Abstract
Electroconvulsive therapy (ECT) has unparalleled antidepressant efficacy, but its cognitive side effects may be persistent. Research suggests that the side effects may be at least partially dissociable from the therapeutic effects of ECT, suggesting that distinct cortical networks may underlie them and introducing a role for focal seizure induction as a means of minimizing side effects. In magnetic seizure therapy (MST), magnetic fields avoid tissue impedance and induce electrical currents confined to superficial cortex, facilitating focal seizure induction. The translational development strategy for MST has included: (1) device development, (2) feasibility in animals and initial human trials, (3) testing in nonhuman primates on safety and mechanisms of action (with neuroanatomical, neurophysiological and cognitive endpoints), (4) safety testing in patients, (5) initial efficacy testing in patients, (6) dosage optimization, and (7) randomized comparison with ECT. These stages have been iterative, with results of early clinical testing prompting device enhancements that were, in turn, tested in nonhuman primates prior to human trials. Safety testing was aided by development of a nonhuman primate model of human ECT, and the validation of a cognitive battery for the monkey that is sensitive to the range of effects of ECT on human memory. Human testing has been facilitated by the development of an international consortium of centers addressing various aspects of technique and dose/response relationships. Challenges facing MST are common to other device based therapies: characterizing dose/response relationships, optimizing efficacy, and developing efficient and reliable methods to induce lasting therapeutic change in the circuitry underlying depression.
Keywords: Magnetic Seizure Therapy (MST), depression, electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation (rTMS), seizure
I. Introduction
This manuscript reviews a new investigational therapeutic intervention, Magnetic Seizure Therapy (MST) and the translational strategy that has been applied to its development. This novel brain stimulation modality has its roots in electroconvulsive therapy (ECT), which was introduced to clinical practice in 1938 by Drs. Cerletti and Bini in Italy, and transcranial magnetic stimulation (TMS), which became widely used as a research tool nearly half a century after ECT. TMS is a non-invasive, focal means to stimulate the superficial cerebral cortex, but at higher dosages, it can induce seizures. MST refers to the intentional induction of a seizure for therapeutic purposes using repetitive transcranial magnetic stimulation (rTMS). Thus, the devices that are used for MST are more powerful than the devices used in rTMS, in that they are capable of sustaining long stimulation trains at high frequencies for the purpose of controlled seizure induction in patients under anesthesia. The aim of this experimental convulsive treatment is to achieve more focal seizure induction that would retain the proven efficacy of ECT in the treatment of depression, while reducing the cognitive side effects typically associated with ECT. The approach to realize this goal has been to take advantage of the focality of magnetic fields to more precisely target the brain circuitry believed to be involved in the pathogenesis of depression, while sparing the neural circuitry implicated in the cognitive side effects.
While ECT remains the most effective and rapidly acting antidepressant available, with an unparalleled spectrum of applications spanning depression, psychosis, mania, catatonia, and movement disorders, the mechanisms of action of ECT and the reason for its unmatched efficacy remain a topic under study. In part, this may be because the discovery that seizures were therapeutic was serendipitous: literally decades of its clinical use have been needed to define its therapeutic spectrum and start unraveling its mechanisms. In contrast, pharmaceutical agents today are subjected to systematic animal testing and staged human investigation to determine dosimetry, efficacy, and side effects prior to routine clinical use. This rational approach to intervention development is only now beginning to be applied to device-based therapies in psychiatry, such as TMS and MST. Here we summarize the translational development strategy as applied to MST, and discuss implications for future studies with this and other device-based therapeutic interventions in psychiatry.
II. Physiologically-Informed Device Development
The introduction of ECT transpired at least three decades prior to the initiation by various countries of mandated, step-wise staged testing of new pharmacological treatments. This is now widely accepted as the standard procedure for the development of new therapeutic drugs, with Stage I- through Stage IV clinical trials typically being conducted before any new treatment reaches the wide market. Because ECT was introduced before the US Food and Drug Administration (FDA) implemented requirements for testing the safety and efficacy of medical devices, it was not subjected during its early development to the types of systematic testing that are the norm today. Decades of clinical use eventually revealed that ECT was more effective for depression than for schizophrenia (its original therapeutic target). Clinical studies began to optimize the procedure to reduce its adverse side effects, such as the introduction of anesthesia and engineering changes in electrical pulse shape (sine wave, to brief pulse, to ultrabrief pulse), modifications in electrode placement (bilateral, right unilateral, bifrontal), and dosage relative to seizure threshold. Eventually these discoveries informed the re-design of the devices, but these improvements took decades to achieve and some are only now entering routine clinical practice outside of academic centers.
The recent approach to rational drug discovery, on the other hand, is taking a more targeted approach. Increasingly, pharmacological medications are being created to interact with specific targets based upon knowledge of pathophysiology and molecular pathways. This rational approach to intervention development could likewise be applied to device development. The first step would be the identification of a clinically salient physiological target. Next would be the development of a purpose-built device designed to reach that physiological target. The ability of the device to achieve the physiological target would then be tested in pre-clinical and clinical studies. These results would be used to refine the design of the device, and eventually the refined device would be subjected to step-wise clinical testing of its safety and efficacy.
This staged, rational developmental approach has been applied to MST (as illustrated in Figure 1). The MST device was designed to reach a specific therapeutic target, guided by the aspects of ECT seizure induction that are postulated to be therapeutic (focal frontal seizure induction) and those postulated to be related to cognitive side effects (medial temporal structures). A purpose-built device was developed to achieve reliable seizure induction under anesthesia; that prototype device was tested in its ability to achieve this physiological target in a nonhuman primate model and in initial trials in humans, its safety and efficacy were piloted, and results informed the redesign of the device to achieve higher output. As reviewed below, work with MST originated with animal studies and has progressed through stages of testing in animals and humans that have resulted in iterative improvements in device design.
Figure 1. Stages of physiologically-informed intervention development as applied to Magnetic Seizure Therapy (MST).
