Abstract
Background/Aims
T-cell responses to HCV antigens have been reported in high-risk HCV seronegative persons, suggesting that an effective cellular immune response might be able to clear infection without the development of antibodies. Such findings, however, could be explained by waning antibody or cross-reactivity to other antigens. To address these issues, we assessed T-cell responses in high-risk, seronegative, young IDUs to multiple peptide mixes spanning the entire HCV genome.
Methods
We evaluated HCV-specific T-cell responses in 26 young (age 18-33 years) aviremic, seronegative IDUs (median duration of injection, 6 years) by interferon-γ ELISpot assay using 429 overlapping HCV peptides pooled in 21 mixes. Seventeen aviremic, seropositive IDUs (spontaneous resolvers) and 15 healthy people were used as positive and negative controls, respectively.
Results
The percentage of patients with HCV-specific cellular immune responses was similar in seronegative and seropositive aviremic IDUs (46% versus 59%, p=0.4), while these responses were not detected in any of the negative controls. Among the seronegative IDUs, 6 (23%) had intermediate to very strong responses to 10-20 peptide mixes and another 6 (23%) had moderately strong responses to 2 to 6 mixes. The 12 seronegative IDUs with HCV-specific T-cell responses had higher demographic and behavioral risk profiles than the 14 IDUs without T-cell responses (estimated risk of HCV infection, 0.47 vs. 0.26, p <0.01).
Conclusions
HCV-specific T-cell responses are common among high-risk, seronegative IDUs. The responses are broad and are associated with risk factors for HCV exposure, suggesting that they reflect true exposure to HCV in seronegative persons.
Introduction
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease that affects >120 million people worldwide and nearly 5 million people in the United States (1-3). Injection drug use is currently the primary mode of HCV transmission in the developed world (4). HCV seroprevalence in long-term injection drug users (IDUs) ranges from 60% to > 90% (5-8). Since the introduction of needle exchange programs and other interventions designed to control bloodborne virus transmission among IDUs, however, HCV seroprevalence estimates in IDUs who have injected drugs for less than 5 years are generally less than 50% (9-12). Nevertheless, incidence rates among uninfected IDUs remain extremely high, ranging from 10% to 40% per year (13-16).
IDUs who have successfully cleared a previous HCV infection, however, have a reduced risk of subsequently developing persistent HCV viremia even if they continue injection drug use (17, 18), suggesting that some IDUs possess immunity that confers at least partial protection from subsequent infection. Viral clearance may even occur in individuals who do not seroconvert. Several groups, for example, have reported cellular immune responses to HCV antigens in antibody-negative persons who may have been exposed to the virus, including healthcare workers (19), spouses (20), and other family members of persons with HCV infection (21). In prison inmates, another high-risk group, viral clearance has been associated with cellular immunity in the absence of seroconversion (22). These findings suggest that cellular immune responses alone may be capable of clearing HCV infection without the development of antibodies.
Cellular immune responses to HCV antigens have been reported in high-risk seronegative IDUs (23, 24). Such findings, however, could be explained by seroreversion (25) or cross-reactivity to other antigens (26). Seroreversion may occur in a substantial proportion of individuals 10 years or more after they clear HCV infection spontaneously. In a study of women who spontaneously cleared HCV infection acquired through contaminated human Rh immunoglobulin, antibody responses were present in 10 of 10 women tested 10 years after exposure but absent in 18 (42%) of 43 women tested 18-20 years after exposure (25). In an IDU study, Mizukoshi et al. reported T-cell responses to multiple HCV antigens in seronegative IDUs in the San Francisco Bay area, but seroreversion could not be excluded because the median age of the IDUs was 44.5 years, and the median duration of injection, 18 years (24). HCV-specific T-cell responses may also be detected in healthy, uninfected persons because of cross-reactivity between an immunodominant epitope in the HCV NS3 protein and the influenza virus neuraminidase protein (26). Other antigens may also cross-react with HCV proteins, and cross-reactivity may be particularly common among IDUs, who are repeatedly exposed to many foreign antigens through nonsterile drug injection. In a second IDU study, Freeman et al. reported cellular immune responses to two HCV polyprotein constructs in seronegative IDUs (23). Approximately one third of the IDUs with positive responses, however, responded only to the construct that included the NS3 region.
In the present study, we evaluated HCV-specific cellular responses in a sample of young aviremic, seronegative IDUs with a relatively short duration of injection drug use recruited in community settings. We studied young IDUs with a short injection history to exclude the possibility of seroreversion in seronegative individuals with T-cell responses. We assessed the breadth of HCV-specific cellular immune responses by measuring the reactivity to 21 peptide mixes encompassing the entire HCV genome. To further assess whether these responses likely resulted from exposure to HCV, we examined the association between behaviors that might facilitate HCV exposure and the presence of HCV-specific cellular responses.
