Abstract
A new type of interaction in which fruit juices diminish oral drug bioavailability through inhibition of uptake transport is the focus of this review. The discovery was based on an opposite to anticipated finding when assessing the possibility of grapefruit juice increasing oral fexofenadine bioavailability in humans through inhibition of intestinal MDR1-mediated efflux transport. In follow-up investigations, grapefruit or orange juice at low concentrations potentially and selectively inhibited in vitro OATP1A2-mediated uptake compared with MDR1-caused efflux substrate transport. These juices at high volume dramatically depressed oral fexofenadine bioavailability. Grapefruit was the representative juice to characterize the interaction subsequently. A volume–effect relationship study using a normal juice amount halved average fexofenadine absorption. Individual variability and reproducibility data indicated the clinical interaction involved direct inhibition of intestinal OATP1A2. Naringin was a major causal component suggesting that other flavonoids in fruits and vegetables might also produce the effect. Duration of juice clinical inhibition of fexofenadine absorption lasted more than 2 h but less than 4 h indicating the interaction was avoidable with appropriate interval of time between juice and drug consumption. Grapefruit juice lowered the oral bioavailability of several medications transported by OATP1A2 (acebutolol, celiprolol, fexofenadine, talinolol, L-thyroxine) while orange juice did the same for others (atenolol, celiprolol, ciprofloxacin, fexofenadine). Juice clinical inhibition of OATP2B1 was unresolved while that of OATP1B1 seemed unlikely. The interaction between grapefruit juice and etoposide also seemed relevant. Knowledge of both affected uptake transporter and drug hydrophilicity assisted prediction of the clinical interaction with grapefruit or orange juice.
Keywords: drug interaction, grapefruit, MDR1, OATP1A2, orange, review
Introduction
Drugs are an essential component of medical therapy. However, the clinical response to them can vary markedly among and within patients and cause adverse effects that result in significant harm in some cases. These unintended and unwanted outcomes often arise when another substance is concomitantly consumed. Food and medication are often taken together. Linking a regular event like food intake with drug administration can improve adherence of the patient to the treatment regimen. However, certain foods can create an interaction through altered activity of a mechanism that is a crucial determinant of systemic drug availability.
The bioavailability and clinical effect of a medication can be determined by drug metabolism mediated by a family of oxidizing enzymes known as cytochrome P450s (CYPs) [1–4]. The specific enzyme, CYP3A4, is particularly significant. It contributes to the inactivation and elimination of an estimated 50% of all drugs. CYP3A4 is located in the simple columnar epithelial cells that line the small intestine and colon (enterocytes) and parenchymal cells that constitute 70–80% of the cytoplasmic mass of the liver (hepatocytes) [3, 4]. Since the systemic availability of orally administered medications involves sequential passage through small intestine and liver, this enzyme is well situated to oxidize drugs during first-pass metabolism. Consequently, the percent of the oral dose of many drugs reaching the systemic circulation unchanged can be markedly minimized. For example, CYP3A4-mediated first-pass metabolism is solely responsible for the normally low mean 15% oral bioavailability of the antihypertensive dihydropyridine calcium channel blocker, felodipine [5]. In the clinical situation where this process becomes manifestly impaired, the clinical conundrum can be sufficiently boosted oral bioavailability to enable excessive systemic drug concentration and overdose-related adverse effects [1–4].
Nearly 20 years ago, we published the seminal report of grapefruit juice–drug interactions [6]. This juice heightened average oral felodipine bioavailability to three-fold. Subsequently, hundreds of original research articles and numerous excellent reviews have been published on this topic [7–29]. It is now evident that a single judicious amount of grapefruit ingested even many hours beforehand has the possibility to affect more than 60 medications. Moreover, adverse event case reports attest to the fact that grapefruit–drug interactions have considerable clinical consequence and scope [30–47]. Warnings are found in many drug product monographs and on a label for application to the prescription vial of affected drugs. The major site of the interaction was the small intestine where enterocytic CYP3A4 underwent irreversible (mechanism-based) inhibition [48]. Grapefruit juice–drug interactions have provided original scientific information that has been translated into improved clinical utilization of a wide range of medications.
More recently, our group provided the first report that grapefruit and orange juices can have the opposite effect of diminishing oral absorption of another class of drugs as a result of inhibition of intestinal drug uptake transport [49]. The clinical concern in this case becomes loss of effectiveness of medications, which can be particularly relevant for those required to treat serious medical conditions, having a narrow therapeutic index or possessing a steep concentration–response relationship. This new category of fruit juice–drug interactions is the focus of this review article.
Background information
There are two major classes of drug transporters [50–55]. The best known belongs to the ATP-binding cassette (ABC) superfamily. They act as efflux transporters and are able to export a diverse range of xenobiotics from the intracellular to the extracellular environment against a high concentration gradient through energy derived from ATP. The P-glycoprotein (ABCB) family is a key member and is composed of the multidrug resistance protein 1 (MDR1 or P-glycoprotein), bile salt export pump (BSEP), multidrug resistance-associated protein (MRP) and breast cancer resistance protein (BCRP).
MDR1 was first identified in tumour cells where it was overexpressed and conferred cross-resistance to many chemotherapeutic agents. MDR1 was later found in several normal tissues (intestine, liver, kidney, blood-brain barrier). Of relevance to this article, the locations of MDR1 at the luminal surface of enterocytes enabled the back transport of cellularly absorbed drug and at the bile canalicular membrane of hepatocytes facilitated drug extrusion from cytoplasm into bile. As a result, MDR1 can act to thwart the systemic availability of orally administered drugs during first-pass.
The common sites for CYP3A4 and MDR1 in the gut and liver pointed towards integrated defence mechanisms against drug absorption albeit via different means [56]. Furthermore, CYP3A4 and MDR1 have overlapping substrate specificities in a number of cases supporting interplay to hinder more effectively drug availability. An example would be the low oral bioavailability of several transplantation anti-rejection drugs (ciclosporin, tacrolimus, serolimus). Also, CYP3A4 and MDR1 often have the same inhibitors. Based on the above, it seemed logical that MDR1 might also be inhibited by grapefruit juice.
Dilute grapefruit juice or ethylacetate extract or components of this juice demonstrated decreased in vitro efflux from human cancer colon cells (CaCo2 cells) of several MDR1 transported substrates (ciclosporin, digoxin, fexofenadine, paclitaxel, rhodamine123, saquinivir, talinolol, vinblastine) [57–69]. Consequently, these data supported the prospect that grapefruit juice would augment the oral bioavailability of a non-metabolized MDR1-transported drug. However, concomitantly consumed grapefruit juice either marginally enhanced (9% increase, P= 0.01) or did not affect the oral pharmacokinetics of digoxin [70, 71]. The quandary was that digoxin intrinsically possesses high oral bioavailability (70–80%) making interpretation of these data debatable. This stimulated a search for a more suitable drug probe.
The antihistamine, fexofenadine, was found to undergo MDR1-mediated in vitro efflux transport [72–74]. Clinically, it was excreted unchanged in humans meaning that grapefruit juice-mediated inactivation of CYP3A4 would not confound the results [75]. The primary route of elimination was biliary secretion consistent with MDR1 having a relevant role in the systemic elimination of this drug. Importantly, fexofenadine had innate oral bioavailability estimated at 33%, which would enable easier detection of a clinical MDR1 inhibitory effect. Furthermore, MDR1 inhibitors (itraconazole, lopinavir, ritonavir, verapamil) increased while inducers (carbamazepine, rifampicin, St John's wort) decreased fexofenadine AUC and Cmax[76–85]. Since it also had good clinical safety over a wide dose range, fexofenadine was possibly a superior clinical drug probe to gauge the inhibitory effect of grapefruit juice on presystemic MDR1 activity.