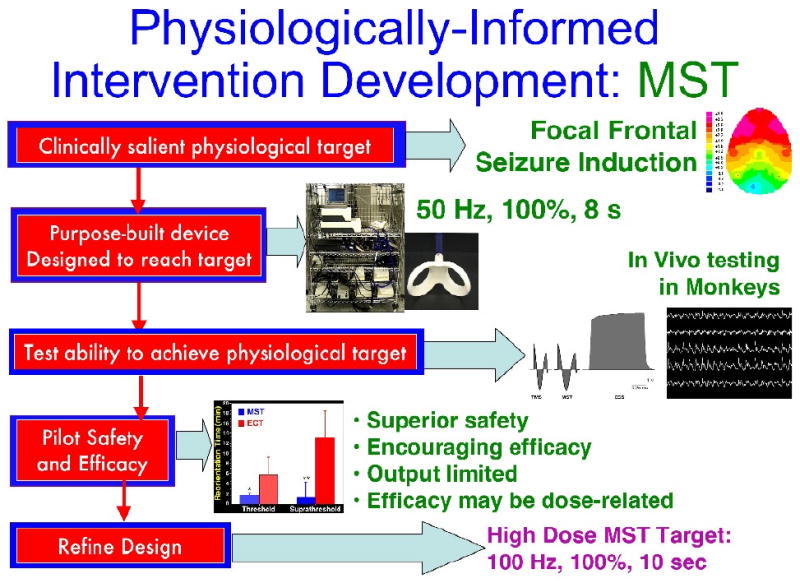
The processes starts with a clinically salient physiological target, which in this case was a pattern of brain activation associated with therapeutic response to ECT, with frontal inhibitory effects as illustrated by frontal delta-band EEG activity (Luber, et al., 2000). A prototype device was designed to achieve this physiological target, which had maximal stimulator output of 50 Hz for 8 seconds, at maximal stimulator output (100% intensity). The ability of this prototype device to achieve the physiological target was tested in nonhuman primates, using intracerebral electrodes to record the strength of the induced electric field of MST, contrasting it with electroconvulsive shock, and to characterize the topography of the induced seizures. The safety and efficacy of this prototype was piloted in depressed patients, yielding supporting evidence of safety relative to ECT and encouraging signs of efficacy in open trials, however there were suggestions that output restrictions may be limiting efficacy. This led to a refined design for a high dose MST device, capable of 100 Hz for 10 seconds at 100% intensity.
III. Rationale for MST
ECT has unparalleled efficacy in the treatment of severe major depression, but its cognitive side effects limit its use (Lisanby, et al., 2000). Extensive research has been conducted in an attempt to improve the risk to benefit ratio of ECT by altering the process of seizure induction, such as electrode placement and electrical parameters of stimulation (Lisanby, 2007). Manipulating these parameters has lessened its side effects, but amnesia is still a major concern for some patients, and may be longer lasting than previously thought (Sackeim, et al., 2007).
There is evidence that the efficacy and side effects of ECT can be at least partly dissociable, as demonstrated by the discovery that high dose right unilateral (RUL) ECT has a comparable efficacy to bilateral (BL) ECT, with a significant decrease in amnesia (Sackeim, et al., 2000). Furthermore, the topographic EEG changes observed during ECT differ from the EEG changes associated with clinical response (Sackeim, et al., 2000). These findings suggest that distinct networks may be associated with ECT efficacy and side effects, and that there may be a role for focal seizure induction to exploit this separation of networks. Research suggests that retrograde amnesia may be related to seizure activity in the medial temporal lobe (Luber, et al., 2000) and consequently, it was proposed that techniques inducing seizure with reduced current spread to this area may cause less retrograde amnesia (Lisanby, 2002, White, et al., 2006). The medial temporal structure specifically associated with amnesia is the hippocampus, which has been shown to be uniquely affected by induced seizures, and to have a low seizure threshold (Lisanby, et al., 2000).
Magnetic stimulation is more focal than direct electrical stimulation because it avoids the impedance of the scalp and skull and results in an induced electric field confined to superficial cortex. This gives more control over the induced electricity and the resultant seizure, and presents the possibility to induce focal seizures in targeted regions while sparing others. Instead of electrical stimulation via metal electrodes in conductive contact with the external surface of the head as used in ECT, the MST device uses a magnetic field with changing flux, which passes unimpeded through the skull and scalp to induce electric current in targeted, specific regions of the cortex. In ECT-induced seizures, control over current density and distribution is limited by impedance of the skull and the resulting shunting of electrical stimulus. Thus, magnetic stimulation has allowed more control over current paths and current density within cortical tissue (Lisanby, et al., 2003).
IV. Intervention Development Platform for MST
The intervention development platform for MST has been supported by an interdisciplinary collaboration, requiring expertise in three core domains: (1) technology development, (2) preclinical testing, and (3) clinical trials. (1) Technology development includes the engineering aspects of device design, modeling (of the circuit topology, coil design, and magnetic and electric field distribution), and in vitro testing (including bench testing and phantom measurements). (2) Preclinical testing includes establishing an animal model, physiological studies (on the electric field distribution and seizure topography), behavioral effects (including cognitive side effects), and neuroanatomical effects (for demonstration of safety and also for mechanisms of action). (3) Clinical testing starts with proof of concept studies, followed by dose-finding studies, randomized controlled trials, and replication studies at other centers.
Feedback from these 3 core domains has influenced iterative MST device improvements. With the results from each domain, we went back to the manufacturer with a new set of requested specifications for device performance and output. The preclinical trials used animal models to characterize the physiology of MST-induced seizures, the cognitive side effects of MST versus ECT, and the safety of MST. The clinical trials have demonstrated the safety and feasibility of MST, shown evidence of superior side effect profile, and promising preliminary evidence for efficacy. These early trials, however, also suggested that the MST device had limited output, and that its efficacy as a therapeutic treatment may be dose-related. As a result, the early-stage devices that produced seizures with 50Hz stimulations applied at 100% power output for 8 seconds, had to be modified through interdisciplinary collaboration and in collaboration with the device manufacturer, to yield a higher-dose stimulation consisting of 10 second10-second duration of 100 Hz at 100% power output (See Figure 2).
Figure 2. Evolution of MST Devices for Non-Human Primate and Clinical Trials.
