Abstract
AIMS
This study was conducted to determine whether atenolol was able to decrease BP level and mitigate BP increase during dynamic resistance exercise performed at three different intensities in hypertensives.
METHODS
Ten essential hypertensives (systolic/diastolic BP between 140/90 and 160/105 mmHg) were blindly studied after 6 weeks of placebo and atenolol. In each phase, volunteers executed, in a random order, three protocols of knee-extension exercises to fatigue: (i) one set at 100% of 1 RM; (ii) three sets at 80% of 1 RM; and (iii) three sets at 40% of 1 RM. Intra-arterial radial blood pressure was measured throughout the protocols.
RESULTS
Atenolol decreased systolic BP maximum values achieved during the three exercise protocols (100% = 186 ± 4 vs. 215 ± 7, 80% = 224 ± 7 vs. 247 ± 9 and 40% = 223 ± 7 vs. 252 ± 16 mmHg, P < 0.05). Atenolol also mitigated an increase in systolic BP in the first set of exercises (100% =+38 ± 5 vs.+54 ± 9; 80% =+68 ± 11 vs. +84 ± 13 and 40% =+69 ± 7 vs.+84 ± 14, mmHg, P < 0.05). Atenolol decreased diastolic BP values and mitigated its increase during exercise performed at 100% of 1 RM (126 ± 6 vs. 145 ± 6 and +41 ± 6 vs.+52 ± 6, mmHg, P < 0.05), but not at the other exercise intensities.
CONCLUSIONS
Atenolol was effective in both reducing systolic BP maximum values and mitigating BP increase during resistance exercise performed at different intensities in hypertensive subjects.
Keywords: atenolol, blood pressure, dynamic resistance exercise, hypertension, β-adrenoceptor blockers
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Dynamic resistance exercise promotes a sizeable increase in blood pressure during its execution in non medicated hypertensives.
WHAT THIS STUDY ADDS
Atenolol not only decreases blood pressure level but also mitigates the increase of blood pressure during dynamic resistance exercise in hypertensive patients. An increase in blood pressure during resistance exercise might be at least in part attributed to an increase in cardiac output.
Introduction
Resistance exercise is characterized by an effort made against a specific opposing force. Dynamic resistance is when a body part moves against this force during contraction, and isometric resistance is when a contraction is not followed by movement [1]. Today, dynamic resistance exercise is recommended for hypertensive subjects in conjunction with aerobic exercise [2]. Nevertheless, during this kind of exercise, blood pressure (BP) increases sharply and expressively [3–18], which might represent a risk for hypertensives. It is known that not only BP values but mainly a sharp rise in BP are risk factors for aneurysm rupture [19–21], which is especially important for hypertensives, who are more prone to have aneurysms [22].
In a previous study [23], we measured intra-arterial BP response to dynamic resistance exercise in hypertensive patients, and systolic BP achieved values as high as 238 ± 12 mmHg. Greater systolic BP levels were reported by Palatini et al. [12]. However, besides the fact that pharmacological therapy is recommended for many hypertensive patients [24], in these previous studies, patients were not receiving any antihypertensive medications. Thus, it is interesting to evaluate whether antihypertensive drugs are able to blunt BP increase during dynamic resistance exercise.
Atenolol is a specific β1-adrenoceptor antagonist that blocks sympathetic activation to the heart, decreasing heart rate (HR), cardiac contractility, cardiac output and BP [25]. The use of β-adrenoceptor blockers was the first line of antihypertensive single-drug therapy for many years, and even nowadays they are commonly used to treat hypertension [24] and other cardiovascular diseases [26], such as coronary artery disease and cardiac heart failure.
The effects of atenolol during aerobic exercise have been extensively studied, and it has been shown that β-adrenoceptor blockade is able to reduce HR and BP maximum values, as well as their increase during aerobic activity [27]. Atenolol effects were also investigated during isometric resistance exercise, and it was shown that β-adrenoceptor blockade decreases maximum BP values but does not affect the increase in BP [28–34]. This absence of effect is probably because an increase in systemic vascular resistance is the main mechanism for BP increase during isometric exercise [18, 35].
Nevertheless, during dynamic resistance exercise, which is the mode of resistance exercise recommended for hypertensives [2, 36], BP increase might be, at least in part, due to an increase in cardiac output [17]. The effects of atenolol during this kind of exercise have been poorly studied. Therefore, this study was designed to test the hypothesis that atenolol can decrease not only BP maximum absolute levels achieved during dynamic resistance exercise, but it can also blunt the increase of BP during this kind of exercise.
We evaluated intra-arterial BP values and increases during dynamic leg-extension exercises performed to failure at three different intensities in hypertensive subjects receiving placebo and atenolol.
Methods
Subjects
Subjects with essential hypertension (stages 1 and 2) were recruited among those treated in the Hypertension Unit of the General Hospital to participate in this study, which was approved by the Ethics Committee of the General Hospital, Medical School, University of São Paulo.
Before enrolment in the study, all subjects underwent routine screening in the hypertension unit according to the international guidelines [24]. This screening included rest and exercise ECG and blood and urine collection. Patients who were receiving antihypertensive medications before enrolment maintained their previous medication during the screening phase. Subjects were excluded from the study if they had any sign of other cardiovascular diseases besides hypertension, and if they presented associated cardiovascular risk factors or target organ damage. None of the subjects was engaged in any regular physical exercise nor had previous experience with resistance exercise.
After screening, all subjects received placebo (lactose 40 mg, corn starch 102 mg, cellulose 5 mg, and magnesium 3 mg) twice daily for 6 weeks, and then they received atenolol therapy (initial dose of 25 mg twice daily, and increased to 50 mg twice daily if blood pressure was above 140/90 mmHg) for another 6 weeks. Subjects were blinded to the therapy in each experiment. Thus, the study was a crossover, nonrandomized, single-blind clinical trial.