Initially, a pilot study was conducted. Two healthy volunteers were tested on 2 days separated by an interval of 1 week. They avoided consumption of fruit juices for 48 h beforehand and fasted from 22.00 h the night prior to each study day. A standardized lunch was provided at 4 h after dosing. Plasma fexofenadine concentrations with grapefruit juice (shown in Figure 1) were lower than those with water for subject 1 (AUC(0,7 h) = 1185 vs. 3032 ng ml−1 h, Cmax= 340 vs. 917 ng ml−1) and Subject 2 (AUC(0, 8 h) = 1427 vs. 2015 ng ml−1 h, Cmax= 307 vs. 461 ng ml−1). The reduction was more in the individual with the highest plasma drug concentrations with water. Thus, preliminary results were opposite to what had been envisaged, likely scientifically novel and potentially clinically important.
Figure 1.
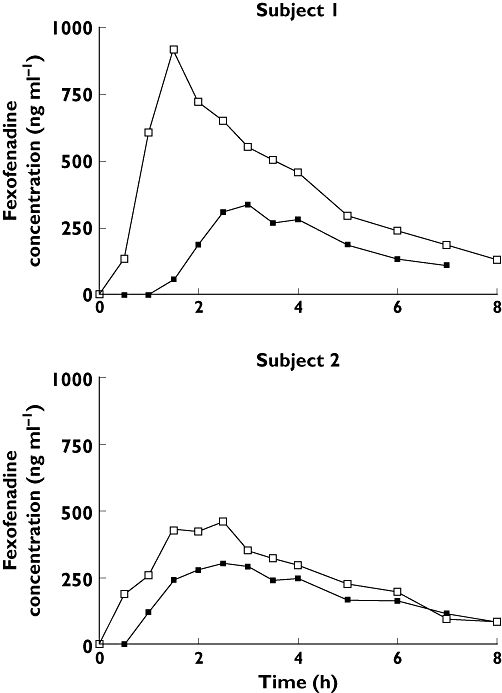
Pilot study results of plasma drug concentration–time profiles of fexofenadine 120 mg for two subjects administered water or grapefruit juice (GFJ) 300 ml. 300 ml water ( ); 300 ml GFJ (
); 300 ml GFJ ( )
)
Fexofenadine is a zwitterion possessing pronounced polarity over a wide pH range from uninterrupted ionization. This would likely afford good solubility of fexofenadine in the fluids of the stomach and small intestine. Consequently, it seemed unlikely that grapefruit juice would act to cause a relevant decrease in fexofenadine dissolution unless this juice interacted with excipients included in this regular release formulation to impair this process. This physicochemical property would also predict low passive intestinal permeability from the intestine into the portal circulation. Add to this MDR1-mediated efflux transport in the gut and liver and it might be expected that the oral bioavailability of fexofenadine would normally be essentially negligible. Since this was clearly not the case, another overriding factor must have played a pivotal role in determining the inherent oral bioavailability of fexofenadine.
Uptake carriers represent the other class of drug transporters [50–55]. Members belong to the solute carrier (SLC) superfamily. They facilitate the translocation of drugs into cells. Organic Anion Transporting Polypeptides (OATPs) constitute an important family of sodium-independent transport proteins. OATP1A2 (OATP-A) was the first identified human member and the initially found uptake transporter for fexofenadine [74]. More recently, an assessment of a range of human uptake transporters (OATP1A2, OATP2B1, OATP1B1, OATP1B3, NTCP, ASBT, OCT1, OAT1, OAT3, OAT4) showed that OATP1A2 was the only one that mediated substantial in vitro uptake [86]. Although more recent findings have indicated that OATP2B1 and OATP1B3 transported fexofenadine, OATP1A2 mediated much greater uptake [87, 88]. Pinch biopsies from healthy humans also showed that OATP1A2 and MDR1 were co-expressed on the luminal membrane of duodenal enterocytes indicating the potential of interplay of opposing vectors for uptake and efflux [86]. Consequently, selective inhibition of OATP1A2 or MDR1 might be expected to cause corresponding actions of decreased or increased intestinal absorption and systemic availability of fexofenadine.
Grapefruit juice interaction studies with fexofenadine
Grapefruit and constituents were examined for decreased MDR1-mediated digoxin or vinblastine efflux using polarized epithelial cell monolayers [49]. Grapefruit juice and homogenized unprocessed segments at 5% regular strength did not alter MDR1 activity. However, an extract of grapefruit peel at 5% strength was inhibitory. The major flavonoid in grapefruit, naringin, was quantified in the juice, segments and extract at 750, 2300, 33 100 µmol l–1, respectively. Pure naringin at 3000 µmol l–1 produced 50% reduction (IC50) of MDR1-mediated transport. Thus, certain parts of grapefruit inhibited in vitro MDR1 activity, possibly as a result of very high naringin content.
Grapefruit juice and naringin were also assessed for reduced OATP-mediated fexofenadine uptake using a transfected cell line [49]. This juice at concentrations ranging from 0.5% to 5% normal strength caused a graded effect on OATP1A2-mediated fexofenadine uptake that ranged from 50% to 90% decrease. Naringin IC50 for OATP1A2 was 3.6 µmol l–1, which was a concentration both hundreds of times lower than that causing equivalent MDR1 inhibition and generally found in grapefruit juice [89]. Thus, grapefruit and naringin were potent and selective in vitro inhibitors of OATP1A2 compared to MDR1.
Orange juice and its contained major flavonoid, hesperidin, were also tested for effect on OATP transport [49]. This juice caused concentration-dependent in vitro inhibition of OATP1A2-mediated uptake of fexofenadine equivalent to that seen with grapefruit juice. Hesperidin IC50 was 2.7 µmol l–1, which was a concentration essentially 50-fold less than that normally occurring in this juice [89]. Thus, a second and more commonly consumed citrus juice and contained flavonoid were potent in vitro inhibitors of OATP1A2 at low relative concentration.
The first formal clinical study assessed single dose maximum juice effect [49]. Grapefruit, orange or apple juice 1200 ml (300 ml consumed with fexofenadine followed by 150 ml at 0.5 h intervals to 3 h) were tested and compared with the equivalent volume of water. Since in vitro results had shown that apple juice at 5% normal strength caused no inhibition of MDR1 drug efflux and some (20%) reduction of OATP1A2 drug uptake, it was included as a possible negative control. All three juices reduced fexofenadine AUC, Cmax and urinary drug excretion (Ae) to 30% – 40% of those with water. Time to Cmax (tmax), elimination drug half-life (t1/2), renal drug clearance (CLR) and urine volume (V) were not altered. Thus, grapefruit, orange and apple juices produced profound reduction in the oral bioavailability of fexofenadine.
To determine juice volume–effect relationships and to clarify the role of inhibition of intestinal OATP1A2 in the interaction, a follow-up clinical investigation was conducted [90]. Grapefruit was selected as the representative juice and tested at more usual (300 ml) and high (1200 ml administered as outlined above) volumes. Grapefruit juice 300 ml concomitantly ingested reduced fexofenadine AUC and Cmax to a mean 58% and 53% of those with the corresponding volume of water. Thus, a commonly consumed volume of grapefruit juice diminished the average oral bioavailability of fexofenadine sufficiently to cause lack of bioequivalence and concern for loss of drug efficacy. Grapefruit juice 1200 ml decreased fexofenadine AUC and Cmax to 36% and 33% of those with the matching volume of water. Since the four-fold higher volume of grapefruit juice caused only a moderately additional decline, this quantity was likely on the upper portion of the juice volume–response curve.
