Abstract
Sun exposure and high prevalence of melanocytic nevi are major risk factors for melanoma, but the relationship between them is not well understood. This study examines the relationship between sun exposure (detailed by anatomic location and history of site-specific sunburns) and the presence of melanocytic nevi on 743 White children in Denver, Colorado. Parental reports of site-specific sunburns were collected annually for 2 years starting at ages 5 to 6 years. In the third year, nevi were counted and mapped by anatomic location. Nevus density was higher for boys (36.0 nevi/m2) than for girls (31.0 nevi/m2; P = 0.04). Nevus density was highest on the face, neck, and lateral forearms and was significantly higher in chronically versus intermittently sun-exposed areas (P < 0.0001). Compared with girls, boys had higher nevus density on the face, neck, and trunk, and lower nevus density on the upper arms and thighs (P < 0.01). In 2 years of reports, most subjects (69%) received at least one sunburn. The face, shoulders, and back were the most frequently sunburned areas of the body. When adjusted for host factors, total number of sunburns was significantly associated with higher total nevus prevalence (P = 0.01 for one burn). Site-specific sunburns were significantly associated with nevus prevalence on the back (P = 0.03 for three or more sunburns), but not on the face, arms, or legs. In this high-risk population, there is evidence for two pathways to nevus accumulation: by chronic sun exposure and by intermittent exposure related to sunburns.
Introduction
Sun exposure is the most important preventable risk factor for malignant melanoma. Epidemiologic evidence suggests that this relationship is complex because accumulated chronic exposure to UV radiation via sunlight may not adequately explain melanoma risk; a pattern of intermittent acute exposure may be more important (1–4). Both chronic UV exposure (as indicated by latitude of residence and outdoor occupation) and intermittent exposure (indicated by outdoor recreation, beach vacations, and sunburns) have been associated with melanoma risk (1). Indeed, chronic and acute exposures are not mutually exclusive etiologic factors and may represent alternate pathways to melanoma (5). Information on the importance of these factors can inform prevention strategies.
The presence of numerous melanocytic nevi is also a major risk factor for melanoma (6–8). Nevi accumulate predominantly during childhood and adolescence, and case control studies indicate that sun exposure during early life is most critical for determining melanoma risk (9, 10). As there is a much shorter latency between sun exposure and the accumulation of nevi than the development of melanoma, childhood nevus density may be a useful intermediate outcome for investigating melanoma risk factors.
Different areas of the body receive different patterns of sun exposure. This fact may be used to investigate the relationship between sun exposure and melanoma risk (11). Similarly, sunburns indicate acute UV exposure (in addition to innate sun sensitivity). There is a strong basis of evidence linking sunburns and melanoma, but there is very little published about the site-specific effects of sunburns (4, 12). It is conceivable that the effect of sunburns on melanoma risk might vary by body site according to the overall pattern of exposure received at that site.
Due to a combination of high altitude (1,600 m) and sunny climate, the population of the state of Colorado is at elevated risk for melanoma. In 1997–2001, age-adjusted annual melanoma incidence rates for non-Hispanic White Coloradans were >30% higher than the overall U.S. incidence rate (13). No studies of the anatomic distribution of childhood nevi have been reported for this population or any other U.S. population, although results from other regions of the world have shown considerable variation (14–16).
This study followed a cohort of Colorado children over 3 years, with two primary objectives: to examine the anatomic distribution of melanocytic nevi on chronically exposed versus intermittently exposed areas of the body and to report on the independent association between site-specific sunburns and nevus prevalence on different areas of the body.
Materials and Methods
This study followed a cohort of 743 White children residing in Colorado for 3 years starting at the ages of 5 to 6 years. Three annual parent interviews provided demographic, phenotypic, and sun exposure data, whereas the third of three annual skin exams provided nevus distribution data.
Study Population
In 2003–2004, a cohort consisting of 1,145 children born in January to September 1998 was recruited from a large health maintenance organization, private pediatric offices, and community locations in the Denver, Colorado metropolitan area. The cohort was formed as part of a randomized controlled trial of a mail-based intervention to promote sun protection behaviors. As part of that study, subjects assigned to the intervention group received eight mailings that promoted sun protection in the springs of 2005 and 2006. Five of these mailings included sun protection products (sunscreen, hats, and swim shirts). Data collection was identical for both the control and intervention groups and both groups were included in the analysis. Among the original cohort of 1,145 children, 980 (86%) had data from all three interviews. Of those with complete interview data, 824 (84%) had complete 2006 skin exam data. Race was determined by parent report and used to limit the analysis to White subjects (both Hispanic and non-Hispanic) because this group is at higher risk for melanoma (17). After 81 (8%) non-White subjects were excluded, the final cohort for this analysis consisted of 743 subjects.
Parent Interviews
Three annual, phone-based parent interviews provided demographic, sun exposure, and sun sensitivity data about the child that was enrolled in the study. Interviews were conducted during the summer months (June-September) in 2004, 2005, and 2006. Interviews were conducted with the parent or legal guardian who “usually takes care of” the child. Whereas this designation could change from year to year, 93% of interviews were completed by a female guardian in 2006. Annual interviews provided information about sex, race and ethnicity, sunburn history, propensity to burn versus tan, and clothing coverage. Sunburn history included the total number of sunburns received in the past 12 months (regardless of severity) and the number of these sunburns received by different parts of the body: face, back, shoulders, chest, arms, and legs. Sunburns reported in 2006 were not included in the analysis to allow for needed lag time in observing effects on nevi. Propensity to burn versus tan was assessed using a four-category scale of the subject’s expected reaction to 1 h of strong sunshine at the beginning of the summer (degree of sunburn the following day relative to the degree of tan 1 week later: painful/none, painful/light, slight/little, none/good). The amount of time spent outside between 11:00 am and 3:00 pm (none, <15, 15–30, 30–60, or >60 min) and the degree to which clothing covered the arms (long-sleeves, short-sleeves, or sleeveless top) were documented from a 24-h recall of the last time the parent spent most of the day with the subject, based on interviews conducted in 2005. The degree to which the upper legs were exposed to sunlight was assessed by a recall of what the subject wore during the last time they went swimming outdoors, also from 2005.