At the third and fourth week of each therapy (placebo and atenolol), auscultatory BP was measured three times during two visits to the laboratory, and the mean of these six measurements was used as the patient's BP level. The first and the fifth Korotkoff's sounds were employed, respectively, to determine systolic and diastolic BP. Subjects continued in the study only if systolic/diastolic BP values when receiving placebo were between 140 and 160/90 and 105 mmHg. Subjects were excluded and drug therapy was prescribed if BP levels were above 160/105 mmHg.
At the fifth week, ECG and respiratory movements were recorded for 10 min with a frequency rate of 500 Hz (WinDaq DI-720, Dataq Instruments, Akron, OH, USA) while the subjects were lying still. A spectral analysis of heart rate variability was performed as previously described [37]. Briefly, on stationary segments of at least 5 min, autoregressive spectral parameters were estimated using Levinson–Durbin recursion, and the order of the model was chosen according to Akaike's criterion (Programma Di Analisi Lineare 8.0, Universitá Degli Studi Di Milano, Milan, Italy). The components were assigned as low- (LF = 0.04–0.15 Hz) and high-frequency (HF = 0.15–0.5 Hz). Both components were reported in normalized units (nu), representing the relative value of each power component in proportion to the total power minus very low-frequency component (VLF = 0–0.04 Hz). Normalized LF and HF components of R–R variability were accepted, respectively, as markers of the cardiac sympathetic and parasympathetic modulations, while the ratio between them (LF : HF) was accepted as the sympathovagal balance [37].
Measurements
Experiments were conducted at the sixth week of each therapy, and intra-arterial BP measurement was employed. During the experiments, BP was measured in the radial artery of the nondominant arm as previously described [38]. After subcutaneous administration of local anaesthetic (2% lidocaine without vasoconstrictor), a 22-gauge catheter (BD-Angiocath, Becton Dickinson, Franklin Lakes, NJ, USA) was inserted into the radial artery, and it was maintained patent by a constant infusion. All procedures were performed in the hospital and by a trained physician.
The catheter was connected to a transducer kit (PX-260, Edwards Life Sciences, Irvine, CA, USA), which was positioned at the level of the fourth intercostal space. A signal amplifier was used (KS3800, Gould Instrument Systems, Valley View, OH, USA), and the signal was acquired on a computer at a sampling frequency of 500 Hz using a data acquisition system (WinDaq DI-720, Dataq Instruments Inc, Akron, OH, USA).
Experiments
In each study phase (placebo and atenolol), all subjects attended two familiarization sessions prior to the experiment in order to learn the correct execution of the knee-extension exercise. In each of these sessions, they performed 10 repetitions of the exercise with the lowest load allowed by the leg extension machine (Physicus PHA 23, São Paulo, Brazil). In addition, 1 week before the experiment, they underwent a 1-repetition-maximum (RM) test following Kraemer & Fry's protocol [39].
For the experiments, subjects were instructed to arrive at the laboratory between 15.00 h and 17.00 h. They were instructed to abstain from any sporadic exercise for the previous 24 h and from smoking for at least 3 h. They ingested a light meal at least 2 h before the experiment, and products containing caffeine and theophylline were not allowed for this meal. All subjects received the last dose of atenolol or placebo in the morning approximately 7 h before the experiments.
After arriving at the laboratory, subjects' auscultatory BP was measured, and the experiment only began if BP was below 160/105 mmHg, which is a secure BP level for beginning exercise, according to the American College of Sports Medicine [40]. Then subjects rested in the supine position while the catheter was inserted into the nondominant radial artery. Afterwards, they moved to the knee-extension machine, where they rested for 10 min while baseline measurements were taken. The subjects then performed, in a random order, three different knee-extension exercise protocols. Each protocol was executed to fatigue, and they consisted of the following: (i) one set at 100% of 1 RM; (ii) three sets at 80% of 1 RM, with a 90 s rest interval between the sets; and (iii) three sets at 40% of 1 RM, with a 90 s rest interval between the sets. A resting period of at least 10 min and long enough to allow BP to return to baseline was taken between the protocols. In each protocol, intra-arterial BP was continuously measured for 3 min before the exercise, during all exercise, and during the 3 min recovery period. For each subject, the protocol order was kept the same in the placebo and atenolol phases.
For each subject, the data collected for 1 min preceding each exercise protocol were averaged and called the pre-exercise value. These values were compared with the highest value achieved during each exercise set (S1, S2, and S3) and to the lowest value obtained during each interval period between the sets (I1, I2, and I3), as previously described [15].
Statistical analysis
Normality and homoscedasticity were checked by Shapiro-Wilks and Levene tests, respectively (SPSS for Windows 13.0; Lead Technologies Inc., Chicago, IL, USA). For each exercise intensity, data were compared by a two-way anova for repeated measures (Statistica for Windows 5.0; Statsoft Inc., Tulsa, OK, USA), establishing therapy (placebo or atenolol) and stages (PRE, S1, I1, S2, I2, S3, I3) as the main factors. The Newman-Keuls post hoc test was employed when necessary. P < 0.05 was set as significant. Data are presented as mean ± SE.
Considering a power of 80%, an alpha error of 5%, and a standard deviation of 3 mmHg for systolic BP, the smallest sample size necessary to detect a difference of 4 mmHg was calculated to be eight subjects.
