Abstract
Type 2 diabetes mellitus (T2DM) is rapidly increasing in prevalence and is a major public health problem. It is a progressive disease which commonly requires multiple pharmacotherapy. Current options for treatment may have undesirable side effects (particularly weight gain and hypoglycaemia) and contraindications, and little effect on disease progression. Incretin based therapy is one of several newer therapies to improve glycaemia and is available in two different forms, dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) agonists. Use of these agents results in a ‘glucose-dependant’ increase in insulin secretion and glucagon suppression resulting in improved glycaemia with low incidence of hypoglycaemia. DPP-4 inhibitors are oral drugs which are weight neutral, while GLP-1 agonists are injected subcutaneously and help promote weight loss while improving glycaemia. GLP-1 agonists have also been shown to increase beta cell mass in rat models. Bariatric surgery is another option for the obese patient with T2DM, with blood glucose normalizing in over half of the patients following surgery. Other therapies in development for the treatment of T2DM include sodium-glucose transporter 2 (SGLT-2) inhibitors, glucagon receptor antagonists, glucokinase activators and sirtuins. In this article, we will review the various existing and emerging treatment options for T2DM.
Keywords: DPP-4, GLP-1, HbA1c, incretin, type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a major global public health problem with an estimated prevalence of 6% (246 million) in 2007 expected to rise to 7.3% (380 million) in 2025 [1]. In the UK, the prevalence of diagnosed diabetes in 2005/2006 was over 2 million [2]. The health, social and economic burden is great and presents a huge challenge to healthcare systems worldwide [3–5].
Normal islet function involves increased insulin secretion by the beta cells and reduced glucagon secretion by the alpha cells in response to hyperglycaemia. In T2DM there is both reduced insulin secretion and a paradoxical increase in glucagon following a meal, and the latter remains high despite hyperglycaemia [6]. T2DM is progressive and the natural history of the disease process starts several years before diagnosis with increasing insulin resistance and beta cell dysfunction. When the dysfunctional/failing beta cells are not able to cope with the increasing insulin resistance, plasma glucose starts to rise and the diagnosis of diabetes can be made (Figure 1). In clinical practice, however, there is commonly a further delay in diagnosis of several years [7]. The progressive nature of the disease results in a gradual increase in glycaemia, and a need for incremental therapy with consequent use of combination therapies. Current national and international guidelines advocate the use of metformin alongside diet and lifestyle measures as initial pharmacotherapy, followed by additional oral therapy and finally insulin [8, 9]. The problem with this strategy is that an increment in therapy only follows a worsening of glycaemic control (‘waiting for failure’ approach) with no effect on declining beta cell function (Figure 2). There is also evidence that hyperglycaemia per se has deleterious effects on beta cell function and insulin action (‘glucotoxicity’). Early tight glycaemic control in T2DM can result in remission of T2DM in a proportion of patients, greater preservation of beta cell function and long term benefits from the point of view of reduced risk of vascular complications [10, 11].
Figure 1.
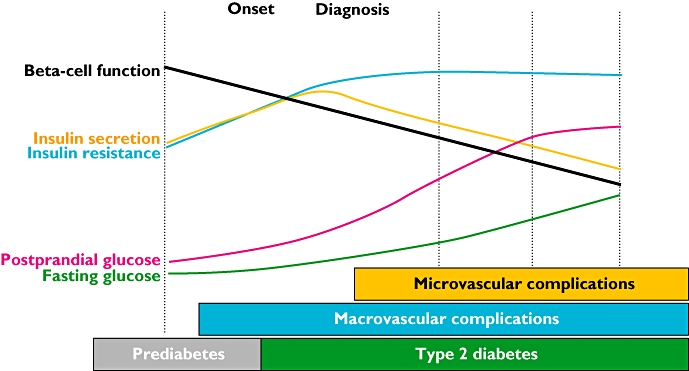
Changing physiology and clinical complications in the natural history of type 2 diabetes. Data extrapolated. Adapted from: Holman RR. Diabetes Res Clin Pract 1998; 40 (Suppl.): S21–5 [162]; Ramlo-Halsted BA, Edelman SV. Prim Care 1999; 26: 771–89 [163]; Nathan DM. N Engl J Med 2002; 347: 1342–9 [164]
Figure 2.
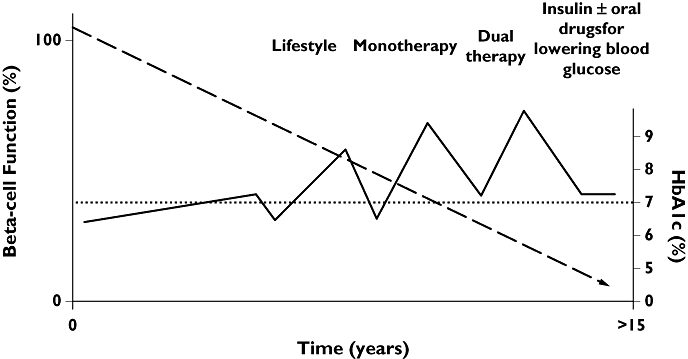
Current therapeutic implications of progressively declining beta-cell function and change in HbA1c in type 2 diabetes. Heine RJ et al. BMJ 2006; 333: 1200–4 [165]
Currently available anti-diabetes agents have some clinical limitations as discussed below, and there is a need for newer therapies with low risk of hypoglycaemia, and lack of weight gain and ideally which also improve beta cell function. These newer therapies should be for use on their own, or in addition to current treatments as combination therapies. The therapies that have recently become available, and those in development, appear to tackle some of these issues, and are discussed below.
Traditionally available anti-diabetes agents
The mode of action of these anti-diabetes agents includes increased insulin secretion – sulphonylureas and insulin secretagogues (meglitinides), improved insulin action – metformin and thiazoledinediones (TZDs) and reduced glucose absorption – alpha glucosidase inhibitors (acarbose). Unless contraindicated or not tolerated, metformin is first line therapy in conjunction with diet and lifestyle in national and international consensus guidelines [8, 9]. It acts by reducing hepatic glucose output and improving peripheral insulin resistance [12]. It is weight neutral with very low risk of hypoglycaemia [13, 14]. Gastrointestinal side effects are common and it is contraindicated because of increased risk of lactic acidosis (0.01 to 0.08 cases per 100 patient-years) in patients with renal, liver and cardiac impairment [12, 15].
Sulphonylureas cause glucose independent closure of the ATP-sensitive K-channels and release of insulin by binding to the SUR1 receptor on pancreatic beta cells. Insulin secretagogues (meglitinides, e.g. nateglinide and repaglinide) work by a similar mechanism to the sulphonylureas on beta cells but are partially glucose dependent and have a quicker onset and shorter duration of action [16]. Weight gain and hypoglycaemia are the main side effects of both sulphonylureas and meglitinides [17, 18], and they need to be used with caution in patients at risk of hypoglycaemia including the elderly and in the context of renal failure [9].