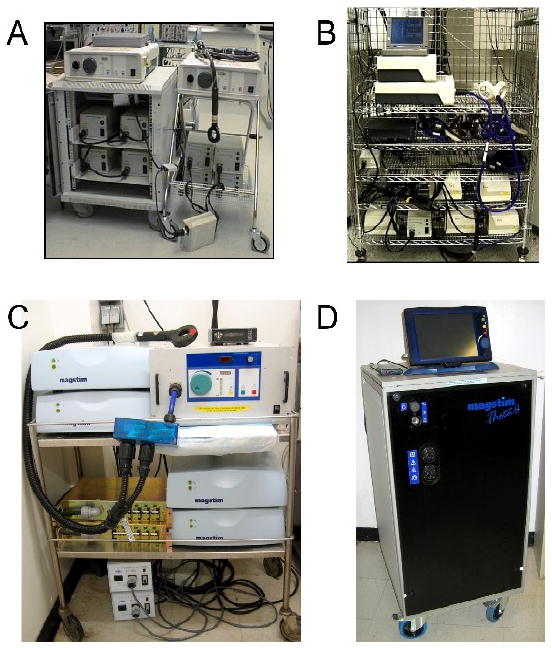
A) First generation nonhuman primate MST device, used in the first MST feasibility trials in rhesus monkeys (Lisanby, et al., 2001) and the first depressed patient (Lisanby, et al., 2001). A conventional rTMS device was expanded by adding 4 charging units, yielding a total of 8 booster modules and a maximal stimulator output of 40 Hz.
B) First generation human MST device used in the first randomized trial of MST in the treatment of depression conducted at Columbia and University of Texas Southwestern Medical Center (Lisanby, et al., 2003, White, et al., 2006). Sixteen charging units were employed, resulting in a maximal stimulator output of 50 Hz, 8 seconds, 100% intensity.
C) Second generation nonhuman primate high dose MST (HD-MST) device used in the first animal studies of HD-MST (Spellman, et al., 2008). A three-phase power supply and other revisions to circuit topology were used to achieve 100 Hz, 10 second trains at 100% intensity.
D) Second generation human HD-MST device used in the first human studies of HD-MST (Kirov, et al., 2008). The Magstim Theta is capable of 1000 pulses per train at 100 Hz and 100% intensity, as well at theta burst stimulation. Three coils can be connected simultaneously to facilitate seizure threshold titration. A touch screen monitor provides an interactive user interface. The self-contained console has a small footprint to fit easily in a typical clinical ECT suite.
V. Technology Development
MST is administered to anesthetized subjects via a custom modified rTMS device that delivers stimuli at convulsive levels. The greatest challenge to early prototypes of an MST device design has been overcoming the anticonvulsant properties of anesthesia. Using anesthetic agents that have a lesser effect on seizure threshold (e.g., etomidate) has reduced this problem somewhat (White, et al., 2006). Further development of the MST device has involved careful consideration of coil design, stimulus parameters, power requirements, heating, and pulse characterization.
Different coil designs have different magnetic field distributions, resulting in variation in seizure threshold, seizure onset, and patterns of seizure spread. We have found the non-focal round coil and the more focal double-cone coil to be reliable in seizure induction, whereas the most focal figure-8 coil has been inefficient in inducing seizure (See Figure 3) (Lisanby, et al., 2003, Lisanby, et al., 2001). Studies are currently exploring how technological changes to coil design can increase the depth of penetration and produce seizure induction from structures deep to the outer surface of the cortex (Deng, et al., 2008, Zangen, et al., 2005). Another potential coil modification is the addition of a metal core that would intensify field strength and allow lower power requirements for the device (Epstein and Davey, 2002).
Figure 3. MST coil design.

Figure 8 coil (left), double cone coil (middle), cap coil (right). The figure 8 coil was least efficient in seizure induction. The Double cone coil conforms well to vertex and midline prefrontal placements and was more efficient in seizure induction. The cap coil, which is a round coil with concave windings to conform closely to the scalp, was highly efficient in inducing seizure with a bilateral placement.
Heating of the coil and other device components occurs during MST, and must be properly handled. These constraints have been lessened through the selection of appropriate heat-tolerant materials and pre-cooling the coil (Lisanby, 2007). The EEG electrodes can also heat up during MST treatment, and nonmetallic EEG electrodes are now available for use. Furthermore, it has also been noted that briefer pulses produce less coil heating, since less energy is needed to cause neuronal depolarization (Jalinous, 2002, Lisanby, 2007, Peterchev, et al., 2007, Peterchev, et al., 2008).
MST devices have larger electrical supply demands than the standard rTMS device, but improvements in design have made the current model more compact and practical in a clinical setting (Lisanby, 2007). Presently, MST devices generate biphasic sinusoidal magnetic pulses because they have higher power efficiency and less coil heating than do monophasic devices (Jalinous, 2002). Studies are currently investigating the relative effectiveness of different TMS pulse characteristics, such as pulse shape and width. Adjusting pulse parameters may improve the efficacy of rTMS, and consequently, MST (Peterchev, et al., 2007, Sommer, et al., 2006, Tings, et al., 2005). Indeed, we recently reported that unidirectional pulses are more efficient in inducing seizure with ECT (Spellman, et al., 2009).
New device designs have increased stimulus output and enabled longer trains of stimulation. Based on experience with ECT, it was suggested that devices which sustain long trains of stimulation may be more efficient when compared to those that generate excessively high frequency for short periods of time (Lisanby, 2007). This lead to the current MST device, which sustains peak output at 10 seconds as opposed to 8 seconds. The newer model has also increased output from 50 Hz to 100 Hz at 100% stimulus intensity. The output was increased to ensure that the MST device could overcome the anticonvulsant effects of anesthesia at sufficient dosages to retain antidepressant efficacy.
The development of the first generation MST device was supported by an NIH grant R01 MH60884, which funded the purchase of the first MST device from the company, according to our requested specifications for device output. The development of the second generation MST device was supported by the competitive renewal of this same NIH grant, which funded the upgrade to the higher output device, according to our specifications.
VI. Translational Development Strategy for MST
The translational development strategy for MST started with demonstrating (a) feasibility in the nonhuman primate and human, (b) testing safety in the nonhuman primate and human, and (c) evaluating efficacy in the human. The developmental process has been iterative, toggling back and forth between the nonhuman primate and human studies at each stage, as reviewed below (and as illustrated in Figure 4).