Results
Sample
Twenty-eight individuals who met the requirements signed the consent form to participate in the study. In the preliminary phase, 11 subjects were excluded because of hypothyroidism (1), cardiac hypertrophy (1), blood pressure levels inconsistent with the study's criteria (4), and personal reasons (5). Thus, 17 subjects fulfilled the inclusion criteria and initiated the study. However, during the placebo phase, one subject was excluded because his BP increased above 160/105 mmHg, and another four subjects were excluded because their BP stayed below 140/90 mmHg. In addition, one subject was not available to continue the study, and one subject was excluded during the atenolol therapy because BP increased above 160/105 mmHg. Thus, 10 subjects completed the entire protocol.
Subject characteristics are shown in Table 1. All subjects had a family history of arterial hypertension, and three of them were smokers. Six of these subjects were receiving drug treatment, including renin-angiotensin system blockers and/or diuretics, before enroling in the study. The atenolol dose was increased to 50 mg twice daily in only one patient, but the results did not change without him.
Table 1.
Subjects' characteristics. Data = mean ± SE
| n | 10 |
| Gender, male/female | 6/4 |
| Age (years) | 46 ± 2 |
| Anthropometrics | |
| Height (m) | 1.67 ± 0.03 |
| Weight (kg) | 78.9 ± 3.7 |
| Body mass index (kg m−2) | 28.1 ± 0.5 |
| Cardiovascular | |
| Systolic blood pressure (mmHg) | 140 ± 4 |
| Diastolic blood pressure (mmHg) | 97 ± 1 |
| Heart rate (beats min−1) | 70 ± 3 |
| Sokolow-Lyon Index (mV) | 2.4 ± 0.2 |
| Cornell Index (mV) | 1.6 ± 0.2 |
| Metabolic | |
| Glycaemia (mmol l−1) | 5.16 ± 0.14 |
| Total cholesterol (mmol l−1) | 5.20 ± 0.31 |
| HDL cholesterol (mmol l−1) | 1.43 ± 0.14 |
| LDL cholesterol (mmol l−1) | 2.83 ± 0.29 |
| VLDL cholesterol (mmol l−1) | 0.99 ± 0.16 |
| Triglycerides (mmol l−1) | 2.17 ± 0.36 |
| Uric acid (µmol l−1) | 389.00 ± 33.71 |
| Others | |
| Creatinine (µmol l−1) | 77.70 ± 4.06 |
| TSH (mlU l−1) | 1.87 ± 0.42 |
| T3 (nmol l−1) | 1.56 ± 0.27 |
| T4 (nmol l−1) | 90.22 ± 15.63 |
| Free T4 (pmol l−1) | 12.07 ± 1.37 |
Atenolol effects at rest
During atenolol therapy, only one subject needed to increase drug dosage, and the results did not change without this patient. Resting heart rate decreased significantly with atenolol (70 ± 3 vs. 62 ± 1 beats min−1, P < 0.05). Moreover, resting systolic and diastolic BP also decreased significantly with atenolol (140 ± 4 vs. 119 ± 2 mmHg and 97 ± 1 vs. 81 ± 1 mmHg, respectively, P < 0.05). Spectral analysis of resting HR variability was performed in eight subjects after placebo and atenolol therapy. Atenolol significantly increased the normalized high-frequency component of HR variability (51 ± 7 vs. 28 ± 7, nu, P < 0.05), and decreased the normalized low-frequency component (38 ± 7 vs. 61 ± 9 nu, P < 0.05) and low- to high-frequency ratio (0.4 ± 0.3 vs. 0.7 ± 0.5, P < 0.05).
Exercise characteristics
Atenolol did not change maximum strength. Thus, exercise at 100, 80, and 40% of 1 RM was performed, respectively, with 64 ± 4 and 63 ± 5 kg, 51 ± 3 and 50 ± 4 kg and 27 ± 2 and 25 ± 2 kg with placebo and atenolol (P > 0.05).
The number of repetitions to failure was also similar between the two therapies during the exercise protocol at 80% of 1 RM (S1 = 10 ± 1 vs. 10 ± 1, S2 = 8 ± 1 vs. 8 ± 1, and S3 = 7 ± 1 vs. 7 ± 1 repetitions, P > 0.05). Moreover, the number of repetitions to failure decreased significantly in sets 2 and 3 in comparison with set 1 (P < 0.05). On the other hand, at the exercise protocol at 40% of 1 RM, the number of repetitions to failure increased slightly but significantly with atenolol (S1 = 20 ± 1 vs. 18 ± 1, S2 = 15 ± 1 vs. 14 ± 1, and S3 = 14 ± 1 vs. 13 ± 2 repetitions, P < 0.05). Moreover, independent of the therapy, the number of repetitions to failure decreased significantly in sets 2 and 3 in comparison with set 1 (P < 0.05).
BP responses to exercise
Figure 1 shows a typical tracing of systolic BP measured in one hypertensive patient during the first set of exercises performed at 80% of 1 RM to failure with placebo and atenolol.
Figure 1.
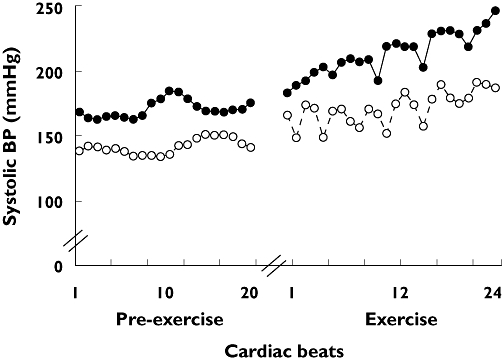
Systolic blood pressure measured for each heartbeat in one hypertensive patient receiving placebo (solid line with solid circles) and atenolol (dotted line with open circles). Measurements were made before and during the first set of knee-extension exercises performed to failure at 80% of 1 RM
Figure 2 shows systolic BP values measured during exercise in the three intensity protocols (panels A, C and E). As expected, systolic BP increased significantly during exercise in all the protocols and returned to pre-exercise values during the intervals (except for I3 in the 80% of 1 RM protocol). Moreover, atenolol significantly decreased systolic BP values measured at all moments (pre-exercise, during the sets and during the intervals).
