Abstract
AIMS
To investigate (i) if kisspeptin administration alters heart rate (HR) or blood pressure (BP) in healthy male and female volunteers, (ii) whether circulating plasma kisspeptin concentrations in healthy pregnant women and women with hypertensive diseases of pregnancy correlate with BP and (iii) whether women with hypertensive diseases of pregnancy have altered plasma kisspeptin concentrations.
METHODS
We have previously reported the effects of administration of kisspeptin-54 on gonadotrophin secretion in healthy male and female volunteers. In these studies, cardiovascular parameters were not a primary endpoint. However, data were also collected on BP and HR for 4 h post administration of kisspeptin-54. Blood samples were taken from 105 women in the third trimester of pregnancy (27 women with hypertensive diseases of pregnancy and 78 controls). Samples were assayed for plasma kisspeptin immunoreactivity (IR).
RESULTS
Administration of kisspeptin was not associated with significant changes in HR or BP in healthy men or women. There was no significant correlation between plasma kisspeptin concentration and BP in healthy pregnant women or in those with hypertensive diseases of pregnancy. No significant differences in plasma kisspeptin-IR concentrations were observed between women with hypertensive diseases of pregnancy and normotensive pregnant controls, plasma kisspeptin concentrations ± SE: controls 2878 ± 157 pmol l−1; pregnancy-induced hypertension 2696 ± 299 pmol l−1 (95% CI vs. controls −514, 878 pmol l−1); pre-eclampsia 3519 ± 357 (95% CI vs. controls −1644, 362 pmol l−1).
CONCLUSIONS
Elevation of plasma kisspeptin-IR is not associated with an alteration in BP in humans.
Keywords: blood pressure, kisspeptin, pre-eclampsia, pregnancy-induced hypertension
“WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Kisspeptin potently stimulates gonadotrophin release in humans and therefore represents a potential therapy for disorders of reproduction. However, ex vivo studies on human tissue suggest that kisspeptin has vasoconstrictive effects. Thus it is important to determine the effects of kisspeptin on blood pressure and heart rate in humans. Furthermore, plasma kisspeptin concentrations increase markedly in pregnancy but it is currently not known if concentrations are altered in hypertensive diseases of pregnancy.
WHAT THIS STUDY ADDS
This study demonstrates that states of elevated kisspeptin are not associated with changes in blood pressure or heart rate in humans. Neither are hypertensive diseases of pregnancy associated with altered circulating kisspeptin. This has implications for the future use of kisspeptin as a therapy.
Introduction
Kisspeptins are the peptide products of the KiSS-1 gene, and are the ligands for the G-protein-coupled receptor GPR54 [1–3]. The kisspeptin/GPR54 system has an important role in reproduction and reproductive development [4–7]. The KiSS-1 and GPR54 genes are highly expressed in the placenta [2, 3, 8] and cultured human trophoblasts have also been shown to secrete kisspeptin [8]. Plasma kisspeptin concentrations are markedly elevated in pregnancy. Horikoshi and co-workers measured circulating concentrations of <2 pmol l−1 in the non-pregnant state, and reported a progressive rise in concentrations during pregnancy to a peak of 9590 ± 1640 pmol l−1 in the third trimester [9]. The physiological role of kisspeptin in pregnancy is currently unclear, although there is evidence to suggest that kisspeptin acts as a regulator of trophoblast invasion [1, 8].
Kisspeptin may have therapeutic potential in manipulating the human hypothalamic pituitary gonadal (HPG) axis for disorders of reproduction. Kisspeptin administration stimulates the HPG axis in rodents and primates [10–18]. We have previously published details of the gonadotrophin and steroid response to kisspeptin administration in human volunteers [10, 12, 12]. This effect appears to be predominantly mediated via the release of gonadotrophin releasing hormone (GnRH) [11, 13, 18–20]. Furthermore, continuous administration of kisspeptin to rats and monkeys results in an initial increase in plasma LH followed by a desensitization of the HPG axis to the effects of kisspeptin possibly due to GPR54 receptor desensitization [21, 22, 23]. Possible therapeutic applications for kisspeptin include its use in infertility and treating hormone dependent malignancies. However, a recent report demonstrates that GPR54 mRNA is expressed in the aorta, coronary artery and umbilical vein and suggests that kisspeptin is a potent vasoconstrictor in human blood vessels ex vivo[24]. Since kisspeptin has potential as a therapeutic agent in disorders of reproduction it is important to determine if kisspeptin administration alters blood pressure or heart rate in vivo in humans. It is also possible that the raised kisspeptin concentrations reported in pregnancy may be associated with hypertensive disorders. Hypertensive diseases are a leading medical problem in pregnancy [25].
