Abstract
Introduction
Hypochlorhydria during Helicobacter pylori infection inhibits gastric Shh expression. We investigated whether acid-secretory mechanisms regulate Shh gene expression through Ca2+i-dependent protein kinase C (PKC) or cAMP-dependent protein kinase A (PKA)-activation.
Method
We blocked Hedgehog signaling by transgenically overexpressing a secreted form of the Hedgehog interacting protein-1 (sHip-1), a natural inhibitor of hedgehog ligands, which induced hypochlorhydria. Gadolinium, EGTA+BAPTA, PKC-overexpressing adenoviruses, and PKC-inhibitors were used to modulate Ca2+i-release, PKC-activity and Shh gene expression in primary gastric cell, organ, and AGS cell line cultures. PKA hyperactivity was induced in the H+/K+-β-cholera-toxin overexpressing mice (Ctox).
Results
Mice that expressed sHip-1 had lower levels of gastric acid (hypochlorhydria), reduced production of somatostatin, and increased gastrin gene expression. Hypochlorhydria in these mice repressed Shh gene expression, similar to the levels obtained with omeprazole treatment of wild-type mice. However, Shh expression was also repressed in the hyperchlorhydric Ctox model with elevated cAMP, suggesting that the regulation of Shh was not solely acid-dependent, but pertained to specific acid-stimulatory signaling pathways. Based on previous reports that Ca2+i-release also stimulates acid secretion in parietal cells, we showed that gadolinium-, thapsigargin- and carbachol-mediated release of Ca2+i induced Shh expression. Ca2+-chelation with BAPTA+EGTA reduced Shh expression. Overexpression of PKC-α, -β and -δ (but not PKC-ε) induced Shh gene expression. In addition, phorbol esters induced a Shh-regulated reporter gene.
Conclusion
Secretagogues that stimulate gastric acid secretion induce Shh gene expression through increased Ca2+i-release and PKC activation. Shh might be the ligand transducing changes in gastric acidity to the regulation of G-cell secretion of gastrin.
Keywords: somatostatin, gastrin, hedgehog interacting protein, PKA, chelation
INTRODUCTION
Sonic Hedgehog (Shh) is expressed by gastric parietal cells1–3, and correlates with several mechanisms related to parietal cell acid secretion. First, Shh stimulates H+/K+-ATPase gene expression and subsequently enhances histamine-stimulated acid secretion by parietal cells4. Second, Shh protein co-localizes with the proton/potassium pump (H+/K+-ATPase)3. Third, gastric acid induces processing of the Shh 45kDa precursor form to the 19kDa biologically active form5. Fourth, blocking gastric acid secretion with a proton pump inhibitor inhibits Shh gene expression2, 6. The latter observation is relevant to the observation that Helicobactor pylori-infection downregulates Shh gene expression apparently through its ability to induce pro-inflammatory cytokines, which inhibit acid secretion6. Parietal cell Shh production is important since tissue-specific deletion of Shh using the Cre-Lox system induces foveolar hyperplasia7, which is reminiscent of the pre-malignant changes induced by H. pylori. Nevertheless, the mechanism by which gastric acid regulates Shh is not well understood.
In gastric cells, the process of acid secretion is associated with protein kinase A (PKA) and protein kinase C (PKC) signaling8. While the elevation in cAMP levels and activation of PKA pertain to histaminic stimulation8, both cholinergic (acetylcholine) and hormonal (gastrin) stimulation of acid secretion increase intracellular calcium (Ca2+i) in parietal cells9, 10. The release of Ca2+i activates several signal transduction pathways including Ca2+-dependent PKC isoforms, α and β11. While the effects of Ca2+i on Shh expression have not been previously investigated, Ca2+-chelation inhibits Indian hedgehog (Ihh) gene expression in chick chondrocytes12. Furthermore, in the chick wing bud, PKC sustains Shh gene expression13. Collectively, these studies suggest that calcium-dependent PKCs, when activated, transduce elevated Ca2+i levels to downstream targets.
Previously, we reported that Shh enhances acid secretion in gastric parietal cells by increasing H+/K+-ATPase gene expression thereby increasing enzyme content4. In this report, regulation of Shh gene expression was examined in vivo and in vitro. Transgenic overexpression of an inhibitor of Hedgehog (Hh) ligands called hedgehog-interacting protein-1 (Hip-1) from parietal cells resulted in hypochlorhydric mice. This permitted us to examine the effect of low gastric acidity on the Shh gene locus (Shh gene expression). Since Shh gene expression was reduced, we hypothesized that the change occurred in response to the hypochlorhydria. Knowing that PKC regulates Shh gene expression during development, we tested the hypothesis that hypochlorhydria reduces Ca2+i and PKC activation, ultimately inhibiting Shh gene expression.
MATERIALS AND METHODS
Generation of transgenic mice
The Hip-1 cDNA lacking the transmembrane domain (sHip-1)14 was subcloned downstream of the H+/K+-ATPase beta-subunit promoter15. The H+/K+-ATPase-Hip transgene was injected into fertilized eggs obtained by mating (C57BL/6 X SJL)F1 (UM Transgenic Core). Transgenic founders were bred to C57BL/6 mice. Ctox mice (line 7) were generated as described previously15.
Omeprazole treatment
Omeprazole (Sigma-Aldrich) stock (80 μmol/ml) was prepared in a 9:1 solution of DMSO:Polyethylene glycol (PEG) 400 (Fluka, Sigma-Aldrich). Intraperitoneal injections of omeprazole (400 μmol/kg) were administered once daily for 3-consecutive days.
Gastric acidity
After opening the stomach along the greater curvature, the gastric contents were collected in 1.5 ml of 0.9% NaCl. The samples were centrifuged to collect a clear supernatant. The hydrogen ion concentration was determined by titrating with 0.005 N sodium hydroxide using the PHM290 pH-Stat Controller titration system (Radiometer, OH).
Western blot analysis
Western blotting was performed according to previously published conditions5 using a 1:200 dilution of goat polyclonal anti-Shh (sc-1194, Santa Cruz, CA), 1:500 GAPDH (Molecular Probes, Invitrogen) antibody, and 1:1000 phospho-PKCα/βII (Thr638/641) antibody (#9375, Cell Signaling, Boston, MA).
