Abstract
Piriformis syndrome, sciatica caused by compression of the sciatic nerve by the piriformis muscle, has been described for over 70 years; yet, it remains controversial. The literature consists mainly of case series and narrative reviews. The objectives of the study were: first, to make the best use of existing evidence to estimate the frequencies of clinical features in patients reported to have PS; second, to identify future research questions. A systematic review was conducted of any study type that reported extractable data relevant to diagnosis. The search included all studies up to 1 March 2008 in four databases: AMED, CINAHL, Embase and Medline. Screening, data extraction and analysis were all performed independently by two reviewers. A total of 55 studies were included: 51 individual and 3 aggregated data studies, and 1 combined study. The most common features found were: buttock pain, external tenderness over the greater sciatic notch, aggravation of the pain through sitting and augmentation of the pain with manoeuvres that increase piriformis muscle tension. Future research could start with comparing the frequencies of these features in sciatica patients with and without disc herniation or spinal stenosis.
Keywords: Piriformis, Sciatica, Diagnosis, Systematic review
Introduction
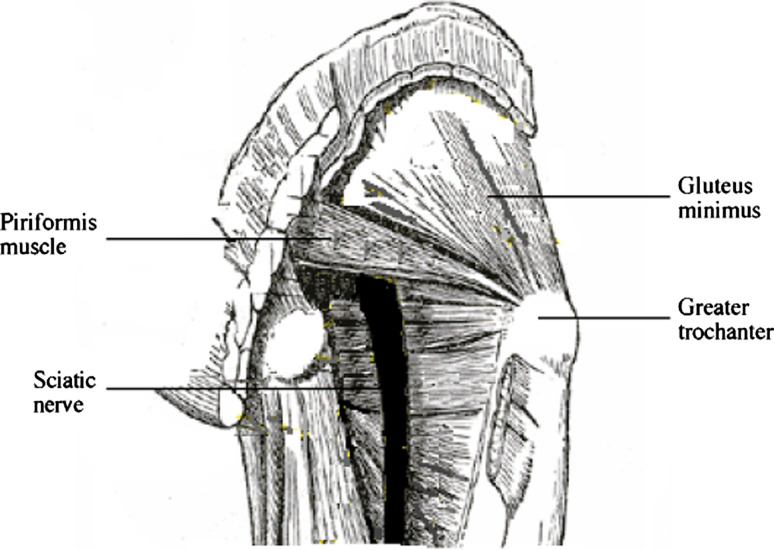
Sciatica is musculoskeletal pain felt in the leg [29] along the distribution of the sciatic nerve and sometimes accompanied by low back pain. Following Mixter and Barr’s work correlating clinical features with operative and histological findings [75], the dominant opinion for decades on the cause of sciatica was nerve root compression by a herniated intervertebral disc (HIVD) [117]. An alternative cause, compression of the nerve trunk by the piriformis muscle (PM), was proposed by Freiberg and Vinke [42] and developed by Robinson [91], who is credited with coining the term piriformis syndrome (PS). The relations between the PM and the sciatic nerve are described in Appendix 1 and illustrated in Fig. 1. Sciatica can arise from other sites too: the lumbar canal (through stenosis), the pelvis (without PM involvement) and along the extra-pelvic journey of the nerve [2].
Fig. 1.
Diagram showing the relations of PM to the sciatic nerve
The existence of PS remains controversial. Only 21 out of 29 physical medicine and rehabilitation specialists surveyed in the USA believed that the condition exists [96]. It has been argued that the syndrome is overdiagnosed [105] and underdiagnosed [30,39]. Fishman et al. [37] attempted to set an operational definition of PS by demonstrating objective electromyography (EMG) findings with symptoms. They found a delay in the H reflex on EMG in the FAIR position (described below) in patients with PS compared to asymptomatic controls. An impressively large number of patients, 918, were studied. However, the study did not establish the accuracy of the H reflex because it lacked symptomatic controls (patients with sciatica, but not PS). Fishman et al. also claimed that response to conservative therapy was greater in patients with a positive test, but scrutiny of their results shows that they did not reach statistical significance. Furthermore, the study design and report did not meet the STARD criteria for a study of diagnostic accuracy [9]. Filler claimed that “large scale formal class A study design” publications, one of which was the study by Fishman et al., had proven the existence of the syndrome [36]. Another of these studies was the one by Filler et al. [36] in which patients with sciatica who responded to local anaesthetic and steroid injections into the PM were classed as confirmed muscle-based PS and those who improved with surgery as surgically confirmed muscle-based PS. MRI neurography in confirmed and surgically confirmed PS patients was reported to have an important predictive value. However, neither of these two studies was actually “class A”. By class A, Filler meant “grade A” studies described by Kent et al. [59]. Both the Fishman and Filler studies failed to meet two of the criteria for grade A studies: that all clinical features be described and that an adequate reference standard be used. Indeed, there is no accepted investigation that can act as the reference standard for PS. Candidates for the role include:
nerve conduction studies (NCS) with the hip flexed, abducted and internally rotated, termed FAIR [38, 97];
NCS with magnetic stimulation [17];
variations on magnetic resonance imaging such as neurography to enhance images of the sciatic nerve [36,68].
However, the validity of these techniques has been vigorously disputed [111]. Hulbert and Doyle [50] and Kirschner et al. [60] have summarised and compared the investigations available.
Proposed mechanisms for PS include: contracture or spasm of the PM from trauma [42,91]; predisposition to nerve compression by congenital variations of the sciatic nerve or PM, in which the sciatic nerve or its divisions pass through the belly or tendinous portions of a normal muscle or the bellies of a bifid muscle [20, 85, 95]; overuse and hypertrophy [10, 21, 84, 112]. Some authors use PS for any sciatica arising from nerve trunk compression, regardless of PM involvement, for example by osteophytes [66], haematomas [104], pseudo-aneurysms [73, 82], endometriotic cysts in the pelvis [28] and prolonged external pressure [23]. Fractures of the femoral neck and of the ischial tuberosity and hip arthroplasty too can cause sciatica [74,123]. Such cases were called secondary PS by Foster [40] and pseudosciatica by others [94].
A systematic review of general population surveys of sciatica found a lifetime prevalence of 12.2–27%, annual 2.2–19.5% and point 1.6–4.8% [62]. The proportion due to HIVD remains uncertain. In a series of 160 sciatica patients, only 131 (82%) had a corresponding HIVD on MRI [58]. Estimates of the ratio of PS to disc herniation come from secondary or tertiary care and vary according to the definitions and selection methods used: <1% [8], 6% [79] and approximately 15% [6]. Those based on contemporaneous coding of diagnoses [8] are less open to selection bias than those based on retrospective reviews of records [6] or recall [79].
Signs specific to PS that have been reported include tenderness of the PM found on external palpation over the greater sciatic notch or on internal palpation per vagina or rectum [30, 91, 120] and tonic external rotation of the hip [99]. Several tests are said to reproduce sciatica by augmenting PM tension, either by passively stretching the muscle, the Freiberg [42] and FAIR tests [121], or by resisted muscle contraction, the Pace [79] and Beatty tests [4] (Table 1).
Table 1.
Specific tests for sciatica
| Name of test | Date first described | Description | Attributed to |
|---|---|---|---|
| Freiberg | 1934 | Passive internal rotation of the hip in extension reproduces pain | Freiberg and Vinke [42] |
| Pace | 1976 | The clinician provides resistance to hip abduction by holding the sitting patient’s knee; reproduces pain | Pace and Nagle [79] |
| Tonic external rotation of hip | 1981 | Visible sign in patient at rest | Solheim [99] |
| FAIR = flexion, abduction and internal rotation of the hip | 1981 | Maintaining the hip in flexion abduction and internal rotation reproduces pain | Solheim [99] |
| Beatty | 1994 | The patient holds the flexed hip in abduction against gravity whilst lying on the unaffected side; reproduces pain | Beatty [4] |
Existing reviews
The literature on PS consists largely of reviews and case studies. Most reviews of PS have been either narrative reviews [13,46,80,88,89,92], sometimes with illustrative case reports [79,120], or case studies accompanied by a review to place them in context.
Silver and Leadbetter [96], identifying 26 cases in 12 studies [1,3,4,11,19,43,53,56,81,95,116,120], calculated frequencies for only three clinical features: ‘neurologic deficit’, the Freiberg sign and the Pace sign. The only systematic review of PS available at the time of our search was confined to non-surgical interventions [26]. Its two trials with positive outcomes were excluded from our review because they did not describe the clinical features sufficiently. Two more reviews have been published since. Hulbert and Deyle highlighted the paucity of evidence for differential diagnosis and treatment [50], but did not identify specific research questions. Kirschner et al. [60] provided a narrative review of botulinum toxin therapy.