The aim of this study was to investigate whether states of elevated circulating kisspeptin concentrations are associated with alterations in heart rate or blood pressure.
Methods
Ethical approval was obtained from the Hammersmith and Queen Charlotte's and Chelsea Hospitals Research Ethics Committee (reference numbers 04/Q0406/80, 04/Q0406/151, 05/Q0406/142). These studies were conducted in accordance with the Declaration of Helsinki and explicit written informed consent was obtained from each volunteer prior to participation.
Assessment of the effect of kisspeptin on pulse and blood pressure in healthy men
Healthy male volunteers received a 90 min intravenous (i.v.) infusion of either saline or varying doses of kisspeptin-54 (0, 0.5, 1, 2, 4 pmol kg−1 min−1). This study was designed with plasma concentrations of reproductive hormones as the primary endpoint and details of the infusion protocol, plasma kisspeptin concentrations achieved and the gonadotrophin response in this study have previously been published [10]. However, analysis of the blood pressure and heart rate data has not previously been communicated. Blood pressure and heart rate were measured every 15 min post administration of kisspeptin for the first 180 min and every 30 min thereafter, using a Trio non-invasive external monitoring device (Datascope, Hoevelaken, Netherlands). The subjects remained supine throughout the study.
Assessment of the effect of kisspeptin on pulse and blood pressure in healthy women
Healthy female volunteers received a subcutaneous (s.c.) injection of kisspeptin-54, following which blood pressure and heart rate were monitored for 4 h. Subjects attended the clinical investigation unit between 08.00 h and 09.00 h in the follicular phase of their menstrual cycle. Kisspeptin-54 dissolved in saline was administered by subcutaneous injection into the abdomen. The doses of kisspeptin used were 0, 0.8, 2.4, 6.4 nmol kg−1. The plasma kisspeptin concentrations achieved and gonadotrophin responses in this study have been previously reported [12]. Again, the primary endpoint in this study was circulating concentrations of reproductive hormones. However, analysis of the blood pressure and heart rate data has not previously been published. Blood pressure and heart rate were measured at times −30, 0, 15, 30, 45, 60, 75, 90, 120, 150, 180, 210 and 240 min after injection, using a Trio non-invasive external monitoring device (Datascope, Hoevelaken, Netherlands). The subjects remained supine throughout the study.
Measurement of circulating kisspeptin concentrations in healthy pregnant women and those with pre-eclampsia or PIH
In this case-control study, volunteers in the third trimester of pregnancy were recruited from the antenatal clinic at Queen Charlotte's and Chelsea Hospital, London, UK. Hypertension was defined as a systolic blood pressure of ≥140 mmHg and a diastolic blood pressure of ≥90 mmHg recorded on two separate occasions within a 24 h period. Pre-eclampsia was defined as hypertension associated with proteinuria occurring after the 20th week of gestation. Proteinuria was defined as urinary protein excretion (assessed on a 24 h urine collection) of 300 mg l−1 or >500 mg 24 h–1. Hypertension occurring after the 20th week of gestation not associated with proteinuria was defined as pregnancy-induced hypertension (PIH). Volunteers with a history of pre-existing hypertension, whether treated or untreated, were excluded. Other exclusion criteria included significant co-morbidity, such as diabetes, cardiac disease or cerebrovascular disease, age <18 years or >45 years, multiple pregnancy and body mass index (BMI) over 30 kg m−2. Additional data on ethnicity, tobacco use and BMI were collected to account for other possible causes of hypertension. Volunteers without any underlying illnesses and with uncomplicated pregnancies in their third trimester were used as controls. Maternal venous blood samples (10 ml) were collected into lithium-heparin tubes (LIP Ltd, Cambridge, UK) containing 5000 kallikrein inhibitor units of aprotinin (0.2 ml Trasylol, Bayer, Newbury, UK). Samples were immediately centrifuged and plasma separated and stored at –20°C until measurement of kisspeptin immunoreactivity (IR).