Figure 2.
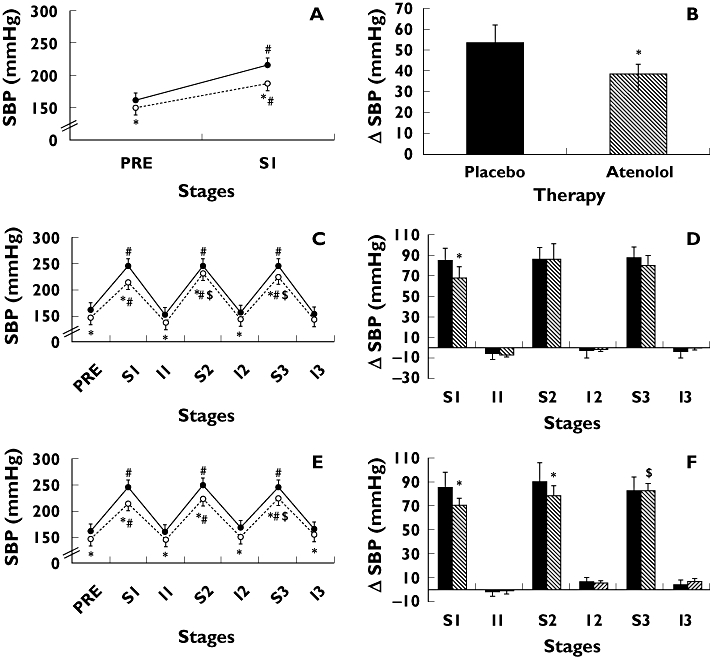
Systolic blood pressure absolute values (SBP) and changes (ΔSBP exercise – pre-exercise) measured pre-exercise (PRE) and during three sets (S1, S2, and S3) and intervals (I1, I2, and I3) of knee-extension resistance exercise performed to failure at 100% (panels A and B), 80% (panels C and D), and 40% (panels E and F) of 1 RM. Measures were taken with subjects receiving placebo (solid line with solid circles or black bars) and atenolol (dotted line with open circles or white bars) in hypertensive subjects. * Significantly different from placebo (P < 0.05). # Significantly different from PRE (P < 0.05). $ Significantly different from S1 (P < 0.05). Data = mean ± SE
Figure 2 shows systolic BP changes measured during exercise in the three intensity protocols (panels B, D, and F). Atenolol significantly blunted the increase in systolic BP observed in the first exercise set at the three exercise intensities (100% of 1 RM =+38 ± 5 vs. +54 ± 9, 80% of 1 RM =+68 ± 11 vs.+84 ± 13, and 40% of 1 RM =+69 ± 7 vs. +84 ± 14 mmHg, respectively, P < 0.05). Moreover, it also blunted the increase of systolic BP during the second exercise set at 40% of 1 RM (+77 ± 9 vs.+89 ± 16 mmHg, respectively, P < 0.05).
Figure 3 shows diastolic BP values and changes measured during exercise in the three intensity protocols. As expected, diastolic BP increased significantly during the exercise sets in all the protocols and returned to pre-exercise values during the intervals. Atenolol did not influence diastolic BP values nor increments during the exercise performed at 80 and 40% of 1 RM, but it decreased diastolic BP and mitigated its increase during exercise performed at 100% of 1 RM (126 ± 6 vs. 145 ± 6 and +41 ± 6 vs. +52 ± 6 mmHg, respectively, P < 0.05).
Figure 3.
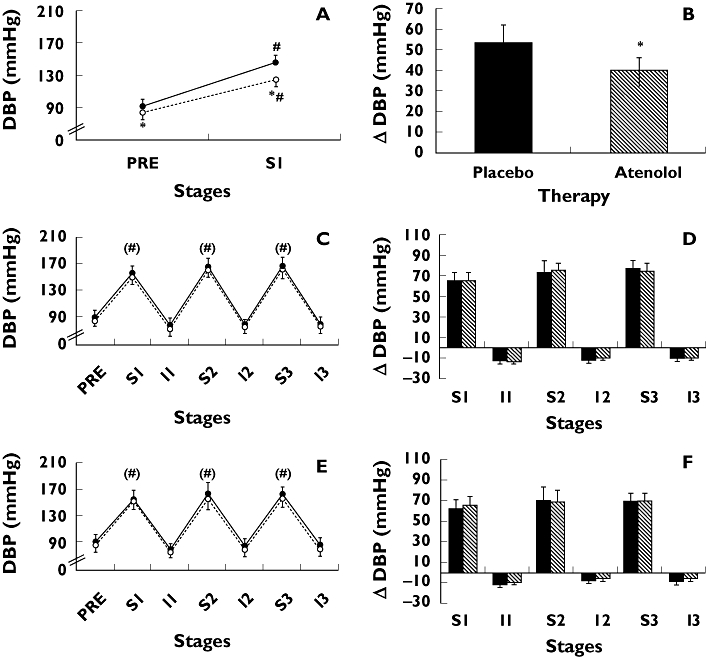
Diastolic blood pressure absolute values (DBP) and changes (ΔDBP exercise – pre-exercise) measured pre-exercise (PRE) and during three sets (S1, S2, and S3) and intervals (I1, I2, and I3) of knee-extension resistance exercise performed to failure at 100% (panels A and B), 80% (panels C and D), and 40% (panels E and F) of 1 RM. Measurements were taken with subjects receiving placebo (solid line with solid circles or black bars) and atenolol (dotted line with open circles or white bars) in hypertensive subjects. * Significantly different from placebo (P < 0.05). # Significantly different from PRE (P < 0.05). () Main effect significance in anova. Data = mean ± SE
HR responses to exercise
Figure 4 shows the HR values and changes measured during exercise in the three intensity protocols. As expected, HR increased significantly during the exercise sets in all protocols and returned to pre-exercise values during the intervals. Moreover, atenolol significantly decreased HR measured at all moments and protocols, and it blunted exercise-induced HR increases during exercise at 100 and 80% of 1 RM, but not at 40% of 1 RM.
