Abstract
Mycobacterium tuberculosis and filarial coinfection is highly prevalent, and the presence of a tissue-invasive helminth may modulate the predominant type 1 T helper (Th1; interferon [IFN]–γ–mediated) response needed to control M. tuberculosis infection. By analyzing the cellular responses to mycobacterial antigens in patients who had latent tuberculosis with or without filarial infection, we were able to demonstrate that filarial infection coincident with M. tuberculosis infection significantly diminishes M. tuberculosis–specific Th1 (interleukin [IL]–12 and IFN-γ) and type 17 T helper (Th17; IL-23 and IL-17) responses related to increased expression of cytotoxic T lymphocyte antigen (CTLA)–4 and programmed death (PD)–1. Blockade of CTLA-4 restored production of both IFN-γ and IL-17, whereas PD-1 blockade restored IFN-γ production only. Thus, coincident filarial infection exerted a profound inhibitory effect on protective mycobacteria-specific Th1 and Th17 responses in latent tuberculosis, suggesting a mechanism by which concomitant filarial (and other systemic helminth) infections predispose to the development of active tuberculosis in humans.
Tissue-invasive helminth parasites infect close to 500 million people worldwide and are associated with strong type 2 T helper (Th2) responses. Lymphatic-dwelling filarial parasites—those responsible for most of the morbidity among these helminth parasites—are long-lived and often induce asymptomatic (or subclinical) infections due, in large part, to the parasites’ ability to manipulate the host immune system and to restrict inflammatory pathology [1]. For example, filarial parasites not only evoke strong Th2 cytokine responses but also induce strong regulatory networks that down-regulate potentially protective type 1 T helper (Th1) responses [2]. Moreover, filarial parasites have been shown to inhibit human dendritic cell (DC) maturation and function, induce alternative activation of macrophages with nitric oxide synthase (Nos2) suppression, down-regulate the expression of Toll-like receptors on antigen-presenting cells and T cells, and induce both apoptosis of dendritic cells, T cells, and natural killer cells and anergy of cognate T cells [3–5].
The immune modulation associated with systemic helminth infections is primarily parasite-antigen specific, but some bystander effects on routine vaccinations, allergic processes, and autoimmune diseases have been noted. Helminth infections are known to impair immune responses to vaccinations with oral cholera [6, 7], tetanus toxoid [8, 9], and bacille Calmette-Guérin (BCG) [10]. Helminth infections are also known to protect against allergic responses, presumably because of their ability to induce regulatory T cells and the production of interleukin (IL)–10 and transforming growth factor (TGF)–β [11, 12]. Finally, helminth infections exert a protective effect against autoimmune diseases by protecting a host from developing autoimmune disease or by relieving symptoms of an established autoimmune process [13, 14]. The impact of helminth infection–induced immune hyporesponsiveness on concomitant infections that occur with high frequency in helminth-endemic areas is not well known. Helminth infections can exert beneficial effects by reducing inflammatory disease associated with infections by Helicobacter pylori [15] and Plasmodium falciparum [16, 17].
Mycobacterium tuberculosis infects ~2 billion people worldwide, with 90% of M. tuberculosis–infected individuals having latent infection. The control of tuberculosis (TB) requires clearly delineated Th1 responses (IL-12, interferon [IFN]–γ, and tumor necrosis factor [TNF]–α) and, to a lesser extent, type 17 T helper (Th17) responses (IL-17 and IL-23), with both Th1 and Th17 cells playing important roles in the induction and maintenance of protective immune responses in mouse models of infection [18, 19] or for prevention of active disease (as seen in latent TB) [20, 21]. During latency, M. tuberculosis is contained in localized granulomas [22]. Mycobacteria-specific T cells mediate delayed-type hypersensitivity reactions to purified protein derivative (PPD), and this reaction (in the absence of demonstrable active infection) is generally considered to indicate latent TB [23].
Because filarial infections and TB are coendemic in many parts of the world, we hypothesized that immune responses in latent TB could be modulated by the regulatory networks induced by chronic, coexisting filarial infections. To this end, we examined the induction of Th1, Th2, and Th17 responses in PPD-positive individuals with or without active filarial infection. We observed that the presence of patent filarial infection profoundly altered the M. tuberculosis–specific responses in individuals with coexisting latent M. tuberculosis infection, an immune modulation mediated by both cytotoxic T lymphocyte antigen (CTLA)–4 and programmed death (PD)–1.