Skin Exams
A skin exam conducted in the summer of 2006 provided information on nevus distribution and phenotypic factors including height, weight, degree of tunning, degree of freckling, and pigmentation of the skin, hair, and eyes. Observation of hair color was aided by the use of hair samples and coded into four categories: red, blond, light brown, and dark brown or black. Eye color was recorded as blue and/or green, brown, or hazel (any combination of brown with another color). Height and weight were measured using standard procedures to enable estimates of total body surface area in square meters using the Mosteller formula (18). Freckling on the face was assessed using a 10-level chart (9). Skin color and degree of tanning were measured using a Minolta Chroma Meter CR-400, which quantifies color using the three-dimension L a b system. Published research supports the use of the L-dimension to describe base skin color (smaller values indicate darker color) and the b-dimension to describe tanning (19, 20). Base skin color was measured on the unexposed, upper medial arm, and degree of tanning was calculated as the difference in b-dimension values between this area and the exposed lateral forearm. Skin color and tanning measures were based on an average of five measurements.
Nevus exams were conducted by a team of seven health care providers who received training from the lead dermatologist of the study. The entirety of the body was examined for nevi, with the exception of the scalp, genitals, and buttocks. Palms and soles were also examined but were excluded from analysis based on the small number of nevi in these areas. Nevi were defined as any size pigmented macules or papules, excluding freckles, café-au-lait macules, and warts. Nevi were differentiated from freckles and café-au-lait macules by the fact that only nevi are raised and that flat, early junctional nevi are dark brown, have regular edges, and do not occur in patches as freckles do. Lentigines were expected to be rare in this young population. Nevi were counted and recorded on a standardized body map. Congenital nevi were counted separately and excluded from overall nevus counts. Duplicate exams were conducted on 66 children by pairs of examiners. All seven examiners participated. Interrater reliability (calculated using the intraclass correlation) was 0.85.
Data Analysis
Nevi of all sizes, both flat and raised, were included in the analysis. In this population, nevi >2 mm in diameter (7% of all nevi) were not numerous enough to be analyzed separately. Nevus density was computed separately for 13 anatomic subsites (face and neck, chest and abdomen, back, lateral upper arms, medial upper arms, lateral forearms and elbows, medial forearms, dorsa of the hands, anterior thighs, posterior thighs, anterior calves and knees, posterior calves, dorsa of the feet) and as a total of these sites. A proportion of total body surface area was attributed to each anatomic site using values from burn area estimation charts adapted to the body site diagram (21). Nevus counts were skewed; therefore, it was necessary to normalize these using natural log-transformed nevus counts + 1.
Anatomic areas were grouped by their expected pattern of sun exposure (14, 22) using a priori definitions that allowed for sex differences in the exposure of the posterior neck, due to hair length. Areas defined as chronically exposed were the face, anterior neck, lateral forearms, dorsa of the hands, and, for boys only, the posterior neck. The trunk, legs, lateral upper arms, medial forearms, and female posterior neck were defined as intermittently exposed areas. The medial upper arms were considered rarely exposed.
Sex differences in nevus density by body site were evaluated via Mann-Whitney U test. Nevus densities in chronic and intermittently exposed areas were compared using a Wilcoxon signed rank test for each sex separately. Univariate comparisons of log-transformed nevus counts by host factor and sunburn levels were made using two-tailed t tests or one-way ANOVAs as appropriate. χ2 analysis was used to compare clothing coverage and time spent outdoors by sex.
Multiple linear regression provided adjusted estimates of the association between sunburns and log-transformed nevus counts. Using site-specific sunburns, separate models were created for the face and neck, back, arms, and legs. Another model was created for total body nevi using the total number of burns received. Whereas sunburns on the shoulders were reported in interviews, nevus count areas did not distinguish shoulders from the chest, back, or upper lateral arms; thus, it was not possible to specifically analyze this area. Sunburns on the chest were too rare to support analysis. To allow for nonlinear effects, sunburn quantity was coded as a set of dummy variables, collapsing those receiving the most sunburns into a single category that included at least 20 subjects. Covariates included in the models were sex, propensity to burn versus tan, base skin color by colorimeter (L-dimension), hair color, eye color, degree of tanning by colorimeter (b-dimension), and freckling on the face. Sex, propensity to burn, hair color, and eye color were entered as dummy variables. Variables were included as cofactors in all models if they were significant predictors (P < 0.05) in any model. The intervention status of subjects in the overarching randomized controlled trial was included in initial models to control for any difference in groups that could arise from receipt of the intervention, and then excluded on the basis that it did not significantly contribute to any model (P > 0.45). All analyses were accomplished using SPSS 13.0.
Results
Over the 2-year reporting period, more than two thirds of subjects received at least one sunburn (Table 1). The face and shoulders were the most frequently sunburned area of the body, followed by the back and arms.
Table 1.