Materials and Methods
Subjects characteristics
Subjects were selected from the Swan Study, which recruited IDUs in Manhattan through a needle exchange program, street outreach, and word-of-mouth. Eligibility criteria were age 18 to 35 years and injection of illicit drugs at least once in the 30 days before enrollment. At enrollment, interviewers administered a standardized questionnaire and blood was drawn for HCV and HIV antibody and HCV RNA testing. HCV antibodies were assayed by second (HCV EIA 2.0, Abbott Laboratories, Abbott Park, IL) and third (HCV 3.0 ELISA and RIBA HCV 3.0, Ortho Clinical Diagnostics, Raritan, NJ) generation testing. The presence of HCV RNA was determined with the discriminatory HCV transcription-mediated amplification (dHCV TMA) assay (Procleix HIV-1/HCV, Chiron, Emeryville, CA (27)), with the lower limit of detection of 100 copies/mL 100% of the time, 10 copies/mL 75% of the time, and 3 copies/mL 40% of the time (each concentration tested with 60 replicates). Aviremic, antibody-negative subjects were offered enrollment in a longitudinal study in which HCV RNA testing was performed biweekly. The protocol was approved by the Weill Cornell Medical College Institutional Review Board and was consistent with the standards established by the Helsinki Declaration of 1975. Written informed consent was obtained from all subjects.
Of 270 IDUs enrolled and tested in the Swan Study from February 2005 through July 2007, PBMCs were available from 26 HCV antibody-negative and 17 HCV antibody-positive participants who repeatedly tested negative for HCV RNA. The 26 HCV antibody-negative IDUs had negative HCV RNA tests on a median of 11 (interquartile range [IQR], 6-16) samples drawn a median of 24 (IQR, 19-40) weeks after the PBMCs were collected. They did not significantly differ from the 126 HCV antibody-negative IDUs who were not included in the present study in age, sex, ethnicity, injection duration, or injection practices. The HCV antibody-positive, RNA-negative IDUs, presumed spontaneous resolvers, were included as positive controls. Fifteen healthy individuals without risk factors for HCV infection were included as negative controls.
Samples
Blood samples were kept at room temperature before separation. PBMCs were isolated using Ficoll-Hypaque density gradient centrifugation within 24 hours of specimen collection and cryopreserved at -150°C.
HCV antigens
A total of 429 18-mer peptides, each overlapping the next by 11 amino acids, which encompass the complete HCV genotype 1a (strain H77) polyprotein, were obtained from NIH AIDS Research & Reference Reagent Program (McKesson BioServices Corporation, Germantown, MD). Peptides were resuspended at 20 mg/ml in dimethyl sulfoxide and were further diluted to 1 mg/ml by adding phosphate-buffered saline (PBS). Peptides were pooled into 21 mixtures (A to U), 20 mixtures containing 21 peptides and one (mixture U) containing 9 peptides.
IFN-γ enzyme-linked immunospot (ELISpot) assay
Ninety-six-well plates (Millipore, Bedford, MA) were coated overnight at 4°C with the primary IFN-γ antibody (mAb 1-D1K, MABTECH, Cincinnati, OH) diluted in sterile PBS to a final concentration of 5 μg/ml. Subsequently, plates were washed 4 times with sterile PBS, blocked for one hour at room temperature with 1% bovine serum albumin (BSA) in PBS, washed 2-3 times with sterile PBS, and blocked for another 30-60 min with cell medium (RPMI medium, 5% human AB serum, and 2 mM L-glutamine). Cryopreserved PBMCs were thawed and plated at 300,000 cells/well in cell medium. Each peptide mix, with individual HCV peptides at a concentration of 2 μg/ml, was added to three replicate wells. Phorbol mystriate acetate in a final concentration of 5 ng/ml, tetanus toxoid (Accurate Chemicals & Scientific Corporation, Westbury, NY) at a final concentration of 5 μg/ml, and Candida albicans (Greer, Lenoir, NC) cellular antigen at a final concentration 10 μg/ml were used as positive controls; all controls were also plated in triplicate. Each plate had at least 6 no-antigen control wells.
After 40 hours of incubation, plates were washed 3 times with PBS, 4 times with PBS/0.01% Tween 20 and then incubated for 2 hours with 1 μg/ml secondary IFN-γ biotinylated antibody (mAb 7-B6-1-Biotin, MABTECH). After rinsing with PBS/0.01% Tween 20 and a one hour incubation with strepatavidin-alkaline phosphatase (Vectastain ABC kit, Vector Laboratories, Burlingame, CA), plates were washed again 3 times with PBS/0.01% Tween 20, 3 times with PBS, and developed using AEC Chromagen/Substrate kit (DBS, Pleasanton, CA). The reaction was stopped by rinsing plates with water. Spots were counted with a KS ELISpot reader (Zeiss, Thornwood, NY). All no-antigen (background) control wells had fewer than 10 spot-forming cells (SFC) per well. Responses were considered positive if, after subtraction of the background, the mean number of SFC was at least 15 and at least the mean + 3 standard deviations of the healthy controls for the respective peptide mix. Responses were classified according to the number of SFC per well over the higher threshold as follows: weak - 1 to 10 SFC, intermediate – 11 to 49 SFC, strong – 50 to 100 SFC, and very strong >100 SFC. Patients were considered positive for HCV-specific cellular responses if more than one peptide mix was positive according to the above criteria.