METHODS
Study population
We studied a group of 30 patients who were tuberculin skin test positive but did not have active filarial infection (hereafter referred to as the PPD+Fil− group) and 30 who were tuberculin skin test positive and had active filarial infection (hereafter referred to as the PPD+Fil+ group) in Tamil Nadu, South India. Age distributions were 19–70 years in the PPD+Fil− group (median age, 39 years) and 14–65 years in the PPD+Fil+ group (median age, 35 years). The PPD+Fil− group had 18 men and 12 women, whereas the PPD+Fil+ group had 19 men and 11 women. Filarial infection was diagnosed by the presence of circulating filarial antigen, first by using the ICT filarial antigen test (Binax) and then by confirming positivity by the Trop Bio Og4C3 enzyme-linked immunosorbent assay (ELISA). All subjects had positive skin test reactivity to intra-dermal tuberculin (2 tuberculin units). A positive tuberculin skin test result was defined as an induration at the site of inoculation of at least 12 mm in diameter, to account for the high prevalence of environmental mycobacteria. This cutoff was based on a rigorous multivariate analysis of 280,000 subjects followed in South India for 15 years in which those with an 8–11-mm tuberculin skin test reaction to PPD-Siebert had a risk for the development of culture-positive TB that was both low and no different from those with a 0–7-mm reaction but in which those with 12-mm or greater reaction had, in comparison, a 3-fold greater relative risk for the development of active TB and a 6.1% lifetime risk [24]. All subjects had normal chest radiograph findings. None of the subjects had pulmonary symptoms (cough, fever, chest pain, or hemoptysis) or sputum that was positive for M. tuberculosis by smear microscopy and culture. Ten (33%) of the 30 subjects in each group had received BCG vaccination, on the basis of the presence of a scar. All individuals were examined as part of a clinical protocol (NCT 01-I-N261) approved by institutional review boards of both the National Institutes of Allergy and Infectious Diseases and the Tuberculosis Research Center, and informed consent was obtained from all participants.
Isolation of peripheral blood mononuclear cells (PBMCs)
Heparinized blood was collected, and PBMCs were isolated by Ficoll diatrizoate gradient centrifugation (LSM; ICN Biomedicals). Erythrocytes were lysed using ACK lysis buffer (Bio-source). Cells were then washed and cultured in Roswell Park Memorial Institute 1640 medium (BioWhittaker) supplemented with 20 mmol/L glutamine (BioWhittaker), 10% heat-inactivated fetal calf serum (Harlan Bioproducts for Science), and 50 μg/mL gentamicin (Mediatech).
Antigens
Mycobacterial PPD (Statens Serum Institute) and M. tuberculosis culture-filtrate antigen (CFA; gift of P. Selvaraj, Tuberculosis Research Center, Chennai, India) were used as the antigenic stimuli, and anti-CD3 antibody was used as the positive control. Final concentrations were 10 μg/mL for PPD and M. tuberculosis CFA and 5 μg/mL for anti-CD3.
In vitro culture
PBMCs were cultured with PPD, M. tuberculosis CFA, or anti-CD3 in 24-well tissue culture plates (Corning) at concentrations of 5 × 106/well. After 24 h, culture supernatants were collected and analyzed for cytokines. For costimulation blocking experiments, we cultured cells in the presence of CTLA-4 immunoglobulin (10 μg/mL) or control immunoglobulin (10 μg/mL) (Ancell) and in the presence of antibody to PDL-1 (the ligand for PD-1; 5 μg/mL) or control mouse IgG2b (5 μg/mL) (eBioscience) for 24, 72, and 120 h.
ELISA
The levels of cytokines in the culture supernatants were measured using Bioplex multiplex cytokine assay system (Bio-Rad). The cytokines analyzed were IL-2, IFN-γ, TNF-α, IL-12p70, IL-4, IL-5, IL-10, IL-13, IL-17, IL-23, IL-6, and IL-1β. IL-23 ELISA was performed using the kit from R&D Systems.
RNA preparation
PBMCs were lysed using the reagents of a commercial kit (QIAshredder; Qiagen). Total RNA was extracted in accordance with the manufacturer’s protocol (RNeasy Mini Kit; Qiagen), and RNA was dissolved in 50 μL of RNase-free water.
cDNA synthesis
RNA (1 μg) was used to generate cDNA by means of TaqMan reverse-transcription (RT) reagents, in accordance with the manufacturer’s protocol (Applied Biosystems). Briefly, random hexamers were used to prime RNA samples for RT by means of MultiScribe reverse transcriptase.
Real-time RT polymerase chain reaction (RT-PCR)
Real-time quantitative RT-PCR was performed with an ABI 7500 sequence detection system (Applied Biosystems) using TaqMan Assays-on-Demand reagents for CTLA-4, PD-1, TGF-β, Foxp3, and an endogenous 18s ribosomal RNA control. Relative transcripts were determined by the formula
where CT is the threshold cycle during the exponential phase of amplification.
Statistical analysis
Comparisons were made using either the Mann-Whitney U test or the Wilcoxon signed-rank test. All statistics were performed using GraphPad Prism for Windows (version 5).
RESULTS
Association between filarial infection and down-regulation of IFN-γ and IL-12 production in latent TB
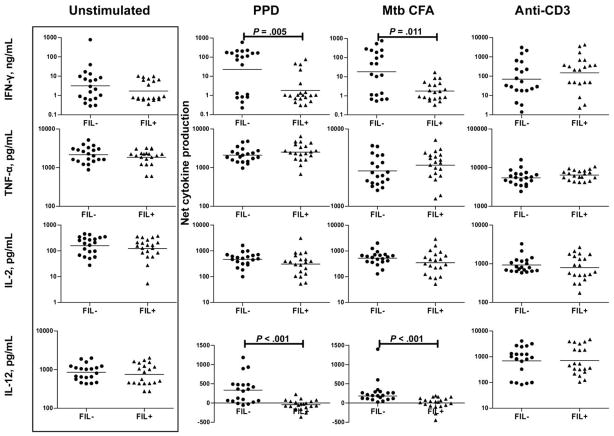
To determine the effect of coexisting filarial infections on the antigen-specific Th1 responses of individuals with positive tuberculin skin test results, we stimulated PBMCs from patients in the PPD+Fil− and PPD+Fil+ groups with PPD or CFA from M. tuberculosis H37 Rv (M. tuberculosis CFA) or anti-CD3 for 24 h and measured the levels of prototypical Th1 cytokines by ELISA (figure 1). First, there were no intrinsic differences in the levels of IFN-γ, TNF-α, IL-2, or IL-12 produced spontaneously or in response to anti-CD3 between the 2 groups. In contrast, the IFN-γ and IL-12 response to PPD or M. tuberculosis CFA was markedly different between the 2 groups, with the PBMCs from the patients in the PPD+Fil+ group showing significantly inhibited production compared with those from patients in the PPD+Fil− group (for IFN-γ, a geometric mean of 1.836 ng/mL in the PPD+Fil+ group vs. 22.68 ng/mL in the PPD+Fil− group for PPD [P = .005] and of 1.770 vs. 18.14 ng/mL for M. tuberculosis CFA [P = .011]; for IL-12, a geometric mean of −112.31 vs. 262.10 pg/mL for PPD [P < .001] and of − 145.35 vs. 227.87 pg/mL for M. tuberculosis CFA [P < .001]). Filarial infection had no significant effect on the production of mycobacterial antigen–driven TNF-α or IL-2.