Frequency distribution of phenotypic factors and 2-y sunburn history by sex in children ages 7 to 8 y, Colorado (n = 743)
| Characteristic | Girls, % (n) | Boys, % (n) |
|---|---|---|
| Sex | 51.8 (385) | 48.2 (358) |
| Base skin color (tertiles of L-dimension)* | ||
| First (darkest) | 40.5 (156) | 25.1 (90) |
| Second (intermediate) | 36.1 (139) | 29.9 (107) |
| Third (lightest) | 23.4 (90) | 45.0 (161) |
| Hair color | ||
| Red | 4.2 (16) | 4.5 (16) |
| Blond | 19.5 (75) | 23.2 (83) |
| Light brown | 29.9 (115) | 20.4 (73) |
| Medium brown to black | 46.5 (179) | 52.0 (186) |
| Eye color | ||
| Blue/green | 48.1 (185) | 46.9 (168) |
| Brown | 29.1 (112) | 32.4 (116) |
| Hazel | 22.9 (88) | 20.7 (74) |
| Freckling on the face† | ||
| None | 31.2 (120) | 34.6 (124) |
| Some | 43.4 (167) | 37.7 (135) |
| Moderate | 20.3 (78) | 20.1 (72) |
| Heavy | 5.2 (20) | 7.5 (27) |
| Propensity to burn vs tan | ||
| Painful burn/no tan | 10.1 (39) | 8.9 (32) |
| Painful burn/light tan | 21.6 (83) | 25.4 (91) |
| Slight burn/little tan | 45.2 (174) | 47.5 (170) |
| No burn/good tan | 23.1 (89) | 18.2 (65) |
| Degree of tan (tertiles of b-dimension difference)‡ | ||
| First (least tan) | 43.1 (166) | 22.6 (81) |
| Second (intermediate) | 36.4 (140) | 30.2 (108) |
| Third (most tan) | 20.5 (79) | 47.2 (169) |
| No. sunburns anywhere on the body | ||
| None | 31.9 (123) | 29.6 (106) |
| 1 | 25.5 (98) | 26.8 (96) |
| 2 | 16.9 (65) | 20.7 (74) |
| 3 | 13.2 (51) | 9.8 (35) |
| 4 | 6.8 (26) | 5.9 (21) |
| 5+ | 5.7 (22) | 7.3 (26) |
| No. sunburns on the face | ||
| None | 54.3 (209) | 55.6 (199) |
| 1 | 20.0 (77) | 22.9 (82) |
| 2 | 11.2 (43) | 10.1 (36) |
| 3 | 7.8 (30) | 5.3 (19) |
| 4+ | 6.8 (26) | 6.1 (22) |
| No. sunburns on the back | ||
| None | 71.9 (277) | 72.3 (259) |
| 1 | 19.5 (75) | 17.3 (62) |
| 2 | 5.5 (21) | 7.0 (25) |
| 3+ | 3.1 (12) | 3.4 (12) |
| No. sunburns on the shoulders | ||
| None | 55.3 (213) | 58.4 (209) |
| 1 | 26.5 (102) | 24.0 (86) |
| 2 | 9.9 (38) | 10.9 (39) |
| 3+ | 8.3 (32) | 6.7 (24) |
| No. sunburns on the chest | ||
| None | 96.4 (371) | 97.5 (349) |
| 1+ | 3.6 (14) | 2.5 (9) |
| No. sunburns on the arms | ||
| None | 84.2 (324) | 85.2 (305) |
| 1 | 8.3 (32) | 9.8 (35) |
| 2+ | 7.5 (29) | 5.0 (18) |
| No. sunburns on the legs | ||
| None | 93.8 (361) | 96.1 (344) |
| 1+ | 6.2 (24) | 3.9 (14) |
| Intervention status | ||
| Intervention | 60.8 (234) | 60.6 (217) |
NOTE: Two-year sunburn history was reported at ages 4 to 5 and 5 to 6 y for the previous 12 mo.
Measured by colorimeter, with L-dimension on the medial upper arm.
By standardized chart.
Measured by colorimeter, as the difference in b-dimension between medial upper arm and lateral forearm.
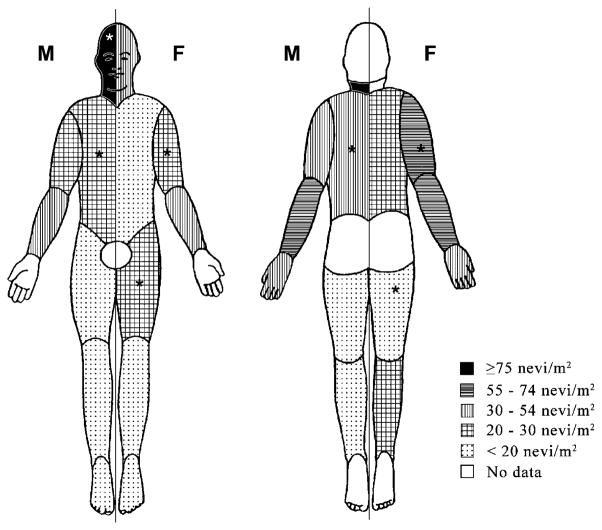
Median total body surface area was 0.99 m2 for males and 0.96 m2 for females. Nevus density was extremely variable over the body, and the distribution of nevi varied according to sex (Table 2; Fig. 1). Total nevus density was higher for boys than for girls. Among boys, nevus density was highest on the face and neck, followed by the lateral forearms. Among girls, nevus density was highest along the lateral arms, and to a lesser extent on the face and neck. Compared with girls, boys had a higher nevus density on the face, neck, and trunk, and lower nevus density on the upper arms and thighs. Nevi were significantly more dense in areas classified as chronically sun exposed compared with intermittently exposed areas (P < 0.0001, for both sexes; Table 2). This difference was more pronounced for boys (2.8-fold) than for girls (2.3-fold) because boys had a higher density of nevi than girls on chronically exposed areas but not on intermittently exposed areas.
Table 2.
Median nevus density (nevi/m2), by body site, exposure pattern, and sex in children ages 7 to 8 y, Colorado (n = 743)
| Body area | % Total body surface area | Median (interquartile range) |
Sex difference, P* | |
|---|---|---|---|---|
| Girls (n = 384) | Boys (n = 355) | |||
| Total | 82.50 | 31.0 (20.4–46.2) | 36.0 (21.8–50.0) | 0.04 |
| Body site | ||||
| Face, neck | 8.00 | 43.7 (25.0–77.5) | 78.4 (46.6–122.6) | <0.0001 |
| Chest | 13.00 | 16.3 (7.9–28.1) | 21.7 (8.8–38.4) | <0.0001 |
| Back | 13.00 | 24.1 (8.6–39.0) | 30.6 (17.2–51.2) | <0.0001 |
| Upper arms, lateral | 3.50 | 58.2 (26.9–118.8) | 31.2 (0.0–84.3) | <0.0001 |
| Upper arms, medial | 4.00 | 27.2 (0.0–71.2) | 25.7 (0.0–49.8) | 0.005 |
| Forearms, lateral (and elbows) | 3.50 | 57.8 (25.9–117.1) | 60.3 (28.0–121.8) | 0.14 |
| Forearms, medial | 3.00 | 37.4 (0.0–98.9) | 36.7 (0.0–83.6) | 0.68 |
| Hands, dorsal | 3.00 | 33.1 (0.0–69.7) | 31.6 (0.0–65.6) | 0.39 |
| Thighs, anterior | 7.75 | 25.2 (0.0–43.8) | 13.3 (0.0–28.2) | <0.0001 |
| Thighs, posterior | 8.25 | 19.0 (9.8–37.0) | 11.0 (0.0–22.7) | <0.0001 |
| Lower legs, anterior (and knees) | 6.25 | 17.6 (0.0–49.6) | 17.4 (0.0–45.4) | 0.49 |
| Lower legs, posterior | 5.75 | 20.0 (0.0–52.3) | 19.8 (0.0–52.0) | 0.92 |
| Feet, anterior | 3.50 | 0.0 (0.0–26.7) | 0.0 (0.0–27.0) | 0.80 |
| Exposure pattern | ||||
| Chronically sun-exposed areas of the body† | Girls: 13.50 Boys: 14.50 |
52.2 (27.0–80.3) | 67.5 (43.4–107.8) | <0.0001 |
| Intermittently sun-exposed areas of the body‡ | Girls: 65.00 Boys: 64.00 |
25.3 (16.7–39.0) | 26.5 (15.0–38.9) | 0.76 |
By Mann-Whitney U test.