Evaluation of data among individuals provided additional insights into the clinical mechanisms. Subjects (n= 9) retested more than 1 year apart had highly reproducible baseline fexofenadine AUCs with water (r2= 0.85) [89]. Thus, the observed five-fold range in fexofenadine AUC among these subjects indicated that it was dependent on characteristics inherent to the individual. Although speculative at this point, these data suggest the possibility that an important genetic rather than environmental component influences the activity of intestinal OATP1A2. In this case, it might be envisioned that greater activity would be associated with higher fexofenadine AUC. Grapefruit juice 300 ml decreased fexofenadine AUC such that the greatest reduction occurred at the highest baseline value (r2= 0.97, n= 12; Figure 2) [90]. Assuming greater effect of an inhibitor at higher transporter activity, one feasible mechanism for the interaction with grapefruit juice at a normally consumed amount would involve a direct inhibitory effect on intestinal OATP1A2.
Figure 2.
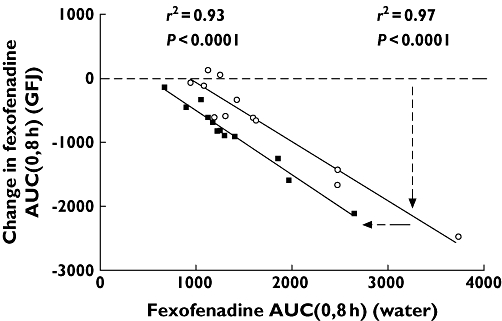
Area under the plasma drug concentration–time profile from 0 to 8 h (AUC(0,8 h)) of fexofenadine 120 mg with water 300 ml or 1200 ml (300 ml with drug followed by 150 ml every 0.5 h until 3.0 h) plotted against the absolute change in this parameter with the matching volume of grapefruit juice for each individual (n= 12). Horizontal dashed line indicates no change in fexofenadine AUC(0,8 h). The lines of best fit (solid lines) were determined by linear regression analysis. Dashed lines with arrows indicate the variable and constant components of the interaction. GFJ 1200 ml ( ); GFJ 300 ml (○) Reprinted with permission from Clinical Pharmacology & Therapeutics
); GFJ 300 ml (○) Reprinted with permission from Clinical Pharmacology & Therapeutics
Grapefruit juice 1200 ml also reduced fexofenadine AUC dependent upon baseline fexofenadine AUC (r2= 0.93) [90]. This line was parallel and left shifted to that for grapefruit juice 300 ml indicating a greater constant effect among individuals. This finding might be interpreted as an additional cause of interaction at high fluid consumption. Substantial juice might decrease the concentration and transit time of drug in the gastrointestinal tract to cause attenuated exposure of fexofenadine to intestinal OATP1A2. This mechanism is supported by a trend towards lower fexofenadine bioavailability with water 1200 ml compared with that with water 300 ml [90]. Since grapefruit juice additionally contains solutes with osmotic activity, this might augment fluid retention to enhance this action. Moreover, this action might provide at least a partial explanation for apple juice at high volume causing decreased oral bioavailability of fexofenadine.
To establish further the validity of the clinical mechanism of direct inhibition of intestinal OATP1A2, constituents in grapefruit juice were tested. The foremost flavonoid in grapefruit, naringin, was a potent in vitro inhibitor of OATP1A2 [89]. Also, two furanocoumarins in grapefruit juice (bergamottin and 6,7-dihydroxybergamottin) reduced the in vitro activity of rat intestinal OATP3, an orthologue to OATP1A2 [49]. Since furanocoumarins are most probably responsible for grapefruit–drug interactions mediated by mechanism-based inactivation of enteric CYP3A4, determination of the involvement of furanoumarins in the clinical inhibition of OATP1A2 activity was ascertained [59, 91–98].
Naringin has been used commercially as a flavouring agent in artificial grapefruit drinks and administered safely to humans in several research studies [99–101]. Grapefruit juice 300 ml, aqueous solution of naringin 1200 µm (same volume and concentration as juice) or water was consumed concomitantly with fexofenadine [89]. Grapefruit juice and naringin solution reduced fexofenadine AUCs to 55% and 75% of that with water, respectively. Thus, naringin was a major causal component in grapefruit juice and likely acted clinically through direct inhibition of enteric OATP1A2. This was the first report to our knowledge of a single specific dietary constituent producing a drug interaction in humans through modulating drug carrier activity. Naringin has sufficient safety, specificity and sensitivity to be a potentially useful inhibitory probe of clinical OATP1A2 activity. Another flavonoid, hesperidin, may be a clinically active constituent in orange juice.
The particulate fraction of grapefruit juice was a major source of furanocoumarins that clinically inactivated CYP3A4 and that contained negligible naringin [96, 102]. Grapefruit juice 300 ml, aqueous re-suspended particulate fraction of juice or water was ingested with fexofenadine [89]. The particulate fraction was also consumed 2 h before fexofenadine. Grapefruit juice control reduced the oral bioavailability of fexofenadine to 57% of that with water. The particulate fraction administered with or 2 h before fexofenadine produced the same plasma drug concentration–time profiles as for water. Thus, furanocoumarins that inactivate CYP3A4 clinically are unlikely to have an inhibitory effect on OATP1A2 in humans.
The duration of inhibitory action was examined [86]. Grapefruit juice 300 ml was consumed with or at two different times before drug administration. Ingestion of grapefruit juice with or 2 h beforehand reduced fexofenadine AUC to 48% and 62% of that with water. Juice at 4 h previously had a fexofenadine AUC that was not different from that with water. Duodenal biopsies obtained 1–2 h following ingestion of grapefruit juice 300 ml or water showed no acute difference in OATP1A2 or MDR1 protein concentrations [86]. Thus, grapefruit juice had a short-term inhibitory effect on fexofenadine absorption that did not result from altered OATP1A2 or MDR1 protein expression. Practically, this type of interaction might be avoided by having a time interval of 4 h between grapefruit juice or possibly orange juice consumption and administration of affected drug.
Scope of the interaction
The above research represented the first to our knowledge of fruit juices interacting with members of the OATP transporter family to reduce the oral bioavailability of a medication. It supported a new type of food-drug interaction of impending clinical importance. The current scientific literature was surveyed to ascertain possible scope. Publications comprehensive enough to report the effect of grapefruit or orange juice on both in vitro drug transport and clinical pharmacokinetics were lacking. However, data combined from several sources enabled a perspective (Table 1).
Table 1.
Fruit juice altered oral drug absorption potentially mediated through inhibition of an organic anion transporting polypeptide
| Clinical studies | ||||
|---|---|---|---|---|
| Inhibitor | ||||
| OATP/drug | Bioavailability(relative to water) | Fruit juice/constituent | Study volume (ml)(−4 to + 4 h) | Total volume (ml) |
| OATP1A2 | ||||
| Acebutolol [103] | 0.93* | Grapefruit | 600 | 2400 [104] |
| Atenolol [103] | 0.60** | Orange | 600 | 3000 [105] |
| Celiprolol [103] | 0.13** | Grapefruit | 600 | 2000 [106] |
| 0.17** | Orange | 600 | 2400 [107] | |
| Ciprofloxacin [108] | 0.78** | Orange | 355 | 355 [109] |
| Fexofenadine [49, 74, 86, 89] | 0.33*** | Grapefruit | 1200 | 1200 [49] |
| 0.36*** | Grapefruit | 1200 | 1200 [90] | |
| 0.58** | Grapefruit | 300 | 300 [90] | |
| 0.55*** | Grapefruit | 300 | 300 [89] | |
| 0.48** | Grapefruit | 300 | 300 [86] | |
| 0.69 | Grapefruit | 480 | 1920 [130] | |
| 0.75* | Naringin 1.2 mm | 300 | 300 [89] | |
| 0.28*** | Orange | 1200 | 1200 [49] | |
| Levofloxacin [108] | 0.93 | Orange | 355 | 355 [131] |
| Talinolol [110] | 0.56*** | Grapefruit | 300 | 300 [111] |
| L-Thyroxine [114–118] | 0.89** | Grapefruit | 600 | 1800 [119] |
| OATP2B1 | ||||
| Glibenclamide [120] | 1.07 | Grapefruit | 600 | 1800 [121] |
| OATP1B1 | ||||
| Pravastatin [123] | 0.92 | Grapefruit | 1200 | 3600 [124] |
| 1.00 | Grapefruit | 500 | 2250 [125] | |
| Pitavastatin [123] | 1.13* | Grapefruit | 500 | 3000 [126] |
| Unknown | ||||
| Etoposide | 0.59* | Grapefruit | 100 | 100 [128] |
P < 0.05,
P < 0.01,
P < 0.001.