TZDs (pioglitazone and rosiglitazone) are peroxisome proliferator activated receptor-γ (PPAR-γ) agonists that improve peripheral insulin sensitivity by increasing peripheral adipose tissue lipogenesis and reducing hepatic fat content and hepatic glucose production [19]. Their main side effects are fluid retention and weight gain, more so when used in combination with insulin. A possible increased risk of myocardial infarction and cardiovascular risk was suggested by a meta-analysis for rosiglitazone [20], but was not confirmed by a recent cardiovascular endpoint study (RECORD) [21]. Also, an increased risk of fracture and heart failure has been found with both rosiglitazone and pioglitazone [22, 23]. TZDs can be used as third line therapy as per NICE guidance, or second line in patients at risk of hypoglycaemia instead of sulphonylureas [9].
Acarbose is an alpha glucosidase inhibitor in the intestinal brush border that prevents breakdown of complex carbohydrates to monosaccharides and reduces postprandial hyperglycaemia [24]. Gastrointestinal side effects are very common and this has prevented wide use [25]. Insulin treatment can be very effective in improving glycaemic control, but the side effects of hypoglycaemia and weight gain reduce its attraction.
Incretins and incretin based therapy
The ‘incretin effect’ was described following the observation that oral glucose produced a greater insulin response than equivalent intravenous glucose [26]. In healthy individuals, 50–70% of the insulin response to a meal is due to secretion of gut related incretin hormones [27]. In patients with T2DM, the incretin effect is reduced, with a lower insulin secretion in response to oral glucose [28].
Glucose-dependant insulotropic polypeptide (GIP) was the first incretin to be discovered, but glucagon like peptide-1 (GLP-1) seems to have a more major role in the incretin effect [29]. GLP-1 is secreted from the L cells in the ileum minutes after food ingestion, suggesting the involvement of neural or endocrine factors rather than direct stimulation [30]. GLP-1 decreases beta cell workload, hence the demand for insulin secretion, by several pancreatic and extra-pancreatic effects. It slows gastric emptying, reducing peak nutrient absorption and insulin demand (beta cell workload) [31]. GLP-1 also decreases postprandial glucagon secretion from pancreatic alpha cells, which helps to maintain the counter regulatory balance between insulin and glucagon, and this has an indirect benefit on beta cell workload, since decreased glucagon secretion will produce decreased postprandial hepatic glucose output [32]. Finally, the direct effect of GLP-1 on the central nervous system results in increased satiety and a reduction of food intake, which in turn reduces beta cell workload [33].
In addition to glucose-dependant stimulation of beta cells, GLP-1 has been shown to stimulate beta cell proliferation in animal models and suppress glucagon release by alpha cells, as well as increasing insulin gene transcription and all steps of insulin biosynthesis [29, 34–36]. In T2DM, GIP concentrations are either normal or increased, while GLP-1 concentrations are usually reduced which makes GLP-1 a more attractive target for therapeutic development [37, 38]. During a 4 h infusion of GLP-1 (7–36 amide) in fasting patients with poorly controlled T2DM, plasma glucose normalized with significantly increased insulin and reduced glucagon concentrations. When glucose concentrations normalized, both insulin and glucagon returned to baseline values with stable blood glucose despite continued GLP-1 infusion emphasizing the ‘glucose sensitive’ nature of this molecule [39].
Circulating concentrations of native GLP-1 and GIP decrease rapidly after secretion because of rapid inactivation, mainly by dipeptidyl peptidase-4 (DPP-4) [40]. Native GLP-1 as a treatment would therefore need to be infused continuously and is therefore of limited clinical utility. There are two alternative approaches to restore the GLP-1 response. One is to protect GLP-1 from inactivation by DPP-4, and the other is to develop GLP-1 receptor agonists that are resistant to DPP-4 and can mimic native GLP-1. Both of these strategies have been introduced into clinical practice with the development of DPP-4 inhibitors and GLP-1 receptor agonists, respectively. Both classes of drug are described as incretin-based therapies and various drugs of these classes are described in detail below.
DPP-4 inhibitors
Sitagliptin is an orally available potent reversible inhibitor of DPP-4 that has a bioavailability of 87%, and is excreted mainly unchanged in the urine [41–43]. The recommended dose of sitagliptin is 100 mg once daily, and the use of sitagliptin (Januvia) 100 mg was approved by the FDA in October 2006 for use as monotherapy and as add-on therapy to sulphonylureas metformin, pioglitazone or rosiglitazone [44]. Sitagliptin-metformin fixed dose combination (Janumet) was approved at the same time [44]. The EMEA approved its use in March 2007 and has recently modified its recommendations to include its use as monotherapy, dual therapy, triple therapy or use in combination with insulin [45]. Sitagliptin is actively secreted in the tubules with the help of transporter proteins including human organic anion transporter-3 (hoat-3), and renal impairment results in a reduced excretion of sitagliptin, so it is recommended that the dose be reduced to 50% in moderate and 25% in severe renal impairment or end stage renal disease on dialysis [46]. However, the EMEA or FDA do not recommend the use of sitagliptin in people with moderate or severe renal impairment [44, 45].
Sitagliptin was largely weight neutral across most studies, and reduced HbA1c by 0.5% to 0.9% as monotherapy, or as add-on therapy to metformin, glimepiride, pioglitazone, glimepiride-metformin combination, insulin or insulin-metformin combination therapy, and it showed non-inferiority when compared with glipizide (5 mg to 20 mg) and rosiglitazone (8 mg) [47–55]. Hypoglycaemia was comparable with placebo in most studies, but there was an increased risk of hypoglycaemia when combined with sulphonylureas or insulin, although the rate of severe hypoglycaemia was low [49, 54]. Fixed dose combination of sitagliptin with metformin allows dual therapy for T2DM with potential for improved compliance, and no weight gain. Sitagliptin is generally well tolerated with few side effects. There have been recent post-marketing reports of anaphylaxis, angioedema and rashes, including Stevens-Johnson syndrome, as well as pancreatitis in patients treated with sitagliptin. Although a causal link to the drug has not been established, the FDA has recently inserted a new warning about pancreatitis with sitagliptin [44]. Sitagliptin undergoes limited oxidative metabolism by cytochrome P450, although it does not induce or inhibit it. This leaves potential for drug–drug interaction, although studies to date have not shown significant drug interactions [56].
Vildagliptin is another potent orally available DPP-4 inhibitor that is metabolized to metabolically inactive components, the main one of which is LAY151, a carboxylic acid metabolite [57]. There was no significant difference in vildagliptin AUC in normal renal function compared with mild, moderate and severe renal impairment [58]. The recommended dose of vildagliptin is 50 mg twice daily and vildagliptin (Galvus) has had an approval letter from the FDA but they have asked for further safety data regarding skin lesions and kidney impairment that were seen in animal studies before obtaining a license. In Europe, the EMEA has given a licence for vildagliptin (Galvus) and Eucreas (vildagliptin-metformin combination) for use of vildagliptin along with metformin, sulphonylureas or a TZD in September 2007, but it is not licensed as monotherapy or for use with insulin [59].