Face, lateral forearms, dorsal hands, anterior neck, and male posterior neck.
Lateral upper arms, medial forearms, trunk, legs, dorsal feet, and female posterior neck.
Figure 1.
Median nevus density (nevi/m2) by body site and sex in children ages 7 to 8 y, Colorado (n = 743). Density for boys is presented on the left side of each body figure, and for girls, on the right. *, body sites and sex where nevus density is significantly higher than in the other sex (P < 0.05).
Univariate comparisons showed that higher prevalence of nevi over the body was associated with total number of sunburns as well as male sex, light skin color, light hair color, blue/green eye color, degree of freckling, intermediate propensity to burn, and tanning (Table 3).
Table 3.
Geometric mean and 95% confidence intervals for total nevus counts by phenotype and 2-y sunburn history and site-specific nevus counts by site-specific sunburn history in children ages 7 to 8 y, Colorado (n =743)
| Geometric mean total nevus counts (95% confidence interval) | P* | |
|---|---|---|
| Sex | ||
| Female | 24.1 (22.6–25.7) | 0.01 |
| Male | 27.1 (25.5–28.9) | |
| Base skin color (tertiles of L-dimension)† | ||
| First (darkest) | 22.0 (20.4–23.8) | <0.0001 |
| Second (intermediate) | 27.2 (25.3–29.2) | |
| Third (lightest) | 27.7 (25.5–30.1) | |
| Hair color | ||
| Red | 14.5 (11.0–18.9) | <0.0001 |
| Blond | 27.3 (24.8–30.0) | |
| Light brown | 27.5 (25.5–29.7) | |
| Medium brown to black | 25.1 (23.4–26.8) | |
| Eye color | ||
| Blue/green | 27.8 (26.0–29.6) | <0.0001 |
| Brown | 21.7 (20.0–23.5) | |
| Hazel | 26.6 (24.2–29.3) | |
| Freckling on the face‡ | ||
| None | 21.3 (19.7–22.9) | <0.0001 |
| Some | 26.6 (24.9–28.4) | |
| Moderate | 29.9 (26.8–33.3) | |
| Heavy | 30.3 (24.2–37.8) | |
| Propensity to burn vs tan | ||
| Painful burn/no tan | 25.4 (20.9–30.7) | <0.0001 |
| Painful burn/light tan | 26.3 (24.0–28.7) | |
| Slight burn/little tan | 27.4 (25.7–29.1) | |
| No burn/good tan | 21.1 (19.1–23.4) | |
| Degree of tan (tertiles of b-dimension)x | ||
| First (least tan) | 22.1 (20.4–23.9) | <0.0001 |
| Second (intermediate) | 26.3 (24.3–28.4) | |
| Third (most tan) | 28.6 (26.5–30.9) | |
| No. sunburns | ||
| None | 21.6 (19.8–23.5) | <0.0001 |
| 1 | 25.9 (23.8–28.2) | |
| 2 | 27.6 (25.1–30.2) | |
| 3 | 28.7 (25.2–32.7) | |
| 4 | 31.5 (25.8–38.4) | |
| 5+ | 27.8 (22.7–33.9) | |
| Intervention status | ||
| Intervention | 25.7 (24.3–27.1) | 0.73 |
| Control | 25.3 (23.4–27.3) | |
| Site-specific geometric mean nevus counts (95% confidence interval) | P* | |
| No. sunburns on the face | ||
| None | 4.4 (4.0–4.8) | 0.37 |
| 1 | 4.8 (4.3–5.5) | |
| 2 | 3.9 (3.2–4.7) | |
| 3 | 4.1 (3.3–5.0) | |
| 4+ | 4.7 (3.5–6.2) | |
| No. sunburns on the back | ||
| None | 2.9 (2.7–3.2) | 0.02 |
| 1 | 3.5 (2.9–4.1) | |
| 2 | 3.8 (2.7–5.2) | |
| 3+ | 4.5 (3.0–6.6) | |
| No. sunburns on the arms | ||
| None | 7.0 (6.5–7.5) | 0.49 |
| 1 | 7.7 (6.3–9.5) | |
| 2+ | 7.9 (6.2–10.0) | |
| No. sunburns on the legs | ||
| None | 5.6 (5.2–6.0) | 0.23 |
| 1+ | 6.9 (5.0–9.4) | |
NOTE: Two-year sunburn history reported at ages 4 to 5 and 5 to 6 y for the previous 12 mo.
Using natural log-transformed nevus counts by one-way ANOVA or t test, as appropriate.
Measured by colorimeter, with L-dimension on the medial upper arm.
By standardized chart.
Measured by colorimeter, as the difference in b-dimension between medial upper arm and lateral forearm.