Research has reached an impasse. Controlled trials of therapy are unlikely to proceed until two conditions are met: a sufficiently high prevalence and a reliable method of diagnosis. Research into prevalence cannot proceed without established diagnostic features. Studies of diagnostic accuracy cannot proceed without a systematic description of the syndrome and a reference standard. Therefore, much research is needed with several study types to evaluate PS. A better understanding of the purported clinical features of PS is the first step. A systematic review of the clinical features of PS is such a step.
Knowledge gained from case studies has limitations. Generalising from particular cases has its dangers and the absence of a comparison group prevents hypothesis testing. Those who promote the concept of levels of evidence allocate case studies next to the bottom level in the hierarchy of evidence [15]. However, case studies have important roles [115]. Discovery begins with finding the unexpected and the stimulation of further research [115]. Evidence of cases and their occurrence is needed before evidence of aetiology or treatment effectiveness can be established [54]. Case reporting can, therefore, lead to more advanced research.
Case studies provide suitable material for systematic reviews, both of the descriptive type [32–35] and meta-analysis. Meta-analyses have covered intervention [25], complication rates of surgery [70, 87,119] and the characteristics and prognosis of tumours [98].
Aims
We had two aims. The first aim was to make the best use of existing evidence to estimate the frequencies of clinical features in patients reported to have PS. Our main research question was, in cases of PS reported in literature, what was the frequency of the symptoms, signs specific to PS and signs looked for in sciatica in general? The second aim was to identify future research questions. We used any study types that reported data relevant to diagnosis.
Methods
The methods were in accord with the PRISMA statement on the conduct of systematic reviews [69].
Search
The search included all studies up to 1 March 2008. The Thomson Dialog NHS facility was used to search four databases: Allied and Complementary Medicine (AMED), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase and Medline. The following search strings were used:
#1 (PIRIFORMIS OR PYRIFORMIS) ADJ SYNDROME.TI, AB
#2 (PIRIFORMIS OR PYRIFORMIS) AND SCIATIC$.TI, AB
#3 1 OR 2
(ADJ = adjacent. The Thomson Dialog NHS facility is no longer available). Additional studies were sought from the references of all retrieved articles.
Inclusion/exclusion
All titles and abstracts were screened independently by two reviewers. Studies were excluded if: they were not about PS; the language was not English, French, Chinese or Spanish; the publication was not a print or Internet biomedical journal; the condition was a complication of hip surgery or fracture. When a disagreement occurred, the report was retrieved.
Retrieved full text articles were screened independently by two reviewers. Studies were included if they satisfied all three criteria: first, the study had to be a case studies report, a narrative review including a case studies report, a study of diagnostic test accuracy or a study of a therapeutic intervention that described clinical features; second, the cases matched the study definition of PS; third, clinical features were described sufficiently for data extraction.
Data extraction
Data was extracted independently by two reviewers. Studies were divided into ‘individual data studies’ (case reports and case series reporting data for each patient) and ‘aggregated data studies’ (case series reporting data aggregated for all patients). Articles were scrutinised for pre-specified features (Appendix 2) chosen from prior knowledge of literature. Two more features (tonic external rotation and tenderness on rectal examination) were added after reading retrieved articles. Several reports of PS have differentiated between buttock pain and low back pain [1,3,4,47,61,77,95,100,112]. Recent European guidelines define low back pain as localised below the ribs and above the inferior gluteal folds [114], which includes the buttock. For this review, we used only those terms in the PS literature, namely, sciatica for pain felt in the leg, buttock pain for pain felt in the buttock itself, and low back pain to mean pain felt in the back but above the buttock.
The rules for data extraction were:
Features stated as present or absent were recorded as positive or negative, respectively.
If the absence of a feature was not explicitly stated, it was recorded as ‘not reported’.
Ambiguous reports, arising from vague or summary phrases, for example, ‘no signs of radiculopathy’, were recorded as ‘uncertain’.
Analysis: individual data studies
Corroboration
The absence of a reference standard test means uncertainty over whether cases truly represent the condition. However, certainty is based on a continuum. Some authors proffer evidence to support their diagnosis, such as response to surgery after a long duration of pain. Such evidence cannot be accepted uncritically, but should be weighed and judged like all evidence. Evidence that supports a plausible cause and effect has been termed as corroborating evidence, without implying an incontrovertible case definition. Any potential corroborating evidence was recorded as free text comment. All comments were then scrutinised and compared to create categories of corroborating evidence.
Calculating frequencies
Choosing the denominator to calculate frequencies from case studies is problematic. Brief clinical records are not written with future publications in mind [54]. The non-reporting of a feature could mean several things: the feature was not sought; the feature was sought, but not recorded; the feature was sought and recorded but not reported. A denominator that includes all cases might underestimate the frequency if a feature had been present but not sought. A denominator that is confined only to studies where a feature was reported, as present or absent, might overestimate the frequency if the authors, believing it to be pathognomonic, selectively report positive cases.
Another potential source of bias is the use of a clinical feature as a criterion for patient selection. This would tend to return a 100% frequency for the feature. Evidence for this bias was found in the aggregated data studies included in the review. Evidence was also found in two individual data studies, but both were excluded on other grounds [7,97]. We decided to calculate frequencies in four ways: all cases; only corroborated cases; only reported cases (i.e. feature explicitly reported as present or absent); only corroborated and reported cases. A feature recorded as uncertain was treated as absent in the analysis. Denominators were calculated using only the sample relevant to a feature: only women for dyspareunia and only cases published after the first description of PS-specific tests (Table 1).
Numerators were calculated by adding the number of patients with positive features. Since the point estimates of percentages were often close to 100, 95% confidence intervals were calculated by first transforming percentages to log odds [106].
Analysis: aggregated data studies
Frequencies for diagnostic features were calculated for each study with the intention to pool data if appropriate.
Analysis: quality assessments
There is no accepted tool for evaluating the quality of case reports in diagnosis. Neither the Centre for Reviews and Dissemination’s guidance on systematic reviews [16] nor the US Agency for Healthcare Research and Quality’s review of rating systems [118] mention case studies. The only grading system discovered was one specific to therapy [122]. We therefore adapted recommendations for case study reports from several sources [14,52,54,109]. Our tool for assessing quality was specific to case reports of PS and diagnosis taking account of (1) completeness of reporting and (2) minimisation of bias (Appendix 3).
Age and sex are vital data. We included the basic components of a pain history (Appendix 3). Studies were categorised according to the number of items reported in the history: good, if two or fewer items were missing; satisfactory, if three or four items were missing; and poor if more than four were missing. For case series, the poorest report was used to categorise the study. History reporting was so poor in aggregated data studies that categorisation was not attempted. Two sets of examinations can reasonably be expected: routine tests for sciatica, such as limited straight leg raising (SLR); and specific tests for PS. The number of routine tests in each study was counted. For case series, the case with the lowest number of reports was taken to represent the study. For PS-specific tests, the presence of at least one specific sign was sought rather than the number because they have changed over time. For case series, it is important that the method of selecting cases be described to minimise bias, for example, by including consecutive cases. We decided against assigning quality scores to perform sensitivity analysis because of the overlap of certain items in the quality assessment and the calculation of frequencies. Two reviewers independently assessed study quality.
Search results
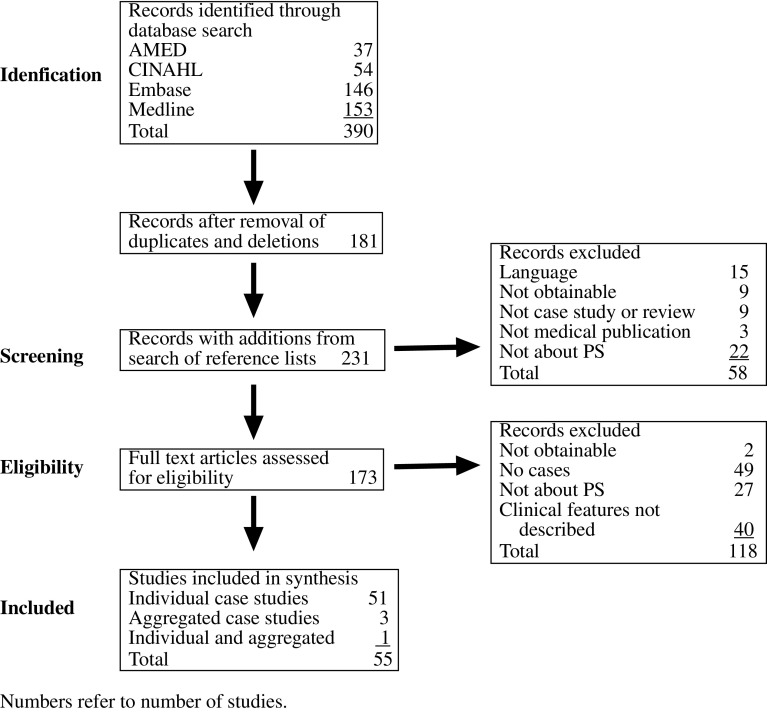
The flow of records is shown in Fig. 2. Studies entered into the synthesis comprised 51 individual data studies (Table 2), 3 aggregated data studies [30,51,71] and 1 combined [30]. Of the individual data studies, 31 were case reports (single case) and 24 case series (two or more cases).