Vildagliptin is well tolerated and largely weight neutral, and has been shown to reduce HbA1c by 0.44 to 1.4% as monotherapy or add-on to metformin, glimepiride, pioglitazone or insulin with a side effect profile comparable with placebo, low incidence of hypoglycaemia and no clinically significant drug interactions [60–69]. There were similar initial reductions in HbA1c with both vildagliptin and rosiglitazone, but the effect was more sustained at 2 years for rosiglitazone compared with vildagliptin [67]. Animal studies have reported cases of skin rash or blisters [58]. Vildagliptin is metabolized mainly in the liver to inactive metabolites, and there have been rare cases reported of hepatitis so liver function monitoring is recommended with discontinuation if AST or ALT rises to more than three times the upper limit of normal. There is a potential for use of vildagliptin in renal impairment as most of it is metabolized in the liver, but current guidelines do not recommend its use in moderate or severe renal impairment [59].
Saxagliptin is another orally available once daily DPP-4 inhibitor that has a higher specificity for DPP-4 than DPP-8 or DPP-9 and a higher potency than sitagliptin or vildagliptin for DPP-4 inhibition [70]. Saxagliptin is metabolized into an active metabolite (BMS-51089) by the cytochrome P450 CYP3A4/5 enzyme, and the metabolite has two fold less potency than the parent molecule. Part of saxagliptin is renally excreted, and there is a modest increase in AUC of saxagliptin and its active metabolite in moderate and severe renal impairment [71]. There is a less than two fold increase in saxagliptin or its metabolite in any grade of hepatic impairment [72]. Saxagliptin (Onglyza) was approved by the FDA in July 2009 and by the EMEA in October 2009 for use as add-on therapy to metformin, sulphonylureas or TZDs, but not as monotherapy, triple therapy or for use with insulin [73].
Saxagliptin is largely weight neutral, generally well tolerated and has a favourable side effect profile with a low incidence of hypoglycaemia. Common side effects include headache, upper respiratory tract infection and urinary tract infection. It has been shown to reduce HbA1c by 0.62% to 0.83% as monotherapy as well as add-on therapy to metformin, sulphonylureas and TZDs [74–79]. Use in moderate or severe renal impairment or severe hepatic impairment is not recommended, and use in moderate hepatic impairment is advised with caution [73]. Ketoconazole is a potent inhibitor and diltiazem a moderate inhibitor of CYP3A4/5, and they both affect the plasma concentration of saxagliptin [80, 81]. Therefore, caution is advised when using drugs that affect the CYP3A4/5 enzyme.
Other DPP-4 inhibitors in development include alogliptin (Takeda) which has recently completed phase 3 trials, and has shown significant HbA1c reductions as monotherapy, and in combination with metformin, glyburide (glibenclamide), pioglitazone and insulin [82–86]. In June 2009, the FDA requested further data, especially related to cardiovascular outcomes so new phase 3 trials are underway with an aim to resubmit for approval in 2 years time. Linagliptin (Boehringer Ingelheim) is currently undergoing phase 3 clinical trials, and phase 3 trials have been suspended for denagliptin (GlaxoSmithKline).
Sitagliptin, vildagliptin and saxagliptin have already been approved for use, with a number of other DPP-4 inhibitors in development. Their main advantage is that they are oral preparations and are weight neutral with a low risk of hypoglycaemia.
GLP-1 agonists
Exenatide is a synthetic version of exendin-4, a salivary protein found in the Gila monster, with 53% homology with native human GLP-1 but is resistant to the action of DPP-4 [87, 88]. Exenatide (Byetta) was initially licensed by the FDA in April 2005 and the EMEA in November 2006 for use as add-on to metformin and/or sulphonylureas. In December 2006 the FDA modified its licence to include use with TZDs with or without metformin [89, 90]. It is recommended as a subcutaneous injection at a dose of 5 µg twice daily for 4 weeks followed by 10 µg twice daily. The main side effects are nausea and vomiting, which is why the drug is initially given at the lower dose. On post marketing surveillance, 30 cases of pancreatitis were reported in patients on exenatide in 2007 and, in 2008, six cases of haemorrhagic or necrotizing pancreatitis were reported. Cases of patients sometimes requiring haemodialysis and renal transplantation have also been reported. The FDA has therefore changed the labelling on the drug to warn about the possibility of pancreatitis, so caution must be exercised particularly in patients at high risk, e.g. those with a history of gall stones, alcoholism and marked hypertriglyceridaemia [90]. The primary route of degradation and elimination of exenatide is renal [91], and there is a 13, 36 and 84% reduction in clearance of exenatide in patients with mild, moderate and end stage renal disease, respectively, compared with subjects with normal renal function [92]. The FDA has inserted a warning advising against use in severe renal impairment and end stage renal disease, and for use with caution in patients with moderate renal impairment or renal transplantation [90]. Antibody formation has been noted in around 40% of patients taking exenatide, and a study of patients re-exposed to exenatide showed no increase in adverse effects or hypersensitivity reactions in antibody positive subjects but data regarding efficacy were inconclusive [93].
Exenatide has been shown to improve glycaemia by around 1.0%, result in a weight reduction of 1.6 kg to 2.8 kg, and with low rates of hypoglycaemia as shown in the three AMIGO trials (AC2993 Diabetes Management for Improving Glucose Outcomes) where exenatide was used for 30 weeks as add-on to metformin and/or sulphonylureas [94–96]. This improvement was maintained in the open labelled 82 weeks and 3 years extension trials [97, 98]. It has also been shown to result in weight loss and improve glycaemia when used as monotherapy [99] and with TZDs [100]. Although not licensed, when used with insulin, it has been shown to allow reduction of insulin dose requirements with weight loss [101–104]. It has also been shown to be non-inferior to insulin glargine in terms of HbA1c reduction in a 16 weeks double-blind crossover study, with the added benefit of weight loss with exenatide [105]. Preclinical studies have shown that exenatide improves beta cell mass and function [106–110]. It has also been shown to improve surrogate markers of beta cell function determined by HOMA-B after 28 days [111].
Liraglutide is a synthetic analogue of human GLP-1 with 97% homology but is resistant to the action of the enzyme DPP-4. Liraglutide (Victoza) has recently been approved by the FDA in January 2010 for use as second line therapy, as monotherapy or as add-on therapy to oral antidiabetes agents [112], while the EMEA approved its use in June 2009, as add-on therapy to metformin and/or sulphonylureas, and TZDs with or without metformin [113]. It is recommended as a subcutaneous once daily injection of 0.6, 1.2 or 1.8 mg, starting at a lower dose to reduce nausea and vomiting. There was no significant effect of renal or hepatic impairment on the safety or side effect profile of liraglutide [114, 115]. The formation of anti-liraglutide antibodies is reported to be low, in 9.3% to 12.7% of patients, with no reported loss of drug activity or efficacy due to this [116].