Total sunburns and back sunburns had a significant positive association with nevi on the total body and the back, respectively. This association was independent of sex, propensity to burn versus tan, skin color, hair color, eye color, freckling, and tanning (Table 4). One or more sunburns anywhere on the body was positively associated with higher total nevus counts (P = 0.01), whereas at least three sunburns on the back was associated with higher nevus counts on the back (P = 0.03) and two sunburns on the back was marginally significant (P < 0.10). In contrast, no number of sunburns on the face, arms, and legs was associated with nevi on these areas. The regression models explained 14% to 24% of the variance in log-transformed nevus counts.
Table 4.
Association between site-specific sunburns and natural log-transformed nevus counts, controlling for sex, phenotype, and propensity to burn versus tan in children ages 7 to 8 y, Colorado (n = 743)
| Predictor (reference category) |
b value (P) |
||||
|---|---|---|---|---|---|
| Body | Face and neck | Back | Arms | Legs | |
| No. site-specific sunburns (0)* | |||||
| 1 | 0.136 (0.01) | 0.068 (0.25) | 0.101 (0.12) | 0.045 (0.64) | 0.034 (0.78) |
| 2 | 0.162 (0.008) | 0.008 (0.92) | 0.188 (0.08) | 0.093 (0.41) | |
| 3 | 0.185 (0.01) | 0.094 (0.33) | 0.312 (0.03) | ||
| 4 | 0.283 (0.002) | 0.137 (0.16) | |||
| 5 | 0.205 (0.02) | ||||
| Sex (female) | |||||
| Male | 0.068 (0.12) | 0.484 (<0.0001) | 0.264 (<0.0001) | −0.140 (0.02) | −0.244 (<0.0001) |
| Base skin color† | 0.011 (0.27) | −0.005 (0.69) | −0.006 (0.60) | 0.020 (0.14) | 0.035 (0.008) |
| Hair color (medium brown to black) | |||||
| Red | −0.849 (<0.0001) | −0.534 (<0.0001) | −0.813 (<0.0001) | −1.176 (<0.0001) | −0.586 (<0.0001) |
| Blond | −0.015 (0.80) | 0.020 (0.76) | 0.059 (0.40) | −0.124 (0.11) | 0.075 (0.31) |
| Light brown | 0.024 (0.64) | 0.111 (0.06) | 0.027 (0.67) | −0.016 (0.82) | 0.005 (0.94) |
| Eye color (brown) | |||||
| Blue/green | 0.144 (0.007) | 0.072 (0.23) | 0.162 (0.01) | 0.230 (0.001) | 0.142 (0.04) |
| Hazel | 0.104 (0.08) | 0.105 (0.12) | 0.140 (0.05) | 0.107 (0.17) | 0.089 (0.25) |
| Freckling on the face‡ | 0.054 (<0.0001) | −0.093 (<0.0001) | 0.047 (<0.0001) | 0.081 (<0.0001) | 0.115 (<0.0001) |
| Propensity to burn vs tan (none/good) | |||||
| Painful burn/no tan | 0.007 (0.94) | 0.036 (0.72) | 0.107 (0.32) | 0.033 (0.78) | 0.000 (0.99) |
| Painful burn/light tan | 0.028 (0.67) | −0.028 (0.71) | 0.082 (0.30) | 0.090 (0.29) | −0.007 (0.94) |
| Slight burn/little tan | 0.113 (0.04) | 0.016 (0.80) | 0.139 (0.04) | 0.147 (0.04) | 0.138 (0.05) |
| Degree of tan (b-dimension difference)§ | 0.036 (0.10) | 0.017 (0.49) | 0.048 (0.07) | 0.064 (0.03) | 0.017 (0.56) |
NOTE: Slopes (b values) and P values for each predictor of nevus counts on five body locations were obtained from multiple linear regression.
Reported at ages 4 to 5 and 5 to 6 y for the previous 12 mo for the specified body site. The highest burn category in each analysis includes subjects having the specified number of burns or greater on that site.
Measured by colorimeter, with L-dimension on the medial upper arm.
By standardized, 10-level chart.
Measured by colorimeter, as the difference in b-dimension between medial upper arm and lateral forearm.
Girls in this population were more likely than boys to wear clothing that provided less coverage of the limbs. Of 346 subjects who spent the day in question outdoors, almost all (94%) wore short-sleeved or sleeveless shirts. Girls were much more likely to report wearing a sleeveless shirt (19%) than were boys (1%; P < 0.0001). Similarly, girls almost always wore swimwear that exposed the thighs (99%), whereas boys were almost the exact opposite in this respect (5%; P < 0.0001). There was no significant difference between boys and girls in the amount of time spent outside during the period of recall (P = 0.62).
Discussion
This study found that the density of melanocytic nevi is greater in chronically sun-exposed areas of the body than those that are intermittently exposed, and that there is a significant association between total sunburns and total nevi on the body. We also found a site-specific relationship between sunburns on the back and nevus prevalence on the back. This study suggests there may be two pathways to nevus accumulation: chronic sun exposure and intermittent exposure related to sunburns.
In general, host factors that were positively associated with nevus prevalence included male sex, light skin, light brown hair, blue/green eyes, freckling, and the propensity to burn slightly and tan lightly compared with other reactions to sun exposure. In contrast, red hair had a statistically significant negative association. These associations have previously been reported (23–25). Degree of tanning, as measured by colorimeter, was positively associated with nevus prevalence in the current study, although this contradicts most findings (9). In contrast to self-reported level of tanning, which is used in most studies and most likely reflects the subjects’ perceptions of their overall darkness of tan, colorimeter-based measurement of tanning is a difference score between tanned and untanned areas of skin. Persons with lighter base skin color may have higher difference scores than those with darker base skin color, although their tanned skin may be lighter.