Statistical analysis
To determine whether positive T-cell responses were associated with exposure to HCV, we first identified indicators of exposure by examining the associations between demographic and behavioral factors and HCV antibody in the 270 eligible IDUs participating in the Swan Study. Eleven demographic and behavioral factors (listed in Table 3) were significantly associated with a higher likelihood of having HCV antibody in univariate analyses using chi-square tests for binary variables and Mantel-Haantzel chi-square tests for trend for ordinal variables. We then examined these 11 factors in the seronegative IDUs, comparing those who had T-cell responses with those who did not. Next, we fitted a multiple logistic regression model to determine independent risk factors for HCV antibody in the 270 eligible study participants. Six of the eleven risk factors were found to independently predict the risk of having HCV antibody (Table 4). The risk estimates for each of these six factors generated by this model were then applied to the demographic and behavioral data from each of the 26 seronegative IDUs in the present study to estimate the expected likelihood of HCV infection for each of them (Table 5). Even though the IDUs in this study were seronegative, the model predicted their risk of having HCV antibody based on the six factors found to predict HCV antibody in the entire cohort. We then compared the predicted risks of HCV infection derived from these analyses in the seronegative IDUs with and without HCV-specific T-cell responses, using Student’s t-test. All statistical analyses were conducted using Statistical Analysis Software (SAS Institute, Cary, N.C.).
Table 3.
Demographic and behavioral risk factors* among seronegative IDUs according to presence or absence of HCV-specific T-cell responses
| Characteristic | T-Cell (+) | T-Cell (-) | OR (95% CI)† | p† |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Total | 12 (46) | 14 (54) | ||
| Age (years)§ | ||||
| 18-25 | 4 (33) | 8 (57) | 1.0 | 0.22 |
| 26-33 | 8 (67) | 6 (43) | 2.67 (0.54, 13.2) | |
| Duration of injection drug use | ||||
| 0-6 years | 6 (50) | 7 (50) | 1.0 | 1.00 |
| 7-18 years | 6 (50) | 7 (50) | 1.0 (0.16, 6.17) | |
| Frequency of injection (past 30 days)§ | ||||
| ≤ 15 times (less often than every other day) | 3 (33) | 6 (43) | 1.0 | 0.43 |
| > 15 times | 9 (53) | 8 (57) | 2.25 (0.42, 12.1) | |
| Ever injected cocaine (or crack)§ | ||||
| No | 2 (17) | 6 (43) | 1.0 | 0.22 |
| Yes | 10 (83) | 8 (57) | 3.75 (0.59, 23.9) | |
| # times used a syringe previously used by another IDU (past 6 months) | ||||
| None | 6 (50) | 6 (43) | 1.0 | 0.88 |
| 1-9 times | 2 (17) | 5 (36) | 0.4 (0.06, 2.93) | |
| 10 or more times | 4 (33) | 3 (21) | 1.33 (0.20, 8.71) | |
| # times divided drugs (to share them) with a used syringe (past 6 months)‡ | ||||
| Never | 6 (50) | 12 (86) | 1.0 | 0.01 |
| 1-3 times | 1 (8) | 2 (14) | 1.0 (0.01, 23.1) | |
| 4 or more times | 5 (42) | 0 (0) | ∞ (1.27, ∞) | |
| # people who had used syringes used to split drugs (past 6 months)‡ | ||||
| None | 6 (50) | 12 (86) | 1.0 | 0.04 |
| 1-3 people | 5 (42) | 2 (14) | 5.0 (0.56, 53.9) | |
| 4-9 people | 1 (8) | 0 | ∞ (0.04, ∞) | |
| # times used cooker after another IDU stuck a used needle in it (past 6 months)§ | ||||
| Never | 6 (50) | 10 (77) | 1.0 | 0.07 |
| 1-3 times | 1 (8) | 2 (15) | 1.2 (0.09, 16.2) | |
| 4 or more times | 5 (42) | 1 (8) | 10.0 (0.4, 250) | |
| Any injection in shooting gallery (past 6 months) | ||||
| No | 10 (83) | 12 (86) | 1.0 | 1.00 |
| Yes | 2 (17) | 2 (14) | 1.2 (0.14, 10.1) | |
| Most common location of injection (past 6 months)§ | ||||
| Home¶ | 4 (33) | 8 (57) | 0.35 (0.08, 1.89) | 0.43 |
| Other places | 8 (67) | 6 (43) | 1.0 | |
| Who gave first injection‡ | ||||
| Self | 1 (8) | 5 (38) | 1.0 | 0.01 |
| Someone < 30 years old | 7 (58) | 8 (62) | 4.38 (0.33, 236) | |
| Someone 30 years or older** | 4 (33) | 0 (0) | ∞ (1.02, ∞) | |
Each of the 11 factors in this table was significantly associated with an increased likelihood of having HCV antibody among the 270 IDUs who participated in the Swan Study.
Odds ratios and P values reflect the association between the characteristic and the presence or absence of HCV-specific T-cell responses (comparing the 12 seronegative IDUs with T-cell responses to the 14 without them).
Factors significantly associated with HCV-specific, interferon-γ T-cell responses
Factors with associations with HCV-specific, interferon-γ T-cell responses that were not significant but are of expected direction and magnitude.
Injections in the home allow more opportunity for the use of safer injection techniques.
IDUs > 30 years old are more likely to have HCV infection than those younger.
Abbreviations: HCV, hepatitis C virus; IDUs, injection drug users; OR, odds ratio; CI, confidence interval.
Table 4.
