Abstract
Purpose
Aspirin and other non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) can inhibit aromatase activity and thus could selectively lower incidence of hormone receptor positive tumors. We assessed whether the association of aspirin and other NSAIDs with postmenopausal breast cancer risk differs by estrogen and progesterone receptor (ER, PR) status of the tumor.
Methods
A population-based cohort of 26,580 postmenopausal women was linked to a SEER Cancer Registry to identify incident breast cancers. Regular use of aspirin and other NSAIDs was reported on a self-administered questionnaire mailed in 1992. Cox proportional hazards models were used to estimate multivariate relative risks (RRs) and 95% confidence intervals (CIs) of breast cancer incidence overall and by ER and PR status, adjusting for multiple breast cancer risk factors.
Results
Through 2005, 1,581 incident breast cancer cases were observed. Compared to aspirin never users, women who regularly consumed aspirin had a lower risk of breast cancer (RR=0.80; 95% CI: 0.71–0.90), and there was evidence for lower risk with increasing frequency of use (RR=0.71 for aspirin use 6 or more times/week versus never use; p-trend=0.00001). Inverse associations for regular aspirin use were observed for ER+ (RR=0.77; 95% CI 0.67–0.89), ER− (RR=0.78; 95% CI 0.56–1.08), PR+ (RR=0.79; 95% CI 0.68–0.92), and PR− (RR=0.73; 95% CI 0.56–0.95) breast cancers. In contrast, use of other NSAIDs was not associated with breast cancer incidence overall (RR=0.95, 95% CI: 0.85–1.07), or by ER or PR status.
Conclusions
Aspirin, but not other NSAID use, was associated with about 20% lower risk of postmenopausal breast cancer and did not vary by ER or PR status of the tumor, suggesting that the hypothesized protective effects of aspirin may either be through cellular pathways independent of estrogen or progesterone signaling, or on tumor microenvironment.
Keywords: breast cancer, aspirin, NSAIDs, hormone receptors, prevention
INTRODUCTION
Breast cancer is the most common noncutaneous cancer, and the second leading cause of cancer-related death among women in the United States [1]. It is estimated that one in eight women will develop breast cancer over her lifetime [2]. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) have received considerable interest as potential cancer chemopreventive agents [3–5]. These agents block inflammation by inhibiting cyclooxygenase (COX) enzymes, leading to lower prostaglandin synthesis. Lowered levels of prostaglandins also inhibit aromatase activity, which in turn leads to lower serum estrogen levels [6, 7], and thus could selectively lower incidence of hormone receptor positive tumors.
Most [8–15] but not all [16–18] epidemiologic studies have found an inverse association between breast cancer and aspirin or other NSAIDs, including an earlier report from this cohort [19]. However, breast cancer is biologically heterogeneous and it is increasingly being recognized that breast cancer risk may vary by molecular characteristics of the tumor, particularly defined by estrogen receptor (ER) and progesterone receptor (PR) status. Epidemiologic studies addressing ER and PR subtype-specific associations between breast cancer and aspirin or other NSAID use are currently more limited, with some studies supporting this hypothesis [8, 11, 20], while others have not [14, 15, 18, 21–24]. However, the analyses in these studies were usually secondary and often based on small numbers of ER and PR defined subtypes.
Here, we update our previously published results for 6 years of follow up [19] to 13 years of follow up, and further present results defined by breast cancer subtype based on hormone receptor status as the primary objective. We hypothesized that aspirin use would continue to be associated with risk of postmenopausal breast cancer overall, and that these associations would be stronger for hormone receptor positive tumors.
Materials and Methods
Study population
The IWHS is a prospective cohort study of 41836 postmenopausal women aged 55–69 years at study entry; full details on study methods have been previously published [19, 25]. The IWHS was reviewed and approved by the institutional review boards of the University of Iowa and the University of Minnesota. A 16-page questionnaire was mailed in 1986 to 99,826 randomly selected women and returned by 41,836 women (42.7% response rate). Follow-up questionnaires were mailed in 1987, 1989, 1992, 1997, and 2004.