The distribution of nevi over the body implicates a strong role for chronic sun exposure in promoting nevus development. Nevi were concentrated on areas of the body classified as chronically exposed to the sun, specifically the face and neck and lateral arms, which supports the idea that chronic sun exposure plays a role in nevogenesis. Most previous research supports the finding that nevus density is higher in chronically sun-exposed areas (arms or face and neck) than in intermittently exposed areas (back, chest, or legs), although there is some variation in this finding, especially when analysis is restricted to nevi >2 mm (14–16, 24–27). Almost all of the nevi in our population were <2 mm (93%). Studies with sufficient detail to describe variation in nevus density along the lateral arms have reported nevus density to decrease from the upper arms to the hands (16, 27). Because this is the opposite pattern than would be expected to result from sun exposure, the existence of some sort of physiologic gradient related to the apparent resistance of the backs of the hands to form nevi in response to UV exposure was proposed. In contrast to these studies, our study found that, among boys, nevus density was higher on the middle (forearm) portion of the arms compared with the upper arms and hands (Table 2). This does not necessarily contradict the existence of a physiologic component, but supports a role for sun exposure because it is consistent with our finding that boys commonly wear shirts with short sleeves.
Sex differences in chronic exposure may explain differences in nevus distribution. For boys, nevi were concentrated on the face and neck, compared with the upper lateral arms and thighs in girls. This pattern is commonly reported, although the lateral upper arm difference is not always detected (16, 24, 25, 27). It has been proposed that sex differences may result from socialization of each gender in the amount of time spent in outdoor recreation and clothing habits (14). Higher nevus density on chronically exposed areas in boys has been attributed to the tendency of boys to spend more time outside, but in our study, boys and girls spent similar amounts of time outside. Because analysis was based on the last day spent mostly with a parent, this would not reflect any differences related to behaviors during other occasions. The fact that girls in this population are more likely to wear clothing that exposes the upper arms and thighs may explain why they have greater nevus density on these areas. Importantly, these results show that sex differences in nevus density are detectable as early as 7 to 8 years of age.
Intermittent acute exposure resulting in sunburns also plays a role in nevus development. Similar to reports in previous studies (19, 25, 28, 29), this study found a positive association of total sunburns with total nevus prevalence on the body. This effect was independent of the effect of major phenotypic factors, including the propensity to burn versus tan, and was significant for one or more sunburns. The magnitude of the association was highest for four sunburns, and leveled off for five or more sunburns, a pattern that has been reported for melanoma (3).
Before this study, extremely little has been published on the site-specific effect of sunburns on nevus development. We found the association between site-specific sunburns and nevi to vary according to body site. In contrast to other body areas, sunburns on the back were significantly associated with back nevi: the association was significant for three or more sunburns, and receiving two sunburns was of borderline significance (P < 0.10). The lack of association on other areas of the body is corroborated by the single other published study of site-specific sunburns and nevi, which reported a marginally significant effect of sunburns on the trunk but not the head and neck, shoulders, arms, or legs (30). Because chronic sun exposure provokes UV-protective adaptations in melanocyte cells, it may moderate the effect of sunburn-inducing UV doses (31). This observation can explain how the infrequently exposed (and consequently maladapted) trunk region is vulnerable to nevogenesis associated with sunburns, whereas the chronically sun-exposed face and neck are not. An alternative explanation is that there is a threshold of nevus density, so that chronically exposed areas, such as the face and neck, reach peak nevus density via chronic exposure, and additional acute exposures resulting in sunburn do not lead to a significant increase in nevi (26). Yet a third possible explanation is that the trunk has an inherent capacity for melanocyte instability, but no specific physiologic explanation for this has yet been proposed (32).
The association of total sunburns with nevi over the whole body apparently contradicts the fact that site-specific associations were detected only on the back. It is possible that our analysis was unable to detect associations on the limbs due to insufficient statistical power related to low frequency of sunburns on the legs and, to a lesser extent, the arms. This would not explain the lack of significant association for the face where sunburns were comparatively frequent. Alternately, a sunburn anywhere on the body may be a marker for acute exposure elsewhere, or the effect of sunburns may extend beyond the exposed area. Specifically, melanocyte populations have been shown to increase outside of the area of sun exposure, which supports the formation of some type of circulating factor in response to UV exposure (33).
The patterns observed in this study between sun exposure and childhood nevi generally support the relationships found between sun exposure and melanoma. Overall, the density of childhood nevi in our population was about half that reported in Australia for children of this age, and this corresponds to differences in adult melanoma rates (13, 24, 34). The higher concentration of childhood nevi on the face and neck in our population matches the distribution of melanoma reported in Australia and melanoma occurring after the age of 50 years in Canada (11, 35). These studies also reflect the low incidence of melanoma on the back of the hands and the tendency of girls to have a higher incidence of melanoma on the limbs than do boys, similar to our results for childhood nevi. However, melanomas in the Canadian population for younger ages were most frequent on the back, and the incidence of melanoma on the lateral arms is not notably high in either the Australian or Canadian study populations. The Canadian study also reported that melanoma incidence was higher on intermittently exposed areas of the body for younger ages, shifting to maximally exposed areas after the of age 50 years. Some of these discrepancies may reflect regional differences because the melanoma distributions correlate to nevus distribution within each population (14, 36).
The relationship between total sunburns and total childhood nevi in our study agrees with a large body of case-control studies that report sunburns in childhood as a risk factor for melanoma (4). One case-control study on the association of site-specific sunburn with melanoma showed that site-specific sunburns were a marginally significant risk factor for melanoma on that site, but the magnitude of the association was similar across body sites (37). It is possible that misclassification of sunburn sites due to the difficulty of recall could have diluted differences between body sites. Prospective data about total sunburns and melanoma on different areas of the body from the Nurses’ Health Study found that there was no association for the face and neck, whereas there were associations for other parts of the body including the trunk, arms, and legs (38). This result corresponds to our finding that site-specific sunburns were related to nevi on the back but not on the face and neck.
One limitation of our study is that chronic and intermittent exposures are interrelated in the sense that sunburn frequency likely correlates with chronic exposure. Site-specific analysis dissociates these effects to some extent by isolating areas that do not receive chronic sun exposure. For the face and neck, however, the sunburn association estimates presented here are not independent of chronic exposure. A second limitation is the lack of sunburn data from the first 4 to 5 years of life; these sunburns are likely to be related to nevus distribution. Thus, the analysis did not include the effect of sunburn during this early period. Some misclassification of body sites by exposure class is expected due to individual variation. For example, forearms may be chronically exposed in some individuals and intermittently exposed in others. As a result, our estimate of the difference between chronic and intermittently sun-exposed areas of the body is likely conservative.