The phase III LEAD studies (Liraglutide Effect and Action in Diabetes) were designed to investigate the efficacy of liraglutide at each step in the treatment continuum from monotherapy to combination with two oral antidiabetes drugs, and comparison with insulin glargine (LEAD 5) and head to head with exenatide (LEAD 6) [117–122]. The LEAD trials showed a reduction in HbA1c of around 1.0% when added to metformin or sulphonylurea monotherapy or combination therapy, a greater reduction of HbA1c than rosiglitazone at doses of 1.2 and 1.8 mg, and a greater reduction in HbA1c than insulin glargine at doses of 1.8 mg. LEAD 6 showed a greater reduction in HbA1c with liraglutide than exenatide with similar weight loss. Liraglutide 1.8 mg was used which is not the common dose anticipated to be used in standard practice, whereas 10 µg of exenatide is the standard dose. Weight loss of 0.2 kg to 2.8 kg in the LEAD trials was seen with liraglutide in comparison with weight gain with sulphonylureas, insulin and TZDs. Preclinical studies have shown that liraglutide increases beta cell mass and inhibits apoptosis [123, 124], It also improves surrogate markers of beta cell function determined by HOMA-B and proinsulin to insulin ratio in patients with T2DM [125].
GLP-1 agonists in development
Exenatide LAR (Eli Lilly) is a once weekly preparation of exenatide and is showing promising results. Exenatide LAR 2 mg has been shown to be generally well tolerated and results in significantly greater improvements in glycaemia compared with exenatide 10 µg twice daily, with no increased risk of hypoglycaemia, and with similar weight loss in a 30 weeks trial [126].
Taspoglutide(Roche and Ipsen), albiglutide (Glaxo-Smithkline) and lixisenatide (Sanofi Aventis) are other GLP-1 agonists that are undergoing phase III trials.
There are therefore a number of GLP-1 agonists in development. The newer agents are subcutaneous injections that can be given less frequently (e.g. once weekly) and result in a ‘glucose dependent’ lowering of blood glucose that results in a low risk of hypoglycaemia while also reducing weight. They have shown an improvement in beta cell function and mass in animal models, and there is the potential that they may influence disease progression in humans but this needs to be tested.
Bariatric surgery
Obesity is strongly associated with diabetes [116]. Diet, lifestyle and medical management have limited efficacy in promoting significant weight loss [127]. Surgery is increasingly seen as a durable option for weight loss with bariatric surgery numbers in the USA increasing from >13 000 in 1998 to >72 000 in 2002 and >100 000 in 2003 [128]. Laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic adjustable gastric banding (LAGB) are the most common bariatric procedures performed worldwide. Gastric bypass and gastric banding result in an average weight loss of 45 kg (60% excess body weight) and 32 kg (46% excess body weight loss), respectively [129], with very low complication rates [130]. General complications related to surgery are thromboembolism, gallstones related to weight loss, incisional hernia, gastrointestinal bleeding and wound related problems [131]. Band slippage and erosion through the stomach wall are complications specific to gastric banding and are surgical emergencies, and have been reported in 1–5% of patients [131]. Gastric bypass can be complicated by problems with the anastamoses including stricturing, leakage, bleeding or internal hernia, in addition to long term vitamin and mineral deficiencies [132, 133]. It is also necessary to be aware of altered drug absorption following bariatric surgery. A recent systematic review has highlighted that a third of drugs have reduced absorption following gastric bypass, and although there is little evidence of reduced drug absorption after gastric banding, there is reduced gastric mixing and drug disintegration so use of liquid or soluble medications may be desirable [134]. Weight loss following bariatric surgery is maintained even after 10 years with reduction in mortality and morbidity [135, 136]. Bariatric surgery slows the progression of impaired glucose tolerance to diabetes [137], and facilitates the remission of diabetes in approximately 80% of subjects following LRYGB and approximately 57% following LAGB [129]. The improvement of glycaemia following LRYGB appears to be independent of and precedes weight loss within days following surgery [137]. Resolution of T2DM following bariatric surgery is less common in older patients and those with a longer duration of diabetes [135]. NICE has recommended bariatric surgery as an option for people with BMI >40 kg m–2 or for those with a BMI of 35–40 kg m–2 and a co-morbidity such as diabetes or hypertension [138]. Bariatric surgery is emerging as a promising therapy for T2DM associated with obesity, but there is a need for randomized controlled trials comparing medical vs. surgical treatment as well as studies on the effect of bariatric surgery on the macro and microvascular complications of T2DM.
SGLT2 inhibitors
The transport of glucose into epithelial cells is mediated by an active co-transport system, the sodium glucose co-transporter (SGLT). SGLT mediates renal tubular glucose reabsorption in humans, and SGLT2 is the isoform that appears to be a better target for therapy, and is exclusively expressed in renal proximal tubules so that therapies targeting SLGT2 ought not to affect other tissues [139]. Selective inhibition of SGLT2 increases urinary glucose excretion by inhibiting renal glucose reabsorption [140]. There are several products currently in development which show promising results of which sergliflozin (Kissei Pharmaceuticals/GlaxoSmithKline) and dapagliflozin (Bristol-Myers Squibb and AstraZeneca) are in advanced clinical trials.
Sergliflozin has been shown to be well tolerated at doses of 50–500 mg for 14 days in healthy human subjects and patients with T2DM, and to increase urinary glucose excretion in a dose dependant manner with low risk of hypoglycaemia [141, 142]. Dapagliflozin as a single daily dose, has been shown to reduce HbA1c, fasting and post prandial plasma glucose as well as reduce weight compared with placebo when used as add-on therapy to metformin alone (at doses of 2.5 mg to 10 mg daily) or as add-on therapy to a combination of insulin and oral antidiabetes agents (at doses of 10 mg and 20 mg) [143, 144]. Side effects including hypoglycaemia and urinary tract infections were comparable across all groups including placebo, although the group on 20 mg dapagliflozin had an increased rate of genital infections (principally vaginal thrush) compared with placebo [143, 144].
Glucagon receptor antagonists
Glucagon is produced by alpha cells in the pancreas and increases hepatic glucose production, and thus increases blood glucose particularly postprandially. Antagonizing the glucagon receptor or immunoneutralization of glucogon reduces hepatic glucose overproduction and in turn leads to improved glycaemic control in diabetic animal models [145–147]. A number of glucagon receptor antagonists have been identified and have been shown to reduce the glucose rise seen with exogenous glucagon administration in healthy and diabetic animals [148–151] as well as healthy humans [152]. These agents may provide a further group of medications targeting post prandial glucose.
Glucokinase activators
Glucokinase is a glucose-sensing enzyme found in the liver and pancreas. Activation of this enzyme promotes hepatic glucose uptake and pancreatic insulin secretion [153]. It is therefore is an ideal target for diabetic therapy, and should produce only glucose dependent effects and reduce the potential for hypoglycaemia [153]. A number of glucokinase activators are currently in development, and with promising preclinical data, some of them have advanced into human clinical trials [154, 155].
Sirtuins
Sirtuins are enzymes that seem to be implicated in many diseases associated with advancing age, such as atherosclerosis and T2DM, and were discovered during research into lifestyle and ageing [156]. Sirtuin activation seems to mimic the effect of dietary restriction [157] and leads to multiple metabolic improvements including enhanced glucose utilization, improved insulin sensitivity and increased exercise tolerance [158–160]. Resveratrol, found in red wine and grapes is an example of a naturally occurring sirtuin activator, and improves the survival of obese mice fed a high calorie diet compared with normal mice [161], and is one of compounds in this class that is under development.