Factors associated with HCV antibody in multiple logistic regression, IDUs participating in the Swan Study (N=270)
| Characteristic | Adjusted OR* (95% CI) | p |
|---|---|---|
| Age (in years) | 1.09 (1.00, 1.19) | 0.04 |
| Duration of injection drug use (in years) | 1.11 (1.02, 1.22) | 0.02 |
| Frequency of injection (past 30 days) | ||
| < 15 times | 1 | 0.03 |
| ≥ 15 times | 2.22 (1.08, 4.55) | |
| Ever injected cocaine/crack | 3.60 (1.63, 7.93) | <0.01 |
| Most common location of injection (past 6 months) | ||
| Home | 0.29 (0.14, 0.57) | <0.01 |
| Another place | 1 | |
| Who gave first injection | ||
| Self | 1 | |
| Someone < 30 years old | 1.16 (0.57, 2.33) | <0.01 |
| Someone ≥ 30 years old | 4.59 (1.70, 12.4) |
Adjusted odds ratios and P values were calculated to examine factors independently associated with HCV antibody positivity among all 270 participants in the Swan Study.
Abbreviations: HCV, hepatitis C virus; IDUs, injection drug users; OR, odds ratio; CI, confidence interval.
Table 5.
Predicted Mean Probability of HCV Infection of HCV Antibody-Negative IDUs by T-Cell Response (N=26)*
| T-cell response | N | Predicted probability of HCV infection |
p | |
|---|---|---|---|---|
| Mean | (95% CI) | |||
| Negative | 14 | 0.26 | (0.14, 0.38) | |
| Positive | 12 | 0.47 | (0.31, 0.62) | <0.01 |
| All patients | 26 | 0.36 | (0.26, 0.46) | |
Probabilities based on the IDUs’ reported demographic and behavioral characteristics and the risk estimates generated by the multivariate model presented in Table 4.
Abbreviations: HCV, hepatitis C virus; IDUs, injection drug users; CI, confidence interval.
Results
Study subjects
Among the 43 aviremic IDUs included in this study (26 HCV seronegative subjects and 17 seropositive controls), the median duration of injection drug use was 7 (range, 1-18) years. Most participants were Caucasian, and their median age was 26 (range, 18-33) years (Table 1). High-risk behaviors for acquisition of blood-borne infections were frequently reported by the members of this cohort. Nearly all (95%) had injected heroin and nearly half (47%) had injected cocaine in the 30 days preceding study enrollment. They had injected drugs frequently (median of 20 heroin injections during the same time period), about half (42%) had injected with syringes previously used by another IDU, and many (30%) had injected drug solution withdrawn from a cooker after another IDU had inserted a used syringe into it.
Table 1.
Characteristics of Seronegative and Seropositive IDUs and Healthy Controls
| HCV RNA Negative IDUs |
Healthy Controls (N=15) | |||
|---|---|---|---|---|
| HCV Ab (−) (N=26) | HCV Ab (+) (N=17) | Total IDUs (N=43) | ||
| Age at sample collection, Median (IQR) | 26 (22,30) | 27 (24,30) | 26 (22.5,30) | 32 (28,39) |
| Gender, N (%) | ||||
| Male | 15 (58) | 12 (71) | 27 (63) | 6 (40) |
| Female | 10 (38) | 4 (24) | 14 (33) | 9 (60) |
| Transgender (M-to-F) | 1 (4) | 1 (6) | 2 (5) | 0 (0) |
| Ethnicity, N (%) | ||||
| White | 16 (62) | 9 (53) | 25 (58) | 12 (80) |
| Black | 1 (4) | 0 (0) | 1 (2) | 2 (13) |
| Hispanic | 5 (19) | 5 (29) | 10 (23) | 0 (0) |
| Other | 4 (15) | 3 (18) | 7 (16) | 1 (7) |
| Duration of injecting (yrs), Median (IQR) | 6 (4,10) | 9 (7,10) | 7 (4,10) | 0 (0,0) |
| Frequency of injection, last 30 days (no.of times), Median (IQR) | 38 (8, 80) | 50 (17, 114) | 41 (15,91) | 0 (0,0) |
| Drugs injected, last 30 days, N(%) | ||||
| Heroin | 25 (96) | 16 (94) | 41 (95) | 0 (0,0) |
| Cocaine | 11 (42) | 9 (53) | 20 (47) | 0 (0,0) |
| Crystal methemphetamine or speed | 0 (0) | 0 (0) | 0 (0) | 0 (0,0) |
Abbreviations: IDUs, injection drug users; HCV, hepatitis C virus; IQR, interquartile range; Ab, antibody.
T-cell responses in IDUs
In order to determine the presence of HCV-specific cellular immune responses, we performed the IFN-γ ELISpot assay using 21 overlapping peptide mixes as antigens. Among the 26 HCV antibody and HCV RNA negative individuals, 12 (46%) had HCV-specific, IFN-γ cellular responses to more than one HCV peptide mix (Fig 1A). There was marked heterogeneity in the strength and breadth of these responses. Six of these subjects (23% of all seronegative IDUs) had intermediate to very strong responses to 10-20 of the antigen mixes (Table 2). Of the remaining 6 subjects, 2 mounted intermediate responses to 6 antigen mixes, 2 subjects had intermediate to strong responses to 4 peptide mixes and 2 subjects weak to intermediate responses to 2 peptide mixes. Of the 17 spontaneous resolvers included as positive controls, 10 (59%) had detectable IFN-γ responses to more than one HCV peptide mix (Fig 1B), which was similar to that observed among the study subjects (46% versus 59%, p = 0.4). These subjects likewise demonstrated marked heterogeneity in the strength and breadth of their responses. Eight of them (47% of all spontaneous resolvers) had intermediate to very strong responses to 8-20 of the antigen mixes. The remaining 2 patients had weak to intermediate responses to 2-3 antigen combinations. Overall, HCV-specific cellular immune responses did not differ significantly in frequency or strength between seronegative and seropositive aviremic IDUs (p = 0.19, Table 2). Of 15 healthy controls, 3 (20%) had weak reactivity to one antigen mix; none met the criteria for HCV-specific cellular responses (Fig 1C).