Risk factor assessment
Risk factor data collected in 1986 included age, level of education, height, weight, relative weight at age 12, age at menarche, age at menopause, number of live births, age at first live birth, family history of breast cancer, use of oral contraceptives, use of hormone therapy, and level of physical activity. The use of aspirin and non-aspirin NSAIDs in the cohort was ascertained on the 1992 questionnaire. Aspirin use was ascertained by asking the respondents "How often do you take aspirin? Examples of aspirin include Bufferin, Anacin, enteric-coated aspirin, Ecotrin, and Excedrin (Do not include acetaminophen, Tylenol, Ibuprofen, Advil): never, less than one per week, one per week, 2–5 per week, and 6+ per week." Use of non-aspirin NSAIDs was ascertained by asking the respondents, "How often do you take other nonsteroidal antiinflammatory drugs or arthritis medicines? Examples include Ibuprofen, Advil, Nuprin, Motrin, Naprosyn, Feldene, and Clinoril (Do not include aspirin, acetaminophen, Tylenol, prednisone, cortisone, Deltasone): never, less than one per week, one per week, 2–5 per week, and 6+ per week." Additional data collected on the 1992 questionnaire included smoking history, lifetime use of alcohol, as well as information on rheumatoid arthritis, and osteoarthritis.
Cohort follow-up
Incident breast cancers through 2005 were identified by linking to the Iowa Cancer Registry, a member of the National Cancer Institute's Surveillance, Epidemiology, and End Results Program [26]. ER and PR status of the tumor was derived from registry reports. Deaths were identified through annual linkage to Iowa state death certificates supplemented by linkage to the National Death Index.
Statistical analysis
Of the 41836 women who responded to the questionnaire in 1986, we excluded women who were premenopausal (n=569); had cancer other than nonmelanoma cancer, (n=6452); had a total or partial mastectomy (n=1884); did not return the 1992 questionnaire (n=8819); or did not answer the aspirin or other NSAID question (n=681). A total of 26,580 participants were included in the analytic cohort (exclusions were not mutually exclusive).
Follow-up for incident events was calculated as the age at completion of the 1992 questionnaire until the age at breast cancer diagnosis, age at move from Iowa, or age at death. If none of these events occurred, a woman was assumed to be alive, cancer-free, and living in Iowa through December 31, 2005. Cox proportional hazards regression analysis was used to estimate relative risks (RRs) and 95% confidence intervals (CIs) for the association of breast cancer risk with aspirin and NSAID use. Incidence was modeled as a function of age [27]. Exposures were characterized by ever use for each agent as well as frequency of use. For the latter analyses, those reporting use of less than one per week or one per week were combined into one group, resulting in categories of never, 1 or less per week, 2–5 per week, and 6 or more per week. For all such analyses, never users were modeled as the referent group. Tests for trend were carried out for each frequency of use variable by ordering the values from lowest to highest and including the resulting variable as a one degree-of-freedom linear term in a Cox proportional hazards model. We also examined combinations of the two agents, defined as follows: use of aspirin only, use of non-aspirin NSAIDs only, use of both, and use of neither. We first assessed aspirin and NSAID associations with overall breast cancer risk.
We also examined associations with risk of breast cancer as defined by subsets according to ER and PR receptor status. For these analyses, the outcome variable was incident receptor status-specific breast cancer, and all other types of breast cancer were considered censored observations at the date of diagnosis.
Statistical analyses modeling overall and hormone receptor-stratified breast cancer risk did adjust for multiple potential confounding factors, as listed in Table 2. All statistical tests were two-sided, and all analyses were carried out using the SAS (SAS Institute, Inc., Cary, NC) and S-Plus (Insightful, Inc., Seattle, WA) software systems.
Table 2.