OATP1A2 is an uptake transporter for a number of medications for which the clinical effect of grapefruit or orange juice is also available. Juice was often consumed before, with and after dosing of drug so that the entire amount ingested during the period of study was often substantial. However, the duration of inhibition of drug absorption was less than 4 h [86]. Thus, the total volume of juice consumed 4 h before to 4 h after dosing was also tabulated for each drug to judge juice volume–effect relationships.
Four OATP1A2 substrates (acebutolol, celiprolol, fexofenadine, talinolol) with grapefruit juice and four (atenolol, celiprolol, ciprofloxacin, fexofenadine) with orange juice had lower oral bioavailability [49, 74, 86, 89, 90, 103–111]. Two drugs (celiprolol, fexofenadine) were studied with both of these juices. Celiprolol, like fexofenadine, had similar reduction in oral bioavailability at equivalent juice volume. This uniformity of effect with grapefruit and orange juices supported a common mechanism of action. Moreover, the size of the interaction for a particular drug with one juice might predict that with the other juice.
The decrease in oral bioavailability differed among OATP1A2 substrates. Since this was apparent at similar juice volume, the extent of the interaction might be principally dependent on the drug. Hydrophilic non-metabolized drugs (atenolol, celiprolol, ciprofloxacin, fexofenadine) were the most affected compared with the lipophilic metabolized drug (acebutolol). Greater polarity and excretion unchanged would be consistent with dependence upon uptake transport, rather than passive diffusion, for intestinal drug absorption. Consequently, OATP1A2 substrates that are primarily renally eliminated unchanged (methotrexate, sotalol) would likely undergo noteworthy reduction in oral bioavailability with grapefruit or orange juice [103, 112]. Both of these drugs have also been used to treat serious medical conditions making the clinical relevance of a marked interaction probable. An OATP1A2 substrate that is mainly metabolized by CYP3A4 (imatinib) would tend to support an increase, rather than a decrease, in oral drug bioavailability when taken with grapefruit juice raising the risk of predictable serious toxicity with this chemotherapeutic agent [113].
β-adrenoceptor blockers are employed in the management of a range of cardiovascular maladies that include hypertension, ischaemic heart disease, cardiac arrhythmias, secondary prevention of myocardial infarction and congestive heart failure. Atenolol, celiprolol and talinolol were tested with grapefruit or orange juice at volumes of 600, 600 and 300 ml consumed during the period of 4 h before to 4 h after drug dosing, respectively [104–107, 111]. The corresponding attenuation of systemic drug availability to 60%, <20% and 56% would be enough to raise the issue of inadequate drug action with a realistically consumed amount of juice, which would be particularly relevant when these β-adrenoceptor blockers are for treatment of a significant cardiovascular condition.
Fluoroquinolone antibiotics possess a wide spectrum of activity, have favourable tolerance and are used to treat a wide variety of infections [109]. Bacterial killing activity is drug concentration-dependent. The optimal effect occurs when the ratio of plasma peak concentration to bacterial minimum inhibitory concentration (MIC) of the fluoroquinolone exceeds 10. The reported decreased oral bioavailability of ciprofloxacin with orange juice raised the possibility that the range of pathogens that might be optimally treated would be reduced. This consequence carries with it the risk of a patient failing treatment and/or evolving a resistant form of the pathogen.
The thyroid hormone, L-thyroxine, has a steep concentration–effect relationship requiring careful dose titration. This is exemplified by the recommendation of no change in brand once a patient has been stabilized on a particular dose as a small alteration in the fractional gastrointestinal absorption of this hormone is regarded as ample to be clinically relevant. It has a long elimination half-life (7 days), which means noticeable accumulation at steady state occurring with a month of repeated administration. L-Thyroxine undergoes carrier-mediated uptake by OATP1A2 as well as by multiple other transporters and it has a small but potentially pertinent reduction in oral bioavailability with grapefruit juice in a single dose study [114–119]. Routine intake of L-thyroxine with grapefruit or orange juice might result in a reduction in steady state systemic concentration sufficient to induce under-treatment.
The uptake carrier, OATP2B1, is expressed on the apical membrane of the enterocyte and thereby might enable the intestinal absorption of certain drugs [50–55]. An in vitro study with the antidiabetic agent, glibenclamide, showed that it was a substrate for OATP2B1 and had impaired intracellular accumulation with grapefruit or orange juice at 5% normal concentration or with naringin, dihydroxybergamottin or bergamottin at corresponding concentrations 100-, 4- and 2-fold less than that normally found in grapefruit juice [120]. In a clinical investigation, glibenclamide with grapefruit juice 600 ml consumed within the timeframe of 4 h before to 4 h after dosing did not result in an oral bioavailability that was different from that with water [121]. Since glibenclamide is normally extensively metabolized, this maybe the explanation for the divergence between in vitro and clinical findings. The oral direct renin inhibitor, aliskerin, is an OATP2B1 substrate, a hydrophilic drug and eliminated unchanged [122]. Aliskerin might be a better drug probe to address the issue of relevant clinical inhibition of intestinal OATP2B1 with grapefruit or orange juice.
Another uptake transporter, OATP1B1, has been identified on the sinusoidal membrane of the hepatocyte and facilitates the intracellular accumulation of HMG-CoA inhibitors (statins) [50–55, 123]. Pitavastatin, pravastatin, rosuvastatin are excreted essentially unchanged. Clinical pharmacokinetic studies showed that pravastatin had no change while pitavastatin had a small increase in oral bioavailability with grapefruit juice [124–126]. Thus, current data do not support a relevant action on the clinical activity of OATP1B1 by grapefruit juice or possibly orange juice. The reason for the absence of an effect is not clear but might be the result of components in grapefruit juice having low affinity for OATP1B1 and/or not attaining sufficient concentration in the circulation. Rosuvastatin is also a substrate of OATP1A2 [127]. The oral bioavailability of rosuvastatin with grapefruit or orange juice has not been reported.
The chemotherapeutic agent, etoposide, had essentially halved oral bioavailability with grapefruit juice 100 ml [128]. Etoposide has an absolute oral bioavailability ranging 47% to 76% with nearly all of it eliminated in urine in the unchanged form [129]. Thus, the pharmacokinetics support that etoposide is a non-metabolized polar drug that would likely require carrier mediated transport for absorption and disposition. However, current information has not yet reported an OATP uptake transporter for it. Regardless of the mechanism of this interaction, etoposide is a commonly used drug in cancer treatment and this magnitude of reduction in oral bioavailability that occurred with a modest volume of grapefruit juice makes the issue of lessened efficacy in this case particularly worrisome.