Conclusion
Improved glucose control long term is needed to reduce vascular complications. Convenient, effective and well tolerated therapies that can be given early in the course of the disease are needed. All of the traditionally available anti-diabetes agents have a place in the management of diabetes reducing the HbA1c by 0.5 to 2%. Insulin is still required when there is significant beta cell failure, and when treatment with oral or injectable therapy fails or is contraindicated. A combination of side effects, contraindications and lack of effect on disease progression or beta cell failure highlight the need for newer therapies. Single drugs are usually not sufficient to maintain glycaemic control with disease progression, and there is a need to combine several treatments. Combination of the traditionally available anti-diabetes agents is common in current practice, and the newer agents can be used in combination with various agents including insulin. The potential pros and cons of diabetes therapies are compared in Table 1.
Table 1.
Pros and cons of diabetes therapies
| Metformin | Sulphonylurea | TZDs | Acarbose | Insulin | DPP-4 inhibitors | Incretin mimetics | |
|---|---|---|---|---|---|---|---|
| Efficacy | ++ | ++ | ++ | + | +++ | ++ (+) | ++ |
| Influence on disease progression | No | No | (?) | No | No | (?) | (?) |
| Cardiovascular outcome studies | Yes | Yes | Yes | No | Yes | No | No |
| Tolerability | Moderate | Moderate | Moderate | Poor | Moderate | Very good | Moderate |
| Weight gain | No | Yes | Yes | No | Yes | No | Weight loss |
| Hypoglycaemia in monotherapy use | No | Yes | No | No | Yes | No (Yes with sulphonyl-urea or insulin) | No (Yes with sulphonyl-urea or insulin) |
Incretin based therapies have been in use for a few years, and NICE has recently updated their guidelines to include these drugs. DPP-4 inhibitors are particularly recommended second line to metformin if there is significant risk of hypoglycaemia and also third line. GLP-1 agonists are recommended as an option in patients with T2DM and severe obesity (BMI >35 kg m–2), or in patients with BMI ≤35 kg m–2 where therapy with insulin would have significant occupational implications or weight loss would benefit other significant obesity-related co-morbidities [9]. Incretin based therapy improves glycaemic control with good tolerability, beneficial effects on weight and low risk of hypoglycaemia. They are therefore attractive options in the treatment of T2DM. GLP-1 also preserves human islet morphology in vitro with preliminary evidence for improved beta-cell function. GLP-1 agonists are given by injection, and have side effects including nausea. Long term safety data for incretin based therapy is obviously not yet as extensive as for the traditionally available anti-diabetes agents so caution must be exercised. Bariatric surgery is a durable option for weight loss, and is associated with reduced insulin concentrations and improved insulin resistance with increased remission of T2DM. Other newer therapies including SGLT2 inhibitors, glucagon receptor antagonists, glucokinase activators and sirtuins are also showing promising results in clinical trials.
Acknowledgments
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
Conflict of interest
MKP has no declarations. AT is a research training fellow supported by the National Institute for Health Research. AT has also won research grants from Sanofi Aventis and Novo Nordisk UK Research Foundation. AHB has received honoraria for lectures and advisory work and research funding from Sanofi-Aventis, Eli Lilly, Novo Nordisk, Servier Laboratories, Takeda, Merck Sharp & Dohme, Bristol Myers Squibb/Astra-Zeneca, Novartis, Roche and GlaxoSmithKline.
REFERENCES
- 1.IDF. The Diabetes Atlas. 2006. IDF (3rd)
- 2.Diabetes UK. Diabetes: State of the Nations. 2006. Diabetes UK.
- 3.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Wanless D. Securing our future health: taking a long-term view. 2002. HM Treasury.
- 5.Jacobson AM. Impact of improved glycemic control on quality of life in patients with diabetes. Endocr Pract. 2004;10:502–8. doi: 10.4158/EP.10.6.502. [DOI] [PubMed] [Google Scholar]
- 6.Muller WA, Faloona GR, Guilar-Parada E, Unger RH. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970;283:109–15. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- 7.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care. 1992;15:815–9. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Buse JB, Davidson MB, Sherwin R, Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE. Type 2 Diabetes CG87 partial update. 2009. NICE.
- 10.Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–6. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 11.Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F, Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–60. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 13.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 14.UKPDS 34. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 15.Tahrani AA, Varughese GI, Scarpello JH, Hanna FW. Metformin, heart failure and lactic acidosis: is metformin absolutely contraindicated? BMJ. 2007;335:508–12. doi: 10.1136/bmj.39255.669444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dornhorst A. Insulinotropic meglitinide analogues. Lancet. 2001;358:1709–16. doi: 10.1016/S0140-6736(01)06715-0. [DOI] [PubMed] [Google Scholar]
- 17.Black C, Donnelly P, McIntyre L, Royle PL, Shepherd JP, Thomas S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD004654.pub2. CD004654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del PS, Bianchi C, Marchetti P. Beta-cell function and anti-diabetic pharmacotherapy. Diabetes Metab Res Rev. 2007;23:518–27. doi: 10.1002/dmrr.770. [DOI] [PubMed] [Google Scholar]
- 19.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 20.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 21.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, Mcmurray JJ. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 22.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–9. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 24.Salvatore T, Giugliano D. Pharmacokinetic-pharmacodynamic relationships of acarbose. Clin Pharmacokinet. 1996;30:94–106. doi: 10.2165/00003088-199630020-00002. [DOI] [PubMed] [Google Scholar]
- 25.Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, Rutten GE, Van WC. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD003639.pub2. CD003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elrick H, Stimmler L, Hlad CJ, Jr, Rai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–82. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 27.Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–8. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 28.Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 29.Ahren B. Gut peptides and type 2 diabetes mellitus treatment. Curr Diab Rep. 2003;3:365–72. doi: 10.1007/s11892-003-0079-9. [DOI] [PubMed] [Google Scholar]
- 30.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 31.Nauck MA, Wollschlager D, Werner J, Holst JJ, Orskov C, Creutzfeldt W, Willms B. Effects of subcutaneous glucagon-like peptide 1 (GLP-1 [7-36 amide]) in patients with NIDDM. Diabetologia. 1996;39:1546–53. doi: 10.1007/s001250050613. [DOI] [PubMed] [Google Scholar]
- 32.Larsson H, Holst JJ, Ahren B. Glucagon-like peptide-1 reduces hepatic glucose production indirectly through insulin and glucagon in humans. Acta Physiol Scand. 1997;160:413–22. doi: 10.1046/j.1365-201X.1997.00161.x. [DOI] [PubMed] [Google Scholar]
- 33.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA. 1987;84:3434–8. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–69. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 36.Drucker DJ. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–8. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 37.Barnett AH. New treatments in type 2 diabetes – a focus on the incretin-based therapies. Clin Endocrinol (Oxf) 2009;70:343–53. doi: 10.1111/j.1365-2265.2008.03396.x. [DOI] [PubMed] [Google Scholar]
- 38.Toft-Nielsen MB, Madsbad S, Holst JJ. Determinants of the effectiveness of glucagon-like peptide-1 in type 2 diabetes. J Clin Endocrinol Metab. 2001;86:3853–60. doi: 10.1210/jcem.86.8.7743. [DOI] [PubMed] [Google Scholar]
- 39.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–4. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 40.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 41.Bergman A, Ebel D, Liu F, Stone J, Wang A, Zeng W, Chen L, Dilzer S, Lasseter K, Herman G, Wagner J, Krishna R. Absolute bioavailability of sitagliptin, an oral dipeptidyl peptidase-4 inhibitor, in healthy volunteers. Biopharm Drug Dispos. 2007;28:315–22. doi: 10.1002/bdd.560. [DOI] [PubMed] [Google Scholar]
- 42.Herman GA, Stevens C, Van DK, Bergman A, Yi B, De SM, Snyder K, Hilliard D, Tanen M, Tanaka W, Wang AQ, Zeng W, Musson D, Winchell G, Davies MJ, Ramael S, Gottesdiener KM, Wagner JA. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–88. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, Mccann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–51. doi: 10.1021/jm0493156. [DOI] [PubMed] [Google Scholar]
- 44.FDA. FDA Sitagliptin. 2009. Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm183768.htm (last accessed April 2010)
- 45.EMEA. Sitaglipin EPAR. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/januvia/januvia.htm (last accessed April 2010.