Figure 4. Translational development strategy applied to MST.
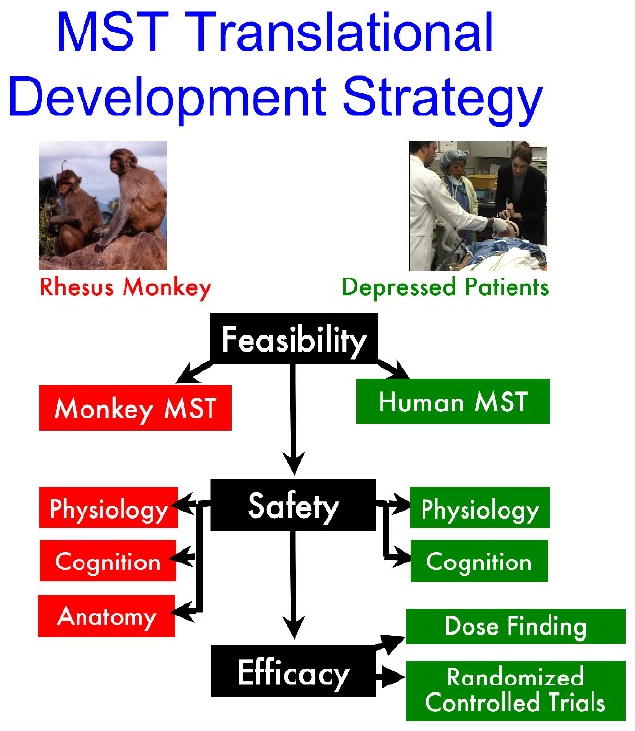
The development of MST progressed through the stages of feasibility testing, safety evaluation, and efficacy trials. At each stage, work toggled back and forth between animal testing in nonhuman primates and clinical trials. Device design was iteratively improved, incorporating results from each stage of development. Animal testing provided the unique opportunity to test the safety of MST using postmortem anatomical studies, while clinical trials provide the unique opportunity to assess efficacy in depressed patients.
VI. A. Feasibility of MST
VI. A. 1. Feasibility of MST in the Nonhuman Primate Model
The first phase in the development of MST was to demonstrate feasibility in an animal model, and then show feasibility in initial proof-of-concept human trials. We selected the rhesus monkey as the animal model for MST feasibility testing after failing to produce seizures in rodents. Monkeys presented a brain-to-coil size ratio that was closer to humans, and the use of pediatric sized coils as opposed to the much smaller rodent coils reduced problems stemming from excessive coil heating. They also have the advantage of being able to perform cognitive tasks with which to access the relative cognitive effects of MST and ECT. In 1998, we performed the first MST-induced seizures in anesthetized rhesus monkeys by applying a custom modified Magstim device, which was capable of an output of 40 Hz at 100% stimulus intensity (Lisanby, et al., 2001). We found the procedure to be reproducible and procedurally quite similar to conventional electroconvulsive shock (ECS) with the exception that post-ictal recovery was exceptionally quick, and post-ictal changes in heart rate and blood pressure did not seem as marked.
VI. A. 2. Feasibility of MST in Depressed Patients
We went on to perform the first feasibility testing of MST in a depressed patient in 2000, in Bern, Switzerland (Lisanby, et al., 2001). The patient was a 20-year-old woman with medication-resistant depression who was referred for inpatient convulsive therapy. She received a course of four MST sessions, followed by ECT. The MST sessions were conducted in the ECT suite using general anesthesia, and occurred at 3 times per week for 4 sessions total. With the exception of earplugs, modified electroencephalographic electrodes, and the use of an MST coil, the procedure was identical to ECT. MST was administered with a pre-cooled coil positioned at the vertex, or a figure-8 coil held to the right prefrontal cortex. The treatments were delivered at 40 Hz and 100% of the maximum stimulator output of the device available at that time. Each MST session successfully induced a tonic-clonic seizure, as was indicated by motor contraction and EEG. Although the seizures resembled those of ECT, there was a remarkable difference favoring MST in post-ictal recovery time, and in the lack of significant side effects. The patient tolerated the treatments well and only reported a mild headache after one treatment. There were no subjective complaints of impaired memory, and the Mini Mental Status Exam score remained at 30/30 throughout the course of MST treatment, although further neuropsychological testing was not performed. Of note the Hamilton Rating Scale for Depression decreased from a baseline of 20 to 13 after the fourth MST treatment.
The same program in Bern, Switzerland later reported the successful treatment of a second patient suffering from medication-resistant major depression (Kosel, et al., 2003). The treatment parameters were similar to those in the first case, and the patient received twelve MST sessions in an inpatient setting. Tonic-clonic seizures were induced for a total of eleven out of the twelve trials. The time taken to regain orientation after treatment was lower than the average reorientation time for all conventional ECT methods. MST also showed a lower cognitive side effect profile when compared to ECT. The patient tolerated the MST well and had no complaints of any cognitive impairment, headache, or muscle pain.
VI. B. Safety of MST
VI. B. 1. Safety of MST in the Nonhuman Primate Model
After demonstrating feasibility, the next step was to systematically examine the safety of the procedure in the monkey model and in the human. In the monkey, we characterized the (1) neurophysiological, (2) cognitive, and (3) anatomical effects of MST.
Neurophysiological studies of MST have shown the electric field and resultant seizure to be more focal than with ECS. By implanting multi-contact intracerebral electrodes, we recorded the electric fields induced in the brain with MST and ECS, and also characterized the resultant seizures. We reported that the electric fields induced by MST within the cortex are less intense and more confined to the superficial cortex than those induced by ECS (Lisanby, et al., 2003, Peterchev, et al., 2007). Specifically, we found MST induced seizures to be less robust, less generalized to deeper brain structures (i.e. hippocampus), and to have less post-ictal suppression. Seizures induced by MST were also found to have a reduced prolactin surge (Morales, et al., 2003) and to have a less immediate post-treatment bradycardia, consistent with less seizure spread to the diencephalons and insula, respectively.