Kisspeptin radioimmunoassay (RIA)
Kisspeptin-IR was measured in plasma using an established RIA as previously described [10]. Briefly, antibody GQ2 was raised in sheep immunized with synthetic human kisspeptin-54 (Bachem UK Ltd, Merseyside, UK) conjugated to bovine serum albumen by glutaraldehyde, and was used at a final dilution of 1:3 500 000. The antibody cross-reacted 100% with human kisspeptin-54, kisspeptin-14, and kisspeptin-10 and less than 0.01% with other related RF amide proteins, including prolactin-releasing peptide, RF amide-related peptide 1 (RFRP1), RFRP2, RFRP3, QRFP43, neuropeptide FF and neuropeptide AF. The assay detected changes of 2 pmol l−1 of plasma kisspeptin-IR with a 95% confidence limit. The intra- and inter-assay coefficients of variation were 8.3 and 10.2%, respectively. The label specific activity was 56 Bq fmol−1.
Data analysis and statistical procedures
Chi squared analysis was used to assess demographic data between the pre-eclampsia, PIH and pregnant control populations. In the studies of administration of varying doses of kisspeptin to healthy volunteers statistical comparisons were made across time and dose using two way anova, with the Bonferroni post hoc correction.
The study of hypertensive diseases of pregnancy was also analyzed by two way anova with the Bonferroni post hoc correction. Statistical power calculations yielded sample sizes of 66 controls and seven experimental subjects in order to detect a difference of 600 pmol l−1 with a control : experimental subject ratio of 1:10, estimated standard deviation of 500, 1–β of 0.8 and α of 0.05.
Linear regression studies were used to assess the relationship between blood pressure and kisspeptin concentrations in pregnancy.
All results are presented as mean ± SE, unless otherwise stated. P < 0.05 was considered statistically significant.
Results
Effects of kisspeptin on pulse and blood pressure in healthy male volunteers
In humans i.v. infusion of increasing doses of kisspeptin-54 into male volunteers did not result in significant changes in diastolic or systolic blood pressure or heart rate, compared with infusion of vehicle (Figure 1).