Fig. 2.
Flow of records
Table 2.
Included studies with individual data
| Study: first author and year (language if not English) | No. in study | No. included in review | No. of routine sciatica signs reported | If signs specific to PS reported | Selection method |
|---|---|---|---|---|---|
| Adams 1980 [1] | 4 | 4 | 4 | Yes | Not described |
| Barton 1991 [3] | 4 | 4 | Uncertain | Yes | Not described |
| Beatty 1994 [4] | 3 | 3 | 0 | Yes | Not described |
| Beauchesne 1997 [5] | 1 | 1 | 4 | No | Not applicable |
| Brown 1988 [11] | 1 | 1 | 3 | Yes | Not applicable |
| Bustamante 2001 [12] | 2 | 1 | 3 | Yes | Not described |
| Chantraine 1990 (French) [18] | 2 | 1 | 0 | Yes | Not described |
| Chen and Wan 1992 [21] | 2 | 2 | 4 | Yes | Not applicable |
| Chen 1992 [19] | 1 | 1 | 4 | Yes | Not applicable |
| Chen 1994 [20] | 1 | 1 | 4 | Yes | Not applicable |
| Chong 2004 [22] | 1 | 1 | 3 | Yes | Not applicable |
| Colmegna 2007 [24] | 1 | 1 | 1 | Yes | Not applicable |
| Dalmau 2005 [27] | 1 | 1 | 0 | Yes | Not applicable |
| Durrani and Winnie 1991 [30] | 1 | 1 | 4 | Yes | Not applicable |
| El-Rubaidi 2003 (Spanish) [31] | 1 | 1 | 2 | No | Not described |
| Foster 2002 [41] | 7 | 7 | 0 | Yes | Not described |
| Freiberg 1937 [43] | 2 | 2 | 1 | Yes | Not described |
| Gandhavadi 1990 [44] | 1 | 1 | 1 | Yes | Not applicable |
| Guyomarc’h 2004 (French) [45] | 3 | 3 | Uncertain | Yes | Not described |
| Hanania 1998 [47] | 6 | 6 | 0 | No | Not described |
| Hopayian 1999 [48] | 3 | 1 | 2 | Yes | Not described |
| Hughes 1992 [49] | 5 | 5 | 1 | Yes | Not applicable |
| Jankiewicz 1991 [53] | 1 | 1 | 1 | Yes | Not applicable |
| Jroundi 2003 (French) [55] | 1 | 1 | 0 | Yes | Not applicable |
| Julsrud 1989 [56] | 1 | 1 | Uncertain | Yes | Not applicable |
| Karl 1985 [57] | 1 | 1 | 1 | Yes | Not described |
| Kobbe 2008 [61] | 2 | 2 | 1 | Yes | Not described |
| Kosukegawa 2006 [63] | 1 | 1 | 4 | No | Not applicable |
| Kouvalchouk 1996 (French) [64] | 4 | 4 | Uncertain | Yes | Not described |
| Ku 1995 [65] | 1 | 1 | 4 | Yes | Not applicable |
| Lee 2004 [67] | 1 | 1 | Uncertain | Yes | Not applicable |
| Lewis 2006 [68] | 14 | 14 | 3 | No | Not described |
| Mayrand 2006 [72] | 1 | 1 | 3 | Yes | Not applicable |
| Molina 2003 [76] | 1 | 1 | 4 | Yes | Not applicable |
| Nakamura 2003 [77] | 2 | 2 | 0 | Yes | Not described |
| Ozaki [78] | 1 | 1 | 4 | Yes | Not applicable |
| Papadopoulos 1990 [81] | 1 | 1 | 4 | Yes | Not applicable |
| Park 1991 [83] | 1 | 1 | 3 | Yes | Not applicable |
| Richardson 1992 [90] | 1 | 1 | 1 | Yes | Not applicable |
| Robinson 1947 [91] | 2 | 2 | 4 | Yes | Not applicable |
| Rossi 2001 [93] | 1 | 1 | 1 | Yes | Not described |
| Sayson 1994 [95] | 1 | 1 | 3 | Yes | Not applicable |
| Solheim 1981 [99] | 2 | 2 | 2 | Yes | Not applicable |
| Spinner 2001 [100] | 1 | 1 | 3 | Yes | Not described |
| Stegbauer 1997 [101] | 1 | 1 | 4 | Yes | Not applicable |
| Stein 1983 [102] | 2 | 1 | 4 | Yes | Not described |
| Synek 1987 [108] | 1 | 1 | 3 | Yes | Not applicable |
| Synek 1987 [107] | 4 | 1 | 4 | No | Not described |
| Turtas 2006 [112] | 1 | 1 | 3 | Yes | Not applicable |
| Vallejo 2004 [113] | 1 | 1 | 1 | Yes | Not applicable |
| Vandertop 1991 [116] | 1 | 1 | 4 | Yes | Not applicable |
| Wyant 1979 [120] | 2 | 2 | 4 | Yes | Not applicable |
Results: quality assessments
Individual data studies
All studies met the criteria of reporting age and sex. The quality of history reporting was good in only 24 studies (Table 3). Commonly missed items were onset of pain, past medical history and evolution of the symptoms. Table 3 also shows that reporting of signs was incomplete with only 40 studies reporting both routine sciatica and PS-specific signs. It is surprising that six studies did not report a single sign specific to PS, despite reporting purported cases. The maximum quality achievable was a good history and a report of both sets of signs. Only 22 studies achieved this.
Table 3.
Summary of history and reported signs in studies with individual data
| Signs | Historya | ||
|---|---|---|---|
| Poor | Satisfactory | Good | |
| None | 1 | 0 | 0 |
| Routine sciatica signs only | 2 | 1 | 2 |
| PS signs only | 2 | 4 | 0 |
| Sciatica and PS signs | 8 | 10 | 22 |
aThe quality of history is graded according to the number of items missing in the report: good ≤2; satisfactory = 3 or 4; poor ≥5. The overall quality is represented by values (history and signs) ranging from poor to maximum achievable (shown in underline, italic, bold, bold italic)
Selection
Of the 20 case series, only 1 reported its inclusion criteria [68]. It described a retrospective study of the records of patients with a mismatch between spinal MRI and their clinical condition referred for MRI neurography, but failed to report how they were selected from such referrals.
Studies with aggregated patient data
Many items in history and examination were missed (Table 4). Only Durrani and Winnie reported how patients were selected, how data were collected, the sex distribution, the mean age and age range and several features [30]. It was a prospective study of consecutive cases seen in a single clinic. Lu et al. [71] reported only the range of ages and Indrekvam and Sudmann [51] reported only the mean age.
Table 4.
Clinical features in aggregated data studies, number (%)
| Study: first author and year (language if other than English) | Lu et a 1985 (Chinese) [71] | Durrani and Winnie 91 [30] | Indrekvam and Sudmann 02 [51] | Filler et al. 2005 [36] |
|---|---|---|---|---|
| No. of cases | 60 | 26 | 19 | 162 |
| Female | 21 (35) | 11 (42) | 15 (79) | Not reported |
| Age range | 17–70 | 25–62 | Not reported | Not reported |
| Age mean | Not reported | 35.5 | 43 | Not reported |
| Buttock pain | 60 (100) | 26 (100) | 19 (100) | (100) |
| Low back pain | Not reported | 13 (50) | Not reported | (42.4) |
| Pain on sitting | Not reported | 15 (58) | Not reported | Not reported |
| Dyspareunia | Not reported | 6 (23) | Not reported | Not reported |
| External tenderness | 54 (90) | 24 (92) | 19 (100) | (70.8) |
| Internal tenderness | Not reported | 26 (100) | Not reported | Not reported |
| Freiberg sign positive | 60 (100) | 9 (35) | 19 (100) | Not reported |
| Pace sign positive | Not reported | 8 (31) | 19 (100) | Not reported |
| Beatty sign positive | Not reported | Not reported | Not reported | Not reported |
| Tonic external rotation of hip | Not reported | 10 (38) | Not reported | Not reported |
| FAIR sign positive | Not reported | Not reported | Not reported | Not reported |
| SLR limited | Not reported | 12 (46) | 5 (23) | (40.7) |
| Reflexes diminished | Not reported | Not reported | Not reported | Not reported |
| Sensation diminished | 24 (40) | Not reported | 10 (53) | Not reported |
| Power diminished | Not reported | Not reported | 3 (16) | Not reported |
Filler et al. [36] recruited from 239 patients with either failed disc surgery or no diagnosis after imaging, selecting those who obtained relief from MRI-guided injection of steroid and local anaesthetic into the PM. They did not describe the sex and age distribution of the selected cases and reported only a few features.