Multivariable-adjusted relative risks for aspirin & other non-aspirin NSAIDs use with breast cancer incidence, overall and stratified by estrogen receptor (ER) and progesterone receptor (PR) status
| All Breast Cancer | ER + | ER− | PR+ | PR− | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Person Years |
No. of Events |
RR†(95% CI) | No. of Events |
RR†(95% CI) | No. of Events |
RR†(95% CI) | No. of Events |
RR†(95% CI) | No. of Events |
RR†(95% CI) | |
| Aspirin Use | |||||||||||
| Never | 84529 | 489 | 1.00 (reference) | 331 | 1.00 (reference) | 61 | 1.00 (reference) | 283 | 1.00 (reference) | 103 | 1.00 (reference) |
| Ever | 222650 | 1092 | 0.80 (0.71, 0.90) | 729 | 0.77 (0.67, 0.89) | 141 | 0.78 (0.56, 1.08) | 627 | 0.79 (0.68, 0.92) | 224 | 0.73 (0.56, 0.95) |
| ≤1/week | 104210 | 535 | 0.87 (0.76, 0.99) | 367 | 0.85 (0.72, 1.00) | 62 | 0.78 (0.53, 1.15) | 317 | 0.88 (0.74, 1.05) | 103 | 0.74 (0.55, 1.01) |
| 2–5/week | 55018 | 268 | 0.78 (0.66, 0.92) | 176 | 0.77 (0.63, 0.94) | 35 | 0.62 (0.37, 1.03) | 155 | 0.80 (0.64, 1.00) | 52 | 0.61 (0.41, 0.91) |
| 6+/week | 63422 | 289 | 0.71 (0.60, 0.83) | 186 | 0.65 (0.53, 0.79) | 44 | 0.91 (0.59, 1.41) | 155 | 0.64 (0.51, 0.80) | 69 | 0.81 (0.57, 1.14) |
| P-trend | 0.00001 | 0.00001 | 0.46 | 0.00005 | 0.11 | ||||||
| Non-Aspirin NSAID Use | |||||||||||
| Never | 185464 | 948 | 1.00 (reference) | 646 | 1.00 (reference) | 119 | 1.00 (reference) | 551 | 1.00 (reference) | 194 | 1.00 (reference) |
| Ever | 121715 | 633 | 0.95 (0.85, 1.07) | 414 | 0.91 (0.79, 1.05) | 83 | 1.16 (0.84, 1.61) | 359 | 0.90 (0.77, 1.05) | 133 | 1.15 (0.89, 1.48) |
| ≤1/week | 57750 | 275 | 0.90 (0.78, 1.05) | 181 | 0.85 (0.71, 1.03) | 37 | 1.09 (0.72, 1.64) | 162 | 0.89 (0.73, 1.08) | 53 | 0.96 (0.69, 1.35) |
| 2–5/week | 25057 | 136 | 1.02 (0.83, 1.25) | 87 | 0.95 (0.74, 1.23) | 22 | 1.50 (0.90, 2.50) | 80 | 1.01 (0.78, 1.32) | 29 | 1.18 (0.76, 1.84) |
| 6+/week | 38907 | 222 | 1.00 (0.84, 1.19) | 146 | 0.97 (0.78, 1.20) | 24 | 1.05 (0.62, 1.78) | 117 | 0.86 (0.68, 1.09) | 51 | 1.48 (1.02, 2.14) |
| P-trend | 0.91 | 0.55 | 0.44 | 0.24 | 0.051 | ||||||
| Combined Use | |||||||||||
| Never | 51950 | 296 | 1.00 (reference) | 200 | 1.00 (reference) | 42 | 1.00 (reference) | 175 | 1.00 (reference) | 61 | 1.00 (reference) |
| Aspirin only | 133514 | 652 | 0.82 (0.70, 0.95) | 446 | 0.83 (0.69, 0.99) | 77 | 0.64 (0.42, 0.97) | 376 | 0.82 (0.67, 1.00) | 133 | 0.75 (0.53, 1.05) |
| NSAID only | 32579 | 193 | 1.00 (0.82, 1.23) | 131 | 1.03 (0.81, 1.32) | 19 | 0.83 (0.46, 1.49) | 108 | 0.97 (0.74, 1.26) | 42 | 1.20 (0.78, 1.86) |
| Both | 89136 | 440 | 0.77 (0.65, 0.91) | 283 | 0.71 (0.58, 0.87) | 64 | 0.87 (0.56, 1.34) | 251 | 0.72 (0.58, 0.90) | 91 | 0.85 (0.59, 1.22) |
Adjusted for age, education, family history of breast cancer, age at menarche, age at menopause, parity/age at first live birth, use of oral contraceptives, use of hormone therapy, body mass index in 1992, body mass index at age 18 years, relative weight at age 12, history of osteoarthritis, history of rheumatoid arthritis, smoking, use of alcohol, smoking, and physical activity level.
