Abstract
Background
Several studies indicate that interstitial and intracapillary monocytes/macrophages (MO) represent a significant proportion of graft-infiltrating cells in renal allografts and that their presence may unfavourably affect clinical outcome. Much less is known about the role of MO in vascular rejection of transplanted kidneys. The aim of our study was to determine the cellular composition of immune cell infiltrates in intimal arteritis and to analyse whether it is associated with features of humoral immunity and impaired graft survival.
Methods
In 34 recipients with vascular rejection, we determined the proportion of intimal and interstitial MO and T-cells (expressed as ratio of CD68- and CD3-positive cells) in immunohistochemically double-labelled slides.
Results
Intimal arteritis is always composed of T-cells and MO with a median CD68/CD3 ratio of 1.03. In 47% of cases, however, T-cells predominate (CD68/CD3 ratio <1). The median interstitial CD68/CD3 ratio is 0.61, with T-cells dominating in 64% of cases. There is no correlation between the cellular composition of arterial and interstitial infiltrates. The proportion of interstitial and arterial MO has no impact on graft survival, and is, in contrast to previous reports on MO in allograft glomerulitis and capillaritis, not associated with C4d staining.
Conclusions
Intimal arteritis in kidney allograft rejection is composed of a mixed infiltrate of MO and T-lymphocytes. In contrast to MO in PTCitis and glomerulitis, the MO in intimal arteritis are not associated with markers of humoral immune response and there are no different allograft outcomes between MO and T-lymphocyte-dominated groups.
Keywords: intimal arteritis, kidney allograft, lymphocyte, macrophage, monocyte
Introduction
Immune cells infiltrating kidney allograft are the most obvious signs of rejection. Based on experimental evidence and the morphologic appearance of inflammatory infiltrates in histology, cellular rejection is assumed to be predominantly T-cell-mediated [1]. Efficacy of therapeutic agents that are primarily aimed at depleting T-cells further supports this view. Nonetheless, numerous studies report that monocytes/macrophages (MO) are also part of the inflammatory infiltrates in the interstitial compartment, tubuli, peritubular capillaries [2,3], glomeruli [4] and even within the arterial intima [5,6]. The Banff Classification [1] defines intimal arteritis as ‘lymphocytic infiltration beneath the endothelium’ and in the latest update to the Banff Classification intimal arteritis is considered to be a variant of T-cell-mediated rejection, categorized as ‘Banff Type II’ rejection [7]. A recent study by Matheson et al., however, seemed to shed a different light on the immunologic mechanisms of vascular rejection. In a small series of allograft biopsies, the authors found that MO are the predominant inflammatory cells in renal allograft intimal arteritis [6]. This somewhat surprising finding has potential therapeutic implications since one could assume that in cases with MO-dominated intimal arteritis, T-cell-depleting therapy might be less efficient than in cases with prominent T-cell infiltration.
It might furthermore be of interest to investigate the contribution of MO in vascular rejection since infiltrates rich in MO have been associated with humoral rejection and unfavourable outcome in other compartments of the kidney [4,8,9].
The aim of our study therefore was to determine the ratio of MO in a larger series of biopsies and to determine whether the cellular composition of intimal arteritis is associated with humoral rejection and an unfavourable outcome.
Material and methods
Patients and biopsies
We initially identified all 181 cases of vascular rejection that were reported between 1997 and 2003, in our renal biopsy database. The original slides of all cases were reviewed in order to confirm the diagnosis and to check whether diagnostically relevant arteries were present on the last section of the original slide series and whether the paraffin block still contained sufficient tissue for further sectioning. At this stage, 104 cases had to be excluded due to the lack of tissue or arteries. The remaining 77 biopsies were cut for immunohistochemical double labelling for CD68 and CD3. In addition, we performed C4d staining on all biopsies. After analysis of immunohistochemically stained slides, 31/77 biopsies had to be excluded due to the lack of arteries. Another four biopsies were also excluded for statistical reasons since they contained less than a total of 15 intimal inflammatory cells (despite lesions having been more prominent in the original slides). A total of 42 biopsies (from 42 patients) could eventually be included into our study.
Immunosuppressive therapy
The majority of patients were on calcineurin-inhibitor-based immunosuppression (94%). Four patients received induction treatment with an anti-lymphocyte polyclonal antibody and three with an anti-IL-2 receptor antibody. Two recipients were subjected to peri-transplant immunoadsorption according to a previously described protocol (Table 1) [10]. Based on the findings in the study biopsy, all patients received anti-cellular rejection treatment (anti-lymphocyte antibodies and/or high dose steroids). A total of 33 patients received one (n = 31) or two (n = 2) courses of anti-lymphocyte serum (ALS) and/or muromonab-CD3 (OKT3). Three patients suffering from C4d-positive rejection were in addition treated by immunoadsorption according to a local protocol [10]. A switch to tacrolimus was performed in 12 patients.