To characterize the neurophysiological effects of chronic exposure to MST, we performed quantitative analyses of ictal expression and post-ictal suppression following a 4-week course of daily ECS, MST, or anesthesia-alone sham (Cycowicz, et al., 2008). Both ECS and MST were given bilaterally at 2.5 × seizure threshold. These scalp EEG results supported our earlier findings from intracerebral electrode recordings demonstrating that MST- and ECS-induced seizures elicit differential patterns of EEG activation. ECS showed significantly more marked ictal expression, and more intense post-ictal suppression than MST in the theta, alpha, and beta frequency bands. However, ECS and MST were indistinguishable in the delta frequency, demonstrating that MST differs in some respects but shares some physiological effects seen with ECS.
To examine the cognitive effects of chronic exposure to MST, it was first necessary to develop and validate a primate model for the amnestic effects of ECT in the monkey. To this end, we developed and tested the Columbia University Primate Cognitive Profile (CUPCP) (Moscrip, et al., 2004). The CUPCP is a three-task cognitive battery presented to rhesus monkeys via a touch sensitive computer monitor. Task 1 measures post-intervention orientation time and long-term memory. Task 2 measures working memory, immediate learning, and retention over a delay. Task 3 measures serial memory and involves 3-item lists to assess learning and memory skills. Anterograde amnesia was indicated by poor retention of a 3-item list learned on the same day after intervention. Retrograde amnesia was indicated by poor performance on lists learned previously to the intervention. Using this animal model, we found that chronic exposure to daily MST results in more benign cognitive side effects than ECS (Moscrip, et al., 2006). Monkeys were slower to complete tasks following ECS versus MST, and were overall less accurate in long and short-term memory and recall tasks post ECS when compared to sham and MST.
Next, we studied the anatomical effects of chronic exposure to MST using neuropathological, stereological, and immunohistochemical methods. We reported that neither MST nor ECS induced signs of neuropathological lesion (Dwork, et al., 2004). Furthermore, stereological cells counts of prefrontal cortex (3 regions) and the dentate gyrus (2 regions) showed no reductions in neuron or glial cell counts following treatment (Dwork, et al., Submitted, Lisanby, et al., 2005). Neuroanatomical studies have also demonstrated differences in the effects of MST and ECS on neuroplasticity. ECS has been shown to increase mossy fiber sprouting in the dentate gyrus of rhesus monkeys after chronic exposure. Such an effect was not found after chronic exposure to MST (Scalia, et al., 2004) using the first generation MST device.
VI. B. 2. Safety of MST in Depressed Patients
The safety of MST in major depression was investigated in a randomized, double-blinded, within-subject comparison of MST to ECT (Lisanby, et al., 2003). Ten inpatients with major depression referred for convulsive therapy were administered MST in two of the first four sessions (random order), and were administered ECT for the remaining sessions. ECT and MST seizure thresholds were titrated in the first two sessions. In the last two sessions, the participants were given suprathreshold doses of ECT and MST, on separate days. Before and after every treatment, a blind rater administered a neuropsychological battery and side-effect rating scale. In all ten participants, seizures were successfully induced with both threshold and suprathreshold level MST. The tonic-clonic seizures resembled those of ECT, except they had a shorter EEG and motor duration than ECT-induced seizures. The ictal power of the MST seizure was also less than that of the ECT sessions. The seizures induced by MST also had a lower ictal EEG amplitude and less post-ictal suppression. After the MST sessions, patients had significantly shorter times to re-orientation, and demonstrated better results on measures of attention, retrograde amnesia, and category fluency. They also reported fewer side effects compared to ECT.
VI. C. Efficacy of MST
Lacking a validated model for antidepressant efficacy in the rhesus monkey that had shown responsiveness to ECS, we examined efficacy in depressed patients. The two openly treated patients in the proof-of-concept feasibility trials both showed clinically significant antidepressant efficacy, but these were not blinded studies (Kosel, et al., 2003, Lisanby, et al., 2001). Subsequently, we conducted a 2-center trial, the aims of which were to provide the first examination of the antidepressant effects of a full course of MST, and to provide the first data on optimization of MST parameters (Lisanby, et al., 2003, White, et al., 2006). This randomized, double-masked controlled trial contrasted two forms of MST that differed in coil type (focal vs. nonfocal) and scalp location (prefrontal cortex vs. vertex). Twenty adults with a major depressive episode were withdrawn from psychotropic medications and randomly assigned to receive MST with a focal double cone coil (DCC) on the midline prefrontal cortex or a nonfocal cap coil on the vertex (coils shown in Figure 3). Seizure threshold was titrated at the first and penultimate treatments, and the remaining treatments were given at the maximal device output (50 Hz, 100% intensity, 8 s duration), three sessions per week. Masked ratings of clinical response were collected at baseline, during the acute treatment phase, and at 2 and 6 month follow-up. Treatment number was titrated to achieve maximal improvement. Masked assessments of neuropsychological functioning were performed at baseline, at each treatment, post treatment, and at 2 months. 20 patients completed the protocol, receiving an average of 9 MST treatments. The 2 coils differed in motor threshold (cap < DCC). While all patients had seizures, the efficiency of seizure induction differed by scalp location (100% at vertex, 12.5% at prefrontal cortex). When the DCC failed to induce a seizure at prefrontal cortex, the coil was moved to the vertex and successfully induced seizures. Seizure threshold increased after the course. Depression scores significantly improved and 53% had at least a 50% improvement. We also compared the dosage requirements for anesthesia, recovery times and electroencephalographic bispectral index (BIS) with simultaneously treated patients receiving ECT. MST was again associated with a decreased orientation time compared to ECT. Interestingly, smaller succinylcholine doses were required during MST sessions. The motor and EEG seizure activity did not differ in this trial, but MST was associated with a reduced variability in the BIS (Lisanby, et al., 2003, White, et al., 2006).
These feasibility data support the safety of administering a course of MST in major depression and show encouraging evidence for efficacy in an open-label setting. However, it is also likely that the therapeutic effects were limited due to under dosing (patients were treated an average of 1.3 × seizure threshold) and suboptimal coil placement. With the substantial increase in seizure threshold across the treatment course, no patient could be treated at the target of 6 times seizure threshold that increases the efficacy of unilateral ECT. Device and coil modifications to rectify these output limitations were therefore undertaken, as reviewed below.