Figure 1.
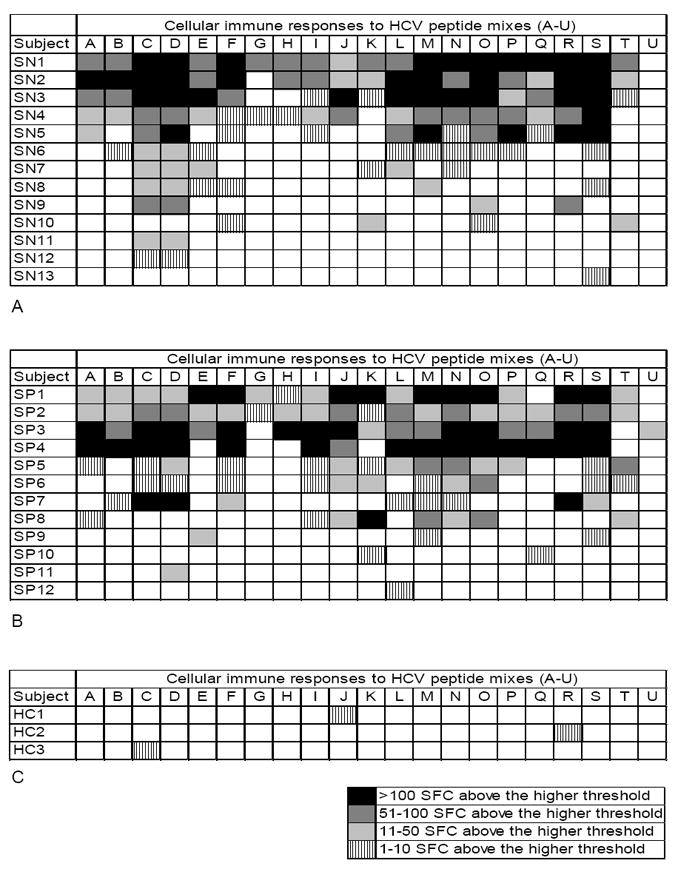
Strength and breadth of hepatitis C virus (HCV)-specific interferon (IFN)-γ responses among A) aviremic, seronegative injection drug users (IDUs), B) aviremic, seropositive IDUs, and C) healthy controls as determined by enzyme-linked immunospot assay. The response was assessed based upon the number of IFN-γ secreting cells per 3 × 105 peripheral blood mononuclear cells after exposure to 21 peptide mixes covering the entire viral polyprotein. After subtraction of the background, a reaction to each mix was considered positive if it had at least 15 spot forming cells (SFC) and at least the mean number of SFC in the healthy controls + 3 standard deviations. Each mix was graded as to the number of SFC observed over the higher threshold: vertical line pattern indicates 1 to 10 SFC, light gray indicates 11 to 50 SFC, dark gray indicates 51 to 100 SFC, and black indicates > 100 SFC. Of 26 analyzed aviremic, seronegative IDUs, 12 had responses to more than 1 mix and were considered positive for HCV-specific cellular responses. Of 17 aviremic, seropositive IDUs, 10 had responses to more than 1 mix and were considered positive for HCV-specific cellular responses. Of 15 normal controls, only 3 had weak responses to only one peptide mix each and none were considered positive for HCV-specific cellular responses. Subjects that had no responses to any peptide mixes are not shown.
Table 2.
Breadth of HCV-specific, interferon –γ responses among seronegative and seropositive IDUs and healthy controls*
| No. positive antigen mixes | HCV RNA Negative IDUs |
Healthy Controls | |
|---|---|---|---|
| HCV Ab (-) | HCV Ab (+) Controls | ||
| 8-20 | 6 (23%) | 8 (47%) | 0 |
| 2-6 | 6 (23%) | 2 (12%) | 0 |
| 0-1 | 14 (54%) | 7 (41%) | 15 (100%) |
Seronegative and seropositive aviremic IDUs did not differ significantly in the number of antigen mixes to which they demonstrated HCV-specific cellular immune responses (p=0.19, Mantel-Haenszel chi-square test for linear trend).
Abbreviations: HCV, hepatitis C virus; IDUs, injection drug users; Ab, antibody
In our study subjects, immune responses were observed to 20 of the 21-peptide pools, suggesting that T-cell responses were generated to a broad, diverse array of epitopes in nearly every region of the HCV polyprotein (Fig 2). The strongest and most frequent responses were observed toward peptide mixes C and D, which are part of the viral envelope glycoproteins 1 and 2 (Figs 1 and 2). Marked responses were also detected toward peptide mixes M, N, O, R and S, which are part of the nonstructural proteins NS4B, NS5A and NS5B. The least immunogenic response was directed toward the peptide mix U that corresponds to C-terminal part of NS5B (Fig 1 and 2). Two subjects (SN11, SN12) had responses exclusively to peptides corresponding to structural proteins.
Figure 2.