Figure 1.
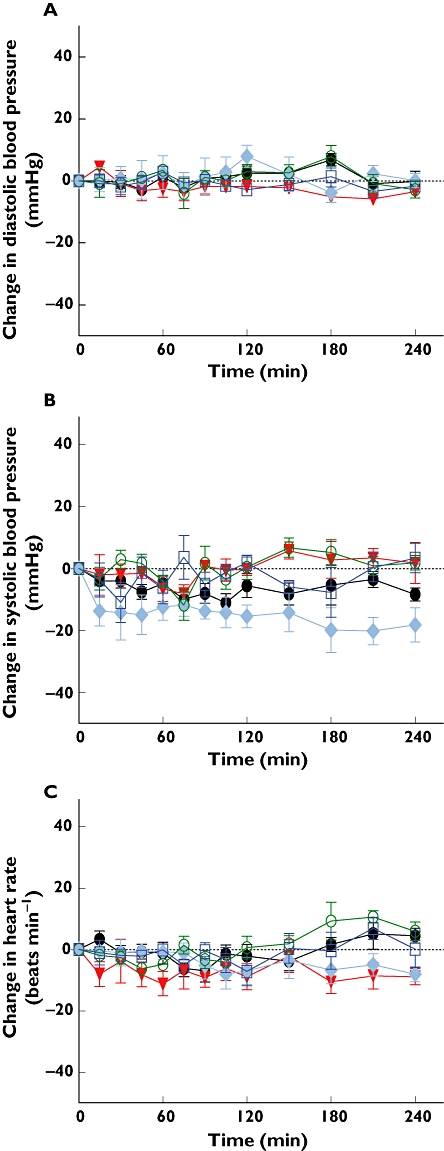
Effect of kisspeptin administration to healthy male volunteers on blood pressure and heart rate. Mean± SE change in (A) diastolic blood pressure (DBP), (B) systolic blood pressure (SBP) and (C) heart rate (HR) during and following i.v. infusion of kisspeptin-54 (0, 0.5, 1, 2, 4 pmol kg−1 min−1) into healthy males. P > 0.05 for all doses of kisspeptin-54 vs. control. 0 pmol kg−1 min−1 ( ); 0.5 pmol kg−1 min−1 (
); 0.5 pmol kg−1 min−1 ( ); 1 pmol kg−1 min−1 (
); 1 pmol kg−1 min−1 ( ); 2 pmol kg−1 min−1 (
); 2 pmol kg−1 min−1 ( ); 4 pmol kg−1 min−1 (
); 4 pmol kg−1 min−1 ( )
)
Effects of kisspeptin on pulse and blood pressure in healthy female volunteers
Administration of increasing doses of kisspeptin-54 by s.c. bolus injection into female volunteers did not result in significant changes in diastolic blood pressure, systolic blood pressure or heart rate (Figure 2).
Figure 2.
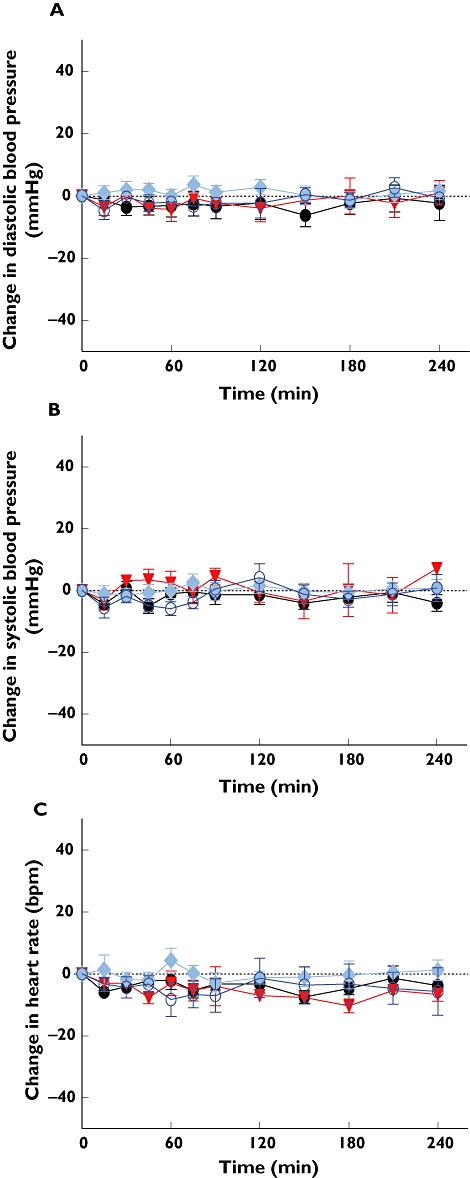
Effect of kisspeptin administration to healthy female volunteers on blood pressure and heart rate. Mean± SE change in (A) diastolic blood pressure, (B) systolic blood pressure and (C) heart rate following s.c. injection of kisspeptin 54 (0, 0.8, 2.4, 2.4, 6.4 nmol kg−1) into healthy females. P > 0.05 for all doses of kisspeptin-54 vs. control. 0.0 nmol/kg ( ); 0.8 nmol/kg (
); 0.8 nmol/kg ( ); 2.4 nmol/kg (
); 2.4 nmol/kg ( ); 6.4 nmol/kg (
); 6.4 nmol/kg ( )
)
Determination of plasma kisspeptin concentrations in women with pre-eclampsia, PIH and normotensive pregnant controls
Table 1 illustrates the demographic data for the pregnant study population. A total of 105 subjects were recruited (controls n= 78, pre-eclampsia = 8, PIH = 19). There was no significant difference between groups in terms of maternal age, gestational age, ethnicity, BMI or tobacco use between groups. There was a statistically significant (P < 0.001) difference in systolic and diastolic blood pressures between the PIH and pre-eclampsia groups compared with controls.