Figure 1.
Association between filarial infection and down-regulation of interferon (IFN)–γ and interleukin (IL)–12 in latent tuberculosis. Peripheral blood mononuclear cells from patients in the purified protein derivative (PPD)+Fil− group (n = 20; tuberculin skin test positive without active filarial infection) and the PPD+Fil+ group (n = 20; tuberculin skin test positive with active filarial infection) were stimulated with PPD, Mycobacterium tuberculosis culture-filtrate antigen (CFA), or anti-CD3 for 24 h, and type 1 T helper cytokine (IL-2, IFN-γ, and tumor necrosis factor [TNF]–α) levels as well as IL-12 levels were measured by enzyme-linked immunosorbent assay. Results are shown as net cytokine production over that in control medium. Bars indicate geometric means. P values were calculated using the Mann-Whitney U test.
Association between filarial infection and up-regulation of IL-4 production in latent TB
Because filarial infections have been associated with the induction of parasite antigen–specific Th2 cytokines [2] and to determine the effect of coexisting filarial infections on the mycobacterial antigen–specific Th2 responses of individuals with positive tuberculin skin test results, we stimulated PBMCs from patients in the PPD+Fil− and PPD+Fil+ group with PPD, M. tuberculosis CFA, or anti-CD3 for 24 h and measured the levels of IL-4, IL-5, and IL-13. As with the Th1 cytokines, there were no significant alterations in the spontaneous or anti-CD3–stimulated production of IL-4, IL-5, or IL-13 (figure 2); however, PPD and M. tuberculosis CFA induced significantly increased production of IL-4 in the PPD+Fil+ group compared with the PPD+Fil− group (geometric mean of 53.12 pg/mL in the PPD+Fil+ group vs. 21.37 pg/mL in the PPD+Fil− group for PPD [P = .010] and of 55.33 vs. 9.66 pg/mL for M. tuberculosis CFA [P < .001]). Filarial infection had no effect on PPD-induced and M. tuberculosis–induced production of IL-5 and IL-13.
Figure 2.
Association between filarial infection and up-regulation of interleukin (IL)–4 in latent tuberculosis. Peripheral blood mononuclear cells from patients in the purified protein derivative (PPD)+Fil− group (n = 20; tuberculin skin test positive without active filarial infection) and the PPD+Fil+ group (n = 20; tuberculin skin test positive with active filarial infection) were stimulated with PPD, Mycobacterium tuberculosis culture-filtrate antigen (CFA), or anti-CD3 for 24 h, and type 2 T helper cytokine (IL-4, IL-5, and IL-13) levels were measured by enzyme-linked immunosorbent assay. Results are shown as net cytokine production over that in control medium. Bars indicate geometric means. P values were calculated using the Mann-Whitney U test.
Association between filarial infection and down-regulation of IL-17 and IL-23 production in latent TB
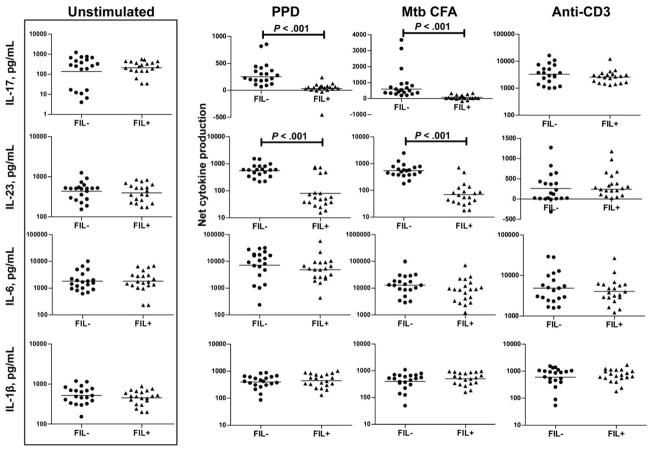
Because IL-17–producing CD4 T cells have been shown to play an important role in the human immune response to mycobacteria [19] and to determine the effect of coexisting filarial infections on the mycobacterial antigen–specific Th17 responses of individuals with positive tuberculin skin test results, we stimulated PBMCs from patients in the PPD+Fil− and PPD+Fil+ groups with PPD, M. tuberculosis CFA, or anti-CD3 for 24 h and measured the levels of IL-17 and its inducing cytokines (IL-23, IL-6, and IL-1β) by ELISA. As with the Th1 cytokines, there were no significant alterations in the spontaneous or anti-CD3–stimulated production of IL-17, IL-23, IL-6, or IL-1β (figure 3). In contrast, as shown in figure 3, PPD and M. tuberculosis CFA induced significantly decreased net production of IL-17 and IL-23 in the PPD+Fil+ group compared with the PPD+Fil− group (for IL-17, geometric mean of −10.58 pg/mL in the PPD+Fil+ group vs. 286.32 pg/mL in the PPD+Fil− group for PPD [P < .001] and of 16.66 vs. 630.5 pg/mL for M. tuberculosis CFA [P < .001]; for IL-23, geometric mean of 79.63 vs. 554.9 pg/mL for PPD [P < .001] and of 69.58 vs. 544.3 pg/mL for M. tuberculosis CFA [P < .001]). Filarial infection had no significant effect on the mycobacterial antigen–driven production of either IL-6 or IL-1β.