Results
There were 26,580 postmenopausal women aged 59 to 77 years at the 1992 baseline in the analytic data set. During 307,178 person-years of follow-up (through 2005), 1581 incident cases of breast cancer were observed. The mean age at diagnosis of breast cancer was 74.8 years (range, 62.0 – 89.1). ER status was available for 1262 of the 1581 breast cancer cases (80%), with 1060 of these ER positive and 202 ER negative. PR status was available for 1237 cases (78%), with 910 of these PR positive and 327 PR negative. Examining combinations of receptor status, 885 cases were ER+PR+, 155 were ER+PR−, 24 were ER−PR+, and 172 were ER−PR−.
As shown in Table 1, women who regularly took NSAIDs only or both NSAIDs and aspirin had a higher BMI, history of osteoarthritis or rheumatoid arthritis, and a history of use of hormone replacement therapy; there were no striking differences for other analyzed breast cancer risk factors.
Table 1.
Selected demographic and breast cancer risk factors according to regular use of aspirin, NSAIDs or both, Iowa Women’s Health Study (1992)
| Regular Use of Aspirin or NSAIDs |
||||
|---|---|---|---|---|
| None (N=4,613) |
Aspirin only (N=11,472) |
NSAIDs only (N=2,862) |
Both (N=7,633) |
|
| Age at baseline (1986), mean ± SD, years | 68.8 ± 4.2 | 68.6 ± 4.2 | 68.3 ± 4.2 | 68.1 ± 4.2 |
| Education, % greater than high school graduate | 38% | 41% | 40% | 41% |
| Age at menarche, mean ± SD, years | 12.9 ± 1.5 | 12.9 ± 1.4 | 12.8 ± 1.5 | 12.8 ± 1.5 |
| Age at menopause, mean ± SD, years | 47.8 ± 6.3 | 48 ± 6.2 | 47.2 ± 6.7 | 47.6 ± 6.4 |
| Body mass index in 1992, mean ± SD, kg/m2 | 26.7 ± 5.0 | 26.6 ± 4.8 | 28.2 ± 5.6 | 27.8 ± 5.2 |
| Body mass index at age 18, mean ± SD, kg/m2 | 21.6 ± 3.0 | 21.5 ± 3.0 | 21.9 ± 3.3 | 21.7 ± 3.1 |
| First degree family history of breast cancer, % | 12% | 12% | 11% | 13% |
| Nulliparous, % | 11% | 9.3% | 8.1% | 7.2% |
| 3+ births and age at first live birth ≤20 years, % | 20% | 20% | 26% | 23% |
| Oral Contraceptive Use, % ever | 17% | 17% | 21% | 22% |
| Hormone therapy use, % ever | 38% | 41% | 51% | 49% |
| Relative weight at age 12, % above average for age and height | 12% | 12% | 15% | 15% |
| Osteoarthritis, % ever | 11% | 11% | 31% | 23% |
| Rheumatoid arthritis, % ever | 7.5% | 6.8% | 19% | 14% |
| Alcohol intake, % none | 84% | 82% | 81% | 80% |
| Ever smoker, % | 28% | 28% | 31% | 29% |
| Physical activity, % no regular | 48% | 45% | 49% | 46% |
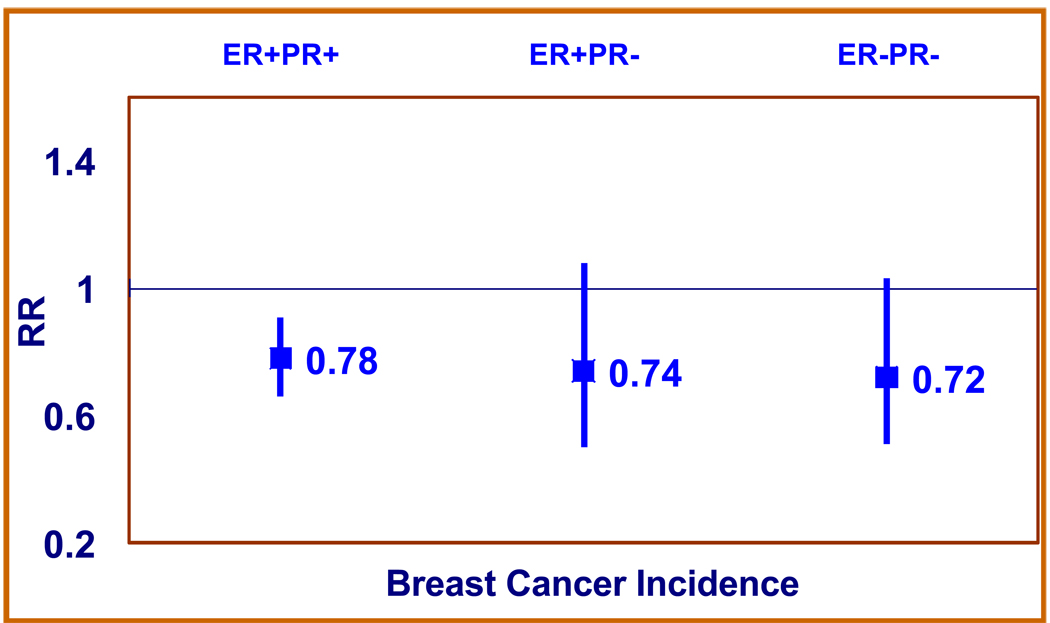
Compared with women who never used aspirin, women regularly consuming aspirin had about 20% lower risk of breast cancer, after adjusting for major breast cancer risk factors (Table 2). Higher frequency of use was associated with a lower risk of breast cancer (RR=0.71 for aspirin use 6+ times/week versus never use; 95% CI: 0.60–0.83) and this trend in frequency of use was highly statistically significant (p-trend=0.00001). Inverse associations for aspirin use (any use versus never use) were observed for ER+ (RR=0.77; 95% CI 0.67–0.89), ER− (RR=0.78; 95% CI 0.56–1.08), PR+ (RR=0.79; 95% CI 0.68–0.92), and PR− (RR=0.73; 95% CI 0.56–0.95) breast cancers, although the inverse association by frequency of use was strongest for ER+ and PR+ tumors. The latter finding was mainly due to the attenuation of the RR estimates in the highest category of intake (6+ per week) for both ER− and PR− tumors. Cross classification by receptor status again showed that point estimate of the association between aspirin and breast cancer did not vary by combination of ER and PR status (Figure 1).