Figure 4.
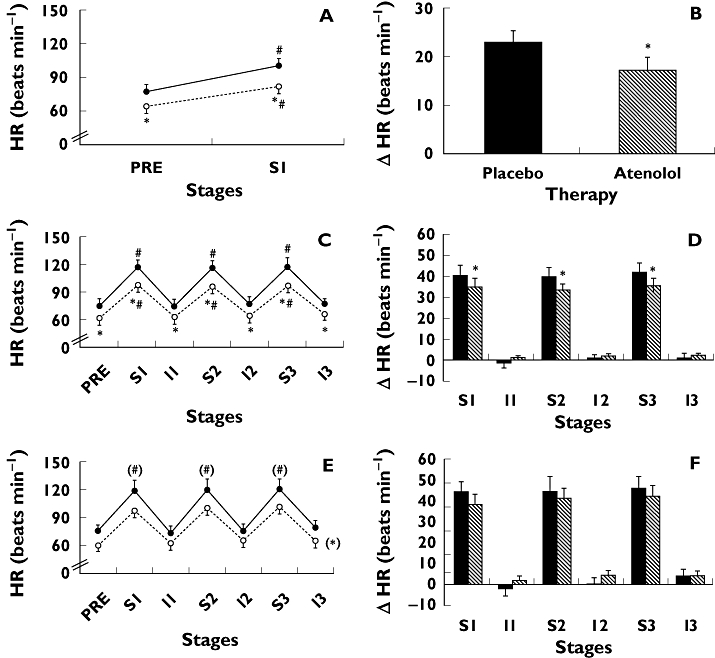
Heart rate absolute values (HR) and changes (ΔHR exercise – pre-exercise) measured pre-exercise (PRE) and during three sets (S1, S2, and S3) and intervals (I1, I2, and I3) of knee-extension resistance exercise performed to failure at 100% (panels A and B), 80% (panels C and D), and 40% (panels E and F) of 1 RM. Measurements were taken with subjects receiving placebo (solid line with solid circles or black bars) and atenolol (dotted line with open circles or white bars) in hypertensive subjects. * Significantly different from placebo (P < 0.05). # Significantly different from PRE (P < 0.05). () Main effect significance in anova. Data = mean ± SE
Discussion
The main scientific contribution of the present study is the description of the selective β1-adrenoceptor blocker's (atenolol) influence on intra-arterial BP responses to different intensities of dynamic resistance exercise conducted to failure in hypertensive subjects. The most important findings were that (i) atenolol decreased systolic BP maximum values achieved during dynamic resistance exercise, (ii) atenolol also blunted the increase in systolic BP during the first set of dynamic resistance exercises of different intensities and (iii) atenolol decreased diastolic BP and mitigated its increase during exercise at 100% of 1 RM.
Some studies have already investigated BP responses to aerobic [41] and isometric resistance exercise [7] in hypertensive patients. However, only two investigations [4, 12] have studied BP responses during dynamic resistance exercise in these patients. In one of them [4], BP was measured indirectly and immediately after the end of the exercise, and in the other [12], intra-arterial BP was measured, but only three hypertensives were studied. In the present study, these limitations were minimized, and results showed that the maximum values of systolic BP achieved with placebo were 215 ± 7 mmHg at 100% of 1 RM, 247 ± 9 mmHg at 80% of 1 RM, and 252 ± 16 mmHg at 40% of 1 RM. These increases might represent a risk to hypertensives.
To our knowledge, this is the first study to investigate the effects of a selective β1-adrenoceptor blocker on BP responses to dynamic resistance exercise. Previous studies have only evaluated the effects of atenolol on isometric resistance exercise [28–34] or the interaction between atenolol and atropine blockades on BP responses to dynamic resistance exercise [42]. In the present study, using only β-adrenoceptor blockade, we demonstrated that atenolol alone was able not only to decrease the absolute value of systolic BP but also to mitigate its increase during dynamic resistance exercise.
Systemic haemodynamic mechanisms responsible for BP increase during resistance exercise are not completely understood, and an increase in systemic vascular resistance is usually cited as the main determinant [17, 18, 35]. Nevertheless, in the present study, atenolol mitigated the increase of systolic BP during resistance exercise. Chronic atenolol use decreases BP mainly by reducing cardiac output due to the negative chronotropic properties of the drug [43]. Thus, the results of the present study suggest that cardiac output might be involved in the increase in BP during dynamic resistance exercise, independent of its intensity. However, in the present investigation, BP was measured on the arm, which would not adequately characterize central pulsatile haemodynamic changes produced by dynamic resistance exercise, as exercise can change arterial compliance [44]. Thus, the possible role of cardiac output on BP increase during resistance exercise emerged from the present results as a hypothesis and should be tested in the future with specific methodologies for this assessment.