Overall, this study offers evidence that nevi may be accumulated in response to both chronic and intermittent exposure, as inferred from body site and sunburn, respectively. Our study is the first longitudinal nevus study of nevus distribution in U.S. children and one of the first to provide data about site-specific sunburns. Our findings are consistent with the theory of two different pathways to melanoma, proposed to explain the anatomic distribution of melanoma subtypes and associated risk factors (5). Melanomas on the intermittently exposed trunk have been associated with earlier average age of incidence (which is taken to indicate high individual susceptibility; refs. 11, 39) and are more likely to be associated with a nevus or to occur in nevus-prone individuals (40, 41). In contrast, melanomas of the face and neck tend to occur later in life (>50 years) and to be associated with solar keratoses, which are indicators of chronic sun exposure (42). Specific mechanisms have not yet been proposed, but melanomas on different parts of the body also seem to have different molecular features (e.g., TP53 on the face and neck and BRAF on intermittently exposed areas), which supports the notion of different pathways to melanoma (5, 43). These observations support the idea that melanoma results either from individual susceptibility (propensity to develop nevi) activated by intermittent exposure or from long-term chronic exposure. Similarly, our results show that nevogenesis may occur in response to both chronic and intermittent exposure to sunlight.
Acknowledgments
We thank Dr. H. Alan Arbuckle, Dr. Joanna Burch, Brenda Mokrohisky, Laura Wilson, and Cathy Sommer for conducting skin exams; Jenny Aalborg for assistance with data collection and management; Chan Zeng for statistical advice; and all of the Colorado Kids Sun Care Program participants and families.
Grant support: National Cancer Institute grant RO1-CA74592.
References
- 1.Elwood JM, Gallagher RP. Sun exposure and the epidemiological aspects of melanoma. In: Gallagher RP, Elwood JM, editors. Epidemiological aspects of cutaneous malignant melanoma. Boston: Kluwer Academic Publishers; 1994. pp. 15–66. [Google Scholar]
- 2.Elwood JM, Gallagher RP, Hill GB, Pearson JCG. Cutaneous melanoma in relation to intermittent and constant sun exposure—the Western Canada Melanoma Study. Int J Cancer. 1985;35:427–33. doi: 10.1002/ijc.2910350403. [DOI] [PubMed] [Google Scholar]
- 3.Elwood JM. Melanoma and sun exposure: contrasts between intermittent and chronic exposure. World J Surg. 1992;16:157–65. doi: 10.1007/BF02071515. [DOI] [PubMed] [Google Scholar]
- 4.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward MK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806–12. doi: 10.1093/jnci/95.11.806. [DOI] [PubMed] [Google Scholar]
- 6.Holman CDJ, Armstrong BK. Pigmentary traits, ethnic origin, benign nevi, and family history as risk factors for cutaneous malignant melanoma. J Natl Cancer Inst. 1984;72:257–66. [PubMed] [Google Scholar]
- 7.Grob JJ, Gouvernet J, Aymar D, et al. Count of benign melanocytic nevi as a major indicator of risk for nonfamilial nodular and superficial spreading melanoma. Cancer. 1990;66:387–95. doi: 10.1002/1097-0142(19900715)66:2<387::aid-cncr2820660232>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Skender-Kalnenas TM, English DR, Heenan PJ. Benign melanocytic lesions: risk markers or precursors of cutaneous melanoma? J Am Acad Dermatol. 1995;33:1000–7. doi: 10.1016/0190-9622(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher RP, McLean DI, Yang P, et al. Suntan, sunburn, and pigmentation factors and the frequency of acquired melanocytic nevi in children. Arch Dermatol. 1990;126:770–6. [PubMed] [Google Scholar]
- 10.Weinstock MA, Colditz GA, Willett WC, et al. Nonfamilial cutaneous melanoma incidence in women associated with sun exposure before 20 years of age. Pediatrics. 1989;84:199–204. [PubMed] [Google Scholar]
- 11.Elwood JM, Gallagher RP. Body site distribution of cutaneous malignant melanoma in relationship to patterns of sun exposure. Int J Cancer. 1998;78:276–80. doi: 10.1002/(SICI)1097-0215(19981029)78:3<276::AID-IJC2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Dennis LK, White E, Lee JAH, McKnight B, Odland P. Constitutional factors and sun exposure in relation to nevi: a population-based cross-sectional study. Am J Epidemiol. 1996;143:248–56. doi: 10.1093/oxfordjournals.aje.a008735. [DOI] [PubMed] [Google Scholar]
- 13.Finch JL, Vu KO, Karp SK, editors. Cancer in Colorado, 1992–2002: incidence and mortality by county [monograph on the Internet] Colorado Department of Public Health and Environment; 2005. [cited 2007 May 15]. Available from: http://www.cdphe.state.co.us/pp/cccr/1992-2002/cccrreports92-02.html/ [Google Scholar]
- 14.Gallagher RP, McLean DI, Yang CP, et al. Anatomic distribution of acquired melanocytic nevi in white children. A comparison with melanoma: The Vancouver Mole Study. Arch Dermatol. 1990;126:466–71. [PubMed] [Google Scholar]
- 15.Harrison SL, Buettner PG, MacLennan R. Body-site distribution of melanocytic nevi in young Australian children. Arch Dermatol. 1999;135:47–52. doi: 10.1001/archderm.135.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Autier P, Boniol M, Severi G, et al. The body site distribution of melanocytic naevi in 6–7 year old European children. Melanoma Res. 2001;11:123–31. doi: 10.1097/00008390-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ries LAG, Harkins D, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2003 [monograph on the internet] Bethesda (MD): National Cancer Institute; 2006. [cited 2007 May 15]. Available from: http://seer.cancer.gov/csr/1975_2003/ [Google Scholar]