Fig. 1.
Multivariable-adjusted relative risks (RR) for aspirin use with breast cancer incidence stratified by estrogen receptor (ER) and progesterone receptor (PR) status.
There was no association with use of non-aspirin NSAIDs with risk of breast cancer (RR=0.95, 95% CI: 0.85–1.07), overall or by ER or PR status, with the possible exception of a suggestive positive trend of non-aspirin NSAIDs use and risk of PR− tumors (p-trend=0.051).
In analysis of combined use of aspirin and other NSAIDs (Table 2), lower risk of breast cancer was observed for aspirin use only (RR=0.82; 95% CI 0.70–0.95) or use of both (RR=0.77; 95% CI 0.65–0.91), but not use of other non-aspirin NSAIDs only. This pattern was fairly consistent for each of the subtypes defined by ER and PR status.
Discussion
In extended follow-up of this prospective, population-based study of postmenopausal women, aspirin use continued to be inversely associated with breast cancer incidence, and a higher frequency of use was associated with a further lower risk. Contrary to our hypothesis, the inverse association did not vary by hormone receptor status (ER or PR) of the tumor. Non-aspirin NSAID use was not associated with breast cancer, overall or by ER or PR status. Adjustment for multiple other breast cancer risk factors did not alter these results.
The study finding of an inverse association between aspirin and breast cancer, both in our previous report [19] and in this update, is consistent with most other case-control [8–11, 13–15] and cohort studies [12, 20, 23], although there are a few exceptions [16–18, 22, 24]. In a meta-analysis of 11 case-control and cohort studies, aspirin use was found to be associated with a 23% lower risk of breast cancer (95% CI: 0.69–0.86). Higher frequency (> 4 times/day) of aspirin use has also been associated with lower risk of breast cancer [28], but the data on duration of aspirin use and breast cancer is not settled. While some studies have reported that aspirin use > 5 years is associated with lower risk of breast cancer [8, 11, 15], a recent meta-analysis of 26 case-control and cohort studies reported no association with duration of use [28]. While most of these studies have been able to adjust for a variety of potential confounders, concerns regarding bias and confounding remain from observational studies. The Women’s Health Study, a randomized, placebo-controlled clinical trial [21], did not find a protective effect of low dose aspirin (100mg) taken on alternate days with risk of breast cancer after an average 10.2 years of follow-up (RR=0.98, 95% CI: 0.89–1.08). However, the trial was not able to address whether higher frequency and dose (> 100 mg/day) of aspirin could lower risk of breast cancer, as suggested by observational studies [13, 29].