VII. Translational Development Strategy for High Dose MST (HD-MST)
VII. A. Rationale for High Dose MST
The feasibility, safety, and efficacy testing reviewed above were conducted with the first generation MST device capable of delivering a total of only 400 pulses at peak intensity and frequency (50 Hz, 100% intensity, 8 second trains). The first efficacy study of this device reviewed above demonstrated that higher output would be needed to optimize efficacy and reliability of seizure induction throughout a complete course of treatment. Studies show that antidepressant response to ECT is dosage sensitive. For example, 6× seizure threshold RUL ECT can match the efficacy of BL ECT, but with cognitive savings (Sackeim, et al., 2000). High dose RUL is achieved by increasing the number of pulses in the pulse train (either through increased frequency, or train duration, or both). This suggests that cognitive side effects might be more related to electric field amplitude than the number of pulses in the train or the resultant seizure. We sought to test whether the same could be true for HD-MST by determining whether we could increase efficacy without a commensurate increase in side effects by increasing the number of pulses in the MST train. We therefore requested the construction of a device capable of sustaining 100 Hz stimulation at 100% maximal stimulator output for 10 second trains, and then subjected this second generation device to the same stages of translational development, putting it back through the stages of feasibility, safety and efficacy testing.
VII. B. Feasibility of HD-MST
We performed the first HD-MST session in a rhesus monkey in 2004, and have gone on to systematically test the neurophysiological, cognitive, and anatomical effects of HD-MST in the nonhuman primate model. HD-MST has been procedurally identical to the first generation MST device, with the exception that the stimulation train is longer. The first patient with depression to receive HD-MST with this device was treated in 2006 in Cardiff, Wales, and the second HD-MST patient was treated in Edinburgh, Scotland. We reported the results of the first 11 cases receiving HD-MST at these 2 centers. The patients reported less confusion following MST treatment versus ECT, and demonstrated a faster orientation time post MST (Kirov, et al., 2008). The treatments were well tolerated without significant adverse events. Scalp EEG recording from one of these initial patients is shown in Figure 5.
Figure 5. Scalp EEG of a patient undergoing high dose MST for treatment of depression.
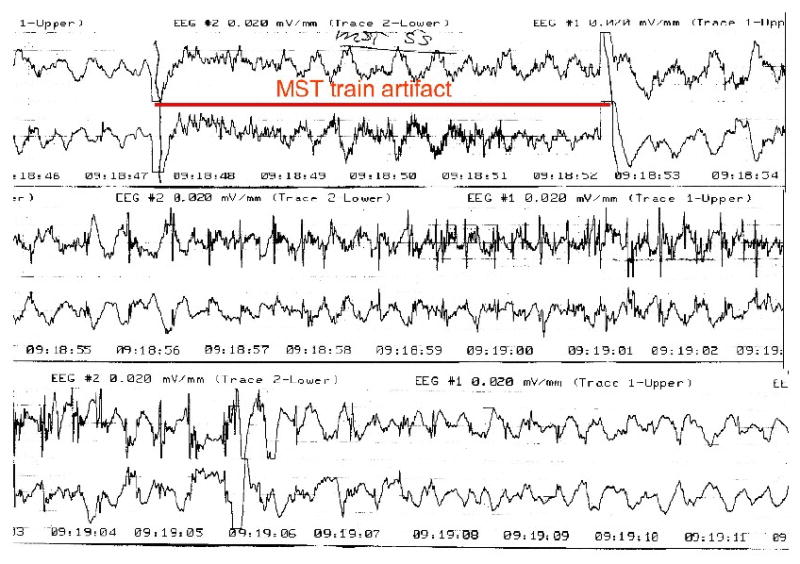
This is a representative 2-channel, bifrontotemporal scalp EEG recording during one of the initial human HD-MST sessions from the case series reported in Kirov et al. (Kirov, et al., 2008). The period of the MST train is marked. Following the train, high frequency spiking can be seen in both channels that evolves into spike-slow wave configuration, and then slow wave activity. This was accompanied by a generalized tonic-clonic seizure as documented by motor convulsion in an unanesthetized limb, employing the cuff-technique.
VII. C. Safety of HD-MST
Since the amplitude of the pulses in HD-MST is identical to those used in the first generation MST device, it was not necessary to repeat the recordings of electric field distribution, as they would be identical on a per-pulse basis.
The first neurophysiological characterization of seizures induced by HD-MST was presented by Cycowicz et al. (Cycowicz, et al., In press). In this study, four rhesus monkeys received 4 weeks of daily treatment with ECS (2.5× seizure threshold), HD-MST (6× seizure threshold), and sham, in a counter-balanced order, and EEGs were recorded during and following the seizure. We found ECS showed significantly more ictal power in all frequency bands than HD-MST.
Cognition was also less affected by HD-MST than ECS. Using an expanded version of the CUPCP, we reported that HD-MST retains cognitive advantages relative to ECS (Spellman, et al., 2008). In this within-subject study, the rhesus monkeys were trained on five cognitive tasks evaluating automatic memory, anterograde learning and memory, spatial and serial working memory, and anterograde and retrograde simultaneous chaining. They were administered daily HD-MST (6× seizure threshold), ECS (2.5 × seizure threshold), and sham for 4 weeks and were tested on the cognitive battery twice daily following treatment. We found HD-MST had a less severe cognitive side-effect profile when compared to ECS. Specifically, the time to complete tasks after HD-MST was not different from sham treatment. Furthermore, subjects took longer to complete tasks following ECS when compared to HD-MST and sham. Treatment effects were also found in four out of six measures of accuracy, in which ECS was worse than sham, but HD-MST was not (Spellman, et al., 2008). It is interesting to note that the degree of cognitive impairment was correlated with higher ictal expression in the scalp EEG (Cycowicz, et al., 2008).