- 18.Mosteller RD. Simplified calculation of body surface area [letter] N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 19.Dwyer T, Blizzard L, Ashbolt R. Sunburn associated with increased number of nevi in darker as well as lighter skinned adolescents of Northern European descent. Cancer Epidemiol Biomarkers Prev. 1995;4:825–30. [PubMed] [Google Scholar]
- 20.Creech LL, Mayer JA. Ultraviolet radiation exposure in children: a review of measurement strategies. Ann Behav Med. 1997;19:399–407. doi: 10.1007/BF02895159. [DOI] [PubMed] [Google Scholar]
- 21.Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352–8. [Google Scholar]
- 22.Armstrong BK. How sun exposure causes skin cancer. In: Hill D, Elwood JM, English DR, editors. Prevention of skin cancer. Boston: Kluwar Academic Publishers; 2004. pp. 89–116. [Google Scholar]
- 23.Dellavalle RP, Johnson KR, Hester EJ, et al. Children with red hair have more freckles but fewer melanocytic nevi: results from a cohort study of 280 three-year-olds. Arch Dermatol. 2005;141:1042–3. doi: 10.1001/archderm.141.8.1042. [DOI] [PubMed] [Google Scholar]
- 24.English DR, Armstrong BK. Melanocytic nevi in children: I. Anatomic sites and demographic and host factors. Am J Epidemiol. 1994;139:390–401. doi: 10.1093/oxfordjournals.aje.a117011. [DOI] [PubMed] [Google Scholar]
- 25.Carli P, Naldi L, Lovati S, La Vecchia C. The density of melanocytic nevi correlates with constitutional variables and history of sunburns: a prevalence study among Italian schoolchildren. Int J Cancer. 2002;101:375–9. doi: 10.1002/ijc.10629. [DOI] [PubMed] [Google Scholar]
- 26.Darlington S, Siskind V, Green L, Green A. Longitudinal study of melanocytic nevi in adolescents. J Am Acad Dermatol. 2002;46:715–22. doi: 10.1067/mjd.2002.120931. [DOI] [PubMed] [Google Scholar]
- 27.MacLennan R, Kelly JW, Rivers JK, Harrison SL. The Eastern Australian childhood nevus study: site differences in density and size of melanocytic nevi in relation to latitude and phenotype. J Am Acad Dermatol. 2003;48:367–75. doi: 10.1016/s0190-9622(03)70143-1. [DOI] [PubMed] [Google Scholar]
- 28.Autier P, Severi G, Pedeux R, et al. Number and size of nevi are influenced by different sun exposure components: implications for the etiology of cutaneous melanoma. Cancer Causes Control. 2003;14:453–9. doi: 10.1023/a:1024961100651. [DOI] [PubMed] [Google Scholar]
- 29.Wiecker TS, Luther H, Buettner P, Bauer J, Garbe C. Moderate sun exposure and nevus counts in parents are associated with development of melanocytic nevi in childhood. Cancer. 2003;97:628–38. doi: 10.1002/cncr.11114. [DOI] [PubMed] [Google Scholar]
- 30.Autier P, Dore JF, Cattaruzza MS, et al. Sunscreen use, wearing clothes, and number of nevi in 6- to 7-year-old European children. European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Natl Cancer Inst. 1998;90:1873–80. doi: 10.1093/jnci/90.24.1873. [DOI] [PubMed] [Google Scholar]
- 31.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–8. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 32.Green A. A theory of site distribution of melanomas: Queensland, Australia. Cancer Causes Control. 1992;3:513–6. doi: 10.1007/BF00052747. [DOI] [PubMed] [Google Scholar]
- 33.Stierner U, Rosdahl I, Augustsson A, Kagedal B. UVB radiation induces melanocyte increase in both exposed and shielded human skin. J Invest Dermatol. 1989;92:561–4. doi: 10.1111/1523-1747.ep12709572. [DOI] [PubMed] [Google Scholar]
- 34.McDermid I, editor. Cancer in Australia 2001 [monograph on the internet] Canberra: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries; 2004. [cited 2007 May 15]. Available from: http://www.aihw.gov.au/publications/index.cfm/title/10083. [Google Scholar]
- 35.Green A, MacLennan R. Etiological Clues from the anatomical distribution of cutaneous melanoma. In: Gallagher RP, Elwood JM, editors. Epidemiological aspects of cutaneous malignant melanoma. Boston: Kluwer Academic Publishers; 1994. pp. 67–79. [Google Scholar]
- 36.Holly EA, Kelly JW, Ahn DK, Shpall SN, Rosen JI. Risk of cutaneous melanoma by number of melanocytic nevi and correlation of nevi by anatomic site. In: Gallagher RP, Elwood JM, editors. Epidemiological aspects of cutaneous malignant melanoma. Boston: Kluwer Academic Publishers; 1994. pp. 159–72. [Google Scholar]
- 37.Chen YT, Dubrow R, Holford TR, et al. Malignant melanoma risk factors by anatomic site: a case-control study and polychotomous logistic regression analysis. Int J Cancer. 1996;67:636–43. doi: 10.1002/(SICI)1097-0215(19960904)67:5<636::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 38.Cho E, Rosner BA, Colditz GA. Risk factors for melanoma by body site for whites. Cancer Epidemiol Biomarkers Prev. 2005;14:1241–4. doi: 10.1158/1055-9965.EPI-04-0632. [DOI] [PubMed] [Google Scholar]
- 39.Whiteman DC, Valery P, McWhirter W, Green AC. Incidence of cutaneous childhood melanoma in Queensland, Australia. Int J Cancer. 1995;63:765–8. doi: 10.1002/ijc.2910630602. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman DC, Parsons PG, Green AC. p53 expression and risk factors for cutaneous melanoma: a case-control study. Int J Cancer. 1998;77:843–8. doi: 10.1002/(sici)1097-0215(19980911)77:6<843::aid-ijc8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Winnepenninckx V, van den Oord JJ. p16INK4A expression in malignant melanomas with or without a contiguous naevus remnant: a clue to their divergent pathogenesis? Melanoma Res. 2004;14:321–2. doi: 10.1097/01.cmr.0000134855.12474.f3. [DOI] [PubMed] [Google Scholar]
- 42.Bataille V, Sasieni P, Grulich A, et al. Solar keratoses: a risk factor for melanoma but negative association with melanocytic nevi. Int J Cancer. 1998;78:8–12. doi: 10.1002/(sici)1097-0215(19980925)78:1<8::aid-ijc2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–30. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]