In conclusion, a new type of drug interaction involving grapefruit or orange juice selectively inhibiting a member of the OATP uptake transporter family (OATP1A2) to diminish oral drug bioavailability has been discussed. This can occur with a single normal amount of grapefruit juice. The major flavonoid, naringin, was a chief causal component in this juice. The duration of inhibition of drug absorption lasted more than 2 h but less than 4 h. OATP1A2 substrates (acebutolol, celiprolol, fexofenadine, talinolol, L-thyroxine) with grapefruit juice and (atenolol, celiprolol, ciprofloxacin, fexofenadine) with orange juice had lower oral bioavailability. The extent of reduction was variable among drugs. However, the effect for a specific drug with one juice was similar to that with the other juice. A greater magnitude of interaction is predicted for an OATP1A2 transported drug that is hydrophilic and normally excreted unchanged rather than lipophilic and extensively metabolized. The interaction involving certain beta-blockers (atenolol, celiprolol, talinolol), ciprofloxacin, L-thyroxine and etoposide has relevant ramifications. Clinical inhibition of OATP2B1 with grapefruit or orange juice remains to be established while that of OATP1B1 with these juices appears unlikely.
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Levy RH, Thummel KE, Trager WF, Hansten PD, Eichelbaum M. Metabolic Drug Interactions. 1st. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 2.Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–21. doi: 10.1056/NEJMra032424. Review. [DOI] [PubMed] [Google Scholar]
- 3.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. Review. [DOI] [PubMed] [Google Scholar]
- 4.Dresser GK, Bailey DG. A basic conceptual and practical overview of interactions with highly prescribed drugs. Can J Clin Pharmacol. 2002;9:191–8. [PubMed] [Google Scholar]
- 5.Edgar B, Regardh CG, Johnsson G, Johansson L, Lundborg P, Löfberg I, Rönn O. Felodipine kinetics in healthy man. Clin Pharmacol Ther. 1985;38:205–11. doi: 10.1038/clpt.1985.160. [DOI] [PubMed] [Google Scholar]
- 6.Bailey DG, Spence JD, Munoz C, Arnold JMO. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–9. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 7.Bailey DG, Arnold JMO, Spence JD. Grapefruit juice and drugs: how significant is the interaction? Clin Pharmacokinet. 1994;26:91–8. doi: 10.2165/00003088-199426020-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ameer B, Weintraub RA. Drug interactions with grapefruit juice. Clin Pharmacokinet. 1997;33:103–21. doi: 10.2165/00003088-199733020-00003. Review. [DOI] [PubMed] [Google Scholar]
- 9.Bailey DG, Arnold JMO, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46:101–10. doi: 10.1046/j.1365-2125.1998.00764.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhr U. Drug interactions with grapefruit juice. Extent, probable mechanism and clinical relevance. Drug Saf. 1998;18:251–72. doi: 10.2165/00002018-199818040-00002. Review. [DOI] [PubMed] [Google Scholar]
- 11.Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–55. doi: 10.2165/00003088-199937030-00003. Review. [DOI] [PubMed] [Google Scholar]
- 12.Kane GC, Lipsky JJ. Drug–grapefruit juice interactions. Mayo Clin Proc. 2000;75:933–42. doi: 10.4065/75.9.933. Review. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt DJ, Patki KC, von Moltke LL, Shader RI. Drug interactions with grapefruit: an update. J Clin Psychopharmacol. 2001;21:357–9. doi: 10.1097/00004714-200108000-00001. Review. [DOI] [PubMed] [Google Scholar]
- 14.Lohezic-Le Devehat F, Marigny K, Doucet M, Javaudin L. Grapefruit and drugs: a hazardous combination? Therapie. 2002;57:432–45. Review. [PubMed] [Google Scholar]
- 15.Lim GE, Li T, Buttar HS. Interactions of grapefruit and cardiovascular drugs: a potential risk of toxicity. Exp Clin Cardiol. 2003;8:99–107. Review. [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–97. doi: 10.2165/00129784-200404050-00002. Review. [DOI] [PubMed] [Google Scholar]
- 17.Dahan A, Altman H. Food-drug interaction: grapefruit juice augments drug bioavailability-mechanism, extent and relevance. Eur J Clin Nutr. 2004;58:1–9. doi: 10.1038/sj.ejcn.1601736. Review. [DOI] [PubMed] [Google Scholar]
- 18.Huang SM, Hall SD, Watkins P, Love LA, Serabjit-Singh C, Betz JM, Hoffman FA, Honig P, Coates PM, Bull J, Chen ST, Kearns GL, Murray MD. Drug interactions with herbals and grapefruit: a conference report. Clin Pharmacol Ther. 2004;7:1–12. doi: 10.1016/j.clpt.2003.07.002. Review. [DOI] [PubMed] [Google Scholar]
- 19.Fujita K. Food-drug interactions via human cytochrome P450 3A (CYP3A) Drug Metabol Drug Interact. 2004;20:195–217. doi: 10.1515/dmdi.2004.20.4.195. Review. [DOI] [PubMed] [Google Scholar]
- 20.Guo LQ, Yamazoe Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol Sin. 2004;25:129–36. Review. [PubMed] [Google Scholar]
- 21.Saito M, Hirata-Koizumi M, Matsumoto M, Urano T, Hasegawa R. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28:677–94. doi: 10.2165/00002018-200528080-00003. Review. [DOI] [PubMed] [Google Scholar]
- 22.Murray M. Altered CYP expression and function in response to dietary factors: potential roles in disease pathogenesis. Curr Drug Metab. 2006;7:67–81. doi: 10.2174/138920006774832569. Review. [DOI] [PubMed] [Google Scholar]
- 23.Sica DA. Interaction of grapefruit juice and calcium channel blockers. Am J Hypertens. 2006;19:768–73. doi: 10.1016/j.amjhyper.2005.11.003. Review. [DOI] [PubMed] [Google Scholar]
- 24.Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 2006;46:1390–416. doi: 10.1177/0091270006294277. Review. [DOI] [PubMed] [Google Scholar]
- 25.Bressler R. Grapefruit juice and drug interactions. Exploring mechanisms of this interaction and potential toxicity for certain drugs. Geriatrics. 2006;61:12–8. Review. [PubMed] [Google Scholar]
- 26.Paine MF, Oberlies NH. Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol. 2007;3:67–80. doi: 10.1517/17425255.3.1.67. Review. [DOI] [PubMed] [Google Scholar]
- 27.Joshi R, Medhi B. Natural product and drugs interactions, its clinical implication in drug therapy management. Saudi Med J. 2008;29:333–9. Review. [PubMed] [Google Scholar]
- 28.Farkas D, Greenblatt DJ. Influence of fruit juices on drug disposition: discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol. 2008;4:381–93. doi: 10.1517/17425255.4.4.381. Review. [DOI] [PubMed] [Google Scholar]
- 29.Gertz M, Davis JD, Harrison A, Houston JB, Galetin A. Grapefruit juice-drug interaction studies as a method to assess the extent of intestinal availability: utility and limitations. Curr Drug Metab. 2008;9:785–95. doi: 10.2174/138920008786049276. Review. [DOI] [PubMed] [Google Scholar]