All studies reported at least one sign specific to PS and one sign in the routine examination for sciatica.
Results: frequencies
Data were usable from a total of 126 patients, 100 in individual data studies and 26 from Durrani and Winnie [30].
Individual data studies
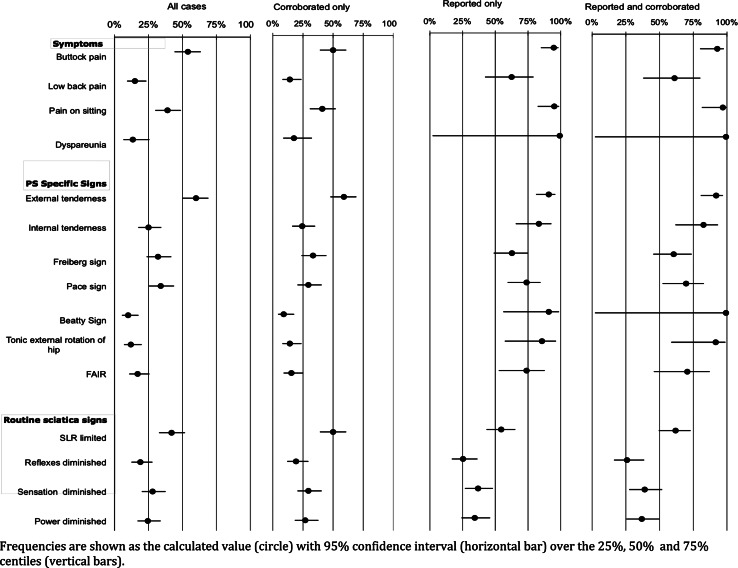
There were 52 women and 48 men with a mean age of 43 years (95% CI 14, 72). Figure 3 shows the frequencies of the clinical features (with 95% CI) for each of the four denominators. Frequencies calculated from all cases (first plot on left) and corroborated cases (second plot from left) were similar (Fig. 3). However, frequencies calculated from reported studies (third plot from the left) were higher than in all studies and corroborated studies. Frequencies calculated from reported studies and reported corroborated studies (plot on furthest right) were similar. Corroboration made little difference to frequency estimates, whereas reporting made a big difference.
Fig. 3.
Frequencies of clinical features from individual data studies
Symptoms
Buttock pain was common and more common than low back pain for all denominators used. The estimates for buttock pain ranged from 50% (corroborated) to 95% (reported) and for low back pain from 14% (corroborated) to 63% (reported). Aggravation of sciatica through sitting was as common as buttock pain, with estimates ranging from 39% (all) to 97% (corroborated and reported). Dyspareunia showed the greatest discrepancy between all cases and reported cases (13–100%, respectively), reflecting the very large proportion of under-reporting in the all cases studies. Therefore, none of the estimates for dyspareunia are reliable.
PS-specific signs
Frequencies were similar for the Freiberg sign, range 32% (all studies) to 63% (reported studies), and the Pace sign, 30% (corroborated) to 74% (reported). The numbers reported for tonic external rotation, FAIR and Beatty signs were small and the proportions of unreported cases high; so estimates are not reliable. External tenderness was common, with a range of 59% (corroborated) to 92% (corroborated and reported). Internal tenderness was frequently unreported, probably because this examination was seldom performed in orthopaedic or neurological practice. The range of estimates was 24% (corroborated studies) to 83% (reported).
Routine signs in sciatica
Limited SLR appeared to be the commonest finding, range 42% (all) to 62% (corroborated and reported), with diminished reflex, sensation and power reaching a maximum of 26, 39 and 37%, respectively.
Combinations of features
The commonest features were further analysed. Three features, pain in the buttock, pain aggravated by sitting and external tenderness were reported together in 22 cases, a frequency of 22% (CI 15–31) for all cases and 31% (CI 21–42) for reported cases. Of these 22, 12 were positive for at least one manoeuvre increasing PM tension.
Aggregated data studies
In three studies, women comprised 35–79% of the series. All four reported 100% frequency for buttock pain, suggesting this was part of their case definition (Table 4). Two reported very few features [51,71], whose frequencies were close to or equal to 100%, suggesting case selection on the basis of these features. Filler [36] reported only frequencies rather than raw data. Pooling was therefore considered inappropriate. Only Durrani and Winnie reported several features (Table 4).
In Durrani and Winnie’s series, the features present in half or more than half the cases were: buttock pain, low back pain, pain aggravated by sitting, external tenderness and internal tenderness. Two further specific signs were tested: Pace and tonic external rotation, which were about as frequent as limited SLR.
Results: corroborating evidence
Of the case studies with individual data, 79 cases had one or other form of corroborating evidence. The categories of corroborating data are shown in Table 5 with examples. The types are not mutually exclusive so that many cases had more than one item of corroboration, illustrated by multiple entries in the examples column. There were reports of congenital anomalies of the PM and/or sciatic nerve, acquired abnormalities of the PM and/or sciatic nerve, but also of normal morphology with response to surgical division of the PM, for example, Barton, case 4 [3].
Table 5.
Types of corroborating data with examples
| Corroborating item | Examples: description and study (first author and year) |
|---|---|
| Nerve conduction studies or electromyography show extraspinal delay | EMG findings suggestive of involvement of the inferior gluteal and peroneal branches of the sciatic nerve; case 3. Hughes et al. 1992 [49] |
| Delayed responses when hip was held in FAIR position; two out of two cases. Nakamura 2003 [77] | |
| Imaging shows structural abnormality: | Hypertrophy of PM; two cases out of two. Chen and Wan 1992 [21] |
| Hypertrophy of PM. Jankiewicz et al. 1991 [53] | |
| T2 hypersignal at the level of PM and sciatic nerve. Jroundi et al. 2003 [55] | |
| Abnormal MRI neurography, suggesting entrapment at the level of the PM; 12 out of 14 cases. Lewis et al. 2006 [68] | |
| Operative findings of abnormalities of PM and/or of sciatic nerve and/or of sciatic nerve impingement | Calcified PM. Beauchesne and Schutzer 1997 [5] |
| Sciatic nerve impinged between PM and short external rotators; case number 1 out of 2. Chen and Wan 1992 [21] | |
| Tendinous band of PM indenting peroneal branch of the sciatic nerve; case number 3 out of 5. Hughes et al. 1992 [49] | |
| Impingement of the sciatic nerve by the PM; six out of seven cases. Foster 2002 [41] | |
| Impingement by the PM or by an associated fibrous band; all four cases that had surgery. Lewis et al. 2006 [68] | |
| Anomalous division of the sciatic nerve with its superior branch passing through the PM; case number 2 out of 4. Kouvalchouk 1996 [64] | |
| Bifurcated sciatic nerve with posterior cutaneous femoral nerve squeezed between the PM and the greater sciatic notch. Ozaki and Muro 1999 [78] | |
| Relief following surgery (long term follow up not reported) | Pain increasing for 9 weeks after fall, relief following excision of calcified muscle. Beauchesne and Schutzer 1997 [5] |
| Seven cases, average duration of pain of 2 years, immediate improvement after division of PM and return to work, and relieved of symptoms at 3–6 months. Foster 2002 [41] | |
| Three cases out of four had surgery. Lewis et al. 2006 [68] | |
| Prolonged post-operative relief (over one year) | Both cases out of two. Chen and Wan 1992 [21] |
| All four cases. Kouvalchouk et al. 1996 [64] | |
| Case 1. Nakamura 2003 [77] | |
| Relief following X-ray guided injection of local anaesthetic and corticosteroid into PM (long term follow up not reported) | Pain for 7 months, free of pain at the 3-month follow-up after two injections. Bustamente [12] |
Incomplete reporting of corroborative data was encountered, for example, omission of the duration of the symptoms [77], operative findings [43] or period of follow-up [5]. Even case series did not report a consistent set of data for all cases in a series [61, 64].
Discussion
Strengths and limitations
The main strength of this study is that it is the first systematic review of the diagnostic features of PS. It is the most comprehensive review of diagnosis, incorporating data from 100 individual cases and aggregated data from another 26. We have extracted data according to pre-specified criteria to cover three important diagnostic areas: symptoms, physical signs specific to PS and signs routinely tested in sciatica.