VII. D. Efficacy of HD-MST
Clinical trials are now being conducted to compare the efficacy of HD-MST with ECT for the treatment of depression. A two-center randomized trial is currently underway at Columbia University Medical Center / New York State Psychiatric Institute and at the University of Texas Southwestern Medical Center, contrasting HD-MST with ultrabrief pulse right unilateral ECT at 6× seizure threshold. There are similar trials underway at Cardiff University in Wales and the Royal Edinburgh Hospital in Scotland. The goal of these trials is to investigate alternate stimulation sites for seizure induction, alternate coil configurations, and to compare MST with conventional ECT. Other work is planned or underway in Germany, Australia, and the US on a different MST device capable of even higher stimulation frequencies.
VIII. Mechanism of Actions
To date there are no studies using functional imaging to examine the mechanisms of action of MST. Since both MST and ECT are convulsive technologies, it is assumed there may be partial overlap in their mechanisms. ECT exerts a range of neurobiological effects on functional brain activation, neurotransmitter systems including those of seratonin, hypothalamic–pituitary–adrenal axis (HPA-axis) normalizing the dexamethasone suppression test, and neuroplasticity. ECT is also a powerful anticonvulsant (Rowny and Lisanby, 2008). The enhanced focality of MST leads to the expectation that MST will exert less of an effect on deeper brain structures. The ability to induce a seizure with MST without exposing deeper brain regions to the direct effects of the induced electric field provides an opportunity to study the relative contribution of the electricity and the seizure in producing the clinical effects seen with ECT. However, the relations between differential impact of seizures on functional brain activation and clinical outcome are not known. Such information will be necessary to inform the development of versions of convulsive therapy with an improved risk/benefit ratio.
IX. Conclusions and Future Directions
The strategy that has been applied to the development of MST illustrates the power of the translational approach to rational device design. The process started with engineering development, followed by animal testing, and finally clinical testing. However, these stages were iterative, with new device designs being re-evaluated in the animal model prior to human testing.
There is much left to be learned about MST, as it is still in the early phases of clinical testing. Ongoing preclinical and clinical trials are currently seeking to answer questions about dosimetry, optimal stimulation site, mechanism of action, and patient selection. The results from these and future studies will inform how MST is delivered at will influence its ultimate clinical role. Future generations of MST technology should be fine-tuned in regards to optimal dosing strategies, coil type and coil position. Developing new coil designs will likely allow enhanced focality of seizure induction and should minimize the spread of seizure, as these are the overall goals of MST. Additionally, the optimal dosage of MST has yet to be established. Although MST is currently performed 3 times per week, as is ECT, there may be a more efficacious frequency of treatment. Furthermore, more work must be done to understand how patients should be selected for treatment. Currently, MST is tested in depressed patient who are referred for convulsive therapy. The patient subgroups that will benefit the most from MST have yet to be identified.
Convulsive therapy is still the most effective and rapidly acting antidepressant available, and is useful for the treatment and management of other conditions where less invasive pharmacological treatments have failed. Work to date suggests that MST has a more benign cognitive side effect profile than ECT, and current studies are comparing its efficacy as an antidepressant treatment to ECT. MST also provides a new tool to study the mechanism of action of ECT. Because it can deliver focal stimulation to specific regions of the brain, it can be used to study the relationship between stimulation site and clinical outcomes. Ultimately, understanding what makes ECT so much more effective and rapidly acting than other available therapies may inform the development of more targeted and safer treatments in the future.
Acknowledgments
The authors appreciate the help of Ms. Yael Cycowicz who assisted with the revision of this paper.
Disclosures: Support for the development of Magnetic Seizure Therapy has come from NIMH R01 MH60884, the Stanley Medical Research Foundation, American Federation for Aging Research / Beeson Scholars Program, the National Alliance for Research on Schizophrenia and Depression (NARSAD), and equipment support from Magstim Company and MagVenture A/S. The authors hold no patents or stocks related to MST.
For work unrelated to MST, Dr. Lisanby has received research support from Neuronetics, Cyberonics, ANS, DARPA, and NYSTAR. Columbia University has filed a patent application for novel TMS technology developed in Dr. Lisanby's laboratory, not related to the topic presented here. Dr. Lisanby Chairs a Data Safety and Monitoring Board for a trial of a cortical stimulator sponsored by Northstar Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cycowicz YM, Luber B, Spellman T, Lisanby SH. Differential neurophysiological effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) in non-human primates. Clinical EEG and Neuroscience. 2008;39 doi: 10.1177/155005940803900309. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Luber B, Spellman T, Lisanby SH. Neurophysiological Characterization of High Dose - Magnetic Seizure Therapy: Comparisons with Electroconvulsive Shock (ECS) and Cognitive Outcomes. Journal of ECT. doi: 10.1097/YCT.0b013e31818dd40a. In press. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Peterchev AV, Lisanby SH. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS) Conf Proc IEEE Eng Med Biol Soc. 2008;2008:5675–5679. doi: 10.1109/IEMBS.2008.4650502. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Arango V, Underwood M, Ilievski B, Rosoklija G, Sackeim HA, Lisanby SH. Absence of histological lesions in primate models of ECT and magnetic seizure therapy. Am J Psychiatry. 2004;161:576–578. doi: 10.1176/appi.ajp.161.3.576. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Romme-Christensen J, Bonde-Larsen K, Scalia J, Underwood MD, Arango V, Pakkenberg B, Lisanby SH. Unaltered Neuronal and Glial Counts in Frontal Cortex and Hippocampus Following Magnetic Seizure Therapy or Electroconvulsive Therapy in a Rhesus Monkey Model Submitted. [Google Scholar]