- 30.Peynaud D, Charpiat B, Vial T, Gallavardin M, Ducerf C. Tacrolimus severe overdosage after intake of masked grapefruit in orange marmalade. Eur J Clin Pharmacol. 2007;63:721–2. doi: 10.1007/s00228-007-0323-3. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi K, Ohtani H, Ikemoto T, Miki A, Hori S, Sawada Y. Possible case of potentiation of the antiplatelet effect of cilostazol by grapefruit juice. J Clin Pharm Ther. 2007;32:457–9. doi: 10.1111/j.1365-2710.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 32.Mazokopakis EE. Unusual causes of rhabdomyolysis. Intern Med J. 2008;38:364–7. doi: 10.1111/j.1445-5994.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 33.Bonin B, Vandel P, Vandel S, Kantelip JP. Effect of grapefruit intake on carbamazepine bioavailability: a case report. Therapie. 2001;56:69–71. [PubMed] [Google Scholar]
- 34.Goldbart A, Press J, Sofer S, Kapelushnik J. Near fatal acute colchicine intoxication in a child. A case report. Eur J Pediatr. 2000;159:895–7. doi: 10.1007/pl00008364. [DOI] [PubMed] [Google Scholar]
- 35.Karch AM. The grapefruit challenge: the juice inhibits a crucial enzyme, with possibly fatal consequences. Am J Nurs. 2004;104:33–5. doi: 10.1097/00000446-200412000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson I. Grapefruit juice caused hypotension. Lakartidningen. 1997;94:112–3. [PubMed] [Google Scholar]
- 37.Lilley LL, Guanci R. Grapefruit and medication. Am J Nurs. 1998;98:10. [PubMed] [Google Scholar]
- 38.Adigun AQ, Mudasiru Z. Clinical effects of grapefruit juice-nifedipine interaction in a 54-year-old Nigerian: a case report. J Natl Med Assoc. 2002;94:276–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Pisarik P. Blood pressure-lowering effect of adding grapefruit juice to nifedipine and terazosin in a patient with severe renovascular hypertension. Arch Fam Med. 1996;5:413–6. doi: 10.1001/archfami.5.7.413. [DOI] [PubMed] [Google Scholar]
- 40.Grande LA, Mendez RD, Krug RT, Verschuyl EJ. Attention grapefruit! Lancet. 2009;373:1222. doi: 10.1016/S0140-6736(09)60289-0. [DOI] [PubMed] [Google Scholar]
- 41.Hermans K, Stockman D, Van den Branden F. Grapefruit and tonic: a deadly combination in a patient with the long QTsyndrome. Am J Med. 2003;114:511–2. doi: 10.1016/s0002-9343(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 42.Lee M, Min DI. Determination of sildenafil citrate in plasma by high-performance liquid chromatography and a case for the potential interaction of grapefruit juice with sildenafil citrate. Ther Drug Monit. 2001;23:21–6. doi: 10.1097/00007691-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Dreier JP, Endres M. Statin-associated rhabdomyolysis triggered by grapefruit consumption. Neurology. 2004;62:670. doi: 10.1212/wnl.62.4.670. [DOI] [PubMed] [Google Scholar]
- 44.Fukatsu S, Fukudo M, Masuda S, Yano I, Katsura T, Ogura Y, Oike F, Takada Y, Inui K. Delayed effect of grapefruit juice on pharmacokinetics and pharmacodynamics of tacrolimus in a living-donor liver transplant recipient. Drug Metab Pharmacokinet. 2006;21:122–5. doi: 10.2133/dmpk.21.122. [DOI] [PubMed] [Google Scholar]
- 45.Pillai U, Muzaffar J, Sen S, Yancey A. Grapefruit juice and verapamil: a toxic cocktail. South Med J. 2009;102:308–9. doi: 10.1097/SMJ.0b013e3181928f81. [DOI] [PubMed] [Google Scholar]
- 46.Bartle WR. Grapefruit juice might still be factor in warfarin response. Am J Health Syst Pharm. 1999;56:676. doi: 10.1093/ajhp/56.7.676. [DOI] [PubMed] [Google Scholar]
- 47.Jürgen Roth H, Wintergalen M. Unusual high levels of ciclosporin in a female patient – the impact of lifestyle? Clin Lab. 2005;51:425–7. [PubMed] [Google Scholar]
- 48.Lown KS, Bailey DG, Fontana RJ, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–53. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dresser GK, Bailey DG, Leake BF, Schwarz UI, Dawson PA, Freeman DJ, Kim RB. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 50.Kim RB. Transporters and drug discovery: why, when, and how. Mol Pharm. 2006;3:26–32. doi: 10.1021/mp050084o. [DOI] [PubMed] [Google Scholar]
- 51.Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–77. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Marzolini C, Tirona RG, Kim RG. Pharmacogenomics of the OATP and OAT families. Pharmacogenetics. 2004;5:273–82. doi: 10.1517/phgs.5.3.273.29831. [DOI] [PubMed] [Google Scholar]
- 53.Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(Suppl 2):1–5. doi: 10.1046/j.1365-2362.33.s2.5.x. [DOI] [PubMed] [Google Scholar]
- 54.Kim RB. Transporters and xenobiotic disposition. Toxicology. 2002;181–182:291–7. doi: 10.1016/s0300-483x(02)00296-2. [DOI] [PubMed] [Google Scholar]
- 55.Tirona RG, Kim RG. Pharmacogenomics of organic anion-transporting polypeptides (OATP) Adv Drug Deliv Rev. 2002;54:1343–52. doi: 10.1016/s0169-409x(02)00077-7. [DOI] [PubMed] [Google Scholar]
- 56.Benet LZ. The drug transporter-metabolism alliance: uncovering and defining the interplay. Mol Pharm. 2009;6:1631–43. doi: 10.1021/mp900253n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takanaga H, Ohnishi A, Matsuo H, Sawada Y. Inhibition of vinblastine efflux mediated by P-glycoprotein by grapefruit juice components in caco-2 cells. Biol Pharm Bull. 1998;21:1062–6. doi: 10.1248/bpb.21.1062. [DOI] [PubMed] [Google Scholar]
- 58.Eagling VA, Profit L, Back DJ. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-1 protease inhibitor saquinavir by grapefruit juice components. Br J Clin Pharmacol. 1999;48:543–52. doi: 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohnishi A, Matsuo H, Yamada S, Takanaga H, Morimoto S, Shoyama Y, Ohtani H, Sawada Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br J Pharmacol. 2000;130:1369–77. doi: 10.1038/sj.bjp.0703433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spahn-Langguth H, Langguth P. Grapefruit juice enhances intestinal absorption of the P-glycoprotein substrate talinolol. Eur J Pharm Sci. 2001;12:361–7. doi: 10.1016/s0928-0987(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 61.Wang EJ, Casciano CN, Clement RP, Johnson WW. Inhibition of P-glycoprotein transport function by grapefruit juice psoralen. Pharm Res. 2001;18:432–8. doi: 10.1023/a:1011089924099. [DOI] [PubMed] [Google Scholar]
- 62.Tian R, Koyabu N, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice on the intestinal efflux of P-glycoprotein substrates. Pharm Res. 2002;19:802–9. doi: 10.1023/a:1016100715125. [DOI] [PubMed] [Google Scholar]
- 63.Xu J, Go ML, Lim LY. Modulation of digoxin transport across Caco-2 cell monolayers by citrus fruit juices: lime, lemon, grapefruit, and pummelo. Pharm Res. 2003;20:169–76. doi: 10.1023/a:1022254617664. [DOI] [PubMed] [Google Scholar]
- 64.Honda Y, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br J Pharmacol. 2004;143:856–64. doi: 10.1038/sj.bjp.0706008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romiti N, Tramonti G, Donati A, Chieli E. Effects of grapefruit juice on the multidrug transporter P-glycoprotein in the human proximal tubular cell line HK-2. Life Sci. 2004;76:293–302. doi: 10.1016/j.lfs.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 66.Tourniaire F, Hassan M, André M, Ghiringhelli O, Alquier C, Amiot MJ. Molecular mechanisms of the naringin low uptake by intestinal Caco-2 cells. Mol Nutr Food Res. 2005;49:957–62. doi: 10.1002/mnfr.200500088. [DOI] [PubMed] [Google Scholar]