The limitations of the study arise from the nature of the literature reviewed. A synthesis of case studies may suffer from either under-reporting, especially of the absence of signs, or over-reporting of the presence of signs. Over-reporting may be a particular problem when signs are being proposed by the author as pathognomonic. We have tackled this problem by calculating frequencies in four ways to provide a range of estimates. This enables comparison of the features with each other. The absence of a reference standard does not diminish the value of these ranges since we found them to be similar in both corroborated and non-corroborated studies. Of the aggregated data studies, the one with the highest quality, Durrani and Winnie, reported frequencies close to those calculated from individual data studies, adding credibility to the findings.
The majority of cases were reported from secondary and tertiary centres, which are more likely to encounter severe or more chronic cases. Therefore, the generalisability to primary care is limited.
Case studies typically present the outcome of treatment as implicit evidence of proof of the diagnosis. However, there are alternative explanations for such improvement, such as natural history, placebo response and observer bias. One strength of our review is that we have made the process explicit and assigned a lesser weight of evidence, support rather than proof, which we have termed corroboration. However, what counts as corroboration itself is open to interpretation and the degree of certainty it can claim is variable. For example, does response to local anaesthetic and steroid into the PM count as evidence of PS or can it, as Tiel [111] has argued, also be expected in cases of more distal nerve impingement? There are instances where evidence even in the absence of a comparison group makes cause and effect seem so probable that a causal relationship is credible [42]. For example, Lewis et al. [68] reported several cases where the results of MRI were supported by findings at operation, followed by relief of symptoms. What such cases cannot do is settle the controversy over the status of PS, but synthesising and making transparent the data does enable judgement on how much weight must be given to them when considering the implications for practice and research.
Implications for practice
The concurrence of several clinical features and the numerous cases with corroborating data add support to the arguments for the existence of the syndrome. Practitioners may consider entertaining the diagnosis in patients with atypical histories [48] or a “negative MRI”. Patients without a diagnosis after imaging still deserve an explanation for their symptoms and hope for their relief. Discussing the possibility of PS with patients in these situations is an option.
Four features appear to be most common: buttock pain, aggravation of sciatica through sitting, external tenderness over the greater sciatic notch and augmentation of the pain with manoeuvres that increase PM tension. These tests are easy to perform within the usual clinical examination. Most practitioners, however, may be less inclined to perform routine internal examination without stronger proof of its accuracy.
This synthesis provides empirical data, which challenge the received wisdom that neurologic deficits and limited SLR are rare [79,103]. It also challenges the belief that the prevalence in women is very much greater [79,91].
It could be argued that there is no value in making a diagnosis where there is no proven treatment. However, the paucity of effective treatment is true of low back pain and sciatica in general. The relief of pain with surgery in carefully selected cases of PS identified in this review has its parallel in the early history of disc decompression by Mixter and Barr. Nevertheless, the high success rates for surgery have been reported only in a small series [41,68,84]. There is limited evidence for non-surgical therapy [26]. Whilst uncertainty about therapy remains, what is certain is that research into therapy is more likely to proceed when the syndrome has been systematically studied.
Implications for research
Filler marshalled imaging and outcome data to argue for PS [36]. Whilst the volume of empirical data he presented deserves attention, we have argued that it does not amount to the highest level of evidence that he claims. Tiel has argued that there are alternative explanations for Filler’s observations: that MR neurography changes are artefactual, that PM injections act by non-specific means and that placebo response may explain treatment success [110]. However, in many case series, patients had not had a placebo response to previous therapies, including disc surgery, before undergoing PM resection. This study will not settle the debate on the existence or rarity of PS, but does permit the formulation of specific research questions.
The significant minority of people with sciatica but no spinal cause (whether HIVD or spinal stenosis) points to the need for research on extraspinal causes of sciatica. Our review raises three questions for research that would progress our understanding of the role of PS in these cases. The first is with respect to whether the features identified here occur significantly more often in patients without a spinal cause than in patients with a proven spinal cause. This would provide stronger evidence that these features represent a condition distinct from sciatica from spinal causes. Unfortunately, data for their frequency in sciatica in general and in HIVD or spinal stenosis in particular are not available because these tests are not routinely conducted. The second question is whether the quartet of buttock pain, pain on sitting, external tenderness and pain with increased PM tension occur significantly together and significantly more commonly in patients without spinal causes than in patients with spinal causes. The third is whether the quartet is accompanied by objective tests of nerve trunk compression, such as imaging or NCS. These three questions are best answered by cross-sectional studies of patients with sciatica.
Further single case reports or small series are unlikely to improve our understanding of PS unless they reveal previously undiscovered aspects of the condition. But, future case studies as well as cross-sectional studies must be more informative. The quality of most case studies reviewed was disappointing. Future studies should report clinical features both comprehensively and explicitly. The items we used for quality assessment provide a framework for such reporting.
Acknowledgments
We thank Mrs Wendy Marsh, Head of Knowledge Services, Ipswich PCT for assistance with retrieval, and Prof. Milos Jenicek and Prof. Paul Glasziou for comments on the assessment of case studies. The study was partly funded by a grant from the Scientific Foundation Board of the Royal College of General Practitioners. The authors are independent of the funding body.
Conflicts of interest statement
None.
Appendix 1: Anatomy of the PM
The PM originates from the pelvic surface of the sacral segments S2–S4, the sacro-iliac joint, the anterior sacro-spinous ligament and the sacro-tuberous ligament. It passes through the greater sciatic notch to insert onto the greater trochanter of the femur. The sciatic nerve exits the pelvis below the belly of the muscle. Many congenital variations exist: the nerve may divide proximally, the nerve or a division of the nerve may pass through the belly of the muscle through its tendons or between the part of a congenitally bifid muscle [85, 86]. The PM externally rotates, abducts and partially extends into the hip.
Appendix 2: Data extraction form for individual patient data
Citation
Type of study
Patient identification number
- Symptoms
- Buttock pain
- Low back pain
- Difficulty in sitting or pain aggravated by sitting
- Dyspareunia
- Signs specific for PS
- External tenderness over the greater sciatic notch
- Internal tenderness of the PM on vaginal or rectal examination
- Freiberg test
- Pace test
- Beatty test
- Tonic external rotation of the hip
- Flexion–adduction–internal rotation (FAIR) painful
- Routine sciatica signs
- Limited SLR or positive Lasegue
- Knee or ankle tendon reflex diminished
- Sensation along dermatomes L4, L5 and S1 diminished
- Power in myotomes L3/L4 and L5/S1 diminished
Appendix 3: Items in the quality assessment in case studies of PS
Description
Were all relevant demographic features, namely, age and sex, described?
Were key features in the history reported? These are onset whether acute or gradual, site of pain, radiation, relieving and aggravating factors, duration, evolution of the condition and past medical history.
Were routine sciatica examinations reported: sensation, power, tendon reflexes, straight leg raising/Lasegue?
Was at least one examination specific for PS reported: external rotation of foot, Freiberg sign, Pace sign, Beatty sign, Flexion–Adduction–Internal Rotation (FAIR) test?
Case definition
-
5.
Was there corroborating evidence?
Selection
-
6.
Applies only for case series
Was the method of selection free of bias, for example, through recruitment of consecutive cases?