Table 1.
Basic immunosuppression and demographic data
| Overall n = 34 (100) |
CD68/CD3<1 n = 16 (47) |
CD68/CD3>1 n = 18 (53) |
P-value | |
|---|---|---|---|---|
| Immunosuppression (initial therapy) | ||||
| Prednisolone | 34 (100) | 16 (100) | 18 (100) | |
| Mycophenolate mofetil | 29 (85) | 13 (81) | 16 (88) | |
| Cyclosporine | 29 (85) | 14 (87) | 15 (83) | |
| Tacrolimus | 3 (9) | 2 (12) | 1 (6) | |
| ALS/OKT3 | 4 (12) | 1 (6) | 3 (17) | |
| IL-2 receptor blockers | 3 (9) | 0 | 3 (17) | |
| FTY720 | 1 (3) | 1 (6) | 0 | |
| BMS (belatacept) | 2 (6) | 0 | 2 (11) | |
| Immunoadsorption | 3 (9) | 1 (6) | 2 (11) | |
| Azathioprine | 4 (12) | 2 (12) | 2 (11) | |
| Recipients | ||||
| Sex (female) | 19 (56) | 9 (56) | 10 (56) | ns |
| Age at biopsy | 46 (40–60) | 52 (36–60) | 46 (41–61) | ns |
| Retransplantation | 9 | 2 | 7 | ns |
| Biopsy time after TX | 14.5 (8–30) | 10.5 (8–15) | 19.5 (8.5–33.5) | ns |
| eGFR (MDRD equation) at biopsy time | 9 (5–24) | 18 (5–32) | 8 (5–14) | ns |
| eGFR (MDRD equation) at 30th day after biopsy | 30 (19–52) | 29 (24–52) | 31 (15–56) | ns |
| Serum creatinine (mg/dL) at biopsy | 4.04 (1.92–5.67) | 4.29 (2.14–5.93) | 3.89 (1.77–5.25) | ns |
| Latest pre-TX PRA >10% | 13 (38) | 6 (38) | 7 (39) | ns |
| HLA mismatch >3 | 14 (41) | 6 (38) | 8 (44) | ns |
| Donors | ||||
| Donor age | 49 (38–58) | 51 (38–57) | 46 (35–59) | ns |
| Type (deceased/living) | 31 (91)/3 (9) | 15 (94)/1 (6) | 16 (89)/2 (11) | ns |
| Cold ischaemia time (in hours) | 13.4 (8–17.25) | 10.67 (8–18.25) | 13.9 (8.82–17.25) | ns |
ALS, anti-lymphocyte serum; OKT3, muromonab; IL-2, interleukin-2; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; TX, transplantation; ns, not significant.
Data are given as counts and percentages (in brackets) or median and interquartile range (range from the 25th to the 75th percentile).
Clinical follow-up
The clinical follow-up after biopsy was available for at least 45 months (mean 84 months, median 84 months, range 45–123 months).
For the assessment of renal function, we used the estimated glomerular filtration rate (eGFR) calculated according to the abbreviated Modification of Diet in Renal Disease (MDRD) equation. To avoid a bias by including artificially low creatinine values in patients under dialysis treatment, we defined an eGFR value of five for dialysis-dependent patients and applied non-parametric statistical calculations.
Immunohistochemistry and immunofluorescence
Immunohistological double labelling for CD3 and CD68 was performed on 2 μm thick paraffin sections. After deparaffinizing and rehydration, antigen retrieval consisted in pressure cooking (1 bar, 20 min) in a 0.01M citrate buffer solution at pH = 6. Blocking of endogenous peroxydase and proteins (Ultravision LP detection system HRP Polymer kit, Lab Vision, Fremont, CA, USA) was carried out. The first monoclonal antibody against CD3 (Clone 7, Lab Vision) was incubated at a dilution of 1/1000 in 1% BSA/PBS solution, 1 h at room temperature. DAB was used as chromogen (ImmunoHisto Peroxidase Detection kit from Pierce, Rockford, IL, USA). The second step of double labelling was performed using an antibody against CD68 (Clone PG-M1, Dako, Glostrup, Denmark) at a dilution of 1/50, incubated over night at 4°C. The Ultravision LP detection system AP Polymer kit (Lab Vision) with Fast-Red substrate pack (ID Labs, London, ON, Canada) for visualization was used. Slides were then slightly counterstained with Mayer’s hemalaun.
C4d staining was performed on paraffin sections utilizing a polyclonal antibody (C4dpAB Biomedica, Vienna, Austria) as previously described [11].