Figure 3.
Association between filarial infection and down-regulation of interleukin (IL)–17 and IL-23 in latent tuberculosis. Peripheral blood mononuclear cells from patients in the purified protein derivative (PPD)+Fil− group (n = 20; tuberculin skin test positive without active filarial infection) and the PPD+Fil+ group (n = 20; tuberculin skin test positive with active filarial infection) were stimulated with PPD, Mycobacterium tuberculosis culture-filtrate antigen (CFA), or anti-CD3 for 24 h, and IL-17, IL-23, IL-6, and IL-1β cytokine levels were measured by enzyme-linked immunosorbent assay. Results are shown as net cytokine production over that in control medium. Bars indicate geometric means. P values were calculated using the Mann-Whitney U test.
Association between filarial infection and increased expression of CTLA-4 and PD-1 in latent TB
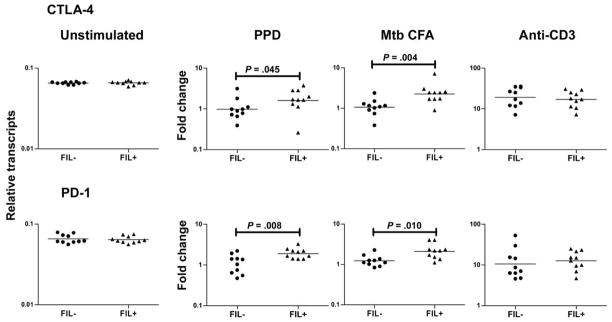
Because expression of CTLA-4 (and to a lesser extent PD-1) have been known to be up-regulated in filarial infections [3, 25] and to determine the role played by these negative costimulatory receptors in the filarial infection–mediated modulation of Th1 and Th17 responses in latent TB, we isolated RNA from cells stimulated with PPD, M. tuberculosis CFA, or anti-CD3 and measured the expression of CTLA-4 and PD-1. As shown in figure 4, we observed increased expression of PPD-induced and M. tuberculosis CFA–induced CTLA-4 and PD-1 messenger RNA (mRNA) in the PPD+Fil+ group compared with the PPD+Fil− group (for CTLA-4, geometric mean fold change of 1.599 in the PPD+Fil+ group vs. 0.9734 in the PPD+Fil− group for PPD [P = .045] and of 2.2267 vs. 1.059 for M. tuberculosis CFA [P = .004]; for PD-1, geometric mean fold change of 1.911 vs. 1.041 for PPD [P = .008] and of 2.105 vs. 1.235 for M. tuberculosis CFA [P = .010]). No difference in baseline or anti-CD3–induced levels of CTLA-4 and PD-1 mRNA was observed.
Figure 4.
Association between filarial infection and up-regulation of cytotoxic T lymphocyte antigen (CTLA)–4 and programmed death (PD)–1 messenger RNA in latent tuberculosis. Peripheral blood mononuclear cells from patients in the purified protein derivative (PPD)+Fil− group (n = 10; tuberculin skin test positive without active filarial infection) and the PPD+Fil+ group (n = 10; tuberculin skin test positive with active filarial infection) were stimulated with PPD, Mycobacterium tuberculosis culture-filtrate antigen (CFA), or anti-CD3 for 24 h, and CTLA-4 and PD-1 levels were measured by real-time reverse-transcription polymerase chain reaction. Results are shown as the fold change over that in control medium. Bars indicate geometric means. P values were calculated using the Mann-Whitney U test.