The reduced increases in systolic BP observed with atenolol during the first set of exercises performed at 100, 80, and 40% of 1 RM were accompanied by reduced increases in HR. However, the magnitude of HR decreases were approximately 4% lower than the decreases in systolic BP (30 vs. 26%, 19 vs. 15%, and 18 vs. 15%, respectively). These differences might be explained by other effects of β1-adrenoceptor blockade, such as the decrease in cardiac contractility. In fact, atenolol has been shown to have negative inotropic effects when acutely administered [25, 45, 46]. However, studies employing atenolol in hypertensives for longer periods (1 to 5 years) did not observe this effect [47, 48], suggesting that the effect is lost with time, as observed in heart failure [49]. As the drug was administered for 6 weeks in the present study, some of this negative inotropic effect might be still present.
Interestingly, atenolol's influence on the increase of systolic BP was greater when exercise was performed at 100% of 1 RM than at 80 and 40% of 1 RM (30% vs. 19 and 18%, respectively). Similarly, although atenolol decreased systolic BP increments in the first set of exercises performed at 80 and 40% of 1 RM, this effect was not so evident during the subsequent sets. These responses suggest that when dynamic resistance exercise is prolonged (e.g. many repetitions per set – 100% vs. 80 and 40% of 1 RM – or in multiple-set protocols – S1 vs. S2 and S3), the maintenance of muscular contraction during exercise might increase isometric components of the exercise, leading to a greater accumulation of metabolites, which might increase peripheral sympathetic nervous activity, provoking vasoconstriction [18, 35]. Thus, under these circumstances, systemic vascular resistance might contribute more effectively towards an increase in systolic BP during dynamic resistance exercise, explaining why atenolol did not have much effect.
Based on the previous discussion, it is possible to suggest that during long-term dynamic resistance exercise (more sets or repetitions), other classes of antihypertensive medications with major actions on peripheral mechanisms might have greater hypotensive effects. Furthermore, if these medications were combined with β1-adrenoceptor blockers, the hypotensive effect might be even greater. These two hypotheses were out of the scope of the present study but should be investigated in the future.
Results from this study might have clinical implications. Because it is known that a sharp increase in BP is a trigger for aneurysm rupture [19–21], the fact that atenolol blunted the increase in BP during resistance exercise suggests that medication (at least atenolol) might decrease the risk of aneurysm rupture during this kind of exercise. Moreover, as many daily activities (carrying groceries, pushing furniture, and so on) have mechanical properties similar to dynamic resistance exercise, medicated patients might be less prone to aneurysm rupture during them. Future studies might investigate these suggestions.
Intra-arterial BP measurement was employed in the present study because the auscultatory indirect method was reported to underestimate intra-arterial BP values in more than 16% during this mode of exercise [13], and other indirect BP measurement techniques have not yet been compared with intra-arterial values during resistance exercise.
The main limitation of the present study was the fixed order for therapy administration (first placebo and then atenolol). This strategy was adopted to avoid an unnecessary artery puncture in a patient with an acceptable BP level in the first experiment with atenolol and with an unacceptable level in the second experiment with placebo. To minimize any possible influence of the experiment order, some strategies were adopted. The volunteers were blinded to the drug used in each experiment, and many adaptation sessions were conducted in both phases of the study. As the decreases in HR and BP observed in the study were similar to the ones previously described in the literature with randomized studies [33], the order of the experiments might have had minor effect on BP results.
The effectiveness of β-adrenoceptor blockade was demonstrated by the fact that, as previously described [32], atenolol decreased resting heart rate by 11% and systolic and diastolic BP by 15 and 16%, respectively. Moreover, spectral analysis of resting HR variability revealed alterations compatible with a clear decrease in cardiac sympathetic modulation [37].
In conclusion, atenolol was effective in decreasing not only the absolute values but also in mitigating the increase of systolic BP during the execution of dynamic resistance exercise performed to failure with different intensities in hypertensive subjects.
Acknowledgments
The authors want to acknowledge the volunteers who contributed to this study. We also thank Josiane Lima de Gusmão and Sandra de Souza Nery for technical assistance and Astra Zeneca for the donation of atenolol. This study was supported by FAPESP (06/52726-1 and 06/06356-8).
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Fleck SJ, Kraemer WJ. Basic principles of resistance training and exercise prescription. In: Fleck SJ, Kraemer WJ, editors. Designing Resistance Training Programs. 3rd. Champaign, IL: Human Kinetics; 2004. pp. 3–12. [Google Scholar]