For automated cell counting of interstitial infiltrates, we performed immunofluorescent double labelling of CD3 and CD68. Pre-treatment of slides including antigen retrieval was identical, but was followed by incubation in a blocking solution (Superblock, ID Labs, London, ON, Canada) for 7 min. The slides were then incubated simultaneously with the two primary antibodies diluted in 1% BSA/PBS (rabbit anti-CD3, dilution 1:100 and mouse anti-CD68, clone PG-M1, dilution 1:30, both from Dako, Glostrup, Denmark) at room temperature for 1 h, followed by incubation with second antibodies diluted in 1% BSA/PBS (goat anti-rabbit IgG Alexa Fluor 488 and goat anti-mouse F(ab)2 fragment Alexa Fluor 594, dilution 1:1000, both from Molecular Probes, Eugene, OR, USA) for 45 min. Nuclear counterstaining was DAPI.
Omission of first antibodies was performed for negative control slides.
Evaluation of intimal immune cells
We counted the number of intimal (but not luminal) CD68-positive MO and CD3-positive lymphocytes in all affected arteries (arterial vessels with at least two continuous layers of myocytes) in needle biopsies and evaluated the five largest arteries in surgical biopsies (Figure 1). The total number of arteries evaluated in this study was 116. The mean number of arteries per biopsy was 3.41 (median 3, range 1–8). For evaluation of data, we calculated the average ratio of CD68- and CD3-positive cells for the whole biopsy. C4d positivity was defined as linear circumferential staining in at least 25% of PTC [11].
Fig. 1.
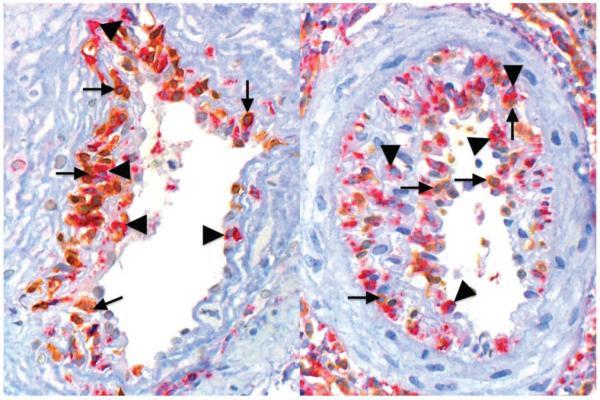
Immunohistochemistry for CD68 (red staining, triangles) and CD3 (brown staining, arrows) of kidney allografts demonstrating T-lymphocyte-(left) or MO-dominated (right) intimal arteritis. Ratios CD68/CD3 were obtained counting manually the number of each cell population within intima of all arteries in each biopsy specimen or of the five biggest arteries in each surgical specimen.
Automated phenotypic characterization of tissue-infiltrating leukocytes by TissueFAXS®
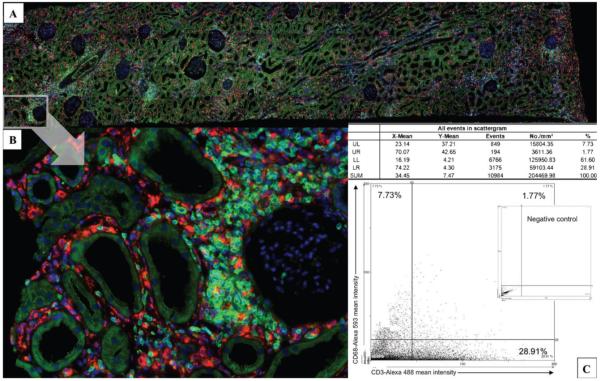
After immunofluorescent staining, tissue sections were scanned using a TissueFAXS® cytometer (TissueGnostics, Vienna, Austria). Entire tissue sections were acquired using a 20× objective lens. Using the system’s built-in autofocus, all fields of view (Figure 2B) were automatically recorded and aligned to one large overview region (Figure 2A). Generally, the entire sample was analysed except non-cortical renal tissue areas and regions with major artefacts. This selection was made manually upon visual control of the digital sample overview.
Fig. 2.
Immunofluorescence for CD68 (Alexa 488, green signal) and CD3 (Alexa 594, red signal). The slides are photographed (A, B) and the cells are electronically counted using by the TissueQuest® analysis software (TissueGnostics). The software identifies graft-infiltrating CD3+ and CD68+ leukocytes; results are visualized using scattergrams (C) and settings for signal positivity are used to evaluate the quantity of CD68- and CD3-positive cells, thus obtaining the interstitial ratio CD68/CD3. For each sample analysed, a negative control slide was available.