References
- 1.Adams JA. The pyriformis syndrome—report of four cases and review of the literature. S Afr J Surg. 1980;18:13–18. [PubMed] [Google Scholar]
- 2.Banerjee T, Hall CD. Sciatic entrapment neuropathy. Case report. J Neurosurg. 1976;45:216–217. doi: 10.3171/jns.1976.45.2.0216. [DOI] [PubMed] [Google Scholar]
- 3.Barton PM. Piriformis syndrome: a rational approach to management. Pain. 1991;47:345–352. doi: 10.1016/0304-3959(91)90227-O. [DOI] [PubMed] [Google Scholar]
- 4.Beatty RA. The piriformis muscle syndrome: a simple diagnostic maneuver. Neurosurgery. 1994;34:512–513. doi: 10.1227/00006123-199403000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Beauchesne RP, Schutzer SF. Myositis ossificans of the piriformis muscle: an unusual cause of piriformis syndrome. A case report. J Bone Joint Surg. 1997;79:906–910. doi: 10.2106/00004623-199706000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Benson ER, Schutzer SF. Posttraumatic piriformis syndrome: diagnosis and results of operative treatment. J Bone Joint Surg Am. 1999;81:941–949. [PubMed] [Google Scholar]
- 7.Benzon HT, Katz JA, Benzon HA, Iqbal MS. Piriformis syndrome: anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98:1442–1448. doi: 10.1097/00000542-200306000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop. 1987;217:266–280. [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broadhurst NA, Simmons DN, Bond MJ. Piriformis syndrome: correlation of muscle morphology with symptoms and signs. Arch Phys Med Rehabil. 2004;85:2036–2039. doi: 10.1016/j.apmr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Brown JA, Braun MA, Namey TC. Pyriformis syndrome in a 10-year-old boy as a complication of operation with the patient in the sitting position. Neurosurgery. 1988;23:117–119. doi: 10.1227/00006123-198807000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante S, Houlton PG. Swelling of the leg, deep venous thrombosis and the piriformis syndrome. Pain Res Manag. 2001;6:200–203. doi: 10.1155/2001/104091. [DOI] [PubMed] [Google Scholar]
- 13.Byrd JWT. Piriformis syndrome. Oper Tech Sports Med. 2005;13:71–79. [Google Scholar]
- 14.Carey TS, Boden SD. A critical guide to case series reports. Spine. 2003;28:1631–1634. doi: 10.1097/01.BRS.0000083174.84050.E5. [DOI] [PubMed] [Google Scholar]
- 15.Centre for Evidence-based Medicine (2009) Oxford Centre for evidence-based medicine—levels of evidence. http://www.cebm.net/index.aspx?o=1025. Accessed 7 July 2009
- 16.Centre for Reviews and Dissemination (2009) Systematic reviews. CRD’s guidance for undertaking reviews in health care. http://www.york.ac.uk/inst/crd/systematic_reviews_book.htm. Accessed 7 July 2009
- 17.Chang CW, Shieh SF, Li CM, Wu WT, Chang KF. Measurement of motor nerve conduction velocity of the sciatic nerve in patients with piriformis syndrome: a magnetic stimulation study. Arch Phys Med Rehabil. 2006;87:1371–1375. doi: 10.1016/j.apmr.2006.07.258. [DOI] [PubMed] [Google Scholar]
- 18.Chantraine A, Gauthier C. The pyriformis syndrome. Ann Readapt Med Phys. 1990;33:347–353. [Google Scholar]
- 19.Chen WS. Sciatica due to piriformis pyomyositis. Report of a case. J Bone Joint Surg Am. 1992;74:1546–1548. [PubMed] [Google Scholar]
- 20.Chen WS. Bipartite piriformis muscle: an unusual cause of sciatic nerve entrapment. Pain. 1994;58:269–272. doi: 10.1016/0304-3959(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen WS, Wan YL. Sciatica caused by piriformis muscle syndrome: report of two cases. J Formos Med Assoc. 1992;91:647–650. [PubMed] [Google Scholar]
- 22.Chong KW, Tay BK. Piriformis pyomyositis: a rare cause of sciatica. Singap Med J. 2004;45:229–231. [PubMed] [Google Scholar]
- 23.Collier FC (1985) Acute monetary sciatica. Lancet 1079 [DOI] [PubMed]
- 24.Colmegna I, Justiniano M, Espinoza LR, Gimenez CR. Piriformis pyomyositis with sciatica: an unrecognized complication of “unsafe” abortions. J Clin Rheumatol. 2007;13:87–88. doi: 10.1097/01.rhu.0000260655.90449.7d. [DOI] [PubMed] [Google Scholar]
- 25.Cook MW, Levin LA, Joseph MP, Pinczower EF. Traumatic optic neuropathy. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1996;122:389–392. doi: 10.1001/archotol.1996.01890160031006. [DOI] [PubMed] [Google Scholar]
- 26.Cramp F, Bottrell O, Campbell H, et al. Non-surgical management of piriformis syndrome: a systematic review. Phys Ther Rev. 2007;12:66–72. [Google Scholar]
- 27.Dalmau-Carola J. Myofascial pain syndrome affecting the piriformis and the obturator internus muscle. Pain Pract. 2005;5:361–363. doi: 10.1111/j.1533-2500.2005.00039.x. [DOI] [PubMed] [Google Scholar]
- 28.Dhote R, Tudoret L, Bachmeyer C, Legmann P, Christoforov B. Cyclic sciatica. A manifestation of compression of the sciatic nerve by endometriosis. A case report. Spine. 1996;21:2277–2279. doi: 10.1097/00007632-199610010-00021. [DOI] [PubMed] [Google Scholar]
- 29.Dionne CE, Dunn KM, Croft PR, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine. 2008;33:95–103. doi: 10.1097/BRS.0b013e31815e7f94. [DOI] [PubMed] [Google Scholar]
- 30.Durrani Z, Winnie AP. Piriformis muscle syndrome: an underdiagnosed cause of sciatica. J Pain Symptom Manage. 1991;6:374–379. doi: 10.1016/0885-3924(91)90029-4. [DOI] [PubMed] [Google Scholar]
- 31.El Rubaidi OA, Horcajadas AA, RodrÌguez RD, Galicia Bulnes JM. Sciatic nerve compression as a complication of the sitting position. Neurocirugia. 2003;14:426–430. doi: 10.1016/s1130-1473(03)70524-7. [DOI] [PubMed] [Google Scholar]
- 32.Ernst E. Manipulation of the cervical spine: a systematic review of case reports of serious adverse events, 1995–2001. Med J Aust. 2002;176:376–380. doi: 10.5694/j.1326-5377.2002.tb04459.x. [DOI] [PubMed] [Google Scholar]
- 33.Ernst E. The safety of massage therapy. Rheumatology. 2003;42:1101–1106. doi: 10.1093/rheumatology/keg306. [DOI] [PubMed] [Google Scholar]
- 34.Ernst E. Ophthalmological adverse effects of (chiropractic) upper spinal manipulation: evidence from recent case reports. Acta Ophthalmol Scand. 2005;83:581–585. doi: 10.1111/j.1600-0420.2005.00488.x. [DOI] [PubMed] [Google Scholar]
- 35.Ernst E. Adverse effects of spinal manipulation: a systematic review. J R Soc Med. 2007;100:330–338. doi: 10.1258/jrsm.100.7.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2:99–115. doi: 10.3171/spi.2005.2.2.0099. [DOI] [PubMed] [Google Scholar]
- 37.Fishman LM, Dombi GW, Michaelsen C, et al. Piriformis syndrome: diagnosis, treatment, and outcome—a 10-year study. Arch Phys Med Rehabil. 2002;83:295–301. doi: 10.1053/apmr.2002.30622. [DOI] [PubMed] [Google Scholar]
- 38.Fishman LM, Zybert PA. Electrophysiologic evidence of piriformis syndrome. Arch Phys Med Rehabil. 1992;73:359–364. doi: 10.1016/0003-9993(92)90010-t. [DOI] [PubMed] [Google Scholar]
- 39.Fishman LM, Schaefer MP. Piriforms syndrome: the piriformis syndrome is underdiagnosed. Muscle Nerve. 2003;28:646–649. doi: 10.1002/mus.10482. [DOI] [PubMed] [Google Scholar]
- 40.Foster MR. Clinical trials for piriformis syndrome. Orthopedics. 1999;22:561–569. doi: 10.3928/0147-7447-19990601-02. [DOI] [PubMed] [Google Scholar]
- 41.Foster MR. Piriformis syndrome. Orthopedics. 2002;25:821–825. doi: 10.3928/0147-7447-20020801-12. [DOI] [PubMed] [Google Scholar]
- 42.Freiberg AH, Vinke TH. Sciatica and the sacro-iliac joint. J Bone Joint Surg Am. 1934;16:126–136. [Google Scholar]
- 43.Freiberg AH. Sciatic pain and its relief by operations on muscle and fascia. Arch Surg. 1937;34:337–350. [Google Scholar]