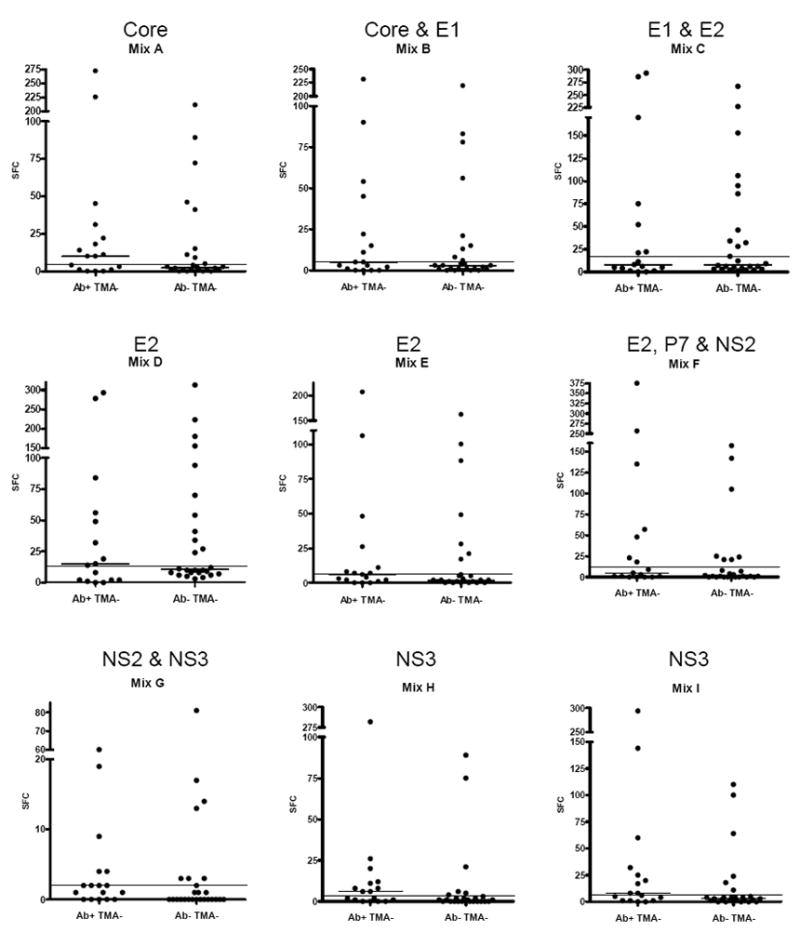
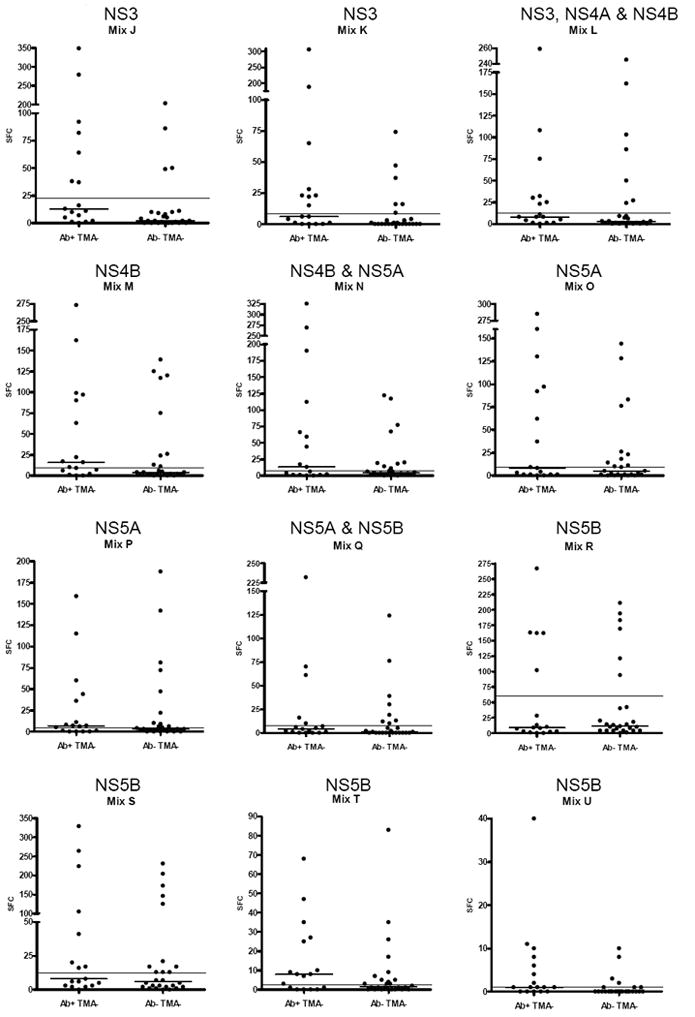
Number of hepatitis C virus (HCV)-specific interferon (IFN)-γ secreting cells in aviremic, seropositive and aviremic, seronegative individuals after incubation with 21 peptide mixes encompassing the entire viral polyprotein. The corresponding viral proteins are indicated above each mix. Each point indicates the mean number of spot-forming cells (SFC) per well for one subject after subtraction of the background. The short horizontal line indicates the mean for each group of subjects. The long horizontal line shows the mean number of SFC + 3 standard deviations in healthy controls. Note differing Y-axis values in each panel.