- Epstein CM, Davey KR. Iron-core coils for transcranial magnetic stimulation. J Clin Neurophysiol. 2002;19:376–381. doi: 10.1097/00004691-200208000-00010. [DOI] [PubMed] [Google Scholar]
- Jalinous R. Principles of magnetic stimulator design. In: Pascual-Leone A, D N, Rothwell J, Wassermann EM, Puri BK, editors. Handbook of Transcranial Magnetic Stimulation. Arnold; London: 2002. pp. 30–38. [Google Scholar]
- Kirov G, Klaus EP, Scott AF, Atkins M, Khalid N, Carrick L, Stanfield A, O'Carrol RE, Mustafa HM, Lisanby SH. Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. The British Journal of Psychiatry. 2008 doi: 10.1192/bjp.bp.107.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosel M, Frick C, Lisanby SH, Fisch HU, Schlaepfer TE. Magnetic seizure therapy improves mood in refractory major depression. Neuropsychopharmacology. 2003;28:2045–2048. doi: 10.1038/sj.npp.1300293. [DOI] [PubMed] [Google Scholar]
- Lisanby SH. Update on magnetic seizure therapy: a novel form of convulsive therapy. J ECT. 2002;18:182–188. doi: 10.1097/00124509-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Lisanby SH. Electroconvulsive therapy for depression. N Engl J Med. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Husain MM, Morales OG, Thornton WL, White PF, Payne N, Rush AJ, Sackeim HA. Controlled clinical trial of the antidepressant efficacy of magnetic seizure therapy in the treatment of major depression. 2003;166 [Google Scholar]
- Lisanby SH, Luber B, Finck AD, Schroeder C, Sackeim HA. Deliberate seizure induction with repetitive transcranial magnetic stimulation in nonhuman primates. Arch Gen Psychiatry. 2001;58:199–200. doi: 10.1001/archpsyc.58.2.199. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA. Safety and feasibility of magnetic seizure therapy (MST) in major depression: randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology. 2003;28:1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Maddox JH, Prudic J, Devanand DP, Sackeim HA. The effects of electroconvulsive therapy on memory of autobiographical and public events. Arch Gen Psychiatry. 2000;57:581–590. doi: 10.1001/archpsyc.57.6.581. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Moscrip T, Morales O, Luber B, Schroeder C, Sackeim HA. Neurophysiological characterization of magnetic seizure therapy (MST) in nonhuman primates. Suppl Clin Neurophysiol. 2003;56:81–99. doi: 10.1016/s1567-424x(09)70212-0. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Pakkenberg B, Dwork AJ, Christensen JR, Larsen KB, Scalia J, Underwood M, Sackeim HA, Arango V. Stereological and neuropathological examination of the safety of electroconvulsive shock (ECS) and magnetic seizure therapy (MST) in nonhuman primates. Biological Psychiatry. 2005;57:147S. [Google Scholar]
- Lisanby SH, Schlaepfer TE, Fisch HU, Sackeim HA. Magnetic seizure therapy of major depression. Arch Gen Psychiatry. 2001;58:303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- Luber B, Nobler MS, Moeller JR, Katzman GP, Prudic J, Devanand DP, Dichter GS, Sackeim HA. Quantitative EEG during seizures induced by electroconvulsive therapy: relations to treatment modality and clinical features. II. Topographic analyses. J ECT. 2000;16:229–243. doi: 10.1097/00124509-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Morales O, Luber B, Kwon E, Ellsasser R, Sackeim HA, Lisanby SH. Prolactin response to convulsive therapy: magnetic seizure therapy (MST) versus electroconvulsive shock (ECS) in nonhuman primates. J ECT. 2003:19–58A. [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. A primate model of anterograde and retrograde amnesia produced by convulsive treatment. J ECT. 2004;20:26–36. doi: 10.1097/00124509-200403000-00007. [DOI] [PubMed] [Google Scholar]
- Moscrip TD, Terrace HS, Sackeim HA, Lisanby SH. Randomized controlled trial of the cognitive side-effects of magnetic seizure therapy (MST) and electroconvulsive shock (ECS) Int J Neuropsychopharmacol. 2006;9:1–11. doi: 10.1017/S146114570500578X. [DOI] [PubMed] [Google Scholar]
- Peterchev A, Kirov G, Ebmeier K, Scott A, Husain M, Lisanby SH. Frontiers in TMS technology development: controllable pulse shape TMS (cTMS) and magnetic seizure therapy (MST) at 100 Hz. Biological Psychiatry. 2007;61:107S. [Google Scholar]
- Peterchev AV, Jalinous R, Lisanby SH. A transcranial magnetic stimulator inducing near-rectangular pulses with controllable pulse width (cTMS) IEEE Trans Biomed Eng. 2008;55:257–266. doi: 10.1109/TBME.2007.900540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowny S, Lisanby SH. Brain Stimulation in Psychiatry. In: Tasman A, Maj M, First MB, Kay J, JA L, editors. Psychiatry. 3rd. Wiley; 2008. [Google Scholar]
- Sackeim HA, Luber B, Moeller JR, Prudic J, Devanand DP, Nobler MS. Electrophysiological correlates of the adverse cognitive effects of electroconvulsive therapy. J ECT. 2000;16:110–120. doi: 10.1097/00124509-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, Fitzsimons L, Moody BJ, Clark J. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32:244–254. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- Scalia J, Lisanby SH, Underwood M, Sackeim H, Dwork AJ, Morales O, Fung E, Arango V. The spatial distribution of mossy fiber sprouting in non-human primate model for electroconvulsive therapy and magnetic seizure therapy. Biological Psychiatry. 2004;55:207S. [Google Scholar]
- Sommer M, Alfaro A, Rummel M, Speck S, Lang N, Tings T, Paulus W. Half sine, monophasic and biphasic transcranial magnetic stimulation of the human motor cortex. Clin Neurophysiol. 2006;117:838–844. doi: 10.1016/j.clinph.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Spellman T, McClintock SM, Terrace H, Luber B, Husain MM, Lisanby SH. Differential effects of high-dose magnetic seizure therapy and electroconvulsive shock on cognitive function. Biol Psychiatry. 2008;63:1163–1170. doi: 10.1016/j.biopsych.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Peterchev AV, Lisanby SH. Focal Electrically Administered Seizure Therapy: A Novel form of ECT Illustrates the Roles of Current Directionality, Polarity, and Electrode Configuration in Seizure Induction. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tings T, Lang N, Tergau F, Paulus W, Sommer M. Orientation-specific fast rTMS maximizes corticospinal inhibition and facilitation. Exp Brain Res. 2005;164:323–333. doi: 10.1007/s00221-005-2253-6. [DOI] [PubMed] [Google Scholar]
- White PF, Amos Q, Zhang Y, Stool L, Husain MM, Thornton L, Downing M, McClintock S, Lisanby SH. Anesthetic considerations for magnetic seizure therapy: a novel therapy for severe depression. Anesthesia & Analgesia. 2006;103:76–80. doi: 10.1213/01.ane.0000221182.71648.a3. [DOI] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]