- 46.Bergman AJ, Cote J, Yi B, Marbury T, Swan SK, Smith W, Gottesdiener K, Wagner J, Herman GA. Effect of renal insufficiency on the pharmacokinetics of sitagliptin, a dipeptidyl peptidase-4 inhibitor. Diabetes Care. 2007;30:1862–4. doi: 10.2337/dc06-2545. [DOI] [PubMed] [Google Scholar]
- 47.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 48.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–43. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 49.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–45. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 50.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 51.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–71. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 52.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–68. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 53.Scott R, Loeys T, Davies MJ, Engel SS. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:959–69. doi: 10.1111/j.1463-1326.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 54.Vilsboll T, Rosenstock J, Yki-Jarvinen H, Cefalu WT, Chen Y, Ling Y, Meehan AG, Katz L, Engel SS, Kaufman KD, Amatruda JM. Sitagliptin, a selective DPP-4 inhibitor, improves glycemic control when added to insulin, with or without metformin, in patients with type 2 diabetes. DiabetesPro. 2009. p. 588. 11 December 2009.
- 55.Williams-Herman D, Johnson J, Teng R, Luo E, Davies MJ, Kaufman KD, Goldstein BJ, Amatruda JM. Efficacy and safety of initial combination therapy with sitagliptin and metformin in patients with type 2 diabetes: a 54-week study. Curr Med Res Opin. 2009;25:569–83. doi: 10.1185/03007990802705679. [DOI] [PubMed] [Google Scholar]
- 56.Herman GA, Bergman A, Wagner J. An overview of the pharmacokinetic profile and the propensity for drug-drug interactions. Diabetologia. 2006;49(Suppl 1):A795. [Google Scholar]
- 57.He YL, Sabo R, Campestrini J, Wang Y, Ligueros-Saylan M, Lasseter KC, Dilzer SC, Howard D, Dole WP. The influence of hepatic impairment on the pharmacokinetics of the dipeptidyl peptidase IV (DPP-4) inhibitor vildagliptin. Eur J Clin Pharmacol. 2007;63:677–86. doi: 10.1007/s00228-007-0312-6. [DOI] [PubMed] [Google Scholar]
- 58.He YL, Sadler BM, Sabo R, Balez S, Wang Y, Campestrini J, Laurent A, Ligueros-Saylan M, Howard D. The absolute oral bioavailability and population-based pharmacokinetic modelling of a novel dipeptidylpeptidase-IV inhibitor, vildagliptin, in healthy volunteers. Clin Pharmacokinet. 2007;46:787–802. doi: 10.2165/00003088-200746090-00006. [DOI] [PubMed] [Google Scholar]
- 59.EMEA. Vildagliptin EPAR. 2009. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/galvus/galvus.htm (last accessed April 2010)
- 60.Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–80. doi: 10.2337/diacare.27.12.2874. [DOI] [PubMed] [Google Scholar]
- 61.Bolli G, Dotta F, Colin L, Minic B, Goodman M. Comparison of vildagliptin and pioglitazone in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Obes Metab. 2009;11:589–95. doi: 10.1111/j.1463-1326.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 62.Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahren B, Byiers S, Shao Q, Dejager S. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11:157–66. doi: 10.1111/j.1463-1326.2008.00994.x. [DOI] [PubMed] [Google Scholar]
- 63.Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naive patients with type 2 diabetes. Horm Metab Res. 2009;41:905–9. doi: 10.1055/s-0029-1234042. [DOI] [PubMed] [Google Scholar]
- 64.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–55. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 65.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–74. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 66.Garber AJ, Foley JE, Banerji MA, Ebeling P, Gudbjornsdottir S, Camisasca RP, Couturier A, Baron MA. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulphonylurea. Diabetes Obes Metab. 2008;10:1047–56. doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenstock J, Niggli M, Maldonado-Lutomirsky M. Long-term 2-year safety and efficacy of vildagliptin compared with rosiglitazone in drug-naive patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2009;11:571–8. doi: 10.1111/j.1463-1326.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 68.Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA(1c) over 1 year in drug-naive patients with Type 2 diabetes. Diabet Med. 2007;24:955–61. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 69.Tahrani AA, Piya MK, Barnett AH. Drug evaluation: vildagliptin-metformin single-tablet combination. Advs Ther. 2009;26:138–54. doi: 10.1007/s12325-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 70.Kirby MS, Dorso C, Wang A, Weigelt C, KOPCH OL, Hamann L, Marcinkeviciene J. In vitro enzymologic characteristics of saxagliptin, a highly potent and selective DPP-4 inhibitor with ‘slow binding’ characteristics. 2008. DPP.
- 71.Boulton D, Tang A, Patel C. Pharmacokinetics of the dipeptidyl peptidase-4 inhibitor saxagliptin in subjects with renal impairment. Endocrine. 2009. Abstracts ECE 2009(20.
- 72.Patel C, Castaneda L, Uli F. Single-dose pharmacokinetics and safety of saxagliptin in subjects with sepatic impairment compared with healthy subjects. 2008. ADA 2008.
- 73.EMEA. Saxagliptin EPAR. 2009. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/onglyza/onglyza.htm (last accessed April 2010)
- 74.Allen E, Hollander P, Li L, Chen R. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with inadequately controlled type 2 diabetes. 2008. EASD. [DOI] [PubMed]
- 75.Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395–406. doi: 10.1111/j.1742-1241.2009.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeFronzo RA, Hissa MN, Garber AJ, Gross JL, Duan RY, Ravichandran S, Chen RS. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649–55. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jadzinsky M, Pfutzner A, Paz-Pacheco E, Xu Z, Allen E, Chen R. Saxagliptin given in combination with metformin as initial therapy improves glycaemic control in patients with type 2 diabetes compared with either monotherapy: a randomized controlled trial. Diabetes Obes Metab. 2009;11:611–22. doi: 10.1111/j.1463-1326.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 78.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–86. doi: 10.1111/j.1463-1326.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 79.Tahrani AA, Piya MK, Barnett AH. Saxagliptin: a new DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Adv Ther. 2009;26:249–62. doi: 10.1007/s12325-009-0014-9. [DOI] [PubMed] [Google Scholar]
- 80.Girgis S, You X, Li L. Effect of diltiazem on the pharmacokinetics of saxagliptin in healthy subjects. 2007. ACC Pharmacology.