Table 1.
Demographic information for pregnant women with pre-eclampsia, PIH and healthy controls
| Characteristic | Controls | Pre-clampsia | PIH |
|---|---|---|---|
| n | 78 | 8 | 19 |
| Maternal age (years) | 33.6 ± 0.6 | 33.9 ± 1.9 | 34.5 ± 0.8 |
| Gestational age (weeks) | 31.6 ± 0.5 | 35.4 ± 1.1 | 34.1 ± 0.9 |
| Ethnicity | |||
| White | 49 | 4 | 11 |
| Asian | 9 | 0 | 1 |
| Black | 8 | 1 | 3 |
| Mixed | 2 | 0 | 3 |
| Other | 7 | 3 | 3 |
| Not known/refused | 3 | 0 | 0 |
| Known tobacco use | 1/66 | 0/8 | 0/19 |
| BMI (kg m−2) | 24.9 ± 0.5 | 26.3 ± 2.4 | 27.3 ± 1.3 |
| Systolic BP (mm Hg) | 110.6 ± 1.3 | 137.5 ± 1.6* | 145.4 ± 2.3* |
| Diastolic BP (mm Hg) | 68.4 ± 1.0 | 91.9 ± 1.9* | 91.4 ± 1.9* |
All women were in the third trimester of pregnancy.
P < 0.001 compared with controls.
Data are presented as mean ± SE.
There was no correlation between plasma kisspeptin concentrations and diastolic blood pressure in normotensive pregnant women, women with PIH or women with pre-eclampsia (Figure 3A). There was no correlation between plasma kisspeptin concentrations and systolic blood pressure in normotensive pregnant women, women with PIH or women with pre-eclampsia (Figure 3B).
Figure 3.

Correlation between plasma kisspeptin concentrations and blood pressure in normotensive pregnant controls, women with pregnancy induced hypertension (PIH) and women with pre-eclampsia. There was no statistically significant correlation (P > 0.05) between plasma kisspeptin-IR and (A) diastolic blood pressure and (B) systolic blood pressure. Controls (•); Pre-eclampsia ( ); PIH (
); PIH ( )
)
Plasma kisspeptin concentrations were 2878 ± 157 pmol l−1 in pregnant controls, 2696 ± 299 pmol l−1 in women with PIH and 3519 ± 357 in women with pre-eclampsia. There were no statistically significant differences between the groups. (95% confidence interval (CI) women with PIH vs. controls −514, 878 pmol l−1, 95% CI women with pre-eclampsia vs. controls −1644, 362 pmol l−1).
Discussion
Kisspeptin has a potential role as a therapeutic agent in disorders of reproduction. However, kisspeptins have been demonstrated to cause potent vasoconstriction in an ex vivo model of the human coronary artery and umbilical vein [24]. We therefore investigated the effects of elevated circulating kisspeptin concentrations on blood pressure and heart rate in vivo in healthy human volunteers.
Healthy male and female volunteers were studied following administration of exogenous kisspeptin-54. The plasma concentrations of kisspeptin-IR achieved, and the resultant effect on circulating gonadotrophins and sex steroid concentrations are published elsewhere [10, 12]. Intravenous infusion of kisspeptin-54 at a rate greater than 0.5 pmol kg−1 min−1 into healthy male volunteers was sufficient to increase significantly plasma LH, FSH and testosterone [10]. In healthy female volunteers studied in the follicular phase of the menstrual cycle, administration by bolus s.c. injection of a dose of kisspeptin-54 greater than 0.8 nmol kg−1 resulted in a significant increase in plasma LH and FSH [12]. However, in both instances, analysis of the effects of kisspeptin-54 on blood pressure and heart rate has not previously been published.