Associations between demographic and behavioral factors with T-cell responses
Among the 270 eligible IDUs participating in the Swan Study, the 11 demographic and behavioral factors listed in Table 3 were significantly associated with HCV antibody in univariate analysis (data not shown). In the 26 seronegative IDUs included in this study, 3 of these factors were significantly associated with HCV-specific T-cell responses, and another 5 of the factors had associations with T-cell responses that were in the expected direction and of similar magnitude as their association with HCV antibody in the larger study sample (Table 3). Thus, most of the demographic and behavioral factors associated with having HCV-specific antibodies among all 270 IDUs in the Swan Study also had associations of similar magnitude and direction with having HCV-specific T-cell responses among the 26 seronegative IDUs in the present study. In contrast, when we examined these relationships in the 17 seropositive spontaneous resolvers, T-cell responses were not associated with any of the risk factors.
Six of the risk factors were independently associated with HCV antibody in multivariate analysis among the 270 eligible Swan Study participants (Table 4). The effect estimates from this model were then applied to generate estimates of the predicted risk of HCV infection for each of the 26 HCV antibody-negative IDUs in the present study based on their data on these six variables. These predicted risk estimates, representing a summary measure of the demographic and behavioral indicators of exposure to HCV for each of the seronegative IDUs in the present study, were significantly higher in the 12 seronegative IDUs who demonstrated HCV-specific T-cell responses than in the 14 without such responses (Table 5).
Discussion
In this study of 26 young, aviremic, seronegative IDUs recruited in New York City, 46% had HCV-specific, IFN-γ cellular responses that suggested prior exposure to HCV. Nineteen (73%) had been injecting for 10 years or less, making seroreversion an unlikely explanation for these findings. The responses were more common in IDUs with demographic and behavioral risk factors for HCV infection, which supports the hypothesis that they were acquired through exposure to HCV. In addition, the detection of a median of 8 responses per subject suggests that they likely represent true immune reactivity to HCV rather than cross-reactivity to other antigens.
Other studies have also detected HCV-specific T-cell responses in seronegative persons at high-risk for viral contact (19-23). Some of these individuals might have had acute HCV infection that spontaneously cleared without seroconvesion (22, 28, 29). Alternative explanations for seronegativity in spontaneous resolvers are delayed development of antibodies (30, 31) or their loss over time (25). Finally, in some high-risk individuals, repeated low-level exposure to HCV may promote the development of cellular immune responses without the development of persistent viremia or serologic responses.
Two previous studies have evaluated cellular immune responses to HCV in IDUs. In one, using whole proteins as antigens, HCV specific T-cell responses were detected in 72% of seronegative IDUs (median age, 27 years; median duration of injection drug use, 7 years) recruited from an Australian primary health care facility (23). In the other, 62% of older seronegative IDUs (median age, 44.5 years; median duration of injection, 18 years) recruited from street settings in the San Francisco Bay area had T-cell responses detected by ELISpot and T-cell proliferation assays using HCV peptides and proteins as antigens (24). Although the seronegative IDUs in the present study were younger (median age, 26 years) and had a shorter duration of injection (median, 6 years), cellular immune responses were still detected in a similar (and statistically indistinguishable) proportion—suggesting that seroreversion was not the explanation. In the San Francisco and the present study, subjects were recruited in community settings to avoid the bias inherent in recruiting IDUs in health care facilities. Taken together, these data suggest that HCV-specific T-cell responses suggesting prior HCV exposure are common among HCV seronegative IDUs, regardless of age, injection duration, or geographic region.
Interestingly, of 17 seropositive individuals who were included in the study as positive controls, only 10 (59%) had positive T-cell responses. This observation suggests that ELISpot assays, like HCV antibodies, may not identify all patients who have mounted effective immune responses to HCV. In these patients, viral clearance may have been achieved through immune responses not detected by IFN-γ ELISpot assays. Alternatively, these responses may have waned over time. In either case, this finding suggests that additional seronegative IDUs may have developed HCV-specific immune responses that were not detected by ELISpot. Consequently, neither cellular nor serological responses may detect all persons with previous HCV exposure. Considering that the overall HCV seroprevalence in the Swan Study was 44% and that we detected HCV-specific T-cell responses in 46% of seronegative IDUs, at least 70% of study participants may have had prior exposure to HCV.
Because peptides were pooled for the ELISpot assay, we do not know the exact epitopes against which T-cell responses were directed. However, certain regions of the genome appear to be more immunogenic. For example, we detected strong responses against peptide mixes C and D in the majority of the patients. These antigen combinations correspond to the C-terminal of the HCV E1 glycoprotein and the N-terminal of the E2 glycoprotein. Marked responses were also detected toward the peptides that correspond to nonstructural proteins, NS4B, NS5A and NS5B. Exposure to intact viral particles alone in the absence of transcription and translation of viral proteins would not be expected to result in immune reactivity to nonstructural proteins. This suggests that the responses we detected likely resulted from productive HCV infection. Two of our patients, however, exhibited reactivity to structural antigens only, and we cannot exclude exposure to viral particles or replicating virus that does not result in productive infection as a cause of these responses.
In summary we found that a substantial proportion of our aviremic, seronegative IDUs had detectable HCV-specific IFN-γ T-cell responses indicating prior HCV exposure. Prospective studies are needed to determine whether such responses are protective against future infection. If they are, they will have important implications for vaccine development.