- 81.Patel C, Boulton D, Brenner E. Effect of ketoconazole on the pharmacokinetics of saxagliptin in healthy subjects. 2007. ACC Pharmacology.
- 82.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–7. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63:46–55. doi: 10.1111/j.1742-1241.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 84.Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11:167–76. doi: 10.1111/j.1463-1326.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 85.Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25:2361–71. doi: 10.1185/03007990903156111. [DOI] [PubMed] [Google Scholar]
- 86.Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145–52. doi: 10.1111/j.1463-1326.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 87.Barnett A. Exenatide. Expert Opin Pharmacother. 2007;8:2593–608. doi: 10.1517/14656566.8.15.2593. [DOI] [PubMed] [Google Scholar]
- 88.Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117:77–88. doi: 10.1016/j.regpep.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 89.EMEA. Exenatide EPAR. 2009. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/byetta/byetta.htm (last accessed 11 December 2009.
- 90.FDA. Exenatide FDA. 2009. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf (last accessed 11 December 2009.
- 91.Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006;49:706–12. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
- 92.Linnebjerg H, Kothare PA, Park S, Mace K, Reddy S, Mitchell M, Lins R. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:317–27. doi: 10.1111/j.1365-2125.2007.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Faludi P, Brodows R, Burger J, Ivanyi T, Braun DK. The effect of exenatide re-exposure on safety and efficacy. Peptides. 2009;30:1771–4. doi: 10.1016/j.peptides.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 95.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 96.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 97.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 98.Ratner RE, Maggs D, Nielsen LL, Stonehouse AH, Poon T, Zhang B, Bicsak TA, Brodows RG, Kim DD. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–28. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 99.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–60. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 100.Zinman B, Hoogwerf BJ, Duran GS, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477–85. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 101.Bhatia R, Viswanathan P, Chaudhuri A, Mohanty P, Bhatia V, Dandona P. Exenatide causes weight loss and a reduction in the insulin dose along with an improvement in HbA1c in Obese type 2 diabetics on insulin. 2006. Available at http://scientificsessions.diabetes.org/Abstracts/index.cfm?fuseaction=Locator.PreviewAbstract&popup=yes&NoLayout=Yes&AbstractID=13438 (last accessed April 2010)
- 102.Hood RC. Exenatide (EXE) Use in T2DM with A1C = 7% 2006. Available at http://scientificsessions.diabetes.org/Abstracts/index.cfm?fuseaction=Locator.PreviewAbstract&popup=yes&NoLayout=Yes&AbstractID=13484 (last accessed April 2010)
- 103.Oyer DS, Crawford R, Shah A, Bettenhausen S. Exenatide in diabetic patients on insulin and TZDs. 2006. Available at http://scientificsessions.diabetes.org/Abstracts/index.cfm?fuseaction=Locator.PreviewAbstract&popup=yes&NoLayout=Yes&AbstractID=15019 (last accessed April 2010)
- 104.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13:444–50. doi: 10.4158/EP.13.5.444. [DOI] [PubMed] [Google Scholar]
- 105.Barnett AH, Burger J, Johns D, Brodows R, Kendall DM, Roberts A, Trautmann ME. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29:2333–48. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 106.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, Parkes DG, Young AA. Exenatide (exendin-4) improves insulin sensitivity and {beta}-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–76. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 107.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52:734–40. doi: 10.2337/diabetes.52.3.734. [DOI] [PubMed] [Google Scholar]
- 108.Tourrel C, Bailbe D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50:1562–70. doi: 10.2337/diabetes.50.7.1562. [DOI] [PubMed] [Google Scholar]
- 109.Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443–52. doi: 10.2337/diabetes.51.5.1443. [DOI] [PubMed] [Google Scholar]
- 110.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 111.Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370–7. doi: 10.2337/diacare.26.8.2370. [DOI] [PubMed] [Google Scholar]
- 112.FDA. FDA Liraglutide. 2010. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf (last accessed April 2010)
- 113.EMEA. Liraglutide EPAR. 2009. Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/victoza/victoza.htm (last accessed 11 December 2009.
- 114.Flint A, Nazzal K, Jagielski P, Segel S, Zdravkovic M. Influence of hepatic impairment on pharmacokinetics of the long-acting human GLP-1 analogue liraglutide. 2007. Diabetes Supplement. [DOI] [PMC free article] [PubMed]
- 115.Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Pharmacokinetics of the long-acting human GLP-1 analogue liraglutide in subjects with renal impairment. 2007. Diabetes Supplement. [DOI] [PMC free article] [PubMed]
- 116.Neumiller JJ, Campbell RK. Liraglutide: a once-daily incretin mimetic for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2009;43:1433–44. doi: 10.1345/aph.1M134. [DOI] [PubMed] [Google Scholar]
- 117.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 118.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 119.Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, During M, Matthews DR. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen FB, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–84. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 124.Sturis J, Gotfredsen CF, Romer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–32. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–94. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 126.Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–50. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 127.Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD004094.pub2. CD004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–17. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 129.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–56. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 130.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 131.Monkhouse SJ, Morgan JD, Bates SE, Norton SA. An overview of the management of morbid obesity. Postgrad Med J. 2009;85:678–81. doi: 10.1136/pgmj.2009.082271. [DOI] [PubMed] [Google Scholar]
- 132.Berger JR. The neurological complications of bariatric surgery. Arch Neurol. 2004;61:1185–9. doi: 10.1001/archneur.61.8.1185. [DOI] [PubMed] [Google Scholar]
- 133.Lee CW, Kelly JJ, Wassef WY. Complications of bariatric surgery. Curr Opin Gastroenterol. 2007;23:636–43. doi: 10.1097/MOG.0b013e3282f094b5. [DOI] [PubMed] [Google Scholar]