- 44.Gandhavadi B. Bilateral piriformis syndrome associated with dystonia musculorum deformans. Orthopedics. 1990;13:350–351. doi: 10.3928/0147-7447-19900301-16. [DOI] [PubMed] [Google Scholar]
- 45.Guyomarc’h HF, Labanere C. Piriformis muscle syndrome: differential diagnosis with sciatalgia in the athlete: three cases and a review of the literature. J Traumatol Surg. 2004;21:133–145. [Google Scholar]
- 46.Hallin RP. Sciatic pain and the piriformis muscle. Postgrad Med. 1983;74:69–72. doi: 10.1080/00325481.1983.11698378. [DOI] [PubMed] [Google Scholar]
- 47.Hanania M, Kitain E. Perisciatic injection of steroid for the treatment of sciatica due to piriformis syndrome. Reg Anesth Pain Med. 1998;23:223–228. doi: 10.1097/00115550-199823020-00020. [DOI] [PubMed] [Google Scholar]
- 48.Hopayian K. Sciatica in the community—not always disc herniation. Int J Clin Pract. 1999;53:197–198. [PubMed] [Google Scholar]
- 49.Hughes SS, Goldstein MN, Hicks DG, Pellegrini VDJ. Extrapelvic compression of the sciatic nerve. An unusual cause of pain about the hip: report of five cases. J Bone Joint Surg Am. 1992;74:1553–1559. [PubMed] [Google Scholar]
- 50.Hulbert A, Deyle GD. Differential diagnosis and conservative treatment for piriformis syndrome: a review of the literature. Curr Orthop Pract. 2009;20:313–319. [Google Scholar]
- 51.Indrekvam K, Sudmann E. Piriformis muscle syndrome in 19 patients treated by tenotomy—a 1- to 16-year follow-up study. Int Orthop. 2002;26:101–103. doi: 10.1007/s00264-001-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jabs DA. Improving the reporting of clinical case series. Am J Ophthalmol. 2005;139:900–905. doi: 10.1016/j.ajo.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jankiewicz JJ, Hennrikus WL, Houkom JA. The appearance of the piriformis muscle syndrome in computed tomography and magnetic resonance imaging: a case report and review of the literature. Clin Orthop Relat Res. 1991;262:205–209. [PubMed] [Google Scholar]
- 54.Jenicek M. Clinical case reporting in evidence-based medicine. Oxford: Butterworth-Heinemann; 1999. [Google Scholar]
- 55.Jroundi L, El Quessar A, Chakir N, El Hassani MR, Jiddane M. The piriformis syndrome: a rare cause of non discogenic sciatica. A case report. J Radiol. 2003;84:715–717. [PubMed] [Google Scholar]
- 56.Julsrud ME. Piriformis syndrome. J Am Podiatr Med Assoc. 1989;79:128–131. doi: 10.7547/87507315-79-3-128. [DOI] [PubMed] [Google Scholar]
- 57.Karl RD, Jr, Yedinak MA, Hartshorne MF, et al. Scintigraphic appearance of the piriformis muscle syndrome. Clin Nucl Med. 1985;10:361–363. doi: 10.1097/00003072-198505000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Karppinen J, Malmivaara A, Tervonen O, et al. Severity of symptoms and signs in relation to magnetic resonance imaging findings among sciatic patients. Spine. 2001;26:E149–E154. doi: 10.1097/00007632-200104010-00015. [DOI] [PubMed] [Google Scholar]
- 59.Kent DL, Haynor DR, Longstreth WTJ, Larson EB. The clinical efficacy of magnetic resonance imaging in neuroimaging. Ann Intern Med. 1994;120:856–871. doi: 10.7326/0003-4819-120-10-199405150-00007. [DOI] [PubMed] [Google Scholar]
- 60.Kirschner JS, Foye PM, Cole JL. Piriformis syndrome, diagnosis and treatment. Muscle Nerve. 2009;40:10–18. doi: 10.1002/mus.21318. [DOI] [PubMed] [Google Scholar]
- 61.Kobbe P, Zelle BA, Gruen GS. Case report: recurrent piriformis syndrome after surgical release. Clin Orthop Relat Res. 2008;466:1745–1748. doi: 10.1007/s11999-008-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konstantinou K, Dunn KM. Sciatica: review of epidemiological studies and prevalence estimates. Spine. 2008;33:2464–2472. doi: 10.1097/BRS.0b013e318183a4a2. [DOI] [PubMed] [Google Scholar]
- 63.Kosukegawa I, Yoshimoto M, Isogai S, Nonaka S, Yamashita T. Piriformis syndrome resulting from a rare anatomic variation. Spine. 2006;31:E664–E666. doi: 10.1097/01.brs.0000231877.34800.71. [DOI] [PubMed] [Google Scholar]
- 64.Kouvalchouk J, Bonnet JM, de Mondenard JP. Piriformis syndrome. Apropos of 4 cases treated by surgery and review of the literature. [French] Rev Chir Orthop Reparatrice Appar Mot. 1996;82:647–657. [PubMed] [Google Scholar]
- 65.Ku A, Kern H, Lachman E, Nagler W. Sciatic nerve impingement from piriformis hematoma due to prolonged labor [letter] Muscle Nerve. 1995;18:789–790. [PubMed] [Google Scholar]
- 66.Kumar B, Sriram KG, George C. Osteophyte at the sacroiliac joint as a cause of sciatica: a report of four cases. J Orthop Surg (Hong Kong) 2002;10:73–76. doi: 10.1177/230949900201000113. [DOI] [PubMed] [Google Scholar]
- 67.Lee EY, Margherita AJ, Gierada DS, Narra VR. MRI of piriformis syndrome. Am J Roentgenol. 2004;183:63–64. doi: 10.2214/ajr.183.1.1830063. [DOI] [PubMed] [Google Scholar]
- 68.Lewis AM, Layzer R, Engstrom JW, Barbaro NM, Chin CT. Magnetic resonance neurography in extraspinal sciatica. Arch Neurol. 2006;63:1469–1472. doi: 10.1001/archneur.63.10.1469. [DOI] [PubMed] [Google Scholar]
- 69.Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.1000100. Accessed 27 March 2010 [DOI] [PMC free article] [PubMed]
- 70.Limongelli P, Khorsandi SE, Pai M, et al. Management of delayed postoperative hemorrhage after pancreaticoduodenectomy: a meta-analysis. Arch Surg. 2008;143:1001–1007. doi: 10.1001/archsurg.143.10.1001. [DOI] [PubMed] [Google Scholar]
- 71.Lu MY, Dong BJ, Ma XY (1985) Piriformis syndrome and its operative treatment: an analysis of sixty cases. Zhonghua wai ke za zhi (Chinese journal of surgery) 23, 483-4, 510 [PubMed]
- 72.Mayrand N, Fortin J, Descarreaux M, Normand MC. Diagnosis and management of posttraumatic piriformis syndrome: a case study. J Manipulative Physiol Ther. 2006;29:486–491. doi: 10.1016/j.jmpt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Merlo IM, Poloni TE, Alfonsi E, Messina AL, Ceroni M. Sciatic pain in a young sportsman. Lancet. 1997;349:846. doi: 10.1016/s0140-6736(97)01120-3. [DOI] [PubMed] [Google Scholar]
- 74.Miller A, Stedman GH, Beisaw NE, Gross PT. Sciatica caused by an avulsion fracture of the ischial tuberosity. a case report. J Bone Joint Surg Am. 1987;69:143–145. [PubMed] [Google Scholar]
- 75.Mixter WJ, Barr JB. Rupture of intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:110–115. [Google Scholar]
- 76.Molina N (2003) Piriformis syndrome. Dyn Chiropr http://www.dynamicchiropractic.com/mpacms/dc/article.php?id=9079. Accessed 4 April 2010
- 77.Nakamura H, Seki M, Konishi S, Yamano Y, Takaoka K. Piriformis syndrome diagnosed by cauda equina action potentials: report of two cases. Spine. 2003;28:E37–E40. doi: 10.1097/00007632-200301150-00022. [DOI] [PubMed] [Google Scholar]
- 78.Ozaki S, Hamabe T, Muro T. Piriformis syndrome resulting from an anomalous relationship between the sciatic nerve and piriformis muscle. Orthopedics. 1999;22:771–772. doi: 10.3928/0147-7447-19990801-09. [DOI] [PubMed] [Google Scholar]
- 79.Pace JB, Nagle D. Piriform syndrome. West J Med. 1976;124:433–439. [PMC free article] [PubMed] [Google Scholar]
- 80.Papadopoulos EC, Khan SN. Piriformis syndrome and low back pain: A new classification and review of the literature. Orthop Clin North Am. 2004;35:65–71. doi: 10.1016/S0030-5898(03)00105-6. [DOI] [PubMed] [Google Scholar]
- 81.Papadopoulos SM, McGillicuddy JE, Albers JW. Unusual cause of ‘piriformis muscle syndrome’. Arch Neurol. 1990;47:1144–1146. doi: 10.1001/archneur.1990.00530100114027. [DOI] [PubMed] [Google Scholar]
- 82.Papadopoulos SM, McGillicuddy JE, Messina LM. Pseudoaneurysm of the inferior gluteal artery presenting as sciatic nerve compression. Neurosurgery. 1989;24:926–928. doi: 10.1227/00006123-198906000-00025. [DOI] [PubMed] [Google Scholar]
