/00024720-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16:277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 23.Ohlin A, Karlsson M, Düppe H, Hasserius R, Redlund-Johnell I. Complications after transpedicular stabilization of the spine. A survivorship analysis of 163 cases. Spine (Phila Pa 1976) 1994;19:2774–2779. doi: 10.1097/00007632-199412150-00007. [DOI] [PubMed] [Google Scholar]
- 24.Patel V, Issever AS, Burghardt A, Laib A, Ries M, Majumdar S. Micro CT evaluation of normal and osteoarthritic bone structure in human knee specimens. J Orthop Res. 2003;21:6–13. doi: 10.1016/S0736-0266(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer BA, Krag MH, Johnson C. Repair of failed transpedicle screw fixation. A biomechanical study comparing polymethylmethacrylate, milled bone, and matchstick bone reconstruction. Spine (Phila Pa 1976) 1994;19:350–353. doi: 10.1097/00007632-199402000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Pihlajämaki H, Myllynen P, Böstman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. J Bone Joint Surg Br. 1997;79:183–189. doi: 10.1302/0301-620X.79B2.7224. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold M, Schwieger K, Goldhahn J, Linke B, Knop C, Blauth M. Influence of screw positioning in a new anterior spine fixator on implant loosening in osteoporotic vertebrae. Spine (Phila Pa 1976) 2006;31:406–413. doi: 10.1097/01.brs.0000199894.63450.70. [DOI] [PubMed] [Google Scholar]
- 28.Renner SM, Lim TH, Kim WJ, Katolik L, An HS, Andersson GB. Augmentation of pedicle screw fixation strength using an injectable calcium phosphate cement as a function of injection timing and method. Spine (Phila Pa 1976) 2004;29:E212–E216. doi: 10.1097/00007632-200406010-00020. [DOI] [PubMed] [Google Scholar]
- 29.Richter M, Wilke HJ, Kluger P, Neller S, Claes L, Puhl W. Biomechanical evaluation of a new modular rod-screw implant system for posterior instrumentation of the occipito-cervical spine: in vitro comparison with two established implant systems. Eur Spine J. 2000;9:417–425. doi: 10.1007/s005860000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohmiller MT, Schwalm D, Glattes RC, Elalayli TG, Spengler DM. Evaluation of calcium sulfate paste for augmentation of lumbar pedicle screw pullout strength. Spine J. 2002;2:255–260. doi: 10.1016/S1529-9430(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 31.Turner AS, Alvis M, Myers W, Stevens ML, Lundy MW. Changes in bone mineral density and bone-specific alkaline phosphatase in ovariectomized ewes. Bone. 1995;17:395S–402S. doi: 10.1016/8756-3282(95)00148-7. [DOI] [PubMed] [Google Scholar]
- 32.Turner AS. The sheep as a model for osteoporosis in humans. Vet J. 2002;163:232–239. doi: 10.1053/tvjl.2001.0642. [DOI] [PubMed] [Google Scholar]
- 33.Viljanen JT, Pihlajamäki HK, Törmälä PO, Rokkanen PU. Comparison of the tissue response to absorbable self-reinforced polylactide screws and metallic screws in the fixation of cancellous bone osteotomies: an experimental study on the rabbit distal femur. J Orthop Res. 1997;15:398–407. doi: 10.1002/jor.1100150312. [DOI] [PubMed] [Google Scholar]
- 34.Wilkes RA, Mackinnon JG, Thomas WG. Neurological deterioration after cement injection into a vertebral body. J Bone Joint Surg Br. 1994;76:155. [PubMed] [Google Scholar]
- 35.Wu ZX, Lei W, Hu YY, Wang HQ, Wan SY, Ma ZS, Sang HX, Fu SC, Han YS. Effect of ovariectomy on BMD, micro-architecture and biomechanics of cortical and cancellous bones in a sheep model. Med Eng Phys. 2008;30:1112–1118. doi: 10.1016/j.medengphy.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki M, Shirota T, Tokugawa Y, Motohashi M, Ohno K, Michi K, Yamaguchi A. Bone reactions to titanium screw implants in ovariectomized animals. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:411–418. doi: 10.1016/S1079-2104(99)70239-8. [DOI] [PubMed] [Google Scholar]
- 37.Zarrinkalam MR, Beard H, Schultz CG, Moore RJ. Validation of the sheep as a large animal model for the study of vertebral osteoporosis. Eur Spine J. 2009;J18:244–253. doi: 10.1007/s00586-008-0813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