Our results suggest that administration of kisspeptin to healthy men and women is not associated with alterations in diastolic blood pressure, systolic blood pressure or heart rate at any of the doses tested. Although future studies are required to evaluate the effects of chronic kisspeptin administration on cardiovascular parameters, these preliminary findings following acute administration are encouraging with reference to the prospect of a kisspeptin/GPR54 based therapy.
Circulating kisspeptin concentrations during the third trimester of pregnancy are elevated approximately 7000-fold over concentrations in non-pregnant humans [9]. These concentrations of plasma kisspeptin-IR are greater than those achieved in our studies following administration of exogenous kisspeptin-54. If kisspeptin has vasoconstrictive effects in humans then it is possible that circulating plasma concentrations of kisspeptin during pregnancy may be elevated in women with pregnancy related disorders of hypertension. We therefore investigated whether there was a correlation between circulating kisspeptin-IR and hypertensive disorders of pregnancy. Our results suggest that plasma kisspeptin-IR is correlated with neither diastolic nor systolic blood pressure in healthy pregnant controls, in women with pregnancy induced hypertension or in women with pre-eclampsia. Moreover, no significant difference in plasma kisspeptin-IR was found between pregnant controls, women with pregnancy induced hypertension and women with pre-eclampsia.
Possible confounding causes of hypertension such as ethnicity and smoking were analyzed and no significant difference was found in those variables. There was a significant difference in SBP and DBP between the hypertensive pregnant groups and the normotensive pregnant group, as expected. There was, however, no correlation between plasma kisspeptin concentrations and diastolic or systolic blood pressure in healthy pregnant controls, women with pregnancy induced hypertension and women with pre-eclampsia. Our results suggest that elevated plasma kisspeptin concentrations do not play a role in the pathogenesis of these hypertensive diseases of pregnancy. In keeping with our findings, a recent study has reported that serum kisspeptin concentrations were slightly lower in the second trimester of women who subsequently developed pre-eclampsia compared with controls [26].
A degree of caution should be exercised in interpreting our results, however. The study was powered to detect a difference in plasma kisspeptin concentrations of 600 pmol l−1 or greater, and therefore the relatively small number of pre-eclampsia patients recruited into the study may not have allowed for the detection of more subtle differences in kisspeptin between the groups. Thus, the usefulness of kisspeptin measurement as a biomarker for hypertensive diseases in pregnancy (particularly in light of the work by Armstrong and co-workers [26]) remains to be determined, and further study with a larger cohort of hypertensive pregnant women would provide a more definitive answer.
Moreover, although there was no statistical difference between the normotensive and hypertensive groups in respect of BMI and gestational age, there was arguably a trend towards greater BMI and gestation in the women with PIH or pre-eclampsia compared with normotensive women. There is little in the literature linking BMI with circulating kisspeptin-IR. In one study of women with polycyctic ovarian syndrome, Panidis and coworkers [27] found a negative correlation between BMI and plasma kisspeptin-IR. The applicability of this finding to a more general, non-polycystic ovarian population remains to be determined. More robust, however, is the positive correlation between gestational age and circulating kisspeptin-IR [9]. Although all the women in our study were in the third trimester of pregnancy, it is possible that the (statistically non-significant) trend towards greater gestational age in the hypertensive women may have masked a subtle difference in circulating kisspeptin-IR, compared with the normotensive controls. More close matching of hypertensive and normotensive women would minimize the impact of gestational age on circulating kisspeptin-IR and may allow for detection of smaller differences in plasma kisspeptin.