In our study, the inverse association between aspirin and breast cancer did not vary by hormone receptor status (ER or PR) of the tumor. Most studies have reported no difference in results based on hormone receptor status [14, 15, 18, 21–24], with the exception of three studies [8, 11, 20]. In the California Teachers Study cohort there was an increased risk of ER−/PR− (RR=1.40; 95% CI: 0.96–2.05) but not ER+/PR+ (RR=0.89; 95% CI: 0.75–1.05) tumors with daily aspirin use [8]. The increased risk of ER−/PR− tumors, which was based on 279 cases, was an unexpected finding. A large case-control study [11] found that ever aspirin use was associated with lower risk of hormone receptor positive (OR=0.74; 95% CI: 0.60–0.93 for ER+PR+, ER+PR− and ER−PR+ tumors combined), but not hormone receptor negative tumors (OR=0.97; 95% CI: 0.67–1.40 for ER−PR− tumors). However, closer examination of those regularly using aspirin (rather than just ever aspirin users) showed that the associations were less discordant for hormone receptor positive (OR=0.71; 95% CI: 0.55–0.93) and negative (OR=0.83; 95% CI: 0.52–1.32) tumors. Even more striking, the results for those using aspirin for > 5 years were nearly identical for hormone receptor positive (OR=0.80; 95% CI: 0.61–1.06) or negative (OR=0.77; 95% CI: 0.46–1.23) tumors. Finally, the AARP Diet and Health Study cohort [20] found that the beneficial effect of aspirin was stronger among ER+ tumors (RR=0.84; 95% CI: 0.71–0.98), as compared to ER− tumors (RR=1.14; 95% CI: 0.81–1.62), but the number of cases were very few in the ER− group (N=52), as compared to ER+ group (N=223), limiting the power of sub-group analysis.
Overall, our findings suggest that the inverse association between aspirin and breast cancer likely does not vary by hormone receptor status (ER or PR) of the tumor within the Iowa Women’s Health Study Cohort. Recently, Holmes et al reported the association between aspirin users and breast cancer survival [30]. The authors reported that women who reported using aspirin 6 to 7 days per week had a significantly lower risk of breast cancer death as compared to no users (Hazard Ratio = 0.36, 95% CI: 0.24 to 0.54), and the association did not differ by hormone receptor status. Thus, the overall evidence supports a protective effect of aspirin on breast cancer that is similar among hormone receptor positive and negative tumors, and thus suggests that the potential mechanism of prevention is likely not directly related to estrogen or progesterone signaling pathways, but perhaps through other pathways such as inflammation pathways. It is also possible that could be that effects of aspirin are on surrounding tissue (tumor microenvironment) and not the tumor itself.
In contrast to aspirin use, non-aspirin NSAID use was not associated with breast cancer incidence. While a few prospective studies have reported that aspirin has a stronger protective effect relative to other non-aspirin NSAIDs [8, 11, 20], others have reported similar benefits [14, 15]. This discrepancy may be due to assessment of type of NSAID used, as unlike aspirin, there are multiple different kinds of non-aspirin NSAIDs. The meta-analysis by González-Pérez et al. [31] found that protective effect of non-aspirin NSAIDs (RR=0.86; 95% CI: 0.73–1.00) on breast cancer was weaker than that of aspirin (RR=0.77; 95% CI: 0.69–0.86), and there was significant heterogeneity in the results based on study design, population studied, and exposure assessment for the non-aspirin NSAIDs. Besides these factors, heterogeneity in the doses, types, and durations of non-aspirin NSAIDs used could also account for variations in observed results, as studies have shown that not all non-aspirin NSAIDs exert similar protective effect against breast cancer [8]. Finally, the observed differences in associations could be also due to the additional biologic effects of aspirin, such as the irreversible inhibition of COX enzymes, as compared to reversible inhibition by non-aspirin NSAIDs.