RESULTS
Hypochlorhydria in sHip-1 expressing mice
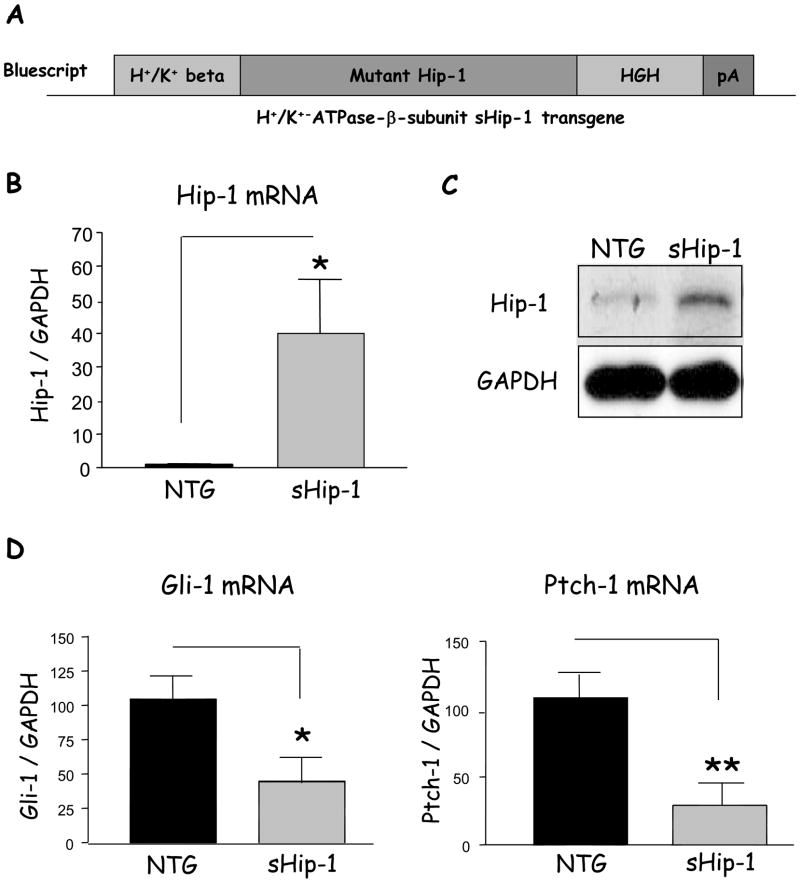
Prior studies of primary parietal cell cultures demonstrated a role for Shh in H+/K+-ATPase gene expression, implicating an indirect effect of Shh on gastric acid production4. Indeed, we recently demonstrated that a proinflammatory cytokine inhibits both gastric acid and Shh gene expression6. Therefore to understand the relationship between gastric acidity and Shh expression in vivo, we generated a transgenic mouse expressing the natural inhibitor of Shh called Hip-1. The secreted form of Hip-1 (sHip-1), which lacks the transmembrane domain, inhibits Hh signaling in the intestine by binding the Hh ligands14. We therefore expressed sHip-1 from the H+/K+-ATPase-β subunit promoter to block Hh signaling in the stomach (Fig. 1A). To confirm expression of the sHip-1 transgene in the stomachs of 2-month-old mice, Hip-1 mRNA expression in non-transgenic mice was compared to transgenic littermates by RT-qPCR. Hip-1 protein was verified by western blotting. sHip-1 mice showed a 40-fold increase in Hip-1 mRNA (Fig. 1B), and a marked increase in Hip-1 protein (Fig. 1C). There was only a slight reduction in size (2.4 kDa) of the sHip-1 transgene compared to endogenous Hip-1, since the deleted transmembrane domain is only 22 amino acids14. The increase in Hip-1 corresponded to a significant reduction in Hh-target gene expression (Gli-1 and Ptch-1) demonstrating efficient suppression of hedgehog signaling (Fig. 1D), and was consistent among 3 different founder lines (Supplementary Fig. 1A–C).
Figure 1. Hip-1 mRNA levels and Shh signaling in the gastric fundus of transgenic mice.
A) The secreted hedgehog interacting protein 1 (sHip-1) transgene, lacking the C-terminal transmembrane domain, was expressed downstream of the H+/K+-ATPase-β subunit promoter, and in frame with the 3′UTR of the human growth hormone (HGH) gene and polyA tail to enhance expression in mammalian cells. The mice expressing the transgene were designated as “sHip-1 mice”. B) Quantitative PCR analysis of Hip-1 mRNA from the sHip-1 mice compared to non-transgenic littermate is shown as the mean +/− SEM for 5 mice per group. C) A representative western blot of Hip-1 protein (85kDa) from a sHip-1 mouse (Founder line 450) compared to non-transgenic littermate is shown. D) Target gene expression of glioma-associated oncogene-1 (Gli-1) and Ptch-1 genes in the sHip-1 fundus versus controls to assay the signaling activity of the Shh pathway (shown is the mean for n = 5 mice per group +/− SEM). P-values are indicated such that * P < 0.05 and ** P < 0.01.
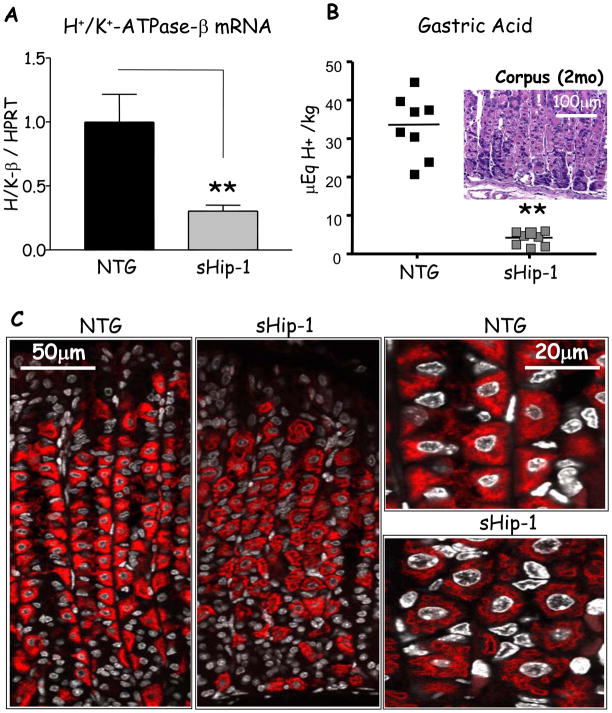
Previous reports have shown that Hh signaling induces H+/K+-ATPase gene expression and enhances histamine-stimulated acid secretion in primary canine parietal cell cultures4. Therefore we examined whether H+/K+-ATPase gene expression was affected by the overproduction of sHip-1. Indeed, H+/K+-ATPase-β subunit mRNA and protein were significantly reduced in 3 founder lines (Fig. 2A, C, and Supplementary Fig. 2), despite the normal morphological distribution of parietal cells observed with H&E staining (Fig. 2B, insert). This correlated with reduced levels of gastric acid in 3 founder lines (Fig. 2B, and supplementary Fig. 1D). In contrast, H+/K+-ATPase-α subunit expression at the protein or mRNA level was not affected (Supplementary Fig. 3).
Figure 2. H+/K+-ATPase-β expression and acid production in sHip-1 mice.
A) H+/K+-ATPase-β mRNA expression in the sHip-1 fundus versus non-transgenic littermate measured by RT-qPCR (shown is the mean for n = 5 mice per group +/− SEM). B) Gastric acidity measured by base titration in sHip-1 versus non-transgenic mice. B) (insert) Histological examination of the sHip-1 gastric mucosa. C) H+/K+-ATPase-β subunit staining (red) in the sHip-1 mice and non-transgenic controls. Low and high-power confocal images are shown in the left and right panels respectively. The DAPI nuclear stain is pseudo-colored in grey. P-values are indicated such that ** P < 0.01.