Acknowledgments
We are grateful to the staff of the Lower East Side Harm Reduction Coalition, Emily Winkelstein and Kelly Szott for assistance with data and sample collection, Lynn Mubita, Leeanne Stratton, Jessica Noack and Hanna Alemayehu for assistance with sample processing, Jihad Obeid, MD for assistance with data management, Dr. Barbara Rehermann for helpful discussions, and our study participants for making this work possible.
Statement of Interests
This study was supported by grants R01DA016159, R01HL076902, and WMC CTSC UL1RR024996 from the National Institutes of Health and with funds received through a commitment made at the Clinton Global Initiative, and the Greenberg Medical Research Institute.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24(Suppl 2):3–8. doi: 10.1055/s-2004-832922. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, Keeffe EB, Balart LA. Advances in Liver Disease: Highlights from the 56th Annual Meeting of the American Association for the Study of Liver Diseases. Rev Gastroenterol Disord. 2006;6:48–61. [PubMed] [Google Scholar]
- 4.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–8. doi: 10.1053/jhep.2002.36389. [DOI] [PubMed] [Google Scholar]
- 5.Tseng FC, O’Brien TR, Zhang M, et al. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users, 1998 to 2000. Hepatology. 2007;46:666–71. doi: 10.1002/hep.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagan H, McGough JP, Thiede H, et al. Syringe exchange and risk of infection with hepatitis B and C viruses. Am J Epidemiol. 1999;149:203–13. doi: 10.1093/oxfordjournals.aje.a009792. [DOI] [PubMed] [Google Scholar]
- 7.Lorvick J, Kral AH, Seal K, Gee L, Edlin BR. Prevalence and duration of hepatitis C among injection drug users in San Francisco, Calif. Am J Public Health. 2001;91:46–7. doi: 10.2105/ajph.91.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users. Medicine (Baltimore) 1995;74:212–20. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Diaz T, Des Jarlais DC, Vlahov D, et al. Factors associated with prevalent hepatitis C: differences among young adult injection drug users in lower and upper Manhattan, New York City. Am J Public Health. 2001;91:23–30. doi: 10.2105/ajph.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology. 2001;34:180–7. doi: 10.1053/jhep.2001.25759. [DOI] [PubMed] [Google Scholar]
- 11.Thorpe LE, Ouellet LJ, Levy JR, Williams IT, Monterroso ER. Hepatitis C virus infection: prevalence, risk factors, and prevention opportunities among young injection drug users in Chicago, 1997-1999. J Infect Dis. 2000;182:1588–94. doi: 10.1086/317607. [DOI] [PubMed] [Google Scholar]
- 12.Garfein RS, Doherty MC, Monterroso ER, et al. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S11–9. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–64. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 14.Thorpe LE, Ouellet LJ, Hershow R, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–53. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 15.Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157:467–71. doi: 10.1093/aje/kwf222. [DOI] [PubMed] [Google Scholar]
- 16.Judd A, Hickman M, Jones S, et al. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ. 2005;330:24–5. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 18.Grebely J, Conway B, Raffa JD, et al. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–45. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 19.Koziel MJ, Wong DK, Dudley D, Houghton M, Walker BD. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J Infect Dis. 1997;176:859–66. doi: 10.1086/516546. [DOI] [PubMed] [Google Scholar]
- 20.Bronowicki JP, Vetter D, Uhl G, et al. Lymphocyte reactivity to hepatitis C virus (HCV) antigens shows evidence for exposure to HCV in HCV-seronegative spouses of HCV- infected patients. J Infect Dis. 1997;176:518–22. doi: 10.1086/517279. [DOI] [PubMed] [Google Scholar]
- 21.Scognamiglio P, Accapezzato D, Casciaro MA, et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–9. [PubMed] [Google Scholar]
- 22.Post JJ, Pan Y, Freeman AJ, et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. J Infect Dis. 2004;189:1846–55. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- 23.Freeman AJ, Ffrench RA, Post JJ, et al. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–7. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 24.Mizukoshi E, Eisenbach C, Edlin BR, et al. Hepatitis C Virus-Specific Immune Responses of Frequently Exposed Long-Term Injection Drug Users. J Infect Dis. doi: 10.1086/589510. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 26.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-Reactivity between Hepatitis C Virus and Influenza A Virus Determinant-Specific Cytotoxic T Cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. Journal of clinical microbiology. 2008;46:499–506. doi: 10.1128/JCM.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morand P, Dutertre N, Minazzi H, et al. Lack of seroconversion in a health care worker after polymerase chain reaction-documented acute hepatitis C resulting from a needlestick injury. Clin Infect Dis. 2001;33:727–9. doi: 10.1086/322619. [DOI] [PubMed] [Google Scholar]
- 29.Bernardin F, Stramer SL, Rehermann B, et al. High levels of subgenomic HCV plasma RNA in immunosilent infections. Virology. 2007;365:446–56. doi: 10.1016/j.virol.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beld M, Penning M, van Putten M, et al. Low levels of hepatitis C virus RNA in serum, plasma, and peripheral blood mononuclear cells of injecting drug users during long antibody-undetectable periods before seroconversion. Blood. 1999;94:1183–91. [PubMed] [Google Scholar]
- 31.Maple PA, McKee T, Desselberger U, Wreghitt TG. Hepatitis C virus infections in transplant patients: serological and virological investigations. J Med Virol. 1994;44:43–8. doi: 10.1002/jmv.1890440109. [DOI] [PubMed] [Google Scholar]