- 67.Lim SL, Lim LY. Effects of citrus fruit juices on cytotoxicity and drug transport pathways of Caco-2 cell monolayers. Int J Pharm. 2006;307:42–50. doi: 10.1016/j.ijpharm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 68.Panchagnula R, Bansal T, Varma MV, Kaul CL. Co-treatment with grapefruit juice inhibits while chronic administration activates intestinal P-glycoprotein-mediated drug efflux. Pharmazie. 2005;60:922–7. [PubMed] [Google Scholar]
- 69.Dahan A, Amidon GL. Grapefruit juice and its constituents augment colchicine intestinal absorption: potential hazardous interaction and the role of P-glycoprotein. Pharm Res. 2009;26:883–92. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 70.Becquemont L, Verstuyft C, Kerb R, Brinkmann U, Lebot M, Jaillon P, Funck-Brentano C. Effect of grapefruit juice on digoxin pharmacokinetics in humans. Clin Pharmacol Ther. 2001;70:311–6. [PubMed] [Google Scholar]
- 71.Parker RB, Yates CR, Soberman JE, Laizure SC. Effects of grapefruit juice on intestinal P-glycoprotein: evaluation using digoxin in humans. Pharmacotherapy. 2003;23:979–87. doi: 10.1592/phco.23.8.979.32881. [DOI] [PubMed] [Google Scholar]
- 72.Perloff MD, von Moltke LL, Greenblatt DJ. Fexofenadine transport in Caco-2 cells: inhibition with verapamil and ritonavir. J Clin Pharmacol. 2002;42:1269–74. doi: 10.1177/009127002762491370. [DOI] [PubMed] [Google Scholar]
- 73.Petri N, Tannergren C, Rungstad D, Lennernäs H. Transport characteristics of fexofenadine in the Caco-2 cell model. Pharm Res. 2004;21:1398–404. doi: 10.1023/b:pham.0000036913.90332.b1. [DOI] [PubMed] [Google Scholar]
- 74.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- 75.Molimard M, Diquet B, Benedetti MS. Comparison of pharmacokinetics and metabolism of desloratadine, fexofenadine, levocetirizine and mizolastine in humans. Fundam Clin Pharmacol. 2004;18:399–411. doi: 10.1111/j.1472-8206.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 76.Sakugawa T, Miura M, Hokama N, Suzuki T, Tateishi T, Uno T. Enantioselective disposition of fexofenadine with the P-glycoprotein inhibitor verapamil. Br J Clin Pharmacol. 2009;67:535–40. doi: 10.1111/j.1365-2125.2009.03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uno T, Shimizu M, Sugawara K, Tateishi T. Lack of dose-dependent effects of itraconazole on the pharmacokinetic interaction with fexofenadine. Drug Metab Dispos. 2006;34:1875–9. doi: 10.1124/dmd.106.011023. [DOI] [PubMed] [Google Scholar]
- 78.van Heeswijk RP, Bourbeau M, Campbell P, Seguin I, Chauhan BM, Foster BC, Cameron DW. Time-dependent interaction between lopinavir/ritonavir and fexofenadine. J Clin Pharmacol. 2006;46:758–67. doi: 10.1177/0091270006288733. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of single and multiple doses of itraconazole on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br J Clin Pharmacol. 2006;62:372–6. doi: 10.1111/j.1365-2125.2006.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimizu M, Uno T, Sugawara K, Tateishi T. Effects of itraconazole and diltiazem on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Br J Clin Pharmacol. 2006;61:538–44. doi: 10.1111/j.1365-2125.2006.02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, Cha IJ, Shin JG. Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther. 2005;78:191–201. doi: 10.1016/j.clpt.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 82.Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther. 2005;77:17–23. doi: 10.1016/j.clpt.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 83.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–21. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 84.Dresser GK, Schwarz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St John's wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- 85.Yamada S, Yasui-Furukori N, Akamine Y, Kaneko S, Uno T. Effects of the P-glycoprotein inducer carbamazepine on fexofenadine pharmacokinetics. Ther Drug Monit. 2009;31:764–8. doi: 10.1097/FTD.0b013e3181bf7db6. [DOI] [PubMed] [Google Scholar]
- 86.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–70. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 87.Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–45. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 88.Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–81. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]
- 89.Bailey DG, Dresser GK, Leake BF, Kim RB. Naringin is a major and selective clinical inhibitor of organic anion transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther. 2007;81:495–502. doi: 10.1038/sj.clpt.6100104. [DOI] [PubMed] [Google Scholar]
- 90.Dresser GK, Kim RB, Bailey DG. Effect of grapefruit juice volume on the reduction of fexofenadine bioavailability: possible role of organic anion transporting polypeptides. Clin Pharmacol Ther. 2005;77:170–7. doi: 10.1016/j.clpt.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Edwards DJ, Bellevue FH, Woster PM. Identification of 6’,7’-dihydroxybergamottin, a cytochrome P450 inhibitor, in grapefruit juice. Drug Metab Dispos. 1996;24:1287–90. [PubMed] [Google Scholar]
- 92.Schmiedlin-Ren P, Edwards DJ, Fitzsimmons ME, He K, Lown KS, Woster PM, Rahman A, Thummel KE, Fisher JM, Hollenberg PF, Watkins PB. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab Dispos. 1997;25:1228–33. [PubMed] [Google Scholar]
- 93.Fukuda K, Ohta T, Oshima Y, Ohashi N, Yoshikawa M, Yamazoe Y. Specific CYP3A4 inhibitors in grapefruit juice: furocoumarin dimers as components of drug interaction. Pharmacogenetics. 1997;7:391–6. doi: 10.1097/00008571-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 94.He K, Iyer KR, Hayes RN, Sinz MW, Woolf TF, Hollenberg PF. Inactivation of cytochrome P450 3A4 by bergamottin, a component of grapefruit juice. Chem Res Toxicol. 1998;1:252–9. doi: 10.1021/tx970192k. [DOI] [PubMed] [Google Scholar]
- 95.Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys. 2000;378:356–63. doi: 10.1006/abbi.2000.1835. [DOI] [PubMed] [Google Scholar]
- 96.Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Role of furanocoumarin derivatives on grapefruit juice-mediated inhibition of human CYP3A activity. Drug Metab Dispos. 2000;28:766–71. [PubMed] [Google Scholar]
- 97.Ohta T, Nagahashi M, Hosoi S, Tsukamoto S. Dihydroxybergamottin caproate as a potent and stable CYP3A4 inhibitor. Bioorg Med Chem. 2002;10:969–73. doi: 10.1016/s0968-0896(01)00362-5. [DOI] [PubMed] [Google Scholar]
- 98.Payne MF, Widmer WW, Hart HL, Pusek SN, Beavers KL, Criss AB, Brown SS, Thomas BF, Watkins PB. A furanocoumarin-free grapefruit juice establishes furanocoumarins as the mediators of the grapefruit juice-felodipine interaction. Am J Clin Nutr. 2006;83:1097–105. doi: 10.1093/ajcn/83.5.1097. [DOI] [PubMed] [Google Scholar]
- 99.Bailey DG, Arnold JMO, Munoz C, Spence JD. Grapefruit juice – felodipine interaction: mechanism, predictability and effect of naringin. Clin Pharmacol Ther. 1993;53:637–42. doi: 10.1038/clpt.1993.84. [DOI] [PubMed] [Google Scholar]
- 100.Bailey DG, Arnold JMO, Strong HA, Munoz C, Spence JD. Effect of grapefruit juice and naringin on nisoldipine pharmacokinetics. Clin Pharmacol Ther. 1993;54:589–94. doi: 10.1038/clpt.1993.195. [DOI] [PubMed] [Google Scholar]