- 134.Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2009 doi: 10.1111/j.1467-789X.2009.00614.x. June 1, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS, Barakat HA, Khazanie PG, Leggett-Frazier N, Long SD. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55:582S–585S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 136.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 137.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, Deramon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.NICE. Obesity Nice Guidance CG43. 2006. Available at http://guidance.nice.org.uk/CG43 (last accessed April 2010)
- 139.Katsuno K, Fujimori Y, Takemura Y, Hiratochi M, Itoh F, Komatsu Y, Fujikura H, Isaji M. Sergliflozin, a novel selective inhibitor of low-affinity sodium glucose cotransporter (SGLT2), validates the critical role of SGLT2 in renal glucose reabsorption and modulates plasma glucose level. J Pharmacol Exp Ther. 2007;320:323–30. doi: 10.1124/jpet.106.110296. [DOI] [PubMed] [Google Scholar]
- 140.Castaneda F, Burse A, Boland W, Kinne RK. Thioglycosides as inhibitors of hSGLT1 and hSGLT2: potential therapeutic agents for the control of hyperglycemia in diabetes. Int J Med Sci. 2007;4:131–9. doi: 10.7150/ijms.4.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hussey EK, Clark RV, Amin DM, Kipnes MS, O'Connor-Seemes L, O'Driscoll EC, Leong J, Murray SC, Dobbins RL, Nunez DJ. Early clinical studies to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of sergliflozin, a novel inhibitor of renal glucose reabsorption, in healthy volunteers and subjects with type 2 diabetes mellitus. 2007. ADA. [DOI] [PubMed]
- 142.Hussey EK, Dobbins RL, Stolz RR, Stockman NL, O'Connor-Seemes L, Kapur A, Murray SC, Nunez DJ. A double-blind randomized repeat dose study to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of three times daily dosing of sergliflozin, a novel inhibitor of renal glucose reabsorption, in healthy overweight and obese subjects. 2007. ADA. [DOI] [PubMed]
- 143.Bailey C, Gross JL, Bastone L, Bastien A, List JF. Dapagliflozin as an add-on to metformin lowers hyperglycaemia in type 2 diabetes patients inadequately controlled with metformin alone. Diabetologia. 2009;52(Suppl 1):S76. 11 December 2009. [Google Scholar]
- 144.Wilding JPH, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. Dapagliflozin pilot study in insulin-resistant T2DM patients. Diabetologia. 2009;52(Suppl 1):S76. 11 December 2009. [Google Scholar]
- 145.Brand CL, Rolin B, Jorgensen PN, Svendsen I, Kristensen JS, Holst JJ. Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia. 1994;37:985–93. doi: 10.1007/BF00400461. [DOI] [PubMed] [Google Scholar]
- 146.Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science. 1982;215:1115–6. doi: 10.1126/science.6278587. [DOI] [PubMed] [Google Scholar]
- 147.Qureshi SA, Rios CM, Xie D, Yang X, Tota LM, Ding VD, Li Z, Bansal A, Miller C, Cohen SM, Jiang G, Brady E, Saperstein R, Duffy JL, Tata JR, Chapman KT, Moller DE, Zhang BB. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes. 2004;53:3267–73. doi: 10.2337/diabetes.53.12.3267. [DOI] [PubMed] [Google Scholar]
- 148.Kodra JT, Jørgensen AS, Andersen B, Behrens C, Brand CL, Christensen ITG, Guldbrandt M, Jeppesen CB, Knudsen LB, Madsen P, Nishimura E, Sams C, Sidelmann UG, Pedersen RA, Lynn FC, Lau J. Novel glucagon receptor antagonists with improved selectivity over the glucose-dependent insulinotropic polypeptide receptor. J Med Chem. 2008;51:5387–96. doi: 10.1021/jm7015599. [DOI] [PubMed] [Google Scholar]
- 149.Madsen P, Kodra JÜT, Behrens C, Nishimura E, Jeppesen CB, Pridal L, Andersen B, Knudsen LB, Valcarce-Aspegren C, Guldbrandt M, Christensen IT, Jørgensen AS, Ynddal L, Brand CL, Bagger MA, Lau J. Human glucagon receptor antagonists with thiazole cores. A novel series with superior pharmacokinetic properties. J Med Chem. 2009;52:2989–3000. doi: 10.1021/jm8016249. [DOI] [PubMed] [Google Scholar]
- 150.Shen DM, Zhang F, Brady EJ, Candelore MR, Las-Yang Q, Ding VD, Dragovic J, Feeney WP, Jiang G, Mccann PE, Mock S, Qureshi SA, Saperstein R, Shen X, Tamvakopoulos C, Tong X, Tota LM, Wright MJ, Yang X, Zheng S, Chapman KT, Zhang BB, Tata JR, Parmee ER. Discovery of novel, potent, and orally active spiro-urea human glucagon receptor antagonists. Bioorg Med Chem Lett. 2005;15:4564–9. doi: 10.1016/j.bmcl.2005.06.101. [DOI] [PubMed] [Google Scholar]
- 151.Rivera N, Everett-Grueter CA, Edgerton DS, Rodewald T, Neal DW, Nishimura E, Larsen MO, Jacobsen LO, Kristensen K, Brand CL, Cherrington AD. A novel glucagon receptor antagonist, NNC 25-0926, blunts hepatic glucose production in the conscious dog. J Pharmacol Exp Ther. 2007;321:743–52. doi: 10.1124/jpet.106.115717. [DOI] [PubMed] [Google Scholar]
- 152.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44:2018–24. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 153.Fyfe M, White J, Taylor A, Chatfield R, Wargent E, Printz R, Sulpice T, Mccormack J, Procter M, Reynet C, Widdowson P, Wong-Kai-In P. Glucokinase activator PSN-GK1 displays enhanced antihyperglycaemic and insulinotropic actions. Diabetologia. 2007;50:1277–87. doi: 10.1007/s00125-007-0646-8. [DOI] [PubMed] [Google Scholar]
- 154.Pal M. Recent advances in glucokinase activators for the treatment of type 2 diabetes. Drug Discov Today. 2009;14:784–92. doi: 10.1016/j.drudis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 155.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 156.Guarente L. Calorie restriction and SIR2 genes – towards a mechanism. Mech Ageing Dev. 2005;126:923–8. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 157.Smith J, Kenney R, Gagne D, Frushour B, Ladd W, Galonek H, Israelian K, Song J, Razvadauskaite G, Lynch A, Carney D, Johnson R, Lavu S, Iffland A, Elliott P, Lambert P, Elliston K, Jirousek M, Milne J, Boss O. Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo. BMC Syst Biol. 2009;3:31. doi: 10.1186/1752-0509-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Mqneur C, Permutt MA, Imai SI. Increased dosage of mammalian Sir2 in pancreatic [beta] cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–17. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 159.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–6. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang WJ. Sirtuins: novel targets for metabolic disease in drug development. Biochem Biophys Res Commun. 2008;373:341–4. doi: 10.1016/j.bbrc.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 161.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, De Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holman RR. Asessing the potential of alpha-glucosidase inhibitors in prediabetic states. Diabetes Res Clin Pract. 1998;40(Suppl):S21–5. doi: 10.1016/s0168-8227(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 163.Ramlo-Halsted BA, Edelman SV. The natural history of type 2 diabetes. Implications for clinical practice. Prim Care. 1999;26:771–89. doi: 10.1016/s0095-4543(05)70130-5. [DOI] [PubMed] [Google Scholar]
- 164.Nathan DM. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342–9. doi: 10.1056/NEJMcp021106. [DOI] [PubMed] [Google Scholar]
- 165.Heine RJ, Diamant M, Mbanya JC, Nathan DM. Management of hyperglycaemia in type 2 diabetes: The end of recurrent failure? BMJ. 2006;333:1200–4. doi: 10.1136/bmj.39022.462546.80. [DOI] [PMC free article] [PubMed] [Google Scholar]