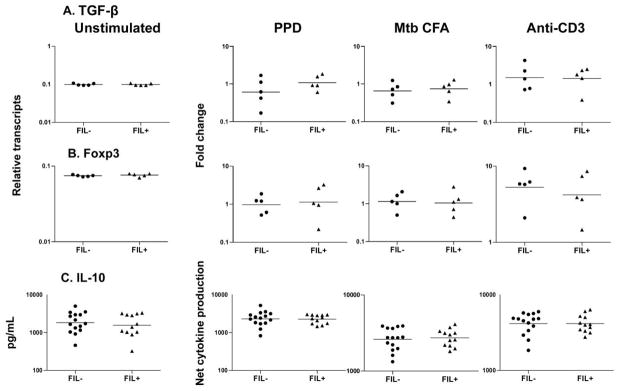
No association between filarial infection and alterations in TGF-β, IL-10, and Foxp3 expression in latent TB
To determine the role played by regulatory factors in filarial infection–mediated down-modulation of Th1 and Th17 responses in latent TB, we measured levels of TGF-β and Foxp3 mRNA by RT-PCR and protein levels of IL-10 by ELISA. As shown in figure 5, no alteration of PPD- and M. tuberculosis CFA-induced TGF-β, IL-10, or Foxp3 was observed in the PPD+Fil+ group compared with the PPD+Fil− group (for TGF-β, geometric mean fold change of 1.075 in the PPD+Fil+ group vs. 0.6072 in the PPD+Fil− group for PPD and of 0.7480 vs. 0.6554 for M. tuberculosis CFA; for IL-10, geometric mean of 2259 vs. 2304 pg/mL for PPD and of 2732 vs. 2618 pg/mL for M. tuberculosis CFA; for Foxp3, geometric mean fold change of 1.142 vs. 0.9770 for PPD and of 1.050 vs. 1.146 for M. tuberculosis CFA). In addition, blockade of TGF-β or IL-10 had no significant effect on cytokine production for patients in the PPD+Fil+ group (data not shown).
Figure 5.
No association between filarial infection and up-regulation of transforming growth factor (TGF)–β messenger RNA (mRNA), Foxp3 mRNA, and IL-10 cytokine levels in latent tuberculosis. Peripheral blood mononuclear cells from patients in the purified protein derivative (PPD)+Fil− group (n = 5–15; tuberculin skin test positive without active filarial infection) and the PPD+Fil+ group (n = 5–10; tuberculin skin test positive with active filarial infection) were stimulated with PPD, Mycobacterium tuberculosis culture-filtrate antigen (CFA), or anti-CD3 for 24 h, and TGF-β mRNA, Foxp3 mRNA, and IL-10 cytokine levels were measured by real-time reverse-transcription polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. Results are shown as fold change or net cytokine production over that in control medium. Bars indicate geometric means. P values were calculated using the Mann-Whitney U test.
Reversal of filarial-associated cytokine down-regulation due to CTLA-4 and PD-1 blockade in latent TB
To determine the functional relationship between the increased expression of CTLA-4 and PD-1 and the down-regulated Th1 and Th17 responses observed in the PPD+Fil+ group, we cultured cells from these patients with PPD or M. tuberculosis CFA in the presence of CTLA-4 or control immunoglobulin (figure 6) or in the presence of anti–PDL-1 or its isotype control (figure 7) and measured the levels of IFN-γ, IL-17, and IL-4 by ELISA at 24, 72, and 120 h. As shown in figure 6, we observed significant increases in PPD-induced and M. tuberculosis CFA–induced production of IFN-γ and IL-17 after 24 h of CTLA-4 blockade (for IFN-γ, geometric mean of 644.8 pg/mL in the presence of CTLA-4 immunoglobulin vs. 285.2 pg/mL in the presence of control immunoglobulin for PPD [P = .002] and of 512.5 vs. 238.1 pg/mL for M. tuberculosis CFA [P = .001]; for IL-17, geometric mean of 1504 vs. 801.4 pg/mL for PPD [P = .002] and of 876.1 vs. 465 pg/mL for M. tuberculosis CFA [P = .021]). CTLA-4 blockade had no significant effect on the production of IL-4. Culturing the cells for longer periods of time (72 and 120 h) made no difference (data not shown). Collectively, these data suggest that CTLA-4–mediated down-regulation of mycobacterial antigen–specific IFN-γ and IL-17 (but not IL-4) occurs in individuals coinfected with filaria and M. tuberculosis.
Figure 6.
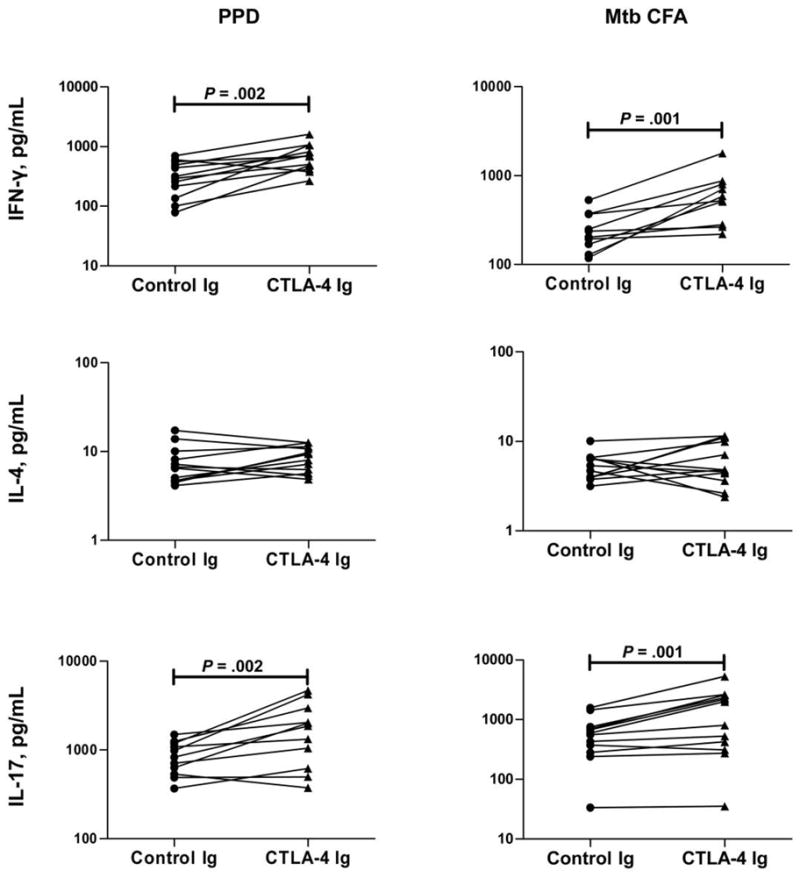
Up-regulation of interferon (IFN)–γ and interleukin (IL)–17 production due to cytotoxic T lymphocyte antigen (CTLA)–4 blockade in latent tuberculosis. Peripheral blood mononuclear cell production of IFN-γ, IL-17, and IL-4 after a 24-h stimulation with purified protein derivative (PPD) or Mycobacterium tuberculosis culture-filtrate antigen (CFA) in the presence of CTLA-4 or control immunoglobulin is shown as line graphs, with each line representing a single patient in the PPD+Fil+ group (n = 12; tuberculin skin test positive with active filarial infection). Results are shown as net cytokine production over that in control medium. P values were calculated using the Wilcoxon signed-rank test.