- 83.Park HW, Jahng JS, Lee WH. Piriformis syndrome—a case report. Yonsei Med J. 1991;32:64–68. doi: 10.3349/ymj.1991.32.1.64. [DOI] [PubMed] [Google Scholar]
- 84.Pecina HI, Boric I, Smoljanovic T, Duvancic D, Pecina M. Surgical evaluation of magnetic resonance imaging findings in piriformis muscle syndrome. Skelet Radiol. 2008;37:1019–1023. doi: 10.1007/s00256-008-0538-0. [DOI] [PubMed] [Google Scholar]
- 85.Pecina M. Contributions to the etiological explanation of the piriformis syndrome. Acta Anat. 1979;105:181–187. [PubMed] [Google Scholar]
- 86.Pokorny D, Jahoda D, Veigl D, Pinskerov V, Sosna A. Topographic variations of the relationship of the sciatic nerve and the piriformis muscle and its relevance to palsy after total hip arthroplasty. Surg Radiol Anat. 2006;28:88–91. doi: 10.1007/s00276-005-0056-x. [DOI] [PubMed] [Google Scholar]
- 87.Raney EM, Freccero DM, Dolan LA, et al. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28:701–704. doi: 10.1097/BPO.0b013e3181875b60. [DOI] [PubMed] [Google Scholar]
- 88.Retzlaff EW, Berry AH, Haight AS, et al. The piriformis muscle syndrome. J Am Osteopath Assoc. 1974;73:799–807. [PubMed] [Google Scholar]
- 89.Rich BSE, McKeag D. When sciatica is not disk disease: detecting piriformis syndrome in active patients. Neurorehabil Neural Repair. 1992;20:104–108. [Google Scholar]
- 90.Richardson RR, Garon JE, Sianis GJ. Sciatic entrapment neuropathy by tendinized muscle—the pyriformis syndrome: a case report. J Neurol Orthop Med Surg. 1992;13:142–145. [Google Scholar]
- 91.Robinson DR. Pyriformis syndrome in relation to sciatic pain. Am J Surg. 1947;73:355–358. doi: 10.1016/0002-9610(47)90345-0. [DOI] [PubMed] [Google Scholar]
- 92.Rodrigue T, Hardy RW. Diagnosis and treatment of piriformis syndrome. Neurosurg Clin N Am. 2001;12:311–319. [PubMed] [Google Scholar]
- 93.Rossi P, Cardinali P, Serrao M, et al. Magnetic resonance imaging findings in piriformis syndrome: a case report. Arch Phys Med Rehabil. 2001;82:519–521. doi: 10.1053/apmr.2001.21971. [DOI] [PubMed] [Google Scholar]
- 94.Rucco V, Onorato A. Common pseudoradicular syndromes (pseudocrural and pseudosciatic pain) Europa Medicophysica. 1998;34:75–83. [Google Scholar]
- 95.Sayson SC, Ducey JP, Maybrey JB, Wesley RL, Vermilion D. Sciatic entrapment neuropathy associated with an anomalous piriformis muscle. Pain. 1994;59:149–152. doi: 10.1016/0304-3959(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 96.Silver JK, Leadbetter WB. Piriformis syndrome: assessment of current practice and literature review. Orthopedics. 1998;21:1133–1135. doi: 10.3928/0147-7447-19981001-12. [DOI] [PubMed] [Google Scholar]
- 97.Slipman CW, Vresilovic EJ, Palmer MA, Lipetz JS, Lenrow D. Piriformis muscle syndrome: a diagnostic dilemma. J Musculoskelet Pain. 1999;7:73–83. [Google Scholar]
- 98.Soga J, Yakuwa Y, Osaka M. Carcinoid syndrome: a statistical evaluation of 748 reported cases. J Exp Clin Cancer Res. 1999;18:133–141. [PubMed] [Google Scholar]
- 99.Solheim LF, Siewers P, Paus B. The piriformis muscle syndrome: sciatic nerve entrapment treated with section of the piriformis muscle. Acta Orthop Scand. 1981;52:73–75. doi: 10.3109/17453678108991762. [DOI] [PubMed] [Google Scholar]
- 100.Spinner RJ, Thomas NM, Kline DG. Failure of surgical decompression for a presumed case of piriformis syndrome. Case report. J Neurosurg. 2001;94:652–654. doi: 10.3171/jns.2001.94.4.0652. [DOI] [PubMed] [Google Scholar]
- 101.Stegbauer CC. Sciatic pain and piriformis syndrome. Nurse Pract. 1997;22:166–180. [PubMed] [Google Scholar]
- 102.Stein JM, Warfield CA (1983) Two entrapment neuropathies. Hosp Pract 18:100A, 100E, 100H [DOI] [PubMed]
- 103.Steiner C, Staubs C, Ganon M, Buhlinger C. Piriformis syndrome: pathogenesis, diagnosis, and treatment. J Am Osteopath Assoc. 1987;87:318–323. [PubMed] [Google Scholar]
- 104.Stevens KJ, Banuls M. Sciatic nerve palsy caused by haematoma from iliac bone graft donor site. Eur Spine J. 1994;3:291–293. doi: 10.1007/BF02226583. [DOI] [PubMed] [Google Scholar]
- 105.Stewart JD. The piriformis syndrome is overdiagnosed. Muscle Nerve. 2003;28:644–646. doi: 10.1002/mus.10483. [DOI] [PubMed] [Google Scholar]
- 106.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. London: Wiley; 2000. [Google Scholar]
- 107.Synek VM. Short latency somatosensory evoked potentials in patients with painful dysaesthesias in peripheral nerve lesions. Pain. 1987;29:49–58. doi: 10.1016/0304-3959(87)90177-1. [DOI] [PubMed] [Google Scholar]
- 108.Synek VM. The pyriformis syndrome: review and case presentation. Clin Exp Neurol. 1987;23:31–37. [PubMed] [Google Scholar]
- 109.Terrasa S, Caccavo T, Ferraris J et al. (2007) Guía para la lectura crítica de una Serie de Casos. Evidencia en la Práctica Ambulatoria. http://www.foroaps.org/files/guia%20de%20serie%20de%20casos.pdf. Accessed 4 April 2010
- 110.Tiel RL. Piriformis and related entrapment syndromes: myth and fallacy. Neurosurg Clin N Am. 2008;19:623–627. doi: 10.1016/j.nec.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 111.Tiel RL, Kline DG. Piriformis syndrome. J Neurosurg Spine. 2006;5:102–104. doi: 10.3171/spi.2006.5.1.102. [DOI] [PubMed] [Google Scholar]
- 112.Turtas S, Zirattu G. The piriformis syndrome: a case report of an unusual cause of sciatica. J Orthop Traumatol. 2006;7:97–99. [Google Scholar]
- 113.Vallejo MC, Mariano DJ, Kaul B, Sah N, Ramanathan S. Piriformis syndrome in a patient after cesarean section under spinal anesthesia. Reg Anesth Pain Med. 2004;29:X364–X367. doi: 10.1016/j.rapm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 114.van Tulder M, Becker A, Bekkering T, et al. Chapter 3. European guidelines for the management of acute nonspecific low back pain in primary care. Eur Spine J. 2006;15(Suppl 2):S169–S191. doi: 10.1007/s00586-006-1071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med. 2001;134:330–334. doi: 10.7326/0003-4819-134-4-200102200-00017. [DOI] [PubMed] [Google Scholar]
- 116.Vandertop WP, Bosma NJ. The piriformis syndrome. A case report. J Bone Joint Surg Am. 1991;73:1095–1097. [PubMed] [Google Scholar]
- 117.Waddell G. The back pain revolution. London: Churchill Livingstone; 2004. [Google Scholar]
- 118.West S, King V, Carey TS et al. (2002) Systems to rate the strength of scientific evidence. Evidence Report/Technology Assessment Number 47. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat1.chapter.70996. Accessed 4 April 2010 [PMC free article] [PubMed]
- 119.West D, Togo A, Kirk AJ. Are bronchoscopic approaches to post-pneumonectomy bronchopleural fistula an effective alternative to repeat thoracotomy? Interact Cardiovasc Thorac Surg. 2007;6:547–550. doi: 10.1510/icvts.2007.159319. [DOI] [PubMed] [Google Scholar]
- 120.Wyant GM. Chronic pain syndromes and their treatment. III. The piriformis syndrome. Can Anaesth Soc J. 1979;26:305–308. doi: 10.1007/BF03006291. [DOI] [PubMed] [Google Scholar]
- 121.Yoon SJ, Ho J, Kang HY, et al. Low-dose botulinum toxin type A for the treatment of refractory piriformis syndrome. Pharmacotherapy. 2007;27:657–665. doi: 10.1592/phco.27.5.657. [DOI] [PubMed] [Google Scholar]
- 122.Young J. Lung volume surgery (LVRS) for chronic obstructive pulmonary disease (COPD) with underlying severe emphysema, A West Midlands Development and Evaluation Committee Report. Birmingham: University of Birmingham; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuen EC, So YT. Sciatic neuropathy. Neurol Clin. 1999;17:617–631. doi: 10.1016/s0733-8619(05)70155-9. [DOI] [PubMed] [Google Scholar]