Shh peptide and H+/K+-ATPase-β reside in the same complex
Since the hedgehog pathway is associated with acid secretion2, 5, 6, and Shh co-migrates with H+/K+-ATPase to the parietal cell apical membrane during acid secretion3, 16, we determined whether Shh and H+/K+-ATPase reside in the same subcellular complex. Indeed, Shh protein immunoprecipitated with the H+/K+-ATPase-β subunit protein (Supplementary Fig. 4A). Shh exists inside the cell as a 45 kDa precursor17, then a 39 kDa protein after removal of the signal peptide. Further processing generates a 27kDa C-terminal form, and a 19kDa biologically active form (ShhN)17. The addition of cholesterol and palmitate to the 19kDa form (ShhNp) modifies the mobility of this form such that it migrates faster in an SDS-gel18. Surprisingly, the H+/K+-ATPase-β protein immunoprecipitated only with the faster-migrating processed Shh form, suggesting that only ShhNp formed a complex with the H+/K+-ATPase-β subunit (Supplementary Fig. 4B).
Increased gastrin expression in sHip-1 mice
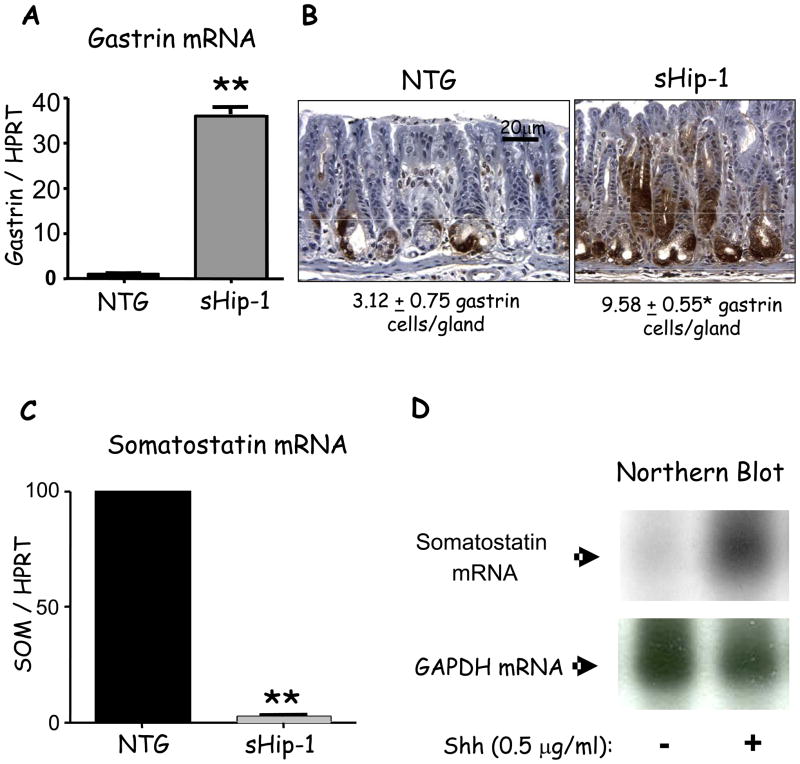
Since the sHip-1 overexpressing mice were hypochlorhydric, we examined whether gastrin gene expression also increased. Indeed, gastrin mRNA levels were significantly elevated (Fig. 3A). Moreover, immunohistochemistry and morphometric analyses revealed a 3-fold increase in the number of gastrin-expressing cells (Fig. 3B). By contrast, somatostatin gene expression was depressed in the sHip-1 mice (Fig. 3C) consistent with elevated gastrin levels. Although suppression of Hh signaling correlated with hypochlorhydria and hypergastrinemia2, 7, we considered the possibility that Hh signaling might regulate gastrin by modulating somatostatin gene expression directly. To test this possibility, enriched cultures of primary D cells were prepared and treated with recombinant Shh. D-cells did not express Shh (Supplementary Fig. 5A), but expressed the Hh receptor Ptch-1 (Supplementary Fig. 5B) demonstrating that they are responsive to Hh ligands. We found an increase in somatostatin gene expression with Shh ligand treatment (Fig. 3D). Therefore, reduced somatostatin levels in sHip-1 mice leading to hypergastrinemia might result from the loss of Shh-mediated D-cell stimulation.
Figure 3. Gastrin and somatostatin mRNA expression levels in sHip-1 mice.
A) Gastrin gene expression relative to hypoxanthine–guanine phosphoribosyltransferase (HPRT) in the sHip-1 antrum versus control mice measured by RT-qPCR. Shown is the mean for n = 3 mice +/− SEM. B) Morphometric quantitation of gastrin protein immunostaining in the sHip-1 antrum versus control mice. C) Somatostatin mRNA expression measured by RT-qPCR in the sHip-1 antrum versus non-transgenic littermate. Shown is the mean for n = 3 mice +/− SEM. D) Northern blot of somatostatin expression in an enriched culture of primary canine D-cells treated with or without 0.5μg/ml recombinant 19kDa Shh peptide. D-cells constitute 70% of the cells in the enriched culture. ** P < 0.01.
Downregulation of Shh mRNA expression in sHip-1 mice
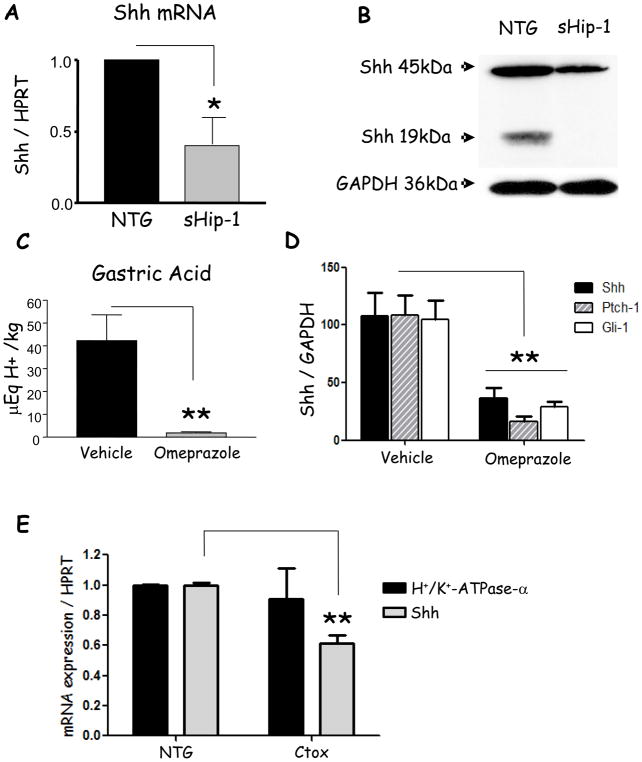
Since expression of sHip-1 was sufficient to inhibit acid secretion and expression of the H+/K+-ATPase enzyme, we examined the effect of acid secretion on endogenous Shh expression. Previously, Suzuki et al. showed in Mongolian gerbils that gastric hypochlorhydria reduces Shh gene expression19. Moreover, we previously reported that acid is required for processing of the nascent Shh peptide to its biologically active form5. However, neither study addresses how acid secretion regulates Shh gene expression. Since gastric acid secretion was reduced in these mice and the Shh locus was still intact in the transgenic mice, we examined the levels of Shh mRNA and protein. Indeed, we found that both Shh mRNA and protein expression were reduced in the sHip-1 mice (Fig. 4A, B). Concluding that the effect on Shh was in response to reduced acid levels6, we treated wild-type mice with omeprazole for 3 days. Consistent with reduced gastric acid in the sHip-1 mice, omeprazole blocked acid secretion (Fig. 4C), Shh gene expression (Fig. 4D), and Hh target genes Ptch-1 and Gli-1 (Fig. 4D). Thus acid secretion is required to sustain Shh mRNA and protein expression5. This observation prompted us to further investigate how acid secretion regulates Shh gene expression.