The strengths of this study include its large, prospective cohort design, ascertainment of cancer incidence using high-quality Surveillance, Epidemiology, and End Results Cancer Registry data, virtually complete follow-up for mortality, and availability of information on multiple risk and protective factors to evaluate potential confounding. The study was population-based, which enhances its external validity.
There are limitations to our data. First, our questionnaire did not assess the type, dose, or duration of aspirin or non-aspirin NSAID use, although we were able to distinguish between use of these two classes of agents, and data on frequency of use was collected. Second, information about ER/PR status of breast cancer was obtained through multiple pathological laboratories, rather than a single reference laboratory. However, SEER reporting of ER and PR status is reasonably valid [32], and the ER/PR distribution in our study was similar to that reported by other studies. Finally, the observational study design has greater potential for uncontrolled confounding than an experimental (i.e., randomized controlled trial) design. However, we did adjust for known multiple confounding variables and the results changed little, adding further strength to our observation. Finally, out results can only be directly generalized to Caucasian women with post-menopausal breast cancer.
In summary, this study suggests that aspirin use may provide chemopreventive benefit with respect to incident breast cancer in postmenopausal women. Risk reduction did not vary by ER or PR status of the tumor, suggesting that the hypothesized protective effects of aspirin might be through pathways linked to inflammation or tumor microenvironment, rather than estrogen or progesterone signaling. However, these observations require confirmation in other studies.
Acknowledgments
We would like to thank Dr. Vered Stearns, Associate Professor, Breast Cancer Program, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, for her expert comments and feedback.
We thank Sondra Buehler for editorial assistance.
This work was supported in part by a grant (R01 CA39742), National Cancer Institute (NCI), USA
Footnotes
Conflict of interest: None.
Contributor Information
Aditya Bardia, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland.
Janet E. Olson, College of Medicine, Mayo Clinic, Rochester, Minnesota
Celine M. Vachon, College of Medicine, Mayo Clinic, Rochester, Minnesota
DeAnn Lazovich, School of Public Health, University of Minnesota, Minneapolis, Minnesota.
Robert A. Vierkant, College of Medicine, Mayo Clinic, Rochester, Minnesota
Alice H. Wang, College of Medicine, Mayo Clinic, Rochester, Minnesota
Paul J. Limburg, College of Medicine, Mayo Clinic, Rochester, Minnesota
Kristin E. Anderson, School of Public Health, University of Minnesota, Minneapolis, Minnesota
James R. Cerhan, College of Medicine, Mayo Clinic, Rochester, Minnesota
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Breast Cancer Facts & Figures 2009–2010. American Cancer Society, Inc.: Atlanta; [Google Scholar]
- 3.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 4.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 5.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 6.Hudson AG, Gierach GL, Modugno F, Simpson J, Wilson JW, Evans RW, Vogel VG, Weissfeld JL. Nonsteroidal anti-inflammatory drug use and serum total estradiol in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:680–687. doi: 10.1158/1055-9965.EPI-07-2739. [DOI] [PubMed] [Google Scholar]
- 7.Gates MA, Tworoger SS, Eliassen AH, Missmer SA, Hankinson SE. Analgesic use and sex steroid hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:1033–1041. doi: 10.1158/1055-9965.EPI-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall SF, Bernstein L, Anton-Culver H, Deapen D, Horn-Ross PL, Mohrenweiser H, Peel D, Pinder R, Purdie DM, Reynolds P, et al. Nonsteroidal anti-inflammatory drug use and breast cancer risk by stage and hormone receptor status. J Natl Cancer Inst. 2005;97:805–812. doi: 10.1093/jnci/dji140. [DOI] [PubMed] [Google Scholar]
- 9.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–205. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Coogan PF, Rao SR, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, Shapiro S. The relationship of nonsteroidal anti-inflammatory drug use to the risk of breast cancer. Prev Med. 1999;29:72–76. doi: 10.1006/pmed.1999.0518. [DOI] [PubMed] [Google Scholar]