Analysis of immunofluorescence samples was done by the TissueQuest® analysis software (TissueGnostics). Details of the method have been described before [12,13]. This method of tissue cytometry is especially qualified to provide phenotypic characterization of tissue-infiltrating leukocytes, as has been shown in several studies [14,15]. In brief, the TissueQuest® analysis software identified graft infiltrating CD3+ and CD68+ leukocytes using a proprietary search algorithm that first identifies DAPI-labelled nuclei and then measures the mean relative fluorescence intensity around each nucleus, taking care that neighbouring nuclei are not considered as ‘cytoplasm’ of the cell. For each cell and fluorescence channel, nine different parameters were determined. The mean relative intensity turned out to be the best parameter to visualize leukocyte populations on resulting scattergrams (Figure 2C) and was used throughout this study to distinguish positive and negative cell populations. For each sample analysed, a negative control slide was available. The fluorescence reactivity determined by TissueQuest® for the control samples defined the level of unspecific staining reactivity and allowed us to determine the individual cut-off value for each sample. Only objects exceeding the fluorescence intensity in the respective channel were accepted as specifically stained and therefore as marker-positive cells. The amount of CD3+ T-lymphocytes and CD68+ macrophages was provided ‘total count’, ‘cells per mm2’ and ‘relative percentage’ for each sample analysed. This analysis also included intimal cells, which, however, on average accounted for <2% of the total cell count and therefore should not have confounding statistical impact.
Statistical analysis
Comparisons between groups were made using chi-square and Fisher exact tests as appropriate. For comparison of continuous variables, Mann–Whitney U-tests were used. Comparison of Kaplan–Meyer survival between groups was performed by the log-rank test using SPSS 10.0.7 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
Results
Of 42 recipients meeting the inclusion criteria, we excluded 8 patients who received lymphocyte-depleting therapy before biopsy resulting in a final study population of 34 patients. Basic demographic data are summarized in Table 1.
Intimal arteritis is always composed of T-cells and MO
For statistical analysis, we defined two groups based on a CD68/CD3 ratio cut-off level of 1, thereby separating cases with intimal arteritis predominated by T-cells (ratio <1) or by MO (ratio >1), respectively. The CD68/CD3 ratio was determined by counting all immunohistochemically stained cells within the intima of cortical arteries. We evaluated a total of 6850 intimal immune cells in collectively 116 arteries (Figure 3).
Fig. 3.
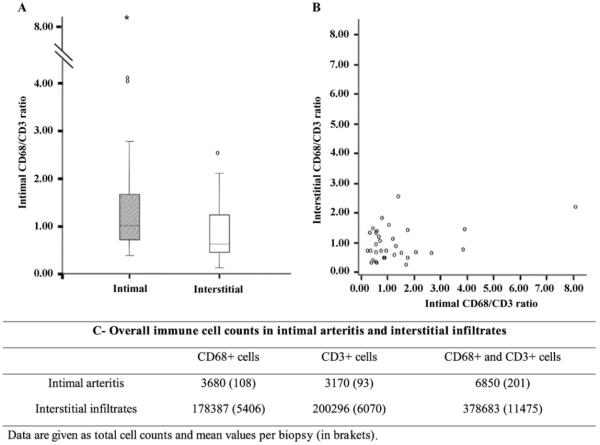
(A) Repartition of the ratios CD68/CD3 in both intimal and interstitial compartments. The median ratio of intimal CD68/CD3 is 1.03 and the interquartile range is 0.73–1.73, whereas the median interstitial CD68/CD3 ratio is 0.61 and the interquartile range is 0.40–1.22. (B) Scattergram of the intimal and interstitial ratios for each case. (C) Overall immune cell counts in intimal and interstitial infiltrates of the 42 cases.
Intimal arteritis always contained both MO and T-lymphocytes. The median ratio of intimal CD68-positive cells/CD3-positive cells was 1.03 (interquartile range, IQR: 0.73–1.73). We detected 18 cases (53%) with an intimal CD68/CD3 ratio of >1 and 16 (47%) with a ratio of <1. In 23.5% of cases, the ratio of monocytic and lymphocytic cells was quite balanced (CD68/CD3 ratio 0.8–1.2). In 76.5% of the cases, we found a clear-cut predominance of either MO (41.2% with CD68/CD3 >1.2) or of T-lymphocytes (35.3% with CD68/CD3 <0.8). In 20.6% of cases (14.7% for MO and 5.9% for T-lymphocytes), we observed a more than 2-fold difference between the two cell populations. Next we analysed whether any morphological lesions were associated with the proportion of intimal MO and T-cells. None of the criteria defined by the Banff Classification and additional morphological features (summarized in Table 2) were unevenly distributed between the groups.
