A feature of aged onset degenerative disease is ubiquitinated protein inclusions. Similar inclusions are found in different tissues ranging from the central nervous, cardiovascular, musculoskeletal and gastrointestinal systems; whether, the same pathomechanism is responsible for the similar pathology in these disparate tissues is not known. To address this question, we explored the pathogenesis of a multi-system degenerative disorder, IBMPFD or inclusion body myopathy (IBM), paget’s disease of the bone (PDB) and fronto-temporal dementia (FTD) of which ubiquitinated inclusions are a key pathological feature in muscle, brain and bone tissue. IBMPFD is caused by mutations in the ubiquitin proteasome system (UPS) chaperone p97/VCP. Previous reports suggest dysfunctional UPS in IBMPFD, however, we find that autophagic protein degradation and autophagosome maturation are diminished in IBMPFD mutant-expressing mice, patients and cell models. Moreover, a loss of p97/VCP function recapitulates the same effects, suggesting that p97/VCP is essential for autophagy. Thus, the degenerative phenotype in IBMPFD and its phenotypic components (IBM, PDB and FTD) may be disorders of impaired autophagy. p97/VCP is likely important in regulating both UPS- and autophagy-mediated protein degradation. This places p97/VCP in a key regulatory position at the intersection of these two proteolytic pathways.
The AAA+ (ATPase associated with a variety of cellular activities) protein p97/VCP is instrumental in coordinating multiple distinct cellular events. These include cell division, organelle biogenesis, nuclear envelope formation and protein degradation via the UPS. p97/VCP performs these functions by binding to specific cofactors that utilize p97/VCP as the “motor” for each task. Of these processes, mediating protein degradation is the most critical for non-dividing terminally differentiated cells such as neurons and skeletal muscle. Indeed, mutations in p97/VCP cause the muscle, brain and bone degenerative disorder, IBMPFD. One prominent feature in IBMPFD patient tissue and transgenic mice are nuclear and cytosolic ubiquitinated protein inclusions. IBMPFD has 100% penetrance, but manifests as three variably presenting phenotypes (i.e. weakness, PDB or dementia). The most prevalent phenotypic feature in IBMPFD is muscle weakness, which affects >90% of p97/VCP mutation carriers. This compares to 50% and 35% of patients phenotypically having PDB and FTD, respectively. Affected skeletal muscle has classic features consistent with a hereditary IBM. These include muscle degeneration, protein inclusions and rimmed vacuoles (RVs) (Figure 1A). Similarly, skeletal muscle-restricted expression of the most common IBMPFD mutation in transgenic mice recapitulates these features with animals acquiring age-associated weakness, accumulation of high molecular weight ubiquitinated proteins, congophilic amyloid inclusions and vacuolation.
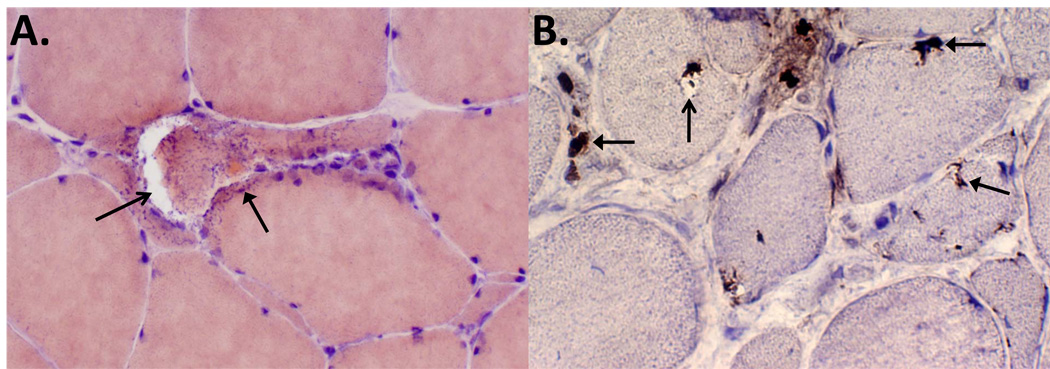
Figure 1.
IBMPFD patient skeletal muscle tissue process for (A) congo red histochemistry or (B) TDP-43 immunohistochemistry. (A) shows an angular fiber with a large rimmed vacuole (open arrow) and an adjacent congophilic (reddish) sarcoplasmic inclusion (closed arrow). (B) demonstrates multiple fibers with TDP-43 inclusions that are perinuclear, subsarcolemmal, sarcoplasmic in healthy appearing, vacuole-containing and degenerating myofibers. The open arrow denotes a vacuole, and closed arrows denote inclusions.
Previous studies suggest that IBMPFD mutations in p97/VCP cause a defect in protein degradation. Specifically IBMPFD mutant-expressing myoblasts accumulate ubiquitinated proteins and the model endoplasmic reticulum associated degradation (ERAD) substrate, ΔF508 CFTR, as cytosolic inclusions. This defect is more specific for protein aggregates which were cleared more slowly from IBMPFD mutant expressing cells and animals. Autophagic markers such as LC3 and p62 do not localize to the polyglutamine aggregates in IBMPFD mutant expressing cells suggesting that IBMPFD mutations in p97/VCP may affect degradation of aggregated proteins.