Figure 4. Shh mRNA expression in sHip-1, omeprazole-treated, and Ctox mice.
A) RT-qPCR demonstrating Shh mRNA expression in the gastric fundus of sHip-1 mice compared to non-transgenic controls. B) Representative western blot of full-length and processed Shh protein in the gastric fundus of sHip-1 mice versus non-transgenic controls. C) Gastric acidity measured by base titration in omeprazole versus vehicle-treated mice. D) RT-qPCR of Shh, Gli-1, and Ptch-1 mRNA expression from the gastric fundus of omeprazole- versus vehicle-treated mice. E) RT-qPCR of Shh and H+/K+-ATPase-α mRNA expression from the gastric fundus of Ctox versus non-transgenic mice. Shown are the means for n = 5 mice per group +/− SEM (for Ctox mice n = 3). P-values are indicated such that * P < 0.05 and ** P < 0.01.
cAMP elevation does not induce Shh gene expression during acid secretion
Previous studies correlated histaminic stimulation of acid-secretion with elevated cAMP levels in the parietal cell20. We therefore investigated the effect of elevated cAMP levels on Shh gene expression in parietal cells. We utilized mice overexpressing cholera toxin, an irreversible stimulator of adenylate cyclase, from the H+/K+-ATPase-β subunit promoter (Ctox mice, line 7), which leads to increased cAMP signaling in parietal cells15. Despite hyperchlorhydria in these mice at 6 weeks of age15, Shh expression was inhibited significantly (Fig. 4E), possibly due to hypogastrinemia. We excluded the possibility that parietal cell atrophy was responsible for the loss of Shh expression by showing that H+/K+-ATPase-α expression was not affected in Ctox mice (Fig. 4E), corroborating previous observations that parietal atrophy does not occur in these mice before they reach 3 months of age15. The downregulation of Shh expression during hyperchlorhydria suggested that the regulation of Shh expression is not related to acid secretion per se, but to signaling processes regulating this process, and that the effect of omeprazole on Shh gene expression might be indirect. We therefore investigated Ca2+i-release and PKC-activation as a potential mechanism for the modulation of Shh gene expression during acid secretion.
Intracellular calcium release modulates Shh expression during acid secretion
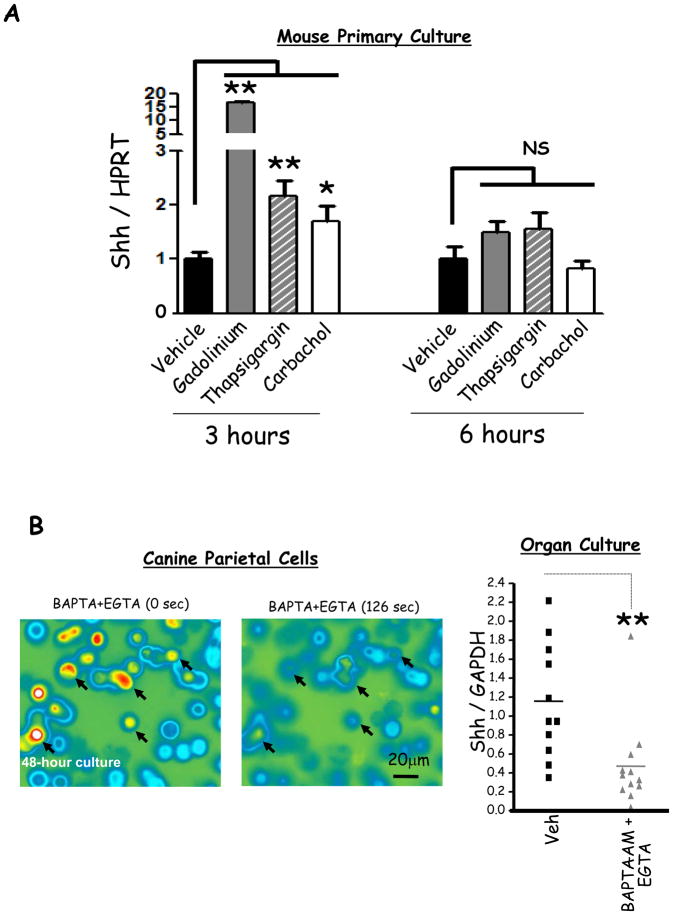
Prior studies have correlated the process of acid-secretion with elevated Ca2+i in the parietal cell. First, cholinergic and hormonal stimulation of acid secretion increases Ca2+i in parietal cells9, 10. Second, thapsigargin- or calcium-sensing receptor (CaSR)-mediated increase in Ca2+i stimulates gastric acid secretion20, 21. Third, gastrin stimulation of acid secretion induces5, while omeprazole-mediated acid inhibition suppresses Shh gene expression (Fig. 4D). We therefore hypothesized that Ca2+i stimulates Shh gene expression. To study the effect of Ca2+i on Shh expression, we treated mouse primary gastric cultures with compounds that increase Ca2+i by three different mechanisms: 1) gadolinium which increases Ca2+i by activating the CaSR21, 2) thapsigargin which inhibits Ca2+-reuptake into the endoplasmic and sarcoplasmic reticulae, and 3) carbachol which elevates Ca2+i via activation of the muscarinic 3 (M3) receptor (Fig. 7). All three compounds increased Shh expression transiently at 3 h, but not at 6 h (Fig. 5A) without affecting cell viability (Supplementary Fig. 6). The induction by Gd3+ was stronger than thapsigargin or carbachol, consistent with the ability of Gd3+ to perform additional functions such as activation of the MAPK pathway and the induction of diacylglycerol (DAG) production22, 23.