Table 2.
Comparison of histomorphological criteria of allograft injury and markers of humoral rejection between T-cell predominant (CD68/CD3 ratio <1) and MO predominant (CD68/CD3 ratio >1) cases
| Overall n = 34 (100) |
CD68/CD3<1 n = 16 (47) |
CD68/CD3>1 n = 18 (53) |
P-value | |
|---|---|---|---|---|
| Banff ’05 criteria | ||||
| g >0 | 17 (50) | 6 (38) | 11 (61) | ns |
| i >0 | 30 (88) | 15 (98) | 15 (83) | ns |
| t >0 | 30 (88) | 14 (88) | 16 (89) | ns |
| v >0 | 34 (100) | 16 (100) | 18 (100) | ns |
| ah >1 | 1 (3) | 1 (6) | 0 | ns |
| cg >1 | 0 | 0 | 0 | |
| ci >1 | 1 (3) | 0 | 1 (6) | ns |
| ct >1 | 0 | 0 | 0 | |
| cv >1 | 2 (6) | 1 (6) | 1 (6) | ns |
| mm | 1 (3) | 0 | 1 (6) | ns |
| Peritubular capillaritis | 30 (88) | 15 (94) | 15 (83) | ns |
| Interstitial rejection (>i1 and >t1) | 1 (3) | 0 | 1 (6) | ns |
| Other lesions | ||||
| Thrombotic microangiopathy | 6 (18) | 1 (6) | 5 (28) | ns |
| Oedema | 20 (59) | 6 (38) | 14 (78) | ns |
| Acute tubular necrosis | 19 (56) | 8 (50) | 11 (61) | ns |
| C4d staining | ||||
| C4d positivity in PTC | 6 (18) | 3 (19) | 3 (17) | ns |
| C4d positivity in glomeruli | 13 (38) | 7 (44) | 6 (34) | ns |
| C4d positivity in arteries | 2 (6) | 1 (6) | 1 (6) | ns |
PRA, panel-reactive antibodies; PTC, peritubular capillaries; ns, not significant.
Data are given as counts and percentages (in brackets) or median and interquartile range (range from the 25th to the 75th percentile).
Intimal MO are not associated with endothelial C4d deposits or panel-reactive antibodies. Markers of humoral immune response such as C4d deposits in PTC or panel-reactive antibody (PRA) levels at transplantation (TX) time did not significantly differ between the groups (Table 2). There was also no difference in HLA mismatches or the number of previous transplantations (Tables 1 and 2).
Interstitial infiltrates
Given the heterogeneous distribution of MO and T-cells within arteries, we wanted to assess whether the compositions of intimal and interstitial immune cells are correlated. Inflammatory infiltrates were investigated in 33 cases (in one case no more sections for additional staining were available) by immunofluorescent double labelling (Figure 2). For comprehensive enumeration of all stained immune cells within the biopsy, we employed a recently developed automated cell counting system [12,13]. We evaluated a total of 378 683 immune cells (for details see Figure 3). The median interstitial CD68/CD3 ratio was 0.61 (IQR: 0.40–1.22). The study population comprised 12 cases (36%) with a CD68/CD3 ratio of >1 and 21 (64%) with a ratio of <1. The composition of intimal inflammatory infiltrates was not correlated with the composition of immune cells in interstitial infiltrates (Spearman’s Rank test, P = 0.5, r2 = 0.014). Comparing CD68/CD3 ratios (>1 versus <1), we also found no statistical correlation between arteries and interstitial space (Fisher exact test, P = 0.59).
Clinical presentation and outcome
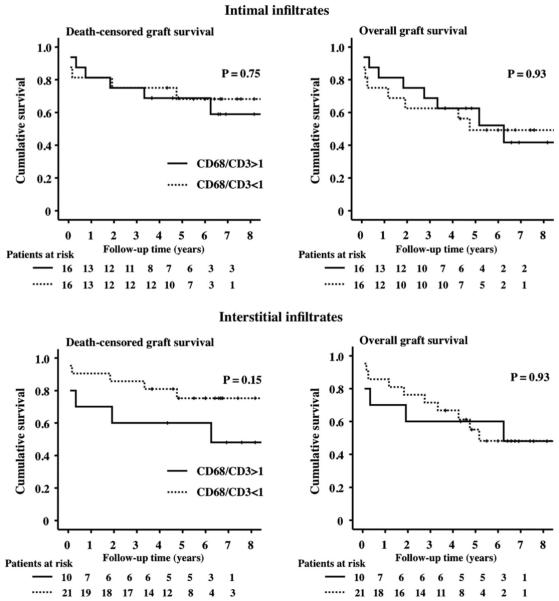
In order to judge whether the composition of intimal or interstitial infiltrates affects response to therapy and outcome, we performed Kaplan–Meyer survival analyses. In two patients, histological analysis was carried out on transplant nephrectomies performed because of critical illness (septicaemia). We therefore excluded these individuals from graft survival analysis. In the remaining 32 patients, we observed no statistically significant differences in death-censored and overall allograft survival for either MO- or lymphocyte-dominated intimal infiltrates (Figure 4, P = 0.75 for death-censored statistics and P = 0.93 for uncensored graft survival). For interstitial infiltrates, we observed a trend towards inferior graft survival when MO dominated the infiltrating cell population. The difference, however, was also not statistically significant (Figure 4, P = 0.15 for death-censored and P = 0.93 for uncensored graft survival).