- 11.Terry MB, Gammon MD, Zhang FF, Tawfik H, Teitelbaum SL, Britton JA, Subbaramaiah K, Dannenberg AJ, Neugut AI. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. JAMA. 2004;291:2433–2440. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 12.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Rahme E, Ghosn J, Dasgupta K, Rajan R, Hudson M. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer. 2005;5:159. doi: 10.1186/1471-2407-5-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Coogan PF, Palmer JR, Strom BL, Rosenberg L. Use of nonsteroidal antiinflammatory drugs and risk of breast cancer: the Case-Control Surveillance Study revisited. Am J Epidemiol. 2005;162:165–170. doi: 10.1093/aje/kwi182. [DOI] [PubMed] [Google Scholar]
- 15.Kirsh VA, Kreiger N, Cotterchio M, Sloan M, Theis B. Nonsteroidal antiinflammatory drug use and breast cancer risk: subgroup findings. Am J Epidemiol. 2007;166:709–716. doi: 10.1093/aje/kwm216. [DOI] [PubMed] [Google Scholar]
- 16.Egan KM, Stampfer MJ, Giovannucci E, Rosner BA, Colditz GA. Prospective study of regular aspirin use and the risk of breast cancer. J Natl Cancer Inst. 1996;88:988–993. doi: 10.1093/jnci/88.14.988. [DOI] [PubMed] [Google Scholar]
- 17.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320:1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs EJ, Thun MJ, Connell CJ, Rodriguez C, Henley SJ, Feigelson HS, Patel AV, Flanders WD, Calle EE. Aspirin and other nonsteroidal anti-inflammatory drugs and breast cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:261–264. [PubMed] [Google Scholar]
- 19.Johnson TW, Anderson KE, Lazovich D, Folsom AR. Association of aspirin and other non-steroidal anti-inflammatory drug use with incidence of post-menopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:1586–1591. [PubMed] [Google Scholar]
- 20.Gierach GL, Lacey JV, Jr, Schatzkin A, Leitzmann MF, Richesson D, Hollenbeck AR, Brinton LA. Nonsteroidal anti-inflammatory drugs and breast cancer risk in the National Institutes of Health-AARP Diet and Health Study. Breast Cancer Res. 2008;10:R38. doi: 10.1186/bcr2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 22.Gill JK, Maskarinec G, Wilkens LR, Pike MC, Henderson BE, Kolonel LN. Nonsteroidal antiinflammatory drugs and breast cancer risk: the multiethnic cohort. Am J Epidemiol. 2007;166:1150–1158. doi: 10.1093/aje/kwm195. [DOI] [PubMed] [Google Scholar]
- 23.Gallicchio L, Visvanathan K, Burke A, Hoffman SC, Helzlsouer KJ. Nonsteroidal anti-inflammatory drugs and the risk of developing breast cancer in a population-based prospective cohort study in Washington County, MD. Int J Cancer. 2007;121:211–215. doi: 10.1002/ijc.22656. [DOI] [PubMed] [Google Scholar]
- 24.Friis S, Thomassen L, Sorensen HT, Tjonneland A, Overvad K, Cronin-Fenton DP, Vogel U, McLaughlin JK, Blot WJ, Olsen JH. Nonsteroidal anti-inflammatory drug use and breast cancer risk: a Danish cohort study. Eur J Cancer Prev. 2008;17:88–96. doi: 10.1097/CEJ.0b013e3282b6fd55. [DOI] [PubMed] [Google Scholar]
- 25.Folsom AR, Kaye SA, Prineas RJ, Potter JD, Gapstur SM, Wallace RB. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131:794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 26.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/csr/1975_2005/ based on November 2007 SEER data submission, posted to the SEER web site, 2008.
- 27.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 28.Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, Zhang WC, Li X. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–150. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 29.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women's Health Initiative. Cancer Res. 2003;63:6096–6101. [PubMed] [Google Scholar]
- 30.Holmes MD, Chen WY, Li L, Hertzmark E, Spiegelman D, Hankinson SE. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma H, Wang Y, Sullivan-Halley J, Weiss L, Burkman RT, Simon MS, Malone KE, Strom BL, Ursin G, Marchbanks PA, et al. Breast cancer receptor status: do results from a centralized pathology laboratory agree with SEER registry reports? Cancer Epidemiol Biomarkers Prev. 2009;18:2214–2220. doi: 10.1158/1055-9965.EPI-09-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]