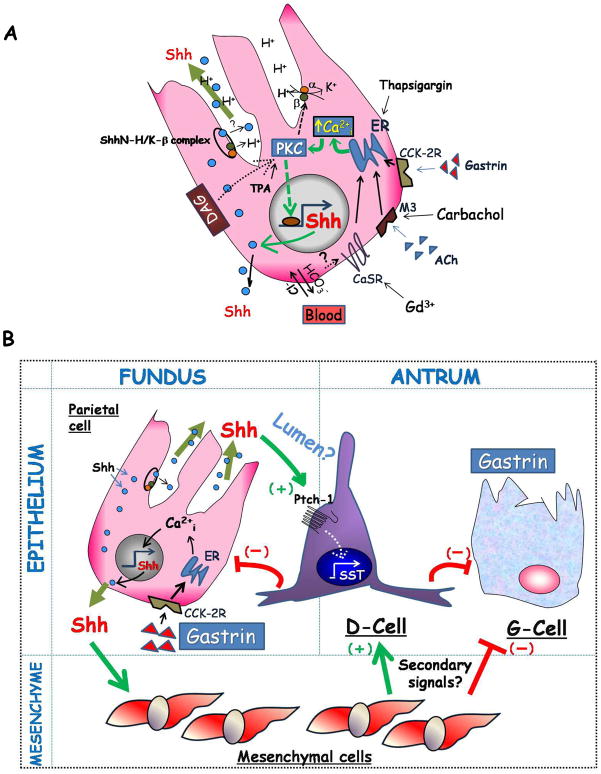
Figure 7. Hypothetical model of Shh in the gastric mucosa.
A) Shown are three mechanisms capable of stimulating acid secretion through an increase in Ca2+i released from the endoplasmic and sarcoplasmic reticulae: 1) hormonal-stimulation with gastrin, 2) cholinergic stimulation with acetylecholine (ACh) through the M3 muscarinic receptor, or 3) alkalinization of the basolateral surface by extruded HCO3− ions leading to the activation of the calcium-sensing receptor (CaSR). Stimulation of acid secretion by histamine release from enterochromaffin-like cells is not shown. Ca2+i-release stimulates acid secretion and Shh gene expression via PKC α and β. PKC-δ might also mediate diacylglycerol (DAG)-induction of Shh gene expression. DAG also stimulates Ca2+i-release and acid secretion. The compounds used in this study, gadolinium (Gd3+), carbachol, thapsigargin and TPA, all stimulated Shh gene expression and are known to increase Ca2+i. Shh protein undergoes processing (intracellular location not defined), migrates to the basolateral membrane, or co-migrates with the H+/K+-ATPase-β subunit to the apical membrane where it is likely to be secreted luminally. B) Luminal Shh potentially targets epithelial cells expressing Ptch-1 such as D-cells to induce non-canonical signaling. In addition, Shh protein reaching the basolateral membrane would target Gli-1-positive mesenchymal cells inducing a secondary signal from the mesenchyme. The outcome of Shh signaling is to induce somatostatin (SST) that in turn inhibits the G-cell and gastrin production, and acid secretion from the parietal cell through paracrine mechanisms.
Figure 5. Effect of Ca2+i release on Shh mRNA expression.
A) Shh mRNA expression in mouse primary fundic culture following gadolinium (Gd3+, 0.5 mM), thapsigargin (1 μM) and carbachol (100 μM) versus vehicle treatment for 3 or 6 hours. Shown is the mean of 6 experiments +/− SEM. B) Left Panel, Fura-2 imaging of canine parietal cells with high levels of Ca2+i after 48-hour culture before and after perfusion with EGTA (4mM) plus BAPTA-AM (10μM). Arrows indicate cultured parietal cells in which Ca2+i were depleted after the perfusion. Right Panel, RT-qPCR of Shh mRNA from mouse fundic organ cultures treated with EGTA (4mM) plus BAPTA-AM (10μM) versus vehicle for 12 hours. Each data point is one mouse. * P < 0.05 and ** P < 0.01.
Prior reports have shown that EGTA and BAPTA-AM treatment of cells depletes extracellular and intracellular Ca2+ respectively24. Therefore we examined whether chelating parietal cell Ca2+i reduced Shh gene expression. We observed that when canine parietal cells were cultured for 48 h, in the absence of any stimulation, they showed a dramatic increase in Ca2+i levels (Supplementary Fig. 7). Hence, we treated these cells with a cocktail of the chelators 48 h post culture. Calcium chelation depleted parietal cell calcium stores as measured by Fura-2 in canine primary cultures (Fig. 5B, left panel) and reduced Shh gene expression in organ cultures from mouse stomachs (Fig. 5B, right panel). We found that EGTA and BAPTA-AM induced intrinsic factor mRNA levels in organ cultures demonstrating that their effect on Shh expression was specific and was not due to general cellular toxicity of the chelators (Supplementary Fig. 8A).
PKC mediates calcium induction of Shh
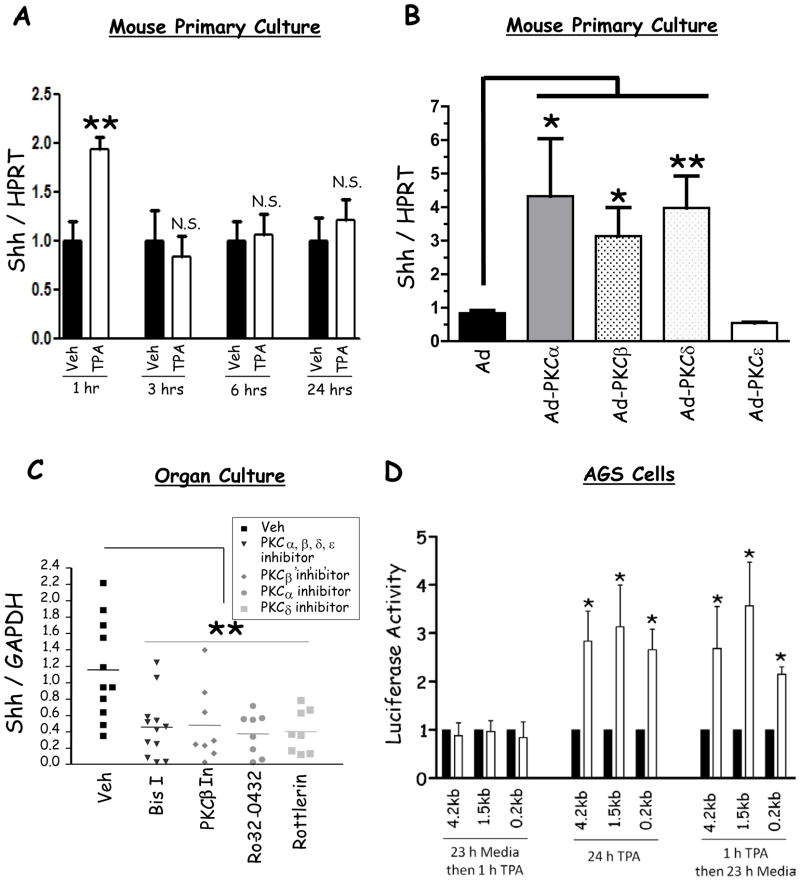
An increase in Ca2+i stimulates several signaling pathways including protein kinase C (PKC)11. Calcium and diacylglycerol (DAG) activate the conventional PKCs (cPKC), PKCα-, βI-, βII-, and γ, whereas only DAG activates the novel isozymes (δ, ε, η, θ and μ). Calcium and DAG do not activate the atypical isozymes (ζ and λ). A recent study showed that PKC stimulates Shh gene expression13. We therefore hypothesized that cPKCs might mediate the effect of calcium on Shh expression. Indeed, treatment of mouse primary gastric cultures with the PKC activator, TPA, transiently induced Shh expression at 1 h, but not after 3, 6, or 24 h of treatment (Fig. 6A).