Fig. 4.
Uncensored and death-censored Kaplan–Meyer survival curves for groups with MO-dominated or T-lymphocytes-dominated intimal or interstitial infiltrates. A trend towards poorer kidney allograft survival is observed when MO dominate in the interstitial infiltrate, which, however, does not reach statistical significance.
In order to evaluate a potential effect of MO numbers on the response to anti-rejection therapy, we calculated the eGFR (MDRD) at the time of biopsy and at 1 month after biopsy for both intimal (Table 1) and interstitial infiltrates [median eGFR at biopsy time: CD68/CD3 <1: 7 (5–17), CD68/CD3 >1: 18 (5–31); median eGFR at 1 month after biopsy: CD68/CD3 <1: 26 (18–52), CD68/CD3 >1: 32 (22–53)]. Estimated GFR values were generally higher after treatment, but the rate of the eGFR increase was not statistically different between patients with low or high proportion of MO within infiltrates.
Discussion
Both cellular and antibody-dependent immune mechanisms may contribute to kidney allograft rejection. Although cellular and humoral immunity are highly interrelated biologically, they still have to be separated diagnostically, since anti-rejection treatment should be aimed at specifically targeting relevant immune mechanisms. Therefore, also the Banff Classification discriminates between cellular and antibody-mediated renal allograft rejection since 2001 [16]. Regarding the mechanisms of cellular rejection, the Banff Classification adheres to the traditional concept that cellular rejection is T-cell-mediated by definition. Further specification of graft-infiltrating cells by immune typing is not implemented into this or other classification systems of rejection and therefore is usually not considered in subsequent therapeutic decisions. This is somewhat surprising since numerous reports indicate that immune cells other than T-cells are commonly infiltrating allografts and likely also have an impact on graft function and outcome. Croker et al. applied the Banff Score of Inflammatory Changes on infiltrating MO (‘macrophage index’, MI) in acute rejection and found that MI was predictive of renal allograft survival and might be a co-factor for the development of chronic allograft nephropathy [17]. The same working group observed a worse outcome when MO infiltrated glomeruli and interstitium and that the MI score correlated with a reduced allograft function at 3 months [9].
Tinckam et al. demonstrated that elevated MO counts within glomeruli in biopsies with acute rejection were predictive of an adverse allograft outcome at 1, 2 and 4 years, which was independent of frequently co-existing humoral rejection [4]. This observation was in accordance with findings of the study from Moreso et al., linking MO in glomeruli to subclinical rejection in protocol biopsies exhibiting interstitial fibrosis and/or tubular atrophy [18]. More recently, Girlanda et al. also described an association of acute allograft dysfunction with monocyte infiltration rather than isolated T-cell infiltration [19].
Among the numerous studies investigating the contribution of MO in various compartments of renal allograft tissue, there are only few reports so far addressing the role of MO in vascular rejection. In a study on transplant nephrectomies, Alpers and co-workers [5] observed that MO were invariably present among intimal inflammatory cells. Quantitation of cells or correlation with clinical findings were, however, not attempted. More recently, Matheson et al. [6] concluded from a study on a small series of biopsies from grafts with vascular rejection (8 cases out of 57 allograft biopsies with various types of rejection) that MO are the predominant inflammatory cells in renal allograft intimal arteritis. This is an intriguing finding given the fact that anti-lymphocytic antibody preparations are the most commonly applied treatment for vascular rejection.