Furthermore, lack of a detectable difference in circulating kisspeptin-IR between normotensive and hypertensive pregnant women does not exclude the kisspeptin/GPR54 system altogether from an aetiological role in pre-eclampsia. It may be, for example, that altered sensitivity to kisspeptin at the level of the receptor or downstream signalling pathways, rather than circulating concentrations of kisspeptin, is more important in affecting BP. It would therefore be pertinent to assess also vascular GPR54 expression and function in placental blood vessels in these patients. Although pre-eclampsia clinically presents after 20 weeks of gestation, the pathophysiology is thought to involve trophoblast invasion and vascular modelling in the placenta in early pregnancy [28–31]. Thus future longitudinal studies measuring kisspeptin concentrations from the first antenatal booking visit throughout gestation with regards to the clinical course of pregnancy and any development of hypertension would be of interest.
In summary, our data suggest that elevation of circulating kisspeptin in healthy men and women does not alter blood pressure or heart rate. Neither did we find a difference in circulating kisspeptin-IR in patients with hypertensive diseases of pregnancy and trimester-matched normotensive controls. These data have important safety implications for the potential clinical use of kisspeptin as a therapeutic tool in the future.
Acknowledgments
We are grateful to all the volunteers who participated in these studies. We would also like to thank all the staff of Queen Charlotte's and Chelsea Hospital, Imperial College London Healthcare NHS Trust, London, UK for their assistance. We are also grateful to the Wellcome Trust for their support and would like to acknowledge the Wellcome Trust/Sir John McMichael Centre for providing the infrastructure for these studies.
Grants and fellowships: Funding for this study was via a Wellcome Trust Clinical Training Fellowship (087180/Z/08/Z, G.M.K.N.). O.B.C. is a NIHR Academic Clinical Lecturer. R.R. is funded by the IPSEN Research Foundation. KGM is supported by a BBSRC New Investigator Award (BB/C515398/1). S.E.K.Z is funded by a Wellcome Trust Clinical Training Fellowship. W.S.D is funded by a HEFCE Clinical Senior Lecturer Award. We are also grateful for support from the NIHR Biomedical Research Centre funding scheme.
Competing interests
There are no competing interests to declare.
REFERENCES
- 1.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le PE, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 2.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–7. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welch DR. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 4.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 5.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 6.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semple RK, Achermann JC, Ellery J, Farooqi IS, Karet FE, Stanhope RG, O'Rahilly S, Aparicio SA. Two novel missense mutations in g protein-coupled receptor 54 in a patient with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:1849–55. doi: 10.1210/jc.2004-1418. [DOI] [PubMed] [Google Scholar]
- 8.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–28. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 9.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, Fujino M. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–9. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 10.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 11.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 12.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–66. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 13.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–8. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 14.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 15.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–97. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 16.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–63. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 17.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 19.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 20.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson EL, Murphy KG, Patterson M, Bewick GA, Stamp GW, Curtis AE, Cooke JH, Jethwa PH, Todd JF, Ghatei MA, Bloom SR. Chronic subcutaneous administration of kisspeptin-54 causes testicular degeneration in adult male rats. Am J Physiol Endocrinol Metab. 2006;291:E1074–82. doi: 10.1152/ajpendo.00040.2006. [DOI] [PubMed] [Google Scholar]
- 22.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–6. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Seminara SB, Pohl CR, Dipietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–70. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 24.Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology. 2007;148:140–7. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- 25.National High Blood Pressure Education Program Working Group. Report of the National High Blood Pressure Education Program Working Group, Report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 26.Armstrong RA, Reynolds RM, Leask R, Shearing CH, Calder AA, Riley SC. Decreased serum levels of kisspeptin in early pregnancy are associated with intra-uterine growth restriction and pre-eclampsia. Prenat Diagn. 2009;29:982–5. doi: 10.1002/pd.2328. [DOI] [PubMed] [Google Scholar]
- 27.Panidis D, Rousso D, Koliakos G, Kourtis A, Katsikis I, Farmakiotis D, Votsi E, Diamanti-Kandarakis E. Plasma metastin levels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85:1778–83. doi: 10.1016/j.fertnstert.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L, van AA. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 29.De WF, Brosens I, Robertson WB. Ultrastructure of uteroplacental arteries. Contrib Gynecol Obstet. 1982;9:86–99. [PubMed] [Google Scholar]
- 30.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, Vanasshe A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 31.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]