- 2.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American colle-ge of sports medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 3.Bezucha GR, Lenser MC, Hanson PG, Nagle FJ. Comparison of hemodynamic responses to static and dynamic exercise. J Appl Physiol. 1982;53:1589–93. doi: 10.1152/jappl.1982.53.6.1589. [DOI] [PubMed] [Google Scholar]
- 4.Harris KA, Holly RG. Physiological response to circuit weight training in borderline hypertensive subjects. Med Sci Sports Exerc. 1987;19:246–52. [PubMed] [Google Scholar]
- 5.Haslam DRS, McCartney N, McKelvie RS, MacDougall JD. Direct measurements of arterial blood pressure during formal weightlifting in cardiac patients. J Cardiopulm Rehabil. 1988;8:213–25. [Google Scholar]
- 6.Hill DW, Butler SD. Haemodynamic responses to weightlifting exercise. Sports Med. 1991;12:1–7. doi: 10.2165/00007256-199112010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hoel BL, Lorentsen E, Lund-Larsen PG. Haemodynamic responses to sustained hand-grip in patients with hypertension. Acta Med Scand. 1970;188:491–5. doi: 10.1111/j.0954-6820.1970.tb08074.x. [DOI] [PubMed] [Google Scholar]
- 8.Lewis SF, Snell PG, Taylor WF, Hamra M, Graham RM, Pettinger WA, Blomqvist CG. Role of muscle mass and mode of contraction in circulatory responses to exercise. J Appl Physiol. 1985;58:146–51. doi: 10.1152/jappl.1985.58.1.146. [DOI] [PubMed] [Google Scholar]
- 9.Ludbrook J, Faris IB, Iannos J, Jamieson GG, Russell WJ. Lack of effect of isometric handgrip exercise on the responses of the carotid sinus baroreceptor reflex in man. Clin Sci Mol Med. 1978;55:189–94. doi: 10.1042/cs0550189. [DOI] [PubMed] [Google Scholar]
- 10.MacDougall JD, McKelvie RS, Moroz DE, Sale DG, McCartney N, Buick F. Factors affecting blood pressure during heavy weight lifting and static contractions. J Appl Physiol. 1992;73:1590–7. doi: 10.1152/jappl.1992.73.4.1590. [DOI] [PubMed] [Google Scholar]
- 11.MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–90. doi: 10.1152/jappl.1985.58.3.785. [DOI] [PubMed] [Google Scholar]
- 12.Palatini P, Mos L, Munari L, Valle F, Del Torre M, Rossi A, Varotto L, Macor F, Martina S, Pessina AC, Dal Palù C. Blood pressure changes during heavy-resistance exercise. J Hypertens Suppl. 1989;7:S72–3. doi: 10.1097/00004872-198900076-00032. [DOI] [PubMed] [Google Scholar]
- 13.Wiecek EM, McCartney N, McKelvie RS. Comparison of direct and indirect measures of systemic arterial pressure during weightlifting in coronary artery disease. Am J Cardiol. 1990;66:1065–9. doi: 10.1016/0002-9149(90)90506-v. [DOI] [PubMed] [Google Scholar]
- 14.Fleck SJ, Dean LS. Resistance-training experience and the pressor response during resistance exercise. J Appl Physiol. 1987;63:116–20. doi: 10.1152/jappl.1987.63.1.116. [DOI] [PubMed] [Google Scholar]
- 15.Lamotte M, Niset G, van de Borne P. The effect of different intensity modalities of resistance training on beat-to-beat blood pressure in cardiac patients. Eur J Cardiovasc Prev Rehabil. 2005;12:12–7. [PubMed] [Google Scholar]
- 16.Lentini AC, McKelvie RS, McCartney N, Tomlinson CW, MacDougall JD. Left ventricular response in healthy young men during heavy-intensity weight-lifting exercise. J Appl Physiol. 1993;75:2703–10. doi: 10.1152/jappl.1993.75.6.2703. [DOI] [PubMed] [Google Scholar]
- 17.Oliver D, Pflugfelder PW, McCartney N, McKelvie RS, Suskin N, Kostuk WJ. Acute cardiovascular responses to leg-press resistance exercise in heart transplant recipients. Int J Cardiol. 2001;81:61–74. doi: 10.1016/s0167-5273(01)00529-0. [DOI] [PubMed] [Google Scholar]
- 18.Seals DR, Chase PB, Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol. 1988;64:2190–6. doi: 10.1152/jappl.1988.64.5.2190. [DOI] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Findlay JM, Ignaszewski AP. Aneurysmal subarachnoid hemorrhage associated with weight training: three case reports. Clin J Sport Med. 1996;6:52–5. doi: 10.1097/00042752-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Watanabe K, Saito A, Matsumura K, Ichikawa M. Circumstances, activities, and events precipitating aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2007;16:25–9. doi: 10.1016/j.jstrokecerebrovasdis.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Vermeer SE, Rinkel GJ, Algra A. Circadian fluctuations in onset of subarachnoid hemorrhage. New data on aneurysmal and perimesencephalic hemorrhage and a systematic review. Stroke. 1997;28:805–8. doi: 10.1161/01.str.28.4.805. [DOI] [PubMed] [Google Scholar]
- 22.Isaksen J, Egge A, Waterloo K, Romner B, Ingebrigtsen T. Risk factors for aneurysmal subarachnoid haemorrhage: the Tromso study. J Neurol Neurosurg Psychiatry. 2002;73:185–7. doi: 10.1136/jnnp.73.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nery SS, Gomides RS, Silva GV, Forjaz CLM, Júnior DM, Tinucci T. Intra-arterial blood pressure response in hypertensive subjects during low- and high-intensity resistance exercise. Clinics. 2010;65:271. doi: 10.1590/S1807-59322010000300006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.Bakris G. An in-depth analysis of vasodilation in the management of hypertension: focus on adrenergic blockade. J Cardiovasc Pharmacol. 2009;53:379–87. doi: 10.1097/FJC.0b013e31819fd501. [DOI] [PubMed] [Google Scholar]
- 26.Follath F. Beta-blockade today: the gap between evidence and practice. Eur Heart J. 2006;8:C28–34. [Google Scholar]
- 27.Lund-Johansen P. The influence of vasodilating beta-blockers on cardiac function and vascular resistance in essential hypertension. Clin Nephrol. 1992;38(Suppl. 1):S78–86. [PubMed] [Google Scholar]