Figure 7.
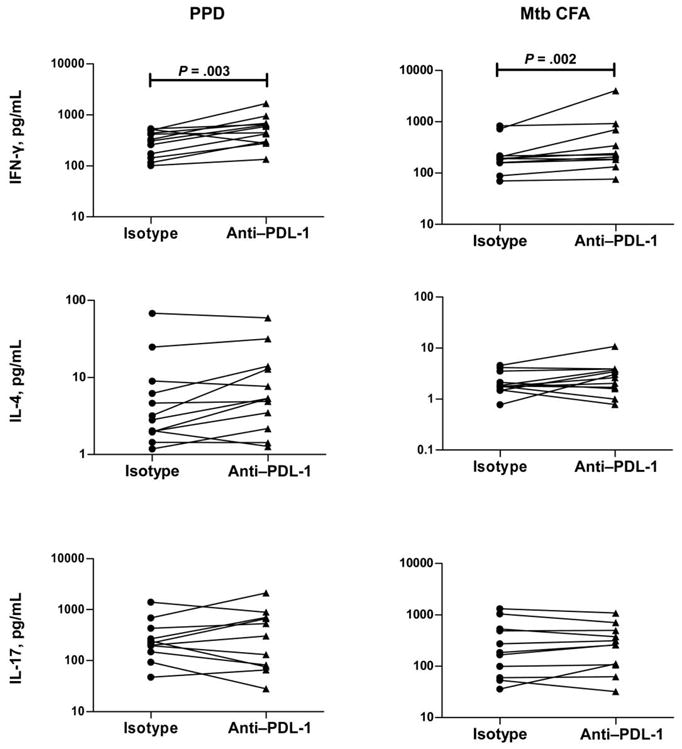
Up-regulation of interferon (IFN)–γ production due to programmed death (PD)–1 blockade in latent tuberculosis. Peripheral blood mononuclear cell production of IFN-γ, interleukin (IL)–17, and IL-4 after a 24-h stimulation with purified protein derivative (PPD) or Mycobacterium tuberculosis culture-filtrate antigen (CFA) in the presence of antibody to PDL-1 (the ligand for PD-1) or control antibody is shown as line graphs, with each line representing a single patient in the PPD+Fil+ group (n = 12; tuberculin skin test positive with active filarial infection). Results are shown as net cytokine production over that in control medium. P values were calculated using the Wilcoxon signed-rank test.
Of interest, the presence of antibody to PDL-1, which is known to block the PD-1/PDL-1 pathway, also caused significant increases in the PPD-induced and M. tuberculosis CFA–induced production of IFN-γ (geometric mean of 481.2 pg/mL in the presence of anti–PDL-1 vs. 275.4 pg/mL in the presence of control antibody for PPD [P = .003] and of 297 vs. 192.3 pg/mL for M. tuberculosis CFA [P = .002]) but not of IL-17 or IL-4 among patients in the PPD+Fil+ group at 24 h (figure 7) and at 72 and 120 h (data not shown), indicating that the PD-1 pathway also plays an important role in the IFN-γ down-regulation observed in individuals coinfected with filaria and M. tuberculosis.
In addition, blockade of CTLA-4 or PD-1 did not significantly enhance the production of IFN-γ and IL-17 among patients in the PPD+Fil− group (data not shown), indicating that these pathways are specifically modulated by filarial infections.
DISCUSSION
Because immune-mediated protection against M. tuberculosis is characterized by strong mycobacterium-specific Th1 responses, it has been postulated that coincident infections with helminth parasites could modulate these immune responses by driving Th2 and/or regulatory T cells that induce anti-inflammatory responses. Indeed, previous studies have shown that the intestinal helminth coinfection is accompanied by lowered in vitro production of IFN-γ and elevated production of IL-10 in individuals with active pulmonary TB [26]. The immunogenicity of BCG vaccination is impaired in helminth-infected individuals, and this is associated with enhanced TGF-β production but not enhanced Th2 responses [27]. In addition, the incidence of lepromatous leprosy (associated with profound mycobacteria-specific anergy) was twice as high in areas where onchocerciasis, another filarial infection, was coendemic [28]; moreover, onchocerciasis has also been shown to modulate immune responses to mycobacterial antigens in children [29]. In a murine model, coinfection with Mycobacterium avium and Schistosoma mansoni resulted in decreased Th1 responses to the mycobacteria in vivo [30]. Furthermore, exposure to filarial parasites modulates the function of human dendritic cells in response to virulent M. tuberculosis infection in vitro [31].