Figure 6. Effect of PKC on Shh expression in mouse primary cells and gastric cell lines.
A) RT-qPCR for Shh mRNA expression before and after TPA treatment for 1, 3, 6, and 24 hours (shown is the mean of 3 experiments +/− SEM). B) RT-qPCR of Shh mRNA from mouse primary fundic cell cultures transduced with adenoviral vectors overexpressing PKC-α, β, δ, ε, and empty vector (shown is the mean of 6 experiments +/− SEM). The cells were treated with 50 MOI of each of the individual adenoviruses. C) RT-qPCR measuring Shh in mouse fundic organ cultures treated with Bisindolylmaleimide I (Bis I, inhibits α, β, δ, and ε), PKC-β inhibitor, Ro-32-0432 (PKC-α inhibitor), and rottlerin (PKC-δ inhibitor). Each data point indicates one mouse. D) Shh-luciferase reporter activity normalized to Renilla luciferase in AGS cells treated with TPA versus vehicle. The figure demonstrates the reporter activity of the 0.2kb, 1.5kb, and 4.2kb promoters after 23 h incubation in media followed by 1 h TPA, 24 h TPA treatment, and 1 h TPA followed by 23 h incubation in media (shown is the mean of 3 experiments +/− SEM). * P < 0.05 and ** P < 0.01.
In order to determine which PKC isoform regulated Shh expression, we overexpressed two cPKC (α and β) and two novel PKC (δ and ε) isozymes using adenoviruses in mouse primary gastric cultures. Due to the reported low expression levels of PKC-α and β isoforms in gastric parietal cells25, we first confirmed that the expression of the activated forms of these isoforms was detectable in canine parietal cells (Supplementary Fig. 9, antibody recognizes both α and β). Ectopic expression of PKC-α, β, or δ induced Shh expression, whereas PKC-ε did not have an effect (Fig. 6B). Thus even among two novel PKC isoforms (δ and ε) normally activated by DAG, only PKC-δ induced Shh.
Since PKC-α, β, and δ induced Shh expression, we next determined whether inhibition of these isozymes in mouse organ cultures inhibited Shh. We used bisindolylmaleimide I (PKC-α, βI, βII, δ, and ε inhibitor), Ro-32-0432 (PKC-α inhibitor), Rottlerin (PKC-δ inhibitor), and PKC-β inhibitor (#539654 from Calbiochem). These inhibitors significantly depressed Shh expression (Fig. 6C), supporting the results observed with the adenoviruses. The observed effect was specific for Shh, since bisindolylmaleimide I treatment did not affect intrinsic factor gene expression (Supplementary Fig. 8B).
Phorbol ester activates Shh promoter activity
Since phorbol esters directly stimulate PKC, we examined whether TPA stimulated Shh gene expression. To map the DNA regions conferring phorbol ester induction of the Shh promoter, we generated Shh-luciferase reporter constructs that contained 4.2, 1.5, and 0.2 kilobases upstream of the transcriptional start site (Supplementary Fig. 10). After transfecting these constructs into the human gastric AGS cell line, and treating the transformants with TPA, we observed significant induction of the 4.2kb, 1.5kb and 0.2kb Shh reporters at 24 h (Fig. 6D), but not at 1 h (Figure 6D). The delay could be attributed to either the time required for the luciferase protein to be produced as previously reported26, or to the possibility that long-term treatment with TPA inhibited PKC activity. To exclude the second possibility, we treated AGS cells for 1 h with TPA followed by 23 h incubation in media, which induced luciferase activity similar to the 24 h treatment (Figure 6D). These results demonstrated that a TPA-responsive enhancer resided within the 0.2kb proximal promoter.
DISCUSSION
In the current study, we set out to understand the relationship between gastric acidity and Shh gene expression. Prior studies reported that Helicobacter-induced chronic gastritis represses Shh gene expression6, 19, 27. Recently, Zavros and coworkers showed that a conditional deletion of the Shh gene locus in parietal cells induces foveolar hyperplasia, an epithelial lesion that recapitulates the pre-malignant changes observed with chronic gastritis7. We have recently reported that the repression of Shh gene expression during chronic gastritis is linked to the loss of gastric acidity mediated by pro-inflammatory cytokines, e.g., IL-1β6. Both Helicobacter infection in vivo and treatment of parietal cell cultures with either IL-1β or TNFα inhibit acid secretion28, 29. Moreover, prior studies have linked parietal cell acid secretion to an increase in intracellular calcium20. None of the prior studies directly link changes in intracellular calcium to Shh gene expression. Therefore we tested the hypothesis that modulating intracellular calcium was the common mechanism by which inflammation-induced hypochlorhydria regulates Shh gene expression.
We increased intracellular calcium by treating primary gastric cell cultures with agents that work through a ligand-receptor mediated mechanism, e.g. carbachol, or that increase or decrease intracellular calcium levels by activating the calcium-sensing receptor, blocking calcium re-uptake mechanisms, or directly chelating calcium (Fig. 7). We previously showed that gastrin stimulates Shh gene expression5. Gastrin has also been shown to elevate Ca2+i in target cells9. Therefore, our results are consistent with the fact that mechanisms which increase Ca2+i will stimulate Shh gene expression. By contrast, chelating intracellular calcium with BAPTA and EGTA inhibited Shh expression. Since changes in intracellular calcium alone were not sufficient to explain the nuclear events, we considered that Ca2+-dependent signal transduction pathways such as protein kinase C were possibly involved. Indeed, we found that overexpression of the Ca2+ or DAG- dependent PKC isoforms were sufficient to stimulate Shh gene expression and that the induction mapped to specific DNA elements. Although not determined here, PKC regulatory signals typically map to AP1 elements that bind Fos-Jun heterodimers30. Helicobacter infection increases AP1 binding to consensus elements31, 32. Yet there are few studies that have addressed the role of AP1-mediated transcription during Helicobacter infection. Indeed, in silico analysis demonstrated several putative AP1 sites within the responsive elements of the Shh promoter.