We therefore wanted to determine the composition of intimal infiltrates in a larger series of biopsies for indication and to investigate a potential impact of intimal MO on the graft outcome, which was not analysed by Matheson and colleagues. Similar to the findings of Matheson who observed a higher total number of MO (n = 98) than of T-cells (n = 46) in the arterial intima, we found more intimal MO (n = 3680) than intimal T-cells (n = 3170). Also, the average cell count per artery in our study was higher for MO (n = 32) than for T-cells (n = 27). In contrast to Matheson we, however, observed that MO were not invariably dominating the intimal inflammatory infiltrate. MO outnumbered T-cells (CD68/CD3 ratio >1) in only 53% of cases and strongly predominated in only 14.7% of the samples (CD68/CD3 ratio >2). This discrepancy might, at least in part, be attributable to different methods and thresholds applied for cell counting. In contrast to Matheson et al., who determined the composition of infiltrating by counting absolute numbers of infiltrating cells per vessel in consecutive serial sections, we directly calculated the ratio of CD68/CD3-positive cells in double-labelled slides. We only counted cells in arteries (not arterioles) and excluded for statistical reasons all cases with less than 15 inflammatory cells per biopsy. In Matheson’s study, the majority of evaluated vessels were arterioles and 5/10 evaluated arteries contained only one to three cells per cross-section. Our data provide evidence for a rather mixed immune cell population in the arterial intima of renal allografts, i.e. both MO and lymphocytes participate in the immune response occurring in the arterial wall.
Based on these results, one could assume that ALS or OKT3 treatment might be less efficient if MO are dominating the intimal infiltrate. In order to address this issue, we examined whether the composition of intimal infiltrates had an impact on the allograft outcome. The Kaplan–Meier analysis of survival did not reveal a worse allograft outcome (censored for patient death) if intimal arteritis was dominated by MO, but showed a trend towards an inferior outcome for biopsies with MO-dominated interstitial infiltrates (Figure 4). The differences, however, did not achieve statistical significance. We also could not observe a difference regarding the response to treatment related to the proportion of MO within intimal and interstitial infiltrates. The lacking effect of MO on the outcome is still somewhat surprising because several studies focusing on tubulointerstitial infiltrates, glomeruli or peritubular capillaries emphasized the deleterious effects on allograft function and survival [4,8,9]. A possible limitation of our study is the limited number of recipients. We only could investigate 34 patients since we had to exclude 8 recipients who received anti-lymphocyte treatment before biopsy, which likely had an artificial effect [20] on the composition of interstitial infiltrates [these patients indeed exhibited a median intimal CD68/CD3 ratio of 1.21 (IQR: 0.82–2.31) and a median interstitial CD68/CD3 ratio of 1.90 (IQR: 1.54–2.20)] and on the outcome. One factor that has been previously shown to have an influence on the composition of infiltrates is antibody-mediated rejection. Several studies (including one of our group) recognized MO infiltration in other compartments of the kidney (peritubular capillaries and/or glomeruli) to be associated with C4d positivity [2,3]. Our present study, however, did not reveal any association of intimal MO predominance with markers of humoral reactivity such as C4d deposits or PRA levels. Alpers reported that the proportion of intimal MO rose with increasing chronic vascular lesions. In our population (mostly early post-TX biopsies), the presence of intimal MO was neither linked to the time between TX and biopsy nor to any kind of histological signs of chronic injury.
Since the proportion of MO within intimal infiltrates was highly variable and was not dependent on any clinical or morphological parameter investigated, we wanted to test whether the composition of intimal cells might correlate with that of interstitial infiltrates. Some studies already examined the composition of inflammatory infiltrates under various conditions in different compartments of renal allografts. In all these studies, interstitial and/or glomerular cells were counted manually either in selected areas [19,21] or including the entire biopsy [18]. In order to avoid a selection bias or a sampling error in our analysis, we did not want to restrict our analysis to (either randomly or specifically) selected areas. Manual counting of all infiltrating cells within a specimen, however, proved to be hardly feasible in densely infiltrated biopsies, which were the rule in our study. Since a single histological section may contain more than 50000 immune cells, we took advantage of the recently developed TissueFAXS®technology for automated cell counting in histological slides [12,13], which we described above. Applying this technology to our study population, we found no correlation between the compositions of inflammatory infiltrates in both compartments. This finding illustrates that immune cells do not homogeneously populate the allograft. It is also in line with the traditional diagnostic principle of separately assessing rejection in different renal compartments, which is also the approach taken by the Banff Classification.
In conclusion, intimal arteritis in kidney allograft rejection is composed of a mixed infiltrate of MO and T-lymphocytes. In contrast to MO in PTCitis and glomerulitis, the MO in intimal arteritis are not associated with markers of humoral immune response. We also did not find different allograft outcomes for MO- or T-lymphocyte-dominated groups. However, this aspect will have to be specifically investigated in a dedicated study on a larger number of kidney allograft biopsies.
Acknowledgements
This study was supported by a grant from the Austrian Science Fund (FWF No. 17451 to H.R.).