Here, examination of antimycobacterial immune responses in patients in the PPD+Fil+ group revealed interesting differences compared with those in the PPD+Fil− group. First, IFN-γ and IL-12 were significantly down-regulated in patients in the PPD+Fil+ group, suggesting that the IL-12/INF-γ pathway in patients with coincident lymphatic filariasis and latent TB is compromised. This has important clinical relevance, in that it is well known that mutations in the IL-12/IFN-γ/Stat1 pathway can lead to disseminated TB and atypical mycobacterial infections in humans [21]; in addition, mice deficient in IL-12 and/or IFN-γ are more susceptible to M. tuberculosis infection than their wild-type controls [18]. IFN-γ is the central effector molecule in the macrophage elimination of bacteria, in that it induces increases in reactive nitrogen and oxygen compounds responsible for bactericidal activity as well as being central in the induction of autophagy, a process recently documented to play a critical role in eliminating mycobacteria within dendritic cells and macrophages [32]. Theoretically, the diminished production of IL-12 and IFN-γ in patients with filarial infection could have a detrimental effect on the control of latent TB and render coinfected individuals more susceptible to developing active disease.
Filarial infections are associated with increased production of IL-4. This cytokine, in addition to inhibiting Th1 responses, has been shown to cause alternative activation of macrophages, resulting in increased arginase-1 and decreased Nos2 activity and reduced nitric oxide production [33]. In addition, IL-4 plays an important role in abrogating autophagy and autophagy-mediated killing of M. tuberculosis in macrophages [34]. Finally, increased IL-4 production by CD8 T cells and γδ T cells has been associated with subsequent development of active TB in healthy contacts of patients with active TB [35]. Thus, the increased IL-4 production seen in filarial infections is another possible mechanism by which there is an increased risk of promoting the development of active TB in coinfected individuals.
In the present study, the presence of an active filarial infection was also associated with diminished production of IL-17 and IL-23 in response to mycobacterial antigens. Although Th17 cells are not as important as IFN-γ–producing Th1 cells in mediating protection against primary M. tuberculosis infection, IL-17 is critical in the induction of the memory response and the mediation of protection against challenge infections and during vaccinations [19]. The lack of M. tuberculosis–induced up-regulation of IL-17 in patients who have latent TB and coexisting filarial infections may be another factor predisposing these individuals to the risk of developing active TB.
Filarial parasites establish persistent infections, and their chronicity is facilitated by mechanisms that dampen immune responses—notably, IL-10 and TGF-β [36]. When we examined the induction of IL-10, TGF-β, and Foxp3 in response to mycobacterial stimulation in individuals who had latent TB with or without filarial infection, there were no significant differences in the production or expression of any of these mediators related to filarial infection status, suggesting that the modulated Th1 and Th17 anti–M. tuberculosis response was not a function of altered IL-10, TGF-β, or Foxp3 expression.
Studies have demonstrated not only that up-regulation of CTLA-4 and PD-1 occurs in chronic infections [37] but also that CTLA-4 and PD-1 expression correlates with T cell exhaustion in viral infections associated with defects in cytokine production, cytotoxicity, and proliferative capacity. CTLA-4 and PD-1 blockade mediates reversal of exhaustion and causes control of viral replication [38, 39]. We and others have shown that filarial infections are associated with up-regulation of CTLA-4 and PD-1 [3, 25]. To elucidate the role played by these coinhibitory receptors in filaria-TB coinfections, we blocked the interaction of CTLA-4 and PD-1 with their ligands and demonstrated that we could reverse the down-regulation of mycobacteria-specific cytokine responses in filaria-infected individuals positive for PPD. Although CTLA-4 blockade significantly increased the production of both Th1 and Th17 cytokines, PD-1 blockade significantly enhanced production only IFN-γ. The difference in induction of cytokine responses by the 2 coinhibitory molecules is likely related to the differences in their expression pattern and kinetics.
There is a great deal of geographic overlap in the regions of high filarial endemicity and susceptibility to TB. Our study clearly demonstrates that coincident filarial infections modulate immune responses to M. tuberculosis. Filaria-induced regulatory networks (specifically CTLA-4 and PD-1) appear to exert a significant bystander/spillover effect on immune responses against mycobacterial antigens in patients with latent TB, with attendant down-regulation of IFN-γ and IL-17 and their upstream cytokines, IL-12 and IL-23, respectively. Although the regulation presumably originates from antigen-presenting cells (because of lowered IL-12 and IL-23 levels), this effect can be reversed at the level of the T cell, given that blockade of either CTLA-4 or PD-1 (both of which act at the T cell level) can overcome the suppression. Our findings not only highlight an increased understanding of the factors that may contribute to the breaking of latency in M. tuberculosis infection but also have significant implications for vaccine efficacy in helminth-endemic countries. Vaccines requiring a Th1 or Th17 response for efficacy may not function optimally in the presence of helminth coinfection. Understanding the pathways (e.g., CTLA-4/PD-1) that helminth infections use to mediate bystander suppression or modulation to exogenous antigens and infections should enable new strategies to antagonize suppression for controlling deleterious infections and optimal boosting of vaccine efficacy.