We observed in this study that both Ca2+-dependent PKCs α and β stimulated Shh gene expression. However, we also observed that Ca2+-independent PKC-δ stimulates Shh gene expression suggesting that other pathways must contribute significantly to the regulation of Shh mRNA expression. Indeed, the activation of PKC-δ is dependent on diacylglycerol (DAG), a second messenger signaling lipid that also stimulates Ca2+i-release9. Importantly, Gd3+ stimulates the activity of the phospholipase C (PLC) enzyme required for the production of DAG33, suggesting that Gd3+ targets both Ca2+i and DAG production. Also, Gd3+ induced Shh gene expression more potently than thapsigargin or carbachol, which could be mediated by Ca2+i-release and DAG production acting synergistically to stimulate Shh expression. It is also important to note that PKC-α and β stimulated Shh expression even though their expression is barely detectable in parietal cells25. We detected low levels of the activated forms of PKCα/βII in isolated canine parietal cells in this study, suggesting that small amounts of these proteins in parietal cells might be functionally sufficient.
This study suggested that the regulation of Shh expression does not specifically pertain to acid secretion per se, but to signalling pathways associated with this process such as calcium-signaling and PKC. Indeed, we observed using the Ctox mice that, despite hyperchlorhydria (induced through a non-calcium mediated mechanism), Shh gene expression was inhibited possibly due to hypogastrinemia leading to lower Ca2+i levels. This suggested that Shh expression is not directly regulated by the efflux of H+ ions from parietal cells, and that the effect of omeprazole on Shh gene expression is possibly indirect. We speculate that omeprazole might be inhibiting Ca2+i-release in parietal cells in vivo, even though this mechanism has not been explored. However, some studies suggest that omeprazole inhibits the alkaline tide between the parietal cell’s basolateral membrane and the blood34, 35, which is necessary for the activation of the CaSR36, and Ca2+i-release21. Thus the inhibition of Ca2+i-release by omeprazole might be a plausible mechanism affecting Shh gene expression requiring further investigation.
The studies here revealed additional information supporting the notion that Shh protein expression is acid-dependent. In the hypochlorhydric stomach of the sHIP mice, the 19 kDa processed ligand was absent. Recent studies using confocal analysis of Shh during parietal cell acid secretion indicate that secretagogues stimulate movement of the ligand to the apical surface3, 16. Since acid secretion only occurs at the canalicular (apical) membrane, we previously concluded that the processing must also occur at the apical membrane5. We show here that the processed form of Shh co-precipitates with the H+/K+-ATPase-β subunit, consistent with the notion that Shh moves, at least in part, to the apical membrane of the parietal cell3, 16.
Despite these observations, the mechanism by which Shh travels from the parietal cell to its target cells in the stomach remains unknown. Recent reports have shown that Shh-responsive cells in the gastrointestinal tract reside in the mesenchyme37. However, some epithelial cells also express the Ptch-1 receptor38 (unpublished observation), suggesting that gastric epithelial cells might respond to Hh ligands in a non-canonical manner by inducing Erk1/239. In this study, we found that D-cells express the Ptch-1 receptor suggesting that they might directly respond to Shh secreted luminally in a non-canonical manner. In contrast, Shh has also been localized to the basolateral membrane of parietal cells suggesting that it is also secreted basolaterally3 to target the mesenchyme. In the latter example, a secondary signal from the mesenchyme might be responsible for regulating gastrin and somatostatin production in G- and D-cells (Fig. 7). Canonical versus non-canonical Hh signaling might explain the disparate temporal yet distinct functions of the ligand.
Despite the evidence that gastric acid stimulated Shh gene expression, this study also showed that Shh is important for maintaining acid secretion, which suggests a feedback mechanism operating between Shh and gastric acid. A recent report shows that conditional deletion of the mouse Shh gene in gastric parietal cells leads to hypochlorhydria and reduced somatostatin expression7. Collectively, these changes induce hypergastrinemia7. Similarly by blocking Hh ligands, the sHip-1 transgenic mice were hypochlorhydric, exhibited reduced somatostatin expression, and developed hypergastrinemia (Fig. 7).
In conclusion, we show here that gastric acid secretion induced Shh gene expression via the release of Ca2+i, which in turn activates calcium-specific PKC-α and PKC-β. These results provide a more detailed molecular mechanism that links Helicobacter infection to regulation of Shh gene expression and gastrin production.
Supplementary Material
Acknowledgments
Grant support: This study was supported by Public Health Service Grants P01-DK62041 (J.L.M.) and R56 DK058312-06A2 (AT).
This work was supported by Public Health Service Grants P01-DK62041 (J.L.M.) and R56 DK058312-06A2 (AT). The generation of the transgenic mice was supported by P30 DK34933 Transgenic Animal Model Core. We thank Professor Jeffrey Molkentin (Cincinnati Children’s Hospital Medical Center) for providing the PKC adenoviruses, and Professors John A. Williams and Margart Westfall for their help and advice on conducting the calcium and PKC experiments. We thank Dr Milena Saqui-Salces for help in preparing the manuscript.
Abbreviations
- Shh
sonic hedgehog
- Ptch-1
Patched-1
- sHip-1
secreted hedgehog-interacting protein 1
- TPA
phorbol-12-myristate-13-acetate
- Ca2+(i)
intracellular calcium
- PKC
protein kinase C
- gadolinium
Gd3+
- DAG
diacylglycerol
Footnotes
Disclosure: The authors have nothing to disclose.
Mohamad El-Zaatari: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
Yana Zavros: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis.
Art Tessier: acquisition of data; analysis and interpretation of data; statistical analysis; technical support.
Meghna Waghray: study concept and design; acquisition of data; analysis and interpretation of data.
Steve Lentz: acquisition of data; technical support.
Deborah Gumucio: study concept and design; critical revision of the manuscript for important intellectual content.
Andrea Todisco: study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding.