- 101.Ameer B, Weintraub RA, Johnson JV, Yost RA, Rouseff RL. Flavanone absorption after naringin, hesperidin and citrus ingestion. Clin Pharmacol Ther. 1996;60:34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 102.Bailey DG, Kreeft JH, Munoz C, Freeman JD, Bend JR. Grapefruit juice-felodipine interaction: effect of naringin and 6’,7’-dihydroxybergamottin in humans. Clin Pharmacol Ther. 1998;64:248–56. doi: 10.1016/S0009-9236(98)90173-4. [DOI] [PubMed] [Google Scholar]
- 103.Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–39. doi: 10.1002/jps.21618. [DOI] [PubMed] [Google Scholar]
- 104.Lilja JJ, Raaska K, Neuvonen PJ. Effects of grapefruit juice on the pharmacokinetics of acebutolol. Br J Clin Pharmacol. 2005;60:659–63. doi: 10.1111/j.1365-2125.2005.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lilja JJ, Raaska K, Neuvonen PJ. Effects of orange juice on the pharmacokinetics of atenolol. Eur J Clin Pharmacol. 2005;61:337–40. doi: 10.1007/s00228-005-0930-9. [DOI] [PubMed] [Google Scholar]
- 106.Lilja JJ, Backman JT, Laitila J, Luurila H, Neuvonen PJ. Itraconazole increases but grapefruit juice greatly decreases plasma concentrations of celiprolol. Clin Pharmacol Ther. 2003;73:192–8. doi: 10.1067/mcp.2003.26. [DOI] [PubMed] [Google Scholar]
- 107.Lilja JJ, Juntti-Patinen L, Neuvonen PJ. Orange juice substantially reduces the bioavailability of the beta-adrenergic-blocking agent celiprolol. Clin Pharmacol Ther. 2004;75:184–90. doi: 10.1016/j.clpt.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 108.Maeda T, Takahashi K, Ohtsu N, Oguma T, Ohnishi T, Atsumi R, Tamai I. Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007;4:85–94. doi: 10.1021/mp060082j. [DOI] [PubMed] [Google Scholar]
- 109.Neuhofel AL, Wilton JH, Victory JM, Hejmanowsk LG, Amsden GW. Lack of bioequivalence of ciprofloxacin when administered with calcium-fortified orange juice: a new twist on an old interaction. J Clin Pharmacol. 2002;42:461–6. [PubMed] [Google Scholar]
- 110.Shirasaka Y, Kuraoka E, Spahn-Langguth H, Nakanishi T, Langguth P, Tamai I. Species difference in the effect of grapefruit juice on intestinal absorption of talinolol between human and rat. J Pharmacol Exp Ther. 2010;332:181–9. doi: 10.1124/jpet.109.159756. [DOI] [PubMed] [Google Scholar]
- 111.Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, Kim RB, Bailey DG, Kirch W. Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther. 2005;77:291–301. doi: 10.1016/j.clpt.2004.11.111. [DOI] [PubMed] [Google Scholar]
- 112.Badagnani I, Castro RA, Taylor TR, Brett CM, Huang CC, Stryke D, Kawamoto M, Johns SJ, Ferrin TE, Carlson EJ, Burchard EG, Giacomini KM. Interaction of methotrexate with organic-anion transporting polypeptide 1A2 and its genetic variants. J Pharmacol Exp Ther. 2006;318:521–9. doi: 10.1124/jpet.106.104364. [DOI] [PubMed] [Google Scholar]
- 113.Hu S, Franke RM, Filipski KK, Hu C, Orwick SJ, de Bruijn EA, Burger H, Baker SD, Sparreboom A. Interaction of imatinib with human organic ion carriers. Clin Cancer Res. 2008;14:3141–8. doi: 10.1158/1078-0432.CCR-07-4913. [DOI] [PubMed] [Google Scholar]
- 114.Fujiwara K, Adachi H, Nishio T, Unno M, Tokui T, Okabe M, Onogawa T, Suzuki T, Asano N, Tanemoto M, Seki M, Shiiba K, Suzuki M, Kondo Y, Nunoki K, Shimosegawa T, Iinuma K, Ito S, Matsuno S, Abe T. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology. 2001;142:2005–12. doi: 10.1210/endo.142.5.8115. [DOI] [PubMed] [Google Scholar]
- 115.Friesema EC, Jansen J, Milici C, Visser TJ. Thyroid hormone transporters. Vitam Horm. 2005;70:137–67. doi: 10.1016/S0083-6729(05)70005-4. [DOI] [PubMed] [Google Scholar]
- 116.Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport by monocarboxylate transporters. Best Pract Res Clin Endocrinol Metab. 2007;21:223–36. doi: 10.1016/j.beem.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport in and out of cells. Trends Endocrinol Metab. 2008;19:50–6. doi: 10.1016/j.tem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 118.Hagenbuch B. Cellular entry of thyroid hormones by organic anion transporting polypeptides. Best Pract Res Clin Endocrinol Metab. 2007;21:209–21. doi: 10.1016/j.beem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 119.Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60:337–41. doi: 10.1111/j.1365-2125.2005.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Satoh H, Yamashita F, Tsujimoto M, Murakami H, Koyabu N, Ohtani H, Sawada Y. Citrus juices inhibit the function of human organic anion-transportingpolypeptide OATP-B. Drug Metab Dispos. 2005;33:518–23. doi: 10.1124/dmd.104.002337. [DOI] [PubMed] [Google Scholar]
- 121.Lilja JJ, Niemi M, Fredrikson H, Neuvonen PJ. Effects of clarithromycin and grapefruit juice on the pharmacokinetics of glibenclamide. Br J Clin Pharmacol. 2007;63:732–40. doi: 10.1111/j.1365-2125.2006.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vaidyanathan S, Camenisch G, Schuetz H, Reynolds C, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP. Pharmacokinetics of the oral direct renin inhibitor aliskiren in combination with digoxin, atorvastatin, and ketoconazole in healthy subjects: the role of P-glycoprotein in the disposition of aliskiren. J Clin Pharmacol. 2008;48:1323–38. doi: 10.1177/0091270008323258. [DOI] [PubMed] [Google Scholar]
- 123.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther. 1999;66:118–27. doi: 10.1053/cp.1999.v66.100453001. [DOI] [PubMed] [Google Scholar]
- 125.Fukazawa I, Uchida N, Uchida E, Yasuhara H. Effects of grapefruit juice on pharmacokinetics of atorvastatin and pravastatin in Japanese. Br J Clin Pharmacol. 2004;57:448–55. doi: 10.1046/j.1365-2125.2003.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ando H, Tsuruoka S, Yanagihara H, Sugimoto K, Miyata M, Yamazoe Y, Takamura T, Kaneko S, Fujimura A. Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin. Br J Clin Pharmacol. 2005;60:494–7. doi: 10.1111/j.1365-2125.2005.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 128.Reif S, Nicolson MC, Bisset D, Reid M, Kloft C, Jaehde U, McLeod HL. Effect of grapefruit juice intake on etoposide bioavailability. Eur J Clin Pharmacol. 2002;58:491–4. doi: 10.1007/s00228-002-0495-9. [DOI] [PubMed] [Google Scholar]
- 129.Fujiwara Y, Ohune T, Okusaki K, Niitani K, Sumiyoshi H, Takemoto Y, Yamaoka N, Yamakido M. Bioavailability of 50- and 75-mg oral etoposide in lung cancer patients. Cancer Chemother Pharmacol. 1996;37:327–31. doi: 10.1007/s002800050392. [DOI] [PubMed] [Google Scholar]
- 130.Banfield C, Gupta S, Marino M, Lim J, Affrime M. Grapefruit juice reduces the oral bioavailability of fexofenadine but not desloratadine. Clin Pharmacokinet. 2002;41:311–8. doi: 10.2165/00003088-200241040-00004. [DOI] [PubMed] [Google Scholar]
- 131.Wallace AW, Victory JM, Amsden GW. Lack of bioequivalence when levofloxacin and calcium-fortified orange juice are coadministered to healthy volunteers. J Clin Pharmacol. 2003;43:539–44. [PubMed] [Google Scholar]