Acknowledgments
We thank National Institutes of Allergy and Infectious Diseases intramural editor Brenda Rae Marshall for editorial assistance and the Tuberculosis Research Center, Epidemiology Unit, Tiruvallur, for valuable assistance in recruiting the patients for this study.
Financial support: Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Nutman TB, Kumaraswami V. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 2001;23:389–99. doi: 10.1046/j.1365-3024.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- 3.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–56. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 4.Nutman TB, Kumaraswami V, Ottesen EA. Parasite-specific anergy in human filariasis. Insights after analysis of parasite antigen-driven lymphokine production. J Clin Invest. 1987;79:1516–23. doi: 10.1172/JCI112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semnani RT, Nutman TB. Toward an understanding of the interaction between filarial parasites and host antigen-presenting cells. Immunol Rev. 2004;201:127–38. doi: 10.1111/j.0105-2896.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper PJ, Chico M, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–80. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper PJ, Chico ME, Losonsky G, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182:1199–206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–8. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 9.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun. 2004;72:2598–604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, Britton S. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis. Vaccine. 2005;23:1326–34. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Smits HH, Hartgers FC, Yazdanbakhsh M. Helminth infections: protection from atopic disorders. Curr Allergy Asthma Rep. 2005;5:42–50. doi: 10.1007/s11882-005-0053-5. [DOI] [PubMed] [Google Scholar]
- 12.Smits HH, Yazdanbakhsh M. Chronic helminth infections modulate allergen-specific immune responses: protection against development of allergic disorders? Ann Med. 2007;39:428–39. doi: 10.1080/07853890701436765. [DOI] [PubMed] [Google Scholar]
- 13.Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune-mediated inflammation. Int J Parasitol. 2007;37:457–64. doi: 10.1016/j.ijpara.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. doi: 10.1385/CRIAI:26:1:35. [DOI] [PubMed] [Google Scholar]
- 15.Fox JG, Beck P, Dangler CA, et al. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 16.Brutus L, Watier L, Hanitrasoamampionona V, Razanatsoarilala H, Cot M. Confirmation of the protective effect of Ascaris lumbricoides on Plasmodium falciparum infection: results of a randomized trial in Madagascar. Am J Trop Med Hyg. 2007;77:1091–5. [PubMed] [Google Scholar]
- 17.Lyke KE, Dicko A, Dabo A, et al. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005;73:1124–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–88. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrington WR, Hawn TR. Mycobacterium tuberculosis, macrophages, and the innate immune response: does common variation matter? Immunol Rev. 2007;219:167–86. doi: 10.1111/j.1600-065X.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland SM. Interferon γ, IL-12, IL-12R and STAT-1 immunodeficiency diseases: disorders of the interface of innate and adaptive immunity. Immunol Res. 2007;38:342–6. doi: 10.1007/s12026-007-0045-8. [DOI] [PubMed] [Google Scholar]
- 22.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261–9. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann SH. Tuberculosis: back on the immunologists’ agenda. Immunity. 2006;24:351–7. doi: 10.1016/j.immuni.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishna S, Frieden TR, Subramani R. Association of initial tuberculin sensitivity, age and sex with the incidence of tuberculosis in South India: a 15-year follow-up. Int J Tuberc Lung Dis. 2003;7:1083–91. [PubMed] [Google Scholar]
- 25.Steel C, Nutman TB. CTLA-4 in filarial infections: implications for a role in diminished T cell reactivity. J Immunol. 2003;170:1930–8. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 26.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert Rev Anti Infect Ther. 2007;5:475–84. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 28.Prost A, Nebout M, Rougemont A. Lepromatous leprosy and onchocerciasis. Br Med J. 1979;1:589–90. doi: 10.1136/bmj.1.6163.589-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, Bradley JE. Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol. 1999;117:517–23. doi: 10.1046/j.1365-2249.1999.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacco R, Hagen M, Sandor M, Weinstock JV, Lynch RG. Established TH1 granulomatous responses induced by active Mycobacterium avium infection switch to TH2 following challenge with Schistosoma mansoni. Clin Immunol. 2002;104:274–81. doi: 10.1006/clim.2002.5263. [DOI] [PubMed] [Google Scholar]
- 31.Talaa KR, Bonawitz RE, Domenech P, Nutman TB. Preexposure to live Brugia malayi microfilariae alters the innate response of human dendritic cells to Mycobacterium tuberculosis. J Infect Dis. 2006;193:196–204. doi: 10.1086/498912. [DOI] [PubMed] [Google Scholar]
- 32.Vergne I, Singh S, Roberts E, et al. Autophagy in immune defense against Mycobacterium tuberculosis. Autophagy. 2006;2:175–8. doi: 10.4161/auto.2830. [DOI] [PubMed] [Google Scholar]
- 33.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 34.Harris J, De Haro SA, Master SS, et al. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–17. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Ordway DJ, Pinto L, Costa L, et al. γλ T cell responses associated with the development of tuberculosis in health care workers. FEMS Immunol Med Microbiol. 2005;43:339–50. doi: 10.1016/j.femsim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 36.King CL, Mahanty S, Kumaraswami V, et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis: preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Invest. 1993;92:1667–73. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 38.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–54. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]