- 28.Cleroux J, Beaulieu M, Kouame N, Lacourciere Y. Comparative effects of quinapril, atenolol, and verapamil on blood pressure and forearm hemodynamics during handgrip exercise. Am J Hypertens. 1994;7:566–70. doi: 10.1093/ajh/7.6.566. [DOI] [PubMed] [Google Scholar]
- 29.Corea L, Valori C, Bentivoglio M, Verdecchia P, Bichisao E. Age and responses to isometric exercise in hypertension: possible predictors of the antihypertensive effect of diuretics and beta-blockers. Int J Clin Pharmacol Ther Toxicol. 1985;23:554–9. [PubMed] [Google Scholar]
- 30.Dreslinski GR, Aristimuno GG, Messerli FH, Suarez DH, Frohlich ED. Effects of beta blockade with acebutolol on hypertension, hemodynamics, and fluid volume. Clin Pharmacol Ther. 1979;26:562–5. doi: 10.1002/cpt1979265562. [DOI] [PubMed] [Google Scholar]
- 31.McInnes GT, Findlay IN, Murray GD, Dargie HJ. Effects of calcium antagonism and beta-blockade on haemodynamic responses to stress. Nephron. 1987;47(Suppl. 1):128–31. doi: 10.1159/000184570. [DOI] [PubMed] [Google Scholar]
- 32.Orlandi C, Fogari R. Effect of chronic atenolol therapy on the cardiovascular response to handgrip in hypertensive patients. Clin Ther. 1983;5:632–7. [PubMed] [Google Scholar]
- 33.Porsti I, Arvola P, Saynavalammi P, Nurmi AK, Metsa-Ketela T, Koskenvuo K, Laitinen LA, Manninen V, Vapaatalo H. Effects of single doses of quinapril and atenolol on autonomic nervous function and exercise capacity in healthy volunteers. Eur J Clin Pharmacol. 1990;38:541–6. doi: 10.1007/BF00278578. [DOI] [PubMed] [Google Scholar]
- 34.Wu SC, Oltrona L, Secchi MB, Mancarella S, Bettazzi L, Civelli M, Sannino C, Loche G, Folli G. [Comparison of the efficacy of monotherapy with a beta-blocker, a diuretic, and ACE inhibitors in the control of blood pressure during stress] Minerva Cardioangiol. 1989;37:323–31. [PubMed] [Google Scholar]
- 35.Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol. 1990;69:407–18. doi: 10.1152/jappl.1990.69.2.407. [DOI] [PubMed] [Google Scholar]
- 36.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 37.Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, Schwartz PJ. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–6. [PubMed] [Google Scholar]
- 38.Baron JF, Camus C, Chemla D, Duranteau R, Grès BD, Coussaye JEL, Mallédant Y, Mantz J, Martin C, Marty J, Ravussin P, Riou B, Teboul JL. [French Society of Anesthesia and Intensive Care. Arterial catheterization and invasive measurement of blood pressure in anesthesia and intensive care in adults] Ann Fr Anesth Reanim. 1995;14:444–53. [PubMed] [Google Scholar]
- 39.Kraemer WJ, Fry AC. Strength testing: development and evaluation of methodology. In: Maud PJ, Foster C, editors. Physiological Assessement of Human Fitness. Champaign, IL: Human Kinetics; 1995. pp. 115–38. [Google Scholar]
- 40.Armstrong L, Balady GJ, Berry MJ, Davis SE, Davy BM, Davy KP, Franklin BA, Gordon NF, I-m L, McConnell T, Myers JN, Pizza FX, Rowland TW, Stewart K, Thompson PD, Wallace JP. Exercise prescription modifications for cardiac patients. In: Whaley MH, Brubaker PH, Otto RM, editors. Acsm's Guidelines for Exercise Testing & Prescription. 7th. Baltimore, MD: Lippincott, Williams & Wilkins, Inc.; 2006. pp. 174–204. [Google Scholar]
- 41.Palatini P. Exercise haemodynamics in the normotensive and the hypertensive subject. Clin Sci (Lond) 1994;87:275–87. doi: 10.1042/cs0870275. [DOI] [PubMed] [Google Scholar]
- 42.Lewis SF, Taylor WF, Bastian BC, Graham RM, Pettinger WA, Blomqvist CG. Haemodynamic responses to static and dynamic handgrip before and after autonomic blockade. Clin Sci (Lond) 1983;64:593–9. doi: 10.1042/cs0640593. [DOI] [PubMed] [Google Scholar]
- 43.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th. New York: McGraw-Hill Companies, Inc.; 2006. pp. 237–95. [Google Scholar]
- 44.Chirinos JA, Segers P, Raina A, Saif H, Swillens A, Gupta AK, Townsend R, Emmi AG, Jr, Kirkpatrick JN, Keane MG, Ferrari VA, Wiegers SE, St John Sutton MG. Arterial pulsatile hemodynamic load induced by isometric exercise strongly predicts left ventricular mass in hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H320–30. doi: 10.1152/ajpheart.00334.2009. [DOI] [PubMed] [Google Scholar]
- 45.Clark BJ. Beta-adrenoceptor-blocking agents: are pharmacologic differences relevant? Am Heart J. 1982;104:334–46. doi: 10.1016/0002-8703(82)90124-7. [DOI] [PubMed] [Google Scholar]
- 46.Rapola JM, Pellinen TJ, Koskinen P, Toivonen L, Nieminen MS. Hemodynamic effects of pindolol and atenolol at rest and during isometric exercise: a noninvasive study with healthy volunteers. Cardiovasc Drugs Ther. 1990;4:737–43. doi: 10.1007/BF01856563. [DOI] [PubMed] [Google Scholar]
- 47.Lund-Johansen P. Haemodynamic long-term effects of atenolol at rest and during exercise in essential hypertension. Postgrad Med J. 1977;53(Suppl. 3):99–101. [PubMed] [Google Scholar]
- 48.Lund-Johansen P. Hemodynamic consequences of long-term beta-blocker therapy: a 5-year follow-up study of atenolol. J Cardiovasc Pharmacol. 1979;1:487–95. doi: 10.1097/00005344-197909000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Doughty RN, Sharpe N. Beta-adrenergic blocking agents in the treatment of congestive heart failure: mechanisms and clinical results. Annu Rev Med. 1997;48:103–14. doi: 10.1146/annurev.med.48.1.103. [DOI] [PubMed] [Google Scholar]