Juanita L. Merchant: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–28. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 2.El-Zaatari M, Grabowska AM, McKenzie AJ, Powe DG, Scotting PJ, Watson SA. Cyclopamine inhibition of the sonic hedgehog pathway in the stomach requires concomitant acid inhibition. Regul Pept. 2008;146:131–9. doi: 10.1016/j.regpep.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Zavros Y, Orr MA, Xiao C, Malinowska DH. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am J Physiol Gastrointest Liver Physiol. 2008;295:G99–G111. doi: 10.1152/ajpgi.00389.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem. 2005;280:15700–8. doi: 10.1074/jbc.M413037200. [DOI] [PubMed] [Google Scholar]
- 5.Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, DLG, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem. 2007;282:33265–74. doi: 10.1074/jbc.M707090200. [DOI] [PubMed] [Google Scholar]
- 6.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, Todisco A, Eaton KA, Merchant JL. Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology. 2010;138:562–72. doi: 10.1053/j.gastro.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA, Miller ML, Lertkowit N, Varro A, Hollande F, Zavros Y. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–61. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urushidani T, Forte JG. Signal transduction and activation of acid secretion in the parietal cell. J Membr Biol. 1997;159:99–111. doi: 10.1007/s002329900274. [DOI] [PubMed] [Google Scholar]
- 9.Tsunoda Y, Takeda H, Asaka M, Nakagaki I, Sasaki S. Initial and sustained calcium mobilizations in the parietal cell during stimulations with gastrin, inositol trisphosphate, phorbol ester and exogenous diacylglycerol. FEBS Lett. 1988;232:83–90. doi: 10.1016/0014-5793(88)80391-0. [DOI] [PubMed] [Google Scholar]
- 10.Chew CS, Ljungstrom M. HCl secretion and [Ca2+]i in cultured parietal cells. J Intern Med Suppl. 1990;732:9–15. doi: 10.1111/j.1365-2796.1990.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 11.Zabrenetzky VS, Bruckwick E, Lovenberg W. Calcium stimulation of protein kinase C in the absence of added phospholipids. Biochem Biophys Res Commun. 1981;102:135–41. doi: 10.1016/0006-291x(81)91499-6. [DOI] [PubMed] [Google Scholar]
- 12.Zuscik MJ, D’Souza M, Ionescu AM, Gunter KK, Gunter TE, O’Keefe RJ, Schwarz EM, Puzas JE, Rosier RN. Growth plate chondrocyte maturation is regulated by basal intracellular calcium. Exp Cell Res. 2002;276:310–9. doi: 10.1006/excr.2002.5527. [DOI] [PubMed] [Google Scholar]
- 13.Lu HC, Swindell EC, Sierralta WD, Eichele G, Thaller C. Evidence for a role of protein kinase C in FGF signal transduction in the developing chick limb bud. Development. 2001;128:2451–60. doi: 10.1242/dev.128.13.2451. [DOI] [PubMed] [Google Scholar]
- 14.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–89. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G970–9. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- 16.El-Zaatari M, Saqui-Salces M, Waghray M, Todisco A, Merchant JL. Sonic hedgehog in gastric physiology and neoplastic transformation: friend or foe? Curr Opin Endocrinol Diabetes Obes. 2009;16:60–5. doi: 10.1097/MED.0b013e328320a821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol. 1995;15:2294–303. doi: 10.1128/mcb.15.4.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–20. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Minegishi Y, Nomoto Y, Ota T, Masaoka T, van den Brink GR, Hibi T. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol. 2005;206:186–97. doi: 10.1002/path.1763. [DOI] [PubMed] [Google Scholar]
- 20.Chew CS, Petropoulos AC. Thapsigargin potentiates histamine-stimulated HCl secretion in gastric parietal cells but does not mimic cholinergic responses. Cell Regul. 1991;2:27–39. doi: 10.1091/mbc.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remy C, Kirchhoff P, Hafner P, Busque SM, Mueller MK, Geibel JP, Wagner CA. Stimulatory pathways of the Calcium-sensing receptor on acid secretion in freshly isolated human gastric glands. Cell Physiol Biochem. 2007;19:33–42. doi: 10.1159/000099190. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Chattopadhyay N, Kifor O, Sanders JL, Brown EM. Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem Biophys Res Commun. 2000;279:363–8. doi: 10.1006/bbrc.2000.3955. [DOI] [PubMed] [Google Scholar]
- 23.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–8. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- 24.Heo JS, Lee MY, Han HJ. Sonic hedgehog stimulates mouse embryonic stem cell proliferation by cooperation of Ca2+/protein kinase C and epidermal growth factor receptor as well as Gli1 activation. Stem Cells. 2007;25:3069–80. doi: 10.1634/stemcells.2007-0550. [DOI] [PubMed] [Google Scholar]
- 25.Chew CS, Zhou CJ, Parente JA., Jr Ca2+-independent protein kinase C isoforms may modulate parietal cell HCl secretion. Am J Physiol. 1997;272:G246–56. doi: 10.1152/ajpgi.1997.272.2.G246. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Kamiya H, Harashima H. Kinetic analysis of protein production after DNA transfection. Int J Pharm. 2005;299:34–40. doi: 10.1016/j.ijpharm.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Shiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobater pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol. 2005;100:581–7. doi: 10.1111/j.1572-0241.2005.41001.x. [DOI] [PubMed] [Google Scholar]
- 28.Beales IL, Calam J. Inhibition of carbachol stimulated acid secretion by interleukin 1beta in rabbit parietal cells requires protein kinase C. Gut. 2001;48:782–9. doi: 10.1136/gut.48.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nompleggi DJ, Beinborn M, Roy A, Wolfe MM. The effect of recombinant cytokines on [14C]-aminopyrine accumulation by isolated canine parietal cells. J Pharmacol Exp Ther. 1994;270:440–5. [PubMed] [Google Scholar]
- 30.Jalava A, Mai S. Fos and Jun form cell specific protein complexes at the neuropeptide tyrosine promoter. Oncogene. 1994;9:2369–75. [PubMed] [Google Scholar]
- 31.Mitsuno Y, Yoshida H, Maeda S, Ogura K, Hirata Y, Kawabe T, Shiratori Y, Omata M. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut. 2001;49:18–22. doi: 10.1136/gut.49.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang YJ, Wu MS, Lin JT, Pestell RG, Blaser MJ, Chen CC. Mechanisms for Helicobacter pylori CagA-induced cyclin D1 expression that affect cell cycle. Cell Microbiol. 2006;8:1740–52. doi: 10.1111/j.1462-5822.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwertz DW, Halverson J. Characterization of phospholipase C-mediated polyphosphoinositide hydrolysis in rat heart ventricles. Arch Biochem Biophys. 1989;269:137–47. doi: 10.1016/0003-9861(89)90094-5. [DOI] [PubMed] [Google Scholar]
- 34.Seidler U, Roithmaier S, Classen M, Silen W. Influence of acid secretory state on Cl(-)-base and Na(+)-H+ exchange and pHi in isolated rabbit parietal cells. Am J Physiol. 1992;262:G81–91. doi: 10.1152/ajpgi.1992.262.1.G81. [DOI] [PubMed] [Google Scholar]
- 35.Wood CM, Schultz AG, Munger RS, Walsh PJ. Using omeprazole to link the components of the post-prandial alkaline tide in the spiny dogfish, Squalus acanthias. J Exp Biol. 2009;212:684–92. doi: 10.1242/jeb.026450. [DOI] [PubMed] [Google Scholar]
- 36.Quinn SJ, Bai M, Brown EM. pH Sensing by the calcium-sensing receptor. J Biol Chem. 2004;279:37241–9. doi: 10.1074/jbc.M404520200. [DOI] [PubMed] [Google Scholar]
- 37.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, Dlugosz AA, Kottmann AH, Gumucio DL. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–28. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–51. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 39.Chang H, Li Q, Moraes RC, Lewis MT, Hamel PA. Activation of Erk by sonic hedgehog independent of canonical hedgehog signalling. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.