Footnotes
Conflict of interest statement. Rupert C. Ecker holds partnership shares and an executive position at Tissuegnostics GmbH/Vienna. He was added to the authors list in appreciation of his valuable help in evaluating interstitial immune cell infiltrates. Rupert C. Ecker or other employees from Tissuegnostics were however not involved in the preparation of the manuscript of this entirely investigator driven study. All other authors declare no conflict of interest.
References
- 1.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working Classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 2.Fahim T, Böhmig G, Exner M, et al. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7:385–393. doi: 10.1111/j.1600-6143.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 3.Magil AB, Tinckam KJ. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63:1888–1893. doi: 10.1046/j.1523-1755.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 4.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68:1866–1874. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 5.Alpers CE, Gordon D, Gown AM. Immunophenotype of vascular rejection in renal transplants. Mod Pathol. 1990;3:198–203. [PubMed] [Google Scholar]
- 6.Matheson PJ, Dittmer ID, Beaumont BW, et al. The macrophage is the predominant inflammatory cell in renal allograft intimal arteritis. Transplantation. 2005;79:1658–1662. doi: 10.1097/01.tp.0000167099.51275.ec. [DOI] [PubMed] [Google Scholar]
- 7.Solez K, Colvin RB, Racusen LC, et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 8.Palomar R, Ruiz JC, Mayorga M, et al. The macrophage infiltration index and matrix metalloproteinase-II expression as a predictor of chronic allograft rejection. Transplant Proc. 2004;36:2662–2663. doi: 10.1016/j.transproceed.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 9.Srinivas TR, Kubilis PS, Croker BP. Macrophage index predicts short-term renal allograft function and graft survival. Transpl Int. 2004;17:195–201. doi: 10.1007/s00147-004-0693-8. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz M, Regele H, Schillinger M, et al. Peritransplant immunoadsorption: a strategy enabling transplantation in highly sensitized crossmatch-positive cadaveric kidney allograft recipients. Transplantation. 2005;79:696–701. doi: 10.1097/01.tp.0000148732.26761.fa. [DOI] [PubMed] [Google Scholar]
- 11.Regele H, Exner M, Watschinger B, et al. Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16:2058–2066. doi: 10.1093/ndt/16.10.2058. [DOI] [PubMed] [Google Scholar]
- 12.Ecker RC, Steiner GE. Microscopy-based multicolor tissue cytometry at the single-cell level. Cytometry A. 2004;59:182–190. doi: 10.1002/cyto.a.20052. [DOI] [PubMed] [Google Scholar]
- 13.Steiner GE, Ecker RC, Kramer G, et al. Automated data acquisition by confocal laser scanning microscopy and image analysis of triple stained immunofluorescent leukocytes in tissue. J Immunol Methods. 2000;237:39–50. doi: 10.1016/s0022-1759(99)00240-9. [DOI] [PubMed] [Google Scholar]
- 14.Chang-Rodriguez S, Ecker R, Stingl G, et al. Autocrine IL-10 partially prevents differentiation of neonatal dendritic epidermal leukocytes into Langerhans cells. J Leukoc Biol. 2004;76:657–666. doi: 10.1189/jlb.0204087. [DOI] [PubMed] [Google Scholar]
- 15.Hoetzenecker W, Meingassner JG, Ecker R, et al. Corticosteroids but not pimecrolimus affect viability, maturation and immune function of murine epidermal Langerhans cells. J Invest Dermatol. 2004;122:673–684. doi: 10.1111/j.0022-202X.2004.22324.x. [DOI] [PubMed] [Google Scholar]
- 16.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 Classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Croker BP, Clapp WL, Abu Shamat AR, et al. Macrophages and chronic renal allograft nephropathy. Kidney Int. 1996;50:S42–S49. [PubMed] [Google Scholar]
- 18.Moreso F, Seron D, O’Valle F, et al. Immunephenotype of glomerular and interstitial infiltrating cells in protocol renal allograft biopsies and histological diagnosis. Am J Transplant. 2007;7:2739–2747. doi: 10.1111/j.1600-6143.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- 19.Girlanda R, Kleiner DE, Duan Z, et al. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8:600–607. doi: 10.1111/j.1600-6143.2007.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallon L, Gagliardini E, Benigni A, et al. Immunophenotypic analysis of cellular infiltrate of renal allograft biopsies in patients with acute rejection after induction with alemtuzumab (Campath-1H) Clin J Am Soc Nephrol. 2006;1:539–545. doi: 10.2215/CJN.01741105. [DOI] [PubMed] [Google Scholar]
- 21.Mengel M, Gwinner W, Schwarz A, et al. Infiltrates in protocol biopsies from renal allografts. Am J Transplant. 2007;7:356–365. doi: 10.1111/j.1600-6143.2006.01635.x. [DOI] [PubMed] [Google Scholar]