However, a defect in protein aggregate and ubiquitinated inclusion degradation fails to account for all of the pathological features seen in IBMPFD patient tissue; specifically rimmed vacuoles (RVs). RVs are accumulations of discontinuous membranous whorls and proteinaceous debris that are presumed to be autophagic in origin; autophagosomal-lysosomal proteins such LC3, p62 and LAMP-1/2 localize to RVs in sporadic and other hereditary IBMs. In addition, several other vacuolar myopathies are due to a loss in key autophagic proteins such as LAMP-2 in Danon disease and the lysosomal enzyme acid maltase in Pompe disease. Similarly, mutations in CHMP2B cause a pathologically similar FTD, fronto-temporal lobar degeneration with ubiquitinated inclusions (FTLD-U); CHMP2B mutations disrupt autophagosome maturation in cell culture and animal models. Finally, mutations in p62 are the most common cause of PDB implicating autophagy in PDB as well.
Therefore, we evaluated the autophagosomal/lysosomal system in IBMPFD mutant-expressing cells, transgenic animals and patient material. LC3 and p62 localize to regions of muscle tissue consistent with RVs in IBMPFD tissue. Additionally, the autophagosome-associated proteins LC3-II and p62 accumulate in transgenic mice harboring the most common IBMPFD mutation, R155H. This is recapitulated in cells stably expressing IBMPFD mutants and in cells treated with siRNA oligonucleotides directed against p97/VCP. These data suggest a decrease in autophagic flux resulting in an accumulation of undegraded LC3-II and p62. To confirm this, we starved control and p97/VCP-siRNA knockdown cells for 4 hours and measured the degradation of LC3-II via immunoblot. LC3-II was degraded in control cells but remained stable in p97/VCP knockdown cells identifying a defect in autophagic flux. Similar results are seen with IBMPFD-mutant expressing cell lines. These data indicate that autophagosomes are indeed being formed, but their degradation and perhaps maturation is defective. We confirmed this using a tandemly tagged mRFP-GFP-LC3 fusion protein. We reasoned that accumulation of autophagosomes because of defective maturation to autolysosomes may explain the genesis of a RV in IBMPFD. In agreement with this, ultrastructural and immunohistochemical analysis of RVs in IBMPFD myopathy mouse and patient skeletal muscle demonstrate that RVs are accumulations of vacuolated structures that contain non-colocalizing autophagosomal and lysosomal puncta.
Defective autophagosome maturation explains the accumulation of ubiquitinated proteins and RVs in IBMPFD; however, another striking feature of IBMPFD muscle and brain tissue that is not easily explained are TAR DNA binding protein-43 (TDP-43) inclusions (Figure 1B). Ubiquitinated inclusions in FTLD-U and amyotrophic lateral sclerosis contain TDP-43. In this those disorders, TDP-43, which is normally nuclear, is absent from the nucleus and instead accumulates as cytosolic inclusions. The same is true for IBMPFD patient muscle. Why this occurs is unknown. We reasoned that an impairment in autophagy may lead to the cytosolic accumulation of TDP-43. Treatment of cultured U20S cells transiently expressing an mCherry-TDP-43 fusion protein with the autophagy inhibitors chloroquine or bafilomycin A1 results in accumulation of cytosolic TDP-43 similar to what is seen when TDP-43 is co-expressed with IBMPFD mutant or dominant negative p97/VCP. Moreover, aged IBMPFD mutant mouse and patient muscle specifically accumulates TDP-43 as sarcoplasmic inclusions. In TDP-43 inclusion bearing myofibers, TDP-43 is absent from the nucleus. This same pathology is seen in mouse muscle when mice are chronically administered chloroquine for 4 weeks to pharmacologically block autophagosome-lysosome fusion. These data demonstrate that TDP-43 pathology in IBMPFD is explained by an impairment in autophagy conferred by IBMPFD mutant p97/VCP.
The UPS and autophagy have traditionally been thought to serve complementary yet distinct roles in protein homeostasis. Specifically, the UPS selectively degrades proteins; whereas, autophagy indiscriminately degrades long-lived proteins. Both of these roles are essential for protein quality control since the UPS prinicipally degrades misfolded proteins shortly after their synthesis and autophagy degrades oxidatively damaged long-lived protein inclusions. However, it is now clear that these two systems coordinate protein degradation. In the setting of normal proteasome activity but impaired autophagy, cells accumulate ubiquitinated protein inclusions, suggesting that autophagy is also needed for the degradation of ubiquitinated proteins. Other studies show that impaired proteasome activity results in a compensatory increase in autophagic activity that is protective to cells. How these two systems communicate is unclear.
The chaperone system needed to “triage” degradation-destined proteins to the UPS or autophagy is slowly being defined. This chaperone system would need to interact with ubiquitinated, misfolded and aggregated proteins, and additionally with components of the UPS and autophagy pathways; sensing the folding state of a protein, and assessing the integrity of the UPS and autophagy. Loss or mutation of this chaperone would presumably lead to impaired degradation of proteins via both the UPS and autophagy, accumulation of ubiquitinated proteins and cell degeneration. Our studies suggest that the chaperone protein, p97/VCP, fulfills many of these criteria. p97/VCP facilitates the degradation of misfolded proteins through ERAD and the UPS, and it is necessary for protein aggregate trafficking and aggresome formation. Our current study now implicates p97/VCP in the regulation of autophagy. Depending upon the state of p97/VCP (i.e., phosphorylated or acetylated) or the availability of p97/VCP cofactors in the cell, we suggest that p97/VCP is ideally suited to sense the proteostatic state of the cell and coordinate the degradation of proteins by the the UPS or autophagy.
Acknowledgements
We thank Robert Baloh, Alan Pestronk, Sara E. Miller and Rodrigo Fuentealba for helpful comments. Funding for this project was from National Institutes of Health (NIH) grants R01AG031867 (to C.C. Weihl), 5K08AG026271 (to C.C. Weihl), Hope Center for Neurological Disorders Pilot grant (to C.C. Weihl) and Washington University Alzheimer’s Disease Research Center grant P50AG05681.